Abstract
In forensic entomology the estimation of the minimum postmortem interval and any other evaluation depend on the accuracy of the species identification. Despite the most common species received a lot of attention in the last years, other species belonging to less common families still remain poorly studied and sometimes their immature stages are not described. The finding of Physyphora alceae (Preyssler, 1791) (Diptera, Ulidiidae) in a decapitated cadaver in Northern Italy allowed the description of the III instar larvae and of the puparium of this species. Further molecular analysis allowed the confirmation of the correct species identification and the monophyly of the genus. In general, phylogenetic analysis confirmed the monophyly of the family, however some issues arised on the real monophyly of the genus Homalocephala. This paper highlights the need of a combination of morphological and molecular identification of the species especially when immatures are collected.
Introduction
In forensic entomology a correct species identification is the conditio sine qua non to answer the investigative questions concerning the peri and post mortem events. To achieve this goal, the morphological approach involving the use of dichotomous keys is the traditional and most used method among the taxonomists. However, sometimes it can be obstructed by the lack of experts and by the limited information concerning the immature stages (i.e. larvae and puparia) (Giordani et al. Citation2018, Citation2019a; Giordani & Vanin Citation2020). Nevertheless, even if specific identification keys are available, a poor state of preservation of the specimens may negatively affect the morphological analysis thus preventing the accurate identification of the samples (Bortolini et al. Citation2018; Pradelli et al. Citation2019).
To overcome such circumstances, from the second half of the 1980s, molecular biology has become a support tool for species identification based on the sequencing of genetic targets proved to be conserved enough to allow the design of universal and specific primers (Folmer et al. Citation1994), and diverse enough to allow species discrimination.
It is worth mentioning that much research has been dedicated to the most common taxa of forensic interest, especially in the families Calliphoridae, Sarcophagidae and Muscidae, whereas little effort has been directed towards less common taxa (Tuccia et al. Citation2016a). These “secondary taxa” collected from a corpse could as well provide important information (Smith Citation1986; Sessa et al. Citation2019; Giordani et al. Citation2019b; Giordani & Vanin Citation2020).
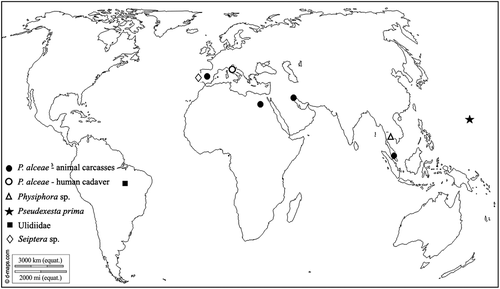
This paper provides the description of the immature stages of the “picture-winged fly” Physiphora alceae (Preyssler, 1791) (Diptera: Ulidiidae) collected from a human cadaver found in Northern Italy. This work wants to contribute in reducing the risk of misidentification of this species that has been reported from carcasses from all over the world () but has been poorly investigated in its forensic applications. The phylogenetic reconstruction for identification purposes based on molecular analysis is also provided.
Figure 1. Records of P. alceae and other Ulidiidae sampled during experiments carried out for forensic purposes (world map from © 2007–2018 d-maps.com)
Case description
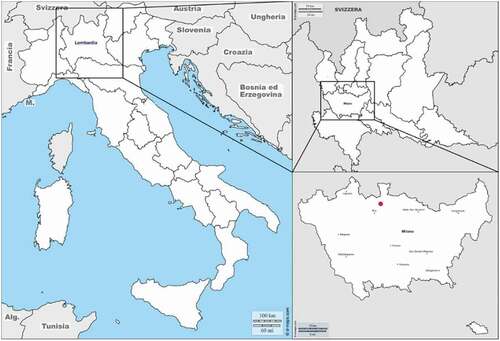
At the beginning of September 2017, the decapitated body of a woman, that was reported missing two months before, was found in a small town within the metropolitan area of Milan (Northern Italy) () in a urban context surrounded by forest and agricultural areas. The body was beheaded, buried in an artificial grave and covered with plastic sheets. A few days later, the missing skull was found in a plastic bag a few kilometers far from the body. Both parts of the cadaver were found in advance decay and partially skeletonized (Caccianiga et al. Citation2020; Mazzarelli et al. Citation2021). The analysis of the insect communities revealed two different colonisation patterns, one for the head composed by species in the families Calliphoridae, Muscidae, Fanniidae and Phoridae whereas the body was colonised by species belonging to the Muscidae, Stratiomyidae, Phoridae and Ulidiidae families.
Figure 2. Metropolitan area of Milan (Italy). The red spot indicates the place where the cadaver of the woman was found (figure modified from © 2007–2018 d-maps.com)
The absence of Calliphoridae, first colonisers of exposed cadavers, suggested the hypothesis of the concealing of the cadaver after the death. Based on the temperature and on the developmental rates of the necrofagous insects collected, an estimation of a minimum Post Mortem Interval (minPMI) in the range of 35–46 days was provided.
Materials and methods
Sample preparation and morphological identification
The adults and the immature stages of the flies (Diptera) were analysed using a Keyence VHX-S90BE digital microscope, equipped with Keyence VH-Z250R and VH-Z20R lens and VHX-2000 Ver. 2.2.3.2 software (Keyence, Japan).
Identification keys from Kameneva and Korneyev (Citation2010) were used to investigate the morphology of the Ulidiidae adults’ specimens, further confirmed by comparison with the collection of the NHM of London and through molecular analysis.
In order to better visualise the diagnostic characters, larvae were diaphanised using a NaOH solution (Tuccia et al. Citation2016b). As no DNA analysis were intended to be performed on puparia, they were carefully cleaned in acetic acid solution and air-dried at room temperature (Pradelli et al. Citation2021). The description of the external characters follows the nomenclature as in Giordani et al. (Citation2018) while the morphology of the cephalopharyngeal skeleton follows McAlpine et al. (Citation1987) and Bjerke et al. (Citation1992).
Molecular identification
Total DNA was extracted from larval and adult specimens using the DNA Investigator Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Prior to extraction, the larva was processed as described in Tuccia et al. (Citation2016b) and incubated overnight in a solution of Proteinase K (0.1 mg/ml final concentration) (Promega, Madison, Wisconsin, USA) and ATL Buffer. A region of the standard barcoding region of the mitochondrial COI gene (658 bp) was amplified using universal LCO-1490 and HCO-2198 primers (Folmer et al. Citation1994). A total reaction volume of 20 μl was prepared using Promega GoTaq® Flexi Polymerase protocol: 4 μl of Colourless GoTaq Flexi Buffer (5×), 2 μl of MgCl2 (25 mM), 0.5 μl of each primer (10 pmol/μl), 0.5 μl of dNTPs Mix (10 mM), 0.25 μl GoTaq DNA Polymerase (5 u/μl) and 2 μl of DNA template. The reactions, including positive and negative controls, were assembled under a Purair PCR-36 laminar flow cabinet (AirScience®, Florida, USA) in order to prevent cross contaminations between samples.
The thermal cycler BioRad C1000 (Bio-Rad Laboratories, Inc.) was used to perform the amplification setting the following program: initial heat activation step at 95°C for 10 min, 35 cycles of 95°C for 1 min, 49.8°C for 1 min, 72°C for 1 min and a final extension step at 72°C for 10 min. Positive and negative DNA templates were used as controls for the reaction. The quality of the reactions was qualitatively assessed by 1.5% (w/v) agarose gel electrophoresis stained with Midori Green Advanced DNA Stain Geneflow Ltd., Lichfield, UK). Amplicons were purified using QIAquick PCR Purification Kit® (Qiagen, Hilden, Germany) following the manufacturer’s instructions, eluted in 40 μl of EB and sequenced by Eurofins Genomics (Ebersberg, Germany). The identity of the sequences was searched by BLAST® (Altschul et al. Citation1990). The nucleotides sequences generated have been uploaded on GenBank [access number: MH686505, MH686506].
Phylogenetic analysis
Fifty-four mtCOI sequences of Ulidiidae (Diptera) with a length over 580 bp were downloaded from BOLD and GenBank databases including 20 sequences of the subfamily Ulidiinae and 34 sequences of the subfamily Otitinae. The same species analysed by Galinskaya and collaborators (Citation2014) in their study of the phylogeny of the family were chosen. Within Ulidiinae, 4 species belonging to Physiphora Fallén, 1810, 2 species belonging to Ulidia Meigen, 1826, 3 species belonging to Timia Wiedemann, 1824, and 3 species belonging to Homalocephala were selected (). Within Otitinae, 3 species belonging to Ceroxys Macquart 1835, 2 species of Otites Latreille, 1804, 2 species belonging to Herina Robineau-Desvoidy, 1830, 4 species belonging to Meliera Becker in 1903, and the species Myennis octopuntata (Coquebert, 1798), Pseudotephritis corticalis (Loew, 1873), Seioptera vibrans (Linnaeus, 1758), and Tetanops sintenisi Becker, 1909 were chosen (). In addition, Psila fimetaria (Linnaeus, 1761) and three species of Drosophila Fallén, 1823 (Drosophila melanogaster Meigen, 1830, Drosophila simulans Sturtevant, 1919, and Drosophila viriles Sturtevant, 1916) were chosen as outgroups (Galinskaya et al. Citation2014).
Table I. Species of Ulidiidae used in the phylogenetic analysis
Table II. mtCOI barcoding sequences used for phylogenetic analysis. Sequences are listed per species, BOLD/GenBank accession number, and geographical origin. * = sequences from this study
Table III. Diptera species from the crime scenes. Pc = puparium closed, Po = puparium open, LIII = third instar larva, A = adult
Two sequences of P. alceae obtained from this work were included to create a final dataset of 61 sequences () that were aligned using the ClustalW software in MEGA7 (Kumar et al. Citation2016). The GTR+F + I+ G4 model was chosen as substitution model among 286 implementing the ModelFinder tool on IQ-Tree v.1.6.10 (Nguyen et al. Citation2015). The best-fit model was chosen based on the highest w-AICc (+0.9647) and on the lowest BIC (13107.8044) values and used to build a Maximum Likelihood phylogentic reconstruction. One thousand bootstrap replicates were inferred setting the ultrafast bootstrap parameter (Hoang et al. Citation2018).
Results
Six dipteran species were identified within the entomological assemblage collected from the cadaver of the woman (), however, according to the purposes of this article only Physiphora alceae (Preyssler, 1791) (Ulidiinae) is described in detail being recorded from a cadaver in Italy for the first time. The other taxa are already known from cadavers and reported from Italian forensic cases or archaeological contexts (Turchetto et al. Citation2001; Turchetto & Vanin Citation2004; Bugelli et al. Citation2015; Giordani et al. Citation2018, Citation2019a, Citation2019b; Giordani & Vanin Citation2020).
Adult description
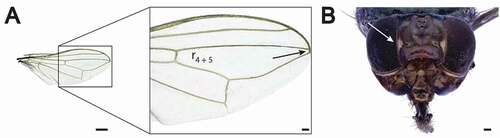
The adults of this species were described in detail by Kameneva and Korneyev (Citation2010). Adult flies can be easily distinguished from the other species because of the colour of their legs, the wing shape and the microtrichose pattern on the frons ()). The legs are black except for the foreleg with creamy-yellow metatarsus ()), midtarsus and hindtarsus ()).
Figure 3. Physiphora alceae adult. (a) dorsal, (b) lateral, (c) ventral view. The black arrow indicates the foreleg with the creamy-yellow metatarsus. Scale bar: 1 mm

Wings are totally hyaline with the cell r4 + 5 almost closed similar to Physiphora longicornis (Hendel, 1909) (Chen & Kameneva Citation2007) ()). Two narrow microtrichose patches on each side of the frons represent a diagnostic character of the species ()).
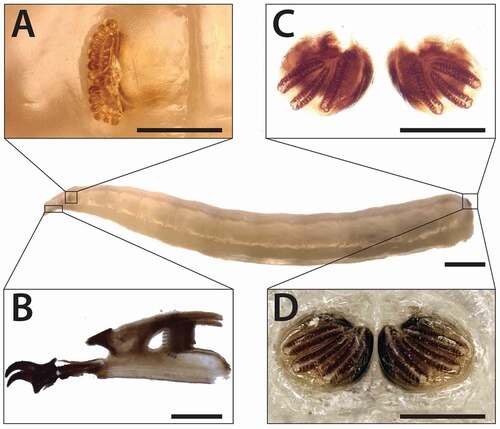
III instar larva description
The body length of third instar larvae in the analysed sample was 8.2 ± 0.3 mm (N = 3) ()). Anterior spiracle is composed by short, flattened stalk bearing 13–15 nodular branches ranged fan-wise with in some specimens a slight median introflection ()). The posterior spiracles, not protruding from the spiracular disc, are very close to each other and show an ovoid-like shape with a thin, dark-brown and close peritreme (difficult to see in the diaphanised specimens) ()). The spiracular scar is not included in the peritreme and is located in the dorsal side of each spiracle. The three respiratory slits have an arcuate radial arrangement and converge towards the spiracular scar. Slits one and two are specular and slightly curving one towards the other at the lower extremity ().
Figure 5. Physiphora alceae III instar larva morphology. (a) anterior spiracle, (b) cephaloskeleton and (c,d) posterior spiracles. Anatomic details have been observed on III instar specimens stored in EtOH 70%. (a, d) and after diaphanisation of soft tissue in NaOH solution (c, b). Scale bars: 1 mm (full length larva), (a) 200 μm (b), 100 μm (a,c,d)
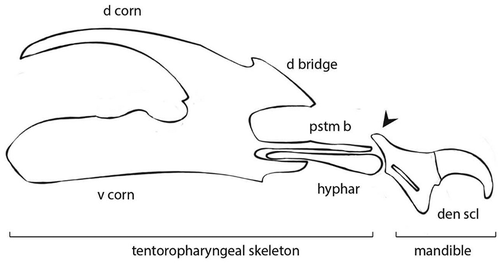
The cephalopharyngeal skeleton varies from light to dark brown in colour and measures, in the analysed specimens 0.85 and 0.28 mm, as maximum length and maximum width respectively (). The mandibles, well sclerotised, have two sharply hooked teeth and a sharp robust dental sclerite. The basal part of the mandible has a robust process postero-dorsally oriented. The parastomal bar and hypopharyngeal sclerite are elongated and slender. Dorsal cornu of the tentoropharyngeal sclerite is arched, slender, large 1/3 of the ventral cornu, without window. The dorsal (anterior) bridge is robust and well sclerotised. The ventral part of the ventral cornu is straight ().
Figure 6. Schematic representation of the cephalopharyngeal skeleton of P. alceae III instar larva. Mandible is composed of dental sclerite (den scl) and its basal part shows a robust process postero-dorsally oriented (black arrow). The hypopharyngeal sclerite (hyphar) links the mandible to the tentoropharyngeal skeleton composed of parastomal bar (pstm b), dorsal bridge (d bridge), dorsal corn (d corn) and ventral corn (v corn)
Puparium description
Puparium. Ochre in colour, measures 4–5 mm in length ()). The anterior extremity of the puparium seen from a lateral view shows a tapered shape ()). Posterior spiracles are very close ()). The same characters observed for the III instar larva are also well conserved in the puparium morphology: oval-rounded posterior spiracles, spiracle scar in the dorsal side and excluded by the peritreme which is complete, thick and varies from dark-brown to black in colour, radial oriented respiratory slits ()). The anterior spiracles are composed of twelve yellowish finger-like lobes. The intersegmental spines of ventral welt of the seventh abdominal segment are distributed in six ordinate and continuous rows except for the third one where spines are grouped by five or six ()). The majority of the spines extend forward towards the anal plate but some others are back oriented. In the two rows closest to the anal division spines are large, generally with single rounded tip while finer, shorter and sharp tipped spines form the remaining rows ()).
Molecular analysis and phylogenetic reconstruction of the Ulidiidae
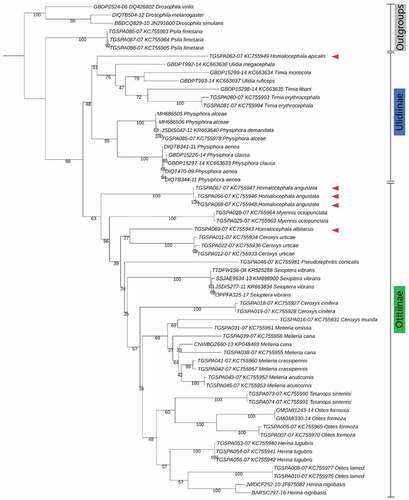
The morphological identification was confirmed by the sequencing of a region of the COI barcoding target. The ClustalW alignment produced a dataset of 61 sequences of equal length (520 nucleotide positions) and counting 307 constant sites and 205 informative sites. The ML tree reported in has a Log-likelihood of −6098.9844. The distinction between the two subfamilies Ulidiinae and Otitinae is well supported by the molecular phylogenesis (BS: 98). The node between the groups Timia-Ulidia and Physiphora is weakly supported by a BS of 48, and both of them are monophyletic. In the first group, no further separation was observed in regards to superior taxonomic levels (genus, species). Within the subfamily Otitinae, the monophyly of the genera is generally well supported while no further well supported clusters distinction can be argued. Additionally, the genus Homalocephala shows an unresolved position. In fact, sequences of species within this genus cluster both in the Ulidiinae (H. apicalis) and in the Otitinae (H. albitarsis and H. angustata) subfamilies, thus showing that the taxonomic resolution of this genus remains unresolved ().
Figure 8. Maximum Likelihood phylogenetic tree. The phylogenetic tree was derived by the alignment of 61 mtCOI barcoding sequences of equal length (520 bp). The displayed topology was obtained upon the selection the best substitution model (Log-likelihood = −6098.9844). The evolutionary distance between Ulidiinae and Otitinae is strongly supported (BS = 98). Red arrows point out to the Homalocephala spp
Discussions
Ecological and forensic observations
The analysis of the necrophagous insect’s community assemblage found on the cadaver in the metropolitan area of Milan (Italy) revealed the presence of the “picture-winged” fly P. alceae, belonging to the subfamily Ulidiinae, which is mainly a tropical taxon (Marshall Citation2012).
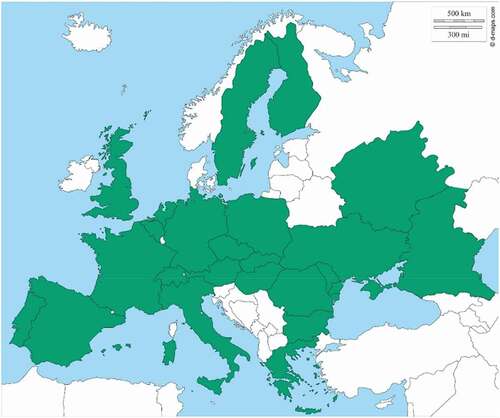
The genus Physiphora Fallén, 1810 includes 16 species widespread in the tropical, subtropical and temperate areas, with the majority of the species inhabiting the Afrotropical regions (Chen & Kameneva Citation2007). In particular, P. alceae is distributed in all the biogeographical regions (Kameneva & Korneyev Citation2010) and in Europe, based on the Fauna Europaea data (https://fauna-eu.org/), it is widespread in the majority of the countries with the exception of the Northern countries such as Iceland, Ireland, Norway, Baltic region, and the west coast of the Balcanic region (). Likely, the absence of P. alceae in the Southern Balcanic regions is potentially due to missing records, as it often occurs.
Figure 9. European distribution of P. alceae. Green: present, white: absent. (Data from Fauna Europaea, https://fauna-eu.org/; figure modified from © 2007–2018 d-maps.com)
In the last 10 years, P. alceae has been found in association with animal carcasses during experiments conducted on field for forensic purposes, while the Italian record here presented seems to be the first case ever related to a human cadaver ().
Table IV. Records of P. alceae and other Ulidiidae sampled during experiments carried out for forensic purposes
The finding of P. alceae on decomposing corpses is in line with the fact that the larvae develop in dung, decaying vegetable matter and compost heaps (Ebejer Citation2015). Most of the details of the biology of this species, however, still remains unclear.
Physiphora alceae as member of the family Ulidiidae can be considered as a taxon that is occasionally present in the crime scenes, together with other Diptera families, such as Heleomyzidae, Trichoceridae, Psycodidae, Drosophilidae, and Milichiidae (Sessa et al. Citation2019; Giordani et al. Citation2019b).
Based on the most recent literature (), it seems that this fly is becoming more and more interesting in forensic entomology, but its potential role is still under investigation. On one hand the morphology of the adults can deceive, as some of the morphological characters are similar to other close-related species (e.g. Physiphora longicornis (Hendel, 1909) (Chen & Kameneva Citation2007)). However, there are no available descriptions of the immature stages that are typically found on a crime scene or on the body. Only a few descriptions of larvae and puparia of Ulidiidae have been published (Bohart & Gressitt Citation1951) but none concerns any species of potential forensic interest or anyway associated with carcasses and cadavers.
In light of these observations, the finding of immature stages of P. alceae in this case offered the opportunity to accurately describe the morphology of the third instar larva and the puparium. In addition, as completion of this investigation, a molecular approach was used to confirm the morphological identification, as a standard practice during the forensic entomology analysis, and to enrich the public database (the mtCOI barcoding sequences are now available on GenBank with the accession numbers MH686505 and MH686506).
Phylogenetic analysis
The molecular results were further corroborated through the phylogenetic approach, mainly referring to the work of Galinskaya et al. (Citation2014) who exhaustively outlined the DNA barcoding of Palearctic Ulidiidae and made a comparison with the morphological data of the family. In contrast to their dataset, a smaller dataset of COI sequences (61) was used, but it was specifically composed of sequences of equal length in order to provide sufficient information for the phylogenetic reconstruction and reduce any potential length bias. In addition, the nucleotide substitution model has been automatically selected using ModelFinder tool on IQ-Tree v.1.6.10 (Nguyen et al. Citation2015), and, unlike the above mentioned authors, testing different substitutions models fell out of the purposes of this work. Our results strongly support the monophyly of the groups Ulidiinae and Otitinae (BS 98, ). The observations related to the species separation are in line with what has been already underlined as well as for the genus Homalocephala. So far, the taxonomic resolution of this genus has not been well defined (Galinskaya et al. Citation2014) due to the high similarity of its morphological features shared between both Ulidiinae and Otitinae species. In addition, the phylogenesis based on the barcoding target seems not to resolve this debate among taxonomists, suggesting that other DNA targets should be tested to increase the resolution power of this analysis. According to our results the species H. angustata and H. albitarsis fall in the cluster of Otitinae while H. apicalis is included in the Ulidiinae cluster. However, this cannot be further elucidated due to the limited number of species of Homalocephala genus and species sequences (3 and 5, respectively) used in this analysis and available in the databases.
Conclusions
The collection of P. alceae from a human cadaver in Northern Italy and its findings on animal carcasses used for decomposition experiments support the hypothesis of a role of this species also as carrion-feeders and open a new prospective of its implication for forensic purposes despite further research about its developmental rate and arrive time on a body are needed.
This work highlights also that the identification of flies of forensic interest necessarily requires the morphological analysis of the specimens which can be confirmed and highly supported by the genetic analysis. Also, when dealing with immatures, none of the two approaches is sufficiently reliable to be used independently by excluding the other a priori, therefore it is highly recommended to use them in combination as previous proposed also for other species (Tuccia et al. Citation2016b).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Mesbah H, Moffatt C, El-Azazy OM, Majeed QA. 2012. The decomposition of rabbit carcasses and associated necrophagous Diptera in Kuwait. Forensic Science International 217:27–31. DOI: 10.1016/j.forsciint.2011.09.021.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215:403–410. DOI: 10.1016/S0022-2836(05)80360-2.
- Baz A, Botías C, Martín-Vega D, Cifrián B, Díaz-Aranda LM. 2015. Preliminary data on carrion insects in urban (indoor and outdoor) and periurban environments in central Spain. Forensic Science International 248:41–47. DOI: 10.1016/j.forsciint.2014.12.012.
- Bjerke J, Anderson A, Freeman T. 1992. Morphology of the larval stages of Tetanops myopaeformis (Röder)(Diptera: Otitidae). Journal of the Kansas Entomological Society 65(1):59–65.
- Bohart GE, Gressitt JL. 1951. Filth-inhabiting flies of Guam. Vol. 204. Honolulu, Hawaii: Bernice P. Bishop Museum.
- Bortolini S, Giordani G, Tuccia F, Maistrello L, Vanin S. 2018. Do longer sequences improve the accuracy of identification of forensically important Calliphoridae species? PeerJ 6:e5962–e5962. DOI: 10.7717/peerj.5962.
- Bugelli V, Forni D, Bassi LA, Di Paolo M, Marra D, Lenzi S, Toni C, Giusiani M, Domenici R, Gherardi M, Vanin S. 2015. Forensic entomology and the estimation of the minimum time since death in indoor cases. Journal of Forensic Sciences 60:525–531. DOI: 10.1111/1556-4029.12647.
- Caccianiga M, Caccia G, Mazzarelli D, Salsarola D, Poppa P, Gaudio D, Cappella A, Franceschetti L, Tambuzzi S, Maggioni L, Cattaneo C. 2020. Common and much less common scenarios in which botany is crucial for forensic pathologist and anthropologists: A series of eight case studies. International Journal of Legal Medicine. Epub ahead of print. PMID: 33341910. DOI: 10.1007/s00414-020-02456-0.
- Chen X-L, Kameneva EP. 2007. A review of Physiphora Fallén (Diptera: Ulidiidae) from China. Zootaxa 1398:15–28. DOI: 10.11646/zootaxa.1398.1.2.
- Ebejer MJ. 2015. The picture-winged flies and related families (Diptera Tephritoidea: Lonchaeidae, Piophilidae, Tephritidae and Ulidiidae) of the Maltese Islands. Bulletin of the Entomological Society of Malta 7:73–91.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3:294–299.
- Galinskaya TV, Suvorov A, Okun MV, Shatalkin AI. 2014. DNA barcoding of Palaearctic Ulidiidae (Diptera: Tephritoidea): Morphology, DNA evolution, and Markov codon models. Zoological Studies 53:51. DOI: 10.1186/s40555-014-0051-1.
- Giordani G, Grzywacz A, Vanin S. 2019a. Characterization and identification of puparia of Hydrotaea Robineau-Desvoidy, 1830 (Diptera: Muscidae) from forensic and archaeological contexts. Journal of Medical Entomology 56:45–54. DOI: 10.1093/jme/tjy142.
- Giordani G, Tuccia F, Floris I, Vanin S. 2018. First record of Phormia regina (Meigen, 1826) (Diptera: Calliphoridae) from mummies at the Sant’Antonio Abate Cathedral of Castelsardo, Sardinia, Italy. PeerJ 6:e4176. DOI: 10.7717/peerj.4176.
- Giordani G, Tuccia F, Zoppis S, Vecchiotti C, Vanin S. 2019b. Record of Leptometopa latipes (Diptera: Milichiidae) from a human cadaver in the Mediterranean area. Forensic Sciences Research 4:341–347. DOI: 10.1080/20961790.2018.1490473.
- Giordani G, Vanin S. 2020. Description of the puparium and other notes on the morphological and molecular identification of Phthitia empirica (Diptera, Sphaeroceridae) collected from animal carcasses. Egyptian Journal of Forensic Sciences 10:13. DOI: 10.1186/s41935-020-00187-2.
- Heo C, Latif B, Kurahashi H, Hayashi T, Nazni W, Omar B. 2015. Coprophilic dipteran community associated with horse dung in Malaysia. Halteres 6:33–50.
- Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35:518–522. DOI: 10.1093/molbev/msx281.
- Kameneva EP, Korneyev VA. 2010. Order Diptera, family Ulidiidae. Arthropod Fauna of the UAE 3:616–634.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33:1870–1874. DOI: 10.1093/molbev/msw054.
- Marshall SA. 2012. Flies: The natural history & diversity of Diptera. Richmond Hill: Firefly Books.
- Martin‐Vega D, Baz A. 2013. Sarcosaprophagous Diptera assemblages in natural habitats in central Spain: Spatial and seasonal changes in composition. Medical and Veterinary Entomology 27:64–76. DOI: 10.1111/j.1365-2915.2012.01028.x.
- Mazzarelli D, Tambuzzi S, Maderna E, Caccia G, Poppa P, Merelli V, Terzi M, Rizzi A, Trombino L, Andreola S, Cattaneo C. 2021. Look before washing and cleaning: A caveat to pathologists and anthropologists. Journal of Forensic and Legal Medicine 79:102137. DOI: 10.1016/j.jflm.2021.102137.
- McAlpine JF, Peterson BV, Shewell G, Teskey H, Vockeroth J, Wood D. 1987. Manual of nearctic Diptera. Vol. 2. Ottawa, ON: Biosystematics Research Institute.
- Monteiro-Filho EDA, Penereiro J. 1987. Estudo de decomposição e sucessão sobre uma carcaça animal numa área do estado de São Paulo. Revista Brasileira De Biologia 47:289–295.
- Morales GE, Wolff M. 2010. Insects associated with the composting process of solid urban waste separated at the source. The Revista Brasileira De Entomologia 54:645–653. DOI: 10.1590/S0085-56262010000400017.
- Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32:268–274. DOI: 10.1093/molbev/msu300.
- Pradelli J, Rossetti C, Tuccia F, Giordani G, Licata M, Birkhoff J, Verzeletti A, Vanin S. 2019. Environmental necrophagous fauna selection in a funerary hypogeal context: The putridarium of the Franciscan monastery of Azzio (northern Italy). Journal of Archaeological Science: Reports 24:683–692.
- Pradelli J, Tuccia F, Giordani G, Vanin S. 2021. Puparia cleaning techniques for forensic and archaeo-funerary studies. Insects 12:104. DOI: 10.3390/insects12020104.
- Prado e Castro C, Arnaldos MI, Sousa JP, García MD. 2011. Preliminary study on a community of sarcosaprophagous Diptera in central Portugal. Entomologia Generalis 33:183–198. DOI: 10.1127/entom.gen/33/2011/183.
- Prado e Castro C, Serrano A, Martins da Silva P, García M. 2012. Carrion flies of forensic interest: A study of seasonal community composition and succession in Lisbon, Portugal. Medical and Veterinary Entomology 26:417–431. DOI: 10.1111/j.1365-2915.2012.01031.x.
- Rumiza A, Khairul O, Zuha R, Heo C. 2010. An observation on the decomposition process of gasoline-ingested monkey carcasses in a secondary forest in Malaysia. Tropical Biomedicine 27:373–383.
- Sawaby RF, El Hamouly H, Abo-El Ela RH. 2018. Diagnosis and keys of the main dipterous families and species collected from rabbit and Guinea pig carcasses in Cairo, Egypt. The Journal of Basic and Applied Zoology 79:10. DOI: 10.1186/s41936-018-0018-6.
- Sessa F, Varotto E, Salerno M, Vanin S, Bertozzi G, Galassi FM, Maglietta F, Salerno M, Tuccia F, Pomara C. 2019. First report of Heleomyzidae (Diptera) recovered from the inner cavity of an intact human femur. Journal of Forensic and Legal Medicine 66:4–7. DOI: 10.1016/j.jflm.2019.05.021.
- Smith KGV. 1986. A manual of forensic entomology. Ithaca: Cornell University Press.
- Tuccia F, Giordani G, Vanin S. 2016a. A general review of the most common COI primers for Calliphoridae identification in forensic entomology. Forensic Science International. Genetics 24:e9–e11. DOI: 10.1016/j.fsigen.2016.07.003.
- Tuccia F, Giordani G, Vanin S. 2016b. A combined protocol for identification of maggots of forensic interest. Science & Justice 56:264–268. DOI: 10.1016/j.scijus.2016.04.001.
- Turchetto M, Lafisca S, Costantini G. 2001. Postmortem interval (PMI) determined by study sarcophagous biocenoses: Three cases from the province of Venice (Italy). Forensic Science International 120:28–31. DOI: 10.1016/S0379-0738(01)00412-1.
- Turchetto M, Vanin S. 2004. Forensic evaluations on a crime case with monospecific necrophagous fly population infected by two parasitoid species. Anil Aggrawal’s Internet Journal of Forensic Medicine and Toxicology 5:12–18.