Abstract
Insectivorous birds are particularly vulnerable to nematodes with heteroxenous life cycles. Although there are many studies on bird filarioids, they mainly focus on economically important or pet bird species, and as a result, the species diversity of these parasites is insufficiently studied. Research on the genus Diplotriaena and their hosts is neglected, although they are globally occurring and dangerous parasites with low specificity to the final host. Here we report the prevalence, invasive intensity and species affiliation of the filarial nematodes of the genus Diplotriaena in a common passerine – Eurasian blackcap (Sylvia atricapilla, L.). In total, 24 first-year individuals of S. atricapilla were caught in Toruń (central Poland) at their breeding grounds in July and August 2019, and after 7 months in captivity, they were killed and dissected. Over 20% of dissected birds were infected with Diplotriaena, and their air sacs were inhabited by 1 to 18 adult worms. Molecular identification of nematode worms was done using the 18S small subunit rRNA gene, and they were identified as D. obtusa. Our study is the first to show the molecular confirmation of the presence of D. obtusa in S. atricapilla.
Introduction
Filarial species represent a relatively small group of parasitic nematodes with a significant impact on human and animal health (Morales-Hojas Citation2009). The most important species from the medical point of view are Wuchereria bancrofti and Brugia spp. causing lymphatic filariasis (elephantiasis) and Onchocerca volvulus responsible for onchocerciasis (river blindness) (Morales-Hojas Citation2009). However, other zoonotic and truly enzootic filariasis were also identified and described previously (Muller & Wakelin Citation2002; Otranto et al. Citation2011; Kemenesi et al. Citation2015). For example, truly enzootic filariasis is caused by Setaria spp., Acanthocheilonema spp. or Eufilaria spp. in ungulates, rodents and birds, respectively (Oloś et al. Citation2019; Binkienė et al. Citation2021; Risch et al. Citation2021). In most cases, filariases are vector-borne infections transmitted by various insects (mosquitos, black flies, locusts) and arachnids (ticks) (Namrata et al. Citation2014; Bravo-Barriga et al. Citation2016; Akramova et al. Citation2017; Heym et al. Citation2019; Aupalee et al. Citation2020; Tokarz et al. Citation2020). As a result, arthropod-eating birds are particularly vulnerable to these parasites. Parasitic worms seem to be more dangerous than other types of parasites, because they may contribute to the death of their avian host. Examples of such parasites include filarial nematodes belonging to the genus Diplotriaena Henry & Ozoux, 1909 (Okulewicz & Sitko Citation2012). Passerine birds ingest the parasite by swallowing an adult Orthoptera infected with the third-stage larvae (L3) (Bain & Vaucher Citation1973). The larvae migrate from the bird intestines through the bile ducts to the liver, where they moult the cuticle. Then the fourth-stage larvae (L4) migrate through the portal vein to the heart, and finally, adult worms settle in the host’s air sacs. Female nematodes lay eggs that are expectorated and end up in the trachea, where they are swallowed and then excreted in the faeces (Cawthorn & Anderson Citation1980). Certain pathogenic effects of Diplotriaena spp. in final hosts have been reported including central nervous system disturbance, marked body mass loss, appetite loss, scarce plumage, subcutaneous emphysema, pneumonia and airsacculitis. The parasite may cause death due to the intensification of the lesions in the host, or sudden death caused by nematode migrations (Keymer Citation1982; Fasina et al. Citation2007; Sterner & Cole Citation2008; Hong et al. Citation2019). Diplotriaena spp. have been recorded in Africa, America, Asia, Australia and Europe (Mackerras Citation1962; Olsen & Braun Citation1971; Fasina et al. Citation2007; Borji & Razmyar Citation2011; Okulewicz & Sitko Citation2012; Fard et al. Citation2015; Hong et al. Citation2019; Rentería-Solís et al. Citation2021). A total of over 70 species have been described (Hong et al. Citation2019), mainly based on morphological features (Chabaud Citation1955; Al-Ankari et al. Citation2003; Okulewicz & Sitko Citation2012; Soomro et al. Citation2016; Bernardon et al. Citation2018; Ceolan Morais et al. Citation2018). There are only few case reports based on molecular identification of these parasites (Vieira et al. Citation2017; Hong et al. Citation2019; Rentería-Solís et al. Citation2021). Binkienė et al. (Citation2021) pointed out that species diversity of bird filarial nematodes is insufficiently investigated and that research on the filarioids mainly focus on economically important or pet birds, and only few of were done on wild birds, including the widely distributed warblers, for instance, belonging to the genus Sylvia Scopoli, 1769 (Passeriformes: Sylviidae). Here we report the species affiliation of the filarial nematode of the genus Diplotriaena, its prevalence and the intensity of invasion in Eurasian blackcap Sylvia atricapilla (Linnaeus, 1758) from central Poland.
Materials and methods
Sampling and necropsy of birds
Birds were caught in Toruń (Poland) (53°00′ N, 18°35′ E) after breeding and at the beginning of autumn migration 2019. Sylvia atricapilla were captured with mist nets and identified at the capture site. Next, the birds were transported to the laboratory where they were kept in captivity for other research purposes for 7 months. During this period S. atricapilla were fed with a mixture of fish feed, hard boiled eggs and supplemented with minerals and vitamins. After completion of experiments, birds were killed by cervical dislocation and immediately dissected. During the necropsy, the presence of parasites was checked only in air sacs, thoracic and abdominal cavities. More precise parasitological post-mortem examinations were not possible because then the bird bodies were subjected to another investigation led by scientists from the Department of Vertebrate Zoology and Ecology, Nicolaus Copernicus University in Toruń, Poland. The research was performed under permits issued by General Directorate for Environmental Protection in Poland (ref. no. DZP-WG.6401.03.144.2018.dł) and by the Local Committee for Ethics in Animal Research in Bydgoszcz, Poland (ref. no. 33/2018).
Morphological identification of parasites
Isolated nematodes were counted, their sex was determined (according to Chabaud (Citation1955) and Bernardon et al. (Citation2018)) and body length was measured (to the nearest 1 mm). Then, few nematode specimens were fixed in ethanol (96%) and frozen (–20°C) for subsequent molecular identification. The remaining individuals isolated from S. atricapilla were leached out for a few minutes in 10% KOH and subjected to further morphological identification. Initial genus identification of the nematodes was done based on the morphological traits and morphometry of the specimens according to available images and descriptions (e.g. Chabaud Citation1955; Vieira et al. Citation2017; Bernardon et al. Citation2018) using a light microscope (Axcio Lab A1) with various magnification (5x, 10x, and 40x). Images were recorded using a digital camera (Axiocam 105 color, Carl Zeiss) and a computer system running Zen software (version 2.3, blue edition). Due to the different visibility of individual morphological features and the condition of the fixed nematodes, the measurements of individual features were taken from a different number of specimens, but not less than three.
DNA extraction, amplification and sequencing
DNA extraction was performed by using NucleoSpin Tissue Kit (Macherey-Nagel, Germany) from adult worms (each originated from another host specimen). A PCR reaction of the 18S small subunit rRNA gene was done by using Nem18SF (5ʹ-CGCGAATRGCTCATTACAACAGC-3ʹ) and Nem18SR (5ʹ- GGGCGGTATCTGATCGCC-3ʹ) primers (Floyd et al. Citation2005). A PCR reaction of each sample was performed in a 20 μl reaction mixture, consisting of 3.2 μl of template DNA, 10.2 μl of ddH2O, 2 μl of 10x buffer B1 (HOT FIREPol®, Solis BioDyne, Estonia), 2 μl of 25 mM MgCl2 (HOT FIREPol®, Solis BioDyne, Estonia), 0.8 μl of each primer, 0.8 μl of 20 mM dNTP (ThermoFisher Scientific, USA), and 0.2 μl of Taq-Polymerase (HOT FIREPol®, Solis BioDyne, Estonia). PCR conditions consisted of 15 min initial denaturation at 95°C; 30 s denaturation at 94°C, followed by 90 s annealing at 55°C, and 90 s elongation at 72°C for 35 cycles; followed by 10 min a final elongation step at 72°C. A 3-μl sample of PCR product was run on a 1.5% agarose gel for 30 min at 100 V to check DNA quality. PCR products were cleaned up by using EPiCC Fast (A&A Biotechnology, Poland). A sequencing reaction was performed based on protocols (reaction mixture, conditions) described previously in Zając and Stec (Citation2020). Sequencing products were cleaned using ExTerminator kit (A&A Biotechnology, Poland) and sequenced in Genomed company (Warsaw, Poland) in both directions. Obtained sequences were deposited in GenBank with the following accession numbers: MW680965, MW680966, MW680967, MW680968.
Data analysis
All obtained sequences were blasted with NCBI BLAST (Altschul et al. Citation1990) to verify species identification. The sequences that resulted in at least 95% identity in NCBI BLAST to our sequences were chosen for the phylogenetic analysis. To visualize relationships between studied species and other nematodes, selected 18S rRNA sequences of D. obtusa (Accession numbers: MT129507, MT129509, MT129513, MT129515, MT129522-523), D. bargusinica (KX583753, KX545341-347), Serratospiculum tendo (MW168997-999), Cyrnea seurati (EU004816), Setaria yehi (KT934942), S. digitata (DQ094175), Mastophorus muris (MN086288-290), Onchocerca cervipedis (KT031393), O. cervicalis (DQ094174), Rumenfilaria andersoni (KT878979, KT907509, KT885224-225), Dirofilaria repens (AB973229), Dipetalonema yatesi (MW192232-233), Oxyspirura petrowi (LC316613) and W. bancrofti (AY843436) were downloaded from GenBank (Bhandari et al. Citation2005; Honisch & Krone Citation2008; Suzuki et al. Citation2015; Vieira et al. Citation2017) and together with sequences obtained in this study were aligned in BioEdit 5.0.0 (Hall Citation1999) with the multiple alignment function of ClustalW (Thompson et al. Citation1994). The obtained alignment (858 bp length) comprised 39 sequences in total. A sequence of Otodistomum cestoides (Trematoda: Azygiidae) was used as an outgroup (Accession number: AJ287553; Cribb et al. Citation2001). Uncorrected pairwise distances within D. obtusa were calculated in MEGA v. 7 (Kumar et al. Citation2016) based on sequences from GenBank and obtained in this study.
Bayesian inference (BI) marginal posterior probabilities were calculated in MrBayes v. 3.2 (Hulsenbeck & Ronquist Citation2001; Huelsenbeck et al. Citation2001) with 1 cold and 3 heated Markov chains for 10 million generations and trees were sampled every 1000 generations. In the BI consensus tree, clades recovered with posterior probability (PP) between 0.95 and 1.00 were considered as well supported. The obtained tree was visualized in FigTree v.1.4.3 programme (http://tree.bio.ed.ac.uk/software/figtree).
Results
Parasitological examination
In total, 24 first-year individuals of S. atricapilla were necropsied, and 5 of them (21%) were infected with nematodes. The invasion intensity ranged from 1 to 18 adult worms in the host air sacs (). Two cases of infection involved only one male nematode, while in the remaining cases the infestation included both sexes ().
Table I. The number of collected filarial nematodes and male to female ratio in Sylvia atricapilla from Torun, Poland
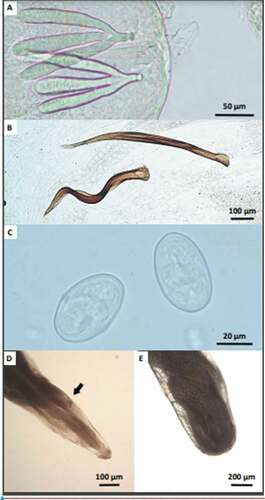
The nematodes were identified as Diplotriaena sp. based on morphological features presented in . Body length of female and male nematodes were 32–46 mm and 13–25 mm, respectively. The nematodes were characterized by a milky white body with a simple mouth without lips. A pair of chitinous tridents (female: 0.128–0.157 mm, male: 0.123–0.152 mm) was observed at the head end of the male and female worm. On the caudal part of the male body, there was a smaller (0.484–0.660 mm) and a larger spicule (0.63–838 mm). Eggs were oval and smooth measuring 0.046–0.048 × 0.028–0.030 mm ().
Table II. Measurements of morpho-taxonomic features of nematodes collected in Sylvia atricapilla (± SE) [mm]
Figure 1. Microscopic images of the body parts of filarial nematodes and their eggs: (a) – tridents on a head region of the male nematode; (b) – spicules on the caudal part of the male nematode; (c) – eggs; (d) – anterior part of the female nematode showing vulva; (e) – caudal part of the female nematode showing a part of the uterus with eggs
Molecular examination
The verification in NCBI BLAST resulted in fitting to D. obtusa (MT129522 (Michalski et al. Citation2021); Query cover: 99%; E-value: 0.00; Percentage identity: 99.20%). All 18S rRNA sequences (755 bp length) obtained in this study were represented by a single and unique haplotype.
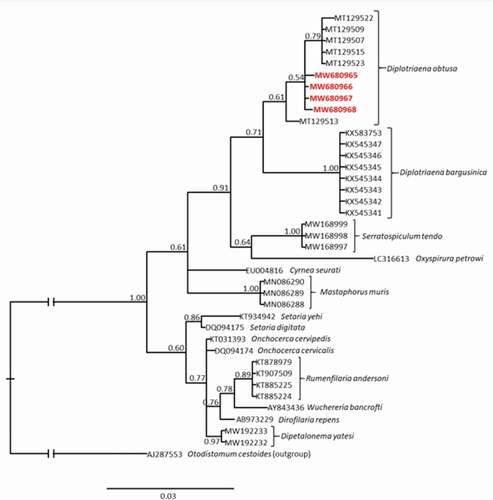
Bayesian (BI) phylogenetic tree showed that all sequences obtained in this study were very similar and clustered together with sequences of D. obtusa collected from the barn swallow Hirundo rustica (Passeriformes: Hirundinidae) in the USA, available in GenBank (Accession numbers: MT129507, MT129509, MT129513, MT129515, MT129522-523; ). In the presented analysis D. obtusa is the most closely related to D. bargusinica (). Intraspecific genetic distance within D. obtusa calculated based on sequences that make up the D. obtusa clade (10 sequences in total) was lower than 0.2%. For the comparison, the genetic distance between D. obtusa and D. bargusinica (single haplotype in the dataset) which is the most closely related species to D. obtusa, was 2.02%.
Figure 2. The Bayesian inference (BI) phylogeny of the selected nematode species constructed based on 18S rRNA sequences. Numbers at nodes indicate Bayesian posterior probability. Bolded and red accession numbers indicate sequences obtained in this study. The scale bar represents 0.03 substitutions per nucleotide position
Discussion
Our report is one of a few in the world and the first one in Poland containing molecular confirmation of species identification of the genus Diplotriaena. The GenBank database currently has sequences of the genus Diplotriaena belonging to only three species, namely: D. anthreptis (Hong and Park, unpublished), D. bargusinica (Vieira et al. Citation2017) and D. obtusa (Rentería-Solís et al. Citation2021; Michalski et al. Citation2021). Our research confirms the presence of D. obtusa in S. atricapilla. Using molecular techniques, D. obtusa was also identified in Cyanistes caeruleus (Passeriformes: Paridae) in Germany (Rentería-Solís et al. Citation2021) as well as in H. rustica and Petrochelidon pyrrhonota (Passeriformes: Hirundinidae) in the United States (Michalski et al. Citation2021). Until today, there were only limited reports on the occurence of the genus Diplotriaena in Poland, i.e. D. tridens in S. atricapilla (Okulewicz & Sitko Citation2012) and S. borin (Passeriformes: Sylviidae) (Okulewicz Citation1982), D. ozouxi in Motacilla flava (Passeriformes: Motacillidae) (Okulewicz Citation2013), D. henryi in Parus major (Passeriformes: Paridae) (Okulewicz Citation1991) and D. obtusa in Delichon urbica and H. rustica (Passeriformes: Hirundinidae) (Jaron Citation1969). All these reports are based solely on the morphological identification of the parasites. The lack of molecular identification of numerous species of the genus Diplotriaena indicates that these parasites are neglected in scientific research.
Research on these parasites is focused on the detection of adult worms in the air sacs or thoracic and abdominal cavities during necropsy of final hosts (Fard et al. Citation2015; Vieira et al. Citation2017; Hong et al. Citation2019; Rentería-Solís et al. Citation2021). Although there are many non-invasive parasitological tests carried out on wild birds (Benedikt Citation2006; DeGroote & Rodewald Citation2010; Luedtke et al. Citation2013; Santiago‐Alarcon et al. Citation2013; Dadam et al. Citation2019; Bichet et al. Citation2020; Fecchio et al. Citation2020; Popescu et al. Citation2020), in the case of Diplotriaena spp. tests based on blood smears may be unreliable due to the periodic presence of microfilariae in the peripheral blood of final hosts (Keymer Citation1982). In addition, due to the lack of molecular characterization of most of the described filarial species, the examination of the adult worms is essential for their specific identification (Binkienė et al. Citation2021). Nevertheless, we agree that the procedure used by Binkienė et al. (Citation2021) to detect different species of filarioid nematodes, which involves taking blood samples and testing them in the field, and then collecting only individuals infected with microfilariae for dissection, allows for a better understanding of the system of filarioid nematodes and wild birds. Examination of the presence of embryonated eggs in the faeces is another non‐invasive diagnostic tool. However, Sterner and Cole (Citation2008) indicated that the investigation is unreliable because other parasites besides air sac worms lay morphologically similar eggs. Additionally, according to our personal observations, the concentration method (flotation in saturated MgSO4 solution) can not detect infection at low intensity, possibly because parasites lay egg periodically. Most research on Diplotriaena spp. was carried out on dead birds found in the field (Fasina et al. Citation2007; Okulewicz & Sitko Citation2012; Fard et al. Citation2015; Hong et al. Citation2019; Rentería-Solís et al. Citation2021), so it is difficult to discuss the true prevalence of this parasite in the environment. Here we had this unique opportunity to observe and analyze infestation in birds randomly sampled from the environment, which were killed for other purposes. The prevalence of D. obtusa in S. atricapilla population from the capture site was quite high and reached over 20%. However, it is still difficult to talk about the true prevalence because we investigated the low number of hosts during one season. This is because we used birds captured for other research purposes. Low intensities of infection (number of worms in air sacs), was probably related to the age of birds, although other factors can not be excluded. For example, the coexistence of other infections can increase or decrease parasitic/pathogen load (Ramsay & Rohr Citation2021). Okulewicz and Sitko (Citation2012) counted even 138 individuals of D. tridens in a dead host, indicating that the invasion may be the direct cause of the host death. Little is known about the real effect of filarial nematodes on the survival, fitness, or reproductive capacity of their wild bird hosts, as opposed to protozoan parasites e.g. of the genus Haemoproteus (Apicomplexa: Haemoproteidae) or Plasmodium (Apicomplexa: Plasmodiidae) (Martínez-de la Puente et al. Citation2010; Lachish et al. Citation2011; Fletcher et al. Citation2019; Romano et al. Citation2019). The progress of research on the intermediate hosts of avian parasites is also limited. The majority of studies on the larvae of Diplotriaena spp. in intermediate hosts come from several decades ago, and we are not aware of the insect species used by the parasite or its distribution and prevalence in intermediate host populations (Bain & Vaucher Citation1973; Cawthorn & Anderson Citation1980; Kabilov Citation1983; Akramova et al. Citation2017). Czajka et al. (Citation2012) emphasize that the roles of vectors in the life cycles of many filarial species and their geographical distribution are largely unknown. In contrast, there is a lot of recent research on the presence of avian apicomplexan parasites in the intermediate hosts (Chakarov et al. Citation2020; Ferreira et al. Citation2020; Taioe et al. Citation2020).
Our report highlights how poorly the system between the genus Diplotriaena and both, final and intermediate hosts has been understood so far. It is also the first study showing the molecular confirmation of the presence of D. obtusa in S. atricapilla. Now we can only assume what the real effects of infestation for the host are, how a wide range of host species is used, what is the parasites’ true prevalence and spread in natural populations of the host. We believe that our work will shed light on the ecology of these parasites, and finally extend our knowledge on the genus Diplotriaena.
Acknowledgements
We would like to thank Anna Kowalczewska, Anna Nowak and Anna Przybylska-Piech for their help in the laboratory and in the field.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akramova FD, Medetov MJ, Shakarboyev UA, Azimov DA. 2017. Locusts (Orthoptera: Acrididae) as intermediate hosts of nematodes Aprocta cylindrica and Diplotriaena isabellina (Filariina: Aproctidae, Diplotriaenidae) in Uzbekistan. Journal of Novel Applied Sciences 6:28–33.
- Al-Ankari A-RS, Elamin EA, Al-Atiya SA, Al-Jabr OA. 2003. First report of Variolepis farciminosa (Cestoda: Hymenolepididae) and Diplotriaena tridens (Nematoda: Diplotriaenoidea) infecting Crested Larks, Galerida cristata, from Hofuf, Al-Ahsa Oasis, Saudi Arabia. Comparative Parasitology 70(1):97–98. DOI: 10.1654/1525-2647(2003)070[0097:FROVFC]2.0.CO;2.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215(3):403–410. DOI: 10.1016/S0022-2836(05)80360-2.
- Aupalee K, Saeung A, Srisuka W, Fukuda M, Streit A, Takaoka H. 2020. Seasonal filarial infections and their black fly vectors in Chiang Mai province, northern Thailand. Pathogens 9(6):512. DOI: 10.3390/pathogens9060512.
- Bain O, Vaucher C. 1973. Développement larvaire de Diplotriaena tridens (Nematoda: Filarioidea) chez Locusta migratoria. Annales de Parasitologie Humaine et Comparee 48(1):81–89. DOI: 10.1051/parasite/1973481081.
- Benedikt V. 2006. Blood parasites in passerine birds in Slovakian East Carpathians. Acta Protozoologica 45:105–109.
- Bernardon FF, Pesenti TC, Pereira Jr J, Müller G. 2018. Ensamblaje de helmintos de Chrysomus ruficapillus (Vieillot, 1819)(Passeriformes: Icteridae) del sur de Brasil. Neotropical Helminthology 12:161–178.
- Bhandari Y, Dabir P, Nandhakumar K, Dayananda KM, Shouche YS, Reddy MVR. 2005. Analysis of polymorphism of 18S rRNA gene in Wuchereria bancrofti microfilariae. Microbiology and Immunology 49(10):909–914. DOI: 10.1111/j.1348-0421.2005.tb03682.x.
- Bichet C, Brischoux F, Ribout C, Parenteau C, Meillère A, Angelier F. 2020. Physiological and morphological correlates of blood parasite infection in urban and non-urban house sparrow populations. PloS One 15(8):e0237170. DOI: 10.1371/journal.pone.0237170.
- Binkienė R, Chagas CRF, Bernotienė R, Valkiūnas G. 2021. Molecular and morphological characterization of three new species of avian Onchocercidae (Nematoda) with emphasis on circulating microfilariae. Parasites & Vectors 14(1):1–19. DOI: 10.1186/s13071-021-04614-8.
- Borji H, Razmyar J. 2011. Detection of Diplotriaena spp. from the body cavity of Myna (Acridotheres tristis) in Mashhad, Iran. Scientia Parasitologica 12:223–225.
- Bravo-Barriga D, Parreira R, Almeida AP, Calado M, Blanco-Ciudad J, Serrano-Aguilera FJ, Pérez-Martín JE, Sánchez-Peinado J, Pinto J, Reina D, Frontera E. 2016. Culex pipiens as a potential vector for transmission of Dirofilaria immitis and other unclassified Filarioidea in Southwest Spain. Veterinary Parasitology 223:173–180. DOI: 10.1016/j.vetpar.2016.04.030.
- Cawthorn RJ, Anderson RC. 1980. Development of Diplotriaena tricuspis (Nematoda: Diplotriaenoidea), a parasite of Corvidae, in intermediate and definitive hosts. Canadian Journal of Zoology 58(1):94–108. DOI: 10.1139/z80-013.
- Ceolan Morais J, de Souza DMF, Gallas M, da Silveira EF, Périco E. 2018. Diplotriaena delirae Pinto & Noronha, 1970 (Nematoda, Diplotriaenidae) in Pitangus sulphuratus (Linnaeus, 1766) (Passeriformes, Tyrannidae) from southern Brazil. CheckList 14(5):823–826. DOI: 10.15560/14.5.823.
- Chabaud A-G. 1955. Remarques sur le cycle évolutif des filaires du genre Diplotriaena et redescription de D. monticelliana (Stossich 1890). Vie et Milieu 6:342–347.
- Chakarov N, Kampen H, Wiegmann A, Werner D, Bensch S. 2020. Blood parasites in vectors reveal a united blackfly community in the upper canopy. Parasites & Vectors 13(1):309. DOI: 10.1186/s13071-020-04177-0.
- Cribb TH, Bray RA, Littlewood DTJ, Pichelin S, Herniou EA. 2001. Relationships of the Digenea–evidence from molecules and morphology. In: Littlewood DTJ, Brayeditors RA, editors. Interrelationships of the Platyhelminthes. London, UK: Taylor & Francis. pp. 186–193.
- Czajka C, Becker N, Poppert S, Jöst H, Schmidt-Chanasit J, Krüger A. 2012. Molecular detection of Setaria tundra (Nematoda: Filarioidea) and an unidentified filarial species in mosquitoes in Germany. Parasites & Vectors 5(1):14. DOI: 10.1186/1756-3305-5-14.
- Dadam D, Robinson RA, Clements A, Peach WJ, Bennett M, Rowcliffe JM, Cunningham AA. 2019. Avian malaria-mediated population decline of a widespread iconic bird species. Royal Society Open Science 6(7):182197. DOI: 10.1098/rsos.182197.
- DeGroote LW, Rodewald PG. 2010. Blood parasites in migrating wood-warblers (Parulidae): Effects on refueling, energetic condition, and migration timing. Journal of Avian Biology 41(2):147–153. DOI: 10.1111/j.1600-048X.2009.04782.x.
- Fard SRN, Khedri J, Ghashghaei O, Radfar MH, Hashemi SH. 2015. Diplotriaena spp. (Nematoda: Diplotriaenidae) from Passer domesticus (Passeridae) and Acridotheres tristis (Sturnidae) in Zabol, Iran. Scientia Parasitologica 16:128–132.
- Fasina FO, Sirdar MM, Scheepers GJ. 2007. Detection of Diplotriaena verminosis in wattled starlings in South Africa. African Journal of Wildlife Research 37(1):101–103. DOI: 10.3957/0379-4369-37.1.101.
- Fecchio A, Chagas CRF, Bell JA, Kirchgatter K. 2020. Evolutionary ecology, taxonomy, and systematics of avian malaria and related parasites. Acta Tropica 204:105364. DOI: 10.1016/j.actatropica.2020.105364.
- Ferreira FC, Santiago-Alarcon D, Braga ÉM. 2020. Diptera vectors of avian haemosporidians: With emphasis on tropical regions. In: Santiago-Alarcon D, Marzal A, editors. Avian malaria and related parasites in the tropics: Ecology, evolution and systematics. Springer International Publishing. Cham, Germany: Springer. pp. 185–250.
- Fletcher K, Träff J, Gustafsson L. 2019. Importance of infection of haemosporidia blood parasites during different life history stages for long-term reproductive fitness of collared flycatchers. Journal of Avian Biology 50(8):e02118. DOI: 10.1111/jav.02118.
- Floyd RM, Rogers AD, Lambshead PJD, Smith CR. 2005. Nematode-specific PCR primers for the 18S small subunit rRNA gene. Molecular Ecology Notes 5(3):611–612. DOI: 10.1111/j.1471-8286.2005.01009.x.
- Hall TA. 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Hall TA, editor. Nucleic acids symposium series. London, UK: Information Retrieval Ltd. pp. 95–98.
- Heym EC, Kampen H, Krone O, Schäfer M, Werner D. 2019. Molecular detection of vector-borne pathogens from mosquitoes collected in two zoological gardens in Germany. Parasitology Research 118(7):2097–2105. DOI: 10.1007/s00436-019-06327-5.
- Hong EJ, Ryu SY, Chae JS, Kim HC, Park JH, Cho JG, Choi KS, Yu DH, Park BK. 2019. Description of Diplotriaena manipoli (Nematoda: Diplotriaenoidea) detected in the body cavity of Garrulus glandarius brandtii from Republic Of Korea. Journal of Veterinary Clinics 36(3):133–138. DOI: 10.17555/jvc.2019.06.36.3.133.
- Honisch M, Krone O. 2008. Phylogenetic relationships of Spiruromorpha from birds of prey based on 18S rDNA. Journal of Helminthology 82(2):129–133. DOI: 10.1017/S0022149X08912359.
- Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. 2001. Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294(5550):2310–2314. DOI: 10.1126/science.1065889.
- Hulsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics 17(8):754–755. DOI: 10.1093/bioinformatics/17.8.754.
- Jaron W. 1969. The helminth parasites of Hirundinidae of the neighbourhood of Warszawa and Olsztyn. Acta Parasitologica Polonica 16:137–152.
- Kabilov TK. 1983. Helminths of vertebrates of Uzbekistan developing with the participation of insects. Tashkent, USSR: Fan. pp. 128.
- Kemenesi G, Kurucz K, Kepner A, Dallos B, Oldal M, Herczeg R, Vajdovics P, Krisztián B, Ferenc J. 2015. Circulation of Dirofilaria repens, Setaria tundra, and Onchocercidae species in Hungary during the period 2011–2013. Veterinary Parasitology 214(1–2):108–113. DOI: 10.1016/j.vetpar.2015.09.010.
- Keymer IF. 1982. Parasitic diseases. In: Petrak ML, editor. Diseases in cage and aviary birds. Philadelphia, PA: Lea&Febiger. pp. 535–598.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870–1874. DOI: 10.1093/molbev/msw054.
- Lachish S, Knowles SC, Alves R, Wood MJ, Sheldon BC. 2011. Fitness effects of endemic malaria infections in a wild bird population: The importance of ecological structure. Journal of Animal Ecology 80(6):1196–1206. DOI: 10.1111/j.1365-2656.2011.01836.x.
- Luedtke B, Moser I, Santiago-Alarcon D, Fischer M, Kalko EK, Schaefer HM, Suarez-Rubio M, Tschapka M, Renner SC. 2013. Associations of forest type, parasitism and body condition of two European passerines, Fringilla coelebs and Sylvia atricapilla. PloS One 8(12):e81395. DOI: 10.1371/journal.pone.0081395.
- Mackerras MJ. 1962. Filarial parasites (Nematoda: Filarioidea) of Australian animals. Australian Journal of Zoology 10(3):400–457. DOI: 10.1071/ZO9620400.
- Martínez-de la Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E, García-Fraile S, Belda EJ. 2010. The blood parasite Haemoproteus reduces survival in a wild bird: A medication experiment. Biology Letters 6(5):663–665. DOI: 10.1098/rsbl.2010.0046.
- Michalski ML, Kadolph E, Roderick CL, Lankton JS, Cole RA. 2021. Diplotriaena obtusa (Nematoda: Diplotriaenidae) from barn swallows (Hirundo rustica) and cliff swallows (Petrochelidon pyrrhonota) collected during mortality events in the Upper Midwest, USA. The Journal of Parasitology 107(4):593–599. DOI:10.1645/19-76.
- Morales-Hojas R. 2009. Molecular systematics of filarial parasites, with an emphasis on groups of medical and veterinary importance, and its relevance for epidemiology. Infection, Genetics and Evolution 9(5):748–759. DOI:10.1016/j.meegid.2009.06.007.
- Muller R, Wakelin D. 2002. Worms and human disease. Wallingford, UK: CABI Publishing. pp. 161.
- Namrata P, Miller JM, Shilpa M, Reddy PR, Bandoski C, Rossi MJ, Sapi E. 2014. Filarial nematode infection in Ixodes scapularis ticks collected from Southern Connecticut. Veterinary Sciences 1(1):5–15. DOI:10.3390/vetsci1010005.
- Okulewicz A. 1982. Nematodes of birds of the family Muscicapidae in Lower Silesia. Wiadomości Parazytologiczne 28:477–482.
- Okulewicz A. 1991. Parasitic nematodes of birds from the Sikor family (Paridae) in Poland. Wiadomosci Parazytologiczne 37:491–498.
- Okulewicz A. 2013. New records of nematodes of passerine migratory birds. Annals of Parasitology 59:135–138.
- Okulewicz A, Sitko J. 2012. Parasitic helminthes — Probable cause of death of birds. Helminthologia 49(4):241–246. DOI: 10.2478/s11687-012-0045-7.
- Oloś G, Nowakowska J, Rojewska S, Welc-Falęciak R. 2019. New findings of Setaria tundra and Setaria cervi in the red deer (Cervus elaphus) in Poland. Parasitology 146(10):1333–1337. DOI: 10.1017/s0031182019000568.
- Olsen OW, Braun CE. 1971. Diplotriaena lagopusi and D. andersoni spp. n. (Diplotriaenidae: Filarioidea) from white-tailed ptarmigan (Lagopus leucurus) in North America. Proceedings of the Helminthological Society of Washington 38:86–89.
- Otranto D, Diniz DG, Dantas-Torres F, Casiraghi M, de Almeida IN, de Almeida LN, dos Santos JN, Furtado AP, de Almeida Sobrinho EF, Bain O. 2011. Human intraocular filariasis caused by Dirofilaria sp. nematode, Brazil. Emerging Infectious Diseases 17(5):863–866. DOI: 10.3201/eid1705.100916.
- Popescu M, Trychta MR, Jackson EG, Selman JB, Houston AE, Collins MD. 2020. Avian haemosporidian prevalence and its relationship to host traits in Western Tennessee. Journal of Ornithology 161(4):995–1010. DOI: 10.1007/s10336-020-01783-8.
- Ramsay C, Rohr JR. 2021. The application of community ecology theory to co‐infections in wildlife hosts. Ecology 102(3):e03253. DOI: 10.1002/ecy.3253.
- Rentería-Solís Z, Peters M, Gawlowska S, Schmäschke R. 2021. Diplotriaena obtusa infection in an Eurasian blue tit (Cyanistes caeruleus) in Germany. Pathology and Phylogenetic analysis. Veterinary Parasitology: Regional Studies and Reports 23:100527. DOI: 10.1016/j.vprsr.2020.100527.
- Risch F, Ritter M, Hoerauf A, Hübner MP. 2021. Human filariasis—contributions of the Litomosoides sigmodontis and Acanthocheilonema viteae animal model. Parasitology Research. DOI: 10.1007/s00436-020-07026-2.
- Romano A, Nodari R, Bandi C, Caprioli M, Costanzo A, Ambrosini R, Rubolini D, Parolini M, Epis S, Saino N. 2019. Haemosporidian parasites depress breeding success and plumage coloration in female barn swallows Hirundo rustica. Journal of Avian Biology 50(2):e01889. DOI: 10.1111/jav.01889.
- Santiago‐Alarcon D, Mettler R, Segelbacher G, Schaefer HM. 2013. Haemosporidian parasitism in the blackcap Sylvia atricapilla in relation to spring arrival and body condition. Journal of Avian Biology 44:521–530. DOI: 10.1111/j.1600-048X.2013.00181.x.
- Soomro B, Ghachal GS, Yusuf SM, Narejo N. 2016. Description of new species Diplotriaena saheefi n. sp. (Nematode: Filariidae) from Jungle myna (Acridotheres fuscus) Wagler; 1827 (Passeriformes: Sturnidae) in district Larkana, Sindh, Pakistan. Journal of Entomology and Zoology Studies 4:579–581.
- Sterner MC, Cole RA. 2008. Diplotriaena, Serratospiculum, and Serratospiculoides. In: Atkinson CT, Thomas NJ, Hunter DB, editors. Parasitic diseases of wild birds. Ames, IA: Wiley-Blackwell. pp. 434–438.
- Suzuki J, Kobayashi S, Okata U, Matsuzaki H, Mori M, Chen KR, Iwata S. 2015. Molecular analysis of Dirofilaria repens removed from a subcutaneous nodule in a Japanese woman after a tour to Europe. Parasite 22:2. DOI: 10.1051/parasite/2015002.
- Taioe MO, Mnisi MC, Chaisi M, Mwale M, Mabunda N, Phetla V, Thekisoe OM. 2020. Mosquito identification and haemosporidian parasites detection in the enclosure of the African penguins (Spheniscus demersus) at the SANBI zoological garden. International Journal for Parasitology: Parasites and Wildlife 13:98–105. DOI: 10.1016/j.ijppaw.2020.08.004.
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22(22):4673–4680. DOI:10.1093/nar/22.22.4673.
- Tokarz R, Tagliafierro T, Ian Lipkin W, Marques AR. 2020. Characterization of a Monanema nematode in Ixodes scapularis. Parasites & Vectors 13(1):371. DOI:10.1186/s13071-020-04228-6.
- Vieira DT, Pegoraro de Macedo MR, Fedatto BF, Müller G. 2017. Morphological, molecular and phylogenetic analyses of Diplotriaena bargusinica Skrjabin, 1917 (Nematoda: Diplotriaenidae). Parasitology International 66(5):555–559. DOI:10.1016/j.parint.2017.04.009.
- Zając KS, Stec D. 2020. Molecular approach to identifying three closely related slug species of the genus Deroceras (Gastropoda: Eupulmonata: Agriolimacidae). Zoological Studies 59:55. DOI: 10.6620/ZS.2020.59-55.
