Abstract
The Ponto-Caspian amphipod Dikerogammarus bispinosus was originally described from the Black Sea basin. Its recent discovery in the Caspian Sea basin was puzzling because it was unknown whether it was an invasive or an overlooked native species in this area. Here, we examined specimens collected from both the Black and Caspian Sea basins by means of molecular species delimitation based on nuclear (28S) and mitochondrial (COI) DNA sequences, as well as scanning electron microscopy (SEM). Our analyses reveal that D. bispinosus comprises three evolutionary independent lineages that are molecularly and morphologically distinct. One lineage occurs throughout rivers in the Black Sea basin, while the other two inhabit the Caspian Sea and were found in sympatry, further reinforcing that they are distinct species. Our time-calibrated phylogeny indicates that these lineages split during the Late Miocene-Pliocene, a period corresponding with the separation of the Black and Caspian basins via the Caucasus mountain uplift. SEM imaging revealed morphological differences with respect to setal patterns on the gnathopod propodi among all three lineages. Therefore, our results clearly indicate not only that D. bispinosus is native in the Caspian region, but that it has been overlooked for a long time. Additional populations covering the entire range of this species complex need to be further studied in order to gain a more complete picture of its evolutionary history and resolve its taxonomy.
Introduction
The Ponto-Caspian region has a complex geological history due to its gradual separation, with recurrent re-connections, from the World Ocean and frequent sea-level fluctuations. These regression/transgression phases lead to recurrent episodes of connection and isolation between different basins within the Ponto-Caspian region. The current Black, Azov, Caspian and Aral Seas, formed in the Pleistocene and early Holocene, are products of this complex geological process (Zenkevich Citation1956; Jones & Simmons Citation1997; Cristescu et al. Citation2003, Figure 1; Popov et al. Citation2006; Hou & Li Citation2018, Figure 2). This dynamic history caused numerous environmental changes (salinity fluctuations) that led to biotic turnover and numerous evolutionary radiations, especially in crustaceans (Cristescu et al. Citation2003; Audzijonyte et al. Citation2006; Hou et al. Citation2014), mollusks (Sands et al. Citation2019a, Citation2019b; Neibera et al. Citation2021), and fish (Neilson & Stepien Citation2009).
Amphipods have greatly diversified in the Ponto-Caspian region (96 endemics), particularly in the Caspian Sea (71 endemic species) (Derzhavin Citation1948; Mordukhai-Boltovskoj Citation1960; Pjatakova & Tarasov Citation1996; Copilaș-Ciocianu & Sidorov Citation2021). The latter is even called the “Crustacean Sea” due to a high diversity of endemic amphipods, mysids and cumaceans (Zenkevich Citation1956; Cristescu & Hebert Citation2005). Ponto-Caspian amphipods are mostly euryhaline species with a high invasive potential, many of which have extended their ranges in the last 60 years (Bij de Vaate et al. Citation2002; Cristescu et al. Citation2004; Copilaș-Ciocianu et al. Citation2021) and are studied quite actively (e.g. Cristescu et al. Citation2004; Grabowski et al. Citation2007; Arbačiauskas et al. Citation2013; Rewicz et al. Citation2015; Copilas-Ciocianu & Sidorov Citation2021).
Dikerogammarus bispinosus Martynov, 1 925 described from the Dnieper River has been considered as native in the lower stretches of rivers that drain to the Black Sea (Martynov, Citation1925; Cărăuşu et al. Citation1955; Jażdżewski & Konopacka Citation1988). Its non-native range stretches westwards along the middle and upper sectors of these rivers, reaching into the Rhine in western Europe (Labat et al. Citation2011). It is also widespread in the upper Danube River, particularly in Germany (Eggers & Martens Citation2001), Austria (Müller & Schramm Citation2001, Borza et al. Citation2015), and in the middle Danube in Hungary and Slovakia (Borza et al. Citation2015). More recently, it has started to expand northeast; it was reported as an invasive species from the Saratov reservoir (Volga River, Russia, Caspian Sea basin) between 2002 and 2006 (Voronin & Yermokhin Citation2004; Filinova & Sonina Citation2012) and from the lower Don (Russia, Black Sea basin) in 2003 (Sayapin Citation2003). It is believed that this species could have dispersed naturally via the Volga-Don canal to the Caspian region or was passively introduced through shipping activity (Copilaș-Ciocianu & Arbačiauskas Citation2018). Yet, evidence of its presence in the Caspian basin before these reports were conflicting as D. bispinosus was considered for a long time a subspecies or synonym of D. villosus (Sowinsky, 1894), and sometimes even D. villosus was erroneously considered as a synonym of D. haemobaphes (Eichwald, 1841) (Pjatakova & Tarasov Citation1996). Mordukhai-Boltovskoj (Citation1960) indicated that D. bispinosus does not occur in the Caspian Sea. Clear morphological features to distinguish these three species as well as molecular comparison were provided quite recently (Müller & Schramm Citation2001; Müller et al. Citation2002; Mamos et al. Citation2021), so D. bispinosus may have been overlooked in the Caspian basin for decades (Tarasov Citation1995; Copilaș-Ciocianu & Arbačiauskas Citation2018). Recently, this species was also reported in the Ural River (Kazakhstan), which drains into the Caspian Sea (Copilaș-Ciocianu & Arbačiauskas Citation2018). Nevertheless, given the currently available data, it has not been possible to determine whether D. bispinosus is an overlooked native or an invader in the Caspian basin (Copilaș-Ciocianu & Arbačiauskas Citation2018).
In this study, we aim to provide insight into the native versus invasive status of D. bispinosus in the Caspian Sea basin by using a phylogeographical approach based on mitochondrial and nuclear markers, combined with Scanning Electron Microscopy (SEM) imaging. We hypothesize that, given the long geological isolation of the Black and Caspian Seas, the local populations of D. bispinosus may belong to different lineages, as observed in several other crustacean species in this region (Cristescu et al. Citation2003; Cristescu & Hebert Citation2005; Audzijonyte et al. Citation2006; Nahavandi et al. Citation2013).
Material and methods
Sampling
Dikerogammarus bispinosus was collected with a hand net and by visual inspection of submerged substrates from three localities on the coast of the Caspian Sea: near Baku (Azerbaijan) and 43 km north of Aktau in Kazakhstan in August 2018, as well as in Yalama (Azerbaijan) in July 2019. Additionally, specimens of D. bispinosus, D. villosus, D haemobaphes and Pontogammarus robustoides (Sars, 1894) that originated from previous sampling campaigns in Austria, Croatia, Ukraine and Hungary, were used as a comparative material (for details see ). Animals were fixed in 96% ethanol. The specimens are stored in the collection of invertebrates at the Department of Invertebrate Zoology and Hydrobiology (University of Lodz, Poland).
Table I. Localities and GenBank accession numbers of specimens used for the current study. N – 54
Morphological identification and SEM imaging
Identity of the collected D. bispinosus was confirmed using the keys by Cărăuşu et al. (Citation1955), Stock (Citation1974), Karaman & Barnard (Citation1979), Stock et al. (Citation1998) and Özbek & Özkan (Citation2011). The diagnostic morphological features for D. bispinosus were defined as follows: pillar-shaped protuberances on first and second urosomites, antenna 2 peduncular segments with tufts of setae longer than the underlying segment and a postero-distal lobe on the basis of the 7th pereopod.
Adult, male individuals used for SEM documentation were dehydrated in an ethanol series, air dried, and sputter-coated with gold (11 nm). Images were produced with a PHENOM PRO X SEM in the Department of Invertebrate Zoology and Hydrobiology, University of Lodz. Given that second antennae and second gnathopods are known to bear important taxonomic features in amphipods, we imaged these appendages for two male individuals per each lineage identified with molecular methods (see below).
DNA extraction and PCR
Samples were processed either in the Canadian Center for DNA Barcoding (CCDB), Guelph, Canada (two individuals, see for details) or in the Department of Invertebrate Zoology and Hydrobiology, University of Lodz (all the remaining individuals).
For samples analysed at the CCDB, a leg was removed from each specimen and transferred into 96 well plates for subsequent DNA extraction (protocol available: https://ccdb.ca/site/wp-content/uploads/2016/09/CCDB_DNA_Extraction.pdf). The standard animal DNA barcode gene region (COI) (Hebert et al. Citation2003) was amplified using the primers LCO1490, 5′-GGTCAACAAATCATAAAGATATTGG-3′ and HCO2198, 5′- TAAACTTCAGGGTGACCAAAAAATCA-3′ (Folmer et al. Citation1994) (https://ccdb.ca/site/wp-content/uploads/2016/09/CCDB_Amplification.pdf/) and bi-directionally Sanger sequenced at place in the CCDB (https://ccdb.ca/site/wp-content/uploads/2016/09/CCDB_PrimerSets.pdf).
For samples analysed at the Department of Invertebrate Zoology and Hydrobiology (University of Lodz), total DNA was extracted using either the Chelex procedure (Casquet et al. Citation2012), or the phenol-chloroform method (see Grabowski et al. Citation2012 for details). The standard animal DNA barcode gene region (COI) (Hebert et al. Citation2003) was amplified using the primers described above (Folmer et al. Citation1994). The amplification of a nuclear fragment of gene 28S rRNA was done with 28 F 5′- TTAGTAGGGGCGACCGAACAGGG-3′ and 28 R 5′-GTCTTTCGCCCCTATGCCCAACTGA-3′ primers published by Hou et al. (Citation2007). The amplification of both gene regions was conducted under the same PCR protocol described by Hou et al. (Citation2007). PCR products (5 μl) were cleaned up with Exonuclease I (20 U/μl; EURx, Poland) and alkaline phosphatase Fast Polar-BAP (1 U/μl, EURx, Poland) treatment, according to the manufacturer’s guidelines and then sequenced (COI – forward direction only, 28S – bi-directional) using standard Sanger method by Macrogen Europe (Netherlands), using the same PCR primers.
Dataset assembly
BLAST search (blast.ncbi.nlm.nih.gov/Blast.cgi) was performed to check for the identity of amplified sequences. The resulting sequences were manually assembled in Geneious v. 10.2.6 (https://www.geneious.com, Kearse et al. Citation2012) and trimmed by removing the remaining parts of the primer regions (length COI: 490–695; 28S: 862–1264) and then submitted to GenBank (Accession: COI: MZ261663-MZ261698; 28S: MZ261642-MZ261662) and simultaneously deposited in the Barcode of Life Data Systems (BOLD) System (Ratnasingham & Hebert Citation2007).
Additional COI and 28S sequences of D. haemobaphes and D. villosus (28 individuals) as well as the outgroup sequence (one individual) of P. robustoides were acquired either from the publicly available datasets deposited in the open databases (GenBank and BOLD) or from own data (see for details).
Relevant voucher information is accessible through the public data set “DS-DIKS” (DOI: http://dx.doi.org/10.5883/DS-DIKS) in the BOLD (http://v4.boldsystems.org).
Phylogenetic analyses
The COI fragment was aligned with MUSCLE (Edgar Citation2004) in MEGA X (Kumar et al. Citation2018) and subsequently amino acid translated to check for stop codons that would indicate pseudogenes. The 28S fragment was aligned online with MAFFT 7 (Katoh & Standley Citation2013) (https://mafft.cbrc.jp/alignment/server/) using the G-INS-I method. Evolutionary models for each gene fragment (including the three codon positions in COI) were selected with PartitionFinder (Lanfear et al. Citation2012) using the greedy algorithm and the Bayesian information criterion.
Gene trees were calculated separately for the COI and 28S fragments. For this, we used the maximum likelihood (ML) method implemented on the W-IQ-TREE webserver (Trifinopoulos et al. Citation2016). Node support was assessed with 1000 ultra-fast bootstrap replicates (Hoang et al. Citation2018) and the Shimodaira-Hasegawa approximate likelihood ratio test (Shimodaira & Hasegawa Citation1999). For COI we used the GTR+G model further partitioned into codon positions, and for 28S the K80+I model. A concatenated analysis of both markers was also run in IQTREE using a partitioned edge-linked model and the GTR+I+G model.
In order to gain a more detailed insight into evolutionary patterns, we generated haplotype networks for each marker. For this purpose we used Haploviewer (Salzburger et al. Citation2011) with input ML phylogenetic trees calculated in MEGA X.
In addition, the number of base differences per site (p-distance) between COI and 28S sequences was calculated in MEGA X, including all codon positions. All positions containing gaps and missing data were eliminated.
Molecular species delimitation and divergence time estimation
We applied three unilocus and one multilocus species delimitation methods to test the hypothesis that D. bispinosus harbors putative cryptic species. The unilocus methods were applied for the COI and 28S fragments separately while the multilocus method was applied to both markers simultaneously. In order to increase the accuracy of the results the dataset consisted of sequences of all the species, i.e., D. haemobaphes, D. villosus and P. robustoides.
The two unilocus methods consisted of two fundamentally different approaches. First, we implemented two distance-based approaches: (1) automatic classification of sequences into putative species clusters based on a barcode gap using the online version of the ASAP software (https://bioinfo.mnhn.fr/abi/public/asap/) (Puillandre et al. Citation2021), distances were computed using the K2p model; (2) Barcode Index Number (BIN), which is a method implemented in BOLD, where newly submitted and already available sequences (COI 5ʹ only) are indexed based on the genetic distance method, and clustered in unique BINs (Ratnasingham & Hebert Citation2013). Second, we applied a tree-based approach which models speciation along the branches of a given phylogenetic tree using the online version of the mPTP software (https://mcmc-mptp.h-its.org/mcmc/) (Kapli et al. Citation2017). As an input we used the ML trees calculated with IQTREE (see above).
For the multilocus species delimitation, we implemented the Bayes factors species delimitation approach (Grummer et al. Citation2014). For this we used a Bayesian implementation of the multispecies coalescent in BEAST 1.8.2 (Drummond et al. Citation2012) with the *BEAST package (Heled & Drummond Citation2010). The species hypotheses proposed by ASAP and mPTP were tested against each other as well as against the null hypothesis of a single species. The marginal log likelihood of each species hypothesis was calculated using path and stepping stone sampling (Baele et al. Citation2012, Citation2013). The marginal likelihood estimator was run for one million generations, 10 paths steps and alpha set to the default 0.3. Model fit was evaluated by calculating Bayes factors (Kass & Raftery Citation1995). A value of at least 10 was considered as strong support against the competing model.
Within this Bayesian multilocus species tree approach, we also estimated divergence times and phylogenetic relationships. The evolutionary models used were TrNef+G, HKY and GTR for the 1st, 2nd and 3rd COI codon positions, respectively, and the K80 + I model for 28S (as determined with PartitionFinder). The molecular clock was calibrated using amphipod-specific substitution rates for both markers (0.01773 ± 0.004 substitutions/site/Ma for COI, and 0.00161 ± 0.0003 substitutions/site/Ma for 28S) estimated for the freshwater families Crangonyctidae and Pseudocrangonyctidae (Copilaş-Ciocianu et al. Citation2019, Citation2020). A lognormal relaxed clock was used for the fast-evolving COI fragment and a strict clock for the slow-evolving 28S. Speciation was modelled using the Birth-Death process. Two independent runs were conducted for 30 million generations, a thinning of 1000, and the first 1000 trees were discarded as burn-in after visual examination in TRACER 1.6 (Rambaut et al. Citation2014). The two runs converged on identical results.
Results
Phylogenetic analyses
The inferred phylogenetic relationships among populations and species are shown in . Three well supported divergent mitochondrial lineages were observed within D. bispinosus and presence of the two previously known lineages of D. haemobaphes was confirmed. These mitochondrial lineages were also distinct at the nuclear level, albeit with lower support (). Although the D. bispinosus lineages are well supported, the exact phylogenetic relationships between them are not very clear at the individual gene level. The concatenated ML analysis also recovered a similar pattern with poorly supported deep nodes. However, the multilocus species tree was well resolved, with each node receiving maximum support ().
Figure 1. Phylogenetic relationships of Dikerogammarus species based on maximum-likelihood gene trees (left) and haplotype networks (right) of the COI and 28S fragments. Numbers above tree branches represent statistical support (ultrafast bootstrap and Shimodaira-Hasegawa approximate likelihood ratio test). Colored boxes next to the trees indicate the results of the species delimitation analyses. Numbers within the mPTP column are posterior probabilities for species support.
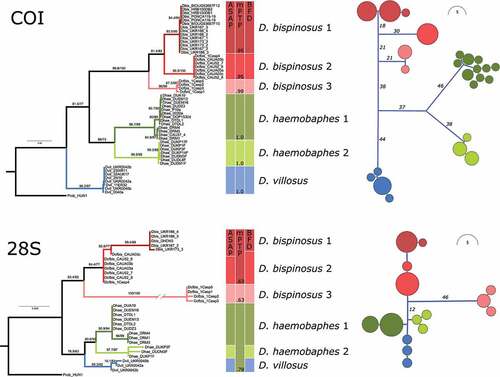
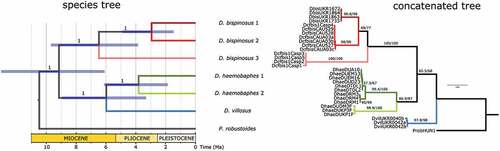
Figure 2. Phylogeny of Dikerogammarus based on a combined COI and 28S dataset. Left, time-calibrated Bayesian species tree. Numbers above branches are posterior probabilities. Right, maximum-likelihood tree based on locus concatenation. Numbers above branches represent statistical support from ultrafast bootstrap and the Shimodaira-Hasegawa approximate likelihood ratio test.
MOTU delimitation
The distance-based ASAP method gave identical results for both the COI (ASAP-score 2, p = 0.00001, threshold distance 0.05) and 28S (ASAP-score 2, p = 0.0185, threshold distance 0.003) fragments by delimiting the same seven MOTUs (Molecular Operational Taxonomic Units): three within D. bispinosus, two within D. haemobaphes and one for D. villosus (). The tree-based mPTP method recovered the same seven MOTUs for COI (probability ≥ 0.95), and only three MOTUs for 28S, lumping together D. bispinosus MOTU 1 and 2, as well as both MOTUs of D. haemobaphes with D. villosus (). However, the three 28S MOTUs had a low probability, ranging from 0.63 to 0.78.
The BIN delimitation method revealed seven BINs, fully congruent with the MOTUs delimited with ASAP and mPTP based on COI. Sequences of D. bispinosus grouped under three BINs: ADM9911 (known already from Europe), ADY3531 (Kazakhstan and Azerbaijan – new BIN), and AEC3943 (Kazakhstan, also new BIN). Dikerogammarus haemobaphes grouped in two BINS (AAX9262, ADB9467), recently presented and discussed in Jażdżewska et al. (Citation2020), and D. villosus grouped under one BIN (AAI9938) which is up to now the only known BIN for this species in BOLD.
For the multilocus MOTU delimitation approach we compared three MOTU hypotheses regarding the number of putative cryptic MOTUs within D. bispinosus: three MOTUs (as suggested by ASAP based on COI and 28S, and mPTP based on COI), two MOTUs (as suggested by ASAP based on 28S), and the null hypothesis of one MOTU. The model that had the highest fit to the data was the three-MOTU hypothesis (ln = −4988.44), followed by the two MOTU (ln = −5002.78) and one MOTU (ln = −5016.96) hypotheses (). The three-MOTU model had a decisive support (>10) versus the two MOTU model (2ln Bayes factors = 28.66) or one MOTU (2ln Bayes factors = 57.04) ().
Table II. Results of Bayes factor species delimitation (BFD) based on Bayesian multilocus analysis of COI and 28S fragments
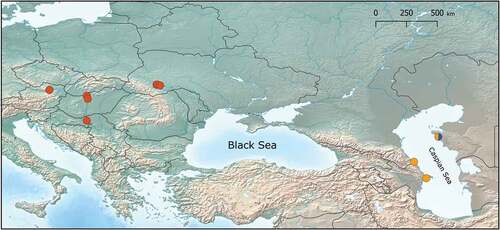
Thus, based on the above results, we define three MOTUs within D. bispinosus: D. bispinosus 1, comprising populations from the Black Sea drainages (Hungary, Austria, Croatia and Ukraine), followed by D. bispinosus 2 and D. bispinosus 3, both apparently restricted to the Caspian basin (). Detailed MOTU distribution is shown on .
Figure 3. Distribution of D. bispinosus MOTUs: D. bispinosus 1 – red; D. bispinosus 2 – yellow; D. bispinosus 3 – blue.
There is a high genetic divergence between the population of D. bispinosus from Europe (Hungary, Austria, Croatia and Ukraine) and the populations from Caspian regions (Kazakhstan and Azerbaijan). The p-distance between D. bispinosus 1 (corresponding to European MOTU) and D. bispinosus 2 (from Kazakhstan and Azerbaijan) and D. bispinosus 3 (Kazakhstan) are 10 and 8% respectively for COI, and 0.6% and 3% for 28S; p-distance between D. bispinosus 2 and D. bispinosus 3 = 12% for COI and 3% for 28S ().
Table III. Estimates of evolutionary divergence between groups (mean p-distances) based on COI gene region (below diagonal) and 28S (above diagonal)
Divergence times
The time-calibrated multilocus species tree indicated a crown age for the Dikerogammarus species of ca. 9.2 Ma (HPD = 13–6 Ma). The species tree was well resolved, each node receiving a maximum support (). The D. bispinosus complex probably started diversifying at ca. 6.5 Ma (HPD = 9.7–3.8 Ma), when MOTU 3 branched off. MOTU 1 and 2 diverged around 2.5 Ma ago (HPD = 4.9–1.7 Ma). D. bispinosus appears to be in a sister relationship to a clade containing both lineages of D. haemobaphes and D. villosus. These latter two species split probably ca. 6 Ma ago. The two D. haemobaphes lineages split around 3.8 Ma ago ().
SEM imaging
The SEM imaging indicated noticeable differences among D. bispinosus MOTUs regarding the setation patterns of the inner side of the second gnathopod propodus (). Specifically, D. bispinosus 1 and 2 had tufts of dense, long and somewhat curled setae along the palmar edge and the posterior side of the propodus, while D. bispinosus 3 had much shorter, sparser and straight setae. D. bispinosus 1 and 2 could be further differentiated by the presence of long setae along the anterior margin of the propodus in the former, while the latter had two to three times shorter setae. Additionally, D. bispinosus 3 had eight setal clusters along the anterior margin of the propodus versus four clusters in D. bispinosus 1 and 2.
Figure 4. SEM pictures of antenna 2 and gnathopod 2 from D. bispinosus 1 (A, B), D. bispinosus 2 (C, D) and D. bispinosus 3 (E, F) respectively.
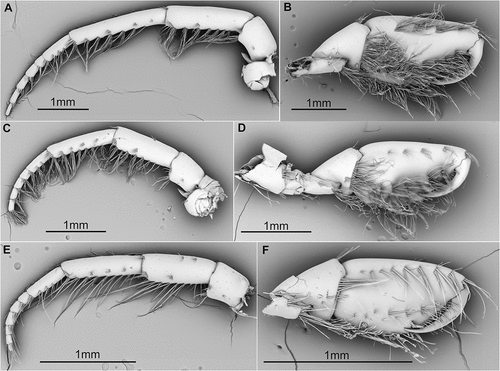
With respect to the second antenna the differences were not so pronounced (). D. bispinosus 1 and 2 appear to have a larger number of setae (>10) than D. bispinosus 3 (max. 5–6) within the setal clusters located on the peduncular segments. Overall, with respect to the studied appendages it appears that D. bispinosus 1 and 2 are more similar to each other than to D. bispinosus 3.
Discussion
Our study reveals that D. bispinosus comprises three evolutionary lineages that are distinct at the molecular, morphological and, partially, at the geographical level. These findings have important implications regarding the taxonomy as well as the alien vs. native status of this species within various geographic areas.
The status (native or invasive) of D. bispinosus in the Caspian basin was highly uncertain due to conflicting reports and fluctuating taxonomy (Copilaș-Ciocianu & Arbačiauskas Citation2018). The most probable invasion pathway is considered from the Black Sea basin, via the Volga-Don canal, to the Caspian basin (Sayapin Citation2003; Voronin & Yermokhin Citation2004; Filinova & Sonina Citation2012). On the other hand, the taxonomy of the invasive Dikerogammarus species was also uncertain until recent molecular studies (Müller et al. Citation2002; Mamos et al. Citation2021) and the possibility was raised that D. bispinosus might have been overlooked for a long time in the Caspian basin (Copilaș-Ciocianu & Arbačiauskas Citation2018). Our study finally settles the issue by indicating that the Caspian and Black Sea basins harbour two and one, respectively, evolutionary independent lineages that have diverged before the Pleistocene. This clearly indicates that D. bispinosus is native to both the Caspian and the Black Sea basins. However, a detailed molecular study of specimens from the Don and Volga rivers is required to confirm the origin of these particular populations.
Geological history and phylogenetic pattern
We consider that the uncovered phylogenetic patterns and associated divergence times coincide with the geological history of the Ponto-Caspian region. In the late Miocene the Paratethys Sea was divided into more or less isolated parts (western part became more brackish, eastern – marine) by the mountain uplift in the Alpine belt (Popov et al. Citation2004; Palcu et al. Citation2021). Based on our molecular clock results, it appears that the divergence of D. bispinosus from the clade encompassing D. haemobaphes and D. villosus took place in this period (). This is also consistent with the timing of several extant Ponto-Caspian radiations (8–12 Mya) observed not only in amphipods (Hou & Sket Citation2016), but also mysid crustaceans and gobiid fishes (Audzijonyte et al. Citation2006; Neilson & Stepien Citation2009). Then, at the end of the Miocene, the uplift of the Caucasus started dividing the Caspian Sea basin from the Azov-Black Sea basin (Zenkevich Citation1963; Cristescu et al. Citation2003, Figure 1(c); Grigorovich et al. Citation2003, Figure 1; Popov et al. Citation2006, Figure 2). This event could be the reason why D. bispinosus 3 separated from D. bispinosus 2 and D. bispinosus 1 (possibly as a result of allopatry). Later, in the early Pliocene, the Caspian Sea was completely separated from the Azov-Black Sea and, since then, the fauna of these two basins developed in reciprocal isolation (Zenkevich Citation1956). However, in the late Pliocene, the recurrent transgressions of the Caspian Sea led to reconnections of the two basins via the Kumo-Manich Strait (Popov Citation1955; Mordukhai-Boltovskoj Citation1960, Table 13; Grigorovich et al. Citation2003, Table 1; Dolukhanov et al. Citation2010; Krijgsman et al. Citation2019, Figure 3(b)). These reconnections (2–3.4 Mya, Grigorovich et al. Citation2003, Table 1) could be the reason for the splitting of the MOTUs D. bispinosus 1 and D. bispinosus 2 (), the latter emerging possibly as consequence of re-colonisation of the Caspian Sea. However, the aforementioned scenario should necessarily be considered with caution because it is also possible that D. bispinosus 3 and the ancestor of clades 1 and 2 evolved in sympatry in the Caspian Sea, and then D. bispinosus 1 evolved as a result of colonization of the Pontic basin. It is not possible to confidently reject either of these scenarios based on the available data. More localities have to be examined in order to gain a more complete picture of the evolutionary history of D. bispinosus.
Remarks on distribution and ecology of D. bispinosus
Dikerogammarus bispinosus was originally described as a subspecies of D. villosus (D. villosus bispinosus), occurring in areas with clean and cold water near deep “pits” (12–22 meters) of the Dnieper River (Martynov Citation1925), which are presently flooded by the Kakhovka reservoir. Dikerogammarus bispinosus samples from the Dnieper River preceded by Martynov (Citation1925) were collected by Dr. Beling and Dr. Markovsky. In their later works Beling (Citation1930, Citation1939) reported a freshwater D. villosus population distributed upstream the Dnieper River (above cities Nikopol, Dniepr, Kremenchuk), while Markovsky (Citation1954) described the distribution of two subspecies for the lower Dnieper, confirming D. v. bispinosus in the area of the modern Kakhovsky reservoir and the nominative D. villosus from the delta and estuary. The latter author reported also an intentional introduction of the nominative D. villosus to the upper Dnieper area (Kyiv). Thus, the distribution of D. bispinosus in the Dnieper River is restricted only to the region it was described from. Yet, this species was also reported from the Dniester and Danube basins.
In the Dniester Basin, D. v. bispinosus was reported to occur upstream from Galich (upper river basin) (Dedyu Citation1967). Our D. bispinosus samples from the Dniester River were also collected in this region.
In the Danube Basin, Cărăuşu (Citation1943) reported D. v. bispinosus from the lower Danube (near Olteniţa). There are also records of D. bispinosus from the middle Danube (Germany, Austria, Hungary, Slovakia, Croatia, Serbia, Romania) (Dudich Citation1947; Borza et al. Citation2015, Citation2017, Citation2021; Brtek Citation2001; Copilaș-Ciocianu & Arbačiauskas Citation2018, Figure 3) where it prefers riverine habitats in the middle stretches of large rivers (Borza et al. Citation2017). To sum up, almost all the historical records of D. bispinosus from the Black Sea basin are of riverine freshwater origin. However, it also occurs along the rocky coast of the Black Sea near Odessa, but that zone is desalinated by coastal runoff (Son et al. Citation2010).
In the Azov region, D. bispinosus is considered as an invasive species with reliable reports known from the lower Don (Sayapin Citation2003). In the Caspian basin it is reported also as an invasive from the middle Volga (Voronin & Yermokhin Citation2004; Filinova & Sonina Citation2012) and from the lower Ural (Copilaș-Ciocianu & Arbačiauskas Citation2018). Some ecological differences between the studied MOTUs can be observed, in particular biotopes where they were retrieved from. While our records of D. bispinosus 1 from riverine freshwater (the Dniester and Danube Rivers) are congruent with literature data, both D. bispinosus 2 and D. bispinosus 3 came from the littoral of Caspian Sea, and are therefore ecologically distinct. This distribution pattern may also be explained by (1) different historical past, (2) the effects of different salinity compositions in the neighboring seas (Beklemishev Citation1922; Mudie et al. Citation2017), especially the high level of sulfates and the low level of chlorides in the Caspian water which controls the distribution of biota through “chloridophobia” (Mordukhai-Boltovskoj Citation1960), and (3) the impact of a stronger competing fauna (in particular, the Mediterranean one), which may displace local species.
The putative ecological differences observed among MOTUs raise the interest to reveal the intra-population affinity of the populations reported from the Don, Volga, and Ural Rivers (Voronin & Yermokhin Citation2004; Filinova & Sonina Citation2012; Copilaș-Ciocianu & Arbačiauskas Citation2018), as well as those occurring in the coastal waters of the Black Sea (Son et al. Citation2010). So far, it appears that D. bispinosus 1 is the only invasive lineage. Additional molecular data from the Volga, Don and Ural rivers would show whether the remaining two lineages extend beyond their native range or not.
Morphological differences of lineages
Until recently there were some issues regarding the taxonomic status of D. bispinosus with respect to D. villosus or D. haemobaphes. Some authors considered it a subspecies of D. villosus (e.g. Cărăuşu et al. Citation1955), while others even went as far as considering that D. villosus (together with D. bispinosus) is a synonym of D. haemobaphes (Pjatakova & Tarasov Citation1996). However, the integrative taxonomic study made by Müller et al. (Citation2002), and based on mitochondrial genomes by Mamos et al. (Citation2021) revealed genetic distinctions among these three species and provided clear morphological features for identification: they differ in number of spines on 1st and 2nd urosomites, length of setation on peduncle and flagellum of 2nd antenna, and length of propodus setation of gnathopods. Within the framework of our research we did not observe clear differences among D. bispinosus MOTUs regarding the second antenna, but there were significant differences regarding the setal patterns on the inner side of the propodus of the second gnathopod (). MOTUs D. bispinosus 1 and 2 seem more similar to each other by having longer and denser setae, which reflects well their phylogenetic relationship (). The setal patterns of gnathopod two in D. bispinosus specimens from the lower Ural (Kazakhstan) published by Copilaș-Ciocianu & Arbačiauskas (Citation2018; Figure 2) are similar to these two.
This suggests that the MOTUs may in fact be distinct species, yet more material from the Black, Azov, and Caspian seas, as well as additional molecular markers and morphometry are needed to fully clarify the taxonomic status of the D. bispinosus lineages identified in this study.
Conclusions
Our study revealed that D. bispinosus comprises three independently evolving lineages which are distinct at the molecular (nuclear and mitochondrial), and morphological level and, partially, geographically. One of these lineages, native to the lower reaches of Pontic rivers, is gradually spreading throughout south-western and Central European rivers, while the two others are apparently restricted to the Caspian Sea. We also clarify that D. bispinosus is not a recent invader in the Caspian Sea, but a native species. Our findings show that even relatively simple pilot molecular research may bring surprising and valuable results also in the case of long-studied invasive species.
Acknowledgements
We would like to thank Krešimir Žganec (University of Zadar) and Mišel Jelić (Varaždin City Museum) for providing the individuals of Dikerogammarus bispinosus from the Drava River (Danube drainage, Croatia). We thank Namig Rzayev (Azerbaijan National Academy of Sciences) and Shabnam Farzali (Firat University) for their assistance in collecting the material in the field, and Dmitry Palatov (Lomonosov Moscow State University) for providing additional material.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arbačiauskas K, Lesutiene J, Gasiunaite ZR. 2013. Feeding strategies and elemental composition in Ponto-Caspian peracaridans from contrasting environments: Can stoichiometric plasticity promote invasion success? Freshwater Biology 58(5):1052–1068. DOI:10.1111/fwb.12108.
- Audzijonyte A, Daneliya ME, Väinölä R. 2006. Comparative phylogeography of Ponto-Caspian mysid crustaceans: Isolation and exchange among dynamic inland sea basins. Molecular Ecology 15(10):2969–2984. DOI:10.1111/j.1365-294X.2006.03018.x.
- Baele G, Lemey P, Bedford T, Rambaut A, Suchard MA, Alekseyenko AV. 2012. Improving the accuracy of demographic and molecular clock model comparison while accommodating phylogenetic uncertainty. Molecular Biology and Evolution 29(9):2157–2167. DOI:10.1093/molbev/mss084.
- Baele G, Li WLS, Drummond AJ, Suchard MA, Lemey P. 2013. Accurate model selection of relaxed molecular clocks in Bayesian phylogenetics. Molecular Biology and Evolution 30(2):239–243. DOI:10.1093/molbev/mss243.
- Beklemishev VN. 1922. Novyye dannyye o faune Aral’skogo morya [New data on the fauna of the Aral Sea]. Russkiy gidrobiologicheskiy zhurnal 1(9–10):276–288.
- Beling DE. 1930. Raboty po yzuchenyiu zhyvotnoho naselenyia porozhystoi chasty r. Dniepr [Study of the animal population of the Dnieper River rapids]. Trudy vtoroho vsesoiuznoho hydrolohycheskoho sezda (20–27 Aprelia 1928 g. Lenynhrad) 3:258–260.
- Beling DE. 1939. Do vyvchenyia biotsenoziv kaminnia i shtuchnykh kamianykh sporud u Dniepri [Studies of biocenoses of stones and man-made stone structures in the Dnieper]. Pratsi Naukovo-Doslidnoho Instytutu Biolohii 3:7–47.
- Bij de Vaate A, Jazdzewski K, Ketelaars HAM, Gollasch S, van der Velde G. 2002. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Canadian Journal of Fisheries and Aquatic Sciences 59(7):1159–1174. DOI:10.1139/f02-098.
- Borza P, Csányi B, Đanić V, Kenderov L, Kladarić L, Lešťáková M, Muc T, Němejcová D, Očadlík M, Paunović M, Rotar B, Szekeres J, Veseli M, Zorić K.2021.Peracarid crustaceans in the River Danube and its tributaries: Results of the 4th Joint Danube Survey.BioInvasions Records 10(3):623–628 in press DOI:10.3391/bir.2021.10.3.12
- Borza P, Csányi B, Huber T, Leitner P, Paunović M, Remund N, Szekeres J, Graf W. 2015. Longitudinal distributional patterns of Peracarida (Crustacea, Malacostraca) in the River Danube. Fundamental and Applied Limnology. Archiv für Hydrobiologie 187(2):113–126. DOI:10.1127/fal/2015/0769.
- Borza P, Huber T, Leitner P, Remund N, Graf W. 2017. Current velocity shapes co-existence patterns among invasive Dikerogammarus species. Freshwater Biology 62(2):317–328. DOI:10.1111/fwb.12869.
- Brtek J. 2001. Contribution to knowledge of amphipods of Slovakia. Acta Rerum Naturalium Musei Nationalis Slovenici 47:65–89.
- Cărăuşu S. 1943. Amphipodes de Roumanie. Gammarides de type Caspien [Amphipods from Romania. Caspian-type gammarids]. Bucurestu: Institutul de Cercetãri Piscicole al României.
- Cărăuşu S, Dobreanu E, Manolache C. 1955. Fauna Republicii Populare Romîne. Crustacea. Amphipoda Forme Salmastre şi de Apă Dulce. Bucharest: Editura Academiei RPR.
- Casquet J, Thebaud C, Gillespie RG. 2012. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Molecular Ecology Resources 12(1):136–141. DOI:10.1111/j.1755-0998.2011.03073.x.
- CCDB. Canadian Centre of DNA Barcode. CCDB protocols. Available: https://ccdb.ca/resources/.
- Copilaș-Ciocianu D, Arbačiauskas K. 2018. First record of Dikerogammarus bispinosus Martynov, 1925 in Kazakhstan: Invasive or overlooked native in the Caspian Sea basin? BioInvasions Records 7(3):285–291. DOI:10.3391/bir.2018.7.3.09.
- Copilaş-Ciocianu D, Borko Š, Fišer C. 2020. The late blooming amphipods: Global change promoted post-jurassic ecological radiation despite Palaeozoic origin. Molecular Phylogenetics and Evolution 143:106664. DOI: 10.1016/j.ympev.2019.106664.
- Copilas-Ciocianu D, Sidorov D. 2021. Taxonomic, ecological and morphological diversity of Ponto-Caspian gammaridean amphipods: A review. Organisms Diversity & Evolution, in press. DOI:10.1007/s13127-021-00536-6
- Copilaş-Ciocianu D, Sidorov DA, Gontcharov AA. 2019. Adrift across tectonic plates: Molecular phylogenetics supports the ancient Laurasian origin of old limnic crangonyctid amphipods. Organisms Diversity & Evolution 19(2):191–207. DOI:10.1007/s13127-019-00401-7.
- Copilaș-Ciocianu D, Sidorov D, Šidagytė-Copilas E. 2021. Global distribution and diversity of alien Ponto-Caspian amphipods. bioRxiv (preprint). DOI:10.1101/2021.07.19.452907
- Cristescu MEA, Hebert PDN. 2005. The “Crustacean Seas” — An evolutionary perspective on the Ponto-Caspian peracarids. Canadian Journal of Fisheries and Aquatic Sciences 62(3):505–517. DOI:10.1139/f04-210.
- Cristescu MEA, Hebert PDN, Onciu TM. 2003. Phylogeography of Ponto-Caspian crustaceans: A benthic – Planktonic comparison. Molecular Ecology 12(4):985–996. DOI:10.1046/j.1365-294X.2003.01801.x.
- Cristescu MEA, Witt JDS, Grigorovich IA, Hebert PDN, MacIsaac HJ. 2004. Dispersal of the Ponto-Caspian amphipod Echinogammarus ischnus: Invasion waves from the Pleistocene to the present. Heredity 92(3):197–203. DOI:10.1038/sj.hdy.6800395.
- Csabai Z, Borza P, Rewicz T, Pernecker B, Berta BJ, Móra A. 2020. Mass appearance of the Ponto-Caspian invader Pontogammarus robustoides in the River Tisza catchment: Bypass in the southern invasion corridor? Knowledge and Management of Aquatic Ecosystems 421(421):9. DOI:10.1051/kmae/2020003.
- Dedyu II. 1967. Amfipody i mizidy basseinov rek Dnestra i Pruta [Amphipods and mysids of Dniester and Prut River Basins]. Moscow: Nauka.
- Derzhavin AN. 1948. Novye formy gammarid iz Kaspiiskogo Morya [New forms of Gammarid from the Caspian Sea. In: Pavlovskyi EN, Shadin VI, editors. Pamiaty akademika Sergeia Aleksandrovicha Zernova. Moscow: USSR Academy of Sciences Publishing House. pp. 280–286.
- Dolukhanov PM, Chepalyga AL, Lavrentiev NV. 2010. The Khvalynian transgressions and early human settlement in the Caspian Basin. Quaternary International 225(2):152–159. DOI:10.1016/j.quaint.2009.10.039.
- Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29(8):1969–1973. DOI:10.1093/molbev/mss075.
- Dudich E. 1947. Die höheren Krebse (Malacostraca) der Mittel-Donau. Fragmenta Faunistica Hungarica 10(4):125–132.
- Edgar RC. 2004. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32(5):1792–1797. DOI:10.1093/nar/gkh340.
- Eggers TO, Martens A. 2001. Bestimmungsschlüssel der Süßwasser-Amphipoda (Crustacea) Deutschlands/A key to the freshwater Amphipoda (Crustacea) of Germany. Lauterbornia 42:1–70.
- Filinova EI, Sonina EE. 2012. Gammaridy poymennykh uchastkov Volgogradskogo vodokhranilishcha [Gamarids of the floodplain areas of the Volgograd Reservoir]. Actual Problems of Studying Crustaceans of Continental Waters: International School-Conference of the Institute of Biology of Inland Waters I.D, Russia. Papanin. Borok 1:303–306.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5):294–299. PMID: 7881515.
- Grabowski M, Bacela K, Konopacka A. 2007. How to be an invasive gammarid (Amphipoda: Gammaroidea) – Comparison of life history traits. Hydrobiologia 590(1):75–84. DOI:10.1007/s10750-007-0759-6.
- Grabowski M, Rewicz T, Bacela-Spychalska K, Konopacka A, Mamos T, Jazdzewski K. 2012. Cryptic invasion of Baltic lowlands by freshwater amphipod of Pontic origin. Aquatic Invasions 7(3):337–346. DOI:10.3391/ai.2012.7.3.005.
- Grigorovich IA, Therriault TW, MacIsaac HJ. 2003. History of aquatic invertebrate invasions in the Caspian Sea. Biological Invasions 5(1/2):103–115. DOI:10.1023/A:1024050824073.
- Grummer JA, Bryson RW, Reeder TW. 2014. Species delimitation using bayes factors: Simulations and application to the Sceloporus scalaris Species Group (Squamata: Phrynosomatidae). Systematic Biology 63(2):119–133. DOI:10.1093/sysbio/syt069.
- Hebert PD, Cywinska A, Ball SL, Dewaard JR. 2003. Biological identifications through DNA barcodes. Proceedings of the Royal Society B: Biological Sciences 270(1512):313–321. DOI:10.1098/rspb.2002.2218.
- Heled J, Drummond AJ. 2010. Bayesian inference of species trees from multilocus data. Molecular Biology and Evolution 27(3):570–580. DOI:10.1093/molbev/msp274.
- Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Molecular Biology and Evolution 35(2):518–522. DOI:10.1093/molbev/msx281.
- Hou ZG, Fu JH, Li SQ. 2007. A molecular phylogeny of the genus Gammarus (Crustacea: Amphipoda) based on mitochondrial and nuclear gene sequences. Molecular Phylogenetics and Evolution 45(2):596–611. DOI:10.1016/j.ympev.2007.06.006.
- Hou Z, Li S. 2018. Tethyan changes shaped aquatic diversification. Biological Reviews 93(2):874–896. DOI:10.1111/brv.12376.
- Hou Z, Sket B. 2016. A review of Gammaridae (Crustacea: Amphipoda): The family extent, its evolutionary history, and taxonomic redefinition of genera. Zoological Journal of the Linnean Society 176(2):323–348. DOI:10.1111/zoj.12318.
- Hou Z, Sket B, Li S. 2014. Phylogenetic analyses of Gammaridae crustacean reveal different diversification patterns among sister lineages in the Tethyan region. Cladistics 30(4):352–365. DOI:10.1111/cla.12055.
- Jażdżewska AM, Rewicz T, Mamos T, Wattier R, Bącela-Spychalska K, Grabowski M. 2020. Cryptic diversity and mtDNA phylogeography of the invasive demon shrimp, Dikerogammarus haemobaphes (Eichwald, 1841), in Europe. NeoBiota 57:53–86. DOI: 10.3897/neobiota.57.46699.
- Jażdżewski K, Konopacka A. 1988. Notes on the Gammaridean Amphipoda of the Dniester River Basin and Eastern Carpathians. Proceedings of the VIth International Colloquium on Amphipod Crustaceans, Ambleteuse, France, 28 June-3 July 1985, pp 72–78.
- Jones RW, Simmons MD. 1997. A review of the stratigraphy of Eastern Paratethys (Oligocene–Holocene), with particular emphasis on the Black Sea. In: Robinson AG, editor. Regional and petroleum geology of the Black Sea and surrounding region. Oklahoma, Tulsa: American Association of Petroleum Geologists. pp. 39–52.
- Kapli P, Lutteropp S, Zhang J, Kobert K, Pavlidis P, Stamatakis A, Flouri T. 2017. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 33(11):1630–1638. DOI:10.1093/bioinformatics/btx025.
- Karaman GS, and Barnard JL 1979. Classificatory Revisions In Gammaridean Amphipoda Crustacea 1. Proceedings of The Biological Society of Washington 9(1):106–165.
- Kass R, Raftery A. 1995. Bayes factors. Journal of the American Statistical Association 90(430):773–795. DOI:10.1080/01621459.1995.10476572.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30(4):772–780. DOI:10.1093/molbev/mst010.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Chris Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649. DOI:10.1093/2Fbioinformatics/2Fbts199.
- Krijgsman W, Tesakov A, Yanina T, Lazarev S, Danukalova G, Van Baak CGC, Agustí J, Alçiçek MC, Aliyeva E, Bista D, Bruch A, Büyükmeriç Y, Bukhsianidze M, Flecker R, Frolov P, Hoyle TM, Jorissen EL, Kirscher U, Koriche SA, Kroonenberg SB, Lordkipanidze D, Oms O, Rausch L, Singarayer J, Stoica M, van de Velde S, Titov VV, Wesselingh FP. 2019. Quaternary time scales for the Pontocaspian domain: Interbasinal connectivity and faunal evolution. Earth-Science Reviews 188:1–40. DOI: 10.1016/j.earscirev.2018.10.013.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35(6):1547–1549. DOI:10.1093/molbev/msy096.
- Labat F, Piscart C, Fontan B. 2011. First records, pathways and distributions of four new Ponto-Caspian amphipods in France. Limnologica 41(4):290–295. DOI:10.1016/j.limno.2010.12.004.
- Lanfear R, Calcott B, Ho SYW, Guindon S. 2012. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29(6):1695–1701. DOI:10.1093/molbev/mss020.
- Mamos T, Grabowski M, Rewicz T, Bojko J, Strapagiel D, and Burzyński A. 2021. Mitochondrial Genomes, Phylogenetic Associations, and SNP Recovery for the Key Invasive Ponto-Caspian Amphipods in Europe. International Journal of Molecular Sciences 22:10300. DOI:10.3390/ijms221910300.
- Markovsky YM. 1954. Fauna bespozvonochnykh nizov’ev rek Ukrainy, usloviya sushchestvovaniya i puti ispol’zovaniya. Chast’ 2. Dneprovsko-Bugskii liman [Fauna of invertebrates of the lower river streams of Ukraine, life conditions and ways of utilization. Part 2. Dnieper-Bug lagoon.]. Kiev: AN USSR.
- Martynov AM. 1925. Gammaridae nizhnego Dnepra [Gammaridae of the Lower Dnepr]. Trudy Vseukrainsoi Gosudarstvennoi Chernomorsko-Azovskoi Nauchno-promyshlennoi Stantsii 1:135–151.
- Mordukhai-Boltovskoj FD. 1960. Kaspiiskaya Fauna v Azovo-Chernomorskom basseine [Caspian fauna in the Azov and Black Seas basin]. Leningrad: Izdatelstvo Akademii nauk USSR.
- Mudie PJ, Marret F, Mertens KN, Shumilovskikh L, Leroy SA. 2017. Atlas of modern dinoflagellate cyst distributions in the Black Sea Corridor: From Aegean to Aral Seas, including Marmara, Black, Azov and Caspian Seas. Marine Micropaleontology 134:1–152. DOI: 10.1016/j.marmicro.2017.05.004.
- Müller JC, Schramm S, Seitz A. 2002. Genetic and morphological differentiation of Dikerogammarus invaders and their invasion history in Central Europe. Freshwater Biology 47(11):2039–2048. DOI:10.1046/j.1365-2427.2002.00944.x.
- Müller J, Schramm S. 2001. A third Dikerogammarus invader is located in front of Vienna. Lauterbornia 41:49–52.
- Nahavandi N, Ketmaier V, Plath M, Tiedemann R. 2013. Diversification of Ponto-Caspian aquatic fauna: Morphology and molecules retrieve congruent evolutionary relationships in Pontogammarus maeoticus (Amphipoda: Pontogammaridae). Molecular Phylogenetics and Evolution 69(3):1063–1076. DOI:10.1016/j.ympev.2013.05.021.
- Neibera MT, Walthera F, Kijashko PV, Mumladzec L, Hausdorf B. 2021. The role of Anatolia in the origin of the Caucasus biodiversity hotspot illustrated by land snails in the genus Oxychilus. Cladistics:1–20. DOI: 10.1111/cla.12479.
- Neilson ME, Stepien CA. 2009. Escape from the Ponto-Caspian: Evolution and biogeography of an endemic goby species flock (Benthophilinae: Gobiidae: Teleostei). Molecular Phylogenetics and Evolution 52(1):84–102. DOI:10.1016/j.ympev.2008.12.023.
- Özbek M, Özkan N. 2011. Dikerogammarus istanbulensis sp. n., a new amphipod species (Amphipoda: Gammaridae) from Turkey with a key for the genus. Zootaxa 2813(1):55–64. DOI:10.11646/zootaxa.2813.1.2.
- Palcu DV, Patina IS, Șandric I, Lazarev S, Vasiliev I, Stoica M, Krijgsman W. 2021. Late Miocene megalake regressions in Eurasia. Scientific Reports 11(1):11471. DOI:10.1038/s41598-021-91001-z.
- Pjatakova GM, Tarasov AG. 1996. Caspian Sea amphipods: Biodiversity, systematic position and ecological peculiarities of some species. International Journal of Salt Lake Research 5(1):63–79. DOI:10.1007/BF01996036.
- Popov GI. 1955. Istoriya Manychskogo proliva v svyazi so stratigrafiyey chernomorskikh i kaspiyskikh chetvertichnykh otlozheniy [Istoriya Manychskogo proliva v svyazi so stratigrafiyey chernomorskikh i kaspiyskikh chetvertichnykh otlozheniy]. Byulleten’ moskovskogo obshchestva ispytateley prirody. Otdel Geologicheskiy 20(2):21–49.
- Popov SV, Rögl F, Rozanov AY, Steiniger FF, Shcherba IG, Kovac M. 2004. Lithological-Paleogeographic maps of Paratethys:10 maps Late Eocene to Pliocene. Courier Forschunginstitut Senckenberg 250:1–46.
- Popov SV, Shcherba IG, Ilyina LB, Nevesskaya LA, Paramonova NP, Khondkarian SO, Magyar I. 2006. Late Miocene to Pliocene palaeogeography of the Paratethys and its relation to the Mediterranean. Palaeogeography, Palaeoclimatology, Palaeoecology 238(1–4):91–106. DOI:10.1016/j.palaeo.2006.03.020.
- Puillandre N, Brouillet S, Achaz G. 2021. ASAP: Assemble species by automatic partitioning. Molecular Ecology Resources 21(2):609–620. DOI:10.1111/1755-0998.13281.
- Rambaut A, Suchard M, Xie D, Drummond AJ. 2014. Tracer v1.6. Available: https://beast.community/tracer.
- Ratnasingham S, Hebert PD. 2013. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PloS One 8(7):e66213. DOI:10.1371/journal.pone.0066213.
- Ratnasingham S, Hebert PDN. 2007. BOLD: The Barcode of life data system (www.barcodinglife.org). Molecular Ecology Notes 7(3):355–364. DOI:10.1111/2Fj.1471-8286.2007.01678.x.
- Rewicz T, Wattier R, Grabowski M, Rigaud T, Bącela-Spychalska K. 2015. Out of the Black Sea: Phylogeography of the Invasive Killer Shrimp Dikerogammarus villosus across Europe. PLoS ONE 10(2):e0118121. DOI:10.1371/journal.pone.0118121.
- Salzburger W, Ewing GB, Von Haeseler A. 2011. The performance of phylogenetic algorithms in estimating haplotype genealogies with migration. Molecular Ecology 20(9):1952–1963. DOI:10.1111/j.1365-294X.2011.05066.x.
- Sands AF, Neubauer TA, Nasibi S, Fasihi Harandi M, Anistratenko VV, Wilke T, Albrecht C. 2019b. Old lake vs. young taxa: A comparative phylogeographic perspective on the evolution of Caspian Sea gastropods (Neritidae: Theodoxus). Royal Society Open Science 6(10):190965. DOI:10.1098/rsos.190965.
- Sands AF, Sereda SV, Stelbrink B, Neubauer TA, Lazarev S, Wilke T, Albrecht C. 2019a. Contributions of biogeographical functions to species accumulation may change over time in refugial regions. Journal of Biogeography 46(6):1274–1286. DOI:10.1111/jbi.13590.
- Sayapin VV. 2003. Bokoplavy (Crustacea, Amphipoda), kak sostavlyayushchiy komponent biologicheskikh resursov Nizhnego Dona [Amphipoda (Crustacea, Amphipoda), as a component of the biological resources of the Lower Don]. PhD thesis, Kuban State University, Krasnodar, Russia.
- Shimodaira H, Hasegawa M. 1999. Multiple comparisons of Log-Likelihoods with applications to phylogenetic inference. Molecular Biology and Evolution 16(8):1114–1116. DOI:10.1093/oxfordjournals.molbev.a026201.
- Son MO, Koshelev AV, Kudrenko SA. 2010. Osobennosti kolonizatsii i obitaniya morskikh i solonovatovodnykh bespozvonochnykh v biotopakh kontura “malyy vodotok – More” [Features of colonization and occurring of marine and brackishwater invertebrates in the contour habitats “small stream sea”]. Mors’kiy Ekologíchniy Zhurnal 3:78–82.
- Stock H, Mirzajani AR, Vonk R, Naderi S, Kiabi BH. 1998. Limnic and brackish water Amphipoda (Crustacea) from Iran. Beaufortia 48(9):173–234.
- Stock JH. 1974. The systematics of certain Ponto-Caspian Gammaridae (Crustacea, Amphipoda). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 70:75–95.
- Tarasov AG. 1995. Fauna vysshikh rakoobraznykh (Crustacea, Malacostraca) reki Ural [Crustacean Fauna (Malacostraca) of the Ural River]. Zoologichesky Zhurnal 74(3):24–34.
- Trifinopoulos J, Nguyen L-T, Von Haeseler A, Minh BQ. 2016. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44(W1):232–235. DOI:10.1093/nar/gkw256.
- Voronin MY, Yermokhin MV. 2004. Stability of the onthogenesis of amphipods (Crustacea, Amphipoda) and the outlook of its usage for biomonitoring of the ecosystems of cooling reservoirs of nuclear power stations. Povolzhsky Ekologichesky Zhurnal 2:123–131.
- Zenkevich L. 1963. Biology of the Seas of the USSR. London: George Allen & Unwin. DOI:10.1038/200617a0.
- Zenkevich LA. 1956. Moria SSSR, ikh fauna I flora. Izdanie 2-e, dopolnenoe [Sea of USSR, their flora and fauna]. 2nd ed. Moscow: Uchpedgiz. extended.
