 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The Betta broodfish supply in Indonesia majorly comes from wild populations and fetches a higher price compared to hatchery cross-breeds, especially Betta rubra. This condition exerts pressure on the wild populations. Therefore, this study aims to examine the diversity, distribution, and conservation status of Betta fish species list in the study area. A total of 422 Betta fishes were collected from January 2019 to January 2021 from 19 of 59 sampling locations surveyed, consisting of four species, namely, Betta imbellis (TL: 14.09–31.98 mm; BW: 0.03–0.39 g), B. splendens (TL: 21.09–40.14 mm; BW: 0.05–0.70 g), B. rubra (TL: 11.35 to 47.80 mm; BW: 0.02–0.90 g), and B. dennisyongi (TL: 20.09–47.81 mm; BW: 0.07–0.91 g). There are four Betta species occurred in Aceh waters, namely B. dennisyongi, B. rubra, B. splendens, and B. imbellis. The results showed that B. dennisyongi was found at 8 sampling locations and had higher local distribution (13.56% LD) followed by B. rubra (8.47% LD), B. splendens (6.78% LD) and B. imbellis which had the lowest distribution and was only found in two locations (3.39% LD). Furthermore, field measurements (in situ) of water quality in the wild habitat showed temperature ranging from 23.7 to 31.9°C while pH ranged from 5.24 to 8.51. Based on the IUCN Redlist data, B. rubra is categorized as being critically endangered.
Introduction
Aceh Province is one of the richest ichthyofaunal regions in Indonesia as over 114 species of freshwater fish have been reported from this area (Muchlisin & Azizah Citation2009). Among these species, 25 reportedly have great potential as ornamental fish including species within the genus Betta popularly known as the fighting fish (Muchlisin Citation2013). Based on Fishbase (Citation2020), 79 species of Betta fish have been described worldwide, where 63 species occur in Asia, of which 52 species are found in Indonesian waters. This fish has a breathing apparatus called labyrinth (Allen & Nicoletto Citation1997; Apriliani et al. Citation2018; Watson et al. Citation2019), and a wide feeding spectrum (Monvises et al. Citation2009), therefore, enabling it to occupy both lotic and lentic freshwater ecosystems (Schindler & Schmidt Citation2006). Furthermore, the adult males provide parental care (Rüber et al. Citation2004; Forsatkar et al. Citation2014) divided into nest-builder and mouthbrooder, usually solitary and territorial (Goldstein Citation2015), have an attractive color pattern and are therefore very popular as aquarium fish (Monvises et al. Citation2009; Thongprajukaew et al. Citation2011; Alderton Citation2012).
The supply of Betta fish in Indonesia, especially in Aceh Province mostly comes from the wild which has a higher selling price compared to cultured population particularly for rare species, such as B. rubra and B. dennisyongi. This condition threatens wild populations and is further exacerbated by habitat perturbations due to land conversion to plantations, climate change, highways, and settlements including in Aceh Province (Low Citation2019b; Nur et al. Citation2020). According to the IUCN data in 2020, a total of 21 Betta species were recorded from inland waters of Sumatra, Indonesia, where 28.6% are critically endangered, least concern (28.6%), endangered (19.0%), vulnerable (19%) and deficient data (4.8%) (IUCN Citation2020).
Studies on the diversity and distribution of Betta fish in the Southeast Asian region have also been reported from several countries, for instance, 12 species were reported from Thailand, namely B. simplex, B. prima, B. pugnax, B. pi (Tan & Ng Citation2005; Tanpitayacoop & Na-Nakorn Citation2005), B. smaragdina (Tan & Ng Citation2005; Monvises et al. Citation2009), B. mahachaiensis, B. siamorientalis (Kowasupat et al. Citation2012a, Citation2012b), B. imbellis, B. splendens (Tan & Ng Citation2005; Kowasupat et al. Citation2014), B. pallida, B. ferox and B. apollon (Panijpan et al. Citation2014). Furthermore, three species were also identified in Cambodian waters, namely B. prima (Tan & Ng Citation2005), B. siamorientalis and B. stiktos (Kowasupat et al. Citation2012a, Citation2014), while two species were reported from Brunei (B. macrostoma and B. akarensis) and two species from Singapore (B. pugnax and B. imbellis) (Tan & Ng Citation2005). In addition, 24 species were found in Malaysian waters namely B. coccina, B. pulchra, B. stigmosus, B. waseri, B. ibanorum, B. macrostoma, B. akarensis, B. chini, B. gladiator, B. lehi, B. taeniata, B. brownorum, B. pi (Tan & Ng Citation2005), B. ocellata, B. persephone, B. tussyae, B. livida, B. hipposideros, B. pugnax (Tan & Ng Citation2005; Kowasupat et al. Citation2014), B. imbellis (Tan & Ng Citation2005; Kowasupat et al. Citation2014), B. kuehnei, B. stigmosa, B. waseri, and B. bellica (Panijpan et al. Citation2014).
Similar studies conducted in Indonesia reported 27 species from Kalimantan, namely, B. breviobesus, B. lehi, B. balunga, B. pinguis, B. obscura, B. pallifina, B. taeniata, B. rutilans, B. brownorum, B. foerschi, B. strohi, B. anabatoides, B. channoides, B. dimidiata (Tan & Ng Citation2005), B. albimarginata, B. edithae, B. enisae, B. unimaculata, B. patoti, B. ocellata (Tan & Ng Citation2005, Citation2006; Sriwattanarothai et al. Citation2010; Panijpan et al. Citation2014), B. antoni, B. krataios, B. mandor, B. compuncta, B. ideii (Tan & Ng Citation2006; Panijpan et al. Citation2014), B. uberis and B. anabantoides (Tan & Ng Citation2005; Sriwattanarothai et al. Citation2010; Panijpan et al. Citation2014), as well as 16 species from Sumatra namely B. bellica, B. simorum, B. pugnax, B. schalleri, B. coccina, B. miniopinna, B. burdigala, B. hipposideros, B. spilotogena, B. renata, B. edithae, B. imbellis (Tan & Ng Citation2005), B. rubra, B. chloropharynx B. falx and B. fusca (Tan & Ng Citation2005; Sriwattanarothai et al. Citation2010; Panijpan et al. Citation2014). Furthermore, one species each was found in Java (B. picta) and Riau archipelago (B. aurigans) (Tan & Ng Citation2005). However, the total number of Betta species in Sumatra Island is probably underestimated as several regions within this island have never been explored intensively. This includes the Aceh Province waters widely known as one of the ichthyofaunal hotspots in Sumatra. Therefore, this study aims to examine the diversity, distribution, and conservation status of Betta fish species list in the study area. This information is crucial for the documentation of the Indonesia ichthyofauna and to plan a better conservation strategy in the future.
Material and methods
Sampling period and location
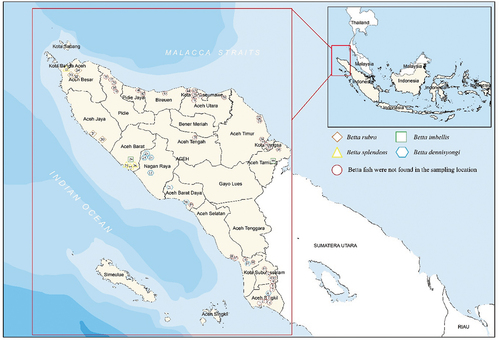
The survey was conducted from January 2019 to January 2021 at 59 sampling sites within Aceh Province, Indonesia, namely Aceh Singkil (8), Kota Subulussalam (5), Aceh Selatan (5), Aceh Barat Daya (3), Nagan Raya (4), Aceh Barat (4), Aceh Jaya (2), Aceh Besar (3), Banda Aceh (1), Pidie (5), Pidie Jaya (2), Bireuen (1), Kota Lhokseumawe (6), Aceh Utara (1), Aceh Tengah (1), Aceh Timur (3), Kota Langsa (3), and Aceh Tamiang (2) (). The description for all sampling sites is presented in , while the field conditions are presented in .
Table I. The GPS coordinates and characteristics of the sites where betta fish were collected
Figure 1. Map of Aceh Province showing sampling sites conducted during January 2019 to January 2021. Aceh Singkil (1–8), Kota Subulussalam (9–13), Aceh Selatan (14–18), Aceh Barat Daya (19–21), Nagan Raya (22–25), Aceh Barat (26–29), Aceh Jaya (30–31), Aceh Besar (32–34), Banda Aceh (35), Pidie (36–40), Pidie Jaya (41–42), Bireuen (43), Kota Lhokseumawe (44–49), Aceh Utara (50), Aceh Tengah (51), Aceh Timur (52–54), Kota Langsa (55–57), and Aceh Tamiang (58–59).
Sampling procedure
An exploratory survey was conducted in this study. The study team collected information from the locals on where the Betta fish are predicted to be found. These locations were then visited to conduct sampling (purposive), followed by random expansion to other nearby locations (random). The fish was sampled using a scoop net. The samples were kept in a plastic bag filled with water and oxygen and were then transported to the Ichthyology Laboratory of Universitas Syiah Kuala in Banda Aceh for further analysis. In the laboratory, the sampled fish was photographed and a total of 20 samples (n = 5 each species) anesthetized by immersing the fish in cold water (4°C) for 2–5 min, then preserved in 10% formalin. Voucher specimens were stored in the Laboratory of Fish Breeding, Universitas Syiah Kuala, Banda Aceh in Indonesia. These procedures were conducted in compliance with Research Ethics Guideline of Universitas Syiah Kuala No. 958/2015.
Water quality parameters such as temperature (°C) were measured using a digital thermometer (Mestek IR01C, Made in China), Dissolved Oxygen (mg L−1) and pH were calculated using a digital water quality checker (Hanna HI 98107, Made in Romania), and GPS coordinate were recorded in-situ during the sampling.
Taxonomic identification
The fish samples were measured using a digital caliper (Mitutoyo CD-6CS, standard errors = 0.01 mm) and weighed using a digital balance (Analytical Balance ME204). The taxonomic status was identified based on morphological and meristic characteristics following Kottelat et al. (Citation1993), Schindler and Schmidt (Citation2006) and Ng and Kottelat (Citation1994). The chin-bar (a thin dark line that extends from the throat area under the snout to the eye, just below the preorbital line), the transverse bars on the dorsal, anal, and caudal fins characteristic follows Tan and Ng (Citation2005).
Local distribution (LD) and conservation status
The local distribution was calculated based on Muchlisin et al. (Citation2015) as follows:
, where Ni.st = Total number of sites where the fishes were found; N.st = Total number of visited sites. In addition, the conservation status of each species was validated and confirmed through the online data in the IUCN Red List database www.iucnredlist.org.
Data analysis
The data of body weight, total length, distribution and water quality parameters are presented in the tables and figures, then analyzed descriptively.
Results
Species composition, distribution, and conservation status
Only 19 sites of the 59 sampling locations surveyed were found to contain Betta fish with a total sample of 422 belonging to four species, namely B. imbellis, B. splendens, B. rubra and B. dennisyongi ( and ). B. imbellis had a total length ranging from 14.09–31.98 mm, and body weight 0.03–0.39 g while B. splendens total length ranged from 21.09–40.14 mm, and body weight 0.05–0.70 g. Furthermore, the B. rubra total length ranged from 11.35 mm to 47.80 mm with 0.02–0.90 g body weight, while B. dennisyongi total length ranged from 20.09–47.81 mm, and body weight of 0.07–0.91 g ().
Table II. The ranges and average total length and body weight of Betta samples were collected from Aceh Province, Indonesia. N = total sample, SD = standard deviation
Figure 3. Betta spp. from Aceh waters, (a) Betta imbellis, (b) Betta splendens, (c) Betta dennisyongi and (d) Betta rubra (Nur et al. Citation2022).

The results showed that B. dennisyongi was widely distributed at eight sites (LD 13.56%), followed by B. rubra (LD 8.47%), B. splendens (LD 6.78%), and B. imbellis which had the lowest distribution and was only found in two locations, namely Aceh Tamiang and Barat (LD 3.39%) and also had the least number of samples (64 individuals), while the largest number of samples was recorded for B. splendens (168 individuals). Based on the IUCN Red List database, among the four species of fighting fish found in Aceh waters, one is categorized as an endangered species (B. rubra), two in the Vulnerable category (B. dennisyongi and B. splendens), and one in the Least Concern category (B. imbellis) ().
Table III. List of Betta species, total number of individuals and distribution at 19 sites in Aceh Province based on sampling sites. Meanwhile, other 34 sites were not displayed in the table because no sample was recorded
Habitat
The in-situ main parameter measurements of water quality showed a temperature ranging from 23.8–31.9°C, where the highest temperature was found in the peat swamp of Aceh Barat, while the lowest was found at Aceh Barat Daya. Furthermore, the pH values ranged from 5.24–8.50, where the lowest pH was recorded in Aceh, while the highest was found at Nagan Raya. Dissolved oxygen (DO) ranged from 4.1 to 9.1 mg L, where the lowest DO was recorded in the oil palm plantation areas in Aceh Selatan District, while the highest was recorded at Aceh Jaya ().
Table IV. Temperature, pH and dissolved oxygen of the respective habitats of Betta in the waters of Aceh, Indonesia
B. imbellis was found in peat swamps, with depths ranging from 50 to 70 cm, particularly among aquatic plant roots ()). In Aceh Barat, B. splendens were found in blackwater peat swamps ()) with depths ranging from 10 to 25 cm, while in Banda Aceh, this species was also found in blackish-brown waters associated with the water plant, Eichhornia crassipes at water depths of 35–70 cm ()). Furthermore, B. rubra in Aceh Besar was found in Blang Bintang and Indrapuri, both habitat areas are tributary streams ()) with a depth of 30–70 cm, characterized by clear and flowing water. B. rubra was found to be frequently associated with Puntius oligolepis, P. binotatus, Danio albolineatus, Rasbora sumatrana, and Lepidocephalichthys hasselti. It was found specifically among plant roots or litter. Meanwhile, in Aceh Jaya, B. rubra was found in irrigation canals and creeks with flowing, clear and slightly brownish-yellow waters at a depth of 10–40 cm ()). Moreover, B. dennisyongi had the widest distribution and lives in several types of waters, for example in Aceh Barat Daya, it was found in mountain springs ()), clear water with depths of 10–15 cm, while in Nagan Raya, this species was found in a drainage channel ()) of clear water with a depth of 5–20 cm. This species was also found in swamps with brownish-yellow water in Aceh Singkil with a depth of 15–40 cm ()), and in tributaries with brownish water with a depth of 20–45 cm in Aceh Selatan ()).
Discussion
A total of four Betta species were recorded during the study, namely Betta imbellis, B. splendens, B. rubra, and B. dennisyongi (). Based on the total number of samples, B. splendens was most predominant (39.81%), followed by B. dennisyongi (27.96%), B. rubra 17.06%, and B. imbellis 15.17%. Although B. splendens were high in numbers, this species was only found in four locations, while B. dennisyongi had a wider local distribution. B. imbellis which had a narrow distribution and the least number of samples, and was only found in two locations (at Krueng Balee and Tanjung Rambut).
Betta fish were found in 19 out of the 59 locations visited, and each location was occupied by only one species. This is probably related to the territorial and habitat preferences of the fish. According to Rainwater (Citation1967) and Bronstein (Citation1982), Betta fish is territorial and aggressive during the spawning season and egg incubation. These territorial and aggressive habits are related to self-defense and offspring protection from predators, competition for mates, and maintaining social hierarchies (Polgardani et al. Citation2017).
The results showed that the total number of fish sampled was dependent on the habitat type. The tributary areas with flowing water (lotic) had less sampled fish compared to stagnant waters (lentic). For instance, B. imbellis and B. splendens were predominant in numbers compared to the other two species where they were more easily caught since the habitats of these species are swamps and ponds where the water is relatively stagnant. Although swamp waters are slightly acidic, these two species adapt well and thriving. Meanwhile, B. rubra and B. dennisyongi were found in locations with flowing waters, hence, the fish were difficult to catch due to their agility and being hidden among the roots of the aquatic plants. These waters generally range from neutral to alkaline.
Based on the survey, B. dennisyongi is the most widely distributed and was found in several habitats. For example, in Aceh Barat Daya, this fish was found in small streams from mountains with clear waters, while in Nagan Raya, it was found in several locations with different habitat types, namely, drainage or irrigation canals, swamps and creeks. In addition, this fish was also found in water puddles in oil palm plantation areas where the water was cloudy and yellowish. Therefore, this study shows that B. dennisyongi is highly adaptable in various types of waters, hence its wider distribution compared to the other three species. This distinctive adaptability is probably related to the presence of a breathing apparatus (labyrinth), which enables oxygen (O2) absorption directly from the atmosphere (Chapman & Chapman Citation1994; Alton et al. Citation2013; Tate et al. Citation2017). The field observations showed that this fish often swim to the water surface, possibly to obtain free oxygen in the air.
B. rubra was found in two districts, namely Aceh Besar and Aceh Kaya. In Aceh Besar this species was found in Blang Bintang and Indrapuri where it occupied creeks and often associated with other fish species such as Puntius oligolepis, P. binotatus, Danio albolineatus, Rasbora sumatrana, and Lepidocephalichthys hasselti. Meanwhile, in Aceh Jaya. this species was also found in tributaries and irrigation canals.
B. splendens is a native fish in the Mekong Delta (Irawan & Afiati Citation2006) and was introduced to Indonesia from Thailand in the 1970s (Dewantoro & Rachmatika Citation2020; Panijpan et al. Citation2020). It is usually found in standing waters, including shallow ponds, swamps, rice fields, creeks, and rivers (Taki Citation1978; Rainboth Citation1996; Jaroensutasinee & Jaroensutansinee Citation2001; Watson et al. Citation2019). Based on the current results, it is suspected that this fish was released either intentionally or unintentionally by hobbyists and managed to successfully breed in the wild in Aceh waters. However, there have been no reports or evaluations of the impact of the presence of B. splendens in Indonesian waters. However, we suspect that the presence of these species may not have an adverse impact on the native Betta species because based on the finding of this study it was shown that there was no more than one species of Betta found at any one location, thus each species of Betta fish inhabits different habitats and does not compete for habitat occupation. However, the adverse impact on other fish species is unknown, and therefore these speculations need clarification by further intensive studies. Meanwhile, B. imbellis is a native species in Indonesia, and usually inhabits puddles and ditches ranging from clear water to yellow-brown (Tan & Ng Citation2005) both in lowland swamps and rice fields. Based on interviews with local hobbyists, B. rubra and B. dennisyongi have high selling prices up to 10–16 USD per pair because these two fish are endemic, B. rubra to Sumatra (Schindler & van der Voort Citation2011), and B. dennisyongi to Aceh (Tan Citation2013). Therefore, they are the main targets for hobbies.
Regarding the conservation status, the IUCN Red List data showed that B. rubra is listed as an endangered species (Low Citation2019a). This species was only recorded in two areas, namely Aceh Besar and Jaya districts. Furthermore, the field observations indicated that the B. rubra habitats in these two areas are threatened by perturbation due to highways and warehousing development. Meanwhile, B. dennisyongi and B. splendens have been listed in the Vulnerable category (Vidthayanon Citation2011; Low Citation2019b), In addition, B. imbellis was listed in the Least concern category (Low Citation2019c). However, based on field observations and interviews with local hobbyists, Betta fish are becoming rare as population continues to decline in the wild as evident in this study where low number of samples were collected. We believe that the status of these species may actually be under greater threat than classified in the IUCN Red List. Therefore, the IUCN conservation status of this species has to be updated immediately. Moreover, there is a need for comprehensive studies on the bioecological aspects as the basis for planning a better conservation strategy and breeding program in the future.
Conclusions
It is concluded that there are four Betta species in the Aceh waters, namely B. splendens, B. imbellis, B. rubra and B. dennisyongi, the last two species being endemic to Aceh and Sumatra. B. rubra is categorized as an endangered species while B. dennisyongi has a wider distribution compared to the other three species although listed in the vulnerable category. In addition, B. imbellis has the narrowest distribution and was found only in Aceh Tamiang and Aceh Barat districts. Meanwhile, B. splendens is an introduced species found in Banda Aceh and Aceh Barat districts, and is categorized as a vulnerable species. However, these four Betta fish were very difficult to find during the course of this study, and possibly the degree of threat has increased. Therefore, the IUCN status of all these fish species needs to be updated. In addition, conservation action for all Betta species that occurs in Aceh has to be initiated immediately to ensure their germplasms are sustained.
Acknowledgements
The authors thank the Rector of Universitas Syiah for supporting this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alderton D. 2012. Bettas and gouramis: Understanding Siamese fighting fish, paradise fish, kissing gouramis, and other anabantoids. Irvine, CA: BowTie Press.
- Allen JM, Nicoletto PF. 1997. Response of Betta splendens to computer animations of males with fins of different length. Copeia 1997:195–199. DOI: 10.2307/1447858.
- Alton LA, Portugal SJ, White CR. 2013. Balancing the competing requirements of air-breathing and display behaviour during male–male interactions in Siamese fighting fish Betta splendens. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 164:363–367. DOI: 10.1016/j.cbpa.2012.11.012.
- Apriliani NS, Supriyati H, Luthfi MJF. 2018. Histological study of respiratory organ of Betta sp. International Conference of Science and Engineering, Yogyakarta. pp. 181–184.
- Bronstein PM. 1982. Breeding, paternal behavior, and their interruption in Betta splendens. Animal Learning & Behavior 10:145–151. DOI: 10.3758/BF03212262.
- Chapman LJ, Chapman CA. 1994. Observations on synchronous air breathing in Clarias liocephalus. Copeia 1994:246–249. DOI: 10.2307/1446696.
- Dewantoro GW, Rachmatika I. 2020. Jenis ikan introduksi dan invasif asing di Indonesia.
- Fishbase. 2020. Species list genus Betta.
- Forsatkar MN, Nematollahi MA, Amiri BM, Huang W-B. 2014. Fluoxetine inhibits aggressive behaviour during parental care in male fighting fish (Betta splendens, Regan). Ecotoxicology 23:1794–1802. DOI: 10.1007/s10646-014-1345-0.
- Goldstein RJ. 2015. The Betta handbook. New York: Barron’s Educational Series.
- Irawan B, Afiati N. 2006. Aggressive behavior of male Siamese fighting fish (Betta splendens Regan) in different colour environment. Aquacultura Indonesiana 7:107–114.
- IUCN. 2020. The IUCN Red List of threatened species. Version 2020-1. Available: https://www.iucnredlist.org.
- Jaroensutasinee M, Jaroensutansinee K. 2001. Bubble nest habitat characteristics of wild Siamese fighting fish. Journal of Fish Biology 58:1311–1319. DOI: 10.1111/j.1095-8649.2001.tb02288.x.
- Kottelat M, Whitten A, Katikasari S, Wirjoatmodjo S. 1993. Ikan air tawar Indonesia bagian Barat dan Sulawesi. Jakarta: Periplus Edition.
- Kowasupat C, Panijpan B, Laosinchai P, Ruenwongsa P, Phongdara A, Wanna W, Senapin S, Phiwsaiya K. 2014. Biodiversity of the Betta smaragdina (Teleostei: Perciformes) in the northeast region of Thailand as determined by mitochondrial COI and nuclear ITS1 gene sequences. Meta Gene 2:83–95. DOI: 10.1016/j.mgene.2013.12.004.
- Kowasupat C, Panijpan B, Ruenwongsa P, Jeenthong T. 2012a. Betta siamorientalis, a new species of bubble-nest building fighting fish (Teleostei: Osphronemidae) from eastern Thailand. Vertebrate Zoology 62:387–397.
- Kowasupat C, Panijpan B, Ruenwongsa P, Sriwattanarothai N. 2012b. Betta mahachaiensis, a new species of bubble-nesting fighting fish (Teleostei: Osphronemidae) from Samut Sakhon Province, Thailand. Zootaxa 3522:49–60. DOI: 10.11646/zootaxa.3522.1.3.
- Low B. 2019a. Betta rubra. The IUCN Red List of threatened species 2019. e.T91310582A91310586.
- Low BW. 2019b. Betta dennisyongi. The IUCN Red List of threatened species 2019. e.T91309581A91309605.
- Low BW. 2019c. Betta imbellis. The IUCN Red List of threatened species 2019. e.T181333A89804943.
- Monvises A, Nuangsaeng B, Sriwattanarothai N, Panijpan B. 2009. The Siamese fighting fish: Well-known generally but little-known scientifically. ScienceAsia 35:8–16. DOI: 10.2306/scienceasia1513-1874.2009.35.008.
- Muchlisin Z. 2013. Potency of freshwater fishes in Aceh waters as a basis for aquaculture development program [Potensi ikan air tawar di Aceh sebagai dasar untuk pengembangan budi daya]. Jurnal Iktiologi Indonesia 13:91–96.
- Muchlisin Z, Akyun Q, Halim A, Rizka S, Sugianto S, Fadli N, Siti-Azizah M. 2015. Ichthyofauna of Tripa peat swamp forest, Aceh Province, Indonesia. Check List 11:1. DOI: 10.15560/11.2.1560.
- Muchlisin Z, Azizah S. 2009. Diversity and distribution of freshwater fishes in Aceh waters, northern Sumatra Indonesia. International Journal of Zoological Research 5:62–79. DOI: 10.3923/ijzr.2009.62.79.
- Ng PK, Kottelat M. 1994. Revision of the Betta-waseri species group (Teleostei, Belontiidae). Raffles Bulletin of Zoology 42:593–611.
- Nur FM, Batubara AS, Eriani K, Tang U, Muhammada AA, Siti-Azizah MN, Wilkes M, Fadli N, Rizal S, Muchlisin ZA. 2020. Effect of water temperature on the physiological responses in Betta rubra, Perugia 1893 (Pisces: Osphronemidae). International Aquatic Research 12. DOI: 10.22034/iar.2020.1900150.1053.
- Nur FM, Batubara AS, Fadli N, Rizal S, Siti-Azizah MN, Muchlisin ZA. 2022. Elucidating species diversity of genus Betta from Aceh waters Indonesia using morphometric and genetic data. Zoologischer Anzeiger 296:129–140. DOI: 10.1016/j.jcz.2021.12.004.
- Panijpan B, Kowasupat C, Laosinchai P, Ruenwongsa P, Phongdara A, Senapin S, Wanna W, Phiwsaiya K, Kühne J, Fasquel F. 2014. Southeast Asian mouth-brooding Betta fighting fish (Teleostei: Perciformes) species and their phylogenetic relationships based on mitochondrial COI and nuclear ITS1 DNA sequences and analyses. Meta Gene 2:862–879. DOI: 10.1016/j.mgene.2014.10.007.
- Panijpan B, Sriwattanarothai N, Laosinchai P. 2020. Wild Betta fighting fish species in Thailand and other Southeast Asian countries. ScienceAsia 46:382–391. DOI: 10.2306/scienceasia1513-1874.2020.064.
- Polgardani NZ, Rouzbehani S, Parsaiyan M. 2017. Male Siamese fighting fish (Betta splendens) recognize resource holding power of opponents. AACL Bioflux 10:977–982.
- Rainboth W. 1996. Fishes of the Cambodian Mekon. Rome: Food & Agriculture Organization.
- Rainwater FL. 1967. Courtship and reproductive behavior of the Siamese fighting fish, Betta splendens Regan (Pisces, Belontiidae). Oklahoma: Oklahoma State University.
- Rüber L, Britz R, Tan HH, Ng PK, Zardoya R. 2004. Evolution of mouthbrooding and life‐history correlates in the fighting fish genus Betta. Evolution 58:799–813. DOI: 10.1111/j.0014-3820.2004.tb00413.x.
- Schindler I, Schmidt J. 2006. Review of the mouth brooding Betta (Teleostei, Osphronemidae) from Thailand, with descriptions of two new species. Zeitschrift für Fischkunde 8:47–69.
- Schindler I, van der Voort S. 2011. Re-description of Betta rubra Perugia, 1893 (Teleostei: Osphronemidae), an enigmatic fighting fish from Sumatra. Raffles Bulletin of Zoology 13:21–32.
- Sriwattanarothai N, Steinke D, Ruenwongsa P, Hanner R, Panijpan B. 2010. Molecular and morphological evidence supports the species status of the Mahachai fighter Betta sp. Mahachai and reveals new species of Betta from Thailand. Journal of Fish Biology 77:414–424. DOI: 10.1016/j.cbpa.2012.11.012.
- Taki Y. 1978. An analytical study of the fish fauna of the Mekong basin as a biological production system in nature. Research Institute of Evolutionary Biology 1:1-77.
- Tan HH. 2013. The identity of Betta rubra (Teleostei: Osphronemidae) revisited, with description of a new species from Sumatra, Indonesia. Raffles Bulletin of Zoology 61:323–330.
- Tan HH, Ng PK. 2005. The fighting fishes (Teleostei: Osphronemidae: Genus Betta) of Singapore, Malaysia and Brunei. Raffles Bulletin of Zoology 13:43–99.
- Tan HH, Ng PK. 2006. Six new species of fighting fish (Teleostei: Osphronemidae: Betta) from Borneo. Ichthyological Exploration of Freshwaters 17:97.
- Tanpitayacoop C, Na-Nakorn U. 2005. Genetic variation of Betta spp. in Thailand by random amplified polymorphic DNA (RAPD) method. Proceedings of 43rd Kasetsart University Annual Conference, 1–4 February, 2005, Thailand: Kasetsart University. Subject: Fisheries. pp. 185–192.
- Tate M, McGoran R, White C, Portugal S. 2017. Life in a bubble: The role of the labyrinth organ in determining territory, mating and aggressive behaviours in anabantoids. Journal of Fish Biology 91(3):723–749. DOI: 10.1111/jfb.13357.
- Thongprajukaew K, Kovitvadhi U, Kovitvadhi S, Somsueb P, Rungruangsak-Torrissen KJA. 2011. Effects of different modified diets on growth, digestive enzyme activities and muscle compositions in juvenile Siamese fighting fish (Betta splendens Regan, 1910). Aquaculture 322:1–9. DOI: 10.1111/j.1574-6968.2011.02314.x.
- Vidthayanon C. 2011. Betta splendens. The IUCN Red List of Threatened Species 2011. e.T180889A7653828.
- Watson C, DiMaggio M, Hill J, Tuckett Q, Yanong R. 2019. Evolution, culture, and care for Betta splendens. Gainesville: EDIS. pp 1–5.

