Abstract
The knowledge of the ecological preferences of threatened species is critical to all conservation programs. Analyses of habitats and ecological parameters of species are necessary to predict future distribution and responses to climate change. Cucujus haematodes Erichson, 1845 (Coleoptera: Cucujidae) is a threatened obligate saproxylic species, listed in the IUCN European Red List of Saproxylic Beetles. After a few decades of apparent absence, the species was recently found in Calabria (Southern Italy) in some good quality biotopes of the Sila National Park in association with Cucujus cinnaberinus (Scopoli, 1763) and Cucujus tulliae Bonacci, Mazzei, Horák & Brandmayr, 2012 (Coleoptera: Cucujidae). Field surveys carried out from 2014 to 2020 in Aspromonte National Park (Calabria) revealed large populations of C. haematodes. Larvae of C. haematodes were collected from under the bark of dead trees in 11 sampling areas within Aspromonte National Park. The presence of larvae of C. haematodes on the non-autochthonous conifer Pseudotsuga menziesii (Mirb.) Franco (Pinales: Pinaceae) was also reported for the first time in Italy.
Introduction
Recent studies report dramatic declines in arthropod populations worldwide and the possible consequences of this serious problem for ecosystems (Hallmann et al. Citation2017). Among arthropods, insects play important ecological roles, and the decline in number of species and individuals is alarming (Crossley et al. Citation2020). About a quarter of insect species are at risk of extinction, and an increasing number of them are included in the Red List of the International Union for Conservation of Nature and Natural Resources (IUCN) (Collen et al. Citation2012). Saproxylic beetles depend on dead and decaying wood for their lifecycle, and thus, land management practices and forest logging are a major threat for them (Cálix et al. Citation2018). Several species of saproxylic beetles are included in the IUCN Red List (https://www.iucnredlist.org/).
The genus Cucujus Fabricius, 1775 (Coleoptera: Cucujidae) includes more than 20 taxa of saproxylic beetles (species and subspecies) distributed in the Holarctic region and Asia (Lee & Pütz Citation2008; Horák & Chobot Citation2009; Bonacci et al. Citation2012). New Cucujus species have been recently reported from Asia and the Indochinese region (Hsiao Citation2020; Jaskuła et al. Citation2021), and among the European species, four have been reported in Italy, C. cinnaberinus (Scopoli, 1763), C. haematodes Erichson, 1845, C. tulliae Bonacci, Mazzei, Horák & Brandmayr, Citation2012 and C. clavipes (Fabricius, 1781). The first three species are officially part of the Italian fauna, but the fourth one, C. clavipes, is apparently an allochthonous one, quoted only once in the Port of Venice in 1968, probably accidentally imported from native North America (Ratti Citation2000; see also, Ratti Citation1986 for the question concerning Cucujus siculus Tournier, 1894, described from Sicily). The first two species, C. cinnaberinus and C. haematodes, are enlisted in the IUCN European Red List of Saproxylic Beetles: in 2018, the IUCN Red List Category for Europe reported C. haematodes as “endangered” (EN) and C. cinnaberinus, indicated as endemic for Europe, as Near Threatened (NT) (Cálix et al. Citation2018). The third Italian species, C. tulliae, is not yet enlisted in the IUCN European Red List of Saproxylic Beetles but only in the Red List of Italian Saproxylic Beetles (Audisio et al. Citation2014; Carpaneto et al. Citation2015).
Among Cucujus species, C. haematodes is considered an aggregate species complex, including the subspecies C. haematodes opacus Leiws, 1888 distributed in Japan and Taiwan, C. haematodes caucasicus Motschulsky, 1845 in the Caucasus, and C. haematodes haematodes Erichson, 1845 in the rest of its range (Horák & Chobot Citation2009). In Europe, the species shows a boreal-montane distribution with an irregular population density (Horák & Chobot Citation2009; Horák et al. Citation2011), mainly in less disturbed forests (Roubal Citation1936), and subsequently considered a relic of virgin forests (Horák et al. Citation2011). Although C. haematodes has a wide distribution in Europe (Horák et al. Citation2009), records of this species in Italy are limited only to Calabrian and Lucan Apennines (Ratti Citation2000; Bonacci et al. Citation2012; Mazzei et al. Citation2018). In the Sila plateau (Calabria), C. haematodes occurs in sympatry with C. cinnaberinus, but with lower abundance (Mazzei et al. Citation2011, Citation2018). The presence of C. haematodes is very important not only for the management of protected areas but also for the conservation status of forests and overall biodiversity (Mazzei et al. Citation2018).
Few data are known on the ecological preferences and distribution of this endangered saproxylic beetle in Europe; therefore, the aim of this study was to collect more data on distribution and choice of host species trees of C. haematodes in the southernmost forest area of Calabria, the territory of Aspromonte National Park.
Materials and methods
Sampling area
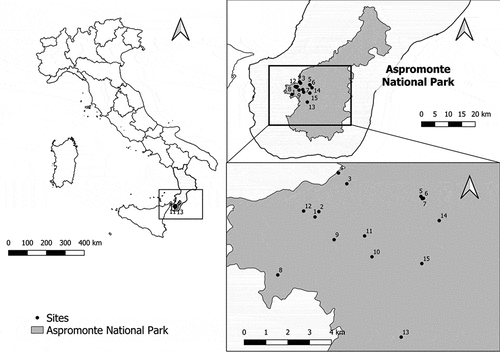
The field research was carried out from 2014 to 2020 in Region Calabria (Southern Italy), in 15 sites of 11 areas within Aspromonte National Park (). The Aspromonte Massif is formed by relict metamorphic assemblages (Pezzino et al. Citation2008) and covers an area of about 3000 km2 with a peculiar vegetation that includes many endemic species. In the territory of the protected area, there are beech forests, Fagus sylvatica L. 1753 (Fagales: Fagaceae), that reach the highest altitudinal limits around 1950 m a.s.l., and pine forests, mostly Pinus nigra var. calabrica Debazac 1965 (Pinales: Pinaceae) extending to 1400–1500 m a.s.l. (Spampinato Citation2014). In some locations, P. nigra is mixed with Abies alba Mill. subsp. apennina (Pinales: Pinaceae) and the introduced species Pseudotsuga menziesii (Mirb.) Franco (Pinales: Pinaceae). The mountain area of Aspromonte National Park is characterized by a temperate climate. According to rainfall and thermal data from ARPACAL (https://www.cfd.calabria.it), Gambarie pluviometer station (code 2470), in the studied period, the annual rainfall ranged from 1718 mm (2014) to 1474 mm (2020), while the annual average temperature was 10.5°C.
Figure 1. Sampling sites in Aspromonte National Park, Calabria (Southern Italy). Maps produced with QGIS software (https://qgis.org).
Sample collection
The sites were randomly selected and only dead trees were checked, a necessary condition for the possible presence of Cucujus spp. individuals. The larvae of Cucujus spp. were collected by hand from under the bark of fallen or standing dead trees (Mazzei et al. Citation2018). The larvae were then placed in separate glass containers with pieces of dead wood and bark, transported to the entomological laboratory of the Department of Biology, Ecology and Earth Sciences (University of Calabria, Arcavacata di Rende, Cosenza, Italy), and placed in a thermostatic chamber at 15°C, 12:12 L:D photoperiod and under humidity 90%. For correct species identification, larvae were reared in the laboratory until the emergence of adults (Bonacci et al. Citation2012). The emerged adults were released back to the sampling areas. Beetles and hymenopterans co-occurring with the cucujids were also collected and identified to the family level using keys (https://sites.google.com/view/mikes-insect-keys/mikes-insect-keys).
Results and discussion
A total of 166 larvae, belonging to the genus Cucujus and at developmental stages from L1 to L5, were collected. The larvae were found on dead logs of A. alba, P. nigra, and Ps. menziesii, in altitude range from 1238 to 1826 m a.s.l. and within an area of 15 km2. All emerged adults belonged to C. haematodes ().
Table I. Characteristics of the sampling areas, tree host species, and insect families associated with C. haematodes larvae
Among the larvae, 45 were found on Ps. menziesii on site 8 (Bisurgi). As far as we know, this is the first report of the presence of C. haematodes on this non-autochthonous conifer species in Italy.
Earlier reports on the distribution of C. haematodes in Europe indicated mountain and foothill areas as its preferred habitat (Ratti Citation2000). In the Primorsky region of eastern Russia, the species was found at lower altitudes (<1000 m a.s.l.) (Horák et al. Citation2011) and in northeastern Kazakhstan (foothills of West Altai Mountains) at 472 m a.s.l. (Szczepański et al. Citation2018). The species is relatively common in lowland areas of North Eastern Poland, especially in the Białowieża Forest (Jaworski et al. Citation2019). The occurrence of C. haematodes at higher altitudes could be related to intensified forestry and competition with C. cinnaberinus (Horák et al. Citation2011).
The results of this study show that, apart from only one larva of C. tulliae found in the Aspromonte National Park (Bonacci et al. Citation2012), C. haematodes is the only Cucujus species recorded in the extreme southern part of Calabria and that A. alba, P. nigra, and Ps. menziesii are its tree hosts. The larvae of this cucujid were found with other insect families associated with dead wood, 15 of Coleoptera (Buprestidae, Carabidae, Cerambycidae, Curculionidae, Elateridae, Eucnemidae, Melandryidae, Mycetophagidae, Nitidulidae, Pyrochroidae, Scarabaeidae, Staphylinidae, Tenebrionidae, Trogossitidae, and Zopheridae) and 2 of Hymenoptera (Formicidae and Vespidae). The larvae of C. haematodes were found in association with dead trees, supporting their ecological role as saproxylic and the fact that the species was not found in areas with few dead trees (Mazzei et al. Citation2018).
This study supports the hypothesis that the southern limit of the distribution of C. haematodes is the Aspromonte National Park, although beech forests are also found in Sicily (Ciccarello et al. Citation2015). The limit may be related to the regional glacial history during the Pleistocene when climatic fluctuations affected the distribution of many species (Avise Citation2000; Hewitt Citation2004) and their genetic diversity (Canestrelli et al. Citation2010, Citation2012). The current ranges of species of the genus Cucujus in Calabria () show a large gap, as found for other species in the same region (Bisconti et al. Citation2018). The gap was probably caused by glacial events in southern areas of the three Mediterranean peninsulas, Italy, Iberia, and the Balkans (Hewitt Citation1996; Taberlet et al. Citation1998). In Calabria, the current gap between the populations of C. haematodes of Sila and Aspromonte is probably due to postglacial events leading to the formation of the Catanzaro Trough (Pezzino et al. Citation2008).
Figure 2. Distribution of Cucujus spp. in Calabria. Full circle: C. cinnaberinus, triangle: C. tulliae, dashed circle: C. haematodes Maps produced with QGIS software (https://qgis.org).
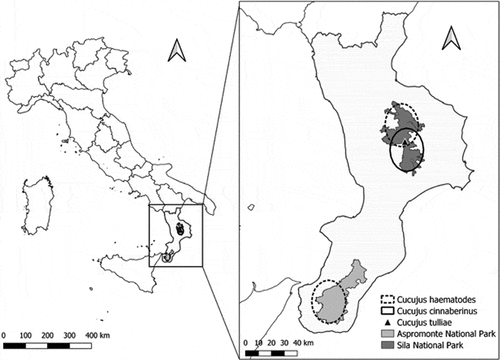
Although several taxa underwent northward dispersal during the interglacial periods (Hewitt Citation2004), this apparently did not occur for the Calabrian populations of genus Cucujus, probably because of thermal barriers. In other geographical areas in Europe, C. haematodes has been recorded at lower altitudes, but in Calabria, the species has been found only at altitudes above 1100 m a.s.l. and only within cold and old-growth forests (Mazzei et al. Citation2011, Citation2018). Currently, three Cucujus species have been recorded in Sila National Park: C. cinnaberinus, C. haematodes, and C. tulliae (Bonacci et al. Citation2012). Except for the larva of C. tulliae previously mentioned (Bonacci et al., Citation2012), only C. haematodes was recorded in the Aspromonte National Park ().
The Calabrian region is a well-known glacial refugium for various species in the Italian peninsula and is considered a reservoir of genetic diversity for several vertebrate taxa such as Bombina pachypus (Bonaparte, 1838; Anura: Bombinatoridae) (Canestrelli et al. Citation2006). Thanks to its geographic characteristics, this region provides one of the best examples of a scenario known as “refugia-within-refugia”, as observed for the peninsula Iberia (Gómez & Lunt Citation2007). For species such as Myodes glareolus (Schreber, 1780; Rodentia: Cricetidae), Dryomys nitedula Pallas, 1778 (Rodentia: Gliridae), and B. pachypus, the Calabrian region provided suitable (although fragmented) habitats during most of the Pleistocene, allowing the long-term survival of relict populations (Senczuk et al. Citation2017; Bisconti et al. Citation2018). The repeated insularization of Sila and Aspromonte Massifs, which strongly affected the population structure of most terrestrial animals living in these areas (Canestrelli et al. Citation2006, Citation2008, Citation2010, Citation2012), may have caused extreme geographic isolation and possibly genetic differentiation also in Cucujus populations. Although the occurrence of a species is known to be determined by many intra- and interspecific interactions as well as abiotic factors (Thompson Citation1994), we advance the hypothesis that the geological and biogeographical past was a key factor determining the current distribution of the species. Climatic changes could alter the dynamics and survival of some forest insect species, mainly specialist ones such as saproxylic beetles. In the case of C. haematodes, climate changes and frequent forest fires could lead to decline of local populations. Further studies are necessary to evaluate the population structure of C. haematodes from Aspromonte and Sila and other European sites, in order to devise the most appropriate strategies in forest environments for species survival.
Acknowledgements
The authors owe thanks to Elvira Castiglione and Francesco Manti for technical assistance in the fieldwork. The manuscript is dedicated to the loving memory of Fernando Angelini, a renowned entomologist and expert in Coleoptera, and of Sergio Tralongo, former director of Aspromonte National Park, who always supported research projects on biodiversity.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Audisio P, Baviera C, Carpaneto GM, Biscaccianti AB, Battistoni A, Teofili C, Rondinini C. 2014. Lista Rossa IUCN dei Coleotteri saproxilici Italiani. Roma: Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare.
- Avise JC. 2000. Phylogeography: The history and formation of species. Cambridge, Massachusetts: Harvard University Press.
- Bisconti R, Aloise G, Siclari A, Fava V, Provenzano M, Arduino P, Chiocchio A, Nascetti G, Canestrelli D. 2018. Forest Dormouse (Dryomys nitedula) populations in southern Italy belong to a deeply divergent evolutionary lineage: Implications for taxonomy and conservation. Hystrix - the Italian Journal of Mammalogy 29:75–79. DOI: 10.4404/hystrix-00023-2017.
- Bonacci T, Mazzei A, Horák J, Brandmayr P. 2012. Cucujus tulliae sp. n. – An endemic Mediterranean saproxylic beetle from genus Cucujus Fabricius, 1775 (Coleoptera, Cucujidae), and keys for identification of adults and larvae native to Europe. Zookeys 212:63–79. DOI: 10.3897/zookeys.212.3254.
- Cálix M, Alexander KNA, Nieto A, Dodelin B, Soldati F, Telnov D, Vazquez-Albalate X, Aleksandrowicz O, Audisio P, Istrate P, Jansson N, Legakis A, Liberto A, Makris C, Merkl O, Mugerwa Pettersson R, Schlaghamersky J, Bologna MA, Brustel H, Buse J, Novák V, Purchart L. 2018. European Red List of saproxylic beetles. Brussels, Belgium: IUCN. Available: https://portals.iucn.org/library/node/47296.
- Canestrelli D, Aloise G, Cecchetti S, Nascetti G. 2010. Birth of a hotspot of intraspecific genetic diversity: Notes from the underground. Molecular Ecology 19:5432–5451. DOI: 10.1111/j.1365-294X.2010.04900.x.
- Canestrelli D, Cimmaruta R, Costantini V, Nascetti G. 2006. Genetic diversity and phylogeography of the Apennine yellow‐bellied toad Bombina pachypus, with implications for conservation. Molecular Ecology 15:3741–3754. DOI: 10.1111/j.1365-294X.2006.03055.x.
- Canestrelli D, Cimmaruta R, Nascetti G. 2008. Population genetic structure and diversity of the Apennine endemic stream frog, Rana italica – Insights on the Pleistocene evolutionary history of the Italian peninsular biota. Molecular Ecology 17:3856–3872. DOI: 10.1111/j.1365-294X.2008.03870.x.
- Canestrelli D, Sacco F, Nascetti G. 2012. On glacial refugia, genetic diversity, and microevolutionary processes: Deep phylogeographical structure in the endemic newt Lissotriton italicus. Biological Journal of the Linnean Society 105:42–55. DOI: 10.1111/j.1095-8312.2011.01767.x.
- Carpaneto GM, Baviera C, Biscaccianti AB, Brandmayr P, Mazzei A, Mason F, Battistoni A, Teofili C, Rondinini C, Fattorini S, Audisio P. 2015. A Red List of Italian saproxylic beetles: Taxonomic overview, ecological features and conservation issues (Coleoptera). Fragmenta Entomologica 47:53–126. DOI: 10.13133/2284-4880/138.
- Ciccarello S, Di Gangi I, Castellano G. 2015. Espansione dei boschi di faggio sui Monti Nebrodi (Sicilia nord-orientale) negli ultimi 50 anni. Quaderni di Botanica ambientale e applicata 26:51–72.
- Collen B, Böhm M, Kemp R, Baillie JEM. 2012. Spineless: Status and trends of the world’s invertebrates. UK: Zoological Society of London.
- Crossley MS, Meier AR, Baldwin EM, Berry LL, Crenshaw LC, Hartman GL, Lagos-Kutz D, Nichols DH, Patel K, Varriano S, Snyder WE, and Moran MD. 2020. No net insect abundance and diversity declines across US long term ecological research sites. Nature Ecology & Evolution 4:1368–1376. DOI: 10.1038/s41559-020-1269-4.
- Gómez A, Lunt DH. 2007. Refugia within refugia: Patterns of phylogeographic concordance in the Iberian Peninsula. In: Weiss S, Ferrand N, editors. Phylogeography of Southern European refugia. Dordrecht: Springer Netherlands. pp. 155–188. DOI: 10.1007/1-4020-4904-8_5.
- Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, de Kroon H. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. DOI: 10.1371/journal.pone.0185809.
- Hewitt GM. 1996. Some genetic consequences of ice ages, and their role in divergence and speciation. Biological Journal of the Linnean Society 58:247–276. DOI: 10.1111/j.1095-8312.1996.tb01434.x.
- Hewitt GM. 2004. Genetic consequences of climatic oscillations in the Quaternary. Philosophical Transactions of the Royal Society. Series B, Biological Sciences 359:183–195. DOI: 10.1098/rstb.2003.1388.
- Horák J, and Chobot K 2009. Worldwide distribution of saproxylic beetles of the genus Cucujus Fabricius, 1775 (Coleoptera: Cucujidae). In: Proceedings of the 5th Symposium and Workshop on the Conservation of Saproxylic Beetles; Buse J, Alexander KNA, Ranius T, Assmann T (Eds) Saproxylic beetles – Their role and diversity in European woodland and tree habitats. Pensoft Publishers: Sofia-Moscow, Russia.
- Horák J, Mertlik J, Chobot K, Kubáň V. 2009. Distribution of a rare saproxylic beetle Cucujus haematodes (Coleoptera: Cucujidae) in the Czech Republic with notes to the occurrence in central Europe. Klapalekiana 45:191–197.
- Horák J, Zaitsev AA, Vávrová E. 2011. Ecological requirements of a rare saproxylic beetle Cucujus haematodes – The beetles’ stronghold on the edge of its distribution area. Insect Conservation and Diversity 4:81–88. DOI: 10.1111/j.1752-4598.2010.00102.x.
- Hsiao Y. 2020. A taxonomic study of Cucujus Fabricius, 1775 from Asia (Coleoptera: Cucujidae), with descriptions of new species and notes on morphological classification. Insect Systematics & Evolution 52:247–297. DOI: 10.1163/1876312X-bja10012.
- Jaskuła R, Kolanowska M, Michalski M, Schwerk A. 2021. From phenology and habitat preferences to climate change: Importance of citizen science in studying insect ecology in the continental scale with American Red Flat Bark Beetle, Cucujus clavipes, as a model species. Insects 12:369. DOI: 10.3390/insects12040369.
- Jaworski T, Plewa R, Tarwacki G, Sućko K, Hilszczański J, Horák J. 2019. Ecologically similar saproxylic beetles depend on diversified deadwood resources: From habitat requirements to management implications. Forest Ecology and Management 449:117462. DOI: 10.1016/j.foreco.2019.117462.
- Lee C-F, Pütz A. 2008. A new species of Cucujus Fabricius, 1775 from China and key to the east–Palaearctic species of the genus (Coleoptera: Cucujidae). Entomologische Zeitschrift 118:211–213.
- Mazzei A, Bonacci T, Contarini E, Zetto T, Brandmayr P. 2011. Rediscovering the ‘umbrella species’ candidate Cucujus cinnaberinus (Scopoli, 1763) in Southern Italy (Coleoptera Cucujidae), and notes on bionomy. Italian Journal of Zoology 78:264–270. DOI: 10.1080/11250003.2010.485210.
- Mazzei A, Bonacci T, Horák J, Brandmayr P. 2018. The role of topography, stand and habitat features for management and biodiversity of a prominent forest hotspot of the Mediterranean Basin: Saproxylic beetles as possible indicators. Forest Ecology and Management 410:66–75. DOI: 10.1016/j.foreco.2017.12.039.
- Pezzino A, Angì G, Fazio E, Fiannacca P, Lo Giudice A, Ortolano G, Punturo R, Cirrincione R, De Vuono E. 2008. Alpine metamorphism in the Aspromonte Massif: Implications for a new framework for the southern sector of the Calabria-Peloritani Orogen, Italy. International Geology Review 50:423–441. DOI: 10.2747/0020-6814.50.5.423.
- Ratti E. 1986. Nota sinonimica a proposito di Cucujus siculus Tournier, 1894 (Coleoptera, Cucujidae). Lavori - Società Veneziana Di Scienze Naturali 11:83–85.
- Ratti E. 2000. Note faunistiche ed ecologiche sui cucuidi italiani (Coleoptera Cucujidae). Bollettino del Museo civico di Storia Naturale di Venezia 50:103–129.
- Roubal J. 1936. Katalog Coleopter (brouku) Slovenska a Podkarpatske Rusi. Dıl II. Bratislava, Slovakia.
- Senczuk G, Colangelo P, De Simone E, Aloise G, Castiglia R. 2017. A combination of long term fragmentation and glacial persistence drove the evolutionary history of the Italian wall lizard Podarcis siculus. BMC Evolutionary Biology 17:6. DOI: 10.1186/s12862-016-0847-1.
- Spampinato G. 2014. Guida alla flora dell’Aspromonte. Reggio Calabria, Italy: Laruffa Editore.
- Szczepański WT, Karpiński L, Plewa R, Hilszczański J, Szołtys H. 2018. First records of Kolibacia squamulata (Gebler, 1830), Cucujus haematodes Erichson, 1845, and Clerus dealbatus (Kraatz, 1879) (Coleoptera: Trogossitidae, Cucujidae, Cleridae) from Kazakhstan. Acta Entomologica Silesiana 26:1–6. DOI: 10.5281/zenodo.1212300.
- Taberlet P, Fumagalli L, Wust-Saucy A-G, Cosson J-F. 1998. Comparative phylogeography and postglacial colonization routes in Europe. Molecular Ecology 7:453–464. DOI: 10.1046/j.1365-294x.1998.00289.x.
- Thompson JN. 1994. The coevolutionary process. Chicago, Illinois: The University of Chicago Press.
