Abstract
The mesophotic zone is a relatively poorly studied area of the Mediterranean Sea, drawing great interest by the scientific community in the last years. This zone represents a connection between the shallow water and the deep-sea communities, in which photophilic framework builders (e.g. coralline red algae) are gradually replaced by heterotrophic ones, such as ahermatypic corals and the bivalve Neopycnodonte cochlear. In this habitat the framework-forming organisms produce a hard substrate with a high topographic complexity, hosting a great biodiversity of secondary structuring taxa like bryozoans. During a survey on coralligenous banks in the mesophotic zone in c. 60 m depth off Gallipoli (southern Apulia), epibiotic aggregations of N. cochlear were found on the fans of the hexacoral Savalia savaglia. In the present paper the diversity of cheilostomatid bryozoans hosted by these bivalve aggregations is described and compared with published information on similar nearby habitats. A total of 48 taxa were found, six of which are newly described: Crassimarginatella matildae sp. nov., Micropora biopesiula sp. nov., Haplopoma celeste sp. nov., Schizomavella (Schizomavella) cerranoi sp. nov., Schizomavella (Calvetomavella) biancae sp. nov., and Schizoporella adelaide sp. nov. The species richness known from the southern Apulian shelf at this depth (47 species) is hereby raised to 83 cheilostomatid bryozoans. Moreover, only 12 species are shared with the other localities studied previously, while 36 are restricted to Gallipoli, supporting the hypothesis of a high rate of exclusivity among Apulian sites in terms of species composition. The differences in faunal composition, and particularly the presence of several new species discovered at Gallipoli, show once more that our knowledge of the bryozoan fauna in certain Mediterranean habitats is still incomplete and warrants further studies.
https://doi.org/urn:lsid:zoobank.org:pub:DDC82039-EF44-4169-8198-C67F60B14BA0
Introduction
In the Mediterranean Sea the bryozoan diversity is relatively high considering the dimensions of the basin: the c. 556 described species represent about 10% of the global bryodiversity (Rosso & Di Martino Citation2016). This number is expected to increase since many habitats and areas in the Mediterranean are still understudied, such as the eastern and southern regions (D’Onghia et al. Citation2015; Rosso & Di Martino Citation2016). Each year, several new species and genera are also being described in the Mediterranean thanks to revision works (e.g. Souto et al. Citation2010a; Vieira et al. Citation2014; Reverter-Gil et al. Citation2016; Berning et al. Citation2019) as well as owing to newly collected material from hardly accessible habitats like dark caves, the mesophotic zone and deep-sea areas (Rosso et al. Citation2018; Citation2020a, Citation2020b). The mesophothic zone is a relatively unexplored area of the Mediterranean Sea, and growing interest by the scientific community in the exploration of this zone is evident in the last years (e.g. Cerrano et al. Citation2019 for a review; Albano et al. Citation2020; Giampaoletti et al. Citation2020). Infact, this zone comprises many different habitats (e.g. part of the coralligenous, various kinds of animal forests and maërl beds) and, due to the limitation of light, the light-dependent, shallow water bioconstructors are gradually replaced by heterotrophic deep-sea communities (see Gori et al. Citation2017). In the mesophotic zone of the southern Italian Apulian region, two peculiar habitats were recently discovered in which invertebrates are the main bioconstructors (Corriero et al. Citation2019; Cardone et al. Citation2020). One of these habitats is constructed by the bivalve Neopycnodonte cochlear (Poli, 1795) and the second is made by two species of scleractinan corals (Phyllangia americana mouchezii (Lacaze-Duthiers, 1897) and Polycyathus muellerae (Abel, 1959)). In these two different habitats the bioconstructions are composed of a multilayered aggregation of dead animals in the centre and an outer layer of presently living organisms, forming a hard substrate with a high topographic complexity, and hosting a huge biodiversity (Corriero et al. Citation2019; Angeletti & Taviani Citation2020; Cardone et al. Citation2020; Giampaoletti et al. Citation2020). In this habitat the bryozoans, as well as other calcified organisms, play an important secondary role in bioconstruction (Cardone et al. Citation2020). In a previous study, the analysis of blocks from these peculiar habitats along the Apulian coasts showed the presence of 50 bryozoan species, 46 of which were Cheilostomatida (Giampaoletti et al. Citation2020).
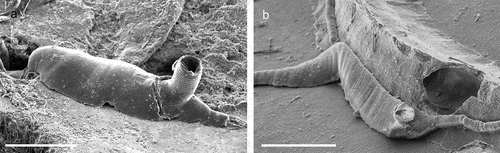
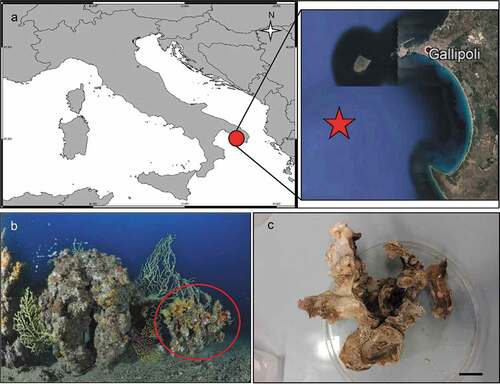
In the frame of the Mediterreanean Mesophotic research project (MESOMED) expeditions, which aim at exploring the Mediterranean mesophotic zone, coralligenous banks were found off Gallipoli (Apulia, Southern Italy). On the banks the outer layer was dominated by the octocoral Paramuricea clavata Risso, 1826 or by the gold coral Savalia savaglia (Bertoloni, 1819). Epibiontic organisms occasionally settle on injured branches of S. savaglia. Particularly the bivalve Neopycnodonte cochlear may form large epibiotic aggregations. The aim of the present paper is to describe the diversity of cheilostomatid bryozoans hosted by these aggregations and compare the results with the bryofauna present at the same depth in the adjoining Apulian areas.
Material and methods
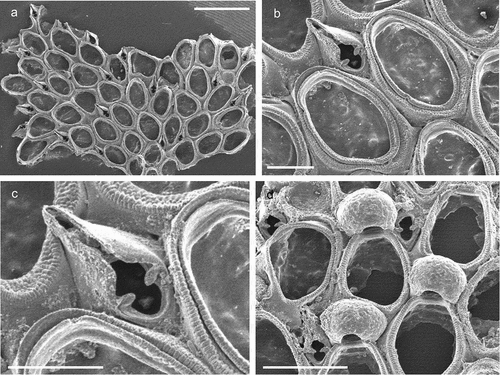
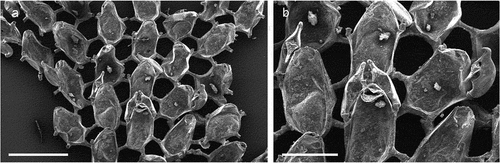
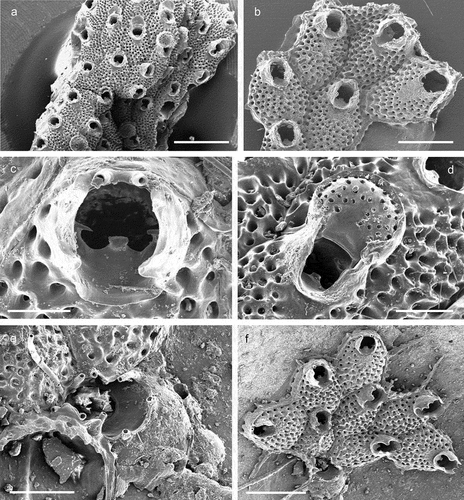
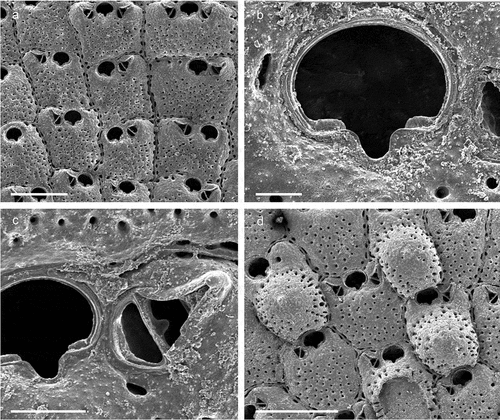
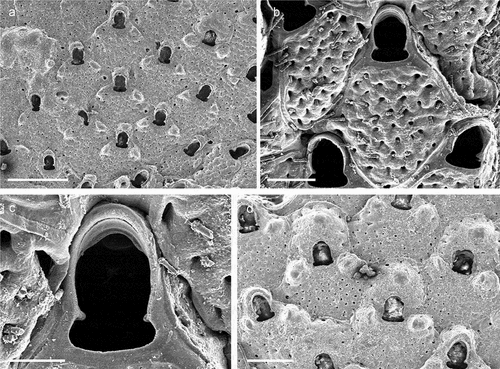
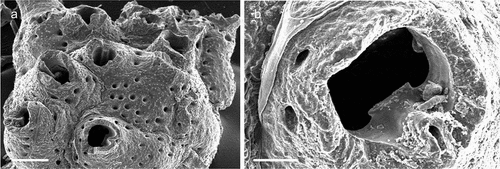
The coralligenous banks at 60 m depth off Gallipoli (Lecce) (40°0ʹ56.20″N; 17°55ʹ17.84″E) along the Ionian Apulian coast ()) were investigated by technical scuba diving on 16 July 2015. The coralligenous banks are up to 2 m in height, towering over a horizontal substrate. The coralligenous is characterised by an outer layer that is dominated by the octocoral Paramuricea clavata Risso, 1826 and the gold coral Savalia savaglia. Epibiontic organisms settle on injured branches of S. savaglia, in particular the bivalve Neopycnodonte cochlear ()). This species starts to colonise dead or damaged portions of the colony (e.g. those inflicted by fishing lines), triggering the development of a very diverse epibiotic community throughout its lifetime as well as post mortem. The bryozoan species studied in the present work live on dead shells of N. cochlear ()) that were still attached to the S. savaglia skeleton. Fourteen groups of shells cemented to each other (about 5–6 shells each) were collected from different S. savaglia colonies and preserved dry. The shell specimens were observed using a Nikon SMZ18 stereomicroscope in order to detect and preliminarly identify the living bryozoan specimens. For all the preliminarily identified bryozoan species, small portions were detached from the shell for detailed analysis of the morphological characters under a scanning electron microscope (SEM). Both untreated and bleached colony portions (using sodium hypochlorite) were mounted on stubs, coated with gold-palladium in a Balzer Union evaporator, and examined with a Philips XL20 SEM. Morphometrics were made on the micrographs using the image software ImageJ (Schneider et al. Citation2012), and are given in the descriptions as mean ± standard deviation, minimum–maximum values, and number of measurements (whenever >2 measurements were taken). Values are in µm unless otherwise noted; abbreviations: L – length, W – width. The type specimens are deposited in the Museo di Storia Naturale di Genova (Italy) labelled with the acronym MSNG. All other examined material is in the first author’s personal collection. Comparative material was examined from the bryozoan collection of the Natural History Museum London (NHMUK).
Figure 1. (a) Map of the Italy with a detail of the Gallipoli coast and studied area detailed (star). (b) A colony of the anthozoan Savalia savaglia colonized by the bivalve Neopycnodonte cochlear (circle) on which the studied bryozoans lived. (c) Group of cemented shells. Scale: C. 1 cm.
The synonymy lists of the respective species are not exhaustive. In the light of recent taxonomic revisions, which revealed that many of the previously accepted bryozoan species are actually species complexes, we here provide only those references in which the species is figured (besides the original publication in which the species was introduced), which allowed verifying their synonymy.
Taxonomic accounts
Phylum Bryozoa Ehrenberg, 1831Class Gymnolaemata Allman, 1856Order Cheilostomatida Busk, 1852Suborder Inovicellina Jullien, 1888Family Aeteidae Smitt, 1868Genus Aetea Lamouroux, 1812Aetea spp.
Material examined
Pica coll: SEM stubs n°18, 40, 58, all bleached colonies.
Remarks
The colonies are too poorly preserved to permit identification to species level, while it is clear (from the budding pattern of the encrusting colony portion) that at least two species are present.
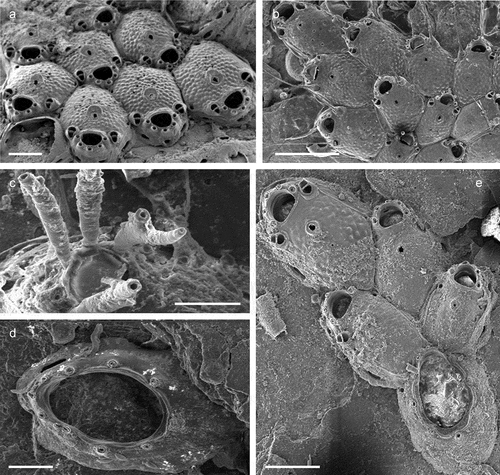
Suborder Flustrina Smitt, 1868Superfamily Calloporoidea Norman, 1903Family Calloporidae Norman, 1903Genus Callopora Gray, 1848Callopora dumerilii (Audouin, Citation1826)
Figure 3. Callopora dumerilii. (a) Colony. (b) Autozooid with avicularia. (c) Close up of maternal zooids. (d) Maternal zooid with ovicell. Scale: (a) 500 µm; (b–d) 200 µm.
Flustra dumerilii Audouin, Citation1826: 240.Callopora dumerilii: Hayward & Ryland Citation1998: 160, figs. 40A–C; Hayward & McKinney Citation2002: 16, figs. 6D–G; Ostrovsky & Schäfer Citation2003: 20, fig. 4; Chimenz Gusso et al. Citation2014: 80, figs. 20a–d.
Material examined
Pica coll: SEM stub n°34 bleached colony; two additional unbleached colonies.
Remarks
The auto- and ovicellate zooids in the colonies studied here usually have six oral spines while in other populations most zooids have only four spines (cf. Hayward & Ryland Citation1998: 160; Hayward & McKinney Citation2002: 18). While Callopora dumerilii usually occurs in shallow waters in the Mediterranean Sea (Hayward & McKinney Citation2002; Chimenz Gusso et al. Citation2014), the present specimens from 65 m presumably mark the deepest record of the species in this region.
Genus Copidozoum Harmer, 1926Copidozoum planum (Hincks, 1880)
Figure 4. Copidozoum planum. (a). Colony. (b) Autozooids with interzooidal avicularium and secondary mural rims, indicating reparative growth by means of intramural budding. (c) Close up of the avicularium. H. Maternal zooids with ovicells. Scale: (a) 1 mm; (b, c) 200 µm; (d) 500 µm.
Membranipora plana Hincks, Citation1880b: 81, pl. 11, fig. 2.
Copidozoum planum: Prenant & Bobin Citation1966: 255, fig. 85; Rosso Citation1996: pl. 2, fig. c; Chimenz Gusso et al. Citation2014: 90, figs. 29a–d; Souto et al. Citation2014: 134, fig. 3B; Reverter-Gil et al. Citation2019: 227, fig. 2c.
Material examined
Pica coll: SEM stub n°56, bleached colony; two additional unbleached colonies.
Remarks
The present specimens are in morphological accordance with the other Mediterranean populations referred to Copidozoum planum.
Genus Corbulella Gordon, 1984Corbulella maderensis (Waters, Citation1898)
Figure 5. Corbulella maderensis. (a) Colony. (b) Autozooid. (c) Vicarious avicularium. (d) Maternal zooids with ovicells. (e) NHMUK 2016.6.9.4, colony from Madeira, Norman collection. Scale: (a) 1 mm; (b, c) 100 µm; (d, e) 200 µm.
Membranipora maderensis Waters, Citation1898: 677, pl. 48, fig. 19.Crassimarginatella maderensis: Harmelin Citation1973: 481, figs 1a–g, 2k, 3h, 4a; Zabala & Maluquer Citation1988: 85, text-fig. 103, pl. 1, fig. G.Corbulella maderensis: Ostrovsky et al. Citation2009: figs. 1e, 3c; Chimenz Gusso et al. Citation2014: 92, figs. 31a–c; Rosso et al. Citation2019a, fig. 5a.
Material examined
Pica coll: SEM stubs n°6 with an unbleached colony; SEM stubs n°7, 9, 15, each with a bleached colony; six addictional unbleached colonies.
Remarks
A comparison of SEM images of the present specimens and material from Madeira (NHMUK 2016.6.9.4, Norman collection), the type locality of Corbulella maderensis, showed no morphological differences.
Genus Crassimarginatella Canu, 1900Crassimarginatella matildae Pica & Berning sp. nov.
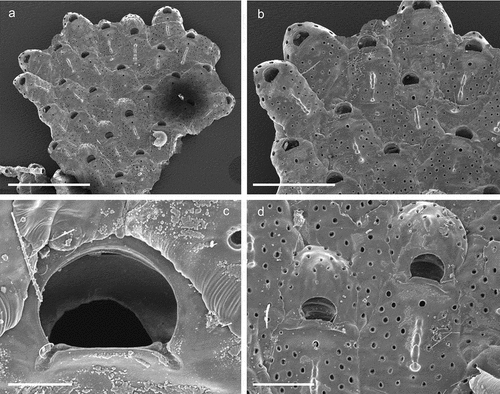
Figure 6. Crassimarginatella matildae sp. nov. (paratype) (a) Colony (SEM stub n°83). (b) Several autozooids and vicarious avicularia (SEM stub n°83). (c) Periancestrular zooids (SEM stub n°83). (d) Maternal zooids with ovicell (SEM stub n°83). (e) Close-up of avicularium (SEM stub n°83). (f) Ancestrula (SEM stub n°8). (g) Early astogenetic zooids and colony growth margin (SEM stub n°83). Scale: (a)2 mm; (b–d) 500 µm; (e, g) 200 µm; (f) 100 µm.
Crassimarginatella crassimarginata: Prenant & Bobin Citation1966: 249 (part), non fig. 83; Harmelin Citation1973: 483 (part), figs. 3e, g (non figs. 3f, i); Zabala & Maluquer Citation1988: 85, text-fig. 104; Harmelin & d’Hondt Citation1993: 69 (part), fig. 8 (non fig. 7); Rosso et al. Citation2017: figs. 5b–c.
Diagnosis
Crassimarginatella with subhexagonal zooids, gymnocyst moderately well developed proximally and narrowing laterally; cryptocyst evenly developed proximally and laterally, narrowing and disappearing only at the very distal end, surface granular to beaded, encircling an oval opesia; zero, two or occasionally three spines on distal zooid margin, two spines in ovicellate zooids. Cystid of vicarious avicularium usually smaller than autozooid but may reach the same size, avicularium with an overall oval appearance, rostrum and mandible distally producing a narrow downcurved mucro; crossbar complete, thick and without columella. Ovicells hyperstomial, ooecium hemispherical; ectooecium smooth, entirely calcified apart from a narrow proximal window of variable shape.
Etymology
Named after the first author’s nephew, Matilda Colapaoli.
Material examined
Holotype
MSNG 62402: one bleached ovicellate colony in the internal portion of a shell, Gallipoli, Lecce (Italy), 40°0ʹ56.20″N, 17°55ʹ17.84″E, depth 60 m; 16 Jul. 2015.
Paratypes
Same data as for holotype. MSNG 62403: one bleached colony with ancestrula; MSNG 62404: unbleached colony; MSNG 62405: one bleached colony; MSNG 62406: bleached colony with ovicells; MSNG 62407: SEM stub n°83, one bleached colony; MSNG 62408: SEM stub n°8, one bleached colony with ancestrula.
Description
Colony encrusting unilaminar, multiserial ()), forming patches of up to 8 mm in diameter. Dried specimens light brown. Zooids flat (L: 590 ± 110, 305–855, 30; W: 403 ± 75, 283–542, 30) and arranged in quincunx, subhexagonal with rounded distal edge, widest usually at about mid-distance, separated by shallow grooves; smooth gymnocyst moderately developed proximally and laterally, narrowing distally ()); cryptocyst encircling a usually oval opesia (L: 374 ± 58, 212–479, 30; W: 241 ± 48, 162–402, 30), relatively evenly developed all around the margin except in proximity of orifice, surface beaded along the upper rim and with circumferential ridges in the lower part ()). Basal walls only marginally calcified; lateral gymnocystal walls well developed, with a single basal pore chamber per neighbouring zooid, communication via uniporous septula surrounded by a large round to oval window. Periancestrular zooids with five short thin orificial spines, these rapidly diminishing to a distal pair in many autozooids ()) with most zooids in the zone of astogenetic repetition lacking spines while few may have three spines; maternal zooids with a single spine at each proximolateral ooecium corner ()).
Colony not precocious. Ooecium hyperstomial, hemispherical, wider than long (L: 169 ± 16, 143–186, 5; W: 282 ± 29, 230–304, 5), closure type (sub-) cleithral. Ectooecium smooth, completely calcified with only a thin transversal suture line or a small foramen above the opening ()).
Avicularium vicarious, surrounded by five autozooids, usually slightly smaller than an autozooid while some may be as large as an autozooid (L: 342 ± 43, 269–399, 15; W: 208 ± 30, 167–267, 14); cystid with variably developed gymnocyst ()). Rostrum (L: 196 ± 26, 133–235, 14) at an acute angle to frontal plane, distally with a relatively long and narrow mucro that is downcurved and incised into the transverse wall of the avicularian cystid; avicularian surface almost equally divided into a distal rostral and a proximal opesial half by a robust and relatively smooth crossbar, semicircular proximal opesia usually slightly wider than the similarly shaped palatal opesia, both framed by a cryptocystal rim similar to that of autozooids ()).
Ancestrula tatiform ()), 230–240 µm long and 296–340 µm wide, more or less circular, with large round opesia and ten evenly spaced spines, surrounded by seven periancestrular zooids ()).
Remarks
Hincks (Citation1880a) described Crassimarginatella crassimarginata from Madeira, which is characterised by avicularia with a distally rounded rostrum and mandible, while oral spines are apparently absent. The nominal species was subsequently recorded also in the Mediterranean Sea (e.g. Gautier Citation1962; Prenant & Bobin Citation1966; Zabala & Maluquer Citation1988). However, although Harmelin (Citation1973) as well as Harmelin and d’Hondt (Citation1993) explicitly noted the differences in avicularium morphology between the Atlantic and Mediterranean forms, the populations have until now been treated as synonymous. As the mucro in the distal rostrum, in which the pointed, sclerotised tip of the mandible comes to rest, is a constant and exclusive character in the Mediterranean population, we here treat the two taxa as specifically distinct, and introduce Crassimarginatella matildae as new species. It is endemic to the Mediterranean Sea while the Alboran Sea is an ecotonal area where both species co-occur (Harmelin & d’Hondt Citation1993).
Genus Ellisina Norman, 1903Ellisina gautieri Fernández Pulpeiro & Reverter Gil, Citation1993
Figure 7. Ellisina gautieri. (a) Colony. (b) Ancestrula and early astogenetic autozooids. (c) Maternal zooid with ovicell. (d) Ancestrula and early astogeny. Scale: (a) 1 mm; (b–d) 200 µm.
Ellisina gautieri Fernández Pulpeiro & Reverter Gil, Citation1993: 98, pl. 2 figs. 1–2.Ellisina cf. antarctica: Harmelin Citation1969: fig. 7; Zabala & Maluquer Citation1988: 81, text-figs. 89–90.Ellisina gautieri: Hayward & Ryland Citation1998: 192, figs. 56C–D; Hayward & McKinney Citation2002: 18, figs. 7E–G; Berning Citation2006: 26, figs. 16–18; Chimenz Gusso et al. Citation2014: 100, figs. 37a–d; Sokolover et al. Citation2016: 446, figs. 5A–B.
Material examined
Pica coll: SEM stubs n°8, 24, each with a bleached colony with ancestrula; SEM stub n°12, unbleached colony with ancestrula; two additional unbleached colonies.
Remarks
The present specimens morphologically agree with the other Mediterranean populations referred to Ellisina gautieri.
Superfamily Flustroidea Fleming, 1828Family Flustridae Fleming, 1828Genus Chartella Gray, 1848?Chartella sp.
Material examined
Pica coll: SEM stub n°51, unbleached colony.
Remarks
The small colony studied is possibly the early astogenetic, encrusting phase of an erect flustrid, which cannot be determined even to genus level owing to the absence of significant characters and comparative material as usually only the erect colony portion of flustrids is described and figured. The zooids are very lightly calcified, c. 0.7 mm long and 0.5 mm wide, and a cryptocyst is not developed. Spines or avicularia were not observed. As Giampaoletti et al. (Citation2020) reported Chartella papyrea (Pallas, 1766) from the mesophotic site of Monopoli in the Adriatic Sea, we doubtfully assign the present specimens to this genus.
Superfamily Buguloidea Gray, 1848Family Bugulidae Gray, 1848Genus Bugula Oken, 1815Bugula sp.
Material examined
Pica coll: one unbleached colony.
Remarks
Only a tiny colony was obtained, which was observed using an optical stereomicroscope in order to confirm the genus as it does not allow to determine the specimen to species level.
Family Beaniidae Canu & Bassler, 1927Genus Beania Johnston, Citation1840Beania mediterranea Souto, Nascimento, Reverter-Gil & Vieira Citation2018
Figure 9. Beania mediterranea. (a) Colony. (b) Autozooids with avicularia. Scale: (a) 1 mm; (b) 500 µm.
Beania mediterranea Souto, Nascimento, Reverter-Gil & Vieira, Citation2018: 1510, figs. 2c, 6a–h.Diachoris buskiana: Waters Citation1879a: 120, pl. 12 fig. 1.Beania magellanica: Prenant & Bobin Citation1966: 555, fig. 191; Zabala Citation1986: 333, fig. 96; Zabala & Maluquer Citation1988: 101, fig. 176; Hayward & McKinney Citation2002: 24, figs. 10A–B; Chimenz Gusso et al. Citation2014: 68, figs. 9a–c.
Material examined
Pica coll: SEM stub n°38, unbleached colony; seven additional unbleached colonies.
Remarks
The specimens from Apulia showed no differences to Beania mediterranea, which was recently described by Souto et al. (Citation2018).
Beania mirabilis Johnston, Citation1840
Beania mirabilis Johnston, Citation1840: 272.Beania mirabilis: Zabala & Maluquer Citation1988: 101, text-fig. 175; Hayward & Ryland Citation1998: 244, text-fig. 79; Hayward & McKinney Citation2002: 26, figs. 10C–D; Chimenz Gusso et al. Citation2014: 69, figs. 10a–d; Rosso et al. Citation2019b, fig. 4E.
Material examined
Pica coll: one unbleached colony.
Remarks
As only a single colony with few zooids was obtained, the specimen was not examined using SEM.
Superfamily Microporoidea Gray, 1848Family Microporidae Vigneaux, 1949Genus Micropora Gray, 1848Micropora biopesiula Pica & Berning sp. nov.
Figure 10. Micropora biopesiula sp. nov. (paratype). (a) Colony centre (SEM stub n°50). (b) Zooid arrangement (SEM stub n°50). (c) Zooid at the colony margin with oval pore-chamber windows (SEM stub n°15). (d) Close-up of autozooidal opesia and opesiules (SEM stub n°50). (e) Maternal zooid with ovicell (SEM stub n°50). (f) Ovicell with the cryptocystal cover of the ovicell-producing distal zooid broken, showing the globular structure of the ooecium underneath (SEM stub n°36). (g) Ancestrula with first-generation autozooid (SEM stub n°40). (h) Ancestrula and early autozooids (SEM stub n°15). Scale: (a, h) 500 µm; (c–e) 100 µm; (f, g) 200 µm.
Micropora coriacea: Rosso Citation1996: pl. 2, fig. f; Chimenz Gusso et al. Citation2014: 104, fig. 41a–d.
Diagnosis
Micropora with zooids that are somewhat longer than wide (L/W = 1.41); cryptocyst granular, perforated by 25–50 pseudopores and two pairs of opesiules: a larger, elongated and funnel-shaped one in the distolateral corners, and a smaller pair of round opesiules proximal to the former; autozooidal opesia slightly wider than long (L/W = 0.61). Gymnocystal bosses lateral to proximal orifice small and usually inconspicuous. Avicularia absent. Ovicell semi-immersed in distal zooid, ooecium hemispherical, about as wide as long (L/W = 1.12), cryptocystal surface granular, proximal margin arched, composed of a broad flat band of smooth gymnocyst with a central suture above which an apex is formed; ooecial orifice dimorphic, slightly larger than in autozooids but with similar length/width ratio (0.63).
Etymology
The name refers to the presence of two pairs of opesiules.
Material examined
Holotype
MSNG 62409: unbleached ovicellate colony, Gallipoli, Lecce (Italy), 40°0ʹ56.20″N, 17°55ʹ17.84″E, depth 60 m, 16 Jul. 2015.
Paratypes
Same data as for holotype. MSNG 62410: unbleached colony with ancestrula; MSNG 62411: small unbleached colony; MSNG 62412: small unbleached colony; MSNG 62413: SEM stub n°15, bleached colony; MSNG 62414: SEM stub n°36, bleached ovicellate colony; MSNG 62415: SEM stub n°40, bleached ancestrula and first zooid; MSNG 62416: SEM stub n°50, bleached colony with ovicells and ancestrula.
Description
Colony encrusting unilaminar, multiserial ()), forming patches of up to 2 cm in diameter. Dried specimens light brown. Zooids irregular in shape (L: 631 ± 57, 551–767, 20; W: 456 ± 71, 343–544, 20), more or less hexagonal to irregular oval ()), slightly longer than wide (L/W = 1.4), separated by a narrow groove or suture; marginal walls slightly rising distal to mid-distance of zooid, mostly composed of granulated cryptocyst except for a distal gymnocystal part where a pair of relatively small rounded bosses are occasionally formed lateral to the proximal opesia ()). Oval pore-chamber windows visible at the colony growing edge, usually two per neighbouring zooid ()). Cryptocyst finely granulated, flat to slightly convex, gently dipping distolaterally before suddenly rising toward the opesia, densely perforated by 25–50 pseudopores ( (b,d)). A pair of deeply recessed opesiules, elongated in shape is placed proximolateral to the orifice (L: 69 ± 11, 51–102, 27; W: 36 ± 7, 20–49, 27) (–d)). A second pair of small rounded opesiules (L: 24 ± 4, 17–33, 27) proximally to the large opesiules ()). Opesia (L: 99 ± 6, 89–110, 20; W: 162 ± 7, 150–176, 20) slightly raised above the zooid surface, D-shaped and wider than long (L/W = 0.6), with a narrow gymnocyst forming the distolateral margin ()). Spines and avicularia absent.
Ovicell (L: 291 ± 12, 276–313, 12; W: 260 ± 15, 233–289, 12) semi-immersed in the frontal shield of the distal zooid ()), ooecium hemispherical, about as wide as long (L/W = 1.1), opesia dimorphic, slightly larger than in autozooids (L: 114 ± 6, 107–128, 13; W: 180 ± 9, 172–194, 13), closure type cleithral. Proximal ooecial margin bordered by a smooth broad rim with a thin, central, longitudinal suture line and an apex forming above it ()).
Ancestrula similar in shape to adult zooid ()), but approximately as long as wide (310 × 280 µm), opesia about twice as wide as long (OpL 60 µm, OpW 130 µm).
First asexually produced zooid budded from distal end of ancestrula, the two second-generation autozooids are produced by both the ancestrula and the 1st generation zooid, oriented at a c. 90° angle; the 3rd-generation zooids grow in the interspaces of this cross-shaped formation, establishing growth of the colony in an opposite direction respect to the polarity of the ancestrula ()).
Remarks
Three Micropora species are known from the modern Mediterranean Sea and eastern Atlantic. Micropora coriacea (Esper, 1791) is the most frequently cited species with an alleged worldwide distribution, which, however, most likely represents a species complex. The species differs from Micropora biopesiula sp. nov. in having only the single distal pair of funnel-shaped opesiules, and usually more pronounced bosses lateral to the proximal orifice margin (e.g. Hayward & Ryland Citation1998: 288, figs 97, 99C–D). Besides having interzooidal avicularia, Micropora normani Levinsen, Citation1909 from the Atlantic coast of Europe has smaller zooids, fewer pores in the cryptocyst, and also only a single pair of opesiules (Hayward & Ryland Citation1998: 290, figs. 98, 99B). Finally, the West African Micropora robusta Cook, Citation1985 also has interzooidal avicularia and just a single pair of opesiules.
Three other Micropora species may exist in the region (see Bock & Gordon Citation2021): Micropora depressa (Moll, Citation1803) and Micropora aculeata (d’Orbigny, 1852), which are both considered as taxa inquirenda, as well as Micropora africana (d’Orbigny, 1852). To our knowledge, none of these species have ever been cited or redescribed since their original description, and their status, as well as their generic assignments, are unclear at present.
As it will be difficult to clarify the status and systematic placement of these three species any time soon, we have decided to describe the present species as new to science in order to raise awareness among biologists that the oft-cited M. coriacea is not the only Micropora species present in the Mediterranean Sea.
Micropora biopesiula sp. nov. has so far only been reported from Italian coasts (Rosso Citation1996; Chimenz Gusso et al. Citation2014). Whether the new species is also present in the eastern Mediterranean cannot be decided at present as images and descriptions are wanting (e.g. Hayward Citation1974).
Genus Mollia Lamouroux, 1816Mollia circumcincta (Heller, Citation1867)
Figure 11. Mollia circumcincta. (a) Colony. (b) Maternal zooids. (c) Calcified ridge on the interior basal wall of a zooid; note the pores in the basal wall, indicating the position of calcified basal attchment tubes. (d) Zooids at the colony growth margin showing the formation of the connecting tubules. Scale: (a) 1 mm; (b–d) 200 µm.
Membranipora circumcincta Heller, Citation1867: 96, pl. 6, fig. 5.Mollia circumcincta: Zabala & Maluquer Citation1988: 91, pl. 2, figs. C–D; Hayward & McKinney Citation2002: 34, figs. 13D–H; Berning Citation2006: 36, figs. 32, 33; Ayari-Kliti et al. Citation2012: 85, pl. 2 fig. 3; Chimenz Gusso et al. Citation2014: 106, figs. 42a–f; Rosso et al. Citation2019a: fig. 5c.
Material examined
Pica coll: SEM stub n°49, one bleached colony; two additional unbleached colonies.
Remarks
Mollia circumcincta [for a recent description based on type material from the Adriatic see Hayward and McKinney (Citation2002: 34)] has been reported only a few times since its discovery, and all records from beyond the central Mediterranean Sea have to be regarded as doubtful. For instance, in the colony from the western Mediterranean figured by Zabala and Maluquer (Citation1988: pl. 2, figs. C, D), the ooecium differs in producing a relatively large area of smoothly calcified ectooecium proximally and laterally that encircles a distally positioned area of exposed nodular endooecium. In Adriatic and NE Ionian M. circumcincta, in contrast, the ectooecium is extremely reduced and restricted to the proximolateral corners of the ooecium. Moreover, the salient distolateral rim framing the zooids is thicker and the nodules covering the cryptocystal surface larger in central Mediterranean populations.
The peculiar calcified ridge on the interior basal wall that was noted in fossil specimens (Berning Citation2006: figs. 32 and 33) are here shown to be present also in the Recent species ()). It is unclear what function the ridge fulfils, and if it is present in all Mollia species or restricted to M. circumcincta.
Family Onychocellidae Jullien Citation1882Genus Onychocella Jullien, Citation1882Onychocella marioni Jullien, Citation1882
Figure 12. Onychocella marioni. (a) Colony. (b) Zooids with an onychocellarium. Scale: (a) 1 mm; (b) 200 µm.
Onychocella marioni Jullien, Citation1882: 277.Onychocella marioni: Zabala & Maluquer Citation1988: 87, pl. 2, fig. A; Rosso Citation1996: pl. 2, fig. b; Ayari-Kliti et al. Citation2012: 86, pl. 2, fig. 5; Chimenz Gusso et al. Citation2014: 110, figs. 45a–c; Achilleos et al. Citation2020: fig. 2; Rosso et al. Citation2020b: 3, figs. 1–3.Onychocella angulosa: Taylor et al. Citation2018: fig. 1a–c.
Material examined
Pica coll: SEM stub n°44, one bleached colony; one additional unbleached colony.
Remarks
While Taylor et al. (Citation2018) have recently summarised the problems existing around the type species of the genus Onychocella, and discussed the potential synonymy of the fossil Onychocella angulosa (Reuss, 1848) and the present-day Onychocella marioni Jullien, Citation1882, Rosso et al. (Citation2020b) thoroughly redescribed and figured the Recent species, to which we assign the Apulian specimens.
Superfamily Cribrilinoidea Hincks, 1879Family Cribrilinidae Hincks, 1879Genus Cribrilaria Canu & Bassler, 1929Cribrilaria cf. hincksi (Friedl, Citation1917)
Figure 13. Cribrilaria cf. hincksi. (a) Colony, (b) Zooids with avicularia and ovicells. (c) Zooid, (d) Interzooidal avicularium, (e) Maternal zooids, (f) Ancestrula and the firstly budded autozooid. Scale: (a) 1 mm; (b) 500 µm; (c, d, f) 100 µm; (e) 200 µm.
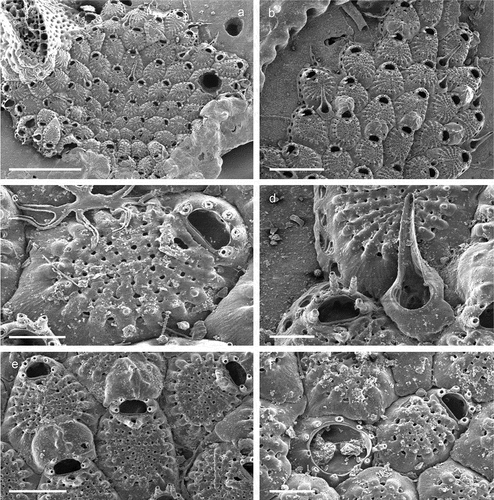
cf. Cribrilina radiata var. hincksi Friedl, Citation1917: 236.Colletosia hincksi: Prenant & Bobin Citation1966: 595, figs. 207–I, –II.Puellina (Cribrilaria) hincksi: Harmelin Citation1988: 31, figs. 5, 9; Zabala & Maluquer Citation1988: 107, text-fig. 216, pl. 6, figs. C–D.Cribrilaria hincksi: Rosso et al. Citation2019b: fig. 5B.non Puellina hincksi: Hayward & McKinney Citation2002: 38, figs. 17A–C.
Material examined
Pica coll: SEM stubs n°47, 72, each with one ovicellate colony.
Remarks
There is quite a confusion concerning the morphology of Cribrilaria hincksi. When introducing the species, Friedl (Citation1917) mentioned (without figuring the material) that his specimens from the northern Adriatic Sea are characterised by “sehr langen dolchförmigen Avicularien” (transl.: very long, dagger-shaped avicularia) that he considered similar to specimens reported from Madeira by Hincks (Citation1880a: 74, pl. 10, fig. 1) as Cribrilina radiata var. (Moll Citation1803). According to Hincks’ figure, the Madeiran species has indeed very long rostra that are apparently positioned between zooids (which is, however, not quite clear from the drawing). In subsequent works (e.g. Prenant & Bobin Citation1966; Harmelin Citation1988; Zabala & Maluquer Citation1988), C. hincksi was regarded as having an extremely elongate avicularium and a serrated rostrum that is recumbent on the distal zooid. The present specimens comply with these characters, but neither ours nor any of the colonies recorded in the above-mentioned publications were from the type locality, the northern Adriatic. Recently, Hayward and McKinney (Citation2002: 38, figs. 17A–C)) did identify specimens from the type locality as P. hincksi, which have, however, comparatively short avicularia that are wedged between zooids, and are therefore unlikely to be conspecific. Thus, without having seen the type material, which is beyond the scope of this paper, it is impossible to define the exact morphology of C. hincksi.
Cribrilaria cf. venusta (Canu & Bassler, Citation1925)
Figure 14. Cribrilaria cf. venusta. (a) Colony. (b) Zooids. (c) Orifice with the proximal border denticulated. (d) Maternal zooids. E. Ancestrula and first-generation autozooid. Scale: (a) 1 mm; (b, d, e) 200 µm; (c) 100 µm.
cf. Puellina venusta Canu & Bassler, Citation1925: 22, pl. 2, fig. 5.Puellina venusta: Harmelin & d’Hondt Citation1993: 68, fig. 6; Hayward & Ryland Citation1998: 336, figs. 119C–D.Cribrilaria crenulata: Harmelin Citation1970: 91, figs. 1i–k, pl. 2 figs. 1–3.Cribrilaria venusta: Harmelin Citation1978: 180, pl. 2, figs. 3–5.Puellina (Cribrilaria) venusta: Bishop & Househam Citation1987: 28, figs. 43–49, 99; Zabala & Maluquer Citation1988: 107, text-fig. 215, pl. 7C–D; Chimenz Gusso et al. Citation2014: 221, figs. 120a–e.
Material examined
Pica coll: SEM stubs n°12, 23, each with one colony with ancestrula; SEM stub n°37, one bleached ovicellate colony; two additional unbleached colonies.
Remarks
The present specimens are within the morphological range observed in other Mediterranean populations referred to Cribrilaria venusta. The original description and image of the species by; Canu and Bassler (Citation1925), however, suggest that both the orifice and ovicell are distinctly smaller in relation to zooid size. A revision of the type material is therefore necessary before the Mediterranean specimens can unequivocally be synonymised with Canu & Bassler’s species from the southern Gulf of Cádiz.
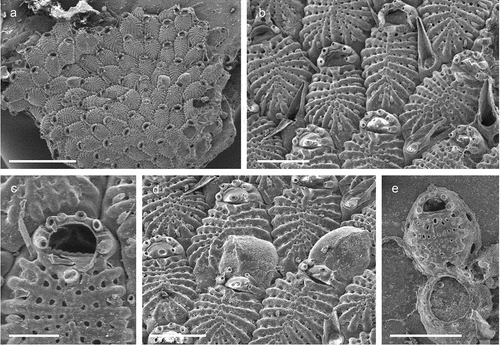
Cribrilaria cf. innominata (Couch, Citation1844)
Figure 15. Cribrilaria cf. innominata. (a) Zooidal arrangement. (b) Autozooid. (c) Zooids and avicularia with inatramural buds, the one to the right with reversed polarity. (d) Avicularium. (e) Maternal zooid with ovicell. (f) Growth margin of colony with newly formed zooids. (g) Ancestrula. (h) Ancestrula and early autozooids. Scale: (a) 500 µm; (b, c, f, h) 200 µm; (d, e, g) 100 µm.
cf. Lepralia innominata Couch, Citation1844: 114, pl. 22, fig. 4.cf. Cribrilaria innominata: Bishop Citation1986: 96, figs. 1–8.cf. Puellina (Cribrilaria) innominata: Bishop & Househam Citation1987: 33, figs. 5 0–58.Puellina (Cribrilaria) innominata: Zabala & Maluquer Citation1988: 108, text-fig. 220, pl. 6E, F; Harmelin Citation1988: 29, fig. 4; Chimenz Gusso et al. Citation2014: 213, figs. 115a–e.
Material examined
Pica coll: SEM stubs n°16, 55, bleached ovicellate colony; SEM stub n°63, bleached colony with ancestrula; seven additional unbleached colonies.
Remarks
Several slight morphological differences extist between the British Cribrilaria innominata (Couch, Citation1844), as redescribed by Bishop (Citation1986), and Mediterranean specimens attributed to this species. For instance, the apophyses that occur at the base of (some of) the oral spines in Mediterranean populations (Harmelin Citation1988; Chimenz Gusso et al. Citation2014) have not been reported in British specimens. Also, in Mediterranean colonies only a single costal pair contributes to the formation of the suboral umbo that proximally frames the lacuna, and there is no median ridge on the costate frontal shield, whereas in British specimens the two distal pairs of costae form the umbo that proximally turns into a short median carina (Bishop Citation1986; Hayward & Ryland Citation1998). Additional comparative SEM studies and genetic analyses are needed before a conclusion as to the status of the Mediterranean specimens can be drawn.
Cribrilaria radiata (Moll, Citation1803)
Figure 16. Cribrilaria radiata: (a) Colony. (b) Detail of zooids and avicularium. (c) Maternal zooids with ovicells. (d) Ovicell. Scale: (a) 1 mm; (b, c) 200 µm; (d) 100 µm.
Eschara radiata Moll, Citation1803: 63, pl. 4, fig. 17.Cribrilaria radiata: Harmelin Citation1970: 80, figs. 1a–c, 3a; pl. 1, figs. 1–3.Puellina (Cribrilaria) radiata: Zabala & Maluquer Citation1988: 107, text-fig. 214, pl. 7. fig. A; Chimenz Gusso et al. Citation2014: 218, figs. 118a–e; Rosso et al. Citation2019a: fig. 5g.
Material examined
Pica coll: SEM stubs n°15, 43, each with one bleached ovicellate colony; five additional unbleached colonies.
Remarks
The Apulian specimens are identical with other Mediterranean records of Cribrilaria radiata.
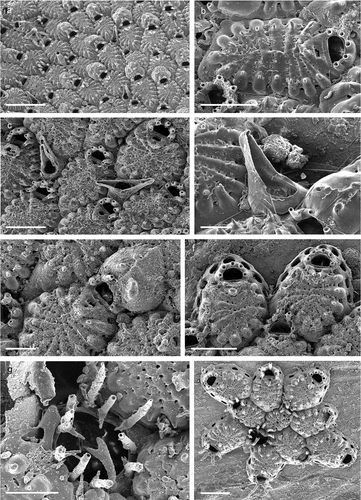
Genus Glabrilaria Bishop & Househam, Citation1987Glabrilaria pedunculata (Gautier, Citation1956)
Figure 17. Glabrilaria pedunculata. (a) Colony. (b) Edge of the colony showing a kenozooid and a maternal zooid with kenozooidal ovicell. (c) Zooids and a kenozooid with pedunculate avicularia. (d) Avicularium. (e) Maternal zooid with avicularia. F. Ancestrula with early autozooids. Scale: (a) 500 µm; (b, c, f) 100 µm; (d) 20 µm; (e) 50 µm.
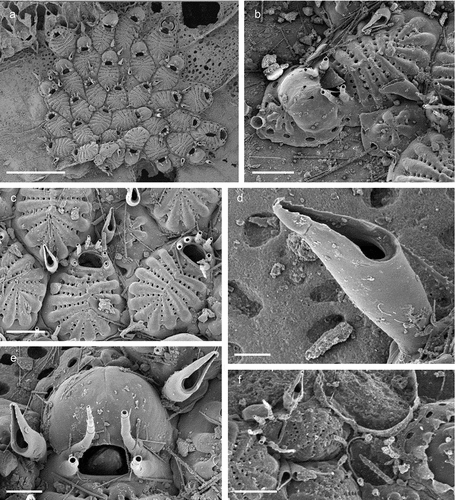
Puellina pedunculata Gautier, Citation1956: 203, fig. 2.Cribrilaria pedunculata: Harmelin Citation1970: 93, figs. 1g–h, pl. 2, fig. 6.Puellina pedunculata: Harmelin Citation1988: 33, figs. 10–11; Harmelin & d’Hondt Citation1993: 69, fig. 9.Puellina (Glabrilaria) pedunculata: Bishop & Househam Citation1987: 55, figs. 95–97; Zabala & Maluquer Citation1988: 107, text-fig. 209, pl. 7, figs. E–F: Chimenz Gusso et al. Citation2014: 215, figs. 116a–c: Rosso et al. Citation2019a: 711, figs. 5e–f.non Cribrilaria pedunculata: Harmelin Citation1978: 188, figs. 5–6.Glabrilaria pedunculata: Rosso et al. Citation2021, fig. 5g.
Material examined
Pica coll: SEM stub n°5 unbleached colony; SEM stub n° 52 unbleached colony with ancestrula; SEM stubs n°8, 9, 10, each with one bleached ovicellate colony; six additional unbleached colonies.
Remarks
The pedunculate avicularia and the kenozooidal ovicell are typical for the genus, and the present specimens fully conform with previous records of Glabrilaria pedunculata.
Superfamily Hippothooidea Busk, 1859Family Hippothoidae Busk, 1859Genus Hippothoa Lamouroux, 1821Hippothoa flagellum Manzoni, Citation1870
Figure 18. Hippothoa flagellum. (a) Zooids covered by spicules of sponges. (b) Orifice. Scale: (a) 200 µm; (b) 20 µm.
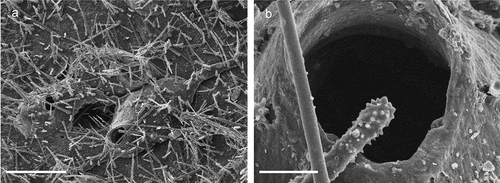
Hippothoa flagellum Manzoni, Citation1870: 328, pl. 2, fig. 5.Hippothoa flagellum: Hayward & Ryland Citation1999: 88, fig. 18; Hayward & McKinney Citation2002: 42, figs. 18F–I.
Material examined
Pica coll: SEM stub n°64, one bleached dead colony; one additional unbleached colony.
Remarks
The colonies encrust a sponge and were, post mortem, covered by a thin veneer of sponge tissue and numerous spicules in turn, giving the colonies a peculiar appearance.
Family Chorizoporidae Vigneaux, 1949Genus Chorizopora Hincks, 1880Chorizopora brongniartii auctt. (Audouin Citation1826)
Figure 19. Chorizopora brongniartii. (a) Colony. (b) Ovicell. (c) Avicularium and kenozooid. (d) Newly formed zooid. Scale: (a) 500 µm; (b, d) 100 µm; (c) 50 µm.
Chorizopora brongniartii: Zabala & Maluquer Citation1988: 141, pl. 19, fig. D; Hayward & McKinney Citation2002: 42, figs. 19A–C; Chimenz Gusso et al. Citation2014: 151, figs. 72a–d.
Material examined
Pica coll: SEM stub n°CHO1 one bleached colony; two additional unbleached colonies.
Remarks
A variety of morphotypes from around the world have been attributed to Chorizopora brongniartii (Audouin, Citation1826). This species complex thus requires revision after fixation of a neotype as the original material has been lost.
Family Haplopomidae Gordon in de Blauwe Citation2009Genus Haplopoma Levinsen, Citation1909Haplopoma celeste Pica & Berning sp. nov.
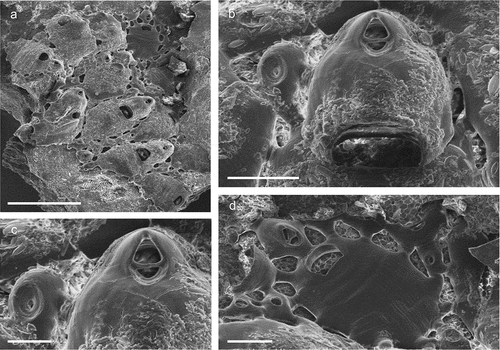
Figure 20. Haplopoma celeste sp. nov. (paratype, SEM stub n°1). (a) Colony. (b) Autozooids. (c) Orifice. (d) Maternal zooids with ovicells. Scale: (a) 1 mm; (b) 500 µm; (c) 50 µm; (d) 200 µm.
Diagnosis
Haplopoma with an evenly perforated frontal shield except for an area around the ascopore, pseudopores bevelled but simple, shield surface smooth but transversely wrinkled and often with a distinct median crest of variable length produced by jointed knobs; orifice broader than long, with a pair of small round condyles; ovicellate zooids of similar size as autozooids; ooecial surface evenly perforated by pseudopores, occasionally with a low median ridge.
Etymology
Named after the first author’s niece, Celeste Beatrix Pica; used as a noun in apposition.
Material examined
Holotype
MSNG 62417: one unbleached colony with ovicells, Gallipoli, Lecce (Italy), 40°0ʹ56.20″N, 17°55ʹ17.84″E, depth 60 m, 16 Jul. 2015.
Paratypes
Same data as for holotype. MSNG 62418: unbleached ovicellate colony; MSNG 62419: unbleached ovicellate colony; MSNG 62420: unbleached colony; MSNG 62421: SEM stub n°1, bleached ovicellate colony; MSNG 62422: SEM stub n°12, bleached colony; MSNG 62423: SEM stub n°81, bleached ovicellate colony; MSNG 62424: SEM stub n°84, bleached colony.
Description
Colony encrusting, unilaminar, multiserial ()), forming small patches of up to 4 mm in diameter. Zooids (L: 569 ± 54, 455–669, 16; W: 326 ± 31, 278–419, 16) elongated pentagonal to hexagonal with a rounded distal end, separated by shallow grooves ()). Gymnocystal frontal shield translucent, slightly convex, surface smooth but with transverse wrinkles and uniformly perforated by some 28–36 bevelled round pseudopores except for an area around the ascopore ()), a short slit is visible distal to each pore using an optical microscope; ascopore circular (23 µm), only slightly larger than the frontal pores ()); proximal to the ascopore a distinct narrow crest of variable length formed by a linear series of more or less jointed knobs is present in most zooids ()). Five oval basal pore chambers on each zooid side, communication pores round and large, widely spaced, three or four per neighbouring zooid ()), basal wall only marginally calcified. Orifice semicircular (L: 66 ± 3, 61–72, 16; W: 102 ± 3, 98–108, 16), wider than long and widest at about mid-distance, proximal margin straight with a pair of small rounded condyles near each corner and distinct notches extending proximolaterally ()).
Ovicellate zooids about the same size as autozooids, ovicells kenozooidal, ooecium hemispherical, partly immersed below colony surface, slightly wider than long (L: 238 ± 8, 229–247, 5; W: 245 ± 15, 230–266, 5), closure type cleithral; smooth ectooecial calcification resembling the frontal shield of the zooid with scattered pores, occasionally with a low central ridge ()).
Ancestrula not observed.
Remarks
The new species is very similar to Haplopoma planum Ryland, Citation1963 as regards the pseudoporous frontal shield, the non-stellate pore type, and the orifice with its condyles, while H. planum lacks the central crest on the zooidal surface, and has distinctly larger zooids, orifices and ovicells (cf. Hayward & Ryland Citation1999: 312, figs. 141A–B, 144). Haplopoma planum is a northern species, however, occurring from Shetland to the Arctic.
The other existing species from the Mediterranean Sea and warm-temperate eastern Atlantic differ more significantly in having only a single row of frontal pseudopores, [Haplopoma impressum (Audouin, Citation1826); Haplopoma sciaphilum Silén & Harmelin, 1976], or stellate pores [Haplopoma bimucronatum (Moll, Citation1803); Haplopoma graniferum (Johnston, Citation1847)], among other character differences.
Together with H. bimucronatum, H. planum and H. sciaphilum, Haplopoma celeste sp. nov. is among the species that are recorded from waters below 50 m depth (or from caves in shallower waters), while the other species are mostly restricted to in the shallow subtidal. To our knowledge, H. celeste has not been recorded before in previous works and is thus only known from the central Mediterranean Sea at depths of 60 m.
Family Romancheinidae Jullien, 1888Genus Escharella Gray, 1848Escharella cf. variolosa (Johnston, Citation1838)
cf. Lepralia variolosa Johnston, Citation1838: 278, pl. 34 fig. 4.cf. Escharella variolosa: Zabala & Malaquer 1988: 125, pl. 13, fig. C; Hayward & Ryland Citation1999: 132, figs. 41, 42B; Chimenz Gusso et al. Citation2014: 158, figs. 77a–d; Souto et al. Citation2014: 140, fig. 4F.
Material examined
Pica coll: two unbleached colony fragments.
Remarks
Only two poorly preserved colony fragments were found, which makes it difficult to precisely identify the species.
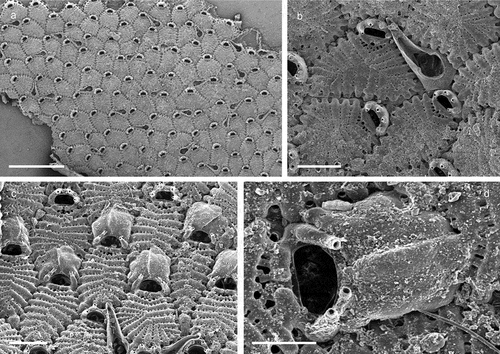
Superfamily Smittinoidea Levinsen Citation1909Family Smittinidae Levinsen Citation1909Genus Prenantia Gautier, Citation1962Prenantia ligulata (Manzoni, Citation1870)
Figure 21. Prenantia ligulata. (a) Colony. (b) Autozooids. (c) Orifice showing two distal oral spines, the lyrula and condyles in an early astogenetic zooid. (d) Maternal zooid with ovicell. (e) Ancestrula. (f) Ancestrula with early autozooids Scale: (a) 1 mm; (b, f) 500 µm; (c, e) 100 µm; (d) 200 µm.
Lepralia ligulata Manzoni, Citation1870: 334, pl. 3, fig. 17.Smittia inerma: Calvet Citation1907: 437, pl. 28, fig. 3.Prenantia inerma: Zabala & Maluquer Citation1988: 122, text-fig. 271, pl. 11, figs. D–E.Prenantia ligulata: Poluzzi Citation1975: 62, pl. 20, fig. 11; Rosso Citation2004, figs. 16–18; Chimenz Gusso et al. Citation2014: 207, figs. 111a–c.
Material examined
Pica coll: SEM stub n°23, small bleached colony; SEM stub n°54, bleached ovicellate colony; SEM stub n°67, bleached colony with ancestrula; one additional unbleached colony.
Remarks
For the sake of consistency, we here continue to follow the most recent works and refer the modern species to the fossil Prenantia ligulata (Manzoni, Citation1870), which would have priority if it should turn out to be synonymous with Prenantia inerma (Calvet, Citation1907), as suggested by Poluzzi (Citation1975). However, the types of both species need to be consulted before this decision can be finalised.
The ancestrula has been observed before but was only incompletely described owing to the periancestrular zooids covering the proximal and lateral gymnocyst (Rosso Citation2004). Here we provide additional information on the morphology of the ancestrula ()). It is about 420 µm long and 290 µm wide, with steep marginal walls rising to an elevated oval area that comprises about the distal two-thirds of the ancestrula. The distinctly raised margin demarcating this area is tightly framed and indented by nine spines that are more or less arching over the frontal surface, the six distal ones of which are relatively closely spaced. The area comprises a broad proximal shelf of smooth cryptocyst that gently slopes towards the centre and narrows distolaterally, and a relatively small, distal, suborbicular opesia. The proximal cryptocystal shelf exhibits a central longitudinal suture.
Family Bitectiporidae MacGillivray, 1895Genus Schizomavella Canu & Bassler, 1917Schizomavella (Schizomavella) cornuta (Heller, Citation1867)
Figure 22. Schizomavella (Schizomavella) cornuta. (a) Colony. (b) Zooids. (c) Orifice with condyles and an oblong suboral avicularium. (d) Spatulate suboral avicularium. (e) Early ontogenetic orifice with with five spines and spatulate suboral avicularium. (f) Ovicell. Scales: (a) 1 mm; (b) 200 µm; (c) 500 µm; (d–f) 100 µm.
Lepralia cornuta Heller, Citation1867: 110, pl. 6, fig. 6.Schizomavella cuspidata: Hayward & Thorpe Citation1995: 665, pl. 2; Reverter Gil & Fernández Pulpeiro Citation1996: 263, figs. 4A–C (only); Hayward & Ryland Citation1999: 286, figs. 131 A, C (not 131B, D).Schizomavella cornuta: Hayward & McKinney Citation2002: 57, figs. 26A–C Reverter-Gil et al. Citation2016: 283, figs. 2–3.
Material examined
Pica coll: SEM stubs n°35, 48, each with one bleached ovicellate colony; seven additional unbleached colonies.
Remarks
The present specimens fully conform with the characters of Schizomavella (Schizomavella) cornuta.
Schizomavella (Schizomavella) cf. linearis (Hassall, Citation1841)
Figure 23. Schizomavella (Schizomavella) cf. linearis. (a) Zooids. (b) Orifice and avicularia. (c) Large adventitious avicularium. (d) Maternal zooid with ovicell and large proximal avicularia. Scale: (a) 500 µm; (b) 100 µm; (c) 50 µm; (d) 200 µm.
cf. Lepralia linearis Hassall, Citation1841: 368, pl. 9, fig. 8.cf. Schizomavella linearis: Hayward & Thorpe Citation1995: 671, pl. 4, figs. a–d; Hayward & Ryland Citation1999: 282, figs. 127–128; Hayward & McKinney Citation2002: 59, figs. 26E–G; Chimenz Gusso et al. Citation2014: 252, figs. 138a–f; Reverter-Gil et al. Citation2016: 304, fig. 11.
Material examined
Pica coll: SEM stub n°69, one bleached ovicellate colony; five additional unbleaced colonies.
Remarks
The Apulian specimens belong to the Schizomavella linearis species complex but differ from all populations hitherto described. Most closely related is the form referred to by Reverter-Gil et al. (Citation2016) as the “pseudolinearis” morphotype from the Adriatic Sea, which has a relatively narrow sinus and avicularia that were reported to be often directed distally (Reverter-Gil et al. Citation2016: 308), although their fig. 11f exclusively shows medially directed avicularia. In the present specimens, the sinus is even narrower, and the avicularia are oriented distomedially to distolaterally. However, despite the large morphological differences between some of the Mediterranean populations and those from the type locality, the British Isles (see Hayward & Thorpe Citation1995; Hayward & Ryland Citation1999), Reverter-Gil et al. (Citation2016) were reluctant to introduce new species owing to the transitional nature of some of the characters, and also because of an absence of a clear geographic pattern in the morphotypes. We have, accordingly, decided to add the present specimens to the species complex and wait for genetic analyses to tackle the issue of their relatedness.
Schizomavella (Schizomavella) subsolana (Hayward & McKinney, Citation2002)
Figure 24. Schizomavella (Schizomavella) subsolana. (a) Colony. (b) Orifice and suboral avicularium. (c) Zooidal orifice showing the sinus. (d) Ovicell. Scales: (a) 500 µm; (b, d) 100 µm; (c) 200 µm.
Schizomavella subsolana Hayward & McKinney, Citation2002: 61, figs. 28A–D.
Material examined
Pica coll: SEM stub n°19, one bleached ovicellate colony; one additional unbleached colony.
Remarks
The present specimen slightly differs from those from the type locality, Rovinj, in the northern Adriatic Sea (Hayward & McKinney Citation2002), in having a less rugose frontal shield with somewhat smaller pores. As all other characters are identical, we consider these differences to have been caused by environmental conditions at greater depth in which the Apulian population was found (60 m vs. 5–20 m off Rovinj).
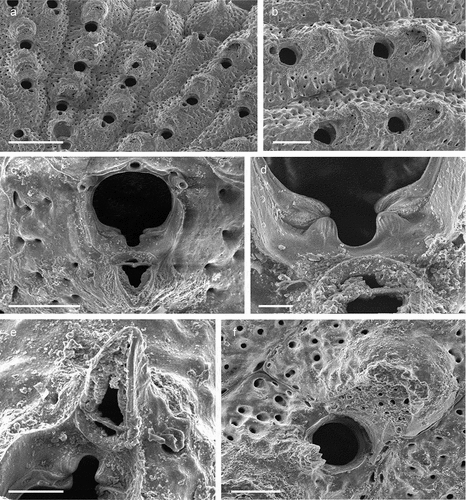
Schizomavella (Schizomavella) cerranoi Pica & Berning sp. nov.
Figure 25. Schizomavella (Schizomavella) cerranoi sp. nov. (holotype). (a) Colony. (b) Ovicellate zooids. (c) Orifice and suboral avicularium. (d) Zooidal orifice showing the sinus and condyles. (e) Avicularium. (f) Ovicell. Scales: (a) 500 µm; (b) 200 µm; (c, f) 100 µm; (d) 20 µm; (e) 50 µm.
Diagnosis
Schizomavella with unilaminar colonies. Zooids rectangular in outline, frontal shield perforated by numerous, relatively small pores. Orifice proximally and laterally surrounded by a thin peristome; primary orifice suborbicular, with a narrowly U-shaped sinus occupying about one-fourth of the proximal margin, condyles relatively long and narrow, with sloping shoulders or running parallel to proximal orifice margin and stopping short of the sinus, no additional structures; distal orifice margin usually with 4 spines persisting thoughout ontogeny in non-ovicellate zooids. Suboral avicularium forning a tall umbo, oriented almost perpendicular to frontal plane, rostrum elongate triangular, crossbar with a small rounded columella. Ovicell marginally covered by secondary calcification of the distal zooid, forming a rugged crest around a small central area of exposed ectooecium that is regularly perforated by small pseudopores.
Etymology
Named after the person who collected the material studied in this paper, Prof. Carlo Cerrano.
Material examined
Holotype
MSNG 62425: SEM stub n°29, one bleached ovicellate colony, Gallipoli, Lecce (Italy), 40°0ʹ56.20″N, 17°55ʹ17.84″E, depth 60 m, 16 Jul. 2015.
Paratypes
Same data as for holotype. MSNG 62426: SEM stub n°58, one bleached colony; MSNG 62427: portion of one bleached ovicellate colony.
Description
Colony encrusting, unilaminar and multiserial ()), developing into a small rounded patch. Autozooids rectangular or irregularly polygonal (L: 460 ± 54, 358–559, 20; W: 414 ± 76, 327–535, 20), in regular radiating lines, separated by fine sutures ()). Frontal shield convex, uniformly perforated by relatively small pores, distally umbonate incorporating a suboral avicularium ()). Orifice partially immersed during ontogeny, surrounded laterally and proximally by a thin, raised peristome that abuts the cystid of the suboral umbo ()); primary orifice rounded, slightly wider than long (L: 116 ± 7, 107–127, 7; W: 101 ± 5, 91–113, 15), sinus narrowly U-shaped (L: 18 ± 2, 16–20, 6; W: 26 ± 3, 22–31, 7), occupying a little less than one fourth of the proximal border (W: 88 ± 5, 81–95, 6), condyles long and relatively narrow, ending short of the sinus, edge sloping or parallel to proximal orifice margin, no proximolateral notches or other structures ()). Usually, three to occasionally four distal oral spines on the distal orifice margin in non-ovicellate zooids, persisting thoughout ontogeny ()). Suboral avicularium (L: 110 ± 0.7, 110–111, 2; W: 50 ± 4, 47–53, 2) almost perpendicular to the frontal plane, the cystid forming a tall structure; rostrum elongate triangular (L: 83 ± 3, 81–86, 3), crossbar complete with small rounded columella, palatal foramen Y-shaped ()). Ovicell acleithral, almost covering the entire frontal shield of the succeeding zooid, about as long as wide or slightly elongated (L: 238 ± 15, 193–314, 20; W: 238 ± 15, 192–260, 20); its margins (particularly distally) covered by relatively smooth secondary calcification of the distal zooid, producing a distinctly raised rugged rim around the exposed ectooecium, which is relatively flattened and regularly perforated by variably shaped pseudopores ()).
Ancestrula unknown.
Remarks
The new species is very closely related to Schizomavella (Schizomavella) subsolana (see above) but differs from it in having a distinctly narrower and deeper sinus. Moreover, Schizomavella (Schizomavella) cerranoi sp. nov. is characterised by larger zooids (particularly their width) and orifices, by a smaller area of exposed ectooecium as well as by having a maximum number of four oral spines. As the two morphotypes apparently occur sympatrically, and as intermediate morphologies were not found in any of the colonies, we have decided to introduce a new species for the present material.
Schizomavella (Schizomavella) cf. tubulata Reverter-Gil, Souto, Novosel & Tilbrook, 2015
Figure 26. Schizomavella (Schizomavella) cf. tubulata. (a) Colony. (b) Zooid. (c) Ancestrula. (d) Ancestrula with early autozooids. Scales: (a) 1 mm; (b) 100 µm; (c) 50 µm; (d) 200 µm.
cf. Schizomavella (Schizomavella) tubulata: Reverter-Gil et al. Citation2016 (nomenclatural availability: 2015): 298, fig. 8; Rosso et al. Citation2019a: fig. 6a.
Material examined
Pica coll: SEM stub n°23, one bleached colony; SEM stub n°30, bleached colony with ancestrula.
Remarks
Only two small immature colonies were obtained. The specimens are very similar to the recently introduced species Schizomavella (Schizomavella) tubulata Reverter-Gil, Souto, Novosel & Tilbrook, 2015 from the Adriatic Sea. Both species have similarly shaped orifice -ith four to five distal spines and a spatulate suboral avicularium oriented almost perpendicular to the frontal plane. In our specimens the early astogenetic zooids show more spines, up to seven, decreasing in number toward the zone of astogenetic repetition. The present colonies differ slightly in lacking a finely denticulate rostrum (cf. Reverter-Gil et al. Citation2016: fig. 8E), in the absence of a peristome encircling the entire orifice, and in the frontal shields with far fewer and smaller pores than in the holotype. However, our colonies are relatively young and immature, in contrast to the originally described mature colonies that showed multilaminar growth. Reference of the Apulian colonies to S. tubulata is thus somewhat doubtful.
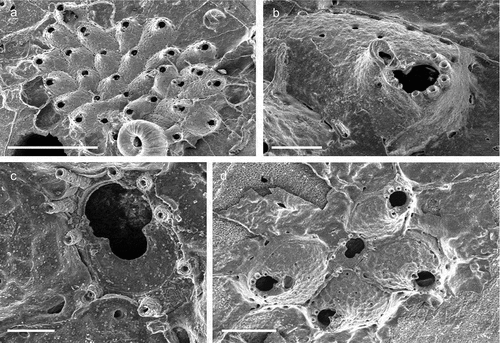
Schizomavella (Calvetomavella) biancae Pica & Berning sp. nov.
Figure 27. Schizomavella (Calvetomavella) biancae sp. nov. (a) Colony (holotype). (b) Zooids with laterofrontal avicularia (holotype). (c) Orifice (holotype). (d) Zooid with small suboral avicularium (holotype). (e) Maternal zooid with ovicell and paired avicularia (holotype). (f) Ancestrula with early astogenetic zooids (paratype). Scales: (a, f) 0.5 mm; (b, d) 100 µm; (c) 50 µm; (e) 200 µm.
Schizomavella discoidea: Hayward & Ryland Citation1999: 280, figs. 123C–D, 126A–C (part, not 126D); López de la Cuadra & García-Gómez Citation2001: figs. 2C–D; De Blauwe Citation2009: 364, figs. 387–388; Chimenz Gusso et al. Citation2014: 249, figs. 136a–c.
Diagnosis
Schizomavella (Calvetomavella) with encrusting unilaminar colonies. Autozooidal frontal shield with a coarsely granular to nodular surface and few small pores around a central imperforate area. Orifice suborbicular in shape and with a narrow and deeply U-shaped sinus as well as broad condyles sloping towards sinus, six or seven oral spines. Avicularia dimorphic: large avicularia often paired and situated along distolateral margins though single latero-suboral ones also occur; oblong in outline, i.e. rostrum distally rounded with slightly raised and serrated edges, palate mostly calcified by a pair of cryptocystal shelves forming central ridges and an elongate foramen at about mid-distance, crossbar with tiny columella. Small avicularia single and usually (latero)suboral though distolateral ones also occur, oval to oblong in outline, slightly raised distal part of rostrum serrated, distal foramen Y-shaped, crossbar with tiny columella. Ovicells relatively large, often covering over half the frontal area of the distal zooid, ooecia flattened globular, ectooecium almost entirely exposed apart from a narrow peripheral rim and with c. 25 rimmed tubaeform pores, ovicellate zooids forming a peristome a with a large U-shaped suboral notch.
Etymology
Named after one of the first author’s nieces, Bianca Colapaoli.
Material examined
Holotype
MSNG 62428: SEM stub n°21, with a bleached ovicellate colony, Gallipoli, Lecce (Italy), 40°0ʹ56.20″N, 17°55ʹ17.84″E, depth 60 m, 16 Jul. 2015.
Paratypes
Same data as for holotype. MSNG 62429: SEM stub n°68, bleached colony with ancestrula; MSNG 62430: bleached ovicellate colony.
Description
Colony encrusting, unilaminar, multiserial ()). Autozooids rectangular to irregular polygonal in shape (L: 433 ± 50, 351–490, 8; W: 328 ± 67, 236–434, 12), slightly convex and separated by a fine suture ()). Frontal shield coarsely granular to nodular, perforated by small round pores (up to 15) mainly located laterally near the suture, suboral area imperforate ()).
Primary orifice slightly elevated (L: 96 ± 3, 91–99, 12; W: 81 ± 3, 75–85, 12) and with six or seven spines in autozooids, about as long as wide, anter suborbicular, proximal margin straight with a narrow and deeply U-shaped sinus (L: 23 ± 2, 21–25, 8; W: 21 ± 3, 13–21, 8) occupying approximately one-fifth to one-fourth of the proximal width (W: 73 ± 4, 64–78, 12) ()). Condyles broad and as long as the proximolateral margin, sloping towards corners of the sinus that are marked by slightly raised and obliquely positioned ridges ()).
Avicularia adventitious, dimorphic: large avicularia usually paired and positioned lateral to the orifice though single and suboral ones also frequently occur, and usually single and small suboral avicularia that may occasionally be positioned distolaterally ()). Large avicularia (L: 157 ± 2, 136–196, 9; W: 42 ± 6, 32–49, 8) ()), pointing proximolaterally, oblong in outline, i.e. rostrum (L: 134 ± 15, 117–167, 9) with parallel margins and distally rounded with a slightly elevated serrated rim; palate mostly calcified by a pair of lateral shelves that form central ridges, producing an elongated enclosed foramen at about mid-distance, foramen immediately distal to crossbar of various irregular shapes, foramen proximal to crossbar D-shaped or oval, complete crossbar usually with a tiny columella. Small avicularia (L: 58 ± 8, 47–66, 5; W: 36 ± 11, 20–47, 5) ()) oval to oblong in outline, rostrum (L: 39 ± 8, 30–49, 5) with a slightly raised and serrated distal margin, Y-shaped distal foramen, foramen proximal to crossbar oval or D-shaped, complete crossbar with a small columella.
Ovicells hyperstomial, relatively large (L: 189 ± 15, 165–208, 6; W: 213 ± 10, 199–227, 6), ooecium somewhat flattened-globular with the ectooecium almost entirely exposed apart from a peripheral rim of frontal calcification by the distal zooid, perforated by about 25 rimmed tubaeform pores ()). Ovicellate zooids develop a peristome with the lateral walls attached to the proximolateral ooecial margins, terminal peristomial margins straight lateral to orifice while proximally abruptly sloping to form a large U-shaped suboral notch ()).
Ancestrula not well preserved, oval in shape, longer than wide, opesia mushroom-shaped surrounded by nine or ten mural spines ()).
Remarks
As noted by Reverter-Gil et al. (Citation2015: 40), who figured material of Schizomavella (Calvetomavella) discoidea (Busk, 1859) from its Madeiran type locality using SEM for the first time, the species is specifically distinct from eastern Atlantic continental shelf and Mediterranean Sea populations, which were historically assigned to that species. Schizomavella (C.) discoidea differs from the present colonies in having larger pores in the frontal shield, a differently shaped peristome in ovicellate zooids (with sloping lateral margins, i.e. the U-shaped proximal notch is not developed), a broader orificial sinus, and in the shape of the avicularian rostrum, which is tapering distally (cf. Reverter-Gil et al. Citation2015: fig. 2). At the present state of knowledge, Schizomavella (Calvetomavella) biancae sp. nov. occurs in the central and western Mediterranean Sea, and along the Atlantic continental shelf of Europe, with the Shetland Islands as its northernmost limit of distribution (cf. Hayward & Ryland Citation1999; Reverter-Gil et al. Citation2015). However, in the absence of SEM images from most publications, the distinction between the new species and the morphologically closely related Schizomavella (Schizomavella) halimedae (Gautier, 1955) is rendered difficult. The two species differ in having large spatulate (even gigantic) avicularia [S. (S.) halimedae] vs. elongated lateral avicularia [S. (C.) biancae sp. nov.], which may, however, be missing in certain colony regions (see also López de la Cuadra & García-Gómez Citation2001: 1723; Reverter-Gil et al. Citation2016: 313). Also, the ooecium is covered by nodular secondary calcification in the former species, whereas the pseudoporous ectooecium is entirely exposed in the latter. As the depth ranges of these species are also largely overlapping, with S. (S.) halimedae likely to occur shallower than S. (C.) biancae sp. nov., which was reported from caves at 13 m to a depth of about 100 m (López de la Cuadra & García-Gómez Citation2001: 1724), it may be that some records of S. (S.) halimedae actually correspond to S. (C.) biancae sp. nov.
Superfamily Schizoporelloidea Jullien Citation1882Family Schizoporellidae Jullien, Citation1882Genus Schizoporella Hincks, 1877Schizoporella magnifica (Hincks, Citation1886)
Figure 28. Schizoporella magnifica. (a) Autozooids. (b) Orifice and lateral avicularia. (c) Ovicells. (d) Ancestrula with early astogenetic autozooids. Scales: (a) 500 µm; (b) 100 µm; (c, d) 200 µm.
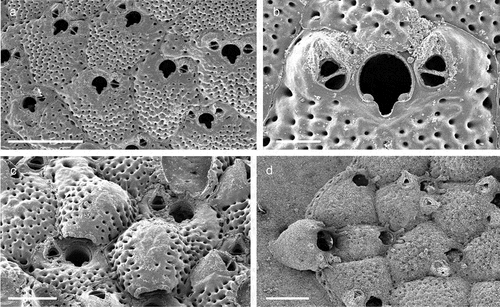
Schizoporella magnifica: Hincks Citation1886: 268, pl. 10, fig. 1; Zabala & Maluquer Citation1988: 133, text-fig. 313, pl. 18, fig. E; Hayward & Ryland Citation1999: 216, figs. 89, 90A; Hayward & McKinney Citation2002: 71, figs. 31F–J; Chimenz Gusso et al. Citation2014: 266, figs. 146a–d.
Material examined
Pica coll: SEM stub n°39, bleached colony with ancestrula; SEM stub n°62, bleached ovicellate colony; two additional unbleached colonies.
Remarks
Schizoporella magnifica (Hincks, Citation1886) occurs throughout the Mediterranean Sea and north to the SW British Isles (Hayward & Ryland Citation1999: 216). The sinus in the present colonies seems to be relatively wide but nevertheless within the range observed in other populations (e.g. Hayward & McKinney Citation2002: fig. 31F, H). Another similar yet specifically distinct Schizoporella species is also present on the Neopycnodonte shells (see below).
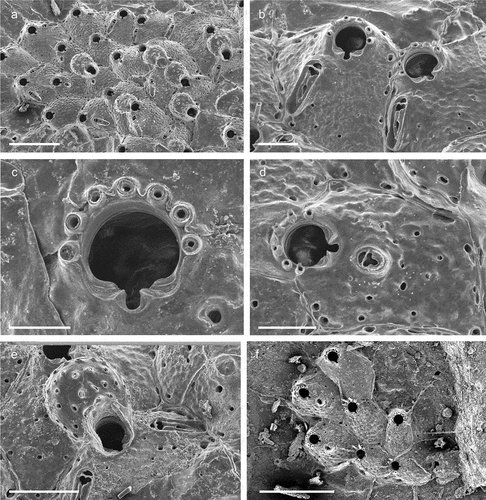
Schizoporella adelaide Pica & Berning sp. nov.
Figure 29. Schizoporella adelaide sp. nov. (holotype). (a) Autozooids. (b) Orifice. (c) Avicularium. (d) Maternal zooids. Scales: (a, b, d) 500 µm; (c) 100 µm.
Diagnosis
Schizoporella with rectangular to oval zooids; frontal shield perforated by c. 40 small round pores, imperforate suboral area prominent but without umbo; orifice slightly wider than long, the short sinus narrowly U-shaped, condyles small, somewhat faceted and short with rounded shoulders. Adventitious avicularia single or double, situated in distolateral corner(s) of zooid, rostrum elongate triangular and gently concave, distal half narrow and with a hooked tip; crossbar with rounded columella. Ovicells occupying almost the entire frontal shield of distal zooid, ooecia marginally perforated, central imperforate area usually with an umbo.
Etymology
Named after the first author’s nephew, Adelaide Colapaoli; used as a noun in apposition.
Material examined
Holotype
MSNG 62431: SEM stub n°80, with one bleached ovicellate colony, Gallipoli, Lecce (Italy), 40°0ʹ56.20″N, 17°55ʹ17.84″E, depth 60 m, 16 Jul. 2015.
Paratypes
Same data as for holotype. MSNG 62432: one bleached colony.
Description
Colony encrusting, multiserial and unilaminar ()). Zooids rectangular to slightly irregular or even oval in shape (L: 545 ± 30, 485–600, 17; W: 495 ± 120, 315–830, 21), separated by a deep groove that is closely juxtaposed by often elongated marginal areolar pores ()). Frontal shield slightly convex, surface relatively smooth yet covered by indistinct granules, pierced by c. 40 small round and irregularly arranged pores, suboral region free of pores and forming the greatest elevation of the autozooid but without producing an umbo ()). Primary orifice slightly broader than long (L: 130 ± 10, 100–160, 28; W: 145 ± 10, 125–175, 27), anter transversely elliptical, proximolateral border straight (W: 115 ± 15, 95–150, 26) with rounded shoulders leading into a short and narrowly U-shaped sinus (L: 30 ± 5, 20–40, 20; W: 30 ± 7, 20–45, 20) that occupies almost one quarter to one third of the entire proximal margin; condyles small but distinctive, edge slightly crenellate and disposed parallel to the proximal border, with rounded or sloping shoulders stopping short of the sinus ()).
Adventitious avicularia single or paired (L: 150 ± 20, 120–180, 11; W: 80 ± 10, 60–105, 13), placed laterally to the orifice and directed distolaterally to laterally, positioned acute to frontal plane; proximal opesia D-shaped, rostrum elongate triangular (L: 100 ± 15, 70–120, 11) with concave margins and a hooked tip, calcified palate restricted to the distinctly narrower distal half; crossbar complete with a well-developed rounded median columella ()).
Ovicells hyperstomial, ooecia globular, slightly longer than wide (L: 440 ± 40, 390–490, 6; W: 390 ± 40, 340–460, 6), occupying almost the entire frontal shield of distal zooid, ooecia marginally perforated by round to slit-like pores except centrally where an umbo is usually developed ()). Ancestrula not observed.
Remarks
Schizoporella adelaide sp. nov. is morphologically similar to Schizoporella magnifica (see above) but can be distinguished from that species owing to a more elongated orifice with a distinctly shorter sinus and condyles, and a crossbar in the avicularium that has a prominent columella. Similarities also exist with the British Schizoporella cornualis Hayward & Ryland, Citation1995, the only other European species that has an avicularium with a columella. While its orifice is rather similar as well (about as long as wide; see Hayward & Ryland, Citation1995: 46), it has distinctly larger condyles than the new species, and also produces a suboral umbo on its frontal shield. Two undescribed species, Schizoporella sp. 2 and sp. 3, which were recorded by Chimenz Gusso et al. (Citation2014) from the southern Italian Island of Ustica are also similar to S. adelaide sp. nov. with regards to the distal pair of avicularia (see also Rosso et al. Citation2019b: fig, 5I). Both species differ from the new species, however, in having a broader sinus, non-crenellate and shorter condyles, and in the absence of a columella in the avicularian crossbar, among other characters.
As no other records could confidently be assigned to Schizoporella adelaide sp. nov., the species must be regarded as endemic to the central Mediterranean Sea at present.
Family Cheiloporinidae Jullien, 1888Genus Hagiosynodos Bishop & Hayward, 1989Hagiosynodos latus (Busk, Citation1856)
Figure 30. Hagiosynodos latus. (a) Colony. (b) Autozooid. (c) Close up of the orifice. (d) Maternal zooids with ovicells. Scales: (a) 500 µm; (b) 100 µm; (c) 50 µm; (d) 200 µm.
Lepralia lata Busk, Citation1856: 309, pl. 10, figs. 1–2.Lepralia kirchenpaueri: Heller Citation1867: 105, pl. 2, fig. 11.Hippopodinella lata: Zabala & Maluquer Citation1988: 136, text-fig. 31 8; Lippi Boncambi et al. Citation1997: 401, fig. 1.Hippopodinella kirchenpaueri: Zabala & Maluquer Citation1988: 136, text-fig. 319, pl. 19, fig. A.Hagiosynodos latus: Hayward and McKinney Citation2002: figs. 35A–D; De Blauwe Citation2009: 380, figs. 406–408; Chimenz Gusso et al. Citation2014: 168, figs. 85a–f.Hagiosynodos kirchenpaueri: Hayward & McKinney Citation2002: 76, figs. 35E–H.Hagionsynodos latus: Berning Citation2006: 97, figs. 123–124
Material examined
Pica coll: SEM stubs n°66, 82, each with one bleached ovicellate colony.
Remarks
The number and identity of Hagiosynodos species present in the Mediterranean Sea is unclear and a matter of debate, and genetic analyses are certainly needed in order to solve this problem. Most of the interspecific differences given to justify the distinction between Hagiosynodos latus (Busk, Citation1856), H. kirchenpaueri (Heller, Citation1867) and H. hadros Hayward & McKinney, Citation2002 are the dimensions of certain characters. However, the more (fossil) material is looked at, the more difficult it is to differentiate between interspecific differences and intraspecific variability in response to (micro)environmental conditions, and to draw a clear line between these species (Schmid Citation1989; Berning Citation2006). Accordingly, the present material is adding to this problem (see ). Whereas some of the characters show dimensions similar to those of H. hadros (zooid length) or H. kirchenpaueri (width of poster), most of the others are intermediate between either H. kirchenpaueri and H. hadros (distance between orifices, orifice length, ovicell width), between H. kirchenpaueri and H. latus (width of anter, distance between frontal shield pores), or in between all of the three (ovicell length). Zooid width, on the other hand, is distinctly greater in the present material than in all of the species reported by Hayward and McKinney (Citation2002).
Table I. Comparison between character dimensions (means, in µm) of the present specimens with the three species reported by Hayward and McKinney (Citation2002)
Concerning qualitative character differences, the present colonies resemble H. latus in the presence of a distal lip (or occasionally a pair of distolateral teeth connected by a narrow shelf), while this character is apparently absent in H. kirchenpaueri and H. hadros (Hayward & McKinney, Citation2002: 79). Thus, whereas skeletal dimensions and relative proportions would vaguely support an assignment of the present colonies to either H. kirchenpaueri or H. hadros, the only distinct character difference argues against this decision. Thus, while we do not reject the likelihood of three distinct species being present in the NE Atlantic and Mediterranean Sea as such, we here stress the inability of distinguishing the species based on morphometry and morphology, and hope that genetic analyses may aid in solving the issue. Accordingly, we here assign the specimens to H. lata and treat the other species as synonymous for now.
Family Phoceanidae Vigneaux, 1949Genus Phoceana Jullien, 1903Phoceana cf. tubulifera (Heller, Citation1867)
Figure 31. Phoceana cf. tubulifera. (a) Colony; (b) Lyrula and condyle. Scales: (a) 200 µm; (b) 50 µm.
cf. Eschara tubulifera Heller, Citation1867: 116.cf. Phoceana tubulifera: Hayward and McKinney Citation2002: 51, figs. 23A–D.
Material examined
Pica coll: SEM stub n°60, one bleached colony.
Remarks
The colony recovered is presumably a part of the encrusting base of an erect colony. Although the zooids are chaotically arranged, possibly as a result of damage to the colony and subsequent reparative budding, the zooids look similar to Phoceana tubulifera (Heller, Citation1867), which has been described from the Adriatic Sea though seldom reported thereafter. The frontal shield is perforated proximally by pseudopores and forms a prominent peristome around the orifice, while the surface is finely granular. Differences to P. tubulifera exist, however, in characters of the orifice. While a lyrula is also present in our material, it is distinctly longer and broader than that figured by Hayward and McKinney (Citation2002: fig. 23c) from the erect portion of the colony. Moreover, condyles were not figured nor reported to be present by these authors, while short and rounded ones exist in our material ()). We have therefore decided to merely confer our colonies to P. tubulifera.
Family Microporellidae Hincks, 1879Genus Microporella Hincks, 1877Microporella appendiculata (Heller, Citation1867)
Figure 32. Microporella appendiculata. (a) Autozooids at the colony growth margin. (b) Zooid. (c) Oral spines. (d) Ancestrula. (e) Ancestrula with early astogenetic autozooids. Scales: (a, e) 200 µm; (b) 500 µm; (c, d) 100 µm.
Lepralia appendiculata Heller, Citation1867: 31, pl. 2, fig. 8.Microporella pseudomarsupiata: Zabala and Maluquer Citation1988: 141, text-fig. 3 35, pl. 19, fig. CMicroporella appendiculata: Hayward and Ryland Citation1999: 294, figs. 134a–b, 135; Chimenz Gusso et al. Citation2014: 187, figs. 100a–b; Di Martino & Rosso Citation2021: 5, fig. 2.
Material examined
SEM stubs n°2, 13, 40, each with one bleached colony with ancestrula; SEM stub n°16, bleached colony; SEM stubs n°32, 49, each with one unbleached colony; five additional unbleached colonies.
Remarks
The present specimens, though mostly small colonies, seem to agree with previous records of M. appendiculata from the Mediterranean Sea (see Di Martino & Rosso Citation2021).
Microporella verrucosa (Peach, Citation1868)
Figure 33. Microporella verrucosa. (a) Autozooids in the encrusting part of the colony. (b) Mandible of the avicularium. (c) Orifice. (d) Avicularium. (e) Ascopore. Scales: (a) 500 µm; (b) 200 µm; (c, d) 50 µm; (e) 200 µm.
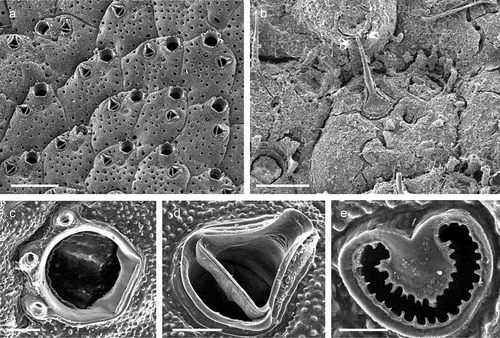
Eschara verrucosa Peach, Citation1868: 116.Diporula verrucosa: Zabala & Maluquer Citation1988: 137, text-fig. 3 23, pl. 19, fig. B; Pouyet & Moissette Citation1992: 66, pl. 10, figs. 1–2; Rosso Citation1996: 216, pl. 5, fig. b; Hayward & Ryland Citation1999: 302, figs. 138C–D, 139; Rosso et al. Citation2014: 202, figs. 3A–C; Achilleos et al. 2019: fig. 2E.Microporella verrucosa: Di Martino & Rosso Citation2021.
Material examined
Pica coll: SEM stub n°53, one unbleached colony; SEM stub n°57, one bleached colony.
Remarks
The fragments here reported are exclusively from the encrusting base of the erect M. verrucosa and lack ovicells. Mediterranean material of this species has recently been redescribed (Di Martino & Rosso Citation2021), yet the types of M. verrucosa as well as of a Pliocene species from Sicily, Eschara lunaris Waters, 1878, need to be revised to test for synonymy between the fossil and Recent forms on the one hand, and the Mediterranean and Atlantic populations on the other hand.
Family Escharinidae Tilbrook, 2006Genus Escharina Milne Edwards, 1836Escharina cf. protecta Zabala, Maluquer & Harmelin, Citation1993 comb. nov.
Figure 34. Escharina cf. protecta. (a) Colony. (b) Autozooids. (c) Ovicellate zooid with extensive peristome. (d) Orifice and avicularia. (e) Crenellate condyles. (f) Spines on autozooid. (g) Maternal zooid. (h) Ancestrula with early astogenetic autozooids. Scales: (a) 1 mm; (b, c, g, h) 200 µm; (d) 100 µm; (e) 20 µm; (f) 50 µm.
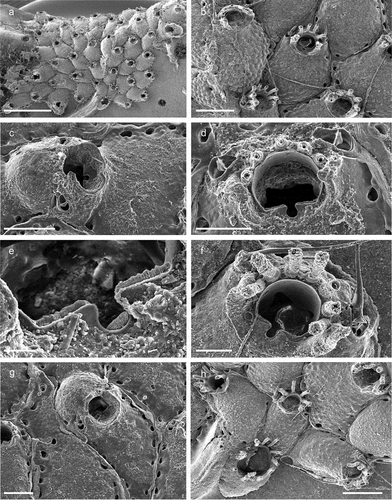
cf. Escharina dutertrei protecta: Zabala, Maluquer & Harmelin, Citation1993: 73, figs. 12a–c, figs. 13–16.Escharina dutertrei protecta: Chimenz Gusso et al. Citation2014: 160, figs. 78a–c.?Escharina dutertrei protecta: Hayward & McKinney Citation2002: 74, figs. 33A–C.
Material examined
Pica coll: SEM stub n°41, unbleached ovicellate colony with ancestrula; SEM stub n°59, bleached ovicellate colony; one additional unbleached colony.
Description
Zooids oval to hexagonal ()), longer than wide (L: 624 ± 86, 487–770, 10; W: 407 ± 81, 315–593, 10), frontal shield slightly convex and vaguely to distinctly nodular, forming a crescent of gymnocystal calcification around proximolateral orifice that is almost level proximally while rising towards the proximal pair of spines to produce a pair of triangular flaps ()); a single row of distinct pores present along the zooecial margin ()); zooecia separated by grooves between slightly raised ridges, each zooid connected via several basal pore chambers. Orifice longer than wide (L: 90 ± 9, 80–104, 5; W: 113 ± 11, 97–123, 5) ()); proximal margin straight or very slightly upturned with very narrow crenellate condyles that parallel the edges; sinus drop-shaped, anter horseshoe-shaped ()). Orifice in ovicellate zooids dimorphic, only little longer but distinctly wider (L: 92–105, 2; W: 128–134, 2); five or seven spines on autozooidal distal margin, two in ovicellate zooids ()).
Avicularia paired, small (L: 70 ± 8, 58–73, 6; W: 34 ± 2, 30–37, 6), positioned in distolateral corners of zooecium, rostrum short and funnel-shaped, directing distomedially, mandible setiform, very long and slender (ML 398 ± 43, 315–439, 6); crossbar complete, straight distally, with a thickened rounded-triangular pivot proximally (–d,f)).
Ovicell kenozooidal, ooecium globular but appearing semi-immersed as soon as the distal zooid has formed ()), wider than long (L: 205 ± 17, 187–220, 3; W: 332 ± 7, 326–340, 3); surface as frontal shield with a narrow proximal band of thickened calcified ectooecium, presumably closed by the operculum; suboral crescent distinctly raised also around proximal aperture while rising lateral to orifice to produce an entire peristome that abutts the proximolateral ooecium.
Ancestrula tatiform ()), longer than wide (L: 377; W: 228), gymnocyst well-developed proximally and abruptly narrowing laterally, opesia pear-shaped (L: 198; W: 142), mural rim with 11 spines, the five proximal ones wider spaced. First autozooid budded distally, which then forms a pair of 2nd generation autozooids laterally.
Remarks
Escharina dutertrei Audouin, Citation1826 was originally described from the Red Sea. As its types are lost, and a neotype has not been selected yet, the species remains ill-defined. This problem led; Zabala et al. (Citation1993) to introduce three new subspecies characterised by a pair of small distal avicularia: the nominotypical Escharina dutertrei dutertrei, Escharina dutertrei haywardi from the boreal Atlantic, and Escharina dutertrei protecta from the Mediterranean Sea and the Azores. At least the Atlanto-Mediterranean subspecies only occur offshore, which, together with the contrast in water temperatures between the boreal Atlantic and the tropical Red Sea, suggests that they are not Lessepsian species. This, in turn, argues against their status as subspecies as they must be regarded as evolutionary entities that are geographically separated from the Red Sea population. Therefore, and in concert with the modern species taxon concept in Bryozoa, in which subtle differences are often considered species-specific, we here regard the three taxa as distinct at species level. Accordingly, while E. dutertrei still needs to be redefined, Escharina haywardi; Zabala, Maluquer & Harmelin, Citation1993 comb. nov. from W Britain is distinguished from the Mediterranean Escharina protecta Zabala, Maluquer & Harmelin, Citation1993 comb. nov. in lacking protective structures around the orifice and in having a different orifice morphology. Based on the image of a specimen of E. protecta from the Azores provided by; Zabala et al. (Citation1993: fig. 14), and owing to the sheer distance between the localities that inhibit a genetic exchange, the central Atlantic population is also likely to be specifically distinct.
The present specimens are very similar to E. protecta but may not be conspecific for the following reasons: there is a distinct area of gymnocystal calcification around the proximolateral orifice of autozooids that forms the outer rim of the protective structure, which is little elevated in the centre while rising distally towards the proximal pair of spines where it forms a pair of small flaps. The flaps are much larger and thicker as well as more distinctly raised and irregularly conical in the autozooids of E. protecta, and apparently not connected by a peristomial rim proximally, and the gymnocystal area also seems to be missing. Only in ovicellate zooids is the peristome similarly developed in both species, although the lateral flaps also appear to be larger in E. protecta. Moreover, the condyles are even narrower and the avicularian mandibles are distinctly longer the present specimens when compared with the images provided by Zabala and Maluquer (Citation1988: pl. 15, fig. C) and Zabala et al. (Citation1993: fig. 12b), respectively. We have therefore decided to describe this morphotype in detail, while an analysis of additional material from different habitats and regions is necessary to test where the boundaries between intra- and interspecific differences lie in this species complex.
Escharina protecta has been reported from the western Mediterranean Sea between depths of 105 and 350 m, as well as in cryptic habitats in shallow waters (6–25 m). The specimens from the central Mediterranean reported by Hayward and McKinney (Citation2002) and Chimenz Gusso et al. (Citation2014), all seem to be of the same morphotype as the colonies presented here while their preferred habitat is similar tot he nominal species, occurring in cryptic environments in shallow waters and down to at least 90 m (Chimenz Gusso et al. Citation2014).
Escharina vulgaris (Moll, Citation1803)
Figure 35. Escharina vulgaris. (a) Colony. (b) Avicularia with mandibles. (c) Maternal zooids with ovicells. (d) Ancestrula with early astogenetic autozooids. Scales: (a, c) 500 µm; (b) 250 µm; (d) 200 µm.
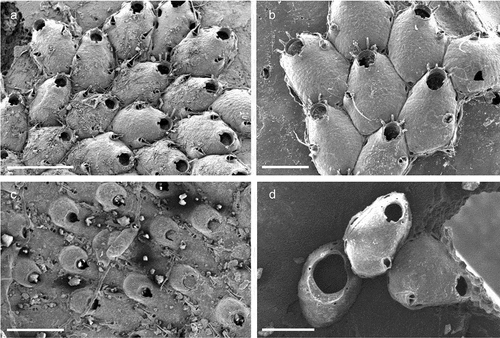
Eschara vulgaris var. a Moll Citation1803: 55, pl. 3, figs. 10A–B.Escharina vulgaris: Zabala & Maluquer Citation1988: 129, text-fig. 290; Rosso Citation1996: 212, pl. 4, fig. e; Hayward & Ryland Citation1999: 236, figs. 100C–D, 101; Hayward & McKinney Citation2002: 72, figs. 32E–I; Chimenz Gusso et al. Citation2014: 161, figs. 79a–d; Rosso et al. Citation2014: 203, figs. 4A–B.
Material examined
Pica coll: SEM stubs n°4, 7, 8 10, each with one bleached ovicellate colony; SEM stub n°24, bleached ovicellate colony with ancestrula; five additional unbleached colonies.
Remarks
There are no apparent differences between the colonies recovered here and other records of Escharina vulgaris from the Mediterranean Sea.
Genus Herentia Gray, 1848Herentia majae Berning, Tilbrook & Rosso, Citation2008
Figure 36. Herentia majae. (a) Early astogenetic colony. (b) Orifice. (c) Ancestrula and firstly budded zooids. (d) Close up of the kenozooidal ancestrula. Scales: (a, c) 200 µm; (b) 50 µm; (d) 100 µm.
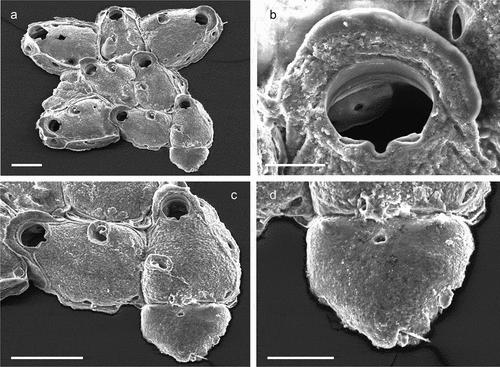
Herentia majae Berning et al. Citation2008: 1519, fig. 2.
Material examined
Pica coll: SEM stub n°33, bleached colony with ancestrula; two additional unbleached colonies.
Remarks
Only early astogenetic colonies were recovered, in which the characters of the adult zooids described by Berning et al. (Citation2008) are not fully developed yet. Based on the small U-shaped sinus as well as on the absence of an areolar pore between the vibraculum and orifice, however, we consider the colonies to belong to H. majae. The ancestrula and early astogeny has not been described before, and we here figure these stages for the first time. The ancestrula is, as in the type species of the genus, Herentia hyndmanni (Johnston, Citation1847), a dome-shaped kenozooid with a completely calcified frontal shield, apart from a central pore and a pair of distolateral spines, the bases of which can vaguely be observed ()). As in H. hyndmanni, the first-generation autozooid is budded distally, which then buds another zooid laterally, while the third-generation zooid is formed in between the two. Even at this stage, differences between H. hyndmanni and H. majae are evident as the early astogenetic zooids in the former already produce the drop-shaped sinus (Berning et al. Citation2008: fig. 1B).
Genus Therenia David & Pouyet, 1978Therenia rosei Berning, Tilbrook & Rosso, Citation2008
Figure 37. Therenia rosei. (a) Zooid arrangement. (b) Autozooid. (c) Autozooidal orifice. (d) Orifice in ovicellate zooid. Scales: (a) 500 µm; (b) 200 µm; (c, d) 100 µm.
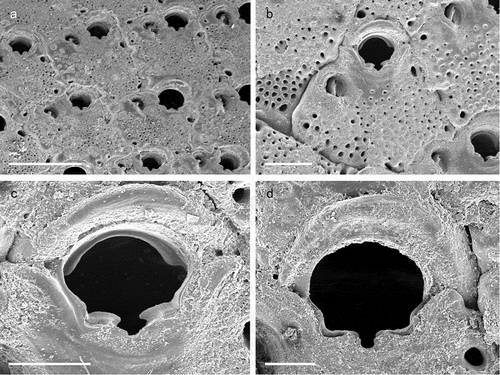
Escharina porosa: Rosso Citation1996: pl. 4, fig. f.Therenia rosei Berning, Tilbrook & Rosso, Citation2008: 1530, fig. 6; Rosso et al. Citation2013: 172, fig. 3e; Sokolover et al. Citation2016: fig 11; Rosso et al. Citation2019a: fig. 6d.
Material examined
Pica coll: SEM stub n°61, bleached ovicellate colony; two additional unbleached colonies.
Remarks
The present colonies can unequivocally be assigned to Therenia rosei Berning Tilbrook & Rosso, Citation2008. Their occurrence on shells at 60 m depth falls within the previously reported habitat range.
Genus Hippomenella Canu & Bassler, 1917Hippomenella mucronelliformis (Waters, Citation1899)
Figure 38. Hippomenella mucronelliformis. (a) Colony. (b) Orifice. (c) Avicularium. (d) Maternal zooid. (e) Ancestrula with early astogenetic autozooids. (f) Close up of the ancestrula. Scale: (a, e) 1 mm; (b, c) 100 µm; (d, f) 200 µm.
Lepralia mucronelliformis Waters, Citation1899: 11, pl.3, figs. 15, 21;Hippomenella mucronelliformis: Brown Citation1949: 513, figs. 1, 2a, b, d, e; Zabala & Maluquer Citation1988: 117; Berning Citation2013: 10, fig. 3; Chimenz Gusso et al. Citation2014: 176, figs. 92a–e; Rosso et al. Citation2019a: fig. 6f; Rosso et al. Citation2021: fig. 5f.
Material examined
Pica coll: SEM stub n°22, bleached colony with ancestrula; SEM stub n°65, bleached ovicellayte colony; two additional unbleached colonies.
Remarks
The present specimens are morphologically indistinguishable from Hippomenella mucronelliformis (Waters, Citation1899) from Madeira, the types of which have recently been imaged and described by Berning (Citation2013). As only little and unbleached material was available for that study, and owing to the few published records and images, we here present additional characters of the species. The ancestrula is tatiform, oval in outline (L: 465; W: 322), with the gymnocyst well-developed and gently sloping proximally while narrowing and steepening distally. The opesia is also oval (L: 262; W: 208) and the mural rim carries some 12 spines. The 1st-generation autozooid is budded distally, which then gives rise to another zooid laterally. The avicularian rostrum is serrated along its entire length in both the long and short forms.
Superfamily Celleporoidea Johnston Citation1838Family Celleporidae Johnston, Citation1838Genus Celleporina Gray, 1848Celleporina cf. canariensis Arístegui Ruiz, Citation1989
Figure 39. Celleporina cf. canariensis. (a) Colony. (b) Zooids. (c, d) Maternal zooid with ovicell. Scale: (a) 1 mm; (b) 500 µm; (c) 100 µm; (d) 200 µm.
cf. Celleporina canariensis Aristegui Ruiz, Citation1989: 147, figs. 3, 7–9.
Celleporina canariensis: Hayward & McKinney Citation2002: 86, figs. 39E–G; Rosso et al. Citation2019a: fig. 6g.
Material examined
Pica coll: SEM stub n°11, bleached ovicellate colony; six additional unbleached colonies.
Remarks
The present specimens, all of which small colonies, are identical with the species recorded as Celleporina canariensis Arístegui, Citation1989 from the Adriatic Sea by Hayward and McKinney (Citation2002). While the large frontal avicularia are lacking in our material, which may be due to the small colony size, all other characters coincide. We have doubts, however, if the Mediterranean population is indeed conspecific with the nominal species from the Canary Islands as the frontal avicularia are not spatulate and the ovicells smaller in relation to zooid size in the Mediterranean population (compare with; Arístegui Ruiz Citation1989: figs 3, 7–9).
Genus Buskea Heller, Citation1867Buskea nitida Heller, Citation1867
Figure 40. Buskea nitida. (a) Colony. (b) Zooids. (c) Close up of the orifice. (d) Maternal zooid. Scales: (a) 500 µm; (b–d) 100 µm.
Buskea nitida: Heller Citation1867: 89, pl. 1, figs. 2–3; Zabala & Maluquer Citation1988: 156, text-figs. 422–424, pl. 24, figs- C – D; Rosso Citation1996: pl. 6, fig. d; Hayward & McKinney Citation2002: 85, figs. 38E–G.Eschara quincuncialis: Norman Citation1867: 204.Buskea quincuncialis: Hayward & Ryland Citation1999: 350, figs. 162C–D, 164.
Material examined
Pica coll: SEM stub n°71, bleached ovicellate colony.
Remarks
The only recovered specimen is a delicate branch fragment composed of 4 alternating series of zooids, and is identical with Buskea nitida Heller, Citation1867 from the Adriatic as reported by Hayward and McKinney (Citation2002), which is arguably regarded as conspecific with Buskea quincuncialis Norman, Citation1867 from Great Britain.
Genus Turbicellepora Ryland, Citation1963Turbicellepora avicularis (Hincks, Citation1860)
Figure 41. Turbicellepora avicularis. (a) Colony. (b) Zooids and avicularia of variable size and shape. (c, d) Avicularia. Scales: (a) 1 mm; (b) 200 µm; (c) 50 µm; (d) 100 µm.
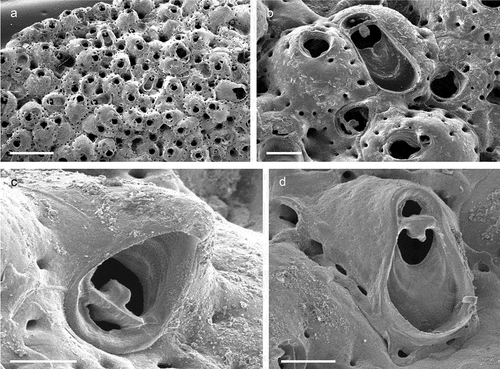
Cellepora avicularis Hincks, Citation1860: 278.Turbicellepora avicularis: Hayward Citation1978: 566, figs. 1, 2A, B, 3, 4I–K, 5A–E, 8, 9; Hayward & Ryland Citation1979: 284, fig. 124; Zabala & Maluquer Citation1988: 161, text figs. 449–451, pl. 27, figs. E–F; Hayward & Ryland Citation1999: 336, figs. 15 5C–D, 156; Hayward & McKinney Citation2002: 90, fig. 41A–D; Chimenz Gusso et al. Citation2014: 295, figs. 164a–f.
Material examined
SEM stubs n°78, 79, each with a fragments of the same bleached ovicellate colony.
Remarks
Only a single, large yet immature colony was recovered, which, based on zooidal and avicularian characters, nevertheless allows us to assign it to Turbicellepora avicularis (Hincks, Citation1860) as reported by Hayward and Ryland (Citation1999) and Hayward and McKinney (Citation2002).
Turbicellepora cf. coronopus (Wood, Citation1844)
Figure 42. Turbicellepora cf. coronopus. (a) Colony. (b) Orifice and small avicularium. (c) Large spatulate avicularium. (d) Maternal zooid. Scales: (a) 500 µm; (b–d) 100 µm.
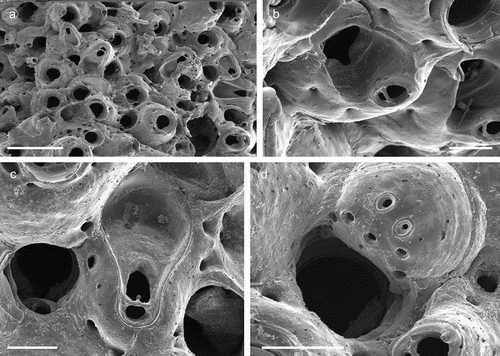
cf. Cellepora coronopus: Wood Citation1844: 18.cf. Turbicellepora coronopus: Hayward Citation1978: 575, figs. 2E, F, 4L, M, 5F, G, 13; Zabala & Maluquer Citation1988: 161, text-figs. 452–454; Chimenz Gusso et al. Citation2014: 298, figs. 166a–d.
Material examined
SEM stubs n°20, 28, 31, each with a bleached ovicellate colony; two additional unbleached colonies.
Remarks
As the species is not well defined, it is difficult to unambiguously assign the present specimens to Turbicellepora coronopus Wood, Citation1844. The type of the nominal species is a Pliocene fossil from Great Britain, which has never been examined using SEM, while the alleged Recent records of the species are confined to the Mediterranean region (Hayward Citation1978). Lagaaij (Citation1952: 137, pl. 15, fig. 8) designated a lectotype and provided an optical image of it, from which details of the orifice and ovicell cannot be observed. At least one character given in the description, however, differs from Recent specimens assigned to T. coronopus: the “normal”, oval, suboral avicularium is reported to be occasionally replaced by a small spatulate one in the type; these avicularia have not yet been recorded in Mediterranean specimens.
Moreover, the only Recent specimens that have been imaged before using SEM (Chimenz Gusso et al. Citation2014) apparently differ in having the suboral avicularium in the centre of the peristome (if single), i.e. directly proximal to the sinus, or on the lateral margin (if paired). In our specimens, as well as in the type of T. coronopus, they are always single (occasionally even absent) and usually slightly offset to the right or left from the proximal peristomial centre. We believe that, besides the lectotype, more material needs to be studied to determine what is to be regarded as intraspecific variability in this species, and if the Mediterranean populations are indeed conspecific with T. coronopus.
Family Phidoloporidae Gabb & Horn, 1862Genus Reteporella Busk, 1884Reteporella cf. couchii (Hincks, Citation1878)
Figure 43. Reteporella cf. couchii. (a) Colony. (b, c) Avicularia. Scales: (a) 200 µm; (b) 50 µm; (c) 100 µm.
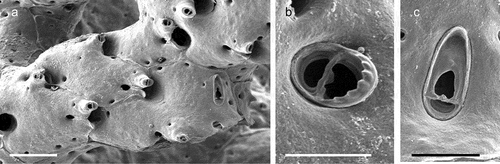
cf. Retepora couchii Hincks, Citation1878: 355, pl. 18, figs. 1–6.Sertella couchii: Zabala & Maluquer Citation1988: 154, text-figs. 414–415, pl. 23, figs. E–F.cf. Reteporella couchii: Hayward & Ryland Citation1996: 107, fis. 1D–E; Hayward & Ryland Citation1999: 370, figs. 173–174; Reverter-Gil et al. Citation2019: 244, figs. 7e–f.Reteporella couchii: Chimenz Gusso et al. Citation2014: 228, figs. 124a–e.
Material examined
Pica coll: SEM stub n°76, bleached colony.
Remarks
While lacking ovicells, the single colony fragment found suggests it may be specifically distinct from Reteporella couchii (Hincks, Citation1878), which was originally described from Great Britain. Although the distinct peristome with its lateral flaps forming a median suture as well as the typical suboral avicularium, which is positioned on an erect cylindrical cystid, are present, both are distinctly shorter than in R. couchii. Whereas these differences may be related to secondary thickening of the frontal surface during ontogeny, the SEM images of additional specimens from the Mediterranean Sea provided by Zabala and Maluquer (Citation1988) and Chimenz Gusso et al. (Citation2014) show the same features. It may thus be possible that a sister species of R. couchii inhabits the Mediterranean Sea.
The species reported as R. couchii by Reverter-Gil et al. (Citation2019) may represent yet another species as the frontal plane of the suboral avicularium is not facing distally but frontally, among other differences.
Reteporella cf. harmeri (Hass, Citation1948)
Figure 44. Reteporella cf. harmeri. (a) Adult colony; (b) Frontal avicularia. (c) Orifice. (a) Avicularia. Scales: (a) 2 mm; (b, d) 200 µm; (c) 50 µm.
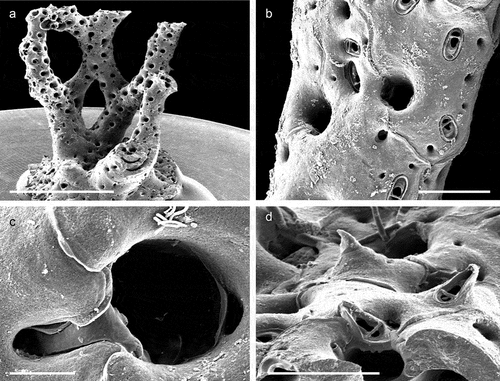
cf. Sertella harmeri: Hass Citation1948: 129, pl. 1, figs. 1–10, pl. 6, fig. 30, pl. 8, fig. 31; Zabala & Maluquer Citation1988: 154, text-figs. 408–409.
Material examined
Pica coll: SEM stub n°45, 75, 74, each with a bleached colony fragments.
Remarks
The only three specimens available, a fragment from close to the colony growth margin as well as two colony bases, are lacking ovicells and are difficult to assign to a species. The species comes closest to the drawings of specimens referred to Reteporella harmeri (Hass, Citation1948) by Zabala and Maluquer (Citation1988). As in Reteporella cf. couchii above, the peristome is well-developed, forming two lateral flaps that merge in the centre, producing a long suture leading to a proximal labial pore. One of the flaps either develops a pointed umbo during early ontogeny or produces a large, elongate triangular and distally hooked avicularium that is facing rather distally and pointing in a frontal to lateral direction. Both the umbo and the avicularian cystid form a slightly protruding and relatively straight edge with three knobs along the proximal peristomial rim. The lateral orifice margins initially carry six spines, while these are lost during ontogeny and the bases gradually covered by secondary calcification. Two additional types of dimorphic avicularia are present and formed during later ontogeny: oblong avicularia on the frontal as well as elongate-triangular ones on the abfrontal surface. Both types are medium-sized, budded anywhere on the surface during ontogeny, and point in various directions. None of the crossbars in the three avicularium types bear a columella. The abfrontal surface is granular.
Whereas most of these characters are shared with the species figured by Zabala and Maluquer (Citation1988), R. harmeri needs revision. As Hass’ types are apparently lost, a neotype needs to be selected, and the species redescribed and imaged using SEM.
Genus Dentiporella Barroso, 1926Dentiporella sardonica (Waters, 1879)
Figure 45. Dentiporella sardonica. (a) Colony. (b) Zooids. (c) Orifice. (d) Adventitious avicularium. (e) Interzooidal avicularium. (f) Maternal zooids. Scales: (a) 1 mm; (b) 500 µm; (c, d) 50 µm; (e) 100 µm; (f) 200 µm.
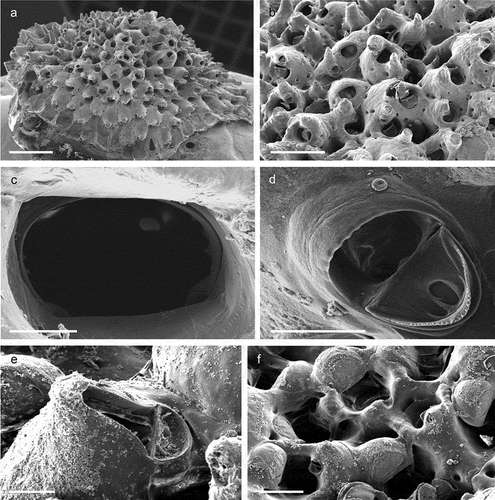
Cellepora sardonica Waters, Citation1879b: 196, pl. 14, figs. 2, 5, 6.Rhynchozoon revelatus: Hayward & McKinney Citation2002: 96, figs. 4 4A–D.Dentiporella sardonica: Zabala & Maluquer Citation1988: 157, text-fig. 432, pl. 27A, B; Souto et al. Citation2010b: fig. 8.
Material examined
Pica coll: SEM stubs n°3, 17, 27, 42, 46, each with one bleached ovicellate colony; two additional unbleached colonies.
Remarks
The present specimens conform with the characters of the lectotype of Dentiporella sardonica, which was recently imaged by Souto et al. (Citation2010b).
Genus Schizotheca Hincks, 1877Schizotheca fissa Busk, Citation1856
Figure 46. Schizotheca fissa. (a) Colony. (b) Zooids. (c) Orifice. (d) Avicularia. (e) Ancestrula. (f) Ancestrula with early astogenetic autozooids. Scales: (a) 500 µm; (b, d, f) 200 µm; (c) 20 µm; (e) 100 µm.
Lepralia fissa: Busk Citation1856: 308, pl. 9, figs. 8–10.Schizotheca fissa: Zabala & Maluquer Citation1988: 150, text-fig. 392, pl. 23, fig. A; Hayward & Ryland Citation1999: 382, figs. 180D, 181; Reverter Gil & Fernández Pulpeiro Citation1998: 48, pl. 2, fig. C; Hayward & McKinney Citation2002: 98, figs. 4 4E–H; Reverter Gil & Fernández Pulpeiro Citation2007: 1935, fig. 3; Chimenz Gusso et al. Citation2014: 278, figs. 153a–e; Rosso et al. 2019, fig. 6i.
Material examined
Pica coll: SEM stubs n°4, 18, 25, each with one bleached colony; three additional unbleached colonies.
Remarks
The present specimens conform in all aspects with Schizotheca fissa (Busk, Citation1856), which is widespread in the Mediterranean Sea and NE Atlantic.
Conclusive remarks
The shell aggregations of Neopycnodonte cochlear collected in the mesophotic zone of the Apulian coast display a relatively large associated biodiversity. We identified 48 cheilostomatid bryozoan species, six of which are newly described in the present paper: Crassimarginatella matildae sp. nov., Micropora biopesiula sp. nov., Haplopoma celeste sp. nov., Schizomavella (Schizomavella) cerranoi sp. nov., Schizomavella (Calvetomavella) biancae sp. nov., and Schizoporella adelaide sp. nov. This number is particularly remarkable when compared with the species richness of other mesophotic habitats from southern Apulia, which were recently presented by Giampaoletti et al. (Citation2020) and Cardone et al. (Citation2020). These authors reported 22 bryozoan species from the site of Monopoli (30–55 m depth) and 18 species from Otranto (45–64 m), both located in the southern Adriatic Sea, as well as 26 species from Santa Maria di Leuca (45–70 m), which is a little south of the locality Gallipoli (60 m) in the Ionian Sea studied herein. The number of species from Gallipoli is even slightly greater than the total number of the three localitites (47 spp.) studied until now (Table SI).
Giampaoletti et al. (Citation2020) already noted a high rate of exclusivity among sites in terms of species composition, which is corroborated by our study as only 12 shared species were reported from Gallipoli, while 35 are restricted to this site and were not recorded by Giampaoletti et al. (Citation2020). Thus, a total of 83 cheilostomatid species have been reported from the southern Apulian mesophotic habitats to date.
A comparison of the data needs to be done with caution, however, as Giampaoletti et al. (Citation2020) did not provide images of the species, and the taxa were apparently identified by optical means only. Without SEM images it is difficult to judge whether morphologically closely related taxa were correctly identified by them. For instance, Microporella marsupiata (Busk, 1860) may be confused with M. appendiculata, and it cannot be ruled out that the colonies they assigned to Schizoporella magnifica belong to that species or to the very similar S. adelaide sp. nov. Moreover, the species Giampaoletti et al. (Citation2020) reported as C. crassimarginata (Hincks, 1880) from Santa Maria di Leuca is likely synonymous with C. matildae sp. nov., and their Schizomavella discoidea (Busk, 1859), reported from all three stations, is probably identical with S. (C.) biancae sp. nov.
The differences in faunal composition between similar and geographically relatively proximate sites in the mesophotic zone, and particularly the presence of several new species discovered at Gallipoli, show once more that our knowledge of the bryozoan fauna in certain Mediterranean habitats, such as the outer shelf and upper slope, is still uncomplete and warrants further studies (Rosso & Di Martino Citation2016).
Supplemental Material
Download MS Excel (14 KB)Acknowledgements
The MESOMED expedition has been led by Carlo Cerrano (Polytechnic University of Marche) in collaboration with Antonio Terlizzi (Salento University), and the logistic support of Andrea Costantini (Costa del Sud Diving Service) Bruno Borrelli, Nicolò Crespi (Portofino Divers) and Ubaldo Pantaleo (Ubica srl).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Achilleos K, Jiménez C, Berning B, Petrou A. 2020. Bryozoan diversity of Cyprus (eastern Mediterranean Sea): First results from census surveys (2011–2018). Mediterranean Marine Science 21(2):228–237. DOI: 10.12681/mms.21201.
- Albano PG, Azzarone M, Amati B, Bogi C, Sabelli B, Rilov G. 2020. Low diversity or poorly explored? Mesophotic molluscs highlight undersampling in the Eastern Mediterranean. Biodiversity and Conservation 29(14):4059–4072. DOI: 10.1007/s10531-020-02063-w.
- Angeletti L, Taviani M. 2020. Offshore Neopycnodonte oyster reefs in the Mediterranean Sea. Diversity 12(3):92. DOI: 10.3390/d12030092.
- Arístegui Ruiz J. 1989. Consideraciones sobre el género Celleporina Gray, 1848 (Ectoprocta: Cheilostomata) en Canarias y descripción de tres especies nuevas: C. canariensis sp. n., C. fragilis sp. n. y C. labiata sp. n. Cahiers de Biologie Marine 30:143–165.
- Audouin JV. 1826. Explication sommaire des planches de polypes de l’Égypte, et de la Syrie, publiées par Jules-César Savigny. Description de l’Égypte, ou recueil des observations et des recherches qui ont été faites en Égypte pendant l’Expédition de l’Armée francaise. Jomard, E.F. C.L.F. Pancoucke, Paris. Description de l’Égypte Histoire Naturelle 1:225–244.
- Ayari-Kliti R, Afli A, Aissa P. 2012. Diversité taxonomique des Bryozoaires Cheilostomes au large du Golfe de Tunis. Bulletin de l’Institut National Des Sciences Et Technologies Mer de Salammbô 39:73–116.
- Berning B. 2006. The cheilostome bryozoan fauna from the Late Miocene of Niebla (Guadalquivir Basin, SW Spain): Environmental and biogeographic implications. Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg 90(7):11–56.
- Berning B. 2013. New and little-known Cheilostomata (Bryozoa, Gymnolaemata) from the NE Atlantic. European Journal of Taxonomy 44:1–25.
- Berning B, Achilleos K, Wisshak M. 2019. Revision of the type species of Metroperiella (Bryozoa, Cheilostomatida), with the description of two new species. Journal of Natural History 53(3–4):141–158. DOI: 10.1080/00222933.2019.1582725.
- Berning B, Tilbrook KJ, Rosso A. 2008. Revision of the north-eastern Atlantic and Mediterranean species of the genera Herentia and Therenia (Bryozoa: Cheilostomata). Journal of Natural History 42(21–22):1509–1547. DOI: 10.1080/00222930802109140.
- Bishop JDD. 1986. The identity of Cribrilaria innominata (Couch, 1844) (Bryozoa, Cheilostomata). Bulletin of the British Museum (Natural History) Zoology 50(2):93–102.
- Bishop JDD, Househam BC. 1987. Puellina (Bryozoa; Cheilostomata; Cribrilinidae) from British and adjacent waters. Bulletin of the British Museum (Natural History), Zoology 53(1):1–63.
- Bock P, Gordon DP. 2021. World list of Bryozoa. Micropora Gray, 1848. World Register of Marine Species. Available: http://www.marinespecies.org/aphia.php?p=taxdetails&id=110944. Accessed Jan 2021 13.
- Brown DA. 1949. On the polyzoan genus Hippomenella Canu & Bassler and its genotype Lepralia mucronelliformis Waters. Journal of the Linnean Society, London (Zoology) 41:513–520. DOI: 10.1111/j.1096-3642.1940.tb02419.x.
- Busk G. 1856. Zoophytology. Quarterly Journal of Microscopical Science 4(16):308–312.
- Calvet L. 1907. Bryozoaires. Expéditions scientifiques du “Travailleur” et du “Talisman” pendant les années 1880–1883. Vol. 8. Paris: Masson et Cie. pp 355–495.
- Canu F, Bassler RS. 1925. Les Bryozoaires du Maroc et de Mauritanie, 1er Mémoire. Mémoires de La Société des Sciences Naturelles du Maroc 10:1–79.
- Cardone F, Corriero G, Longo C, Mercurio M, Tarantini SO, Gravina MF, Pierri C. 2020. Massive bioconstructions built by Neopycnodonte cochlear (Mollusca, Bivalvia) in a mesophotic environment in the central Mediterranean Sea. Scientific Reports 10(1):1–16. DOI: 10.1038/s41598-020-63241-y.
- Cerrano C, Bastari A, Calcinai B, Di Camillo C, Pica D, Puce S, Valisano L, Torsani F. 2019. Temperate mesophotic ecosystems: Gaps and perspectives of an emerging conservation challenge for the Mediterranean Sea. The European Zoological Journal 86(1):370–388. DOI: 10.1080/24750263.2019.1677790.
- Chimenz Gusso C, Nicoletti L, Bondanese C. 2014. Briozoi. Biologia Marina Mediterranea 21(suppl.):1–366.
- Cook PL. 1985. Bryozoa from Ghana. A preliminary survey. Annales / Musée Royal de l’Afrique Centrale, Sciences Zoologiques, Tervuren 238:1–315.
- Corriero G, Pierri C, Mercurio M, Nonnis Marzano C, Tarantini SO, Gravina MF et al. 2019. A Mediterranean mesophotic coral reef built by non-symbiotic scleractinians. Scientific Reports 9:3601. DOI: 10.1038/s41598-019-40284-4.
- Couch RQ. 1844. A Cornish fauna: Being a compendium of the natural history of the country, intended to form a companion to the collection in the Royal Institution of Cornwall … part 3. The zoophytes and calcareous corallines. Truro: Royal Institution of Cornwall.
- D’Onghia G, Capezzuto F, Cardone F, Carlucci R, Carluccio A et al. 2015. Macro- and megafauna recorded in the submarine Bari Canyon (southern Adriatic, Mediterranean Sea) using different tools. Mediterranean Marine Science 16(1):180–196. DOI: 10.12681/mms.1082.
- de Blauwe H. 2009. Mosdiertjes van de Zuidelijke bocht van de Noordzee: Determinatiewerk voor België en Nederland. Oostende: Vlaams Instituut voor de Zee. pp 443.
- Di Martino E, Rosso A. 2021. Seek and ye shall find: New species and new records of Microporella (Bryozoa, Cheilostomata) in the Mediterranean. Zookeys 1053:1–42. DOI: 10.3897/zookeys.1053.65324.
- Fernández Pulpeiro E, Reverter Gil O. 1993. Le genre Ellisina (Bryozoa, Cheilostomida) sur les côtes européennes: Description d’Ellisina gautieri sp. nov. Cahiers de Biologie Marine 34(1):93–101.
- Friedl H. 1917. Bryozoen der Adria. Zoologischer Anzeiger 49(9):225–240.
- Gautier YV. 1956. Résultats scientifiques des campagnes de la “Calypso”. Part 5. Bryozoaires. Annales de l’Institut Océanographique 32(2):189–225.
- Gautier YV. 1962. Recherches écologiques sur les bryozoaires chilostomes en Méditerranée occidentale. Recueil des Travaux de la Station Marine d’Endoume 38(24):1–434.
- Giampaoletti J, Cardone F, Corriero G, Gravina MF, Nicoletti L. 2020. Sharing and distinction in biodiversity and ecological role of bryozoans in Mediterranean mesophotic bioconstructions. Frontiers in Marine Science 7:1040. DOI: 10.3389/fmars.2020.581292.
- Gori A, Bavestrello G, Grinyó J, Dominguez-Carrió C, Ambroso S, Bo M. 2017. Animal forests in deep coastal bottoms and continental shelf of the Mediterranean Sea. In: Rossi S, Bramanti L, Gori A, Orejas C, editors. Marine animal forests: The ecology of benthic biodiversity hotspots. Cham, Switzerland: Springer International Publishing, pp. 207–233.
- Harmelin JG. 1969. Bryozoaires des grottes sous-marines obscures de la région marseillaise. Faunistique et écologie. Tethys 1:793–806.
- Harmelin JG. 1970. Les Cribrilaria (Bryozoaires Chilostomes) de Méditerranée; systématique et écologie. Cahiers de Biologie Marine 11:77–98.
- Harmelin JG. 1973. Les bryozoaires des peuplements sciaphiles de Méditerranée: le genre Crassimarginatella Canu (Chilostomes Anasca). Cahiers de Biologie Marine 4:471–492.
- Harmelin JG. 1978. Sur quelques Cribrimorphes (Bryozoa, Cheilostomata) de l’Atlantique Oriental. Tethys 8(2):173–192.
- Harmelin JG. 1988. Espèces affines microsympatriques chez Puellina (Bryozoa, Cheilostomata) et description d’espèces nouvelles. Zoologica Scripta 17(1):25–38. DOI: 10.1111/j.1463-6409.1988.tb00084.x.
- Harmelin JG, d’Hondt JL. 1993. Transfers of bryozoan species between the Atlantic Ocean and the Mediterranean Sea via the Strait of Gibraltar. Oceanologica Acta 16(1):63–72.
- Hass H. 1948. Beitrag zur kenntnis der Reteporiden. Zoologica 101:1–138.
- Hassall AH. 1841. Supplement to a catalogue of Irish zoophytes. Annals and Magazine of Natural History 7(1):363–373. DOI: 10.1080/03745484109442710.
- Hayward PJ. 1974. Studies on the cheilostome bryozoan fauna of the Aegean Island of Chios. Journal of Natural History 8(4):369–402. DOI: 10.1080/00222937400770321.
- Hayward PJ. 1978. Systematic and morphological studies on some European species of Turbicellepora (Bryozoa, Cheilostomata). Journal of Natural History 12:551–590. DOI: 10.1080/00222937800770411.
- Hayward PJ, McKinney FK. 2002. Northern Adriatic bryozoa from the vicinity of Rovinj, Croatia. Bulletin of the American Museum of Natural History 270:1–139. DOI: 10.1206/0003-0090(2002)270<0001:NABFTV>2.0.CO;2.
- Hayward PJ, Ryland JS. 1979. British Ascophoran Bryozoans. In: Kermack DM, Barnes RSK, editors. Synopses of the British Fauna. London: Academic Press for the Linnaean Society, vol. 14. pp. 1–312.
- Hayward PJ, Ryland JS. 1995. The British species of Schizoporella (Bryozoa: Cheilostomatida). Journal of Zoology 237:37–47.
- Hayward PJ, Ryland JS. 1996. Some British Phidoloporidae (Bryozoa: Cheilostomatida). Zoological Journal of the Linnean Society 117:103–112. DOI: 10.1111/j.1096-3642.1996.tb02150.x.
- Hayward PJ, Ryland JS. 1998. Cheilostomatous Bryozoa. Part 1. Aeteoidea - Cribrilinoidea. Synopses of the British Fauna (New Series) 10:1–366.
- Hayward PJ, Ryland JS. 1999. Cheilostomatous Bryozoa. Part 2. Hippothooidea - Celleporoidea. Synopses of the British Fauna (New Series) 14:1–416.
- Hayward PJ, Thorpe JP. 1995. Some British species of Schizomavella (Bryozoa: Cheilostomatida). Journal of Zoology 235:661–676. DOI: 10.1111/j.1469-7998.1995.tb01776.x.
- Heller C. 1867. Die Bryozoen des adriatischen Meeres. Verhandlungen der Kaiserlich-Königliche Zoologisch-botanischen Gesellschaft in Wien 17:77–136.
- Hincks T. 1860. Zoophytology. Descriptions of new Polyzoa from Ireland. Quarterly Journal of Microscopical Science 8(32):275–280.
- Hincks T. 1878. Notes on the genus Retepora, with descriptions of new species. Annals and Magazine of Natural History 1(5):353–365. DOI: 10.1080/00222937808682345.
- Hincks T. 1880a. Contributions towards a general history of the marine Polyzoa. Part I. Madeiran Polyzoa. Annals and Magazine of Natural History Ser. 5 6:69–80.
- Hincks T. 1880b. Contributions towards a general history of the marine Polyzoa. Part II. Foreign Membraniporina. Annals and Magazine of Natural History Ser. 5 6:81–92.
- Hincks T. 1886. The Polyzoa of the Adriatic: A supplement to Prof. Heller’s ‘Die Bryozoen des Adriatischen Meeres’, 1867. Annals and Magazine of Natural History ( ser. 5) 17:254–271, DOI: 10.1080/00222938609460142.
- Johnston G. 1838. A history of the British zoophytes. Edinburgh: W.H. Lizars. pp 341.
- Johnston G. 1840. Miscellanea Zoologica. Description of a new genus of British zoophyte. Annals and Magazine of Natural History ( ser. 1) 5:272–274, DOI: 10.1080/00222934009496822.
- Johnston G. 1847. A history of the British zoophytes. 2nd ed., Vol. I. London: Van Voorst. pp 488.
- Jullien J. 1882. Note sur une nouvelle division des Bryozoaires Cheilostomiens. Bulletin de La Société Zoologique de France 6:271–285.
- Lagaaij R. 1952. The Pliocene Bryozoa of the low countries and their bearing on the marine stratigraphy of the North Sea region. Mededelingen van de Geologische Stichting 5:6–233.
- Levinsen GMR. 1909. Morphological and systematic studies on the cheilostomatous Bryozoa Vol. Copenhagen: Nationale Forfatterers Forlag. pp. 1–431.
- Lippi Boncambi F, Nicoletti L, Chimenz C. 1997. Quante specie di Hippopodinella Barroso 1924 (Bryozoa, Cheilostomida) esistono nel Mediterraneo? Biologia Marina Mediterranea 4(1):401–404.
- López de la Cuadra CM, García-Gómez JC. 2001. New and little known ascophoran bryozoans from the Western Mediterranean, collected by ‘Fauna Ibérica’ expeditions. Journal of Natural History 35:1717–1732. DOI: 10.1080/002229301317092414.
- Manzoni A. 1870. Bryozoi fossili Italiani. Quarta Contribuzione. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Wien (Abteilung 1) 61(1):323–349.
- Moll JPC. 1803. Eschara, ex zoophytorum, seu, phytozoorum ordine pulcherrimum ac notatu dignissimum genus, novis speciebus auctum, methodice descriptum et iconibus ad naturam delineatis illustratum. Vindobonae: Camesiniana. pp 1–70.
- Norman AM. 1867. Report of the comittee appointed for the purpose of exploring the coasts of the Hebrides by means of the dredge. Part 2. On the Crustacea, Echinodermata, Polyzoa, Actinozoa and Hydrozoa. Report of the British Association for the Advancement of Science London 1866:193–206.
- Ostrovsky AN, Nielsen C, Vávra N, Yagunova EB. 2009. Diversity of brood chambers in calloporid bryozoans (Gymnolaemata, Cheilostomata): Comparative anatomy and evolutionary trends. Zoomorphology 128(1):13–35. DOI: 10.1007/s00435-008-0070-8.
- Ostrovsky AN, Schäfer P. 2003. Ovicell structure in Callopora dumerilii and C. lineata (Bryozoa: Cheilostomatida). Acta Zoologica 84(1):15–24. DOI: 10.1046/j.1463-6395.2003.00121.x.
- Peach CW. 1868. On a new British Eschara. Journal of the Royal Institution of Cornwall 3:116–117.
- Poluzzi. 1975. I Briozoi Cheilostomi del Pliocene della Val d’Arda (Piacenza, Italia). Memorie della Società italiana di scienze naturali 21(2):37–77.
- Pouyet S, Moissette P. 1992. Bryozoaires du Pliocène d’Altavilla (Sicile-Italie): Révision de la collection Cipolla, nouvelles données, paléoécologie. Palaeontographica, Abteilung A 223:19–101.
- Prenant M, Bobin G. 1966. Bryozoaires. 2” partie: Chilostomes Anasca. Faune de France 68:1–647.
- Reverter Gil O, Fernández Pulpeiro E. 1996. Some species of Schizomavella (Bryozoa, Cheilostomatida) from the Atlanto-Mediterranean region. Cahiers de Biologie Marine 36:259–275.
- Reverter-Gil O, Berning B, Souto J. 2015. Diversity and systematics of Schizomavella species (Bryozoa: Bitectiporidae) from the bathyal NE Atlantic. PLoS ONE 10(10):e0139084. DOI: 10.1371/journal.pone.0139084.
- Reverter-Gil O, Fernández-Pulpeiro E. 1998. Algunos Briozoos Cheilostomados recolectados en la Ría de Ferrol (N.O. España). Boletín de La Real Sociedad Española de Historia Natural (Sección Biología) 94(1/2):51–60.
- Reverter-Gil O, Fernández-Pulpeiro E. 2007. Species of genus Schizotheca Hincks (Bryozoa, Cheilostomata) described in the Atlantic-Mediterranean region, with notes on some species of Parasmittina Osburn. Journal of Natural History 41(29–32):1929–1953. DOI: 10.1080/00222930701515520.
- Reverter-Gil O, Souto J, Novosel M, Tilbrook KJ. 2016. Adriatic species of Schizomavella (Bryozoa: Cheilostomata). Journal of Natural History 50(5–6):281–321. DOI: 10.1080/00222933.2015.1062153.
- Reverter-Gil O, Souto J, Trigo JE. 2019. New species and new recors of Bryozoa from Galicia (NW Spain). Journal of Natural History 53(3–4):221–251. DOI: 10.1080/00222933.2019.1582815.
- Rosso A. 1996. Popolamenti e tanatocenosi a briozoi di fondi mobili circalitorali del Golfo di Noto (Sicilia, Italia). Naturalista Siciliano 20:189–225.
- Rosso A. 2004. Two new species of Phylactella (Bryozoa Cheilostomatida) from the Mediterranean area belonging to the P. labrosa (Busk) complex of species. Journal of Natural History 38(20):2655–2668. DOI: 10.1080/00222930310001647325.
- Rosso A, Di Martino E. 2016. Bryozoan diversity in the Mediterranean Sea: An update. Mediterranean Marine Science 17(2):567–607. DOI: 10.12681/mms.1706.
- Rosso A, Di Martino E, Gerovasileiou V. 2020a. Revision of the genus Setosella (Bryozoa: Cheilostomata) with description of new species from deep-waters and submarine caves of the Mediterranean Sea. Zootaxa 4728(4):401–422. DOI: 10.11646/zootaxa.4728.4.1.
- Rosso A, Di Martino E, Pica D, Galanti L, Cerrano C, Novosel M. 2018. Non-indigenous bryozoan species from natural and artificial substrata of Mediterranean submarine caves. Marine Biodiversity 48(3):1345–1355. DOI: 10.1007/s12526-016-0602-2.
- Rosso A, Gerovasileiou V, Di Martino E. 2020b. Really onychocellids? Revisions and new findings increase the astonishing bryozoan diversity of the Mediterranean Sea. Journal of Marine Science and Engineering 8:904. DOI: 10.3390/jmse8110904.
- Rosso A, Gerovasileiou V, Sanfilippo R, Guido A. 2019a. Bryozoan assemblages from two submatrine caves in the Aegean Sea (Eastern Mediterranean). Marine Biodiversity 49:707–726. DOI: 10.1007/s12526-018-0846-0.
- Rosso A, Sanfilippo R, Guido A, Gerovasileiou V, Taddei Ruggiero E, Belmonte G. 2021. Colonisers of the dark: Biostalactite‐associated metazoans from “lu Lampiùne” submarine cave (Apulia, Mediterranean Sea). Marine Ecology 42(1):e12634. DOI: 10.1111/maec.12634.
- Rosso A, Sanfilippo R, Sciuto F. 2014. Open-shelf, soft-bottom bryozoans from the Ciclopi Marine Protected Area (E Sicily, Mediterranean). In: Rosso A, Wyse J, Patrick N, Porter Joanne S, editors. Bryozoan studies 2013. Trento: Museo delle Scienze. pp. 199–211.
- Rosso A, Sanfilippo R, Sciuto F, Serio D, Catra M, Alongi G, Viola A, Leonardi R. 2019b. Preliminary information on bryozoans associated with selected infralittoral algae communities from Sicily (eastern Mediterranean). Australasian Palaeontological Memoir 52:115–129.
- Rosso A, Sanfilippo R, Taddei Ruggiero E, Di Martino E. 2013. Faunas and ecological groups of Serpuloidea, Bryozoa and Brachiopoda from submarine caves in Sicily (Mediterranean Sea). Bollettino della Società Paleontologica Italiana 52(3):167–176.
- Rosso A, Sanfilippo R, Vertino A, Zibrowius H. 2017. Hanging coral gardens of a Tyrrhenian submarine cave from Sicily (Italy). Bollettino della Società Paleontologica Italiana 56(1):1–12.
- Ryland JS. 1963. The species of Haplopoma (Polyzoa). Sarsia 10:9–18.
- Schmid B. 1989. Cheilostome Bryozoen aus dem Badenien (Miozän) von Nußdorf (Wien). Beiträge zur Paläontologie von Österreich 15:1–101.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9:671–675. DOI: 10.1038/nmeth.2089.
- Sokolover N, Taylor PD, Ilan M. 2016. Bryozoa from the Mediterranean coast of Israel. Mediterranean Marine Science 17(2):440–458. DOI: 10.12681/mms.1390.
- Souto J, Fernández-Pulpeiro E, Reverter-Gil O. 2010a. The genus Amathia Lamouroux (Bryozoa, Ctenostomata) in Iberian waters. Cahiers de Biologie Marine 51:181–195.
- Souto J, Nascimento KB, Reverter-Gil O, Vieira LM. 2018. Dismantling the Beania magellanica species complex (Bryozoa, Cheilostomata): Two new species in European waters. Marine Biodiversity 49:1505–1518. DOI: 10.1007/s12526-018-0925-2.
- Souto J, Reverter-Gil O, De Blauwe H, Fernandez-Pulpeiro E. 2014. New records of bryozoans from Portugal. Cahiers de Biologie Marine 55(1):129–150.
- Souto J, Reverter-Gil O, Fernández-Pulpeiro E. 2010b. Gymnolaemate bryozoans from the Algarve (southern Portugal): New species and biogeographical considerations. Journal of the Marine Biological Association of the United Kingdom 90:1417–1439. DOI: 10.1017/S0025315409991640.
- Taylor PD, Martha SO, Gordon DP. 2018. Synopsis of ‘onychocellid’ cheilostome bryozoan genera. Journal of Natural History 62(25–26):1657–1721. DOI: 10.1080/00222933.2018.1481235.
- Vieira LM, Spencer Jones ME, Winston JE, Migotto AE, Marques AC. 2014. Evidence for polyphyly of the genus Scrupocellaria (Bryozoa: Candidae) based on a phylogenetic analysis of morphological characters. PLoS ONE 9(4):e95296. DOI: 10.1371/journal.pone.0095296.
- Waters AW. 1879a. On the Bryozoa (Polyzoa) of the Bay of Naples. Annals and Magazine of Natural History ( Ser. 5) 3:114–126, DOI: 10.1080/00222937908682488.
- Waters AW. 1879b. On the Bryozoa (Polyzoa) of the Bay of Naples (continued). Annals and Magazine of Natural History ( Ser. 5) 3:192–202, DOI: 10.1080/00222937908694085.
- Waters AW. 1898. Observations on the Membraniporidae. Journal of the Linnean Society (Zoology) London 26:654–693. DOI: 10.1111/j.1096-3642.1898.tb01741.x.
- Waters AW. 1899. Bryozoa from Madeira. Journal of the Royal Microscopical Society 19(1):6–16. DOI: 10.1111/j.1365-2818.1899.tb00139.x.
- Wood SV. 1844. Descriptive catalogue of the zoophytes from the Crag. Annals and Magazine of Natural History 13:10–21. DOI: 10.1080/03745484409442561.
- Zabala M. 1986. Fauna dels Briozous dels Països Catalans. Institut d’Estudis Catalans, Arxius de la Secció de Ciéncies, Barcelona 84:1–836.
- Zabala M, Maluquer P. 1988. Illustrated keys for the classification of Mediterranean Bryozoa. Treballs Del Museu de Zoologia 4:1–294.
- Zabala M, Maluquer P, Harmelin JG. 1993. Epibiotic bryozoans on deep-water scleractinian corals from the Catalonia slope (western Mediterranean, Spain, France). Scientia Marina 57(1):65–78.