Abstract
This article clarifies two morphological problems, the first regarding all Apochela Schuster, Nelson, Grigarick and Christenberry 1980, and the second the genus Milnesium Doyère, 1840. Among Eutardigrada Richters, 1926, Apochela have four claws per leg, while Parachela Schuster, Nelson, Grigarick and Christenberry 1980 have two double claws per leg. Until now, the four claws per leg of Apochela have been denominated as if they had derived from two parachelan-like double claws undergoing branch separation. However, morphological and molecular data indicate the relative phylogenetic distance of Apochela from Parachela as sister groups; the ancestor of Eutardigrada was probably similar to a primitive heterotardigrade in having four separate claws per leg, which organised and differentiated in couples but without fusion in Apochela, while they fused to form proper double claws only in Parachela. If this hypothesis is correct (Apochela would have retained four separate claws per leg as plesiomorphic character), the denomination of apochelan claws must be adapted to reflect it. The second problem regards the valvular system present between buccal tube and pharyngeal bulb in the genus Milnesium. Until now, only one valvular system type was known, attributed to Milnesium tardigradum (today sensu lato), or to Milnesium cf. tardigradum. We reveal a second type recognisable in many species. The two types are described, discussing their possible correlation with life stage, sex, some morphological and morphometric traits, mounting medium, geographic distribution and currently available phylogenetic trees; the valvular system type is probably species-specific and we attribute to most species of the genus the correct type. Our conclusions are preliminary, but, in any case, the present study indicates that the exact morphology of the discussed portion of the bucco-pharyngeal apparatus in Milnesium is still not well known.
Introduction
According to the most accepted classification of Tardigrada Doyère (Citation1840), the Eutardigrada Richters (Citation1926) are a class including two orders: Apochela Schuster et al. (Citation1980) (containing the single family Milnesiidae Ramazzotti Citation1962) and Parachela Schuster, Nelson, Grigarick and Christenberry, 1980, including all the other Eutardigrada. Guil et al. (Citation2019), on a molecular basis, proposed to elevate the Apochela to class level with the name Apotardigrada, and to give the Parachela the old class name Eutardigrada; those authors also elevated to the order level the four superfamilies of Parachela. However, Morek et al. (Citation2020b) considered the proposal of Guil et al. (Citation2019) unjustified and re-established the previous taxonomy.
Concerning this problem and also taking into consideration the perplexities expressed by Fleming and Arakawa (Citation2021), in the present paper we follow the traditional phylogeny and terminology, also because we are above all interested in two morphological problems, one regarding all Apochela (independently from the systematic rank of this taxon) and the second regarding only the genus Milnesium Doyère (Citation1840).
After having studied tardigrades for many years, we noticed with surprise two problems regarding their morphology in strong need of a clarifying discussion. The first problem regards the origin (with the relative phylogenetic implications), and the consequent denomination, of the claws of the Apochela; as a matter of fact, we realised that in the literature different terms have been used for the apochelan claws, albeit usually referring to them as “branches”, not “claws”. The second problem regards a portion of the bucco-pharyngeal apparatus of the species of the genus Milnesium, and more precisely the valvular system between the buccal tube and the pharyngeal bulb, of which, until now, only one type has been described; while studying and measuring many specimens of multiple species of said genus, we noticed in some of them a morphology incompatible with the valvular system thus far known.
Material and methods
Microscopy and imaging
Observations were made under a × 100 objective (× 1000 magnification), in oil immersion, of a DM1000 LED Leica Phase Contrast Microscope equipped with a micrometer for measurements; these are given in micrometres (μm). Photographs were taken using a Canon IXUS 185 digital camera installed on the above-mentioned microscope; the photographs produced were handled and improved, and the plates arranged, using Adobe Photoshop 2021 software; this was used also to create the drawing in .
Material examined
We examined 138 specimens, belonging to 22 species of the genus Milnesium, deposited in the Pilato and Binda collection (Department of Biological, Geological and Environmental Sciences of the University of Catania, Italy) (). We also examined numerous images from the literature to evaluate the valvular system type of many species; these data are given in .
Table I. Examined species of Milnesium deposited in the Pilato and Binda collection, with type specimens, if any, indicated (if none, the geographic provenance is given), slide numbers and number of specimens analysed. If not specified, specimens were of undetermined age and sex (likely females)
Table II. Species of Milnesium by valvular system type, with indication of examined specimens and/or figures (from the literature or the present paper) on which the system attribution is based. Types means holotype and paratypes; slide numbers are as indicated in
Table
Data sources for –VI
Data sources for are indicated in the tables themselves; i.e. observations from material of the Pilato and Binda collection (according to ), and literature data (relevant references in the tables).
Table III. Species of the genus Milnesium of which young and adult specimens, and/or males and females, have been observed (in the present study or the literature, as indicated). Types means holotype and paratypes; slide numbers are as indicated in
Table IV. Species with type 1 valvular system, correlated to body size, pt (= “percentual tube”, i.e. percentual ratio of the given structure with the buccal tube length (Pilato Citation1981)) of buccal tube standard width, and mounting medium. Data source indicates the literature sources and/or material examined by us (as indicated in ) to determine the valvular system type and obtain other data (body size, pt of buccal tube standard width, and mounting medium); the provided references are species’ original descriptions, or redescriptions (as specified), or refer to cultured strains (as specified). Body and buccal tube measurements, unless otherwise indicated, are from the corresponding cited literature source and refer to the specimen(s) with photograph(s) published in the same paper, in order to match the precise specimen with determined valvular system type, with its measurements; “our measurement(s)” refers to measurements taken by us on the previously indicated slide(s). Mounting media: AAF = fixed in acetic acid end mounted in Faure medium; Hoyer’s = Hoyer’s medium; PVA = polyvinyl alcohol; PVLF = polyvinyl lactophenol
Table V. Species with type 2 valvular system, correlated to body size, pt of buccal tube standard width, and mounting medium. Data source indicates literature sources and/or material examined by us (as indicated in ; types mean holotype and paratypes) to determine the valvular system type and obtain other data (body size, pt of buccal tube standard width, and mounting medium); the provided references are species’ original descriptions, or redescriptions (as specified). Body and buccal tube measurements, unless otherwise indicated, are from the corresponding cited literature source and refer to the specimen(s) with photograph(s) published in the same paper, in order to match the precise specimen with determined valvular system type, with its measurements; “our measurement(s)” refers to measurements taken by us on the previously indicated slide(s). Mounting media: AAF = fixed in acetic acid end mounted in Faure medium; Hoyer’s = Hoyer’s medium; PVA = polyvinyl alcohol; PVLF = polyvinyl lactophenol; “Polivinil” is what Ramazzotti, in 1962, wrote on his slides containing the type material of M. granulatum (the exact medium is unknown)
Figure 1. Claws of the hind legs of a Milnesium sp. to demonstrate the new nomenclature. Arrows: primary (or main) claws; arrowheads: secondary claws. Black arrows: posterior primary claws; white arrows: anterior primary claws; black arrowheads: posterior secondary claws; white arrowheads: anterior secondary claws. For legs I–III, “external” (or “lateral”) and “internal” (or “medial”), instead of “posterior” and “anterior”, will be used to indicate the single claws.
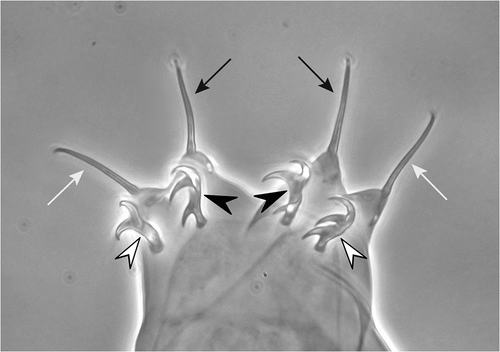
Figure 2. Valvular system of the tardigradum type (type 1); arrows indicate the cuticular folds in the tract connecting buccal tube and pharynx; arrowheads in (b) and (c) indicate the flaps. Scale bars: 20 µm. All slides deposited in the Pilato and Binda collection. (a) Paratype of Milnesium almatyense Tumanov (Citation2006) (Slide No. 5106). The flaps are not well visible while the flexible tract between buccal tube and pharyngeal bulb, with some folds (arrows), is well visible. (b) Valvular system of a specimen of the neotype series of Milnesium tardigradum Doyère (Citation1840) (Slide No. 5489). One flap (arrowhead) and the flexible tract between buccal tube and pharyngeal bulb, with some folds (arrows), are well visible. This flexible tract is caudally dilated the pharyngeal bulb being closer to the buccal tube than in (a). (c) Valvular system of a paratype of Milnesium inceptum Morek et al. (Citation2019) (Slide No. 5821). One flap (arrowhead) and the flexible tract between buccal tube and pharyngeal bulb, with some folds (arrows), are well visible. This flexible tract is caudally dilated, the pharyngeal bulb being closer to the buccal tube than in (a). (d) Valvular system of a specimen of the neotype series of Milnesium tardigradum Doyère (Citation1840) (Slide No. 5489). Two flaps (arrowheads) and the flexible tract between buccal tube and pharyngeal bulb, with some folds (arrows), are well visible. This flexible tract is caudally completely dilated, the pharyngeal bulb being pushed onto the buccal tube.
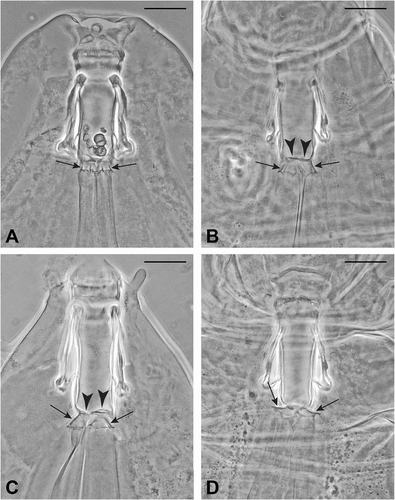
Figure 3. Valvular system of the shilohae type (type 2). Two flaps (arrowheads) and two conical thickenings (arrows) are visible, while the third ones are not in focus. The drawing (of Milnesium shilohae) is based on ) of Meyer (Citation2015).
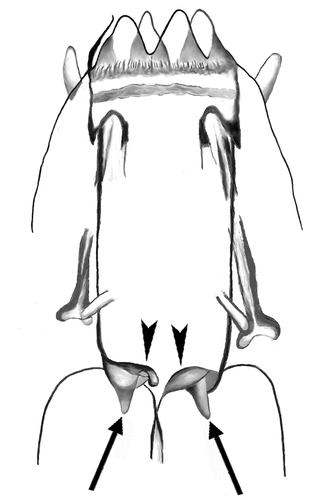
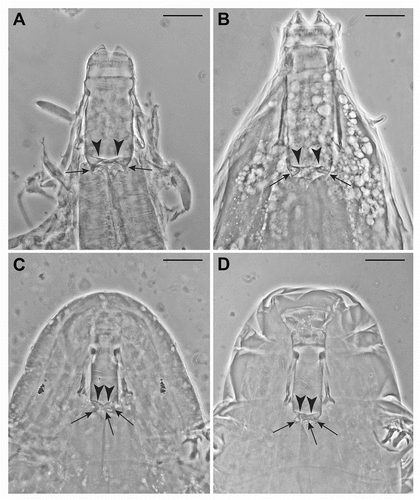
Figure 4. (a–c). Valvular system of the shilohae type (type 2); arrows indicate the conical projections; arrowheads indicate the flaps. Scale bars: 20 µm. All slides deposited in the Pilato and Binda collection. (a) Holotype of Milnesium tetralamellatum Pilato and Binda (Citation1991) (Slide No. 3704). Two flaps (arrowheads) and two conical projections (arrows) are visible. The third ones are not in focus. (b) Holotype of Milnesium brachyungue Binda and Pilato (Citation1990) (Slide No. 3940). Two flaps (arrowheads) and two conical projections (arrows) are visible. The third ones are not in focus. (c) Specimen of Milnesium sp. 4 (Slide No. 5703). Two flaps (arrowhead) are clearly visible, and the three flexible conical projections (arrows) are partially visible, despite the partially reduced distance between buccal tube and pharyngeal bulb. (d) Paratype of Milnesium reticulatum Pilato et al. (Citation2002) (Slide No. 4851). Two flaps (arrowhead) are visible, and three flexible conical projections (arrows) are also visible (the third in the middle, barely) despite the reduced distance between buccal tube and pharyngeal bulb.
For , the information on the status of each species regarding cuticle, peribuccal lamellae, claw configuration (indicated according to Michalczyk et al. Citation2012) and geographic distribution was taken from Morek et al. (Citation2016, Citation2018, Citation2019, Citation2020a, Citation2020b, Citation2020c), Young et al. (Citation2016), Schlabach et al. (Citation2018), Kaczmarek et al. (Citation2019), Moreno-Talamantes et al. (Citation2019, Citation2020), Surmacz et al. (Citation2019), Morek and Michalczyk (Citation2020), Nelson et al. (Citation2020a) and Sugiura et al. (Citation2020).
Table VI. Correlation between valvular system type (type 1 = tardigradum type; type 2 = shilohae type), eventual cuticular sculpture, number/configuration of the peribuccal lamellae, claw configuration, and ecozones in which each species results recorded. ° = species recorded in an ecozone different from that of the locus typicus. The fossil species M. swolenskyi has been excluded because it is not ecologically comparable with the other species’ actual zoogeography
Results and discussion
Origin of the claws of the Apochela and their denomination
Among Tardigrada, the Eutardigrada of the order Parachela have, typically, two double claws per leg, considering as clearly secondarily derived conditions those of Necopinatidae Ramazzotti and Maucci (Citation1983) and of some Hexapodibiidae Cesari et al. (Citation2016), having reduced or absent claws; and the slight claw branch separation of only the external/posterior claws of a few taxa (e.g. in the family Ramazzottiidae Sands, McInnes, Marley, Goodall-Copestake, Convey & Linse, 2008 according to Zawierucha et al. 2018, and in Grevenius deflexus (Mihelčič, 1960) according to Ramazzotti & Maucci Citation1983).
No known heterotardigrade has double claws, and, as a consequence, double claws should be considered derived from the fusion, two by two, of the claws of an ancestor having four claws per leg. In each double claw, a “basal portion”, a “primary branch” and a “secondary branch” are recognised, although the way they are joined to one another differs according to different models. Because in many cases (not all) the primary branch is connected to the secondary branch more or less far from the base of the double claw, the denominations “primary branch” and “base + secondary branch” are also used.
In contrast to the Parachela, the Milnesiidae (the only family of the order Apochela) have four claws per leg arranged in a quadrangle, and until now all tardigradologists, us included, have used for their claws a denomination arising from a very old traditional hypothesis in which the four claws per leg of the Milnesiidae were derived from two double claws by a secondary separation of their branches. Very probably, many of those who have used this denomination did not believe in (or even know about) that old hypothesis, but we stress here that this denomination has an origin and a meaning, from that discussed hypothesis; therefore, if this hypothesis proves to be wrong, the denomination should be changed in favour of a better one reflecting the true origin and meaning of apochelan claws.
We cannot indicate exactly the origin of that old hypothesis. Doyère (Citation1840, p. 283) wrote, regarding Milnesium,
Ongles au nombre de quatre à chaque patte, dont deux terminaux simples et en forme de filaments allongés crochus à l’extrémité, portés chacun sur un mamelon distinct; deux situés en dessous et en dedans, l’antérieur divisé en trois crochets fortement courbés, le postérieur en deux [four claws on each leg, of which two terminal in the shape of simple elongated filaments with hooked ends, each held on a separate relief; two present below and more internally, the anterior with three strongly curved hooks, the posterior with two].
Thulin (Citation1928, p. 229) wrote, “Terminalkrallen […] sind von den Basalkrallen völlig separiert” (Terminal claws […] are completely separated from the basal claws). We do not know whether Cuénot was the first to assert it, but we found that he was absolutely explicit in 1932 in writing (Cuénot Citation1932, p. 5), “une griffe grêle et une massive, relièes du reste par une mince band de cuticle, équivalent à une diplogriffe ordinaire dont les deux branches sont tres largement séparées et différenciées” (one slender and one stout claw, connected by a thin cuticular stripe, equivalent to an ordinary diploclaw of which the two branches are largely separate and differentiated).
Again Cuénot (Citation1949, pp. 57–58) also explicitly wrote, on the claws of Milnesium, “Chaque diplogriffe a une branche longue et une branche massive à 3 éperons” (each diploclaw has a long branch and a massive branch with three spurs). Bertolani (Citation1982, p. 26) wrote, on the Apochela, “Il ramo principale della diplounghia è nettamente separato dal secondario” (the main branch of the diploclaw is clearly separate from the secondary one). Ramazzotti and Maucci (Citation1983, translation by Beasley Citation1995, p. 43) also considered the four claws of each leg of the Milnesiidae to be the homologue of two double claws, writing,
In the genus Milnesium, the claws have the shape shown in Fig. 9, F; here also there are two double claws for each leg, but with widely spaced, clearly distinguishable branches. The main branch of each double claw is long and thin, with the usual accessory points at the tip, while the secondary branch is thicker, and bears 2, 3, or 4 curved spurs.
Kinchin (Citation1994) also followed the tradition; on p. 34 he wrote, “In the Apochela, the primary and secondary branches are both attached directly to the legs”, and on p. 127 he wrote, on the claws of Milnesium, “the claws are unusual in that the primary and secondary branches are inserted upon the cuticle separately”. Similarly, Bertolani and Grimaldi (Citation2000, p. 105) wrote, “Each leg with two ‘double’ claws, each claw with a main branch […] separated by a basal claw”. Yang (Citation2003, p. 239) also defined as “double claws” the claws of the species Milnesium dujangensis Yang (Citation2003) (species considered invalid by Morek et al. Citation2016). That old tradition (affecting the apochelan claw naming) is still living; Guil et al. (Citation2019) wrote on p. 14, in the definition of the Apochela (that they called Apotardigrada because considered them a class), “Claws with completely separated primary and secondary branches”. Likewise, Nelson et al. (Citation2020b, p. 510) wrote,
In the eutardigrade order Apochela (Milnesiidae), the two claw branches are separate from each other. The primary branch is long and thin and has accessory points, while the secondary branch is short, stout, and usually bears two or three hooks or spurs. […] In the eutardigrade order Parachela, the primary and the secondary branches are connected and usually arise from a common basal tract.
Lastly, Morek et al. (Citation2020b) expressed the discussed hypothesis in writing,
claws both in apochelans and parachelans are arranged into pairs: external and internal on legs I–III and anterior and posterior on legs IV. It is true that the primary branches and secondary branches in Apochela are separated, but there are examples of separation of the claw branches in some Parachela too.
Therefore, all of the above authors implicitly attributed to the apochelan claws an origin from double claws through a secondary separation of the branches.
Some authors have used the denomination “primary branch” for the longer distal claws, and “base + secondary branch” for the shorter basal claws furnished with at least two tips (e.g. Binda & Pilato Citation1990; Michalczyk et al. Citation2012; Ciobanu et al. Citation2014, Citation2015; Londoño et al. Citation2015; Meyer Citation2015; Roszkowska et al. Citation2015; Morek et al. Citation2020a, Citation2020b, Citation2020c). Other researchers, or the above-mentioned authors in other papers, have used the denominations “primary branch” and “secondary branch” (e.g. Morek et al. Citation2016, Citation2018, Citation2019; Young et al. Citation2016; Schlabach et al. Citation2018; Kaczmarek et al. Citation2019; Moreno-Talamantes et al. Citation2019; Surmacz et al. Citation2019). In the descriptions of Milnesium minutum Pilato and Lisi (Citation2016), Milnesium sandrae Pilato and Lisi (Citation2016), and of Milnesium validum Pilato et al. (Citation2017), the denominations used by the authors were “primary claws” and “secondary claws”, but without a discussion of this choice. We must also record that Marley et al. (Citation2011, p. 54) wrote, on the claws of the Apochela, that they “possess four claws per leg (two simple and two compound/branched, arranged in a quadrangle)”, but the authors did not discuss the denomination of those claws.
In our opinion, the denominations (“primary branch” and “secondary branch”, or “primary branch” and “base+secondary branch”) used to date are inopportune because they arise from the above-mentioned ancient – and in our opinion incorrect – hypothesis of the origin attributed to the claws of the Apochela. We do not accept that hypothesis for the following reasons.
Although Morek et al. (Citation2020b, p. 12) wrote that “all molecular phylogenies show that Apochela and Parachela are sister clades that are both equally related to all Heterotardigrada”, Apochela seem to have retained some plesiomorphic characters in common with Heterotardigrada, which were instead lost or modified in the other Eutardigrada (i.e. presence of cephalic papillae, and four separate claws on each leg; see further below). The idea of Morek et al. (Citation2020b) is today generally accepted, but until recently studies suggested that within Eutardigrada, the Parachela were more derived and less closely related to Heterotardigrada, while Apochela were more primitive and more closely related to Heterotardigrada (although keeping the higher affinity between Apochela and Parachela as Eutardigrada). Those studies were published about 10 years ago, but this does not mean they must be totally wrong. We can record here some citations:
Marley et al. (Citation2011, p. 54): “the cephalic papillae of Apochela suggest a closer alignment with the Heterotardigrada, a conclusion strongly supported by molecular evidence (Jørgensen & Kristensen Citation2004; Kiehl et al. 2007; Sands et al. 2008b; Jørgensen et al. Citation2010)”.
Wełnicz et al. (Citation2011, p. 37): “The position of M. tardigradum is consistent with previous molecular and morphological results and indicates a basal position of these species within the Eutardigrada”.
Guil and Giribet (Citation2012), p. 21: “When studying the sensitivity to outgroup choice, the class Eutardigrada was monophyletic under only one combination of outgroups; all other combinations placed the eutardigrade order Apochela as sister to the class Heterotardigrada”; p. 32: “Eutardigrada was paraphyletic in most other analyses as the order Apochela was sister to Heterotardigrada”; pp. 32–33: “Eutardigrade monophyly was controversial because the order Apochela (represented in this study by several Milnesium species; Table II; Fs. 1 and 8) was sister group to the class Heterotardigrada in many of the outgroup combinations (trees not shown)”; pp. 44–45: “The class Eutardigrada was not monophyletic under most outgroup combinations (Table IV) […] because Milnesium was sister to heterotardigrades. Guidetti et al. (Citation2009) suggested Milnesium tardigradum to be a fast-clock species and postulated this to be a long-branch attraction problem, although this was not demonstrated; our study, including more outgroups and more ingroup species, finds the same result”.
We can add that, very recently, Guil et al. (Citation2019) published a phylogenetic analysis whose conclusion was to separate Apochela and Parachela as distinct classes (with the names Apotardigrada and Eutardigrada, respectively); these authors also elevated to order level the four superfamilies of Parachela. Fleming and Arakawa (Citation2021) expressed their perplexity with this action, and Morek et al. (Citation2020b) even proposed to abolish those conclusions, but all this represents a debate with different opinions, and more comprehensive studies are necessary to see if this debate is concluded or still in progress.
As regards strictly morphology, we agree with Marley et al. (Citation2011) on the evaluation of the presence in Apochela of cephalic papillae as a possible character linking to Heterotardigrada; furthermore, we note that Nichols et al. (Citation2006, p. 53) performed a cladistic analysis in which Milnesiidae was found to be “the most basal eutardigrade family”.
The hypothesis that the apochelan claws derive from parachelan, or parachelan-like, double claws through secondary, complete separation of the branches, is, according to Morek et al. (Citation2020b), supported from the molecular point of view; however, apart from the differing opinions and results in the literature cited above, it looks to us poorly sustainable, because it would oblige us to imagine improbable, unparsimonious ancestors and evolutionary paths from a morphological point of view.
It is broadly believed that Heterotardigrada should have retained more plesiomorphic characters than the more modified Eutardigrada, so that the ancestor of the latter should have had four aligned claws per leg and cephalic papillae. Within Eutardigrada, Apochela have cephalic papillae, absent in Parachela, and it has sometimes been asked (e.g. by Marley et al. Citation2011), still without a definitive answer, whether this may represent a primitive character linking to Heterotardigrada; more importantly, heterotardigrades never have double claws (present as a rule in Parachela and questioned in Apochela), and there is not even trace of a tendency to couple the claws in any heterotardigrade.
If the criticised hypothesis (that the claws of the Apochela derived from double claws) were true, the first eutardigrade (from which Apochela and Parachela derived) would have had already double claws (thus, a secondary complete separation of the claw branches in Apochela must be hypothesised). Regarding cephalic papillae, since these structures are present in heterotardigrades, parsimony leads us to think, as our best hypothesis, that the ancestor of Eutardigrada should still have had some form of cephalic papillae, that were then retained/modified by Apochela and lost by Parachela.
The questioned ancestor of Eutardigrada, already having proper double claws but still with cephalic papillae, is not connectable to any known heterotardigrade or parachelan, living or fossil. Furthermore, the supposed secondary separation of the claw branches of the Apochela looks to us very questionable, for many reasons: it is true that in a few Parachela a secondary separation of the claw branches occurred, but (1) this did not occur in basal stems; (2) it occurred only in groups with asymmetric claw arrangements on each leg; (3) only one of the two double claws of each leg has separated branches, and the branches remain close and with a connection (although not sclerified); (4) the double claw global structure, although with separated branches, is still recognisable and perfectly linkable to similar groups with proper double claws; and (5) in Apochela, the two “branches” of each “double claw” are totally separated like single, independent claws, each one borne by its own leg prominence, and arranged in a quadrangle; therefore, this would mean a very deep evolutionary reversal according to the hypothesis we are criticising. All this, in addition to the fact that Apochela have a symmetric claw arrangement, leads us to think that apochelan claws are simply in a more primitive state than parachelan double claws, and that, after the Apochela stem onset, such claws followed a different evolutionary path (arranging in a quadrangle) with respect to the path that in Parachela led to proper double claws.
We therefore hypothesise, in a way that looks to us more parsimonious, that Eutardigrada derived from a primitive heterotardigrade-like ancestor with cephalic papillae (the rule in Heterotardigrada) similar or not in number and arrangement to living heterotardigrades, and with four separated, aligned claws per leg (which is very common in this class). This ancestor could have given origin to a phyletic line characterised by a tendency to reduce and/or modify the sensorial appendages and to modify the arrangement of the claws. In this way, a descendant of this line became so different from the original ancestor that it can be considered a “protoeutardigrade”, i.e. the ancestor of a new class (the Eutardigrada). From it, all eutardigrades derived, and subdivided into two sister lines: the orders Apochela and Parachela. The Apochela maintained cephalic papillae (perhaps with modifications), and four separate claws but these were arranged in a quadrangle and differentiated two by two; the more modified (in terms of those characters) Parachela lost any form of primordial sensorial appendages, and the four claws per leg differentiated and fused two by two, forming two (symmetrical or asymmetrical) double claws per leg.
In conclusion, in our opinion, the claws of the Apochela appear in a primitive condition with respect to the double claws of the Parachela; they cannot be considered, and named, “branches” because each of them seems to be primitively “a claw”. If so, we find it inopportune to use for structures in a primitive condition denominations traditionally attributed to the same structures in a derived condition. As a consequence, we suggest for the claws of the Apochela the following denominations: “primary (or main) claws” for the distal, longer and more slender claws; and “secondary claws” for the basal, shorter and stouter claws. If necessary, one can of course add the specification “external” (or “lateral”) and “internal” (or “medial”) for the claws of legs I–III, and “posterior” and “anterior” for the claws of the hind legs ().
Valvular system in the connecting tract between buccal tube and pharyngeal bulb in the species of the genus Milnesium
It is known that in the species of the genus Milnesium a flexible tract is present connecting the buccal tube and the phayngeal bulb, and Tumanov (Citation2006), in the description of some new species, specified that when measuring the buccal tube length the flexible portion must also be measured, but he did not furnish a detailed description of that a flexible portion. We have been studying species of Milnesium and encountered the problem of the measurement of the buccal tube including the flexible caudal portion, and this induced us to examine carefully that portion of the bucco-pharyngeal apparatus in many species of that genus.
Some authors (Marcus Citation1929; Dewel & Clark Citation1973; Guidetti et al. Citation2012) pointed out that between the buccal tube and the pharyngeal bulb, a valvular system is present preventing any backflow of food from the pharynx. Marcus (Citation1929) reported a valvular system formed by three separate folds; Dewel and Clark (Citation1973) furnished a more detailed description of the valvular system in Milnesium tardigradum Doyère (Citation1840), and reported a cuticular fold between the buccal tube and pharyngeal bulb with three flap-like extensions more or less long. Guidetti et al. (Citation2012), who worked on specimens mounted “in polyvinyl lactophenol or in Faure-Berlese fluids”, described (on p. 131) the bucco-pharyngeal apparatus of Milnesium cf. tardigradum, and wrote, as regards the valvular system,
the end of the buccal tube of Milnesium is characterized by three triangular expansions located […] one ventrally and two dorso-laterally. Between these expansions, three flexible “laminar cuticular flaps” are present, each bearing a radial laminar fold on its external side. These flaps, also identified by Dewel and Clark (Citation1973), work as valves to close the buccal tube.
Unfortunately, we are not sure what species was examined by Dewel and Clark (Citation1973) and Guidetti et al. (Citation2012) because the former authors referred to Milnesium tardigradum when, in 1973, all the Milnesiidae (today 45 species) were attributed to that species, and Guidetti et al. (Citation2012) referred to Milnesium cf. tardigradum. Dewel and Clark (Citation1973) and Guidetti et al. (Citation2012) each referred to only one species of the genus; they clearly described the same type of valvular system and traditionally it has been assumed that it was the same throughout the genus. However, we examined many species of the genus and realised that two types of valvular system are recognisable. We describe them here, identifying the first type as “tardigradum type” or simply “type 1” and the second as “shilohae type” (we chose this denomination because in fig. 2B of the description of Milnesium shilohae of Meyer Citation2015 the valvular system is perfectly visible) or simply “type 2”.
We stress here that we attribute to Milnesium tardigradum the characters of the valvular system that we noticed in specimens of the neotype series kindly sent to us by Łukasz Kaczmarek (Adam Mickiewicz University in Poznań, Poland) and deposited in the Pilato and Binda collection; these characters perfectly agree with those described and visible in illustrations in Dewel and Clark (Citation1973) and Guidetti et al. (Citation2012).
In the tardigradum type (type 1) (–(d); Guidetti et al. (Citation2012, fig. 16e–f); more figures in the literature are listed in ), the general morphology is rather simple: the three sclerified caudal expansions of the buccal tube are relatively short, and the flaps (), arrowheads) are delicate; a simple flexible tract is present between buccal tube and pharyngeal bulb with some longitudinal folds (), arrows); this tract being flexible, a little shifting is possible of the pharyngeal bulb with a consequent change of the shape of the connecting tract that usually appears caudally bell-shaped, opening like an umbrella, when the distance between pharyngeal bulb and buccal tube decreases (), arrows; and very clearly visible in Surmacz et al. (Citation2019, ) relative to that in Milnesium pseudotardigradum Surmacz et al. Citation2019). When the distance between pharyngeal bulb and buccal tube is minimal, the flexible tract usually appears completely dilated and shortened (the “umbrella” flattens) and the pharyngeal bulb is in contact with the terminal portion of the sclerified buccal tube wall (), arrows). However, in a few cases, the flexible connecting tract may crumple inside the lumen of the buccal tube/pharyngeal bulb when these organs are pushed onto each other; if this situation is taken to extremes, the type of valvular system becomes undeterminable.
We recognised, through personal observations and/or from original description images, this type of valvular system in M. alabamae Wallendorf and Miller, Citation2009; M. almatyense Tumanov, Citation2006; M. alpigenum Eherenberg, Citation1853 (redescribed by Morek et al. Citation2019); M. beasleyi Kaczmarek et al., Citation2012; M. beatae Roszkowska et al., Citation2015; M. berladnicorum Ciobanu et al., Citation2014; M. dornensis Ciobanu et al., Citation2015; M. inceptum Morek et al., Citation2019; M. katarzynae Kaczmarek et al., Citation2004; M. kogui Londoño et al., Citation2015; M. longiungue Tumanov, Citation2006; M. pacificum Sugiura et al., Citation2020; M. pentapapillatum Morek et al., Citation2020b; M. pseudotardigradum Surmacz et al., Citation2019; M. reductum Tumanov, Citation2006; M. tardigradum; M. variefidum Morek et al., Citation2016; and M. wrightae Kaczmarek et al., Citation2019.
In the second type of valvular system (the shilohae type, type 2), the general morphology is slightly more complex: the flaps (see , derived from Meyer (Citation2015, f. 1B), and (a–c), arrowheads) are thicker, and the three sclerified caudal expansions of the buccal tube are prolonged forming three evident conical, flexible projections (see , based on Meyer (Citation2015, f. 1B) relative to Milnesium shilohae; and , arrows), which seem to become part of the flexible connection between buccal tube and pharyngeal bulb (where, instead, no longitudinal fold is visible). It seems also that these conical projections, being flexible, may participate in forming a valve that shuts the buccal tube, isolating it from the pharyngeal bulb, at least when buccal tube and pharyngeal bulb are more or less pushed onto each other () arrows), because in this case the flexible connecting tract and the projections never open outwards, going instead inside the lumen. When this is taken to extremes (i.e. the conical projections are no longer distinguishable), it becomes impossible to distinguish this valvular system type from type 1 in the same situation.
We recognised the type 2 valvular system, by personal observations and/or from original description images, in M. antarcticum Tumanov, Citation2006; M. barbadosense Meyer and Hinton, Citation2012; M. brachyungue Binda and Pilato, Citation1990; M. burgessi Schlabach et al., Citation2018; M. eurystomum Maucci, Citation1991; M. fridae Moreno-Talamantes et al., Citation2020; M. granulatum Ramazzotti, Citation1962; M. jacobi Meyer and Hinton, Citation2010; M. reticulatum Pilato et al., Citation2002; M. shilohae Meyer, Citation2015; M. tetralamellatum Pilato and Binda, Citation1991; M. tumanovi Pilato et al., Citation2016; M. validum Pilato et al., Citation2017; M. vorax Pilato et al., Citation2016; and M. zsalakoae Meyer and Hinton, Citation2010.
The differences between the two types are therefore the following: type 1 is simpler and has thinner flaps than type 2; the three sclerified caudal expansions of the buccal tube are relatively short in type 1, while they are prolonged into three non-sclerified conical projections in type 2, probably becoming part of, not separated from, the connecting tract between buccal tube and pharyngeal bulb; this connecting tract is provided with simple longitudinal cuticular folds in type 1, which are absent in type 2 (where the conical projections are); when buccal tube and pharyngeal bulb are more or less pushed onto each other, the type 1 connecting tract usually opens like an umbrella, while that of type 2 always goes inside those organs’ lumen. The two valvular system types become indistinguishable only when buccal tube and pharyngeal bulb are completely pushed onto each other and the whole connecting tract crumples inside their lumen, which is a rare case for type 1 (because the connecting tract usually opens outwards), while it is the rule for type 2, in which, in this extreme case, the conical projections go totally inside the lumen and are no longer distinguishable (not even their caudal tips).
Unfortunately, the available specimens and/or photographs of some species do not allow us to identify the type of valvular system with absolute certainty. These species are: M. argentinum Roszkowska et al., Citation2015; M. asiaticum Tumanov, Citation2006; M. bohleberi Bartels et al., Citation2014; M. cassandrae Moreno-Talamantes et al., Citation2019; M. krzysztofi Kaczmarek and Michalczyk, Citation2007; M. lagniappe Meyer et al., Citation2013; M. matheusi Kaczmarek et al., Citation2019; M. minutum Pilato and Lisi, Citation2016; M. sandrae Pilato and Lisi, Citation2016; and M. swansoni Young et al., Citation2016. In this group it is necessary to include also M. quadrifidum Nederström, 1919 (valid according Morek et al. Citation2016) and M. swolenskyi Bertolani and Grimaldi, Citation2000, of which no photographs are available.
In all the valid species of Milnesium are reported, each with the relative type of valvular system. We indicate the material we examined, and the photograph, or photographs, from the literature on which we based our determination of the type of valvular system when possible.
As can be seen from , a problem seems to arise with M. eurystomum (but only this species); it seems to have type 1 in the material of Morek et al. (Citation2020a), or rather, in their GB.005 strain from Scotland, shown in –7 of that paper (no photographs are available of the other three populations studied in that work), while it appears to be of type 2 in Maucci’s holotype and in a specimen we have (Slide No. 1815) from Greenland, close to the locus typicus. However, we have some doubts on the attribution by Morek et al. (Citation2020a) of their photographed specimens to the species. Not only is that material from Scotland, but the identification also appeared to be more an assumption than a certainty, because those authors wrote (pp. 466–467),
The morphological and molecular species delimitation gave incongruent results […]. The classical morphological delineation as well as ITS-2 [Internal Trascribed Spaced-2] data (both in bPTP [Poisson tree processes model] and ABGD [Automatic Barcode Gap Discovery]) indicated that the four analysed populations represent a single species. On the other hand, the PCA [principal component analysis] analysis as well as the bPTP analysis based on COI [Cytochrome Oxidase C subunit I] and the ABGD analysis based on the concatenated ITS-2þCOI sequences suggested the presence of two to three species. Importantly, however, the putative species indicated by these methods did not overlap. Given these strong incongruences and overall weak support for multiple species, we adopted the conservative scenario and concluded that all four populations represent M. eurystomum.
Regarding morphometry, they wrote (Morek et al. Citation2020a, p. 466), “the statistical analysis of morphometric traits returned strongly incongruent results meaning that the populations could not be confidently split using morphometry. Consequently, we concluded that our four populations represent a single species and the observed variability is intraspecific”, but we stress that if they studied, as we suspect, extremely similar species together (so similar as to be mistaken for the same species, which is not so rare in Eutardigrada), it is possible they had similar morphometric characters also.
Therefore, although we cannot state with certainty that the discussed population from Scotland is not attributable to that species, we at least have doubts and believe this problem should be clarified before concluding on correct diagnosis. If that population is not M. eurystomum sensu strictu, the problem disappears, but the case would represent an example of different valvular system types in very closely related species (this will be discussed further); if, instead, the diagnosis proved to be correct, it would represent the only such example among the 33 valid species in which we were able to identify the valvular system, making the possibility of intraspecific variability of the trait very low (see further discussion below).
We investigated a possible correlation of valvular system types with age and sex; in we indicate the species for which young and adult specimens, and/or both males and females, have been studied. We include species of, according to the present study, dubious valvular system type, because this uncertainty depends only on the available material we studied and available photographs from the literature (e.g. not well in focus), but the authors who examined those species usually studied and measured many specimens, and thus had the ability to experiment with focus and notice possible differences (see further below).
Males have been found in eight species, while young and adult specimens have been found in 19 species (based on clear assessment by authors, or on the publication of very ample population body size range, clearly indicating the representation of multiple life stages). The material we studied, and the literature sources including photographs of both young and adult specimens, and/or photographs of both males and females, clearly suggest non-correlation in all cases. also lists the studies without photographs in which young and adults (according to body size), and/or both males and females, were found. This is because, often, many specimens were studied in those works, and therefore it is likely that the authors who observed and measured them would have noticed, at least sometimes, differences in the valvular system type, and this has never happened. It is true that nobody until now has noticed the existence of type 2, but we stress that we did so only because our study involved comparatively measuring many different species, so that, when focusing the caudal end of the buccal tube to take these measurements, we gradually realised the species were not all equivalent. Probably, no previous researchers realised this because they studied and measured one population at a time, and the population was homogeneous and without differences among specimens (see further below regarding our conclusion that the trait is species-specific or at least population-specific). We therefore conclude that valvular system type does not depend on life stage or sex.
We also tested a possible correlation with body size (as a confirmation of the previous verification) or buccal tube width, and whether valvular system types could be merely an artifact of the chosen mounting medium. In the two types of valvular system are correlated with the above-mentioned features; to avoid mistakes, body length, buccal tube standard width and mounting media in the tables refer to the individual specimens for which we determined the valvular system type, examined by us or photographed in the cited literature sources; the “Data source” column in each table, identifies each separate “case” (meaning for each species a slide, or slides of the same population, mounted in the same mounting medium).
We were able to examine 43 cases in total in which the valvular system type was recognisable: 27 for type 1, and 16 for type 2. For simplicity, in the following text, body length and pt of buccal tube standard width are indicated, respectively, as BL and BTSW.
Regarding the type 1 valvular system (), BL ranges from 307 to 898 µm, and BTSW from 25.5 to 48.2 µm; therefore, there seems to be no evidence of correlation, which agrees with the results of , considering that the young and adult specimens of each species can show significant differences in BTSW, so that no correlation with life stage is, at least partially, also no correlation with BTSW. Regarding mounting media, 70.4% of the cases (19/27) were mounted in Hoyer’s medium, 14.8% (4/27) in polyvinyl lactophenol, 3.7% (1/27) in polyvinyl alcohol and 11.1% (3/27) fixed in acetic acid and mounted in Faure fluid. Thus, this type of valvular system would appear much more correlated to Hoyer’s medium than to polyvinyl lactophenol (nothing can be said on the other fluids, due to the very low numbers), but see further discussion below.
As for type 2 (), BL ranged from 245 to 847 µm, and BTSW from 29.8 to 64.1 µm, indicating evidence of non-correlation of the two variables with valvular system type 2; this, again, is in agreement with the results shown in . Regarding mounting media, 18.8% of the cases (3/16) were mounted in Hoyer’s medium, 62.5% (10/16) in polyvinyl lactophenol, 12.5% (2/16) in polyvinyl alcohol and 6.3% (1/16) in “Polivinil” (according to Michalczyk et al. (Citation2012), this word was written on the slide by Ramazzotti, who did not give further mounting medium information in his papers); no specimens with the type 2 valvular system were fixed in acetic acid and mounted in Faure fluid. This type of valvular system would thus appear to be correlated more with polyvinyl lactophenol than with Hoyer’s medium (again, nothing can be said regarding the other fluids, due to the very low numbers), but we have doubts in concluding that such correlations (for both types) indicate with certainty that the valvular system type is just an artifact, as we discuss thoroughly below.
Comparing data on BL and BTSW between the two valvular system types ( vs ), we have a type 1 BL range of 307–898 µm vs a type 2 BL range of 245–847 µm, and a type 1 BTSW range of 25.5–48.2 µm vs a type 2 BTSW range of 29.8–64.1 µm; we therefore conclude that data of the two valvular system types for both characters can be considered congruent and the differences (between type 1 BL vs type 2 BL; and between type 1 BTSW vs type 2 BTSW) should depend on a lack of data, also taking into account the results of (considering BL highly correlated, and BTSW often correlated, with life stage).
As shown, the most frequently used mounting media are Hoyer’s and polyvinyl lactophenol; other media occur in too few cases and are therefore discarded for reliable comparisons. Indeed, the comparison between the main mounting media might also prove to be unreliable, due to a difference between the two “samples” and the consequent risk of random statistical fluctuations: Hoyer’s is used more often, in 22/43 cases, while polyvinyl lactophenol appears in only 14/43 cases. Also the number of total cases of each valvular type shows differences – 27/43 for type 1, and 16/43 for type 2 – again, with a risk of random statistical fluctuations due to the difference between the two “samples”. As seen, type 1 cases mounted in Hoyer’s medium account for 70.4% (19/27), while mounted polyvinyl lactophenol represent 14.8% (4/27); in contrast, type 2 has 18.8% (3/16) of cases mounted in Hoyer’s medium, and 62.5% (10/16) in polyvinyl lactophenol. Such percentages would seem to indicate a correlation of type 1 with Hoyer’s medium, and of type 2 with polyvinyl lactophenol. However, in addition to our perplexity regarding possible misleading statistical effects due to the very different “sample” sizes (both for type 1 vs type 2, and for Hoyer’s medium vs polyvinyl lactophenol), there are many considerations that induce us to think that the valvular system type should not be correlated to mounting medium used:
If it were an artifact, why does each type of valvular system appear the same in different species, sometimes also mounted in different media (see further below)? The morphology of each type does not change among the species that show it, even in the case that they are mounted in different media (only the frequencies of type/medium apparently change).
It is hard to believe that the apparent morphology of type 1 can become that of type 2 (and vice versa; compare with ) just by artefactual effect. How can the evident conical projections of type 2 disappear, and its more robust flaps be reduced, passing into type 1? This latter appears very simple and with a very “clean” connecting tract between buccal tube and pharynx, with only some cuticular folds, never seen in type 2.
Each species was found always associated with its particular valvular system type independently of the mounting medium used; we have only one (apparent?) exception, which is M. eurystomum, discussed above. As indicated in , M. almatyense was mounted in two or three types of fluids (depending on whether our specimens from Sicily are or are not truly M. almatyense), and M. tardigradum, M. granulatum and M. reductum were each mounted in two types of fluids. Furthermore, Guidetti et al. (Citation2012), in their careful morphological analysis, worked on specimens mounted “in polyvinyl lactophenol or in faure-berlese fluids” without reporting differences. To support the hypothesis that the valvular system types are merely an artifact, it would be necessary to admit that each apparent morphology of the two valvular system types should be acquired without differences using different mounting media.
We have two slides (in polyvinyl lactophenol) with both valvular system types but only in different species: slide No. 2775 with two specimens of M. tardigradum (type 1), two of M. cf. almatyense (type 1), and the holotype of M. vorax (type 2); slide No. 3746 with two specimens of very different unidentified species, one with type 1 and the other with type 2.
No population or slide (with many specimens) of the same species was ever found showing different morphology. We have examined (): M. reticulatum (44 specimens), M. tardigradum (16), M. brachyungue (10), M. inceptum (9), M. tetralamellatum (7), M. alpigenum (6), M. validum (6); also, several papers (e.g. for the six species with photographs, of which the valvular system type was determinable, of ) show photographs of more than one specimen without differences in their valvular morphology; to this total must be added the numerous papers in which the authors reported a high number of analysed (and often also measured) specimens without mentioning any differences (the importance of this point was already raised while discussing the evaluation of male/female and young/adult specimens). Considering that mounting media showed not more than about 70% apparent correlation, the artifact hypothesis would necessarily mean that in the same slide or population of a species in the same medium, some specimens, at least sometimes, should be found with different apparent morphology with respect to the numerous other specimens; this has never happened.
Therefore, all considered (including the problem of M. eurystomum), valvular system types appear to be species-specific, or at least population-specific; in the latter case, it must be stressed that uniformity within each population should not depend (always) on parthenogenetic strains, males being present in several cases. Our assumption implies that the differences to date found in the possible correlation of the valvular system type with the mounting medium should depend on a lack of data and chance, for all the reasons and considerations already expressed.
In the probable case that valvular system types are species-specific, we tried to match them with the status of other characters (cuticle, peribuccal lamellae and claws) and the geographic distribution of all species, in . Excluding the species for which the valvular system type is undetermined, we ascertained that there is no correlation between the type of valvular system and the characters of the cuticle; in fact, all possible cuticle states (smooth, reticulated or pseudoporous) can be found both in species with the tardigradum type (type 1) valvular system and in species with the shilohae type (type 2).
As regards peribuccal lamellae number and configuration, a possible correlation with type of valvular system is difficult to determine; this is because of the still high number of species with six lamellae in which the exact configuration has not yet been described (whether 6 equal or “4 + 2” or (2 + 2) + 2). With current data, the “4 + 2” is present in 10 of 18 (55.6%) species with the tardigradum type valvular system, while it is apparently absent in those with the shilohae type, but those latter have 11/15 species with six lamellae of uncertain configuration. The presence of only four (developed) lamellae seems to be limited to the species with the shilohae type of valvular system, but it is a rare configuration, and among the species with uncertain valvular system, two have probably four lamellae (M. lagniappe and M. swansoni), and two have an unknown number of lamellae (M. krzysztofi and M. quadrifidum); therefore, some of these might well have four lamellae but possess the tardigradum type of valvular system.
No clear correlation can be evidenced to date between the valvular system type and the claw configuration. The most common configurations, [2-3]-[3-2] and [3-3]-[3-3], are shared by the two groups of species; among the other configurations, few would be exclusive of one group or the other, but those configurations are less common. Thus it could be just an effect of a lack of data, and, more importantly, it must be considered that claw configuration may be variable even within the same species depending on life stage. In conclusion, we believe that no correlation exists between claw configuration and valvular system type.
All these facts confirm that the grouping of species of Milnesium on the basis of morphological characters is still a pending problem, and in particular the two old groups (tardigradum, believed to have a smooth cuticle, and granulatum with a sculptured cuticle) are not valid, as already stated by Morek et al. (Citation2016) and Morek and Michalczyk (Citation2020). In addition to cuticle characters, the number and configuration of peribuccal lamellae and the claw configuration also seem to be useless to divide the genus Milnesium into monophyletic species groups.
Another issue to be analysed is the possible correlation between the type of valvular system of the various species and their geographic distribution, since very recently Morek and Michalczyk (Citation2020) and Morek et al. (Citation2021) pointed to a possible correlation between geographic distribution and phylogenetic grouping of the species of the genus Milnesium. We follow a traditional division into ecozones (Udvardy Citation1975; Schultz Citation2000): Afrotropical, Antarctic, Australasian, Indomalayan, Nearctic, Neotropical, Oceanian and Palearctic; the latter has been divided into Western Palearctic and Eastern Palearctic due to the great extent of the territory, in order not to obscure possible differences. The following evaluations are based only on the species whose valvular system type could be determined.
No reliable evaluation can be made on Afrotropical, Antarctic, Australasian, Indomalayan, Nearctic, Neotropical and Oceanian ecozones due to the scarcity of data, although it the presence of species with both types of valvular systems is ascertained in the Afrotropical and in the Neotropical, with a total of six and seven species, respectively, more or less equally divided into the two valvular system types in each region. In the Nearctic, in contrast, among seven species only two (28.6%) have the tardigradum valvular system type, while the remaining five (71.4%) have the shilohae type; further studies are necessary to ascertain whether this type 2 is in fact dominant in this region.
The subdivision we tried to apply to the Palearctic ecozone did not reveal significant differences; therefore, we here discuss the entire ecozone: it counts 16 species, of which 13 (68.4%) possess the tardigradum type, while three (15.7%) have the shilohae type; thus, in this region the first type of valvular system would appear to be dominant with the currently available data.
Putting all these data together, it seems, at least in some ecozones, that the geographic distribution of species having one or the other type of valvular system may be not random, but we did not find any region that, to a reliable approximation, can indicate an example of geographic isolation of species with one or the other valvular system type; however, it must be stressed that in the analysis carried out by Morek and Michalczyk (Citation2020) there also was no isolation because exceptions existed. Our geographic evaluations are in any case preliminary, until most ecozones are better explored, and the species with dubious valvular system type can be determined.
As a last attempt, we tried to match our data with the numerous molecular phylogenies available in the recent literature, but we found that most of the sequenced species had type 1. Also, the very few sequenced species with type 2 fell within trees among several other species having type 1, which would mean a lack of correlation between molecular phylogenetic trees and valvular system type. However, data today are still very incomplete; thus, it would be interesting to repeat the test once more species are sequenced (especially those with type 2), and, again, once those with dubious valvular system type have been determined.
To sum up, all these uncertainties and non-correspondences should not be discouraging, both because in many cases more data must be acquired before conclusions are drawn and because it is already known (e.g. Morek & Michalczyk Citation2020) that in the genus Milnesium many morphological characters are not correlated with one another and with geography or phylogeny. The valvular system type might be correlated to other factors, not taken into consideration due to a lack of data, such as detailed characteristics of the microenvironment and/or the type of diet; we ascertained a non-correlation with buccal tube width, while Roszkowska et al. (Citation2016) found a correlation between diet and width of the buccal tube, but this is too little from which to jump to conclusions, because a correlation between valvular system type and diet still cannot be excluded, in a way not contradicting these two provisional data points. It is possible that the species of each ecozone known today are not well representative of the whole possible variety of these two factors, and indeed others; thus, future, more in depth research might significantly change the evaluations we could draft provisionally with the current data.
Conclusions
Our clarifications on the claws of the Apochela allow us to eliminate the contradiction between their traditional denomination which is based on an ancient, questionable hypothesis (the supposed derivation of the claws of Apochela from double claws) and, on the other hand, the more probable origin of the Apochela (and all Eutardigrada) from an ancestor with four separate claws per leg.
The description of an until now unnoticed second type of valvular system between buccal tube and pharyngeal bulb in some species of Milnesium adds a new trait to be considered in the genus, but, like many novelties, still leaves some questions open, requiring further studies.
The discussed case of M. eurystomum is peculiar and must be verified; it leads us to think that the trait may depend on one or a few genes that can easily vary within very closely related species, explaining the supposed non-significance from the phylogenetic point of view, and the non-correlation with all analysed features. We expressed doubts on the diagnosis of Morek et al. (Citation2020a), but if it proved to be correct, it would represent until now the only case of intraspecific variability of the trait (but highly probably interpopulational and not intrapopulational).
In any case, the present study indicates that the exact morphology of the discussed portion of the bucco-pharyngeal apparatus in Milnesium must be studied better, because former studies on it have now proved not to give a complete picture of the exact, detailed morphology of that area. In fact, even in the improbable case that valvular system types should prove to be an artifact, the present study has described structures until now never noticed (i.e. the conical projections of type 2) which should be correctly interpreted, thoroughly investigated (also through scanning electron microscopy) and described, to gain complete knowledge of the valvular system and, more generally, of the tract connecting the buccal tube and pharyngeal bulb in the genus Milnesium. Our conclusions are therefore preliminary, due to a lack of data on many previously mentioned aspects, but can give impetus to new investigations by tardigradologists.
Compliance with ethical standards
All procedures were in accordance with the national laws.
Acknowledgements
We thank the following colleagues who kindly sent us material for the Pilato and Binda collection: D. V. Tumanov (Saint Petesburg University, Russia) for paratypes of M. almatyense, M. antarcticum, M. asiaticum, M. longiungue and M. reductum; Ł. Kaczmarek (Adam Mickiewicz University in Poznań, Poland) and Ł. Michalczyk (Jagellonian University of Krakow, Poland) for specimens of the neotype series of M. tardigradum and paratypes of M. beasleyi; the latter colleague again, for paratypes of M. inceptum and M. alpigenum; and R. Bertolani (University of Modena and Reggio Emilia, Italy) for a specimen of M. eurystomum (formerly belonging to W. Maucci). We are also grateful to the Editor and the anonymous reviewers who improved this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bartels PJ, Nelson DR, Kaczmarek Ł, Michalczyk Ł. 2014. The genus Milnesium (Tardigrada: Eutardigrada: Milnesiidae) in the great smoky mountains national park (North Carolina and Tennessee, USA) with the description of Milnesium bohleberi sp. nov. Zootaxa 3826(2):356–368. DOI: 10.11646/zootaxa.3826.2.5.
- Beasley CW. 1995. The phylum Tardigrada. Third Edition by G Ramazzotti and W. Maucci. English Translation. Abilene, TX: Published by the translator at McMurry University. pp. 1–1014.
- Bertolani R. 1982. Tardigradi (Tardigrada). Guide per il riconoscimento delle specie animali delle acque interne italiane. CNR AQ/1/168 (15). pp. 1–104.
- Bertolani R, Grimaldi D. 2000. A new eutardigrade (Tardigrada: Milnesiidae) in amber from the Upper Cretaceous (Turonian) of New Jersey, and Grimaldi D, editor. Studies on fossils in amber, with particular reference to the cretaceous of New Jersey. Backhuys Publishers. pp. 103–110.
- Binda MG, Pilato G. 1990. Tardigradi della Terra del Fuoco e Magallanes. Milnesium brachyungue, nuova specie di tardigrado Milnesiidae. Animalia 17:105–110.
- Cesari M, Vecchi M, Palmer A, Bertolani R, Pilato G, Rebecchi L, Guidetti R. 2016. What if the claws are reduced? Morphological and molecular phylogenetic relationships of the genus Haplomacrobiotus May, 1948 (Eutardigrada, Parachela). Zoological Journal of the Linnean Society 178:819–837. DOI: 10.1111/zoj.12424.
- Ciobanu D, Roszkowska M, Kaczmarek Ł. 2015. Two new tardigrade species from Romania (Eutardigrada, Milnesiidae, Macrobiotidae) with some remarks on secondary sex characters in Milnesium dornensis sp. nov. Zootaxa 3941(4):542–564. DOI: 10.11646/zootaxa.3941.4.4.
- Ciobanu DA, Zawierucha K, Moglani I, Kaczmarek Ł. 2014. Milnesium berladnicorum sp. n. (Eutardigrada, Apochela, Milnesiidae), a new species of water bear from Romania. Zookeys 429:1–11. DOI: 10.3897/zookeys.429.7755.
- Cuénot L. 1932. Tardigrades. Faune de France 24:1–96.
- Cuénot L. 1949. Les Tardigrades, and Grassé P, editor. Traité de Zoologie. Paris. Vol. 6, pp 39–59.
- Dewel RA, Clark jr. WH. 1973. Studies on the tardigrades. II. Fine structure of the pharynx of Milnesium tardigradum Doyère. Tissue & Cell 5(1):147–159. DOI: 10.1016/s0040-8166(73)80012-6.
- Doyère LMF. 1840. Memoire sur les tardigrades. I. Annales des Sciences Naturelles, Paris S. 2(14):269–362.
- Eherenberg CG. 1853. Diagnoses novarum formarum. Verhandlungen der Königlich Preussische Akademie der Wissenschaften zu Berlin 8:526–533.
- Fleming JF, Arakawa K. 2021. Systematic of Tardigrada: A reanalysis of tardigrade taxonomy with specific reference to Guil et al. (2019). Zoologica Scripta 50:376–382. DOI: 10.1111/zsc.12476.
- Guidetti R, Altiero T, Marchioro T, Sarzi Amadè L, Avdonina AM, Bertolani R, Rebecchi L. 2012. Form and function of the feeding apparatus in Eutardigrada (Tardigrada). Zoomorphology 131:127–148. DOI: 10.1007/s00435-012-0149-0.
- Guidetti R, Schill RO, Bertolani R, Dandekar T, and Wolf M. 2009. New molecular data for tardigrade phylogeny, with the erection of Paramacrobiotus gen. nov. Journal of Zoological Systematics and Evolutionary Research 47(4):315–321. DOI:10.1111/j.1439-0469.2009.00526.x.
- Guil N, Giribet G. 2012. A comprehensive molecular phylogeny of tardigrades-adding genes and taxa to a poorly resolved phylum-level phylogeny. Cladistics 28:21–49. DOI: 10.1111/j.1096-0031.2011.00364.x.
- Guil N, Jorgensen A, Kristensen RM. 2019. An upgrated comprehensive multilocus phylogeny of the Tardigrada tree of life. Zoologica Scripta 48(1):1–18. 10.111/zsc.12321.
- Jørgensen A, Faurby S, Hansen JG, Møbierg N, Kristensen RM. 2010. Molecular phylogeny of Arthrotardigrada (Tardigrada). Molecular Phylogenetics and Evolution 54:1006–1015. DOI: 10.1016/j.ympev.2009.10.006.
- Jørgensen A, Kristensen RM. 2004. Molecular phylogeny of Tardigrada-investigation of the monophyly of Heterotardigrada. Molecular Phylogenetics and Evolution 32:666–670. DOI: 10.1016/j.ympev.2004.04.017.
- Kaczmarek Ł, Grobys D, Kulpa A, Bartylak T, Kmita H, Kepel M, Kepel A, Roszkowska M. 2019. Two new species of the genus Milnesium Doyère, 1840 (Tardigrada, Apochela, Milnesiidae) from Madagascar. ZooKeys 884:1–22. DOI: 10.3897/zookeys.884.29469.
- Kaczmarek Ł, Jakubowska N, Michalczyk Ł. 2012. Current knowledge on Turkish tardigrades with a description of Milnesium beasleyi sp. nov. (Eutardigrada: Apochela: Milnesiidae, the granulatum group). Zootaxa 3589:49–64. DOI: 10.11646/zootaxa.3589.1.3.
- Kaczmarek Ł, Michalczyk Ł. 2007. A new species of Tardigrada (Eutardigrada: Milnesiidae): Milnesium krzyzstofi from Costa Rica (Central America). New Zealand Journal of Zoology 34:297–302. DOI: 10.1080/03014220709510088.
- Kaczmarek Ł, Michalczyk Ł, Beasley CW. 2004. Milnesium katarzynae sp. nov. a new species of eutardigrade (Milnesiidae) from China. Zootaxa 743:1–5. DOI: 10.11646/zootaxa.743.1.1.
- Kinchin IM. 1994. The biology of tardigrades. London: Portland Press. pp. 1–186.
- Londoño R, Daza A, Caicedo M, Quiroga S, Kaczmarek Ł. 2015. The genus Milnesium (Eutardigrada: Minesiidae) in the Sierra Nevada de Santa Marta (Colombia), with the description of Minesium kogui sp. nov. Zootaxa 3955(4):561–568. DOI: 10.11646/zootaxa.3955.4.7.
- Marcus E. 1929. Tardigrada. In: Bronns HG, editor. Klassen und Ordnungen des Tierreichs. Vol. 5. Leipzig: Akademische Verlagsgesellschaft. pp. 1–608.
- Marley NJ, McInnes SJ, Sands CJ. 2011. Phylum Tardigrada: A re-evaluation of the Parachela. Zootaxa 2819:51–64. DOI: 10.11646/zootaxa.2819.1.2.
- Maucci W. 1991. Tre nuove specie di Eutardigradi della Groenlandia meridionale. Bollettino del Museo Civico di Storia Naturale di Verona 15:279–289.
- Meyer HA. 2015. Water bears (Phylum Tardigrada) of Oceania, with the description of a new species of Milnesium. New Zealand Journal of Zoology 1–14. DOI: 10.1080/03014223.2015.1062402.
- Meyer HA, Hinton JG. 2010. Milnesium zsalakoae and Milnesium jacobi, two new species of Tardigrada (Eutardigrada: Milnesiidae) from the southwestern USA. Proceedings of the Biological Society of Washington 123(2):113–120. DOI: 10.2988/09-29.1.
- Meyer HA, Hinton JG. 2012. Terrestrial Tardigrada of the Island of Barbados in the West Indies, with the description of Milnesium barbadosense sp. n. (Eutardigrada: Apochela: Milnesiidae). Caribbean Journal of Science 46(2–3):194–202. DOI: 10.18475/cjos.v46i2.a8.
- Meyer HA, Hinton JG, Dupré MC. 2013. Milnesium lagniappe, a new species of water bear (Tardigrada, Eutardigrada, Apochela, Milnesiidae) from the southern United States. Western North American Naturalist 73(3):295–301. DOI: 10.3398/064.073.0305.
- Michalczyk Ł, Wełnicz W, Frohme M, Kaczmarek Ł. 2012. Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3154:1–20. DOI: 10.11646/zootaxa.3154.1.1.
- Morek W, Blagden B, Kristensen RM, Michalczyk Ł. 2020a. The analysis of inter- and intrapopulation variability of Milnesium eurystomum Maucci, 1991 reveals high genetic divergence and a novel type of ontogenetic variation in the order Apochela. Systematics and Biodiversity 18(5):461–479. DOI: 10.1080/14772000.2020.1771469.
- Morek W, Ciosek CA, Michalczyk Ł. 2020b. Description of Milnesium pentapapillatum sp. nov. from Kazakhstan, with an amendment of the diagnosis of the order Apochela (Tardigrada) and abolition of the class Apotardigrada. Zoologischer Anzeiger 288:107–117. DOI: 10.1016/j.jcz.2020.07.002.
- Morek W, Gąsiorek P, Stec D, Blagden B, Michalczyk Ł. 2016. Experimental taxonomy exposes ontogenetic variability and elucidates the taxonomic value of claw configuration in Milnesium Doyère, 1840 (Tardigrada: Eutardigrada: Apochela). Contribution to Zoology 85(2):173–200. DOI: 10.1163/18759866-08502003.
- Morek W, Michalczyk Ł. 2020. First extensive multilocus phylogeny of the genus Milnesium (Tardigrada) reveals no congruence between genetic markers and morphological traits. Zoological Journal of the Linnean Society 188(3):681–693. DOI: 10.1093/zoolinnean/zlz040.
- Morek W, Stec D, Gąsiorek P, Sumarcz B, Michalczyk Ł. 2018. Milnesium tardigradum Doyère, 1840: The first integrative study of interpopulation variability in a tardigrade species. Journal of Zoological Systematics and Evolutionary Research 1–23. DOI: 10.1111/jzs.12233.
- Morek W, Surmacz B, López-López A, Michalczyk Ł. 2021. “Everything is not everywhere”: Time-calibrated phylogeography of the genus Milnesium (Tardigrada). Molecular Ecology 30:3590–3609. DOI: 10.1111/mec.15951.
- Morek W, Surmacz B, Michalczyk Ł. 2020c. Novel integrative data for two Milnesium Doyère, 1840 (Tardigrada: Apochela) species from Central Asia. Journal of Zoological Systematics and Evolutionary Research 96(2):499–514. DOI: 10.3897/zse.96.52049.
- Morek W, Suzuki AC, Schill RO, Georgiev D, Yankova M, Marley NJ, Michalczyk Ł. 2019. Redescription of Milnesium alpigenum Ehrenberg, 1853 (Tardigrada: Apochela) and a description of Milnesium inceptum sp. nov., a tardigrade laboratory model. Zootaxa 4586(1):35–64. DOI: 10.11646/zootaxa.4586.1.2.
- Moreno-Talamantes A, León-Espinosa GA, García-Aranda MA, Flores-Maldonado JJ, Kaczmarek Ł. 2020. The genus Milnesium Doyère, 1840 in Mexico with description of a new species. Annales Zoologici (Warszawa) 70(4):467–486. DOI: 10.3161/00034541ANZ2020.70.4.001.
- Moreno-Talamantes A, Rozkowska M, García-Aranda MA, Flores-Maldonado JJ, Kaczmarek Ł. 2019. Current knowledge on Mexican tardigrades with a description of Milnesium cassandrae sp. nov. (Tardigrada: Milnesiidae) and discussion on the taxonomic value of dorsal pseudoplates in the genus Milnesium. Zootaxa 4601(5):501–524. DOI: 10.11646/zootaxa.4691.5.5.
- Nelson DR, Adkins Fletcher R, Guidetti R, Roszkowska M, Grobys D, Kaczmarek Ł. 2020a. Two new species of Tardigrada from moss cushions (Grimmia sp.) in a xerothermic habitat in northeast Tennessee (USA, North America), with the first identification of males in the genus Viridiscus. PeerJ 8:e10251. DOI: 10.7717/peerj.10251.
- Nelson DR, Guidetti R, Rebecchi L, Kaczmarek Ł, McInnes S. 2020b. Phylum Tardigrada. In: Damborenea C, Rogers DC, Thorp J, editors. Thorp and Covich’s freshwater invertebrates. Vol. 5(15). New York: Academic Press, pp. 505–522. DOI: 10.1016/B978-0-12-804225-0.00015-0.
- Nichols PB, Nelson DR, Garey JR. 2006. A family level analysis of tardigrade phylogeny. Hydrobiologia 558:53–60. DOI: 10.1007/s10750-005-1414-8.
- Pilato G. 1981. Analisi di nuovi caratteri nello studio degli Eutardigradi. Animalia 8:51–57.
- Pilato G, Binda MG. 1991. Milnesium tetralamellatum, new species of Milnesiidae from Africa (Eutardigrada). Tropical Zoology 4:103–106. DOI: 10.1080/03946975.1991.10539480.
- Pilato G, Binda MG, Lisi O. 2002. Notes on tardigrades of the Seychelles with description of two new species. Bollettino Delle Sedute dell’Accademia Gioenia Di Scienze Naturali, Catania 35(261):503–517.
- Pilato G, Lisi O. 2016. Milnesium minutum and Milnesium sandrae, two new species of Milnesiidae (Tardigrada, Eutardigrada, Apochela). ZooKeys 580:1–12. DOI: 10.3897/zookeys.580.6603.
- Pilato G, Sabella G, D’Urso V, Lisi O. 2017. Two new species of Eutardigrada from Victoria Land, Antarctica. Zootaxa 4137(3):541–558. DOI: 10.11646/zootaxa.4317.3.6.
- Pilato G, Sabella G, Lisi O. 2016. Two new species of Milnesium (Tardigrada: Milnesiidae). Zootaxa 4132(4):575–587. DOI: 10.11646/zootaxa.4132.4.9.
- Ramazzotti G. 1962. Tardigradi del Cile, con descrizione di quattro nuove specie e di una nuova varietà. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano 101:275–287.
- Ramazzotti G, Maucci W. 1983. Il Phylum Tardigrada. Memorie dell’Istituto Italiano di Idrobiologia Marco De Marchi 41:1–1012.
- Richters F. 1926. Tardigrada. In: Kukenthal W, and Krumbach T, editors. Handbuch der Zoologie. Vol. 3. Berlin, Leipzig: Walter de Gruyter & Co., pp 1–68.
- Roszkowska M, Bartels PJ, Goldyn B, Ciobanu DA, Fontoura P, Michalczyk Ł, Nelson D, Ostrowska M, Moreno-Talamantes A, Kaczmarek Ł. 2016. Is the gut content of Milnesium (Eutardigrada) related to buccal tube size? Zoological Journal of the Linnean Society 178:794–803. DOI: 10.1111/zoj.12459.
- Roszkowska M, Ostrowska M, Kaczmarek Ł. 2015. The genus Milnesium Doyère, 1840 (Tardigrada) in South America with description of two new species from Argentina and discussion of the feeding behaviour in the family Milnesiidae. Zoological Studies 54:1–17. DOI: 10.1186/s40555-014-0082-7.
- Schlabach S, Donaldson E, Hobelman K, Miller WR, Lowman MD. 2018. Tardigrades of the Canopy: Milnesium burgessi nov. sp. (Eutardigrada: Apochela: Milnesiidae) a new species from Kansas. Transactions of the Kansas Academy of Science 121(1–2):39–48. DOI: 10.1660/062.121.0204.
- Schultz J. 2000. Handbuch der Ökozonen. Stuttgart: Eugen Ulmer GmbH & Co. pp. 1–577. DOI: 10.1002/mmnz.20040800119.
- Schuster RO, Nelson DR, Grigarik AA, Cristenberry D. 1980. Systematic criteria of the Eutardigrada. Transactions of the American Society of Microscopical Society 99(3):284–303. DOI: 10.2307/3226004.
- Sugiura K, Minato H, Matsumoto M, Suzuki AC. 2020. Milnesium (Tardigrada: Apochela) in Japan: The first confirmed record of Milnesium tardigradum s.s. and description of Milnesium pacificum sp. nov. Zoological Science 37(5):476–495. DOI: 10.2108/zs190154.
- Surmacz B, Morek W, Michalczyk Ł. 2019. What if multiple claw configurations are present in a sample? A case study with the description of Milnesium pseudotardigradum sp. nov. (Tardigrade with unique developmental variability). Zoological Studies 58(32):1–15. DOI: 10.6620/ZS.2019.58-32.
- Thulin G. 1928. Über die Phylogenie und das System der Tardigraden. Hereditas 11: 207–266.
- Tumanov DV. 2006. Five new species of the genus Milnesium (Tardigrada, Eutardigrada, Milnesiidae). Zootaxa 1122:1–23. DOI: 10.11646/zootaxa.1122.1.1.
- Udvardy MDF. 1975. A classification of the biogeographical provinces of the world. IUCN Occasional Paper, 18. Morges, Switzerland: IUCN, pp. 1–48.
- Wallendorf M, Miller WR. 2009. Tardigrades of North America: Milnesium alabamae nov. sp. (Eutardigrada: Apochela: Milnesiidae) a new species from Alabama. Transactions of the Kansas Academy of Science 112(3–4):181–186. DOI: 10.1660/062.112.0404.
- Wełnicz W, Grohme MA, Kaczmarek Ł, Schill RO, Frohme M. 2011. ITS-2 and 18S rRNA data from Macrobiotus polonicus and Milnesium tardigradum (Eutardigrada, Tardigrada). Journal of Zoological Systematics and Evolutionary Research 49(Suppl. 1):34–39. DOI: 10.1111/j.1439-0469.2010.00595.
- Yang T. 2003. Two new species and three new records of the Tardigrada (Heterotardigrada, Echiniscidae; Eutardigrada, Milnesiidae, Macrobiotidae, Hypsibiidae). Acta Zootaxonomica Sinica 28(2):235–240.
- Young A, Chappell B, Miller W, Lowman M. 2016. Tardigrade of the three Canopy: Milnesium swansoni sp. nov. (Eutardigrada: Apochela: Milnesiidae) a new species from Kansas, U.S.A. Zootaxa 4072(5):559–568. DOI: 10.11646/zootaxa.4072.5.3.
