Abstract
The occurrence, relative abundance and bathymetric distribution of the deep Mediterranean heterobranch fauna were evaluated as a result of an extensive visual census down to 1825 m depth, carried out along the western Italian coasts, covering a latitudinal range of about 600 nautical miles. Observations were conducted using Remotely Operated Vehicles (ROVs) in 551 sites, focusing on the deepest part of the continental shelf, the shelf edge and the upper bathyal zone. The analysis of over 508 hours of video footage and more than 27,000 high-resolution photographs allowed to explore about 594,000 m2 of rocky outcrops and nearby soft seafloors in four main coastal areas (Ligurian Sea, north-central Tyrrhenian Sea, southern Tyrrhenian Sea, and Sicily Channel) and twelve offshore seamounts. Thirty-six species of heterobranchs, for a total of 559 records, were identified. Sixteen were only sporadically spotted (< 1% of the total observations), while three (Paraflabellina ischitana, Tritoniidae nd and Peltodoris atromaculata) contributed each for more than 10% of the records. An extension of the known Mediterranean bathymetric distribution was reported for 80% of the observed species, with many typically shallow-water taxa being also found in mesophotic environments (40–200 m). The observed marked decrease in diversity and abundance suggested that Mediterranean heterobranchs are mainly a coastal benthic group. From the geographical point of view, the heterobranch fauna present in the Ligurian Sea appeared significantly different from that observed in the Tyrrhenian Sea and the Sicily Channel, and this latitudinal pattern was discussed. Particular attention was given to single out the bathymetric distribution of the food sources of the most frequently observed species, supporting stenophagy as a limiting factor for their colonisation of the deep sea.
Introduction
The Mediterranean malacofauna is considered one of the best-known in the world (Oliverio Citation2003). In particular, Heterobranchia (Mollusca, Gastropoda) have been significantly studied in the last 50 years, mainly thanks to the advent of scientific SCUBA diving, which has led to a profusion of studies improving our understanding of the species richness, ecology, phylogeny and geographic distribution (e.g., Vicente Citation1967; Schmekel & Portmann Citation1982; Thompson et al. Citation1990; Templado & Villanueva Citation2010; Trainito & Doneddu Citation2014; Furfaro & Mariottini Citation2016; Zenetos et al. Citation2016; Betti et al. Citation2017). Up to date, more than 600 species of heterobranchs have been described from the Mediterranean Sea (Trainito & Doneddu Citation2014; Parera et al. Citation2020). Nevertheless, the available information is skewed towards SCUBA diving depth range (from the surface to around 40 m), while very little is known about the composition and distribution of the heterobranch fauna living in the deepest part of the Mediterranean continental shelf, shelf edge and slope, down to the bathyal habitat.
Most of the available data on deep Mediterranean heterobranchs targets the soft-bottom fauna of the North-West Mediterranean Sea, studied mainly thanks to catches made by destructive gears, such as dredges and trawling nets. Vicente (Citation1967) reported 21 species between 45 and 450 m inside the Gulf of Marseille (France), scattered on detritic, maërl and mud, with only the cephalaspidean Philine monterosati Monterosato, Citation1874 found below 120 m. In an extensive survey off the coasts of Blanes (Spain), Ros (Citation1975) reported 34 heterobranch species from 40 to 250 m, observed on detritic, sand and mud. This faunal census was later updated to 47 species by Domènech et al. (Citation2006), who provided qualitative information on the frequency of occurrence of species found between 50 and 300 m in the same area, indicating the cephalaspideans Gastropteron rubrum (Rafinesque, 1814) and Scaphander lignarius Linnaeus, 1758 and the nudibranch Tethys fimbria Linneus, 1767 as the most frequent deep taxa. Bouchet (Citation1977) reported two bathyal nudibranch species (Baptodoris cinnabarina Bergh, 1884 and Doris alboranica Bouchet, Citation1977) from the Alboran Sea (500–910 m). Cattaneo-Vietti (Citation1991) recorded 32 species, mostly observed sporadically, from the trawling grounds of the continental shelf and slope of the Ligurian and northern Tyrrhenian seas down to 700 m. Among the most widespread species, there were: Philine quadripartita Ascanius, 1772 and T. fimbria, mainly linked to the terrigenous mud biocoenosis, Pleurobranchaea meckeli (Blainville, 1825) and Doris pseudoargus Rapp, 1827 in the coastal detritic one, while Pleurobranchus membranaceus (Montagu, 1816) and Kaloplocamus ramosus (Cantraine, 1835) frequently appeared in the shelf-edge detritic biocoenosis, characterised by the crinoid Leptometra phalangium (Müller, 1841) and by the seapen Funiculina quadrangularis (Pallas, 1766). Scaphander lignarius was also commonly collected, from coastal detritic to bathyal mud. Finally, B. cinnabarina was found in the bathyal mud biocenosis of the slope, feeding on the sponge Thenea muricata (Bowerbank, 1858) (Bouchet Citation1977; Cattaneo-Vietti Citation1991). Lastly, a survey conducted in the Cretan Sea (eastern Mediterranean basin) reported nine species living on soft bottoms between 40 and 700 m (Koutsoubas et al. Citation2000). These included some new findings for this habitat, such as the acteonid Crenilabium exile (Jeffreys, 1870), the cephalaspidean Roxaniella jeffreysi (Weinkauff, 1866) and the sacoglossan Ascobulla fragilis (Jeffreys, 1856).
On the other hand, information on the Mediterranean heterobranch fauna of mesophotic and bathyal rocky environments is scarce. Recently, Tritonia callogorgiae Chimienti, Furfaro and Taviani, 2020, living on the deep alcyonacean Callogorgia verticillata (Pallas, 1766), was described on the basis of specimens photographed and collected by Remotely Operated Vehicle (ROV) at 420–430 m depth, off the Montenegro margin (Chimienti et al. Citation2020). Overall, deep data mainly come from reports of technical divers exploring the upper mesophotic range, but are often based on fortuitous findings, focused on restricted areas, and do not allow for a comprehensive interpretation of the ecology and biogeography of the species.
In the last fifteen years, several ROV surveys were carried out along a large part of the Italian coasts, from the Ligurian Sea to the Sicily Channel, targeting the characterisation of the megabenthic biocoenoses dominated by structuring species thriving between 30 and 1825 m on hardgrounds and nearby soft seafloors (e.g., Bo et al. Citation2012, Citation2021; Cau et al. Citation2015; Angiolillo et al. Citation2016, Citation2021; Altobelli et al. Citation2017; Enrichetti et al. Citation2019; Moccia et al. Citation2021). Such habitats constitute Vulnerable Marine Ecosystems (VMEs) (Del Mar Otero & Marin Citation2019), attracting a rich faunal biodiversity; however, the associated macrofauna was never specifically targeted. These ROV datasets gave a chance, for the first time, to assess the large-scale bathymetric and geographic distribution of a significant number of mesophotic and bathyal Mediterranean heterobranchs and investigate the ecological traits affecting deep-sea colonisation.
Materials and methods
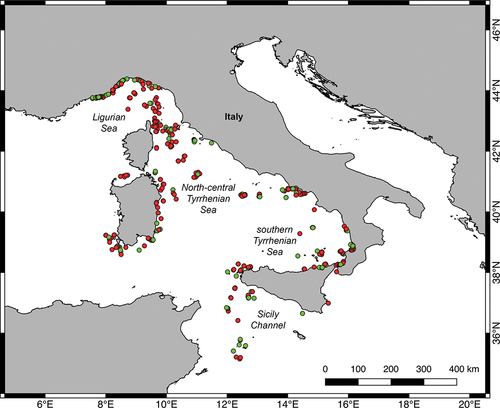
In total, 551 ROV videos, each corresponding to a different dive and locality, were examined to analyse the large-scale distribution of the heterobranchs present on the rocky bottoms (and soft-bottom surroundings) of the mesophotic and bathyal zone. In total, 502 ROV footages were obtained from explorative campaigns conducted in four Mediterranean coastal areas (Ligurian Sea, north-central Tyrrhenian Sea, southern Tyrrhenian Sea and Sicily Channel), between 30 and 775 m depth (). In addition, 12 offshore seamounts, six in the Ligurian basin (namely Penelope, Ulisse, Janua, Spinola, Occhiali and Santa Lucia), four in the central Tyrrhenian Sea (Vercelli, Cialdi, Etruschi and Baronie), and two in the southern Tyrrhenian Sea (Palinuro and Marsili) were explored thanks to 49 ROV dives between 60 and 1825 m depth (). Overall, the ROV tracks covered about 594,000 m2 of the seafloor for over 508 h of video footage. Besides, more than 27,000 photos taken by high-resolution cameras were analysed, helping identify the smallest and cryptic specimens. Surveys were conducted over several years (2007–2019).
Figure 1. QGIS map of the study area with location of the ROV dives considered in this work. Dives with at least one record of heterobranchs are indicated by green dots, otherwise, they are indicated by red dots.
Five depth ranges (30–50 m; 51–100 m; 101–150 m; 151–200 m; >201 m) were defined to depict the bathymetric distribution patterns of the species. Dives extending for a broad depth range (more than 50 m) were divided into parts according to the identified ranges so that the final considered number was 883 (817 for coastal regions and 66 for seamounts).
ROV footage was analysed using Apple’s Final Cut Pro X software. The analysis allowed recording heterobranchs larger than 2 cm, identified by external morphological features at the lowest possible taxonomic level, thanks to field guides and specialistic scientific literature. The currently valid scientific species names were checked on the World Register of Marine Species (WORMS). The number of individuals has been counted for each species, and the exact depth at which they were found was reported. Quantitative data about specimens were normalised as the number of individuals per 100 dives for each large geographic area and depth range. Putative food sources (identified based on the literature and the position of the molluscs) were also reported together with any other ecological note (e.g., type of habitat, phenotype, mating events and presence of eggs). The putative associations were explored for four species or groups of species through linear regressions considering the frequency of occurrence (per 100 dives) of the food sources and their consumers.
Significant differences in the percentage diversity between depth ranges were investigated with a Kruskal–Wallis test (data not normally distributed, n = 37 for each depth range considering 50–100 m, 101–200 m and > 201 m). In order to verify potential differences among the species according to geographic distribution and depth, the whole dataset (expressed as the number of specimens per 100 dives, n = 5 per each species per each depth range) was square-root transformed to underweight abundant taxa and analysed by Principal Component Analysis (PCA) (Bray–Curtis similarity). In order to test for significant differences in the patterns of relative abundance of heterobranchs (total, cladobranchs and dorids, separately) in the four considered macro-areas (LS, Ligurian Sea; NCT, north-central Tyrrhenian Sea; ST, southern Tyrrhenian Sea; SC, Sicily Channel) at the different depth ranges (30–50 m, 51–100 m, 101–150 m, 151–200 m, > 201 m), a two-way PERMANOVA (Bray–Curtis similarity) was performed on the whole dataset (expressed as the number of specimens per dives, n = 7–114 per depth range).
Analyses were carried out with PAST Statistics Software® (Hammer et al. Citation2001).
Results
Diversity and occurrence of the heterobranch fauna
The heterobranch fauna recorded in the analysed ROV footage accounted for 559 specimens identified in 28 genera and 36 species (
Table I. List of the heterobranch species found in this study. Number of specimens recorded per depth range and geographic area is given. LS, Ligurian Sea; NCT, north-central Tyrrhenian Sea; ST, southern Tyrrhenian Sea; SC, Sicily Channel
Table II. Sampling location for each recorded species in the twelve explored seamounts. The numbers in parenthesis are the depth (in m) of each record. LS, Ligurian Sea; NCT, north-central Tyrrhenian Sea; ST, southern Tyrrhenian Sea. Seamounts: Ul, Ulisse; Pe, Penelope; Ja, Janua; Oc, Occhiali; Sp, Spinola; St. L, St. Lucia; Ve, Vercelli; Ba, Baronie; Ci, Cialdi; Et, Etruschi; Pa, Palinuro; Ma, Marsili
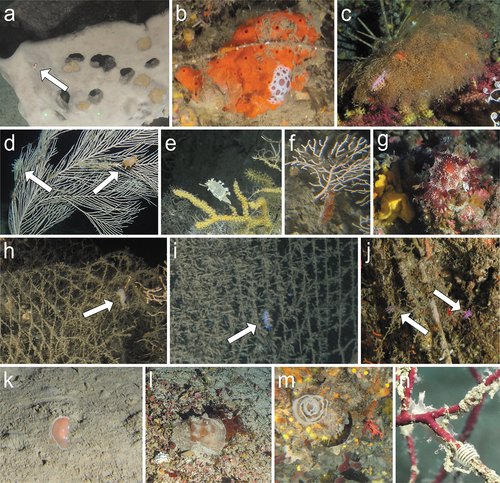
Figure 2. In situ ROV images of the 20 most represented species. (a) Thuridilla hopei on a bed of green algae in the coralligenous (Ponza, 75 m); (b) Umbraculum umbraculum on a silted rocky bank on the shelf edge (Graham Bank, 88 m); (c) Pleurobranchus testudinarius in a cavity of a rocky outcrop (Lampedusa, 108 m); (d) Fjordia lineata feeding on an hydrozoan growing on a heavily silted rocky outcrop of the shelf edge (Genova Portofino, 100 m); (e) Caloria elegans feeding on opportunistic hydrozoans growing on a dead gorgonian on a silted bank (Gulf of Naples, 110 m); (f) Facelina sp. on a silted offshore rocky outcrop (St. Eufemia, 122 m); (g) Pruvotfolia pselliotes crawling on mud (Bordighera Canyon, 50 m); (h) Flabellina affinis moving on a rich coralligenous bed (Ponza, 76 m); (i) Paraflabellina ischitana feeding on Eudendrium sp. within a red coral forest (SW Sardinia, 85 m); (j) Antiopella cristata feeding on the bryozoan Reteporella spp. (Tyrrhenian Calabria, 75 m); (k) two specimens of Luisella babai mating on detric mud (Patti Gulf, 82 m); (l) Tritonia callogorgiae feeding on the anthozoan Callogorgia verticillata in a bathyal environment dominated by fossil hard corals (Ulisse Seamount, 500 m); (m) Diaphorodoris alba moving in a coralligenous environment (SW Sardinia, 85 m); (n) two specimens of Felimare orsinii mating on a keratose sponge (Savona, 45 m); (o) a large specimen of Felimare picta moving on a sponge bed (Imperia, 65 m); (p) two specimens of Felimare tricolor feeding on a keratose sponge (Genova Punta Manara, 65 m); (q) Felimare cf. villafranca crawling on a silted rocky bed on the outer shelf (Tuscan Archipelago, 75 m); (r) Felimida luteorosea moving in the understory of a gorgonian forest on a silted offshore rocky outcrop (St. Eufemia, 83 m); (s) Felimida purpurea on an encrusting sponge of a rocky outcrop (Gulf of Naples, 80 m); (t) a large specimen of Peltodoris atromaculata in a deep coralligenous environment (Egadi Archipelago, 57 m).

All the taxa identified at the species level were previously reported in Mediterranean waters. Following the classification proposed by Bianchi et al. (Citation2012), the biogeographic origin of the studied fauna is as follows: 59% of the taxa are Atlantic-Mediterranean species, 25% are (sub)tropical Atlantic, 13% are Boreo-Atlantic, while one species is cosmopolitan.
Bathymetric distribution of the heterobranch fauna
Heterobranch records were made between 30 and 500 m, with the shallowest finding being Peltodoris atromaculata and the deepest Tritoniidae nd.
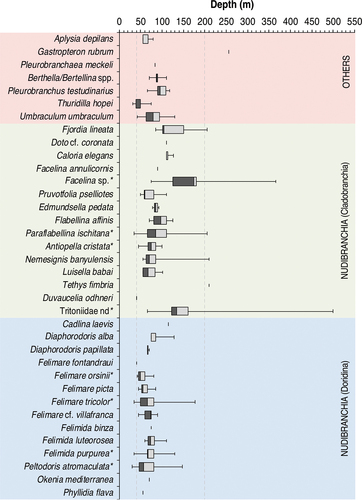
The highest diversity was observed between 30 and 100 m depth (86.5% of the recorded species). This datum significantly decreased in the depth range 101–200 m (51.4%) and dropped at greater depths (18.9%) (Kruskal–Wallis, H = 25.13, Hc = 33.57, p < 0.001). The species recorded below the mesophotic zone (>200 m) were G. rubrum and the cladobranchs Fjordia lineata (Lovén, 1846), Facelina sp., P. ischitana, Nemesignis banyulensis (Portmann & Sandmeier, 1960), T. fimbria and Tritoniidae nd (). Among these taxa, G. rubrum and T. fimbria were exclusively found in the deepest depth range, even if with a single record each. On the other hand, P. ischitana and N. banyulensis were mainly observed in the 50–100 m depth range, F. lineata in the 100–200 m, while Facelina sp. and Tritoniidae nd were consistently present below 200 m. No dorids were observed below 200 m ().
Figure 3. Bathymetric box plots of the 36 heterobranch species observed in this study. Dash lines indicate the limits of the mesophotic zone (40–200 m). * refers to the most commonly recorded species.
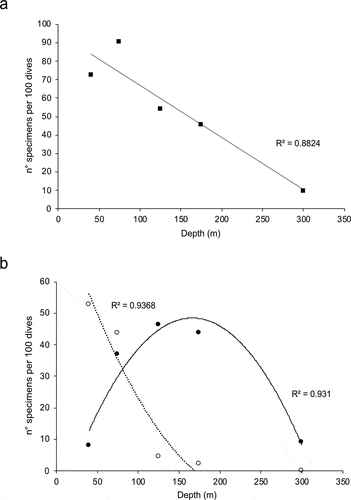
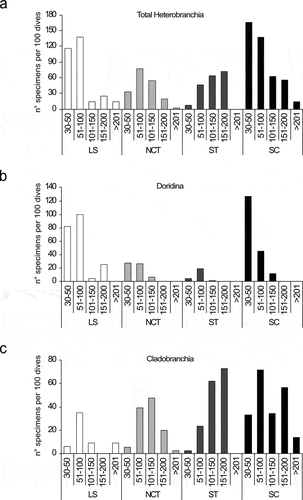
The trend of relative abundances of all recorded specimens (n° specimens/100 dives) showed a marked decrease with depth ()). This trend was particularly evident for dorids, while cladobranchs showed a mesophotic peak of abundance ()).
Geographic distribution of the heterobranch fauna
Heterobranchs were reported in 25% of the total dives, with the highest percentage frequency of occurrence in the Ligurian Sea and Sicily Channel (33% of the total dives), followed by the north-central Tyrrenian Sea (22%), southern Tyrrhenian Sea (20%) and seamounts (15%).
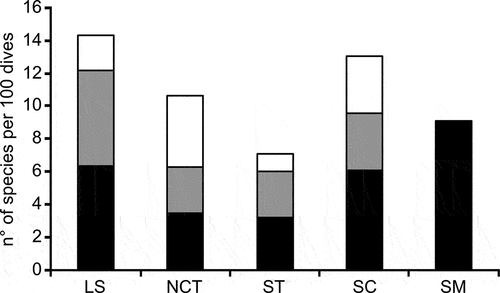
The expected species diversity percentage (number of species per 100 dives) decreased according to latitude from the Ligurian basin (14 species), to the southern Tyrrhenian Sea (7 species), with a second peak in the Sicily Channel (13 species) (). The ratio between the taxa of the two Nudibranchia suborders was balanced in the Ligurian and Tyrrhenian seas (6:6, 3:3, 3:3, for Cladobranchia and Doridina, respectively). On the other hand, the Cladobranchia were dominant in the Sicily Channel (6:3). The fauna of seamounts was composed of only six species, all cladobranchs (; ).
Figure 5. Expected number of species (per 100 dives) of Cladobranchia (black), Doridina (grey) and other Heterobranchia (white) according to the considered macro-areas: LS, Ligurian Sea; NCT, north-central Tyrrhenian Sea; ST, southern Tyrrhenian Sea; SC, Sicily Channel; SM, seamounts.
The four coastal assemblages showed differences also in terms of relative abundance. In the PCA analysis, the first two axes explained 54% of the total variability observed in the dataset (expressed as the number of specimens per 100 dives) (C1: 36.2%, C2: 18.7%) (). Based on this analysis, depth played a significant role in distinguishing regional groups. The Ligurian area is distinct from the north-central and southern Tyrrhenian and Sicily Channel areas. The biplot showed that a relatively large number of taxa, including most Felimare species, P. ischitana and P. atromaculata drove the mesophotic faunas, while Tritoniidae nd and Facelina sp. drove the deeper ones.
Figure 6. PCA analysis of the species composition and frequency dataset (based on the number of specimens per 100 dives) of the heterobranch assemblages of the four main considered macro-areas (red, Ligurian Sea; blue, north-central Tyrrhenian Sea; grey, southern Tyrrhenian Sea; Orange, Sicily Channel) at all depth ranges (from right to left on C1 axis).
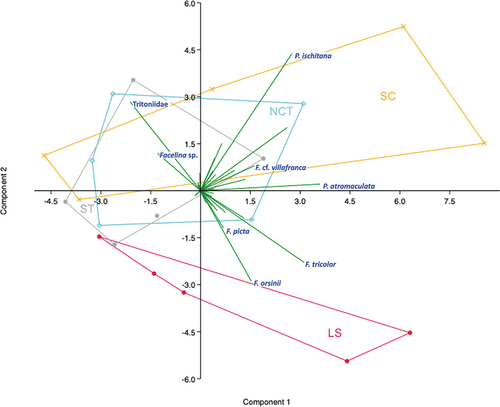
The Ligurian heterobranch fauna showed, indeed, some peculiarities. The cladobranchs Facelina sp., P. ischitana and Tritoniidae nd, elsewhere relatively frequent, were rare in the Ligurian dataset. On the contrary, Luisella babai (Schmekel, 1872) and the sacoglossan T. hopei were commoner sightings. Regarding dorids, F. orsinii and Felimare picta (Philippi, 1836) were observed only in the Ligurian Sea, while F. tricolor was predominant in this area, and F. purpurea was more rarely reported ().
Latitude and depth significantly contributed to the differences observed in the relative abundance patterns of the heterobranch fauna (). In particular, a marked decrease was observed between the shallower depth ranges (30–100 m) and the deeper ones (>100 m) in the Ligurian Sea and the Sicily Channel ()), explained by significantly higher abundances of dorids above 100 m (), ). Cladobranchs, instead, mainly contributed to the mesophotic and deep faunas of the Tyrrhenian regions and Sicily Channel (), ).
Table III. Results of the statistical analyses
Figure 7. Bathymetric and geographical patterns of relative abundance (expressed as the number of specimens per 100 dives) of (a) total Heterobranchia, (b) Doridina, and (c) Cladobranchia.
Overall, not many records were made on the seamounts. Heterobranchs were observed in half of the sites, with a total of 17 specimens (). Six species were identified: A. cristata was the solely reported above 100 m depth (Vercelli Seamount, 70 m depth), while other four cladobranchs (F. lineata, Facelina sp., P. ischitana and N. banyulensis) were found between 100 and 210 m, and Tritoniidae nd was observed down to 460–500 m (Penelope and Ulisse seamounts), being the deepest records of this taxon ().
Ecological remarks on mesophotic and deep-sea heterobranchs
Almost all sightings of heterobranchs were made in environments characterised by coralligenous formations (48% of the total observations) and rocky hardgrounds (46%) (these latter including large boulders and silted outcrops on the outer shelf and upper slope, and dead coral frameworks, especially on bathyal seamounts). The remaining records were made in detritic environments (4%) (including also rhodolith beds) and soft bottoms (2%) (mud and sand), both mainly found in the proximity of the hardgrounds (, ). Dorids contributed to 53% of the total records in coralligenous habitats, while cladobranchs were responsible for 67% of the records on rocky outcrops and 68% of the total occurrences on detritic. Other Heterobranchia, instead, contributed for 54% of the records on soft bottoms.
Table IV. Ecological observations of the heterobranchs target of this study. Legend to habitats (listed in order of frequency for each species): R, rocky bottom; C, coralligenous; S, soft bottom; D, detritic
Figure 8. Ecological observations based on ROV footage. (a) Felimare tricolor on the keratose sponge Spongia lamella (Schultze, 1879) (Savona, 80 m); (b) Peltodoris atromaculata feeding on the demosponge Haliclona fulva (Spotorno, 57 m); (c) Paraflabellina ischitana feeding on the hydrozoan Eudendrium cf. racemosum (Pantelleria, 102 m); (d-g) various phenotypes of Tritoniidae nd on the alcyonaceans Callogorgia verticillata (d, S. Eufemia, 122 m, probably Tritonia callogorgiae), Acanthogorgia hirsuta (e), Graham Bank 166 m, probably T. callogorgiae), Eunicella cavolini (f), Gulf of Naples, 115 m, probably Marionia blainvillea), and in the coralligenous environment (g), Cetraro, 65 m, probably M. blainvillea); Facelina sp. (h), Gulf of Naples, 125 m), P. ischitana (i–j), Gulf of Naples, 130 m), and Flabellina affinis (j), SW Sardinia, 115 m) crawling on lost fishing gear covered in hydrozoans; (k) a swimming specimen of Gastropteron rubrum (Bisagno Canyon, 256 m); (l) two mating Pleurobranchus testudinarius on a rhodolith bed (Lampedusa, 95 m); (m) egg ribbon of P. atromaculata (Spotorno, 57 m); (n) Egg mass of Duvaucelia odhneri on the alcyonacean Leptogorgia sarmentosa (Capo Mortola, 40 m).
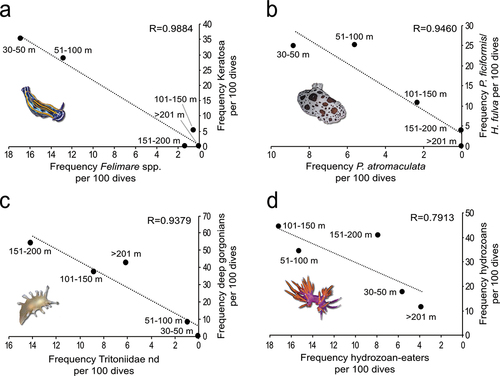
About 47% of the sightings were represented by the heterobranchs on exclusive or recurrent organisms, which could be identified as possible food sources (). The sacoglossan T. hopei was strictly linked to shallow-water photosynthetic algal communities ()). Antiopella cristata was observed on the bryozoans Reteporella spp. ()), while Duvaucelia odhneri Tardy, 1963 was observed on the gorgonian Leptogorgia sarmentosa (Esper, 1791), and U. umbraculum was three times observed feeding on massive white sponges ()). However, most of the associations regarded four taxa or closely related groups of species, whose relation to the presence of their putative food sources with depth was explored (). The strongest correlation was found between the dorids Felimare spp. and keratose sponges, peaking between 30 and 100 m and almost disappearing below 130 m (), 9(a)). Similarly, the dorid P. atromaculata showed an overlap in the occurrence of its food sources, the sponges Petrosia ficiformis (Poiret, 1789) and Haliclona fulva (Topsent, 1893), both disappearing below the shelf edge (), 9(b)). The occurrence of the cladobranch Tritoniidae nd was linked to the presence of deep-sea gorgonians, especially below 100 m ( (d)-(g), 9(c)). The specimens were observed feeding on a large variety of anthozoans, including C. verticillata (46% of the observed associations), Acanthogorgia hirsuta Gray, 1857 (25%), Bebryce mollis Philippi, 1842 (12%), and, to a lesser extent, Eunicella cavolini (Koch, 1887) (7%), Villogorgia bebrycoides (Koch, 1887) (7%), and Paramuricea clavata (Risso, 1826) (3%). No strong relationship was observed between the hydrozoan-eater cladobranchs (belonging to the families Facelinidae, Flabellinidae, and, less represented, Coryphellidae, Myrrhinidae and Samlidae) and large colonial hydrozoans (such as Plumularoidea, Sertularioidea, Bougainviillidae, Eudendriidae, Haleciidae, Lafoeidae, Campanulariidae) (), 9(d)). About 20% of these heterobranchs were found crawling on lost fishing gear, feeding on hydrozoans colonising nets and lines ( (h)–(j)). The species most commonly observed in these cases were Facelina sp. and P. ischitana. This latter, as well as Flabellina affinis (Gmelin, 1791) and Caloria elegans (Alder & Hancock, 1845), were also observed on hydrozoans colonising dead gorgonians ()).
Figure 9. Relationships between heterobranchs and their food sources explored by linear regressions based on the frequency of occurrence per 100 dives of: (a) Felimare spp. (including Felimare fontandraui, Felimare orsinii, Felimare picta, Felimare tricolor, and Felimare cf. villafranca) and keratose sponges (including Cacospongia mollior Schmidt, 1862, Dysidea spp.,Ircinia spp., Sarcotragus spp., and Spongia spp.), (b) Peltodoris atromaculata and the sponges Petrosia ficiformis and Haliclona fulva, (c) Tritoniidae nd and the deep alcyonaceans Callogorgia verticillata, Acanthogorgia hirsuta, and Bebryce mollis and (d) hydrozoan-eaters cladobranchs (including Caloria elegans, Edmundsella pedata, Facelina annulicornis, Facelina sp., Fjordia lineata, Flabellina affinis, Luisella babai, Nemesignis banyulensis, Paraflabellina ischitana, and Pruvotfolia pselliotes) and arborescent hydrozoans.
Considering soft-bottom species, the scattered records regarding the cephalaspidean G. rubrum, the pleurobranchids P. meckeli and Pleurobranchus testudinarius Cantraine, 1835, and the nudibranch T. fimbria provided no evidence of feeding activity ( (k)–(l)).
The 883 dives covered all months except for March and December; however, observations were mainly concentrated from June to September, accounting for 79% of the total dives. The summer observations included most of the dives with sightings of heterobranchs (about 87%) (). Among the species with the highest number of records, P. atromaculata, P. ischitana and Tritoniidae nd were also observed during spring (May) and autumn (October), with one winter record (January) for the latter species (370 m). During summer, egg ribbons were observed near specimens of U. umbraculum, C. elegans, D. odhneri, Tritoniidae nd, Felimare cf. villafranca (Risso, 1818), and P. atromaculata ( (m, n), ). Finally, F. orsinii, L. babai and P. testudinarius were observed during mating between June and November ().
Discussion
Deep-sea heterobranchs have received relatively poor attention compared with their coastal counterpart, particularly those taxa that do not possess a shell remaining in the sediments or that live on hardgrounds unsuitable for traditional destructive sampling gear. These latter species, however, generally present a size compatible with the modern ROV image resolution; therefore, their distribution and ecology could be investigated with targeted studies. So far, only a handful of papers specifically dealt with this group for taxonomic or ecological purposes, in the Atlantic and Pacific oceans down to 2300 m (NRDC, Citation2014; Valdés et al. Citation2018; Zhang & Zhang Citation2021) and in the Mediterranean Sea down to 420 m (Chimienti et al. Citation2020).
This study enriches the knowledge on deep hard bottom Mediterranean heterobranchs with a novel large-scale approach that includes a bathymetric range embracing the mesophotic and upper bathyal zone and a latitudinal transect of about 600 nautical miles touching all western coasts of Italy and some offshore locations.
ROV technology does not allow for a comprehensive evaluation of the diversity of the subclass Heterobranchia. It misses part of the diversity of this group, especially burrowing taxa with external shells found in sediments (e.g., Acteonoidea, Pyramidelloidea, Valvatoidea) (e.g., Monterosato Citation1874, Citation1890; Jeffreys Citation1882; Smriglio & Mariottini Citation1996; Bogi & Galil Citation2004, Citation2013). In addition, regarding epifaunal species, it is limited in the observation of very small specimens and in the detection of fine external morphological features. Finally, it is not efficient in the collection of a large number of individuals for anatomical studies. Nevertheless, numerous heterobranchs belonging to the taxa considered in this study (Sacoglossa, Umbraculida, Aplysiida, Cephalaspidea, Pleurobranchida, and Nudibranchia) display morphological and ecological traits detectable by ROV and sufficient for a reasonably good identification. As a result, 36 species were identified in this analysis, of which 13 adds up to the known deep fauna of the basin, previously accounting for 59 taxa found in mesophotic and bathyal environments (40–910 m) (e.g., Vicente Citation1967; Ros Citation1975; Bouchet Citation1977; Cattaneo-Vietti Citation1991; Koutsoubas et al. Citation2000; Domènech et al. Citation2006) (). This result supports the use of ROV, despite its limitations, as an effective tool to characterise deep macrofauna, especially on hardgrounds that are unsuitable for other sampling devices.
Table V. Literature (black) and updated (red) bathymetric distribution of the heterobranch species (referring to most taxa of the former “Opistobranchia” group) known to live in mesophotic and bathyal environments of the Mediterranean Sea (73 total species). Literature data include Vicente (Citation1967), Bouchet (Citation1977), Cattaneo-Vietti (Citation1991), Koutsoubas et al. Citation2000, Trainito and Doneddu, Citation2014, OPK website, and present study. Only data reporting depths of living organisms were considered. Legend: H, hardgrounds, S, soft bottoms; *, species found in the present study; °, species reported here for the first time in the mesophotic/bathyal depth range.The dashed line before the first value indicates that the species is present from near the surface at an unspecified depth. Grey data refer to the wide depth ranges reported in Ros (Citation1975) and Domènech et al. (Citation2006)
Among the most abundant taxa, only two were considered challenging in terms of taxonomic identification. Facelina sp. is a 3 cm long cladobranch strongly resembling the recently re-established shallow-water taxon Facelina vicina (Bergh, 1882), for long identified as Facelina bostoniensis (Couthouy, 1838) (Carmona Citation2020). With respect to shallow-water specimens, it is paler, as also reported by Mastrototaro et al. (Citation2017) from bathyal muds (473–552 m) off Ibiza (Balearic Sea). Its collection is mandatory to clarify its identification, possibly as a new species.
Tritoniidae nd represented the second challenging case in terms of identification. Of the 69 records, five could be attributed to Marionia blainvillea (Risso, 1818) mainly due to their dark red colouration and common occurrence in coralligenous environments (65–155 m) associated with E. cavolini and P. clavata, as previously reported in the literature (McDonald & Nybakken Citation1999; Ponti et al. Citation2016). Half of the remaining records could be attributed to T. callogorgiae due to their generally lighter colouration (from white to yellow, to orange) and the fact that they were associated with the alcyonacean C. verticillata in bathyal habitats as described (Chimienti et al. Citation2020). However, the other records included specimens with overlapping characteristics. Overall, the impossibility of verifying the anatomical characteristics distinguishing the two taxa and the similar morphology in the ROV footage does not allow for a conclusive identification (hence Tritoniidae nd), especially in the putative overlapping depth range. If the records of T. callogorgiae are correct, then the known bathymetric distribution of this species should be extended (110–500 m), and its putative diet should also include five other gorgonians, both shallow and deep ones. Species identification and genus-level taxonomic assignments of Family Tritoniidae are still debated and under investigation (Korshunova & Martynov Citation2020; Moles et al. Citation2021); a revision of Mediterranean Tritoniidae, including a study of the type material of M. blainvillea, is necessary to disentangle the distinction between these two species.
The heterobranch fauna reported in this study is mainly constituted by Atlantic-Mediterranean species usually found in shallow-water coralligenous biocoenoses (e.g., Ballesteros Citation2006; Furfaro & Mariottini Citation2016; Trainito & Doneddu Citation2016). Although a bias exists due to the lack of precise depth locations of the findings in many literature sources, the observations provided here extend the known bathymetric distribution of 29 of the recorded species (). This result supports the ability of some Mediterranean species, traditionally considered of shallow waters (0–40 m), to colonise at least the upper mesophotic depth range (40–80 m), with a conspicuous number also extending in the lower mesophotic (80–200 m). Due to the progressive decrease in the availability of hardgrounds with depth, these species can be found on the scattered roches du large (sensu Pérès & Picard Citation1964; Bo et al. Citation2012; Montefalcone et al. Citation2021) of the outer shelf and shelf break, but may also be found in offshore coarse detritic environments, including rhodolith and maërl beds (Vicente Citation1967). The ability of hard-bottom heterobranchs to colonise unconsolidated, coarse sediments, where they feed on small opportunistic sessile species, has also been suggested in shallow-water environments (Betti et al. Citation2017). As a response to the changing habitat, an impoverishment of the diversity and abundance of heterobranchs is observed according to the bathymetric gradient, although with some differences between the two main Nudibranchia suborders. The dorids are driven by a higher abundance of upper mesophotic species such as Felimare spp., while the cladobranchs are mainly represented by species with a lower mesophotic peak of occurrence, such as P. ischitana, F. lineata, Facelina sp. and Tritoniidae nd. The most typical bathyal species in the Mediterranean basin (>200 m) occupy detritic or muddy bottoms following a higher availability of these habitats (Vicente Citation1967; Cattaneo-Vietti Citation1991). Nonetheless, most of these species are eurybathic (), with only T. callogorgiae, among the hard-bottom species, showing a clear specialisation for deep habitats, probably driven by a diet based on anthozoans forming forests on the mesophotic and bathyal hardgrounds (Bo et al. Citation2012; Angeletti et al. Citation2014; Chimienti et al. Citation2020). As observed for numerous other deep marine animal taxa (Bianchi et al. Citation2012), various heterobranchs extending in the deepest ranges have a Boreo-Atlantic origin (e.g., F. lineata, P. membranaceus, S. lignarius, and the family Tritoniidae), hence are cold-affinity species. Interestingly, numerous species reaching high depths in the Mediterranean basin (e.g., T. fimbria, P. quadripartita, P. meckeli, D. pseudoargus, F. lineata) are also found in very shallow and cold waters (such as the north and the central Adriatic Sea or canyon areas subjected to upwelling currents), with a marked seasonality (Zenetos et al. Citation2016; Betti et al. Citation2017; Betti Citation2021).
The scenario obtained from the latitudinal analysis of the findings showed that the heterobranch fauna present along the Ligurian continental shelf is significantly different from the Tyrrhenian and Sicilian ones. This situation could be attributed to the distinctive northern distribution of some Felimare species and the paucity of Facelina sp., P. ischitana and Tritoniidae nd, more represented in the southern regions. Cattaneo-Vietti (Citation1991) suggested that the narrow extension of the Ligurian continental shelf and the occurrence of a patchwork of habitats could be listed among the reasons explaining the high species richness of the deep soft-bottom heterobranch fauna in this region and the wider bathymetric ranges of the species. Overall, a marked decrease in biodiversity was observed from North to South, with a secondary high peak of species richness in the Sicily Channel. This latter is indeed an important boundary area, known for its complex geomorphologic and oceanographic conditions, ultimately leading to a hotspot of biodiversity (Bianchi & Morri Citation2000; Evans et al. Citation2016; Altobelli et al. Citation2017).
The specific richness of the seamounts appears modest: this may be related to the relative paucity of dives carried out in the offshore locations and to the fact that surveys started at 60 m, excluding therefore the possibility to compare the 30–50 m depth range, which, in the coastal areas, hosted a distinctive heterobranch assemblage adapted to coralligenous environments with high biological coverage. Moreover, the investigated seamounts are generally dominated by soft-bottom habitats, limiting the observations of heterobranchs by ROV. The sightings are concentrated on the peaks, which, due to more turbulent conditions, are dominated by lush communities of filter-feeders (Bo et al. Citation2011, Citation2021), attracting the most commonly observed tritoniid species. Nevertheless, the low diversity and abundance can also be related to another factor. It is reasonable to think that these offshore structures, located between 23 and 70 nautical miles from the shore (Bo et al. Citation2021), are not significantly interested by the coastal larval flux. This process seems particularly effective for the investigated dorids, which shows a sort of costaphily (sensu Cornelius Citation1992). Such pattern would be related to a low dispersal ability, which, in some cases (e.g., F. cf. villafranca, F. orsinii, C. laevis) is supported by direct development (Thompson Citation1967; Ortea et al. Citation1996; Coelho & Calado Citation2010). The different larval dispersal abilities showed by heterobranchs (ranging from <10 km to 10,000 km) (Todd et al. Citation1998), alongside local hydrodynamic patterns, may affect the species composition of the assemblages, especially in offshore locations (Todd Citation1981; Fritts-Penniman et al. Citation2020).
The observed bathymetric and geographic distribution patterns of the heterobranch assemblages are the result of numerous biological and ecological factors, including the physiology of the species and their adaptation to depth, the habitat complexity, the presence of suitable substrates, the larval dispersal ability, but also depends on the availability of food sources. The widespread stenophagy of many heterobranch taxa (e.g., Nudibranchia, Sacoglossa) (McDonald & Nybakken Citation1997; Megina et al. Citation2002; Gemballa & Schermutzki Citation2004), in fact, has a crucial role in influencing their evolutionary processes (e.g., Jensen Citation1997; Goodheart et al. Citation2017) and the availability of the food sources may drive the composition and distribution of the assemblages (e.g., Betti et al. Citation2017; Canessa et al. Citation2021).
The strongest trophic relationship is found between the species belonging to the genus Felimare and the keratose sponges they exclusively feed on (McDonald & Nybakken Citation1997), co-occurring down to 130 m and then completely disappearing. These species are particularly abundant in the Ligurian Sea, where keratose-dominated sponge grounds are widely documented at mesophotic depths (Enrichetti et al. Citation2020). A similar situation involves P. atromaculata, whose distribution mirrors that of its favourite food sources, the sponges P. ficiformis and H. fulva (Gemballa and Schermutzki, Citation2004), largely present in all the considered areas, but never below 150 m depth.
Another strong relationship occurs between T. callogorgiae and deep gorgonians. The species belonging to the family Tritoniidae are known to be specialised anthozoan-feeders (Schmekel & Portmann Citation1982; McDonald & Nybakken Citation1999) and have been worldwide reported as typical inhabitants of the deep sea (e.g., Valdés Citation2006; Valdés et al. Citation2017, Citation2018; Chimienti et al. Citation2020; Penney et al. Citation2020). The observed ability of T. callogorgiae to feed on different alcyonaceans could explain its success in the hardbottom Mediterranean coral communities, which are greatly diffused (Chimienti et al. Citation2018). The high reproductive potential of tritoniids (Vayssière Citation1901) may also concur in explaining the wide distribution of this species.
On the contrary, a weak relationship was detected between the hydrozoan-feeder cladobranchs and their food source, partially due to the limited possibility of identifying hydrozoan species exclusively through ROV images. Interestingly, however, the most stenophagous species, F. affinis and P. ischitana, known to feed exclusively on Eudendrium hydrozoans in shallow waters (Schmekel & Portmann Citation1982; Hirano & Thompson Citation1990; Furfaro & Mariottini Citation2016), seem to shift their feeding preferences on other hydrozoans when reaching the outer shelf or offshore seamounts, where Eudendrium spp. seem rarer. Paraflabellina ischitana, for example, has been observed on a wide range of other hydrozoans (e.g., Plumularioidea, Bougainvilliidae, Haleciidae) between 150 and 200 m. On the other hand, the complete lack of sightings of a very common shallow-water Eudendrium-eater, Cratena peregrina (Gmelin, 1791) (Di Camillo et al. Citation2012; Lombardo & Marletta Citation2020), suggests that not all species are able to adjust their diets. The depth-related food-shifting ability could also be shared by nudibranchs targeting other taxa. For example, A. cristata, known to eat various bryozoans (e.g., Bugulidae, Celleporidae) in shallow waters (Schmekel & Portmann Citation1982; Urgorri & Besteiro Citation1984), was observed feeding on Reteporella spp. in deep coralligenous environments (50–85 m). The dorid B. cinnabarina is known to eat the sponge T. muricata (Bouchet, Citation1977; Cattaneo-Vietti Citation1991) in deep bathyal muds, necessarily turning to other sponge species in shallow and mesophotic waters (Ballesteros & Valdés Citation1999). Potentially, a similar situation could also exist for anthozoan-feeders such as tritoniids.
Both hydrozoans and bryozoans include small, fast-growing opportunistic species, which easily colonise the surface of seafloor macro-litter, such as lost fishing gear (Enrichetti et al. Citation2021). These objects may be locally abundant and indirectly act as poles of attraction for generalist consumers. It is the case of Facelina sp. and P. ischitana commonly observed crawling on lost nets and lines as well as skeletons of dead gorgonians. Their occurrence on the St. Lucia Seamount may reflect the high fishing impact of the site, including high densities of lost gear and mass mortalities of structuring species (Bo et al. Citation2014).
Ultimately, the stenophagy of many heterobranch species, representing one of the key factors of their evolutionary success in shallow waters, may represent a limit in their expansion towards the deeper ecosystems. This is also supported by the fact that most of the species known to dwell in the deepest depth ranges (> 300 m) (), besides being adaptated to soft-bottom habitats, are also distinctly euribathic and euryphagous (e.g., Philine spp., S. lignarius, P. meckeli, T. fimbria) (Thompson Citation1976; Schmekel & Portmann Citation1982; Cattaneo-Vietti et al. Citation1993).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgements
The datasets used in this study were obtained thanks to multiple ROV campaigns conducted by the University of Genova, ISPRA, University of Cagliari and Conisma between 2007 and 2018. Financing entities included EU, Ministero dell’Università e della Ricerca, Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Calabria and Sardinia Regions. The authors would like to thank Ph.D. Francesco Enrichetti (UniGE) for the GIS map rendition and Prof. Nardo Vicente (Université Paul Cézanne) for the help in the bibliographic research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Altobelli C, Perzia P, Falautano M, Consoli P, Canese S, Romeo T, Andaloro F. 2017. Mediterranean banks in EBSA area: Hotspots of biodiversity under threat. Marine Environmental Research 131:57–68. DOI: 10.1016/j.marenvres.2017.09.005.
- Angeletti L, Taviani M, Canese S, Foglini F, Mastrototaro F, Argnani A, Trincardi F, Bakran-Petricioli T, Ceregato A, Chimienti G, Mačić V, Poliseno A. 2014. New deep-water cnidarian sites in the southern Adriatic Sea. Mediterranean. Marine Science 15:263–273.
- Angiolillo M, Gori A, Canese S, Bo M, Priori C, Bavestrello G, Salvati E, Erra F, Greenacre M, Santangelo G. 2016. Distribution and population structure of deep-dwelling red coral in the Northwest Mediterranean. Marine Ecology 37(2):294–310. DOI: 10.1111/maec.12274.
- Angiolillo M, La Mesa G, Giusti M, Salvati E, Di Lorenzo B, Rossi L, Canese S, Tunesi L. 2021. New records of scleractinian cold-water coral (CWC) assemblages in the southern Tyrrhenian Sea (western Mediterranean Sea): Human impacts and conservation prospects. Progress in Oceanography 197:102656. DOI: 10.1016/j.pocean.2021.102656.
- Ballesteros E. 2006. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanography and Marine Biology: An Annual Review 44:123–195.
- Ballesteros M, Valdés A. 1999. Redescripción de Baptodoris cinnabarina Bergh, 1884 (Opisthobranchia, Doridina, Platydorididae) y discusión taxonómica de otras especies del género Baptodoris Bergh. Iberus 17:27–35.
- Betti F. 2021. Il regno dei nudibranchi. Imola (BO): Ed. La Mandragora. pp. 192.
- Betti F, Bava S, Cattaneo-Vietti R. 2017. Composition and seasonality of a heterobranch assemblage in a sublittoral, unconsolidated, wave-disturbed community in the Mediterranean Sea. Journal of Molluscan Studies 83:325–332. DOI: 10.1093/mollus/eyx019.
- Bianchi CN, Morri C. 2000. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for future research. Marine Pollution Bulletin 40:367–376. DOI: 10.1016/S0025-326X(00)00027-8.
- Bianchi CN, Morri C, Chiantore M, Montefalcone M, Parravicini V, Rovere A. 2012. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In: Stambler N, editor. Life in the Mediterranean Sea: A look at habitat changes. New York: Nova Science Publishers. pp. 1–55.
- Bo M, Bava S, Canese S, Angiolillo M, Cattaneo-Vietti R, Bavestrello G. 2014. Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biological Conservation 171:167–176. DOI: 10.1016/j.biocon.2014.01.011.
- Bo M, Bertolino M, Borghini M, Castellano M, Covazzi Harriague A, Di Camillo CG, Gasparini G, Misic C, Povero P, Pusceddu A, Schroeder K, Bavestrello G. 2011. Characteristics of the mesophotic megabenthic assemblages of the Vercelli seamount (North Tyrrhenian Sea). PLoS One 6:e16357. DOI: 10.1371/journal.pone.0016357.
- Bo M, Canese S, Spaggiari C, Pusceddu A, Bertolino M, Angiolillo M, Giusti M, Loreto MF, Salvati E, Greco S, Bavestrello G. 2012. Deep coral oases in the South Tyrrhenian Sea. PloS One 7:e49870. DOI: 10.1371/journal.pone.0049870.
- Bo M, Coppari M, Betti F, Enrichetti F, Bertolino M, Massa F, Bava S, Gay G, Cattaneo-Vietti R, Bavestrello G. 2021. The high biodiversity and vulnerability of two Mediterranean bathyal seamounts support the need for creating offshore protected areas. Aquatic Conservation: Marine and Freshwater Ecosystems 31:543–566. DOI: 10.1002/aqc.3456.
- Bogi C, Galil BS. 2004. The bathybenthic and pelagic molluscan fauna off the Levantine coast, eastern Mediterranean. Bollettino Malacologico 39:79–90.
- Bogi C, Galil BS. 2013. New molluscan records from the eastern Mediterranean bathyal. Marine Biodiversity Records 6:e19. DOI: 10.1017/S1755267212001285.
- Bouchet P. 1977. Opisthobranches de profondeur de l’Ocean Atlantique: II-Notaspidea et Nudibranchiata. Journal of Molluscan Studies 43:28–66.
- Canessa M, Bavestello G, Cattaneo-Vietti R, Furfaro G, Doneddu M, Navone A, Trainito E. 2021. Rocky substrate affects benthic heterobranch assemblages and prey/predator relationships. Estuarine, Coastal and Shelf Science 261:107568. DOI: 10.1016/j.ecss.2021.107568.
- Carmona L. 2020. Investigating the amphiatlantic status of Facelina bostoniensis (Couthouy, 1838) (Nudibranchia: Aeolidida). Journal of Molluscan Studies 86:64–71. DOI: 10.1093/mollus/eyz034.
- Cattaneo-Vietti R. 1991. Bathymetric distribution of soft-bottom opisthobranchs along the Ligurian and Tuscany continental slope (Western Mediterranean). Proc. 10th Intern. Malacol. Congress, 1989, Tubingen. pp. 327–334.
- Cattaneo-Vietti R, Burlando B, Senes L. 1993. Life history and diet of Pleurobranchaea meckelii (Opisthobranchia: Notaspidea). Journal of Molluscan Studies 59:309–313. DOI: 10.1093/mollus/59.3.309.
- Cau A, Follesa MC, Moccia D, Alvito A, Bo M, Angiolillo M, Canese S, Paliaga EM, Orrù PE, Sacco F, Cannas R. 2015. Deepwater corals biodiversity along roche du large ecosystems with different habitat complexity along the south Sardinia continental margin (CW Mediterranean Sea). Marine Biology 162:1865–1878. DOI: 10.1007/s00227-015-2718-5.
- Chimienti G, Angeletti L, Furfaro G, Canese S, Taviani M. 2020. Habitat, morphology and trophism of Tritonia callogorgiae sp. nov., a large nudibranch inhabiting Callogorgia verticillata forests in the Mediterranean Sea. Deep Sea Research Part I:Oceanographic Research Papers 165:103364. DOI: 10.1016/j.dsr.2020.103364.
- Chimienti G, Bo M, Mastrototaro F. 2018. Know the distribution to assess the changes: Mediterranean cold-water coral bioconstructions. Rendiconti Lincei. Scienze Fisiche E Naturali 29:583–588. DOI: 10.1007/s12210-018-0718-3.
- Coelho R, Calado G. 2010. Spawn and early development of NE Atlantic species of Hypselodoris (Gastropoda: Opisthobranchia). Iberus 28:63–72.
- Cornelius PF. 1992. Medusa loss in leptolid Hydrozoa (Cnidaria), hydroid rafting, and abbreviated life-cycles among their remote-Island faunae: An interim review. ScientiaMarina 56:245–261.
- Del Mar Otero M, and Marin P. 2019. 46 conservation of Cold-Water Corals in the Mediterranean: Current status and future prospects for improvement. In: Orejas C, Jiménez C, editors. Mediterranean Cold-Water Corals: Past, present and future. Vol. 9. Cham: Springer. pp. 535–545.
- Di Camillo CG, Betti F, Bo M, Martinelli M, Puce S, Vasapollo C, Bavestrello G. 2012. Population dynamics of Eudendrium racemosum (Cnidaria, Hydrozoa) from the north Adriatic Sea. Marine Biology 159:1593–1609. DOI: 10.1007/s00227-012-1948-z.
- Domènech A, Avila C, Ballesteros M. 2006. Opisthobranch molluscs from the subtidal trawling grounds off Blanes (Girona, north-east Spain). Journal of the Marine Biological Association of the United Kingdom 86:383–389. DOI: 10.1017/S0025315406013233.
- Enrichetti F, Bavestrello G, Betti F, Coppari M, Toma M, Pronzato R, Canese S, Bertolino M, Costa G, Pansini M, and Bo M. 2020. Keratose‐dominated sponge grounds from temperate mesophotic ecosystems (NW Mediterranean Sea). Marine Ecology 41: e12620.
- Enrichetti F, Bavestrello G, Betti F, Rindi F, Tregrosso A, Bo M. 2021. Fate of lost fishing gears: Experimental evidence of biofouling colonisation patterns from the northwestern Mediterranean Sea. Environmental Pollution 268:115746. DOI: 10.1016/j.envpol.2020.115746.
- Enrichetti F, Dominguez-Carrió C, Toma M, Bavestrello G, Betti F, Canese S, Bo M. 2019. Megabenthic communities of the Ligurian deep continental shelf and shelf break (NW Mediterranean Sea). PLoS One 14:e0223949. DOI: 10.1371/journal.pone.0223949.
- Evans J, Aguilar R, Alvarez H, Borg JA, Garcia S, Knittweis L, and Schembri PJ. 2016. Recent evidence that the deep sea around Malta is a biodiversity hotspot. Rapport du Congrès de la Commission Internationale pour l’Exploration Scientifique de la Mer Méditerranée 41:463.
- Fritts-Penniman AL, Gosliner TM, Mahardika GN, Barber PH. 2020. Cryptic ecological and geographic diversification in coral-associated nudibranchs. Molecular Phylogenetics and Evolution 144:106698. DOI: 10.1016/j.ympev.2019.106698.
- Furfaro G, Mariottini P. 2016. Check-list of the Nudibranchs (Mollusca Gastropoda) from the biodiversity hot spot “Scoglio del Corallo” (Argentario Promontory, Tuscany). Biodiversity Journal 7:67–78.
- Gemballa S, Schermutzki F. 2004. Cytotoxic haplosclerid sponges preferred: A field study on the diet of the dotted sea slug Peltodoris atromaculata (Doridoidea: Nudibranchia). Marine Biology 144:1213–1222. DOI: 10.1007/s00227-003-1279-1.
- Goodheart JA, Bazinet AL, Valdés A, Collins AG, Cummings MP. 2017. Prey preference follows phylogeny: Evolutionary dietary patterns within the marine gastropod group Cladobranchia (Gastropoda: Heterobranchia: Nudibranchia). BMC Evolutionary Biology 17:1–14. DOI: 10.1186/s12862-017-1066-0.
- Hammer Ø, Harper DA, Ryan PD. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:1–9.
- Hirano YJ, Thompson TE. 1990. Flabellinid nudibranchs from the Bay of Naples, with a description of a new species, Flabellina ischitana. Journal of Molluscan Studies 56:345–354. DOI: 10.1093/mollus/56.3.345.
- Jeffreys JG. 1882. Notes of the Mollusca procured by the Italian exploration of the Mediterranean in 1881. Annals and Magazine of Natural History 5:27–35. DOI: 10.1080/00222938209459661.
- Jensen KR. 1997. Evolution of the Sacoglossa (Mollusca, Opisthobranchia) and the ecological associations with their food plants. Evolutionary Ecology 11:301–335. DOI: 10.1023/A:1018468420368.
- Korshunova T, Martynov A. 2020. Consolidated data on the phylogeny and evolution of the family Tritoniidae (Gastropoda: Nudibranchia) contribute to genera reassessment and clarify the taxonomic status of the neuroscience models Tritonia and Tochuina. PLoS One 15:e0242103. DOI: 10.1371/journal.pone.0242103.
- Koutsoubas D, Tselepides A, Eleftheriou A. 2000. Deep sea molluscan fauna of the Cretan Sea (Eastern Mediterranean): Faunal, ecological and zoogeographical remarks. Senckenbergiana Maritima 30:85–98. DOI: 10.1007/BF03042958.
- Lombardo A, Marletta G. 2020. New data on the seasonality of Flabellina affinis (Gmelin, 1791) and Cratena peregrina (Gmelin, 1791)(Gastropoda Nudi-branchia) in the Ionian Sea, Central Mediterranean. Biodiversity Journal 11:1045–1053. DOI: 10.31396/Biodiv.Jour.2020.11.4.1045.1053.
- Mastrototaro F, Chimienti G, Acosta J, Blanco J, Garcia S, Rivera J, Aguilar R. 2017. Isidella elongata (Cnidaria: Alcyonacea) facies in the western Mediterranean Sea: Visual surveys and descriptions of its ecological role. The European Zoological Journal 84:209–225. DOI: 10.1080/24750263.2017.1315745.
- McDonald GR, and Nybakken JW. 1997. List of the worldwide food habits of nudibranchs. The Veliger 40. https://escholarship.org/uc/item/0g75h1q3
- McDonald GR, Nybakken JW. 1999. A worldwide review of the food of nudibranch mollusks. Part II. The suborder Dendronotacea. The Veliger 42:62–66.
- Megina C, Carballo JL, Cervera JL, García-Gómez JC. 2002. The diet of Platydoris argo (Gastropoda: Nudibranchia) and the dietary specialization of sponge eating dorids. Journal of Molluscan Studies 68:173–179. DOI: 10.1093/mollus/68.2.173.
- Moccia D, Cau A, Bramanti L, Carugati L, Canese S, Follesa MC, Cannas R. 2021. Spatial distribution and habitat characterisation of marine animal forest assemblages along nine submarine canyons of Eastern Sardinia (central Mediterranean Sea). Deep Sea Research Part I: Oceanographic Research Papers 167:103422. DOI: 10.1016/j.dsr.2020.103422.
- Moles J, Berning MI, Hooker Y, Padula V, Wilson NG, Schrödl M. 2021. Due South: The evolutionary history of Sub-Antarctic and Antarctic Tritoniidae nudibranchs. Molecular Phylogenetics and Evolution 162:107209. DOI: 10.1016/j.ympev.2021.107209.
- Montefalcone M, Tunesi L, Ouerghi A. 2021. A review of the classification systems for marine benthic habitats and the new updated Barcelona Convention classification for the Mediterranean. Marine Environmental Research 169:105387. DOI: 10.1016/j.marenvres.2021.105387.
- Monterosato TA. 1874. Recherches Conchyliologiques effectuées au cap Santo Vito, en Sicile. Journal de Conchyliologie 22:243–282.
- Monterosato TA. 1890. Conchiglie della profondità del mare di Palermo. Il Naturalista Siciliano 9:181–191.
- Natural Resources Defense Council (NRDC). 2014. The Atlantic’s deep sea treasures: Discoveries from a new frontier of ocean exploration. https://www.nrdc.org/sites/default/files/atlantic-deep-sea-treasures-IB.pdf.
- Oliverio M. 2003. The Mediterranean molluscs: The best-known malacofauna of the world … so far. Biogeographia 24:195–208.
- Ortea J, Valdés A, and García-Gómez JC. 1996. Revisión de las especies atlánticas de la familia Chromodorididae (Mollusca: Nudibranchia) del grupo cromático azul: Review of the atlantic species of the family Chromodorididae (Mollusca: Nudibranchia) of the blue chromatic group. Avicennia 1:1–165.
- Parera A, Pontes M, Salvador X, Ballesteros MBV. 2020. Sea-slugs (Mollusca, Gastropoda, Heterobranchia): The other inhabitants of the city of Barcelona (Spain). Butlletí de la Institució Catalana d’Historia Natural 84:75–100.
- Penney HD, Hamel JF, Mercier A. 2020. Range extension of two deep-sea nudibranchs, Tritonia newfoundlandica and Doridoxa ingolfiana (Mollusca: Gastropoda: Heterobranchia), in eastern Canada. The Canadian Field-Naturalist 134:165–170. DOI: 10.22621/cfn.v134i2.2443.
- Pérès JM, and Picard J. 1964. Nouveau manuel de bionomie benthique de la mer Méditerranée. Vol. 31. Aix-en-Provence: Recueil des travaux de la Station Marine d’Éndoume. pp. 1–137.
- Ponti M, Grech D, Mori M, Perlini RA, Ventra V, Panzalis PA, Cerrano C. 2016. The role of gorgonians on the diversity of vagile benthic fauna in Mediterranean rocky habitats. Marine Biology 163:120. DOI: 10.1007/s00227-016-2897-8.
- Ros J. 1975. Opistobranchios deI litoral ibérico. Lnv. Pesq 39:269–372.
- Schmekel L, and Portmann A. 1982. Opisthobranchia des Mittelmeeres. Opisthobranchia des Mittelmeeres, Nudibranchia und Saccoglossa. In: Fauna e flora del Golfo di Napoli, Monografia della Stazione Zoologica di Napoli. Vol. 40. Berlin, Heidelberg: Springer-Verlag. pp. 1–410.
- Smriglio C, Mariottini P. 1996. Central Tyrrhenian Sea Mollusca: XI. Description of Callostracon tyrrhenicum sp. nov. (Gastropoda, Acteonidae) and remarks on the other Mediterranean species of the family Acteonidae d’Orbigny, 1835. Basteria 60:183–193.
- Templado J, Villanueva R. 2010. Table S14. Checklist of the Phylum Mollusca. Supplementary material File S2. In: Coll M., Piroddi C., Steenbeek J., Kaschner K., Ben Rais Lasram F., Aguzzi J., Ballesteros E., Bianchi C. N., Corbera J., Dailianis T., Danovaro R., Estrada M., Froglia C., Galil B. S., Gasol J. M., Gertwagen R., Gil J., Guilhaumon F., Kesner-Reyes K., Kitsos M. S., Koukouras A., Lampadariou N., Laxamana E., Lòpez-Fé De La Cuadra C. M., Lotze H. K., Martin D., Mouillot D., Oro D., Raicevich S., Rius-Barile J., Saiz-Salinas J. I., San Vicente C., Somot S., Templado J., Turon X., Vafidis D., Villanueva R., Voultsiadou E. The biodiversity of the Mediterranean Sea: Estimates, patterns and threats. PLoS One 5:e11842. DOI: 10.1371/journal.pone.0011842.
- Thompson T. 1967. Direct development in a Nudibranch, Cadlina laevis, with a discussion of developmental processes in Opisthobranchia. Journal of the Marine Biological Association of the United Kingdom 47:1–22. DOI: 10.1017/S0025315400033518.
- Thompson TE. 1976. Biology of Opisthobranch Molluscs. Vol. 1. London: The Ray Society. pp. 207.
- Thompson TE, Cattaneo R, Wong YM. 1990. Eastern Mediterranean Opisthobranchia: Dotidae (Dendronotoidea), Arminidae and Madrellidae (Arminoidea). Journal of Molluscan Studies 56:393–413. DOI: 10.1093/mollus/56.3.393.
- Todd CD. 1981. The ecology of nudibranch molluscs. Oceanography and Marine Biology: An Annual Review 9:141–234.
- Todd CD, Lambert WJ, Thorpe JP. 1998. The genetic structure of intertidal populations of two species of nudibranch molluscs with planktotrophic and pelagic lecithotrophic larval stages: Are pelagic larvae “for” dispersal? Journal of Experimental Marine Biology and Ecology 228:1–28. DOI: 10.1016/S0022-0981(98)00005-7.
- Trainito E, Doneddu M. 2014. Nudibranchi del Mediterraneo. 2a edizione, riveduta ed ampliata. Ed. Milano: Il Castello. pp. 192.
- Trainito E, Doneddu M. 2016. Contribution to the knowledge of the molluscan fauna in the Marine Protected Area Tavolara-Punta Coda Cavallo: Sacoglossa, Umbraculida, Pleurobranchomorpha, Anaspidea, Cephalaspidea, Thecosomata (Gastropoda; Heterobranchia). Bollettino Malacologico 53:79–89.
- Urgorri V, Besteiro C. 1984. La alimentacion de los moluscos nudibranquios de Galicia. Iberus 4:51–58.
- Valdés A. 2006. Marionia tedi Ev. Marcus, 1983 (Nudibranchia, Tritoniidae) in the Gulf Of Mexico: First record of an Opisthobranch Mollusk from Hydrocarbon Cold Seeps. Gulf and Caribbean Research 18:41–46. DOI: 10.18785/gcr.1801.05.
- Valdés A, Lundsten L, Wilson NG. 2018. Five new deep-sea species of nudibranchs (Gastropoda: Heterobranchia: Cladobranchia) from the Northeast Pacific. Zootaxa 4526:401–433. DOI: 10.11646/zootaxa.4526.4.1.
- Valdés A, Murillo FJ, McCarthy JB, Yedinak N. 2017. New deep-water records and species of North Atlantic nudibranchs (Mollusca, Gastropoda: Heterobranchia) with the description of a new species. Journal of the Marine Biological Association of the United Kingdom 97:303–319. DOI: 10.1017/S0025315416000394.
- Vayssière AJBM. 1901. Étude comparée des Opisthobranches des côtes française de l’Océan Atlantique et de la Manche avec ceux de nos côtes Méditerranéennes. Bulletin Scientifique de la France Et de la Belgique 34:281–315.
- Vicente N. 1967. Contribution à l’étude des gastéropodes opisthobranches du Golfe de Marseille: I. Systématique, écologie, biologie. Recueil Des Travaux de la Station Marine d’Endoume 42:133–179.
- Zenetos A, Macic V, Jaklin A, Lipej L, Poursanidis D, Cattaneo-Vietti R, Beqiraj S, Betti F, Poloniato D, Kashta L, Katsanevakis S, Crocetta F. 2016. Adriatic ‘opisthobranchs’ (Gastropoda, Heterobranchia): Shedding light on biodiversity issues. Marine Ecology 37:1239–1255. DOI: 10.1111/maec.12306.
- Zhang S, and Zhang S. 2021. Tritonia iocasica sp. nov., a new tritoniid species from a seamount in the tropical western Pacific (Heterobranchia: Nudibranchia). Journal of Oceanology and Limnology 39: 1817–1829.