Abstract
Regeneration is a post-embryonic developmental process common in Metazoa, which, despite obvious taxa-specific differences, can often share common principles and patterns. Among these, the distalization and (proximal) intercalation model successfully describes most animal regeneration phenomena. Stellate echinoderms (Crinoidea, Asteroidea, and Ophiuroidea) are particularly practical models for regeneration studies as the proximo-distal regrowth of their “segmental” arms, including the inner “continuous” yet homologous structures, i.e. radial water canal, radial nerve cord, and somatocoel, provide a unique opportunity to investigate the existence of evolutionarily shared regenerative patterns. In the present work, we comparatively examined the anatomy of arm regeneration in four stellate echinoderm species – the crinoid Antedon mediterranea, the asteroids Echinaster sepositus and Coscinasterias tenuispina, and the ophiuroid Amphipholis squamata. We observed that in all the models the distal elements, i.e. the apical blastema of crinoids, and terminal ossicle and tube foot of asteroids and ophiuroids, form in an early stage, followed by the proximal region, which develops in the proximal-to-distal direction. In all arms, the continuous structures develop before discrete lateral structures (e.g. ossicles and tube feet), and appear to provide materials that make the subsequent development possible. Overall, the model inferred from our study is compatible with those previously proposed for other animal models that involve processes of distalization and intercalation. The evidence of shared patterns suggests that at least some overall regeneration mechanisms have ancient origins and are well conserved throughout echinoderm and animal evolution. This study could help shed light on those evolutionarily conserved principles (patterns) among metazoan regeneration.
1. Introduction
Regeneration is the restoration of lost body parts but the term can be, and has been, applied to intrinsically different processes, such as, for example, physiological or homeostatic regeneration of tissues and cell turn-over (Bely & Nyberg Citation2010). While the latter is a relatively “simple” phenomenon, probably present in any metazoan, the former requires the activation of complex developmental programmes and their orchestration at different biological levels, from tissues/cells to gene expression (Candia Carnevali Citation2006; Tanaka & Reddien Citation2011; Ben Khadra et al. Citation2017; MacCord & Maienschein Citation2019; Bideau et al. Citation2021; Candia Carnevali et al. Citation2022).
If we focus specifically on regeneration at the whole organism level, such as in the case of complex structures (limbs) or even of the whole body, some features specific to this phenomenon are identifiable among different organisms (Bely & Nyberg Citation2010; Bideau et al. Citation2021; Rinkevich et al. Citation2022). Regeneration typically follows a series of precise developmental processes, which include the activation and mobilization of progenitor cells towards the wound, as well their positioning, proliferation and differentiation, all these requiring the expression of multiple specific genes and a fine, often nerve-mediated, orchestration (Brockes & Kumar Citation2008; Kumar et al. Citation2008; Lengfeld Citation2009; Czarkwiani et al. Citation2016; Pirotte et al. Citation2016; Tanaka Citation2016; Ben Khadra et al. Citation2018; Lai & Aboobaker Citation2018; Ferrario et al. Citation2020).
Regenerative abilities are often coupled with some features of embryogenesis and reproduction but, unlike other developmental phenomena, they present a unique phylogenetic distribution (Rinkevich et al. Citation2022). A certain ability to regrow lost body parts is known in many phyla throughout all the animal kingdom, irrespective of their phylogenetic position (Sánchez Alvarado Citation2000; Chen et al. Citation2011; Lai & Aboobaker Citation2018; Coffman Citation2019), although Ecdysozoans generally display a limited regenerative ability (Bely & Nyberg Citation2010; Bideau et al. Citation2021).
Although regeneration is not necessarily a prerogative of “simpler” animals, it is quite rare for large/complex organisms, to possess the ability to regenerate extended body portions or, even more uncommon, a whole individual from small body fragments (=whole-body regeneration) (Thouveny & Tassava Citation1997; Candia Carnevali Citation2006; Dolmatov Citation2020; Elchaninov et al. Citation2021). Notable exceptions are, among protostomes, Annelida (apart from Hirudinea) and, among deuterostomes, a few species of Hemichordata (Dawydoff Citation1909; Tweedell Citation1961), Tunicata (Rinkevich et al. Citation1995) and Echinodermata (e.g. Linckia spp.; Brockes & Kumar Citation2008), the phylum to which the experimental models of the present research belong to.
In this perspective, echinoderms constitute an extremely peculiar taxon as, although they display high complexity, they provide an extraordinary diversity and number of regeneration-competent species (virtually the majority of the phylum members). Some of these species (e.g. Antedon mediterranea, Apostichopus japonicus, and Holothuria glaberrima) represent historical and well-established models of regeneration (García-Arrarás et al. Citation1998; Candia Carnevali & Bonasoro Citation2001; Quiñones et al. Citation2002; Sun et al. Citation2011; Nieves-Ríos et al. Citation2020). Furthermore, echinoderms show close phylogenetic affinity to vertebrates (see Supplementary Materials Figure S1; Laumer et al. Citation2019), thus opening the scenario of possible shared mechanisms between the two taxa. Additionally, echinoderms are “pre-duplication” clades within the deuterostomes (Meyer & Schartl Citation1999; Dehal & Boore Citation2005; Kassahn et al. Citation2009), meaning that they have a single copy per each gene and this facilitate any genetic analysis addressed to unravel the molecular mechanisms underpinning regeneration. Indeed, although the molecular approach has long been hampered in the study of echinoderm regeneration, in recent decades both gene expression studies (single genes or high-throughput transcriptomics) and proteomic approaches have started to fill in this gap of knowledge, providing a large amount of valuable information (for recent reviews see Franco et al. Citation2013; Ben Khadra et al. Citation2018; Dolmatov Citation2021; Medina-Feliciano & García-Arrarás Citation2021).
In the present research, we specifically focused on the regrowth of the three main continuous structures running along each arm of stellate echinoderms (crinoids, asteroids and ophiuroids), namely the Radial Nerve Cord (RNC), the Radial Water Canal (RWC), and the somatocoel (see Supplementary Materials Figure S2), since they are believed to play a key role during the regenerative process (Mladenov et al. Citation1989; Candia Carnevali & Bonasoro Citation2001; Mooi et al. Citation2005; Ben Khadra et al. Citation2018). In particular, during the regenerative process, the RNC extends in length before the other structures and coordinates their development (Moss et al. Citation1998; Thorndyke et al. Citation2000; Thorndyke & Candia Carnevali Citation2001; Sugni et al. Citation2010; Byrne et al. Citation2019; Byrne Citation2020). Indeed, the nerve dependence of echinoderm regeneration has been confirmed several times and with different methods (Thorndyke & Candia Carnevali Citation2001; Candia Carnevali Citation2006). A particular attention has been recently addressed to nervous system regeneration in an asteroid species representative in regeneration research (Coscinasterias muricata), allowing a better understanding of the interaction between neural components and other arm tissues during regenerative development also through production of specific neural factors (Byrne et al. Citation2019; Byrne Citation2020). The importance of nerves during the regeneration process is also observed in other metazoan, where it plays an important role in providing local and systemic signals as well as sources of nerve-related cells (Kumar & Brockes Citation2012; Sinigaglia & Averof Citation2019). For example, in salamanders, nerves are crucial for blastema cell proliferation during limb regeneration (Miller et al. Citation2019). The same is true in arthropod limbs (Maruzzo & Bortolin Citation2013).
In the present study, we have taken into consideration both historical (Antedon mediterranea - Crinoidea), and recent experimental models (Echinaster sepositus and Coscinasterias tenuispina -Asteroidea) and Amphipholis squamata (Ophiuroidea), as representatives of different stellate echinoderm taxa. By means of a (multi-taxa) comparative anatomy approach, we attempt to find shared patterns/mechanisms of regenerative development to provide a possible evolutionary interpretation of complex structure (i.e. arm) regeneration in this phylum. Most research is indeed usually focused on one species/taxa, necessarily limiting the identification of common principles. In particular, we followed the process of arm regeneration at different stages in order to describe, reconstruct, via light and electron microscopy analyses, and subsequent manual 3D reconstructions, the modes and times of tissue regeneration.
The identification of common principles in echinoderm regeneration could help the development of a general model that might be extrapolated to other Metazoa, thus improving our understanding of this highly complex phenomenon (Lai & Aboobaker Citation2018).
2. Materials and methods
2.1. Animal sampling and maintenance
Adult specimens of Antedon mediterranea (defined as those with mean diameter 12 cm) were collected in Noli Gulf (Savona) and Le Grazie Gulf (La Spezia, Liguria, Italy). Adult specimens of Echinaster sepositus (mean diameter 12 cm) were collected in the Marine Protected Area of Portofino (Genoa, Liguria, Italy), at a depth of 5–8 m. Adult specimens of Coscinasterias tenuispina (mean diameter 12 cm) and adult specimens of Amphipholis squamata (mean diameter 1 cm) were collected in the Marine Protected Area Isola di Bergeggi (Savona, Liguria, Italy). All animals, at least 10 for each species, were placed in different 50 L aerated aquaria containing Artificial Sea Water (ASW) (37‰ salinity as in the Mediterranean Sea, at water temperature 17°C, as mean value of the sampling sites) and sediment (rocks and sand) at the bottom in order to recreate the natural environment where these species live, and they were left to acclimatize for at least 2 weeks prior to regeneration tests. Salinity (using a Milwaukee refractometer), temperature (using a mercury thermometer), and animal health were checked daily each morning. Other chemical ASW parameters, such as pH (mean value 8), concentration of carbonate (GH mean value 8, KH mean value 6), nitrite (0 mg/L), and nitrate (mean value 100 mg/L) were checked once a week using aquarium test strip and promptly adjusted when needed by changing or adding fresh ASW. Animals were fed throughout the experiment (acclimatization + regeneration period) as follow: starfish were fed once a week with a piece of cuttlefish (1 cubic centimetre), and with live mussels (Coscinasterias only); brittle stars and crinoids were fed three times a week with the commercial products Phyto Reef and Snow Reef (SHG), approximately 1 mL of each product for each 50 L tank.
2.2. Regeneration tests
Amputations were performed as previously described in the literature for the species used in this study or for related species (Candia Carnevali et al. Citation1995a; Ben Khadra et al. Citation2015a; Czarkwiani et al. Citation2016; Ferrario et al. Citation2018). Different regeneration time-points were selected for the four species used in this study, based on our knowledge of diverse echinoderm regeneration rates and in order to compare their early and advanced regenerative phases, which occur with different timings depending on the species (Ben Khadra et al. Citation2018).
To reproduce the natural conditions of autotomy in the distal arm amputation in A. mediterranea, pressure was exerted on a distal syzygial articulation with a sterile scalpel, as described in the literature (Candia Carnevali et al. Citation1995a). The specimens were then placed in different tanks according to the prefixed stage of regeneration, namely 3 days (d), 2 weeks (w), and 4 w post-amputation (p.a.). After these time-points, corresponding to early (3 d p.a.) and advanced regenerative phases (2 and 4 w p.a.; Candia Carnevali & Bonasoro Citation2001), regenerating arms were collected following the same procedure.
Arm amputation in C. tenuispina and E. sepositus was performed as described elsewhere (Ben Khadra et al. Citation2015a, Citation2015b) with a common sterile razor blade approximately at the distal third of the arm to standardize the level of amputation in all experimental animals. Later, starfish specimens were placed back in their original tanks. After 3 weeks (w), 6 w, 10 w, 16 w, and >>16 w p.a., the regenerating arms were collected in the same way approximately half a centimetre proximally to the amputation plane. In total, 2–5 samples were collected from each species and for each stage of regeneration, corresponding to early and advanced regenerative phases (Ben Khadra et al. Citation2015a, Citation2015b, Citation2018). To avoid excessive stress, only one arm sample was collected from each individual.
To ensure a precise and sharp amputation, due to the high level of motility of brittle star arms, each specimen of A. squamata was placed in a petri dish under a stereomicroscope, and anesthetized with a solution of 3.5% MgCl26H2O in 1:1 distilled water (DW) and filtered ASW, as described for Amphiura filiformis (Czarkwiani et al. Citation2016). Immobile arms were amputated with a sterile scalpel precisely at the junction between the third and the fourth segment counting from the central disc to standardize the level of amputation in all experimental animals. After this, specimens of the same stage of regeneration were placed together in larger petri dishes covered by gauze held by rubber bands, to allow free circulation of water, and placed back in the tanks. After letting the animals regenerate their arms for 1 day (d), 2 d, 3 d, 5 d, 1 week (w), 2 w, or 3 w, corresponding to the repair phase, and the early and advanced regenerative phases (Czarkwiani et al. Citation2016; Ben Khadra et al. Citation2018; Ferrario et al. Citation2018), the regenerating arms were collected, amputating the arm at the junction with the central disc. In total, 10 samples were collected for each regenerative stage in order to compare their early and advanced regenerative phases, which occur with different timings depending on the species.
2.3. Sample fixation and embedding for light microscopy
In order to obtain optimal samples for microscopy observations and imaging and according to previous literature, paraffin embedding was used for large samples (starfish) (Ben Khadra et al. Citation2015a, Citation2015b), whereas epoxy resin embedding for small samples (brittle stars and crinoids; Sugni et al. Citation2010; Ferrario et al. Citation2018). Indeed, resin embedding is not recommended for samples thicker than 0.3–0.5 mm, as those of E. sepositus, due to the low penetration depth of this embedding medium. Regenerating samples of the four species were fixed and embedded following different procedures depending on the arm diameter. Crinoids and brittle stars possess tiny arms suitable for epoxy resin embedding and subsequent ultramicrotome sectioning, whereas starfish arms are bigger in diameter and therefore perfectly suitable for paraffin embedding and subsequent microtome sectioning. Different protocols are detailed below.
A. mediterranea samples were fixed in 2% glutaraldehyde, 1.2% sodium chloride in 0.1 M sodium cacodylate tri-hydrate buffer for 4–5 hours at 4°C, washed in the same buffer for 3 hours and then in a solution of 1% osmium tetroxide in 0.1 M sodium cacodylate for 2 hours at room temperature (RT). Afterwards, samples were washed in DW, and left overnight in a decalcifying solution (2% L-ascorbic acid and 0.3 M sodium chloride in DW; Dietrich & Fontaine Citation1975). Then, samples were left in 2% uranyl acetate in 25% ethanol for 2 hours at RT, washed in 25% ethanol to remove residual uranyl acetate and dehydrated in an increasing ethanol series. After dehydration, samples were washed in propylene oxide, in propylene oxide/Epon Araldite 812 in different proportions (3:1, 1:1, 1:3, pure resin), embedded in pure resin in a rubber mold, and left for 3 days at 65°C to polymerize.
C. tenuispina and E. sepositus samples were fixed in Bouin fixative (75 mL saturated picric acid, 25 mL formaldehyde 37%, 5 mL glacial acetic acid), which also acted as a decalcifying solution, and left therein for at least 1 month at 4°C. Then, samples were repeatedly washed in tap water and dehydrated in an increasing ethanol series. After dehydration, samples were washed twice in xylene, in xylene/paraffin solution in different proportions (3:1, 1:2, 1:3, pure paraffin) and then embedded in pure paraffin.
The fixation and resin embedding procedure for A. squamata samples was similar to that of crinoids, with only small optimizations in the fixation step as follows: fixation for 2 hours with 1.4% sodium chloride and 1% uranyl acetate. See Supplementary Materials for Transmission Electron Microscopy (TEM) analyses.
2.4. Sample sectioning and staining
The specific technique of sectioning and staining was chosen for each species on the base of compatibility with the inclusion medium.
Starfish and brittle star samples embedded in paraffin were sectioned with a steel blade on a rotary microtome (Leitz 1512) into both longitudinal and 8 μm thick cross sections for the starfish, and into 7 μm thick cross sections for the brittle stars. Slides were then stained following the Milligan trichrome staining technique (Milligan Citation1946) and mounted with Eukitt®.
A. mediterranea and A. squamata samples embedded in resin were cut with a glass blade using a Reichert Ultracut E into both longitudinal and cross sections of 1 μm in thickness. Slides were immediately treated with sodium methoxide, methanol, and ethanol, stained with 1% crystal violet in DW and 1% basic fuchsin in DW and mounted with Eukitt®.
Both paraffin and resin sections were observed under a Jenaval light microscope provided with a Leica EC3 camera and Leica Application Suite LAS EZ Software (version 1.8.0). C. tenuispina paraffin sections were observed also under a NanoZoomer S60 (Hamamatsu).
The photographs of the observed sections were compiled into plates with the GNU Image Manipulation Program 2.8 (GIMP).
2.5. Isolation of brittle star skeletal elements for scanning electron microscopy (SEM)
SEM analysis was performed for A. squamata in order to help the precise reconstruction of the gross arm anatomy in a non-regenerating situation, which, to the best of our knowledge, has never been thoroughly described in the literature. Therefore, two non-regenerating arms were immersed in a 0.1 M solution of NaOH for 4 days at 4°C to dissolve the organic tissue. Gentle mechanical pressure was applied to help separate the skeletal elements (arm plates, spines, and vertebrae). After several washings with DW to remove the excess of NaOH, the isolated skeletal elements were left to air dry, placed on a stub and sputter-coated (Sputter Coater Nanotech) with pure gold for observation under a scanning electron microscope (SEM LEO-1430).
SEM analysis of crinoids and starfish skeletal elements was not performed since ossicle morphology and anatomy in these classes are already well described in the literature (Gislén Citation1924; Hyman Citation1955).
3. Results
3.1. Non-regenerating arm anatomy
The general arm anatomy of the models used in the present study has already been described for Antedon mediterranea (Candia Carnevali & Bonasoro Citation2001) and Echinaster sepositus (Ben Khadra et al. Citation2015a); therefore, it does not need to be described here in great detail, but only briefly for the sake of comparison among classes. Coscinasterias tenuispina anatomy and morphology closely resemble that of the previously described Marthasterias glacialis (Daviddi Citation2014). To the best of our knowledge, the internal arm anatomy of Amphipholis squamata has not been described in great detail to date. It is therefore presented here in more detail than that of the other models.
3.1.1. Antedon mediterranea (Crinoidea)
The anatomy of crinoids () diverges considerably from that of starfish () and brittle stars () having preserved some plesiomorphic characters of all the extant taxa. The arms, branching from the central calyx, are divided into segments with ossicles enclosing muscles, ligaments, and the brachial nerve (entoneural component), the most aboral of the continuous structures. The oral portion of the segments contains not only four coelomic canals derived from the somatocoel (the medial aboral coelom and genital canal, and the two lateral subtentacular coeloms), but also the Radial Water Canal (RWC), the most oral continuous structure. On both sides of the ambulacral groove, the arm branches into pinnules that have the same internal structure as the arm itself. In the pinnules and in the edges of the ambulacral groove, the RWC gives rise to the oral tube feet (for further details see Hyman Citation1955).
Figure 1. Schematic reconstruction of the non-regenerating arm anatomy of a generic crinoid (A) and a generic asteroid (B), both in cross section. The oral side is on the top in the crinoid and on the bottom in the asteroid. In both schemes elements labelled in red are muscles. An image of the whole organism is provided as outlined below; the red line represents the plane of the arm that is shown in cross section. Abbreviations: a, ampulla of tube foot; ae, ambulacral epithelium; ag, ambulacral groove; am, ambulacral muscle; ac, aboral coelom; ao, ambulacral ossicle; bm, brachial muscle; bn, brachial nerve; bo, brachial ossicle; ct, connective tissue; ed, epidermis; ep, entoneural plexus; gc, genital canal; li, ligament; pa, papula; pc, pyloric caeca; po, parietal ossicle; rnc, radial nerve cord; rwc, radial water canal; s, spine; sc, somatocoel; tc, subtentacular coelom; tf, tube foot; wvs, water vascular system. Made with GIMP.
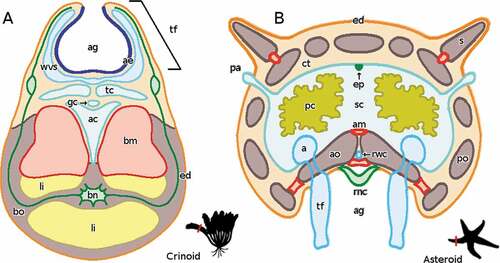
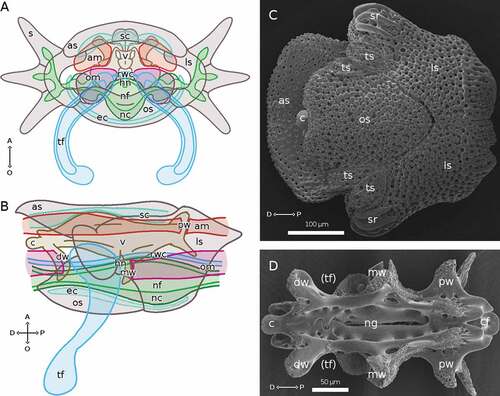
Figure 2. Non-regenerating anatomy of a typical arm segment of A. squamata. (A) Arm segment in distal view, drawn to show the internal anatomical features. (B) Arm segment in lateral view, drawn to show the internal anatomical features. (C) SEM micrograph of an arm segment in oral view, to show all skeletal elements. (D) SEM micrograph of an isolated vertebra in oral view; (tf) marks the space where the base of a tube foot is located. (B, D) Distal end shown at left. Abbreviations: A, aboral side; am, aboral muscle; as, aboral plate; c, distal condyle of vertebra; cf, condylar fossa of vertebra; D, distal side; dw, distal wing of vertebra; ec, epineural canal; hn, hyponeural component of the RNC; ls, lateral plate; mw, medial wing of vertebra; nc, cellular ectoneural component of the RNC; nf, fibrous ectoneural component of the RNC; ng, RNC groove of vertebra; O, oral side; om, oral muscle; os, oral plate; P, proximal side; pw, proximal wing of vertebra; rwc, radial water canal; s, spine; sr, spine ridges; sc, somatocoel; tf, tube foot; ts: tentacle scale; v, vertebra. A and B made with GIMP.
3.1.2. Echinaster sepositus and Coscinasterias tenuispina (Asteroidea)
The body wall of a starfish arm, covered by the epidermis, contains a number of parietal ossicles (). An ambulacral groove, composed of adjacent ambulacral ossicles, homologous to brittle star vertebrae, runs along the oral side of each arm. The three continuous arm structures are present in the same aboral-oral distribution as in brittle stars (see below) with the somatocoel as the most aboral, the Radial Nerve Cord (RNC) as the most oral and the RWC in between. Differently from brittle stars, the somatocoel is very large, and contains the gonads and the pyloric caeca branching from the stomach; a poorly developed entoneural nerve or plexus runs along its aboral surface. The RWC is hosted between the ambulacral ossicles with the tube feet, ending in suckers, extending into the ambulacral groove and their corresponding ampullae in the somatocoel. Differently from E. sepositus, in C. tenuispina the pairs of tube feet overlap so as to form four parallel rows. The RNC (hyponeural and ectoneural components) runs along the ambulacral groove, and, differently from brittle stars, it is directly exposed to the environment. The RWC ends distally with a terminal tube foot, which orally hosts, in its distal end, the typical neuro-photosensitive organ of starfish, called optic cushion. Both structures are protected by the overlying terminal ossicle, which does not completely surround them as does, instead, that of brittle stars.
3.1.3. Amphipholis squamata (Ophiuroidea)
Observ-ations via light microscopy and TEM analysis of non-regenerating arms of A. squamata were performed to understand and describe the arm anatomy of this species in standard conditions, which is otherwise poorly described in the literature (for TEM analyses see Supplementary Materials, Figure S3).
As is typical of brittle stars, the arms, branching from the central disc, are organized into segments covered by the epidermis. In each segment, embedded in the dermal layer, there are a central vertebral ossicle and the dermal arm plates: one oral, one aboral, and two lateral, those latter bearing ridges into which three pairs of spines are inserted ()). As in other brittle stars, the vertebrae have a very complex morphology (), extending through all the segments’ length. At the distal end, a condyle fits into a socket on the proximal end of the following vertebral ossicle. Three pairs of lateral wing-like extensions are visible, to which muscles are attached. The proximal-most pair extends aborally, and the aboral muscles are attached to its sides. The oral muscles, instead, are attached to the distal face of the distal-most pair and to the proximal face of the middle pair (). Tube feet emerge between the oral and lateral arm plates () and their bases are protected by a pair of small tentacle scales. The aboral arm plate protrudes distally more than the oral one, fitting into the one of the following segments (). SEM analysis shows the specific stereomic structure of each of these skeletal elements ().
In a typical cross section of a segment, the visible structures are, from the aboral to the oral side, the aboral arm plate, the somatocoel, the vertebral ossicle, the RWC, the RNC, the epineural canal (surrounding the RNC, homologous to the ambulacral groove of starfish), and the oral arm plate (). The distal-most arm segment has only one skeletal element, the cone-shaped terminal ossicle, surrounding the terminal tube foot (see, e.g. . The somatocoel, the most aboral of the three continuous structures, runs through the whole arm just below the aboral arm plate and it varies in width within each segment, descending along the lateral arm plates in correspondence of the spines, and narrowing between adjacent segments. The second continuous structure, the RWC, runs from the central disc to the distal tip of the arm, where it protrudes out of the body wall as the terminal tube foot. A pair of tube feet branches out laterally in each segment (); while related species, such as Amphiura filiformis, have pseudoampullae, in A. squamata these are not present. The RNC, the most oral continuous structure, also runs from the nervous ring in the central disc into the terminal tube foot, forming, at the centre of each segment, a swelling, called pseudoganglion (Figure S3(A)), from which smaller ganglia depart towards the bases of spines and tube feet, and narrowing in the intergangliar region at the level of the tube feet bases. The ectoneural cellular component is much more abundant in the pseudoganglion than in the intergangliar region (Figure S3(A)) and fibres run in both proximal-distal and aboral-oral directions along the whole RNC length.
3.2. Regeneration of the continuous arm structures
3.2.1. Nerve cord
3.2.1.1. Antedon mediterranea
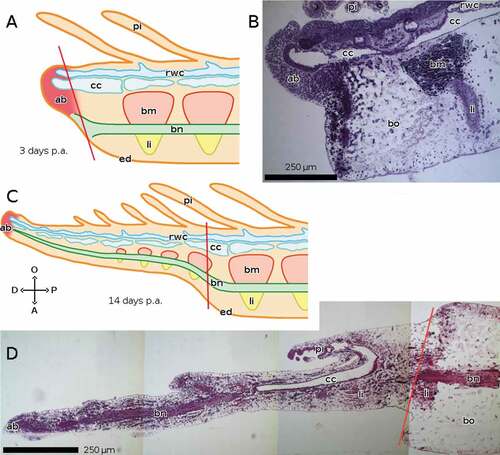
In A. mediterranea, in the early phase of regeneration, around 3 days post-amputation (p.a.), the brachial nerve stops at the plane of amputation, although it starts emitting nervous fibres into the regenerating tip on its oral side, which is mostly formed by the apical blastema. Note that, unlike the RNC of the other described species, the brachial nerve of Crinoidea is aboral (). In the advanced phase, by 14 days p.a., the brachial nerve reaches the end of the regenerate, again ending in correspondence with the apical blastema. It bends orally, thus entering the regenerate, which has a smaller size. Repeated muscles and ligaments form around the nerve. Lateral branches of the brachial nerve project into the pinnules ().
Figure 3. Sagittal sections of arms of A. mediterranea during regeneration. The distal end of the regenerate is on the left in both cases. (A, B) 3 days p.a. (early regeneration) ((A) schematic drawing). (C, D) 14 days p.a. (advanced regeneration) ((C) schematic drawing). Staining of B and D: crystal violet and basic fuchsin. Abbreviations: A, aboral side; ab, apical blastema; bm, brachial muscle; bn, brachial nerve; bo, brachial ossicle; cc, coelomic canals; D, distal side; ed, epidermis; li, ligament; O, oral side; P, proximal side; pi, pinnula; rwc, radial water canal. Red lines mark the planes of amputation. A and C made with GIMP.
3.2.1.2. Echinaster sepositus
The repair phase of regeneration in E. sepositus occurs over 3 days, with the formation of a new complex epidermis covering the plane of amputation about 1–2 days p.a. By that point, the RNC has already healed, and in the distal-most part the cellular component alone is visible (Ben Khadra et al. Citation2015a). At 3 weeks p.a., the RNC of E. sepositus already reaches the end of the regenerate, which is less than 1 mm long. Both the cellular and the fibrous components are visible, although the former is still visibly thicker than the latter, and the optic cushion is still missing ().
Figure 4. Sagittal sections of arms of E. sepositus during regeneration. The distal end of the regenerate is on the left in all cases. (A-C) 3 weeks p.a. ((B) schematic drawing, (C) detail). (D, E) 6 weeks p.a. ((E) schematic drawing). (F, G) >>16 weeks p.a. ((G) schematic drawing). The * in F marks an artifact: the coelothelium detached from the overlying tissue during preparation, which does not correspond to any anatomical feature in the living animal. Staining of A, C, D and F: Milligan trichrome technique. Abbreviations: A, aboral side; a, ampulla; am, ambulacral muscle; ao, ambulacral ossicle; ct, connective tissue; D, distal side; ed, epidermis; lwc, lateral water canal; mg, mucous gland; nc, cellular component of the RNC; nf, fibrous component of the RNC; O, oral side; oc, optic cushion; P, proximal side; pc, pyloric caeca; po, parietal ossicle; rnc, radial nerve cord; rwc, radial water canal; sc, somatocoel; tf, tube foot; to, terminal ossicle; ttf, terminal tube foot. Red lines mark the planes of amputation. B, C, E and G made with GIMP.
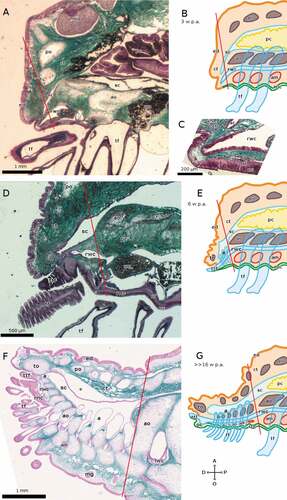
At 6 weeks p.a., the RNC of E. sepositus is swelling at the distal end, and the fibrous component has become thicker than the cellular component in the proximal region of the regenerate ().
In the advanced phase of regeneration (>>16 weeks p.a.), the RNC of E. sepositus ()) has assumed its definitive form with a well-differentiated optic cushion, although it is not yet the definitive size as the regenerate is around 4 mm long.
3.2.1.3. Coscinasterias tenuispina
By 3 weeks p.a., the RNC of C. tenuispina is much more developed than that of E. sepositus at the same time-point, the fibrous component being thicker than the cellular one, and the optic cushion being already visible as a red spot at the distal-oral end of the regenerate, which is about 1 mm long ().
Figure 5. Sagittal and cross sections of arms of C. tenuispina during regeneration. The distal end of the regenerate is on the left in all cases. (A–C) 3 weeks p.a. ((A) sagittal section, (B) schematic drawing, (C) cross section). (D–G) 6 weeks p.a. ((D) sagittal section, (E) schematic drawing, (F, G) cross sections). The arrowhead in D marks a putative mass of coelomocytes in the lumen of a tube foot. (H–J) >>16 weeks p.a. ((H, J) cross sections, (I) schematic drawing). Staining of A, C, D, F, G and H: Milligan trichrome technique. Abbreviations: A, aboral side; a, ampulla; ao, ambulacral ossicle; as, ambulacral spine; ct, connective tissue; D, distal side; ed, epidermis; hs, hyponeural sinus; lm, lower ambulacral muscle; lwc, lateral water canal; nc, cellular component of the RNC; nf, fibrous component of the RNC; O, oral side; oc, optic cushion; P, proximal side; pa, papula; pe, pedicellaria; po, parietal ossicle; rnc, radial nerve cord; rwc, radial water canal; s, spine; sc, somatocoel; tf, tube foot; to, terminal ossicle; ttf, terminal tube foot; um, upper ambulacral muscle. Red lines mark the planes of amputation. B, E and I made with GIMP.
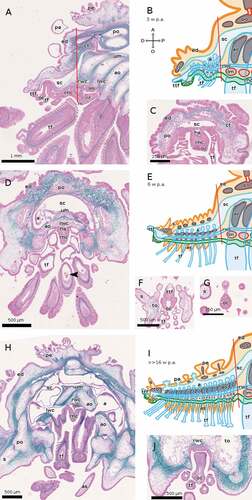
At 6 weeks p.a., the regenerate of C. tenuispina is over 2 mm long; its RNC has taken on its definitive structure, with the cellular component forming only a thin layer orally to the thicker fibrous component (). The pigment-cup ocelli of the optic cushion are well visible ().
In the advanced phase of regeneration (>>16 weeks p.a.), the RNC of C. tenuispina () has also assumed its definitive form with a well-differentiated optic cushion, and is around 10 mm long.
3.2.1.4. Amphipholis squamata
In the brittle star A. squamata, none of the continuous structures grows at all in the first day p.a., in which regeneration is limited to the growth of new epidermis over the wound and the accumulation of undifferentiated/rearranging tissue (). After that, the RNC is the first continuous structure to grow, starting, between 1 and 3 days p.a., with the cellular component of the ectoneural system, whereas the fibrous and hyponeural components do not extend (). At this point, the RNC is already surrounded by a new epineural canal. Between 3 and 5 days p.a. the regenerative bud forms, protruding from the oral side of the stump. The cellular component of the RNC extends throughout the length of the bud, whereas the fibrous and hyponeural components only start differentiating proximally ()). Between 5 and 7 days p.a., all the components of the RNC are differentiated along the complete length of the regenerating arm. In addition, the RNC thickness increases in the tracts that will become the pseudoganglia; this is the first trace of arm segmentation. The neuropile (the continuous fibrous component) is also completely differentiated (). By 14 days p.a., the differentiation between pseudoganglia and intergangliar tracts is evident. The presence of pseudoganglia and muscle are diagnostic of this stage, in which arm segmentation is complete, although the distal-most segments are still not completely differentiated (). The RNC has taken its definitive form: the only difference it shows between 14 and 21 days p.a. is its increased size ().
Figure 6. Sagittal sections of arms of A. squamata during repair and early regenerative stages, each shown as histological section (left) and as schematic drawing (right). The distal end of the regenerate is on the left in all cases. (A, B) 0–1 days p.a. (C, D) 1–3 days p.a. The arrowhead in C marks the growth of new cellular ectoneural tissue enveloping the end of the RWC. (E, F) 3–5 days p.a. Staining of A, C and E: crystal violet and basic fuchsin. Abbreviations: A, aboral side; am, aboral muscles; as, aboral plate; ct, coelothelium; D, distal side; es, epineural sinus; hn, hyponeural component of the RNC; li, ligament; nc, cellular ectoneural component of the RNC; nf, fibrous ectoneural component of the RNC; O, oral side; om, oral muscles; os, oral plate; P, proximal side; rwc, radial water canal; sc, somatocoel; ut, undifferentiated tissue; v, vertebra. Red lines mark the planes of amputation. B, D and F made with GIMP.
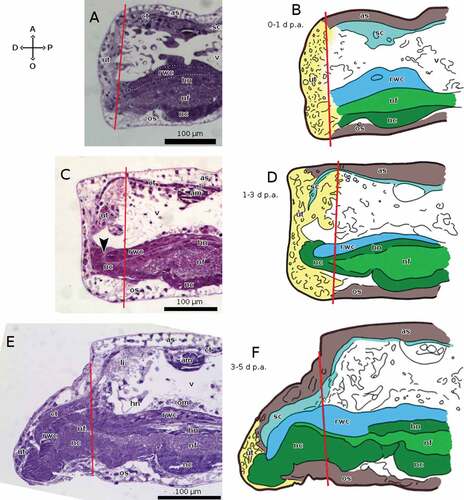
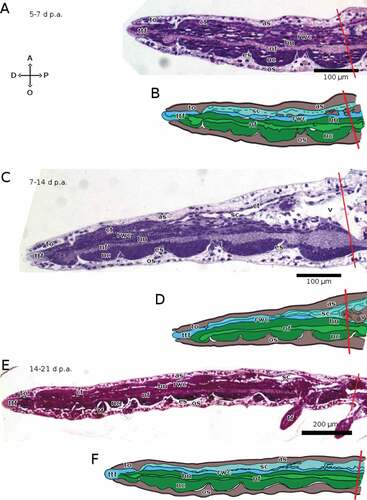
Figure 7. Sagittal sections of arms of A. squamata during advanced regenerative stages, each shown as histological section (above) and as schematic drawing (below). The distal end of the regenerate is on the left in all cases. (A, B) 5–7 days p.a. (C, D) 7–14 days p.a. (E, F) 14–21 days p.a. Staining of A, C and E: crystal violet and basic fuchsin. Abbreviations: A, aboral side; as, aboral plate; ct, coelothelium; D, distal side; es, epineural sinus; hn, hyponeural component of the RNC; nc, cellular ectoneural component of the RNC; nf, fibrous ectoneural component of the RNC; O, oral side; os, oral plate; P, proximal side; rwc, radial water canal; sc, somatocoel; tf, tube foot; to, terminal ossicle; ttf, terminal tube foot; v, vertebra. Red lines mark the planes of amputation. B, D and F made with GIMP.
3.2.2. Radial water canal
3.2.2.1. Antedon mediterranea
In A. mediterranea, later in the early phase of regeneration, around 3 days p.a., the RWC extends into the apical blastema of the regenerate, although lateral canals are still missing; the ambulacral groove reaches the blastema as well (). In the advanced phase, around 14 days p.a., as pinnules are starting to grow at the sides of the ambulacral groove, lateral water canals project into them from the RWC to form the tube feet on their oral surface ().
3.2.2.2. Echinaster sepositus
Regarding starfish, in E. sepositus the RWC contracts very quickly after amputation to limit the loss of internal fluid, enabling even the distal-most tube feet to retain their inner pressure (Ben Khadra et al. Citation2015a). At 3 weeks p.a., the RWC of E. sepositus extends against the regenerating body wall just beyond the plane of amputation, still without protruding outside (); its inner coelomic epithelium is thicker than that of the stump ().
At 6 weeks p.a., the terminal tube foot of E. sepositus is fully formed; the proximal tube feet are developing, their ampullae being clearly visible in the somatocoel, but the tube feet are wholly enclosed within the arm ().
In the advanced phase of regeneration (>>16 weeks p.a.), the tube feet of E. sepositus reach the outside and have similar proportions to those of the stump, though the suckers are only present in the most proximal ones ().
3.2.2.3. Coscinasterias tenuispina
At 3 weeks p.a., the RWC of C. tenuispina already emerges as terminal tube foot, though it is very short and partially hidden/protected by the body wall; four rows of small tube feet have already formed in the proximal-most part of the regenerate ().
At 6 weeks p.a., the tube feet are well developed: they are still much smaller than those in the stump, but they are proportionally longer, and they end in suckers. Putative masses of coelomocytes are visible in the lumen of the tube feet ().
In the advanced phase of regeneration (>>16 weeks p.a.), the regenerate has many more pairs of tube feet but their structure has otherwise changed very little (). Overall, in both species the regenerating vascular system is similar to the mature structure, except for its size.
3.2.2.4. Amphipholis squamata
In the brittle star A. squamata, the RWC starts growing after the RNC, between 3 and 5 days p.a., when it starts extending into the regenerative bud, aborally to the RNC (). Between 5 and 7 days p.a., the RWC reaches the arm’s distal end, and protrudes from it as the terminal tube foot (). By 14 days p.a., the RWC starts extending laterally to form the paired tube feet in the proximal-most segments of the regenerating arm. Over the following week of regeneration, the tube feet develop in the remaining regenerating segments in a proximo-distal direction. By 21 days p.a. they are present in all segments except the last one, which has only the terminal tube foot ().
3.2.3. Somatocoel
3.2.3.1. Antedon mediterranea
In A. mediterranea, already in the early phase of regeneration, around 3 days p.a., the coelomic canals that comprise the somatocoel of Crinoidea extend into the apical blastema more distally than the RWC; they do not need to bend, as the coelomic canals, unlike the somatocoel of the other examined species, have an oral position in the arm (). In the advanced phase, around 14 days p.a., the coelomic canals continue growing into the regenerate () and project into the pinnules.
3.2.3.2. Echinaster sepositus
In E. sepositus, the somatocoel is perhaps the first major anatomical structure to react to amputation, by contracting oro-aborally within the first hour to limit coelomic fluid loss, via the contraction of muscle fibres in the coelothelium. Furthermore, coelomocytes form a clot near the plane of amputation (Ben Khadra et al. Citation2015a). Although at 3 weeks p.a. the somatocoel of E. sepositus does not extend far past the plane of amputation, it already shows an evident bend in oral direction, including in the distal-most region of the stump, without reaching the body surface. All the bending tract is very narrow, and the coelothelium is significantly thickened ().
At 6 weeks p.a., the somatocoel of the E. sepositus regenerate also hosts the ampullae (). In the advanced phase of regeneration (>>16 weeks p.a.), the somatocoel of both starfish species is not entirely rectilinear yet, as the regenerate is still smaller than the stump and located on the oral side ().
3.2.3.3. Coscinasterias tenuispina
A contraction of the somatocoel similar to E. sepositus is also visible at 3 weeks p.a. in C. tenuispina, although in this case the somatocoel in the proximal-most part of the regenerate broadens into a space that contains the ampullae of the regenerating tube feet ().
At 6 weeks p.a., the somatocoel is broadening; it still contains only the ampullae, as the pyloric caeca have not yet regrown into it (). In the advanced phase of regeneration (>>16 weeks p.a.), it also starts protruding out of the body wall with the papulae before E. sepositus ().
3.2.3.4. Amphipholis squamata
In the brittle star A. squamata, in the first day p.a., the somatocoel ends abruptly at the wound (), but in the following 2 days it starts bending in an oral direction, as clearly visible in sagittal sections (). This enables it to penetrate into the regenerative bud, which appears on the oral side of the stump and only extends proximally to the RNC, which always represents the distal-most regenerating structure in the bud, between 3 and 5 days p.a. (). After 5 days p.a., the somatocoel shows little change in morphology other than in its size. In fact, TEM analysis shows that, at the distal part of the regenerating bud, somatocoel cell bodies are flat and elongated, but no signs of new cilia are visible (data not shown).
4. Discussion
4.1. The role of continuous structures in arm regeneration
Overall this study could help shed light on the mechanisms previously proposed for animal models belonging to other phyla that involve processes of distalization and intercalation as echinoderms occupy a strategic position within the organisms with a significant ability to regenerate being anatomically much more complex than most regeneration-competent invertebrates, but simpler than the phylogenetically closely related vertebrates. The chronological primacy of continuous structures (RNC, somatocoel, and RWC) over peripheral ones (muscles, ossicles, tube feet, spines) might suggest that the former play a critical signalling or organizing role in the development of the latter (Ben Khadra et al. Citation2017, Citation2018; Byrne et al. Citation2019). The following paragraphs propose a discussion on the putative role/importance of the three continuous arm structures involved in regeneration.
4.1.1. Nerve cord
In A. mediterranea, the brachial nerve plays a key role during regeneration (Candia Carnevali Citation2006). Although the brachial nerve shows modest regrowth in the first regenerative phase (Candia Carnevali et al. Citation1993), it provides a fundamental contribute by supplying and transporting most of the migratory cellular elements towards the amputation region. Among these elements in particular, Candia Carnevali et al. (Citation1993) suggested that granular cells could be interpreted as sort of glial elements with a specific trophic role during the regeneration of the brachial nerve. Proliferative activity along the nerve is particularly intense in the first 2 days p.a. (Candia Carnevali et al. Citation1995a). Furthermore, the brachial nerve and its distal extremity are surrounded by myocytes and collagen fibres which, before organizing into the new bands of muscles and ligaments, start to concentrate around these parts (Candia Carnevali & Bonasoro Citation2001). Certain granular amoeboid coelomocytes are a likely source of growth factors that regulate the successive development; the migration of such cells also occurs along the brachial nerve (Thorndyke & Candia Carnevali Citation2001). This latter feature appears to be a peculiarity of crinoids, but a heavy involvement of the RNC in the processes of regeneration is clear in all studied echinoderms (Ben Khadra et al. Citation2018, and see below).
As shown in the present study, the regenerate in brittle stars starts developing around the severed RNC. The greater development of the ophiuroid nervous system compared to other echinoderm classes might be correlated with the speed of their regeneration (Sköld & Rosenberg Citation1996; Fujita Citation2001; Biressi et al. Citation2010; Czarkwiani et al. Citation2016; Piovani et al. Citation2021). Thorndyke et al. (Citation2000), noting a strong proliferative activity in the epithelium of the RNC as well in as the coelothelium, pointed out that elongation of the RNC is a priority over its growth in mass, especially in burrowing brittle stars that use arms to gather food from seawater. This means that even the RNC itself appears to grow along the arm axis before extending laterally. It could be significant that, in A. squamata, it is the cellular component of the ectoneural complex of the RNC that extends first, not the (simpler) fibrous one as might superficially be assumed; the regeneration of new neurons and glial cells is a critical part of the regrowing process.
In the two starfish species analysed in this study, the proportion of RNC thickness composed of cells decreases with time, as its growth is outpaced by that of the fibrous layer. A study of the sea cucumber Holothuria glaberrima showed that the glial cells of the RNC start dividing after amputation and directly produce neurons as daughter cells (Mashanov et al. Citation2013). In the asteroid Asterina gibbosa it was observed that removal of the RNC stops arm regeneration altogether (Thorndyke & Candia Carnevali Citation2001) and for this reason it can be said that the whole process of arm regeneration is ultimately nerve-dependent.
In general, nerve supply appears necessary for correct and complete regenerative development of a structure throughout animal phyla. While regeneration begins even in structures with partially or entirely removed nerves, the regenerate is invariably distorted, incomplete, or otherwise abnormal (Seifert et al. Citation2012).
4.1.2. Somatocoel and water vascular system
As is often the case in metazoans that lack extensive blastocoel-derived circulatory vessels (Ruppert & Carle Citation1983; Muñoz-Chapuli et al. Citation2005), coelom-derived compartments, such as both the somatocoel and the water vascular system, take up many of the functions of a circulatory system. These include the emergency reaction after wounding, in order to produce clotting, and thereby closing the wound, with the coelomocytes (Moss et al. Citation1998; Pinsino et al. Citation2007; Gorshkov et al. Citation2009; Ferrario et al. Citation2018; Smith et al. Citation2018; Andrade et al. Citation2021). However, the importance of coelomic compartments is not limited to the performance of this function in the early repair phase. As mentioned above, cells from the somatocoel are important for new tissue formation in all echinoderms (Rieger & Lombardi Citation1987; Candia Carnevali et al. Citation1995b; Dolmatov Citation2020; Piovani et al. Citation2021). This means that a very precocious development of the new somatocoel is critical to the process of regeneration, being important for the transport of coelomocytes.
Accordingly, the very first recognizable structures in the apical blastema of A. mediterranea are the coelomic compartments. The blastemal cells seem to derive mainly from the migrating amoebocytes already employed in the previous repairing process. The coelomic structures regrow at first in the form of a solid cord of coelomocytes and only after do they undergo a cavitation process, forming open channels (Candia Carnevali et al. Citation1993).
Significant cellular proliferation can be observed in the coelothelium (to a level comparable to that of the blastema) as well flow of coelomocytes (produced from the coelothelium) along the coelomic canals in the first 3 days p.a.; similar proliferation occurs at the same time in the RWC epithelium as well, although not as intensely (Candia Carnevali et al. Citation1995a; Candia Carnevali & Bonasoro Citation2001). Biressi et al. (Citation2010) described the proliferative mass of tissue found in the earliest stages of regeneration of the brittle stars A. filiformis and O. longicauda as being formed by migrating cells brought to the amputation site by the coelom, and clustering around the distal end of the RNC. Similar observations were made in the starfish Asterias rollestoni (Fan et al. Citation2011).
The RWC is closely associated with the RNC, especially at the level of the terminal tube foot, where they are closest. In A. squamata, the new RWC begins to regenerate totally embedded in the growing RNC. While the latter has a critical role of signalling and organization, the former could have the task of transporting proliferating cells: masses of cells expressing a large amount of the morphogenetic molecule afBMP2/4 can be found in the RWC of A. filiformis, presumably set aside for regeneration following autotomy (Bannister et al. Citation2008).
The contribution of the coelomic canals to regeneration is not limited to cellular transport. The coelomic epithelium itself can act as a major producer of new tissue in all stellate classes (Ben Khadra et al. Citation2018). In asteroids, cells from the coelothelium and the epithelium of the RWC are apparently transferred to the site of regeneration to de-differentiate and proliferate (Mladenov et al. Citation1989; Thorndyke & Candia Carnevali Citation2001; Candia Carnevali Citation2006). A very clear thickening of these same epithelia was evident in the first stage of regeneration of E. sepositus as described in this study. In the coelothelium and RWC epithelium of M. glacialis, Guatelli (Citation2017) described a layer of vesicular cells with an extensive apparatus for protein synthesis, presumably involved in the production of growth factors and/or new cells.
4.2. Continuous and non-continuous arm structures
The continuous arm structures have a particular role in the overall body organization of echinoderms. Mooi et al. (Citation2005) distinguished an axial region (sensu Mooi), corresponding to the radia, which, in the course of ontogenesis, extends in a proximal-to-distal direction, and an extraxial region (sensu Mooi), corresponding to the interradia, which grows laterally from the former. According to Mooi’s definition – although not to the one employed elsewhere in this study – the axial structures include the arm segments of ophiuroids and the ambulacral ossicles of asteroids, as well as the RNC and the RWC. These are characterized by internal bilateral symmetry and a linear, sequential development, as opposed to the isotropic arrangement of interradial plates and ossicles, or the asymmetrical placement of other structures, such as anus and madreporite. In this model, axial development is determined by a fixed “ocular” element, corresponding to the terminal ossicle of asterozoans (), which grows away from the disc or calyx, leaving behind itself a row of axial elements (sensu Mooi), proximally older and distally younger. These expand sideways from the central axis of the radius. This process is consistent both with the regeneration model of distalization and intercalation (Agata et al. Citation2003, Citation2007) and with the ontogenetic influence of the axis on the periphery hitherto described, which might consist largely in providing positional information to their surroundings.
Gathering this information and combining them with the existing literature enabled us to build a simple general model () of regeneration in stellate echinoderms - that, considering phylogenetic relationships, might be valid also in the armless echinoids and holothuroids. The main characteristics of the process are:
The distal extremity of the arm develops first, and regulates the formation of the rest, growing away from the centre of the body;
The continuous arm structures form on the trail of the distal extremity, and regulate the formation of the peripheral structures;
Both continuous and peripheral structures develop in a proximo-distal direction, as the ones closer to the centre of the body formed before those farther away and are thus more differentiated.
Figure 8. Simplified summary diagram of the hypothetical process of regeneration in echinoderms. The question mark indicates hypothesised relations; yellow arrows indicate a developmental regulatory relation. The distal extremity of the arm is the first to form, and it regulates the formation of the other structures as it grows farther from the central body (Agata et al. Citation2007). The continuous structures of the arm, namely somatocoel, radial water canal (RWC) and radial nerve cord (RNC), form in proximo-distal sequence on the trail of the distal elements, and in turn, particularly the RNC, regulate the formation of the surrounding peripheral structures, such as muscles, ossicles, and tube feet (Mooi et al. Citation2005). The cells involved in arm regeneration appear to be provided by the continuous structures, deriving from the epithelium of somatocoel and RWC (e.g. Candia Carnevali & Bonasoro Citation2001), coelomocytes from these same structures (e.g. Biressi et al. Citation2010), dedifferentiated myocytes (Candia Carnevali & Bonasoro Citation2001), and (in crinoids) amoebocytes along the RNC (Thorndyke & Candia Carnevali Citation2001). Made with GIMP.
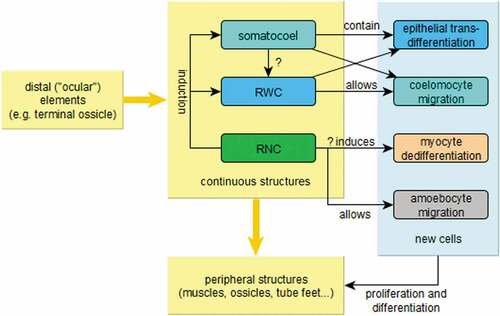
Figure 9. Schematic illustrations of the principles at work in echinoderm arm regeneration. The arm is modelled after a generic asterozoan, although the model also applies to crinoids. A: oral or aboral view; the stump and the distal structures (especially the latter) influence the intermediate regions, most importantly the continuous (axial, sensu Mooi) structures, which in turn influence the surrounding peripheral (extraxial, sensu Mooi) structures. The arrows represent the direction and importance of induction signalling, not necessarily the direction of growth. B: lateral view; somatocoel and RWC act as channels of migration for coelomic and undifferentiated cells that will form the new tissues. The same function is provided by dedifferentiated myocytes from the most distal muscles in the stump. In crinoids, granular amoebocytes (probably mostly regulatory) crawl along the brachial nerve. Masses of coelomic cells and the RNC also have a regulatory role, expressing Hox genes (which determine the proximo-distal axis of echinoderm radia; see David & Mooi Citation2014) and growth factors (Thorndyke & Candia Carnevali Citation2001). Made with GIMP.
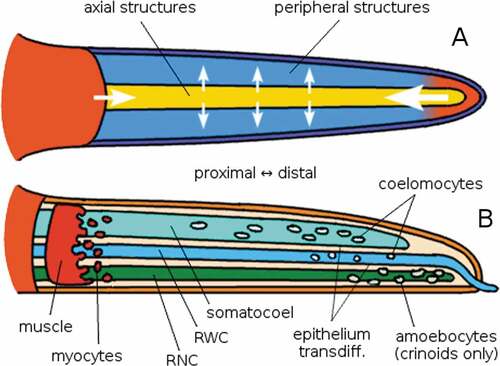
The nerve cord (i.e. the brachial nerve in crinoids, and the RNC in asterozoans; ) seems to be the primary signalling centre, whereas the somatocoel and the RWC epithelia are mainly involved as a source of cells contributing to repair and regeneration (Candia Carnevali & Bonasoro Citation2001; Candia Carnevali Citation2006; Ben Khadra et al. Citation2017, Citation2018; Ferrario et al. Citation2018; Byrne et al. Citation2019; Byrne Citation2020). These same structures are also the main channels of transport both of the cells that will physically take part in the construction of new tissues and of the cells expressing growth factors () (Candia Carnevali & Bonasoro Citation2001; Candia Carnevali Citation2006). In crinoids, the brachial nerve is also a major site of cell migration (Candia Carnevali et al. Citation1993).
4.3. Commonalities and differences among classes
In this study, the processes of distalization and intercalation described by Agata et al. (Citation2003, Citation2007) have been observed in all classes: the distal extremity of the arm is always the first to regenerate/differentiate (distalization). This latter then regulates the development of the intermediate region as it grows away from the central body in a proximo-distal direction (intercalation), with proximal structures maturing earlier than distal ones, with the exception of the extremity.
Wound repair occurs within a few days after amputation in all three classes. In asteroids, which have a particularly wide somatocoel, it is associated with an evident contraction of the body walls limiting coelomic fluid loss; in crinoids and ophiuroids, the same contraction is carried out separately by the muscular lining of the main canals, especially the RWC (Mladenov et al. Citation1989; Ben Khadra et al. Citation2018). In particular, in all three classes, the aboral and the oral plates fall back towards the injured area, in the first hours of regeneration, probably to close the somatocoel and the RWC in order to limit the loss of fluids. This is associated with a flow of coelomocytes towards the injured area, causing clotting and removal of pathogens (Gorshkov et al. Citation2009). In A. squamata, the terminal tube foot and the terminal ossicle are clearly visible (after 7 days of regeneration) when most arm segments are only slightly differentiated. In E. sepositus, it precedes the emergence of the regenerated tube feet. In C. tenuispina, it occurs before the earliest time-point studied here, but in the closely related Marthasterias glacialis the terminal tube foot appears a week before the earliest tube feet (Daviddi Citation2014). In A. mediterranea the distal region formed in the early stage is not an already well-differentiated structure/tissue such as in asteroids and ophiuroids, but a blastema, a localized pool of undifferentiated cells.
In all model species regeneration proceeds from the continuous arm structures, only secondarily extending to outer or lateral structures, such as muscles and ossicles, and finally to appendages, such as spines, papulae, and pinnules. For example, the RNC of A. squamata is already well structured in pseudoganglia and divided into cellular and fibrous components before spines and tube feet even begin to form. In E. sepositus, papulae do not appear until the new tube feet and ambulacral ossicles have already developed (Byrne et al. Citation2019).
Regeneration appears to be associated in all classes with a degeneration of muscle tissue in the stump, particularly in the distal-most area. Previous studies have hypothesized morphallactic de-differentiation and re-arrangement of stump muscle as one possible source of new material during regeneration (Candia Carnevali et al. Citation1998; Biressi et al. Citation2010; Ben Khadra et al. Citation2017). Whereas the contractile apparatus of the muscle cells could be recycled as source of macromolecules when found within phagocytes, it is not yet clear whether the nucleus undergoes the same fate, or originates a pluripotent cell (Ben Khadra et al. Citation2018). A similar degradation of proximal limb muscle is also observed in the regeneration of arthropod legs even if, in this case, it is currently unclear whether new muscles derive from former muscles that have undergone histolysis or from immigrant blastocytes of unknown origin (Maruzzo & Bortolin Citation2013). In all echinoderms cells for regeneration are mainly recruited via dedifferentiation, whereas the use of primarily undifferentiated cells has been documented for crinoids only (Candia Carnevali Citation2006; Dolmatov Citation2020; Ferrario et al. Citation2020).
As the antero-posterior axis of bilateral animals (as determined by the sequential expression of Hox genes) is homologous to the oro-aboral axis of echinoderms, and not to the proximo-distal axis of the radia (Arenas-Mena et al. Citation2000; David & Mooi Citation2014), the process described here should be compared to regeneration of appendages in other phyla (e.g. amphibian limbs), rather than of the main body axis. In particular, the distal-less gene, expressed along the proximo-distal axis of body appendages in several bilaterian phyla, including vertebrate and arthropod limbs, is also expressed along the radia of echinoderms, particularly in the WVS (Panganiban et al. Citation1997; Mooi et al. Citation2005), as are engrailed and other genes (Byrne et al. Citation2005, Citation2018).
While the modes of regeneration – particularly in the asterozoan classes (Ophiuroidea and Asteroidea) – show remarkable similarities, a key difference between crinoid and asterozoan models is the different level of organization of the distal region, the first to be regenerated (). Indeed, in crinoids, this is a true localized blastema, formed by the proliferation of coelomocytes and amoebocytes (Candia Carnevali & Bonasoro Citation2001; Ben Khadra et al. Citation2018), whereas asteroids and ophiuroids develop differentiated organs along the entire arm, already at an early stage. The terminal ossicle always appears simultaneously with the terminal tube foot, suggesting either a developmental or functional dependence of one on the other. In starfish, the optic cushion also occurs at the same time. summarizes the main similarities and differences in regeneration among stellate classes.
Table I. Comparison of the key features of regeneration in the stellate echinoderm classes. RNC = radial nerve cord; RWC = radial water canal
4.4. Conclusions and future perspective
The times and mechanisms of regeneration of all model species analysed in this research were consistent with those described in past studies. The concepts of distalization and intercalation (Agata et al. Citation2007) and axial (continuous) vs extraxial (non-continuous) region (Mooi et al. Citation2005) could be integrated into a coherent general model. Regeneration always starts with a wound repair phase. However, as proposed by Agata et al. (Citation2003, Citation2007) and recently suggested also for stellate echinoderms (Ben Khadra et al. Citation2018; Piovani et al. Citation2021), the earliest structure to be formed is the distal end of the arm, which apparently acts as a signalling centre and organizer for the development of the adjacent cells. This is similar to what has been described in the embryo development literature (Martinez Arias & Steventon Citation2018). Then, the intermediate regions of the continuous structures take shape from the interaction of the distal extremity with the stump. The cells of the nerve cord are the first to grow, probably having a central signalling role, but RWC and somatocoel follow immediately, transporting stem or dedifferentiated cells to the site of regeneration. At any given time, the proximal region is always more developed than the distal region.
As this study focused entirely on anatomy and histology, future research should include similar comparative research based on molecular data, particularly gene expression and the presence of growth factors. Because of their role in the organization of body axes during embryogenesis, promising targets include for example Hox genes (Duboule Citation2007), as suggested by preliminary studies on the expression of these gene cluster in E. sepositus arm regeneration (Ben Khadra et al. Citation2014); indeed, it would be interesting to investigate whether the body axes organizing role of these genes is conserved along evolution and between two different developmental processes (embryonic/regenerative). Another possibility is selective removal of structures that have key roles in regeneration, although this might prove difficult when such structures are wholly internal as in the case of crinoids. Lastly, another aspect to be considered in future research would be understand if the arm regeneration model of stellate echinoderms proposed here is also generalizable to the “armless” echinozoa. In this case, the analysis should be carried out mainly on the radial nerve cords and the radial canals of the water vascular system as it is rather difficult to trace the correspondence anatomy of the somatocoel, which is much wider than that present in the arm of a stellate echinoderm (Hyman Citation1955).
Authors’ contributions
FB, MS and CF conceived the study. AA, MC, CF and FB carried out the experiments on all species. AA realised schematic reconstructions. AA, MC, CF and FB wrote the manuscript. All authors analysed the data, contributed to and approved the final manuscript.
Supplemental Material
Download TIFF Image (15 MB)Supplemental Material
Download TIFF Image (8.8 MB)Supplemental Material
Download TIFF Image (4.7 MB)Supplemental Material
Download MS Word (3.1 MB)Acknowledgements
We are grateful to the Marine Protected Areas of Portofino and Bergeggi (Ligurian Sea, Italy) for permission to collect experimental animals. The authors wish to thank the scuba divers who helped with specimen collection, the Unitech NOLIMITS, imaging facility at the University of Milan, for the use of microscopes, and the anonymous reviewers’ valuable comments that improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
References
- Agata K, Saito Y, Nakajima E. 2007. Unifying principles of regeneration I: Epimorphosis versus morphallaxis. Development, Growth & Differentiation 49:73–78. DOI: 10.1111/j.1440-169X.2007.00919.x.
- Agata K, Tanaka T, Kobayashi C, Kato K, Saitoh Y. 2003. Intercalary regeneration in planarians. Developmental Dynamics 226:308–316. DOI: 10.1002/dvdy.10249.
- Andrade C, Oliveira B, Guatelli S, Martinez P, Simões B, Bispo C, Ferrario C, Bonasoro F, Rino J, Sugni M, Gardner R, Zilhão R, Coelho AV. 2021. Characterization of coelomic fluid cell types in the starfish Marthasterias glacialis using a flow cytometry/imaging combined approach. Frontiers in Immunology 12:641664. DOI: 10.3389/fimmu.2021.641664.
- Arenas-Mena C, Cameron AR, Davidson EH. 2000. Spatial expression of Hox cluster genes in the ontogeny of a sea urchin. Development 127(21):4631–4643. DOI: 10.1242/dev.127.21.4631.
- Bannister R, McGonnell IM, Graham A, Thorndyke MC, Beesley PW. 2008. Coelomic expression of a novel bone morphogenetic protein in regenerating arms of the brittle stars Amphiura filiformis. Development Genes and Evolution 218:33–38. DOI: 10.1007/s00427-007-0193-9.
- Bely AE, Nyberg KG. 2010. Evolution of animal regeneration: Re-emergence of a field. Trends in Ecology and Evolution 25(3):161–170. DOI: 10.1016/j.tree.2009.08.005.
- Ben Khadra Y, Ferrario C, Di Benedetto C, Said K, Bonasoro F, Candia Carnevali MD, Sugni M. 2015a. Wound repair during arm regeneration in the red starfish Echinaster sepositus. Wound Repair and Regeneration 23(4):611–622. DOI: 10.1111/wrr.12333.
- Ben Khadra Y, Ferrario C, Di Benedetto C, Said K, Bonasoro F, Candia Carnevali MD, Sugni M. 2015b. Re-growth, morphogenesis, and differentiation during starfish arm regeneration. Wound Repair and Regeneration 23(4):623–634. DOI: 10.1111/wrr.12336.
- Ben Khadra Y, Said K, Thorndyke M, Martinez P. 2014. Homeobox genes expressed during echinoderm arm regeneration. Biochemical Genetics 52(3–4):166–180. DOI: 10.1007/s10528-013-9637-2.
- Ben Khadra Y, Sugni M, Ferrario C, Bonasoro F, Coelho AV, Martinez P, Candia Carnevali MD. 2017. An integrated view of asteroid regeneration: Tissues, cells and molecules. Cell and Tissue Research 370(1):13–28. DOI: 10.1007/s00441-017-2589-9.
- Ben Khadra Y, Sugni M, Ferrario C, Bonasoro F, Oliveri P, Martinez P, Candia Carnevali MD. 2018. Regeneration in stellate echinoderms: Crinoidea, Asteroidea and Ophiuroidea. Results and Problems in Cell Differentiation 65:285–320.
- Bideau L, Kerner P, Hui J, et al. 2021. Animal regeneration in the era of transcriptomics. Cellular and Molecular Life Sciences 78:3941–3956. DOI: 10.1007/s00018-021-03760-7.
- Biressi ACM, Zou T, Dupont S, Dahlberg C, Di Benedetto C, Bonasoro F, Thorndyke M, Candia Carnevali MD. 2010. Wound healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): Comparative morphogenesis and histogenesis. Zoomorphology 129(1):1–19. DOI: 10.1007/s00435-009-0095-7.
- Brockes JP, Kumar A. 2008. Comparative aspects of animal regeneration. Annual Review of Cell and Developmental Biology 24:525–549. DOI: 10.1146/annurev.cellbio.24.110707.175336.
- Byrne M. 2020. The link between autotomy and CNS regeneration: Echinoderms as non-model species for regenerative biology. BioEssays 42:1900219. DOI: 10.1002/bies.201900219.
- Byrne M, Cisternas P, Elia L, Relf B. 2005. Engrailed is expressed in larval development and in the radial nervous system of Patiriella sea stars. Development Genes and Evolution 215(12):608–617. DOI: 10.1007/s00427-005-0018-7.
- Byrne M, Koop D, Morris VB, Chui J, Wray GA, Cisternas P. 2018. Expression of genes and proteins of the pax-six-eya-dach network in the metamorphic sea urchin: Insights into development of the enigmatic echinoderm body plan and sensory structures. Developmental Dynamics 247(1):239–249. DOI: 10.1002/dvdy.24584.
- Byrne M, Mazzone F, Elphick MR, Thorndyke MC, Cisternas P. 2019. Expression of the neuropeptide SALMFamide-1 during regeneration of the seastar radial nerve cord following arm autotomy. Proceedings of the Royal Society B 286(1901):20182701. DOI: 10.1098/rspb.2018.2701.
- Candia Carnevali MD. 2006. Regeneration in echinoderms: Repair, regrowth, cloning. Invertebrate Survival Journal 3(1):64–76.
- Candia Carnevali MD, Bonasoro F. 2001. Microscopic overview of crinoid regeneration. Microscopy Research and Technique 55(6):403–426. DOI: 10.1002/jemt.1187.
- Candia Carnevali MD, Bonasoro F, Lucca E, Thorndyke MC. 1995a. Pattern of cell proliferation in the early stages of arm regeneration in the feather star Antedon mediterranea. Journal of Experimental Zoology 272(6):464–474. DOI: 10.1002/jez.1402720608.
- Candia Carnevali M, Bonasoro F, Patruno M, and Thorndyke MC. 1998. Cellular and molecular mechanisms of arm regeneration in crinoid echinoderms: the potential of arm explants. Dev Gene Evol 208:421–430. DOI:10.1007/s004270050199.
- Candia Carnevali MD, Bonasoro F, Wilkie IC. 1995b. Coelom and tinkering in echinoids. In: Lanzavecchia G, Valvassori R, Carnevali C, editors. Body cavities: Phylogeny and function. Vol. 8. Selected Symposia and Monographs U.Z.I. Modena: Mucchi Editore. pp. 135–165.
- Candia Carnevali MD, Lucca E, Bonasoro F. 1993. Mechanisms of arm regeneration in the feather star Antedon mediterranea: Healing of wound and early stages of development. Journal of Experimental Zoology 267:299–317. DOI: 10.1002/jez.1402670308.
- Candia Carnevali MD, Sugni M, and Bonasoro F. 2022. Regeneration potential in echinoderms: Revisiting the regeneration concept. In: Saleuddin S, editor. Physiology of Invertebrates from Limited Taxa. Burlington, Canada: Apple Academic Press, Inc. in press.
- Chen L, Wang Z, Ghosh-Roy A, Hubert T, Yan D, O’Rourke S, Bowerman B, Wu Z, Jin Y, Chisholm AD. 2011. Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron 71(6):1043–1057. DOI: 10.1016/j.neuron.2011.07.009.
- Coffman JA. 2019. Regenerative potential across species: An eco-evo-devo perspective. In: Palacios D, editor. Epigenetics and regeneration. London, United Kingdom, San Diego, United States, Cambridge, United States, Oxford, United Kingdom: Academic Press, Elsevier. pp. 197–214.
- Czarkwiani A, Ferrario C, Dylus DV, Sugni M, Oliveri P. 2016. Skeletal regeneration in the brittle star Amphiura filiformis. Frontiers in Zoology 13:18. DOI: 10.1186/s12983-016-0149-x.
- David B, Mooi R. 2014. How Hox genes can shed light on the place of echinoderms among the deuterostomes. EvoDevo 5(1):22–40. DOI: 10.1186/2041-9139-5-22.
- Daviddi A. 2014. Microscopic anatomy of arm-tip regeneration in the starfish Marthasterias glacialis (Linneaus, 1758) following traumatic amputation. Master thesis, Università degli Studi di Milano.
- Dawydoff C. 1909. Beobachtungen über den Regenerationsprozess bei den Enteropneusten. Zeitschrift für wissenschaftliche Zoologie 93:237–305.
- Dehal P, Boore JL. 2005. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biology 3(10):e314. DOI: 10.1371/journal.pbio.0030314.
- Dietrich HF, and Fontaine AR. 1975. A decalcification method for ultrastructure of echinoderm tissues. Stain Technology 50(5):351–354. DOI:10.3109/10520297509117086.
- Dolmatov IY. 2020. Variability of regeneration mechanisms in echinoderms. Russian Journal of Marine Biology 46:391–404. DOI: 10.1134/S106307402006005X.
- Dolmatov IY. 2021. Molecular aspects of regeneration mechanisms in Holothurians. Genes (Basel) 12(2):250. DOI: 10.3390/genes12020250.
- Duboule D. 2007. The rise and fall of Hox gene clusters. Development 134:2549–2560. DOI: 10.1242/dev.001065.
- Elchaninov A, Sukhikh G, Fatkhudinov T. 2021. Evolution of regeneration in animals: A tangled story. Frontiers in Ecology and Evolution 9:621686. DOI: 10.3389/fevo.2021.621686.
- Fan T, Fan X, Du Y, Sun W, Zhang S, Li J. 2011. Patterns and cellular mechanisms of arm regeneration in adult starfish Asterias rollestoni Bell. Journal of the Ocean University of China 10:255. DOI: 10.1007/s11802-011-1837-y.
- Ferrario C, Ben Khadra Y, Czarkwiani A, Zakrzewski A, Martinez PF, Colombo G, Bonasoro F, Candia Carnevali MD, Oliveri P, Sugni M. 2018. Fundamental aspects of arm repair phase in two echinoderm models. Developmental Biology 433:297–309. DOI: 10.1016/j.ydbio.2017.09.035.
- Ferrario C, Sugni M, Somorjai IML, Ballarin L. 2020. Beyond adult stem cells: Dedifferentiation as a unifying mechanism underlying regeneration in invertebrate deuterostomes. Frontiers in Cell and Developmental Biology, Section Stem Cell Research 8:587320. DOI: 10.3389/fcell.2020.587320.
- Franco C, Soares R, Pires E, Koci K, Almeida AM, Santos R, Varela Coelho A. 2013. Understanding regeneration through proteomics. Proteomics 13(3–4):399–721. DOI: 10.1002/pmic.201370033.
- Fujita T. 2001. Arm regeneration frequency in bathyal populations of Ophiura sarsii off northern Japan. In: Baumiller TK, Féral JP, David B, editors. Echinoderm research 2001. Abingdon/Exton (PA)/Tokio: Balkema publishers Lisse. pp. 221–227.
- García-Arrarás JE, Estrada-Rodgers L, Santiago R, Torres II, Díaz-Miranda L, Torres-Avillán I. 1998. Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). The Journal of Experimental Zoology 281:288–304. DOI: 10.1002/(SICI)1097-010X(19980701)281:4<288::AID-JEZ5>3.0.CO;2-K.
- Gislén T. 1924. Echinoderm studies. The articulations of the arm-joints in the crinoids. Zoologiska Bidrag från Uppsala 9:1–330.
- Gorshkov AN, Blinova MI, Pinaev GP. 2009. Ultrastructure of coelomic epithelium and coelomocytes of the starfish Asterias rubens L. in norm and after wounding. Cell and Tissue Biology 3(5):477–490. DOI: 10.1134/S1990519X09050113.
- Guatelli S. 2017. Coelomic epithelium and coelomocytes of Marthasterias glacialis (Asteroidea) in normal and regenerating arm-tips: Microscopic anatomy and proteomics characterization. Master thesis, Universita’ degli Studi di Milano.
- Hyman LH. 1955. The invertebrates. Vol. IV. Echinodermata. New York: McGraw-Hill.
- Kassahn KS, Dang VT, Wilkins SJ, Andrew C, Perkins AC, Ragan MA. 2009. Evolution of gene function and regulatory control after whole-genome duplication: Comparative analyses in vertebrates. Genome Research 19:1404–1418. DOI: 10.1101/gr.086827.108.
- Kumar A, Brockes JP. 2012. Nerve dependence in tissue, organ, and appendage regeneration. Trends in Neurosciences 35(11):691–699. DOI: 10.1016/j.tins.2012.08.003.
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. 2008. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318:772–777. DOI: 10.1126/science.1147710.
- Lai AG, Aboobaker AA. 2018. EvoRegen in animals: Time to uncover deep conservation or convergence of adult stem cell evolution and regenerative processes. Developmental Biology 433:118–131. DOI: 10.1016/j.ydbio.2017.10.010.
- Laumer CE, Fernández R, Lemer S, Combosch D, Kocot KM, Riesgo A, Andrade SCS, Sterrer W, Sørensen MV, Giribet G. 2019. Revisiting metazoan phylogeny with genomic sampling of all phyla. Proceedings of the Royal Society B: Biological Sciences 286:20190831. DOI: 10.1098/rspb.2019.0831.
- Lengfeld T. 2009. Multiple Wnts are involved in Hydra organizer formation and regeneration. Developmental Biology 330:186–199. DOI: 10.1016/j.ydbio.2009.02.004.
- MacCord K, Maienschein J. 2019. Understanding regeneration at different scales. Elife 8:e46569. DOI: 10.7554/eLife.46569.
- Martinez Arias A, Steventon B. 2018. On the nature and function of organizers. Development 145(5):dev159525. DOI: 10.1242/dev.159525.
- Maruzzo D, and Bortolin F. 2013. Arthropod regeneration. In: Minelli A, Boxshall G, and Fusco G, editors. Arthropod biology and evolution. Berlin: Springer-Verlag. pp. 149–169.
- Mashanov VS, Zueva OR, García-Arrarás JE. 2013. Radial glial cells play a key role in echinoderm neural regeneration. BMC Biology 11:49. DOI: 10.1186/1741-7007-11-49.
- Medina-Feliciano JG, García-Arrarás JE. 2021. Regeneration in echinoderms: Molecular advancements. Frontiers in Cell and Developmental Biology 9:768641. DOI: 10.3389/fcell.2021.768641.
- Meyer A, Schartl M. 1999. Gene and genome duplications in vertebrates: The one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Current Opinion in Cell Biology 11:699–704. DOI: 10.1016/S0955-0674(99)00039-3.
- Miller BM, Johnson K, Whited JL. 2019. Common themes in tetrapod appendage regeneration: A cellular perspective. EvoDevo 10:11. DOI: 10.1186/s13227-019-0124-7.
- Milligan M. 1946. Trichrome stain for formalin-fixed tissue. American Journal of Clinical Pathology 10(6):184. DOI: 10.1093/ajcp/16.11_ts.184.
- Mladenov PV, Bisgrove B, Asotra S, Burke RD. 1989. Mechanisms of arm-tip regeneration in the sea star, Leptasterias hexactis. Roux’s Archives of Developmental Biology 198(1):19–28. DOI: 10.1007/BF00376366.
- Mooi R, David B, Wray GA. 2005. Arrays in rays: Terminal addition in echinoderms and its correlation with gene expression. Evolution & Development 7(6):542–555. DOI: 10.1111/j.1525-142X.2005.05058.x.
- Moss C, Hunter AJ, Thorndyke MC. 1998. Patterns of bromodeoxyuridine incorporation and neuropeptide immunoreactivity during arm regeneration in the starfish Asterias rubens. Philosophical Transactions of the Royal Society of London B: Biological Sciences 53(1367):421–436. DOI: 10.1098/rstb.1998.0220.
- Muñoz-Chapuli R, Carmona R, Guadix JA, Macías D, Pérez-Pomares JM. 2005. The origin of the endothelial cells: An evo-devo approach for the invertebrate/vertebrate transition of the circulatory system. Evolution & Deevelopment 7(4):351–358. DOI: 10.1111/j.1525-142X.2005.05040.x.
- Nieves-Ríos C, Alvarez-Falcón S, Malavez S, Rodriguez-Otero J, García-Arrarás JE. 2020. The nervous system component of the mesentery of the sea cucumber Holothuria glaberrima in normal and regenerating animals. Cell and Tissue Research 380:67–77. DOI: 10.1007/s00441-019-03142-3.
- Panganiban G, Irvine SM, Lowe C, Roehl H, Corley LS, Sherbon B, Grenier JK, Fallon JF, Kimble J, Walker M, Wray GA, Swalla BJ, Martindale MQ, Carroll SB. 1997. The origin and evolution of animal appendages. PNAS 94(10):5162–5166. DOI: 10.1073/pnas.94.10.5162.
- Pinsino A, Thorndyke MC, Matranga V. 2007. Coelomocytes and post-traumatic response in the common sea star Asterias rubens. Cell Stress & Chaperones 12(4):331–341. Dec. DOI: 10.1379/CSC-288.1.
- Piovani L, Czarkwiani A, Ferrario C, Sugni M, Oliveri P. 2021. Ultrastructural and molecular analysis of the origin and differentiation of cells mediating brittle star skeletal regeneration. BMC Biology 19(1):9–16. DOI: 10.1186/s12915-020-00937-7.
- Pirotte N, Leynen N, Artois T, Smeets K. 2016. Do you have the nerves to regenerate? The importance of neural signalling in the regeneration process. Developmental Biology 409:4–15. DOI: 10.1016/j.ydbio.2015.09.025.
- Quiñones JL, Rosa R, Ruiz DL, Garcia-Arrarás JE. 2002. Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Developmental Biology 250:181–197. DOI: 10.1006/dbio.2002.0778.
- Rieger RM, Lombardi J. 1987. Ultrastructure of coelomic lining in echinoderm podia: Significance for concepts in the evolution of muscle and pertitoneal cells. Zoomorphology 107:191–208. DOI: 10.1007/BF00312261.
- Rinkevich B, Ballarin L, Martinez P, Somorjai I, Ben-Hamo O, Borisenko I, Berezikov E, Ereskovsky A, Gazave E, Khnykin D, et al. 2022. A pan-metazoan concept for adult stem cells: The wobbling Penrose landscape. Biological Reviews 97(1):299–325. Online ahead of print. DOI: 10.1111/brv.12801.
- Rinkevich B, Shlemberg Z, Fishelson L. 1995. Whole-body protochordate regeneration from totipotent blood cells. Proceedings of the National Academy of Sciences of the United States of America 92(17):7695–7699. 15. DOI: 10.1073/pnas.92.17.7695.
- Ruppert EE, Carle KJ. 1983. Morphology of metazoan circulatory systems. Zoomorphology 103:193–208. DOI: 10.1007/BF00310477.
- Sánchez Alvarado A. 2000. Regeneration in the metazoans: Why does it happen? Bioessays 22(6):578–590.
- Seifert AW, Monaghan JR, Smith MD, Pasch B, Stier AC, Michonneau F, Maden M. 2012. The influence of fundamental traits on mechanisms controlling appendage regeneration. Biological Reviews 87(2):330–345. DOI: 10.1111/j.1469-185X.2011.00199.x.
- Sinigaglia C, Averof M. 2019. The multifaceted role of nerves in animal regeneration. Current Opinion in Genetics and Development 57:98–105. DOI: 10.1016/j.gde.2019.07.020.
- Sköld M, Rosenberg R. 1996. Arm regeneration frequency in eight species of Ophiuroidea (Echinodermata) from European Sea areas. Journal of Sea Research 35(4):353–362. DOI: 10.1016/S1385-1101(96)90762-5.
- Smith LC, Arizza V, Barela Hudgell MA, Barone G, Bodnar AG, Buckley KM, Cunsolo V, Dheilly NM, Franchi N, Fugmann SD, Furukawa R, Garcia-Arraras J, Henson JH, Hibino T, Irons ZH, Li C, Lun CM, Majeske AJ, Oren M, Pagliara P, Pinsino A, Raftos DA, Rast JP, Samasa B, Schillaci D, Schrankel DS, Stabili L, Stensväg K, Sutton E. 2018. Echinodermata: The complex immune system in echinoderms. In: Cooper E, editor. Advances in comparative immunology. Cham: Springer. DOI: 10.1007/978-3-319-76768-0_13.
- Sugni M, Wilkie IC, Burighel P, Candia Carnevali MD. 2010. New evidence of serotonin involvement in the neurohumoral control of crinoid arm regeneration: Effects of parachlorophenylanine and methiothepin. Journal of the Marine Biological Association of the United Kingdom 90(3):555–562. DOI: 10.1017/S0025315409990531.
- Sun L, Chen M, Yang H, Wang T, Liu B, Shu C, Gardiner DM. 2011. Large scale gene expression profiling during intestine and body wall regeneration in the sea cucumber Apostichopus japonicus. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 6:195–205.
- Tanaka EM. 2016. The molecular and cellular choreography of appendage regeneration. Cell 165:1598–1608. DOI: 10.1016/j.cell.2016.05.038.
- Tanaka EM, Reddien PW. 2011. The cellular basis for animal regeneration. Developmental Cell 21(1):172–185. DOI: 10.1016/j.devcel.2011.06.016.
- Thorndyke MC, Candia Carnevali MD. 2001. Regeneration neurohormones and growth factors in echinoderms. Canadian Journal of Zoology 79(7):1171–1208.
- Thorndyke MC, Patruno M, Moss C, Beesley PW, Mallefet J. 2000. Cellular and molecular bases of regeneration in brittle stars. Echinoderms 2000: Proceedings of the 10th International Conference, 31 January–4 February, Dunedin: Taylor & Francis.
- Thouveny Y, Tassava RA. 1997. Regeneration through phylogenesis. In: Ferretti P, Géraudie J, editors. Cellular and molecular basis of regeneration: From invertebrates to humans. New York: John Wiley and Sons. pp. 9–43.
- Tweedell KS. 1961. Regeneration of the enteropneust, Saccoglossus kowalevskii. The Biological Bulletin 120:118–127. DOI: 10.2307/1539342.
