Abstract
In this paper, samples of mosses and lichens collected from Ivohibory Forest (south-central Madagascar) were studied in search for tardigrades. In total, 39 tardigrade taxa were identified, 21 of which are new records for Madagascar. Moreover, one of these is a new species for science. Bryodelphax beniowskii sp. nov., described here based on morphological analysis. It is most similar to Bry. olszanowskii and Bry. parvuspolaris, but differs from them mainly by ventral plates arrangement and some other morphometric characters.
Introduction
Madagascar is the world’s fourth-largest Island located in the Indian Ocean around 400 km to the east of the eastern shores of Africa. It stretches between ~12° to ~26°S latitude, occupying in total ca. 590,000 km2. Although originally a part of Gondwana, Madagascar Plate broke away from it together with Antarctic, Indian and Australian plates. Madagascar finally separated from the Seychelles and India ca. 66–90 million years ago, leading to its current form. Due to this unique history, the Island is sometimes called a microcontinent (de Wit Citation2003; Kusky et al. Citation2007).
Madagascar is characterised by a tropical climate with two climatic zones, each with its own distinct type of vegetation formations. The eastern side of the Island is covered in various evergreens, while its western part is mostly covered by deciduous flora. These zones are divided by a central mountain range, stretching along north-south axis of the Island (Du Puy & Moat Citation1996). Both zones are divided into several regions, each of which has distinctive climatic characteristics and a set of unique habitats.
Madagascar is characterised by high biological endemism, estimated at > 90% for terrestrial vertebrates and > 80% for vascular plants, and many of the taxa recorded on the Island are only found in one or very few localities (Goodman & Benstead Citation2003; Wilmé et al. Citation2006; Callmander et al. Citation2011). Currently, there are few taxa that are believed to be leftovers from the time when Madagascar was part of Gondwana, while most are assumed to be results of influx from Africa and Asia and subsequent speciation (Yoder & Nowak Citation2006; Warren et al. Citation2010; Buerki et al. Citation2013).
The study area, i.e. Ivohibory Forest is located in south-central Madagascar (approximately 22.598830S, 46.720841E, Ivohibe District, Fianarantsoa Province). It is predominantly covered by humid rainforest, situated on the eastern slopes of a hill located on the dry side of the main mountain range. It is estimated to cover an area of 1,400 ha, with an elevation ranging between 900 and 1,500 m above sea level. The forest is surrounded by man-made savannah, as well as micropatches of dry forest (Wright & Houlihan Citation2017).
The phylum Tardigrada consists of ca. 1,400 species (Guidetti & Bertolani Citation2005; Degma & Guidetti Citation2007; Degma et al. Citation2021) that can be found in terrestrial, freshwater and marine environments around the world (Ramazzotti & Maucci Citation1983 and later translation by Beasley Citation1995; McInnes Citation1994; Nelson et al. Citation2015). Our knowledge on the diversity and distribution of terrestrial tardigrades in Madagascar is very poor. Up to this day, 23 species were reported from this Island, including 10 for which this is their type locality, i.e., Cornechiniscus madagascariensis Maucci, Citation1993, Echiniscus succineus Gąsiorek & Vončina, Citation2019, Kristenseniscus walteri (Pilato & Lisi, Citation2003), Macrobiotus madegassus Maucci, Citation1993, Macrobiotus porifini Kuzdrowska, Mioduchowska, Gawlak, Bartylak, Kepel, Kepel & Kaczmarek, Citation2021, Mesobiotus fiedleri Kaczmarek, Bartylak, Stec, Kulpa, Kepel, Kepel & Roszkowska, Citation2020a, Milnesium matheusi Kaczmarek, Grobys, Kulpa, Bartylak, Kmita, Kepel, Kepel & Roszkowska, Citation2019, Mil. wrightae Kaczmarek, Grobys, Kulpa, Bartylak, Kmita, Kepel, Kepel & Roszkowska, Citation2019, Paramacrobiotus experimentalis Kaczmarek, Mioduchowska, Poprawa & Roszkowska, Citation2020b, Pseudechiniscus angelusalas Roszkowska, Grobys, Bartylak & Kaczmarek, Citation2020 (Maucci Citation1993; Pilato & Lisi Citation2003; Bartylak et al. Citation2019; Gąsiorek & Vončina Citation2019; Gąsiorek et al. Citation2019a; Kaczmarek et al. Citation2019; Grobys et al. Citation2020, Citation2020a, Citation2020b; Roszkowska et al. Citation2020; Kuzdrowska et al. Citation2021).
In the present paper, 122 samples collected in Ivohibory Forest area were studied and 39 tardigrade taxa are reported, seven of which are taxa not recorded outside of Madagascar. Furthermore, a new species for science of the genus Bryodelphax Thulin, Citation1928 is described based on morphological characters.
Materials and methods
Samples and sample processing: Samples of lichens and mosses were collected in the Ivohibory Forest in June 2017 (permits No 122/17/MEEF/SG/DGF/DSAP/SCB.Re and 150NEV06/MG17). Later they were packed in paper envelopes, dried at a temperature of ca. 30°C and sent to the laboratory at the Faculty of Biology, Adam Mickiewicz University in Poznań, Poland. There, tardigrades and their eggs were extracted from the samples and studied following the protocol of Dastych Citation1980b.
Microscopy and imaging: Adult specimens and eggs for light microscopy were mounted on microscope slides in Hoyer’s medium and secured with a coverslip and then examined under an Olympus BX41 phase contrast light microscope (PCM) with an Olympus SC50 digital camera (Olympus Corporation, Shinjuku-ku, Japan).
All figures were assembled in Inkscape 1.0.1. For deep structures that could not be fully focused on a single photograph, a series of 2–10 images were taken every ca. 0.5 μm and then manually assembled into a single deep-focus image in Adobe Photoshop 21.0.2.
Morphometrics and morphological nomenclature: Measurements of all morphological traits are given in micrometers [μm]. Structures were measured only if their orientation was suitable. Body length was measured from the anterior extremity to the end of the body, excluding the hind legs. The lengths of the claw branches were measured from the base of the claw to the top of the branch. The sp index is the ratio of the length of a given structure to the length of the scapular plate (scp) expressed as a percentage (Dastych Citation1999). It was later proposed as the psc index by Fontoura and Morais (Citation2011). The ventral plate configuration in Bryodelphax species is given according to Kaczmarek et al. (Citation2012). The genus abbreviations follow Perry et al. (Citation2019).
Morphometric data were handled using the “Echiniscoidea” template available from the Tardigrada Register (Michalczyk & Kaczmarek Citation2013). Tardigrade taxonomy is according to Bertolani et al. (Citation2014), Vecchi et al. (Citation2016), Gąsiorek et al. (Citation2019b, Citationc; Citation2021c), Stec et al. (Citation2020a, Citationb, Citation2021).
Comparative material: For the identification and differentiation of the new species, we used the key by Gąsiorek et al. (Citation2020). We also analysed and compared our new species with the type material of Bry. aaseae Kristensen, Michalczyk & Kaczmarek, Citation2010, Bry. asiaticus Kaczmarek & Michalczyk, Citation2004, Bry. brevidentatus Kaczmarek, Michalczyk & Degma, Citation2005, Bry. olszanowskii Kaczmarek, Parnikoza, Gawlak, Esefeld, Peter, Kozeretska & Roszkowska, Citation2018b and Bry. parvuspolaris Kaczmarek, Zawierucha, Smykla & Michalczyk, Citation2012.
Other species were identified based on original descriptions, redescriptions (Meyer Citation2016; Gąsiorek et al. Citation2018, Citation2019b) and keys by Ramazzotti and Maucci (Citation1983), Biserov (Citation1998), Claxton (Citation1998), Kaczmarek et al. (Citation2006), Fontoura and Pilato (Citation2007), Michalczyk and Kaczmarek (Citation2010), Morek et al. (Citation2016), Kaczmarek and Michalczyk (Citation2017), Kaczmarek et al. (Citation2017), Kaczmarek et al. (Citation2020a), Tumanov (Citation2020).
Results
In total, 1873 specimens and 314 eggs were found in 73 samples. They were identified as belonging to 39 different taxa (see , for more details).
Table I. Tardigrades found in the present study, in alphabetical order (* – species new for Madagascar; species new for science recorded in Ivohibory samples collected during the same expedition (described in current and previous papers) in bold)
Table II. Measurements [in μm] and sp values of selected morphological structures of males of Bryodelphax beniowskii sp. nov. mounted in Hoyer’s medium (sp—ratio of the length of a given structure to the length of the scapular plate (scp) expressed as a percentage (sp = length of the structure × 100⁄ length scapular plate (scp))
Taxonomic account of the new speciesPhylum: Tardigrada Doyère Citation1840Class: Heterotardigrada Marcus Citation1927Order: Echiniscoidea Richters Citation1926Family: Echiniscidae Thulin, Citation1928Genus: Bryodelphax Thulin, Citation1928
Bryodelphax beniowskii sp. nov. Bartylak, Kayastha, Roszkowska & Kaczmarek (, )
Material examined
Five specimens: holotype (female) and four paratypes (all females) mounted on microscope slides in Hoyer’s medium.
Figure 1. Bryodelphax beniowskii sp. nov.: A—dorsal projection of the entire animal, arrows indicate divisions of paired and median plates into anterior and posterior parts, arrowheads indicate supplementary plates near median plates (holotype), B—ventral projection of the entire animal, asterisk indicates gonopore (holotype). Scale bars in [μm]. All PCM.
![Figure 1. Bryodelphax beniowskii sp. nov.: A—dorsal projection of the entire animal, arrows indicate divisions of paired and median plates into anterior and posterior parts, arrowheads indicate supplementary plates near median plates (holotype), B—ventral projection of the entire animal, asterisk indicates gonopore (holotype). Scale bars in [μm]. All PCM.](/cms/asset/88e004aa-48cb-4b6f-8f59-be40f6c4d15e/tizo_a_2042404_f0001_b.gif)
Description of the females (for measurements and statistics, see ): Body yellow or light orange in living specimens (transparent after mounting on microscope slides in Hoyer’s medium) (, B). Eyes absent or not visible after mounting. Small and conical primary and secondary clavae. Cirri interni and externi with poorly developed cirrophores. Cirri interni always shorter than cirri externi. Only lateral cirri A present apart from head appendages. Cirri A of length typical for Bryodelphax, i.e., up to 25% of the total body length.
Dorsal sculpture visible in PCM as black pillars and white pores. Distinctly thicker and darker margins of dorsal plates and internal margins of facets in plates present, as originally reported by Gąsiorek (Citation2018). Pores large and easily detectable and distributed unevenly in scapular plate (6–16 pores/100 μm2, x = 10, N = 15 (in total of 5 specimens, three different plate portions for each specimen were measured), ) and in caudal (terminal) plate (0–17 pores/100 μm2, x = 9, N = 15 (in total of 5 specimens, three different plate portions for each specimen were measured) ). In other plates, pore density much lower (0–6 pores/100 μm2, x = 3, N = 15 (in total of 5 specimens, three different plate portions for each specimen were measured)). In caudal plate pillars 0.3–0.8 μm in diameter and pseudopores 0.6–0.9 μm in diameter (). Paired and median plates divided horizontally by smooth stripes into anterior and posterior parts (, 2B). Median plates 1 and 2 divided by transverse stripes, median plate 3 undivided. Median plate 2 is the largest among all median plates. In addition, paired plates I and II are divided horizontally into two parts. Twelve poorly visible supplementary plates near median plates 1–3 (, 2B).
Figure 2. Bryodelphax beniowskii sp. nov.: A—pores on scapular plate (holotype); B—pores on paired and median plates, arrow indicates division of paired plate into anterior and posterior parts, arrowheads indicate supplementary plates near median plates (holotype) C—pores on terminal plate (holotype). Scale bars in [μm]. All PCM.
![Figure 2. Bryodelphax beniowskii sp. nov.: A—pores on scapular plate (holotype); B—pores on paired and median plates, arrow indicates division of paired plate into anterior and posterior parts, arrowheads indicate supplementary plates near median plates (holotype) C—pores on terminal plate (holotype). Scale bars in [μm]. All PCM.](/cms/asset/58eeca03-9384-4e1e-8c7c-90f9ca70c663/tizo_a_2042404_f0002_b.gif)
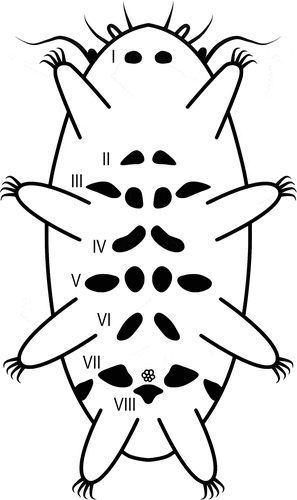
Ventral side with eight rows of faint greyish plates (formula VIII:2-2-4-2-4-2-4-1) (). First row with two plates situated at the level of head plate, i.e. in front of legs I, second row with two plates and third row with four plates situated between legs I and II, fourth row with two plates and fifth row with four plates situated between legs II and III, sixth row with two plates situated in line of legs III, seventh row with four plates situated in line with the gonophore and eighth row with one plate situated behind the gonophore. Three triangular genital plates (parts of rows seventh and eighth) surrounding the gonopore (two lateral, in line with the gonopore and the third one posterior to the gonopore (, 5C). Pillars (0.3–0.5 μm) and pores (0.4–0.5 μm) on ventral plates visible under PCM.
Figure 3. Bryodelphax beniowskii sp. nov.: ventral plates. Roman numerals indicate the rows in which the ventral plates are arranged, arrows indicate patches of granulation around gonopore (holotype). Scale bars in [μm]. PCM.
![Figure 3. Bryodelphax beniowskii sp. nov.: ventral plates. Roman numerals indicate the rows in which the ventral plates are arranged, arrows indicate patches of granulation around gonopore (holotype). Scale bars in [μm]. PCM.](/cms/asset/2e823219-bec5-4a21-bb4b-9ae25addfd90/tizo_a_2042404_f0003_b.gif)
Figure 4. Configuration of ventral plates in Bryodelphax beniowskii sp. nov. Roman numerals indicate the rows in which the ventral plates are arranged.
Papilla-like structure on leg I hardly visible under PCM (), papillae on leg IV present (). External claws smooth, but internal ones with spurs facing downward and positioned close to the claw bases (). Female gonopore with the typical six-petal rosette (, 5C). Males, juveniles and eggs not found.
Figure 5. Bryodelphax beniowskii sp. nov.: A—claws on leg I; arrowhead indicates papilla (paratype), B—claws of leg III (paratype), C—gonopore surrounded by patches of granulation; arrowhead indicates papilla on leg IV, asterisk indicates gonopore, arrows indicate patches of granulation (holotype). Scale bars in [μm]. All PCM.
![Figure 5. Bryodelphax beniowskii sp. nov.: A—claws on leg I; arrowhead indicates papilla (paratype), B—claws of leg III (paratype), C—gonopore surrounded by patches of granulation; arrowhead indicates papilla on leg IV, asterisk indicates gonopore, arrows indicate patches of granulation (holotype). Scale bars in [μm]. All PCM.](/cms/asset/454dd85c-cb2a-4abe-b198-80ec6ceecc4a/tizo_a_2042404_f0005_b.gif)
Remarks
Presence of pores on ventral plates () makes this species the first one in the genus Bryodelphax to have this distinct morphological characteristic.
Type locality
46°43’14.1”E, 22°37’04.4”S, ca. 1198 m asl, Fianarantsoa Province, Ivohibory Forest, lichens on the tree trunk, 04.06.2017, coll. Marta Kepel and Andrzej Kepel.
Type depositories
Holotype (slide MAD98/3) and four paratypes (slides: MAD98/4, MAD98/5, MAD98/6 and MAD98/7) are deposited at the Department of Animal Taxonomy and Ecology, Institute of Environmental Biology, Adam Mickiewicz University in Poznań, Poland.
Etymology
Bryodelphax beniowskii was chosen to commemorate Mateusz Maurycy Michał Franciszek Serafin August Beniowski (in Hungarian: Benyovszky, in Slovak: Beňovský), a military officer, adventurer and writer, national hero in Poland, Hungary and Slovakia. In the 18th century, he organised two expeditions to Madagascar, where he was killed and buried.
Differential diagnosis
Within the weglarskae group (distinguished due to presence of ventral plates), only Bry. olszanowskii Kaczmarek, Parnikoza, Gawlak, Esefeld, Peter, Kozeretska & Roszkowska, Citation2018b and Bry. parvuspolaris Kaczmarek, Zawierucha, Smykla & Michalczyk, Citation2012 have eight plate rows (Gąsiorek et al. Citation2020) which is why only these two are considered for differential diagnosis. Adult females of Bry. beniowskii sp. nov. differ from:
1. Bry. olszanowskii, reported only from the type locality in Antarctic (Kaczmarek et al. Citation2018ab) by: different ventral plate formula (VIII:2-2-4-2-4-2-4-1 in the new species vs. VIII:4-1-1-2-2-2-2-2 in Bry. olszanowskii), presence of papilla-like structure on leg I and absence of dentate collar on legs IV.
2. Bry. parvuspolaris known from the type locality in Spitsbergen (Kaczmarek et al. Citation2012) by: different ventral plate formula (VIII:2-2-4-2-4-2-4-1 in the new species vs. VIII:1-1-2-2-2-2-2-1 in Bry. parvuspolaris), higher sp of cirrus internus (33.4–40.1 in the new species vs. 22.4–26.3 in Bry. parvuspolaris), higher cirrus int/ext length ratio (63%–75% in the new species vs. 43%–53% in Bry. parvuspolaris), presence of papilla-like structure on leg I, presence of papilla on legs IV and absence of dentate collar on legs IV.
Discussion
In recent years, the number of known tardigrade taxa from Madagascar almost doubled from 13 in 2003 to 23 at the time of the current study (Maucci Citation1993; Pilato & Lisi Citation2003; Bartylak et al. Citation2019; Kaczmarek et al. Citation2019; Gąsiorek & Vončina Citation2019; Gąsiorek et al. Citation2019a; Grobys et al. Citation2020; Kaczmarek et al. Citation2020a; Citation2020b; Roszkowska et al. Citation2020; Kuzdrowska et al. Citation2021). In this study we present further 21 taxa (including one new for science) reported from Madagascar. However, the taxonomic status and distribution of some of newly reported species need to be clarified.
Bryodelphax beniowskii sp. nov. is the second species of the genus Bryodelphax reported from Madagascar, but it should be mentioned that Bry. parvulus Thulin, Citation1928 was reported from the Island long time ago (Maucci Citation1993). Taking into consideration that the type locality of Bry. parvulus is in Lapland (Thulin Citation1928) and since that time many new species of this genus were described (Degma et al. Citation2021), its presence in Madagascar needs confirmation.
Three species found in present study were previously known only from Africa, i.e., Diphascon zaniewi Kaczmarek & Michalczyk, Citation2004, Minibiotus africanus Binda & Pilato, Citation1995 and Ramazzottius szeptycki (Dastych, Citation1980a). These species should be temporarily considered as species restricted to Africa.
Furthermore, three species, i.e., Mil. argentinum Roszkowska, Ostrowska & Kaczmarek, Citation2015, Min. pseudostellarus Roszkowska, Stec, Ciobanu & Kaczmarek, Citation2016 and Doryphoribius amazzonicus Lisi, Citation2011 were up to now reported only from South America and their presence in Madagascar might suggest a long distance, anthropogenic dispersal. A similar distribution was recently presented for Minibiotus pentannulatus Londoño, Daza, Lisi & Quiroga, Citation2017 which was described from South America and later reported in South Africa by Stec et al. Citation2020a). Similarly, Pam. savai (Binda & Pilato, Citation2001) reported here were recorded from distant locality in Sri Lanka. Considering these discoveries, these species can be potentially considered long-distance migrants.
Echiniscus cavagnaroi Schuster & Grigarick, Citation1966 and Kri. tessellatus (Murray, Citation1910) are known from tropical or subtropical regions and should be considered as likely pantropical species. Moreover, it should also be mentioned that Kri. walteri (Pilato & Lisi, Citation2003) recorded from Madagascar is very similar to Kri. tessellatus.
Echiniscus manuelae da Cunha & do Nascimento Ribeiro, Citation1962 has a disjunct Afrotropical-Neotropical distribution (Gąsiorek et al. Citation2021a). The next member of the genus Echiniscus, i.e., Ech. testudo (Doyère, Citation1840) is considered as species with wide distribution. Reports of this species from localities in Africa, South and North America or New Zealand may suggest a cosmopolitan distribution of this taxon, probably associated with anthropogenic dispersal (Jørgensen et al. Citation2007; Gąsiorek et al. Citation2019d).
Specimens of Hys. dujardini found in the present study fit recent redescription of this species (Gąsiorek et al. Citation2018). Its distribution is now very unclear. This species was considered cosmopolitan in the past (McInnes Citation1994), but almost all records of this taxon need a confirmation based on modern taxonomy.
Two taxa were identified as Mur. cf. hastatus and Hys. cf. convergens. These species have a very unclear taxonomic position and a redescription of both taxa, from the type localities, is necessary. Both species were described from Europe (Murray Citation1907a; Urbanowicz Citation1925) and it is highly probable that Madagascan specimens should be considered as different species, however this confirmation will only be possible after revision. Another taxon Macrobiotus cf. sandrae is extremely similar to Mac. azzunae Ben Marnissi, Cesari, Rebecchi & Bertolani, Citation2021 from Tunisia and without molecular studies unambiguous identification is problematic (Ben Marnissi et al. Citation2021).
The other taxa i.e., Ursulinius cf. cameruni, Hys. cf. scabropygus, Crenubiotus sp., Macrobiotus cf. drakensbergi, Mil. cf. beatae, Mil. cf. dornensis and Min. cf. intermedius reported as new for Madagascar fauna were identified based on small number of specimens and for some of them eggs (which are crucial for correct identification) were missing. Presence of Urs. cameruni (Iharos, Citation1969) and Mac. drakensbergi Dastych, Citation1993 on Madagascar is highly probable as both species were described from Africa (Mcinnes et al. Citation2017).
We also identified many specimens only to the genus level or to the group of species i.e. Ramazzottius sp., Doryphoribius sp., Mac. hufelandi group, Mesobiotus sp., Pam. richtersi group and Pseudechiniscus (Meridioniscus) sp. Correct identification of these individuals was not possible due to lack of eggs or small number of specimens. It should also be stated that some of the specimens of Mac. hufelandi group, Mesobiotus sp. or Pam. richtersi group may belong to species reported from Madagascar in previous studies or in the present study, however, without eggs, it is not possible to say so with certainty.
In summary, tardigrade fauna of Madagascar is very poorly known but seems to be very rich because even in a very small area studied in the present research, many new species for science were found (Kaczmarek et al. Citation2019; Bartylak et al. Citation2019; Roszkowska et al. Citation2020; Kaczmarek et al. Citation2020a, b; Kuzdrowska et al. Citation2021, as well as the present study), altogether with many species new for Madagascar.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bartylak T, Kulpa A, Grobys D, Kepel M, Kepel A, Kmita H, Gawlak M, Grabiński W, Roszkowska M, Kaczmarek Ł. 2019. Variability of Echiniscus tristis Gąsiorek & Kristensen, 2018—is morphology sufficient for taxonomic differentiation of Echiniscidae? Zootaxa 4701(1):1–24. DOI:10.11646/zootaxa.4701.1.1.
- Beasley CW (1995) The Phylum Tardigrada. Third Edition by G. Ramazzotti and W. Maucci, English Translation. Published by the translator at McMurry University, Abilene, USA, 1–1014 pp.
- Ben Marnissi J, Cesari M, Rebecchi L, Bertolani R. 2021. Integrative description of a new Tunisian tardigrade species, Macrobiotus azzunae sp. nov. (Eutardigrada, Macrobiotidae, hufelandi group). European Journal of Taxonomy 758:122–146. DOI: 10.5852/ejt.2021.758.1429.
- Bertolani R, Guidetti R, Marchioro T, Altiero T, Rebecchi L, Cesari M. 2014. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Molecular Phylogenetics and Evolution 76:110–126. DOI: 10.1016/j.ympev.2014.03.006.
- Bertolani R, Rebecchi L. 1993. A revision of the Macrobiotus hufelandi group (Tardigrada, Macrobiotidae), with some observations on the taxonomic characters of eutardigrades. Zoologica Scripta 22(2):127–152. DOI:10.1111/j.1463-6409.1993.tb00347.x.
- Binda MG, Guglielmino A. 1991. Tardigradi dell’Africa. VI. Macrobiotus polygonatus nuova specie di eutardigrado del Kilimangiaro (Tanzania). Animalia 18:223–227.
- Binda MG, Pilato G. 1993. Ridescrizione di Echiniscus reticulatus Murray, 1905 e descrizione di Echiniscus cirinoi, nuova specie di Tardigrado della Tanzania. Animalia 20:55–58.
- Binda M, Pilato G. 2001. Macrobiotus savai and Macrobiotus humilis, two new species of tardigrades from Sri Lanka. Bollettino delle sedute dell’Accademia di Scienze Naturali, Catania 34:101–111.
- Biserov VI. 1998. Tardigrades of the Caucasus with a taxonomic analysis of the genus Ramazzottius (Parachela: Hypsibiidae). Zoologischer Anzeiger 236:139–159.
- Buerki S, Devey DS, Callmander MW, Phillipson PB, Forest F. 2013. Spatio-temporal history of the endemic genera of Madagascar: Evolution of the Endemic Madagascan Flora. Botanical Journal of the Linnean Society 171(2):304–329. DOI:10.1111/boj.12008.
- Callmander MW, Phillipson PB, Schatz GE, Andriambololonera S, Rabarimanarivo M, Rakotonirina N, Raharimampionona J, Chatelain C, Gautier L, Lowry PP. 2011. The endemic and non-endemic vascular flora of Madagascar updated. Plant Ecology and Evolution 144(2):121–125. DOI:10.5091/plecevo.2011.513.
- Ciobanu DA, Roszkowska M, Kaczmarek Ł. 2015. Two new tardigrade species from Romania (Eutardigrada: Milnesiidae, Macrobiotidae), with some remarks on secondary sex characters in Milnesium dornensis sp. nov. Zootaxa 3941(4):542–564. DOI:10.11646/zootaxa.3941.4.4.
- Claps MC, Rossi GC. 1988. Tardígrados de Argentina VI. Iheringia (Série Zoología) 67:3–11.
- Claxton SK. 1998. A revision of the genus Minibiotus (Tardigrada: Macrobiotidae) with descriptions of eleven new species from Australia. Records of the Australian Museum 50(2):125–160. DOI:10.3853/j.0067-1975.50.1998.1276.
- Cuénot L. 1929. Description d’un tardigrade nouveau de la faune francaise. Archives D’anatomie Microscopique 25:121–125.
- da Cunha AX, du Nascimento Ribeiro F. 1962. A fauna de tardígrados da Ilha da Madeira. Memorias e estudos do Museu Zoologico da Universidade de Coimbra 279:1–24.
- Dastych H. 1980a. Hypsibius szeptycki sp. nov., a new species of Tardigrada from South Africa. Bulletin de l’Académie Polonaise Des Sciences 27:505–508.
- Dastych H. 1980b. Niesporczaki (Tardigrada) Tatrzańskiego Parku Narodowego. Monografie Fauny Polski 9:1–232.
- Dastych H. 1993. A new genus and four new species of semiterrestrial water-bears from South Africa (Tardigrada). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 90:175–186.
- Dastych H. 1997. Some notes on morphology of Echiniscus tessellatus Murray, 1910 (Tardigrada). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 94:73–79.
- Dastych H. 1999. A new species of the genus Mopsechniscus Du-Bois Reymond Marcus, 1944 (Tardigrada) from the Venezuelan Andes. Acta Biologica Benrodis 10:91–101.
- de Wit MJ. 2003. Madagascar: Heads it’s a continent, tails it’s an Island. Annual Review of Earth and Planetary Sciences 31(1):213–248. DOI:10.1146/annurev.earth.31.100901.141337.
- Degma P, Bertolani R, Guidetti R (2021) Actual checklist of Tardigrada species. (2009-2021, Edition 40th: 19-07-2021). 10.25431/11380_1178608
- Degma P, Guidetti R. 2007. Notes to the current checklist of Tardigrada. Zootaxa 1579(1):41–53. DOI:10.11646/zootaxa.1579.1.2.
- Doyère L. 1840. Mémoire sur les Tardigrades. Annales des Sciences Naturelles 14:269–361.
- Du Puy DJ, Moat J (1996) A refined classification of the primary vegetation of Madagascar based on the underlying geology: Using GIS to map its distribution and to assess its conservation status. In: Proceedings of the International Symposium on the “Biogeographie de Madagascar”., Editions de l’ORSTOM, Paris, September 1995, pp. 205–218.
- Fontoura P, Morais P. 2011. Assessment of traditional and geometric morphometrics for discriminating cryptic species of the Pseudechiniscus suillus complex (Tardigrada, Echiniscidae). Journal of Zoological Systematics and Evolutionary Research 49(s1):26–33. DOI:10.1111/j.1439-0469.2010.00594.x.
- Fontoura P, Pilato G. 2007. Diphascon (Diphascon) faialense sp. nov. a new species of Tardigrada (Eutardigrada, Hypsibiidae) from the Azores and a key to the species of the D. pingue group. Zootaxa 1589(1):47–55. DOI:10.11646/zootaxa.1589.1.4.
- Gąsiorek P. 2018. New Bryodelphax species (Heterotardigrada: Echiniscidae) from Western Borneo (Sarawak), with new molecular data for the genus. Raffles Bulletin of Zoology 66:371–381. DOI: 10.5281/zenodo.5358943.
- Gąsiorek P, Bochnak M, Vončina K, Michalczyk Ł. 2021a. Phenotypically exceptional Echiniscus species (Heterotardigrada: Echiniscidae) from Argentina (Neotropics). Zoologischer Anzeiger 294:210–228. DOI: 10.1016/j.jcz.2021.08.003.
- Gąsiorek P, Jackson KJ, Meyer HA, Zając K, Nelson DR, Kristensen RM, Michalczyk Ł. 2019a. Echiniscus virginicus complex: The first case of pseudocryptic allopatry and pantropical distribution in tardigrades. Biological Journal of the Linnean Society 128:789–805. DOI: 10.1093/biolinnean/blz147.
- Gąsiorek P, Kristensen RM. 2018. Echiniscidae (Heterotardigrada) of Tanzania and Uganda. Tropical Zoology 31(3):131–160. DOI:10.1080/03946975.2018.1477350.
- Gąsiorek P, Morek W, Stec D, Michalczyk Ł. 2019b. Untangling the Echiniscus Gordian knot: Paraphyly of the “arctomys group” (Heterotardigrada: Echiniscidae). Cladistics 35(6):633–653. DOI:10.1111/cla.12377.
- Gąsiorek P, Stec D, Morek W, Marnissi J, Michalczyk Ł. 2017a. The tardigrade fauna of Tunisia, with an integrative description of Bryodelphax maculatus sp. nov. (Heterotardigrada: Echiniscidae). African Zoology 52(2):77–89. DOI:10.1080/15627020.2017.1297688.
- Gąsiorek P, Stec D, Morek W, Michalczyk Ł. 2017b. An integrative redescription of Echiniscus testudo (Doyère, 1840), the nominal taxon for the class Heterotardigrada (Ecdysozoa: Panarthropoda: Tardigrada). Zoologischer Anzeiger 270:107–122. DOI: 10.1016/j.jcz.2017.09.006.
- Gąsiorek P, Stec D, Morek W, Michalczyk Ł. 2018. An integrative redescription of Hypsibius dujardini (Doyère, 1840), the nominal taxon for Hypsibioidea (Tardigrada: Eutardigrada). Zootaxa 4415(1):45–75. DOI:10.11646/zootaxa.4415.1.2.
- Gąsiorek P, Stec D, Morek W, Michalczyk Ł. 2019c. Deceptive conservatism of claws: Distinct phyletic lineages concealed within Isohypsibioidea (Eutardigrada) revealed by molecular and morphological evidence. Contributions to Zoology 88(1):78–132. DOI:10.1163/18759866-20191350.
- Gąsiorek P, Vončina K. 2019. New Echiniscidae (Heterotardigrada) from Amber Mountain (Northern Madagascar). Evolutionary Systematics 3(1):29–39. DOI:10.3897/evolsyst.3.33580.
- Gąsiorek P, Vončina K, Ciosek J, Veloso M, Fontoura P, Michalczyk Ł. 2021b. New Indomalayan Nebularmis species (Heterotardigrada: Echiniscidae) provoke a discussion on its intrageneric diversity. Zoological Letters 7(1):6. DOI:10.1186/s40851-021-00172-0.
- Gąsiorek P, Vončina K, Degma P, Michalczyk Ł. 2020. Small is beautiful: The first phylogenetic analysis of Bryodelphax Thulin, 1928 (Heterotardigrada, Echiniscidae). Zoosystematics and Evolution 96(1):217–236. DOI:10.3897/zse.96.50821.
- Gąsiorek P, Vončina K, Michalczyk Ł. 2019d. Echiniscus testudo (Doyère, 1840) in New Zealand: Anthropogenic dispersal or evidence for the ‘Everything is Everywhere’ hypothesis? New Zealand Journal of Zoology 46(2):174–181. DOI:10.1080/03014223.2018.1503607.
- Gąsiorek P, Vončina K, Zając K, Michalczyk Ł. 2021c. Phylogeography and morphological evolution of Pseudechiniscus (Heterotardigrada: Echiniscidae). Scientific Reports 11(1):7606. DOI:10.1038/s41598-021-84910-6.
- Goodman SM, Benstead JP. 2003. The natural history of Madagascar. Journal of Mammalogy 84:813–814.
- Grobys D, Roszkowska M, Gawlak M, Kmita H, Kepel A, Kepel M, Parnikoza I, Bartylak T, Kaczmarek Ł. 2020. High diversity in the Pseudechiniscus suillus–facettalis complex (Heterotardigrada: Echiniscidae) with remarks on the morphology of the genus Pseudechiniscus. Zoological Journal of the Linnean Society 188(3): 733–752. DOI:10.1093/zoolinnean/zlz171
- Guidetti R, Bertolani R. 2005. Tardigrade taxonomy: An updated check list of the taxa and a list of characters for their identification. Zootaxa 845(1):1–46. DOI:10.11646/zootaxa.845.1.1.
- Iharos G. 1969. Tardigraden aus Mittelwestafrika. Opuscula Zoologica Budapest 9:115–120.
- Jørgensen A, Møbjerg N, Kristensen RM. 2007. A molecular study of the tardigrade Echiniscus testudo (Echiniscidae) reveals low DNA sequence diversity over a large geographical area. Journal of Limnology 66(1s):77–83. DOI:10.4081/jlimnol.2007.s1.77.
- Kaczmarek Ł, Bartylak T, Stec D, Kulpa A, Kepel M, Kepel A, Roszkowska M. 2020a. Revisiting the genus Mesobiotus Vecchi et al., 2016 (Eutardigrada, Macrobiotidae) – Remarks, updated dichotomous key and an integrative description of new species from Madagascar. Zoologischer Anzeiger 287:121–146. DOI: 10.1016/j.jcz.2020.05.003.
- Kaczmarek Ł, Gawlak M, Bartels PJ, Nelson DR, Roszkowska M. 2017. Revision of the Genus Paramacrobiotus Guidetti et al., 2009 with the description of a new species, re-descriptions and a key. Annales Zoologici 67(4):627–656. DOI:10.3161/00034541ANZ2017.67.4.001.
- Kaczmarek Ł, Grobys D, Kulpa A, Bartylak T, Kmita H, Kepel M, Kepel A, Roszkowska M. 2019. Two new species of the genus Milnesium Doyère, 1840 (Tardigrada, Apochela, Milnesiidae) from Madagascar. ZooKeys 884:1–22. DOI: 10.3897/zookeys.884.29469.
- Kaczmarek Ł, Kosicki J, Roszkowska M. 2018a. Tardigrada of Bory Tucholskie National Park, Zaborski Landscape Park, and their surroundings (Pomerania Province, Poland). Turkish Journal of Zoology 42:6–17. DOI: 10.3906/zoo-1705-44.
- Kaczmarek Ł, Michalczyk Ł. 2004. Notes on some tardigrades from South Africa, with the description of Diphascon (Diphascon) zaniewi sp. nov. (Eutardigrada: Hypsibiidae). Zootaxa 576(1):1–6. DOI:10.11646/zootaxa.576.1.1.
- Kaczmarek Ł, Michalczyk Ł. 2009. Redescription of Hypsibius microps Thulin, 1928 and H. pallidus Thulin, 1911 (Eutardigrada: Hypsibiidae) based on the type material from the Thulin collection. Zootaxa 2275(1):60–68. DOI:10.11646/zootaxa.2275.1.4.
- Kaczmarek Ł, Michalczyk Ł. 2010. The genus Echiniscus Schultze 1840 (Tardigrada) in Costa Rican (Central America) rain forests with descriptions of two new species. Tropical Zoology 23:91–106.
- Kaczmarek Ł, Michalczyk Ł. 2017. The Macrobiotus hufelandi group (Tardigrada) revisited. Zootaxa 4363(1):101–123. DOI:10.11646/zootaxa.4363.1.4.
- Kaczmarek Ł, Michalczyk Ł, Degma P. 2005. A new species of Tardigrada Bryodelphax brevidentatus sp. nov. (Heterotardigrada: Echiniscidae) from China (Asia). Zootaxa 1080(1):33–38. DOI:10.11646/zootaxa.1080.1.3.
- Kaczmarek Ł, Michalczyk Ł, Guidetti R. 2006. Description of the new species Calcarobiotus (C.) longinoi sp. nov. (Eutardigrada, Macrobiotidae) from Costa Rica with the diagnostic key to the genus Calcarobiotus. Italian Journal of Zoology 73(3):247–253. DOI:10.1080/11250000600831642.
- Kaczmarek Ł, Michalczyk Ł, McInnes SJ. 2014. Annotated zoogeography of non-marine Tardigrada. Part I: Central America. Zootaxa 3763(1):1–62. DOI:10.11646/zootaxa.3763.1.1.
- Kaczmarek Ł, Michalczyk Ł, Mcinnes SJ. 2015. Annotated zoogeography of non-marine Tardigrada. Part II: South America. Zootaxa 3923(1):1–107. DOI:10.11646/zootaxa.3923.1.1.
- Kaczmarek Ł, Parnikoza I, Gawlak M, Esefeld J, Peter H-U, Kozeretska I, Roszkowska M. 2018b. Tardigrades from Larus dominicanus Lichtenstein, 1823 nests on the Argentine Islands (maritime Antarctic). Polar Biology 41(2):283–301. DOI:10.1007/s00300-017-2190-4.
- Kaczmarek Ł, Roszkowska M, Poprawa I, Janelt K, Kmita H, Gawlak M, Fiałkowska E, Mioduchowska M. 2020b. Integrative description of bisexual Paramacrobiotus experimentalis sp. nov. (Macrobiotidae) from Republic of Madagascar (Africa) with microbiome analysis. Molecular Phylogenetics and Evolution 145:106730. DOI: 10.1016/j.ympev.2019.106730.
- Kaczmarek Ł, Zawierucha K, Buda J, Stec D, Gawlak M, Michalczyk Ł, Roszkowska M. 2018c. An integrative redescription of the nominal taxon for the Mesobiotus harmsworthi group (Tardigrada: Macrobiotidae) leads to descriptions of two new Mesobiotus species from Arctic. PLOS ONE 13(10):e0204756. DOI:10.1371/journal.pone.0204756.
- Kaczmarek Ł, Zawierucha K, Smykla J, Michalczyk Ł. 2012. Tardigrada of the Revdalen (Spitsbergen) with the descriptions of two new species: Bryodelphax parvuspolaris (Heterotardigrada) and Isohypsibius coulsoni (Eutardigrada). Polar Biology 35(7):1013–1026. DOI:10.1007/s00300-011-1149-0.
- Kiosya Y, Vončina K, Gąsiorek P. 2021. Echiniscidae in the Mascarenes: The wonders of Mauritius. Evolutionary Systematics 5(1):93–120. DOI:10.3897/evolsyst.5.59997.
- Kristensen RM, Michalczyk Ł, Kaczmarek Ł. 2010. The first record of the genus Bryodelphax (Tardigrada: Heterotardigrada: Echiniscidae) from Easter Island, Rapa Nui (Pacific Ocean, Chile) with the description of a new species, Bryodelphax aaseae. Zootaxa 2343(1):45–56. DOI:10.11646/zootaxa.2343.1.4.
- Kusky TM, Toraman E, Raharimahefa T. 2007. The Great Rift Valley of Madagascar: An extension of the Africa–Somali diffusive plate boundary? Gondwana Research 11(4):577–579. DOI:10.1016/j.gr.2006.11.009.
- Kuzdrowska KA, Mioduchowska M, Gawlak M, Bartylak T, Kepel A, Kepel M, Kaczmarek Ł. 2021. Integrative description of Macrobiotus porifini sp. nov. (Macrobiotidae) from Madagascar and its phylogenetic position within the hufelandi group. The European Zoological Journal 88(1):375–389. DOI:10.1080/24750263.2021.1883752.
- Li X-C, Wang D-Y, Wang L. 2008. The Tardigrada fauna of Hainan Island (Asia: China) with descriptions of two new species. The Raffles Bulletin of Zoology 56:293–305.
- Lisi O. 2011. Remarks on Doryphoribius flavus (Iharos, 1966), and description of three new species (Tardigrada, Hypsibiidae). Zootaxa 2834(1):17–32. DOI:10.11646/zootaxa.2834.1.2.
- Londoño R, Daza A, Lisi O, Quiroga S. 2017. New species of waterbear Minibiotus pentannulatus (Tardigrada: Macrobiotidae) from Colombia. Revista Mexicana de Biodiversidad 88(4):807–814. DOI:10.1016/j.rmb.2017.10.040.
- Marcus E. 1927. Zur Anatomie und Ökologie mariner Tardigraden. Zoologische Jahrbücher. Abteilung für Systematik, Ökologie und Geographie der Tiere 53:487–558.
- Maucci W. 1993. Prime notizie su tardigradi ”terrestri” del Madagascar con descrizione di tre specie nuove. Bollettino del Museo Civico di Storia Naturale, Verona 17:381–392.
- McInnes SJ. 1994. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. Journal of Natural History 28:257–352. DOI: 10.1080/00222939400770131.
- Mcinnes SJ, Michalczyk Ł, Kaczmarek Ł. 2017. Annotated zoogeography of non-marine Tardigrada. Part IV: Africa. Zootaxa 4284(1):1–74. DOI:10.11646/zootaxa.4284.1.1.
- Meyer HA. 2016. Re-description of Echiniscus cavagnaroi Schuster & Grigarick, 1966 (Tardigrada: Heterotardigrada: Echiniscoidea: Echiniscidae) from type material, with new records from Hawaii and Bermuda. Zootaxa 4121(5):575–582. DOI:10.11646/zootaxa.4121.5.7.
- Michalczyk Ł, Kaczmarek Ł. 2010. Description of Doryphoribius dawkinsi, a new species of Tardigrada (Eutardigrada: Hypsibiidae) from the Costa Rican highlands, with the key to the genus Doryphoribius. Zootaxa 2393(1):46–58. DOI:10.11646/zootaxa.2393.1.4.
- Michalczyk Ł, Kaczmarek Ł. 2013. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. Journal of Limnology 72(1s):175–181. DOI:10.4081/jlimnol.2013.s1.e22.
- Morek W, Gąsiorek P, Stec D, Blagden B, Michalczyk Ł. 2016. Experimental taxonomy exposes ontogenetic variability and elucidates the taxonomic value of claw configuration in Milnesium Doyère, 1840 (Tardigrada: Eutardigrada: Apochela). Contributions to Zoology 85(2):173–200. DOI:10.1163/18759866-08502003.
- Moreno-Talamantes A, Roszkowska M, García-Aranda MA, Flores-Maldonado JJ, Kaczmarek Ł. 2019. Current knowledge on Mexican tardigrades with a description of Milnesium cassandrae sp. nov. (Eutardigrada: Milnesiidae) and discussion on the taxonomic value of dorsal pseudoplates in the genus Milnesium Doyère, 1840. Zootaxa 4691(5):501–524. DOI:10.11646/zootaxa.4691.5.5.
- Murray J. 1907a. XXIV.—Scottish Tardigrada, collected by the Lake Survey. Transactions of the Royal Society of Edinburgh 45(3):641–668. DOI:10.1017/S0080456800011777.
- Murray J. 1907b. XII.-Some South African Tardigrada. Journal of the Royal Microscopical Society 27(5):515–524. DOI:10.1111/j.1365-2818.1907.tb01665.x.
- Murray J. 1910. Tardigrada. In: British Antarctic Expedition 1907–09. Reports on the Scientific Investigations. London: William Heinemann. pp. 83–185.
- Nelson DR, Guidetti R, Rebecchi L. 2015. Phylum Tardigrada. In: Thorp J, Rogers C, editors. Thorp and Covich’s Freshwater Invertebrates. London: Elsevier. pp. 347–380. DOI: 10.1016/B978-0-12-385026-3.00017-6.
- Perry E, Miller WR, Kaczmarek Ł. 2019. Recommended abbreviations for the names of genera of the phylum Tardigrada. Zootaxa 4608(1):145–154. DOI:10.11646/zootaxa.4608.1.8.
- Pilato G, Lisi O. 2003. Echinscus walteri, new species of tardigrade from Madagascar. Bollettino del Museo di Storia Naturale di Verona 27:65–70.
- Plate L. 1889. Beiträge zur Naturgeschichte der Tardigraden. Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere 3:487–550. DOI: 10.5962/bhl.part.1265.
- Ramazzotti G, Maucci W. 1983. Il Phylum Tardigrada. III edizione riveduta е aggiornata. Memorie dell’Istituto Italiano di Idrobiologia 41, 1–1012.
- Richters F. 1926. Tardigrada. In: Kükenthal W, Krumbach T, editors. Handbuch der Zoologie. Berlin and Leipzig: de Gruyter. pp. 58–61.
- Roszkowska M, Grobys D, Bartylak T, Gawlak M, Kmita H, Kepel A, Kepel M, Parnikoza I, Kaczmarek Ł. 2020. Integrative description of five Pseudechiniscus species (Heterotardigrada: Echiniscidae: the suillus-facettalis complex). Zootaxa 4763(4):451–484. DOI:10.11646/zootaxa.4763.4.1.
- Roszkowska M, Ostrowska M, Kaczmarek Ł. 2015. The genus Milnesium Doyère, 1840 (Tardigrada) in South America with descriptions of two new species from Argentina and discussion of the feeding behaviour in the family Milnesiidae. Zoological Studies 54(1):12–28. DOI:10.1186/s40555-014-0082-7.
- Roszkowska M, Stec D, Ciobanu DA, Kaczmarek Ł. 2016. Tardigrades from Nahuel Huapi National Park (Argentina, South America) with descriptions of two new Macrobiotidae species. Zootaxa 4105(3):243–260. DOI:10.11646/zootaxa.4105.3.2.
- Schuster RO, Grigarick AA (1966) Tardigrada from the Galápagos and Cocos Islands. Proceedings of the California Academy of Sciences 34, 315–328.
- Stec D, Kristensen RM, Michalczyk Ł. 2020a. An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger 286:117–134. DOI: 10.1016/j.jcz.2020.03.007.
- Stec D, Vecchi M, Calhim S, Michalczyk Ł. 2021. New multilocus phylogeny reorganises the family Macrobiotidae (Eutardigrada) and unveils complex morphological evolution of the Macrobiotus hufelandi group. Molecular Phylogenetics and Evolution 160:106987. DOI: 10.1016/j.ympev.2020.106987.
- Stec D, Vecchi M, Maciejowski W, Michalczyk Ł. 2020b. Resolving the systematics of Richtersiidae by multilocus phylogeny and an integrative redescription of the nominal species for the genus Crenubiotus (Tardigrada). Scientific Reports 10(1):19418. DOI:10.1038/s41598-020-75962-1.
- Suzuki A, Heard L, Sugiura K. 2018. Tardigrada of Mikurajima. Mikurensis 7:3–8.
- Thulin G. 1928. Über Die Phylogenie Und Das System Der Tardigraden. Hereditas 11(2–3):207–266. DOI:10.1111/j.1601-5223.1928.tb02488.x.
- Tibbs L, Emanuels A, Miller WR. 2016. Tardigrades of the canopy: Argentine species Milnesium beatae Roszkowska, Ostrowska and Kaczmarek, 2015 (Eutardigrada, Milnesidae) discovered in the trees of Kansas, U.S.A. Transactions of the Kansas Academy of Science 119(2):173–178. DOI:10.1660/062.119.0207.
- Tumanov DV. 2020. Integrative description of Mesobiotus anastasiae sp. nov. (Eutardigrada, Macrobiotoidea) and first record of Lobohalacarus (Chelicerata, Trombidiformes) from the Republic of South Africa. European Journal of Taxonomy 726:102–131. DOI: 10.5852/ejt.2020.726.1179.
- Urbanowicz C. 1925. Sur la variabilité de. Macrobiotus oberhaeuseri. Bulletin Biologique de la France et de la Belgique (Paris) 59:124–142.
- Vecchi M, Cesari M, Bertolani R, Jönsson KI, Rebecchi L, Guidetti R. 2016. Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebrate Systematics 30(4):303–322. DOI:10.1071/IS15033.
- Warren BH, Strasberg D, Bruggemann JH, Prys-Jones RP, Thébaud C. 2010. Why does the biota of the Madagascar region have such a strong Asiatic flavour? Cladistics 26(5):526–538. DOI:10.1111/j.1096-0031.2009.00300.x.
- Wilmé L, Goodman SM, Ganzhorn JU. 2006. Biogeographic Evolution of Madagascar’s Microendemic Biota. Science 312(5776):1063–1065. DOI:10.1126/science.1122806.
- Wright PC, Houlihan PR (2017) Vohibory Rainforest: Biological Inventory Report – September 27th to October 12th 2016. 23pp.
- Yoder AD, Nowak MD. 2006. Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell. Annual Review of Ecology, Evolution, and Systematics 37(1):405–431. DOI:10.1146/annurev.ecolsys.37.091305.110239.
- Zawierucha K, Dziamięcki J, Jakubowska N, Michalczyk Ł, Kaczmarek Ł. 2014. New tardigrade records for the Baltic states with a description of Minibiotus formosus sp. n. (Eutardigrada, Macrobiotidae). ZooKeys 408:81–105. DOI: 10.3897/zookeys.408.6612.
