Abstract
Due to its high toxicity and bioaccumulation tendency, tebuconazole (TBZ) is one of the ten substances posing the highest risk of harmful effects in aquatic ecosystems. The liver, a key compartment for xenobiotics detoxification, is also the organ in which TBZ mainly accumulates in fish. Herein, we investigated for the first time the morpho-functional changes induced in zebrafish (Danio rerio) liver after a short-term exposure (48, 96, and 192 hours) to a low, environmentally relevant concentration of TBZ (5 µg/L) to disclose the early effects under a realistic exposure scenario. We revealed that pathological alterations with varying degrees of severity could be detected in all the examined samples. The injuries become intense and irreversible with increased exposure time involving both hepatocytes and vascular components based on the degree of tissue changes. The main morphological alterations were: parenchyma dyschromia, macrophages infiltration, congestion of blood vessels, and sinusoids. TBZ exposure also resulted in a significant decrease in glycogen contents and hepatocyte dimensions, and the modulation of superoxide dismutase, an early indicator of oxidative stress. We demonstrated that even a very low dose of TBZ affects hepatic morphology and function, disrupting liver homeostasis and physiology.
Introduction
Due to the pervasive implementation of modern agricultural practices, tonnes of pesticides are applied worldwide every year, and the global pesticide application has increased by 35% between 2000 and 2015 (FAO Citation2018; Rani & Shanker Citation2018). According to the European Commission and the United States Geological Survey (USGS), about 500 active ingredients, spanning through 18 classes of pesticides, are listed as approved by Regulatory Authorities in different countries (Maggi et al. Citation2019). Given their massive use and potential to affect the structure and function of aquatic habitats, pesticides raise a great concern worldwide and are recognized as priority pollutants in the environment (Rani & Shanker Citation2018; Perez-Rodriguez et al. Citation2019; Tauchnitz et al. Citation2020; European Commission Citation2020a). Fungicides are an extensively employed category of pesticides, accounting for a sales volume of 45% in the E.U (European Commission Citation2020b) and their use is forecast to increase with an annual growth rate of 5.95% owing to global climate change and the invasion of fungal pathogens (Research and Markets Citation2020).
Tebuconazole (TBZ) is a broad-spectrum systemic fungicide widely and repeatedly applied during the growing season on a variety of crops (Fustinoni et al. Citation2014; Youness et al. Citation2018; Othmène et al. Citation2020). It is among the world’s best-selling fungicides, and its application is expected to grow further in the next few years (Fustinoni et al. Citation2014; Chang et al. Citation2020). Once applied, TBZ accumulates in the soil, with a half-life of up to 600 days, and easily reaches the aquatic environment through post precipitation spraying or surface runoff (Šudoma et al. Citation2021). Indeed, it is one of the most frequently found fungicides in water samples (Hvězdová et al. Citation2018; Silva et al. Citation2019; de Souza et al. Citation2020; Škulcová et al. Citation2020), with concentrations ranging from 0.6 to 200 μg/L in surface waters (Chang et al. Citation2020; Li et al. Citation2020).
The antifungal activity of TBZ, as for other azoles, is related to the depletion of ergosterol and consequent disruption of cell wall integrity (Youness et al. Citation2018) achieved through the interaction with the sterol 14-demethylase, an enzyme belonging to the cytochrome P450 (CYP) family (e.g., CYP51). The inhibitory effect is not restricted to fungal CYPs, and it leads to the modulation of nontargeted cytochrome P450 enzymes including CYPs involved in steroidogenesis (e.g., CYP19) and xenobiotic detoxification (e.g., CYP1A), thus acting as an endocrine disruptor. TBZ also exerts other endocrine-related effects, interfering with the thyroid system in mammals and fish (Lv et al. Citation2017; Li et al. Citation2019).
Tebuconazole is among the ten substances for which the risk of harmful effects in aquatic ecosystems is highest (Zubrod et al. Citation2019) and meets the E.U. criteria to be considered a persistent and toxic substance (European Union Citation2015). In addition, TBZ has been classified by the Environmental Protection Agency (EPA) as a possible human carcinogen based on harmful effects observed in mice which included severe hepatic impairment (Perez-Rodriguez et al. Citation2019).
A systematic overview of available literature furnishes substantial evidence of numerous detrimental effects induced by TBZ in fish (Ferreira et al. Citation2010; Andreu‐Sánchez et al. Citation2011; Toni et al. Citation2011a, Citation2011b; Liu et al. Citation2016; Lutnicka et al. Citation2016; Altenhofen et al. Citation2017; Li et al. Citation2019; Macirella et al. Citation2019; Perez-Rodriguez et al. Citation2019; Çilingir Yeltekin et al. Citation2020; Li et al. Citation2020). Since in fish, the liver is the major site of accumulation, biotransformation, and excretion of contaminants, it is not surprising that in zebrafish, the bioaccumulation rate of TBZ is higher in this organ than in others (Li et al. Citation2019). Despite this, the effects of TBZ on this organ in fish have been the subject of a relatively limited number of studies (Ferreira et al. Citation2010; Toni et al. Citation2011a, Citation2011b; Ferreira et al. Citation2012; Li et al. Citation2019; Chang et al. Citation2020; Li et al. Citation2020) which, moreover, evaluate hepatotoxic damage mainly based on biochemical tests. The morphological liver injuries induced by TBZ remain largely overlooked (Ferreira et al. Citation2010; Boran et al. Citation2012; Tabassum et al. Citation2016; Bonomo et al. Citation2021), and literature data are limited to an overall histological evaluation in only two species (Ferreira et al. Citation2010; Li et al. Citation2020). It must be stressed that all literature data on fish refer to chronic trials or exposures to high TBZ concentrations. Conversely, due to repeated applications of fungicides in agricultural practices, the most likely exposure scenario to TBZ is short-term exposure to low concentrations alternating with a period of non-exposure (Zubrod et al. Citation2019).
Information from tests at high concentrations can certainly provide information on the effects of fungicides; however, it is essential to test concentrations putatively found in natural ecosystems (Krewski et al. Citation2010) when evaluating the toxic potential of chemicals. Accordingly, here we investigated the effects of acute exposure to an environmentally relevant concentration of TBZ in zebrafish (Danio rerio). This species has become an important vertebrate model due to its small body size, easy husbandry, short generation time, and physiological and morphological similarities to mammals. These peculiarities make zebrafish an ideal model for toxicological research to clarify the effects of chemicals (Macirella et al. Citation2019) on human and eco-system health. We examined for the first time the morpho-functional liver alterations induced by TBZ in Danio rerio, to better clarify noxious effects even after exposure to a very low dose and contribute to the implementation of risk assessment procedures. To obtain a more reliable evaluation, both frequency and degree of histological lesions were assessed according to two different grading systems thus allowing an objective comparison of experimental groups.
Given the key role of liver hepatocytes in glucose storage and metabolism, carbohydrate content may be a sensitive indicator of hepatocyte’s functional status (Gharaei et al. Citation2011; Ostaszewska et al. Citation2018). We, therefore, used two typical histometric parameters (diameter of hepatocytes and glycogen granules’ content) to assess hepatocytes’ structural and functional integrity.
It is well acknowledged that exposure to azole fungicides, and other pesticides, induce oxidative stress, defined as an imbalance between the prooxidant-antioxidant defenses, leading to potential biological systems damage (Qiu et al. Citation2019). The amount of ROS is controlled by several factors within the cellular environment, and all living organisms possess multilevel enzymatic and nonenzymatic defense systems (Stephenie et al. Citation2020). The induction of anti-oxidant enzymes reflects the activation of defense mechanisms in organisms to counteract ROS toxicity (Sadauskiene et al. Citation2020). Primary antioxidant enzymes are a cluster of proteins building the first line of defense against ROS and include superoxide dismutase (SOD), representing the most powerful antioxidant in the cell (Stephenie et al. Citation2020). Hence, we evaluated superoxide dismutase (SOD) expression and modulation as an indicator of oxidative stress induction, also performing a semiquantitative analysis.
Materials and methods
Fish maintenance
Zebrafish adults (n = 36; length, 3.5 ± 0.5 cm and weight, 0.43 ± 0.06 g) were purchased from a tropical fish store. During the acclimatization period of 2 weeks in the laboratory, we maintained animals in two aquaria filled with dechlorinated tap water (36 L) and equipped with oxygenation and filter systems. Fish were kept under light/dark (L.D.) cycle of 14:10 and fed daily with commercial fish food. The acclimatization period was performed keeping all the water parameters constant according to the standard guidelines (temperature = 26 ± 0.5°C, pH = 7.3, conductivity = 300 µs/cm, dissolved oxygen = 8 ± 1 mg/L, hardness = 100 mg/L CaCO3) (Avdesh et al. Citation2012); no mortality was recorded during the acclimatization period.
Experimental design
TBZ stock solution was made by dissolving the fungicide (Tebuconazole purity 99.5%, CAS number: 107534-96-3; Sigma-Aldrich Chemie, Steinheim, Germany) in 100 µL of acetone. The stock solution was diluted in dechlorinated tap water to reach the nominal concentration of 5 µg/L that corresponds to 0.02% of the median lethal concentration at 96 hours (LC5096h) (Andreu‐Sánchez et al. Citation2011). Moreover, the selected concentration was chosen considering the environmental contamination range of TBZ worldwide (0.6 to 200 μg/L), and it is a realistic concentration in surface waters (Liu et al. Citation2016; Chang et al. Citation2020).
For each experimental unit (48, 96, and 192 hours), six fish were randomly assigned to 6 L aquaria filled with the exposure solutions using a semi-static system. The control group (n = 18) was transferred into 18 L aquaria containing dechlorinated tap water with the same amount of acetone used for TBZ dilution. Renewal of 100% of volume aquaria with clean dechlorinated tap water or clean dechlorinated tap water containing the opportune concentration of TBZ was performed every 96 hours following the standard procedure guidelines (ASTM Citation2002). The concentration of TBZ in each tank was verified via gas chromatography with a nitrogen-phosphorus detector (GC-NPD) according to the APAT CNR IRSA 5060 Man. 29/03 water analytical method. An independent accredited laboratory performed the validation of analytical methods. For chemical analysis, water samples from each exposure tank were collected at the beginning and within 12 hours of the complete renewal of the test solution.
During the experiment, fish were fed on alternate days; food waste and debris were removed daily using a fine mesh. The water quality parameters (i.e., temperature, pH, conductivity, dissolved oxygen, hardness, and photoperiod) were periodically monitored and maintained constant as reported for the acclimatization period.
At the selected time points (48, 96, and 192 hours), fish were anesthetized using ethyl 3-aminobenzoate methanesulfonate (20 mg/L MS 222, Sandoz, Sigma-Aldrich, St. Louis, MO, USA). Excised liver samples from each experimental unit, including the control, were processed for light and confocal microscopy.
Animal care, experiments, and animal sacrifice were conducted according to the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes (Council of Europe No. 123, Strasbourg, 1985). For each experimental unit, including the control, two replicates were conducted.
Histology
Liver samples were fixed for 4 hours by direct immersion in 4% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in phosphate-buffered saline solution (PBS 0.1 M, pH 7.2, 4°C) and post-fixed in osmium tetroxide (1% in PBS) for 2 hours. Samples were dehydrated in graded ethanol, placed in propylene oxide, and embedded in Epon-Araldite (Araldite 502/Embed 812, Electron Microscopy Sciences). Semi-thin sections (1 µm thickness), obtained using a Leica UltraCut UCT (Leica Microsystems, Wetzlar, Germany), were stained with toluidine blue and observed under an LM Leitz Dialux 20 E.B. (Leica Microsystems, Wetzlar, Germany).
Histopathological and morphometric analysis
Graph Pad Prism 8.00 for Windows (GraphPad Software, Inc., San Diego, CA, USA) was used to run all statistical analyses.
Mean assessment values and degree of tissue changes
For each animal of both the control and TBZ exposed groups, three nonsequential semi-thin sections (toluidine blue-stained) were analyzed for semiquantitative histological analyses. Each section was observed under an LM Leitz Dialux 20 E.B. (Leica Microsystems, Wetzlar, Germany) at different magnifications (20×, 40×, 100×) to establish histopathological changes. The degree of histopathological changes was evaluated following the grading system of Pal et al. (Citation2012) with some modifications. Based on the frequency of histological manifestation, a numerical value was assigned to each lesion (Table S1). The grading system used was: grade 1 = no pathological alterations (-), grade 2 = mild to moderate pathological alterations (+, ++), grade 3 = severe pathological alterations (+++). This grading system was used to establish the mean assessment values (MAV) of the organ for each treatment group, including the control. The nonparametric Kruskal-Wallis test was used to compare mean values with p < 0.0001 as a significance level.
The degree of tissue changes (DTC) was determined based on the extent of lesions and their likelihood of recovery. Histopathological lesions were grouped into 3 progressive stages (Stage I, II, III) of liver function impairment according to Paulo et al. (Citation2012) and Marcon et al. (Citation2015) with some modifications.
Stage I indicated fewer damages to the tissue; Stage II indicated more drastic alterations that may interfere with the natural liver function; Stage III indicated severe and permanent changes. For all groups, DTC values were calculated for each animal (n = 12) using the following equation (Marcon et al. Citation2015): DTC = (1 x ƩI) + (10 x ƩII) + (100 x ƩIII), where I, II, and III referred to the number of lesions observed in 10 images coming from each animal of all treatment groups, including the control. The average DTCs were estimated and used to assessed tissue damage based on five classes: i) 0–10 indicated normal tissue structure, ii) 11–20 indicated mildly damaged tissue, iii) 21–50 indicated moderately damaged tissue, iv) 50–100 indicated severely damaged tissue, and v) more than 100 indicated severely and irreversibly damaged tissue. Ordinary one-way ANOVA was performed using Tukey’s multiple comparison test to compare mean values with p < 0.0001 as a significance level.
Analysis of glycogen granules distribution
To analyze the area occupied by glycogen granules, five sections of each animal of all treatment groups, including the control, were chosen and photographed at 40x magnification; the obtained images (n = 5) were analyzed using the method described by Pereira et al. (Citation2014) with slight modifications. The glycogen granules were isolated using a plug-in image analysis program, ImageJ (NIH, developed at the National Institutes of Health, a part of the U.S. Department of Health and Human Services), and the total area occupied by the granules was quantified in each image. The arithmetic means were calculated, and the values were statistically compared using the one-way ANOVA followed by Tukey’s multiple comparisons test (at a significance level of 0.001). Data were checked for normality (Shapiro-Wilk test) and homogeneity of variances (Bartlett’s test) and presented as mean ± standard deviation.
Analysis of hepatocytes size
The dimensions of hepatocytes were measured on semi-thin sections (toluidine blue-stained) using an image analysis program (NIH, developed at the National Institutes of Health, a part of the U.S. Department of Health and Human Services). For each animal of all treatment groups, including the control, 4 liver photographs (100x magnification) were used to measure the lengths of 15 randomly selected hepatocytes; dimensions were taken passing through the nucleus of the hepatocyte. Data were statistically compared using the Kruskal-Wallis test followed by Dunn’s Multiple Comparison tests (at a significance level of 0.001). Bartlett’s test was used for homogeneity of variances, and data were presented as mean ± standard deviation.
Measurement of plasma oxidative status
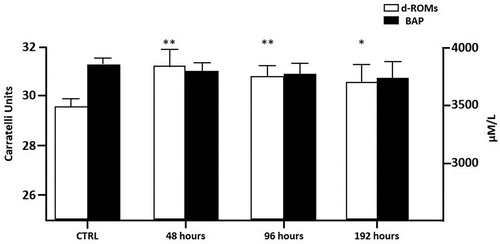
For each animal of both the control and TBZ exposed groups (n = 6), plasma samples were collected to evaluate oxidative status using a free radical analyzer system and a spectrophotometric device reader (FREE Carpe Diem, Diacron International, Grosseto, Italy) (La Russa et al. Citation2020, Citation2021). The diacron-reactive oxygen metabolite (dROMs) test was used to analyze the total amount of hydroperoxides in plasma samples following the manufacturer’s instruction (Diacron International S.R.L., Italy). Briefly, 20 µl of plasma was placed in a cuvette containing the R2 reagent adding subsequently 20 µl of R1 reagent. The color change in the cuvette was optically measured and recorded in U. CARR (Carratelli Units), where 1 U. CARR corresponds to 0.08 mg/L of hydrogen peroxide.
Total plasma antioxidant capacity was measured using a biological antioxidant potential (BAP) kit according to the manufacturer’s instruction (Diacron International S.R.L., Italy). Briefly, 50 μL of R2 reagent containing trivalent iron was added in a cuvette containing the R1 reagent. After gently mixing, 10 μl of plasma serum was added to the cuvette, and the color-development was measured using the FREE Carpe Diem. Results were expressed in µmol/L.
All data were statistically compared using the one-way ANOVA followed by the Bonferroni multiple comparisons test. Results have been expressed as the mean ± standard deviation.
Immunohistochemistry and quantification of SOD expression
Liver samples for immunohistochemical analyses were fixed for 48 hours by direct immersion in Bouin’s solution. After dehydration in an increasing ethanol series, samples were cleared in xylene and embedded in paraffin wax (mean fusion point = 56°C). Samples were cut (10 µm thickness) using a rotary microtome Leica RM 2125 RT (Leica Microsystems, Wetzlar, Germany) and mounted on positively charged slides. Tissue sections were deparaffinized, washed several times in PBS (0.1 M, pH 7.2, 4°C), raised for 30 minutes with 20% normal goat serum at room temperature, and finally incubated over-night at 4°C with a mouse monoclonal anti-Cu-Zn SOD1 (Santa Cruz Biotechnology, Inc., CA) at a working dilution of 1:100. On the following day, slides were washed several times in PBS and incubated for 30 minutes at room temperature in the dark with a fluorescein isothiocyanate-conjugated-globulin goat anti-mouse (Sigma- Aldrich Chemical Co., St. Louis, MO, USA) at a working dilution of 1:100. Propidium iodide (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) at a working dilution of 1:200 was applied on washed slides to label cell nuclei. Finally, slides were washed in PBS, mounted, and observed under a Leica TCS SP2 Confocal Laser Scanning Microscope (Leica Microsystems, Wetzlar, Germany).
SOD expression in TBZ exposed groups was compared to the control group using immunolabelled slides that were randomly observed and photographed under a Leica TCS SP2 confocal microscope. Managing an image analysis software (NIH, developed at the National Institutes of Health; Bethesda, MD, USA), we analyzed the area positively expressing SOD belonging to a field of 875 × 875 pixels (350 × 350 µm) and compared the data using the one-way ANOVA followed by Bonferroni’s multiple comparison tests (at a significance level of 0.001). Data were checked for normality (D’Agostino & Pearson omnibus normality test) and homogeneity of variances (Bartlett test) and presented as mean ± standard deviation.
Results
During the whole experiment, no mortality was recorded in the control or the TBZ exposed groups. In all samples from TBZ exposed groups, some histological injuries of the liver were observed.
Control group
The liver morphology of Danio rerio has been previously described (Macirella et al. Citation2016), and in the present work, only a brief overview will be furnished. The liver parenchyma, in which the hepatocytes were orderly arranged, shows a homogeneous and compact appearance. The vein with different sizes and shapes and minute bile channels were scattered through /within hepatocytes ()).
Figure 1. Light micrographs of Danio rerio liver under basal condition. (a) The liver parenchyma shows a homogeneous structure with hepatocytes orderly arranged. (b) A continuous endothelium lines the sinusoids, and the space of Disse extends between the hepatocytes and the sinusoids wall. Note erythrocytes and few macrophages in the lumen of the sinusoids. (c) The veins with different sizes and shapes are scattered through the hepatocytes. (d) Bile ducts are surrounded by a simple cuboidal epithelium. h = hepatocyte, n = nucleus, arrow = glycogen granules, arrowhead = lipid droplet, bd = bile duct, ds = Disse’s space, e = erythrocyte, m = macrophage, s = sinusoid, v = vein.
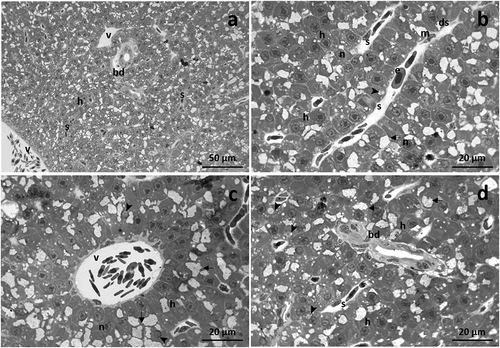
Hepatocytes formed a cord-like structure surrounding a network of sinusoids. Sinusoids are lined by a continuous endothelium which, along with hepatocytes, originate the space of Disse ()). Erythrocytes could be seen along with few macrophages in both sinusoid and vessel lumen (). Hepatocytes showed a large nucleus, usually centrally located, and a prominent nucleolus. Numerous glycogen granules were scattered in the cytoplasm, and some lipid droplets could also be observed ()). With further magnification, the simple cuboidal epithelium that borders the bile ducts was more clearly visible ()).
TBZ exposed groups
After 48 hours of exposure to TBZ, the liver’s architecture changed, and wide lysis zones were scattered throughout the parenchyma. It was possible to observe the parenchyma dyschromia in the hepatic tissue due to the emergence of both necrotic and apoptotic events ()). In some areas, necrotic hepatocytes showed a poor and lightly stained cytoplasm. On the contrary, apoptotic hepatocytes exhibited a deeply stained cytoplasm and degenerated or pyknotic nuclei ()).
Figure 2. Light micrographs of Danio rerio liver after 48 (a-d) and 96 (e-j) hours of exposure to TBZ. (a) Note the parenchyma dyschromia of the hepatic tissue and the appearance of wide lysis areas (la). (b) Necrotic cells have a poor and lightly stained cytoplasm (asterisks), while apoptotic hepatocytes show a deeply stained cytoplasm and degenerated or pyknotic nuclei (white arrow). Note the congestion of sinusoids. (c) Blood vessels are congested, and aggregates of highly pigmented melano-macrophages appear in the parenchyma. (d) Note the degeneration of cuboidal epithelium lining the bile ducts. (e) The parenchyma dyschromia increases, and numerous sinusoids are scattered in the tissue. (f) Apoptotic hepatocytes show a deeply stained cytoplasm and degenerated nuclei (black arrow); note the immigrate macrophages and the congested sinusoids. (g) Blood vessels are occluded, and the macrophages proliferate in their lumen. (h,i) Detail of the degeneration of the cuboidal cells lining the bile ducts. (j) Numerous necrotic hepatocytes show a poor, pale-stained cytoplasm (star). h = hepatocyte, n = nucleus, arrow = glycogen granules, s = sinusoid, v = vein, bd = bile duct, m = macrophage, mc = melanomacrophage complex, black arrowhead = lipid droplet.
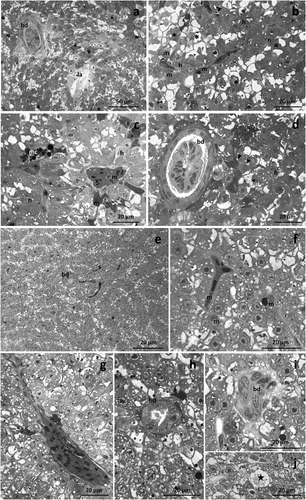
Both sinusoids and vessel lumen was congested, in which macrophages were frequently detected (). Numerous macrophages also migrated in the parenchyma, where they originated aggregates of highly pigmented melano-macrophages at several points ()). The cuboidal epithelium lining the bile ducts was strongly modified ()).
After 96 hours of exposure to TBZ, the histological architecture of the hepatic parenchyma was severely modified. The dyschromia became more evident, and the number of sinusoids scattered in the tissue increased ()). Numerous apoptotic hepatocytes characterized by a deeply stained cytoplasm and degenerated nuclei were detected in the tissue ()). Macrophages proliferated in the lumen of both vessels and sinusoids or migrated in the liver parenchyma. Moreover, the appearance of numerous blood cells in the lumen of sinusoids and vessels resulted in their congestion (). The bile ducts were severely modified, and in particular, the cuboidal cells degenerated (). Numerous necrotic hepatocytes were easily recognized by their poor, pale-stained cytoplasm ()).
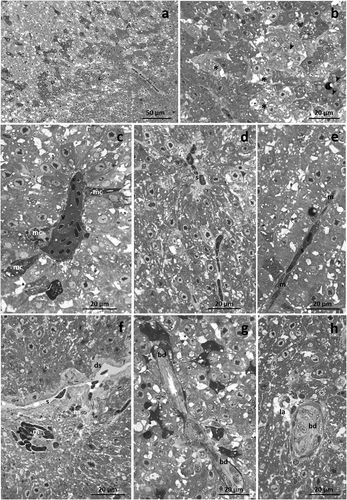
Liver morphology appeared markedly altered after 192 hours of TBZ exposure, and the intensity of degenerative phenomena significantly increased. Both slightly-stained and deeply-stained hepatocytes were detected, enhancing the dyschromia of the hepatic parenchyma (). A drastic decrease in glycogen granules could also be noted ()). Congested blood vessels and sinusoids were detected, and macrophages dispersed in their lumen were conspicuous (). Moreover, close to the occluded blood vessels, pigmented melano-macrophages complexes were noted ()). Disse’s space was often dilated, and immigrated macrophages, arranged in clusters, were frequently dispersed in the liver tissue ()). Bile ducts displayed a strongly modified architecture, and several broad lysed areas were visible ().
Figure 3. Light micrographs of Danio rerio liver after 192 hours of exposure to TBZ. (a) The dyschromia of the liver parenchyma further increases, and glycogen granules are less frequent. (b) Note the intensification in the number of slightly-stained (black asterisk) and deeply-stained (white asterisk) hepatocytes. (c) Blood vessels are congested, and pigmented melano-macrophage complexes (mc) are frequently detected. (d,e) Occluded sinusoids are filled with numerous macrophages. (f) Note the dilation of Disse’s space (ds) and the numerous immigrated macrophages dispersed in the parenchyma. (g,h) Detail of bile duct degeneration and lysed areas (la) dispersed in the parenchyma. s = sinusoid, v = vein, m = macrophage, bd = bile duct, arrow-head = lipid droplet.
Histometric analysis
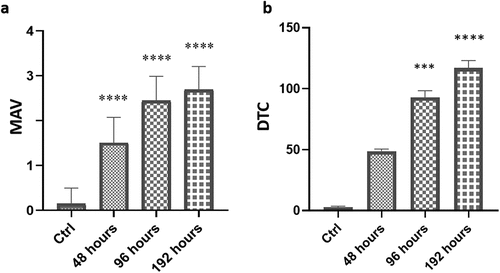
Semiquantitative analysis of MAV and DTC value of all treatment groups, including the control, is shown in respectively. Compared to the control, the MAV value was significantly higher in all treated groups (****p < 0.0001). In particular, the average values of MAV increased with the increasing time of exposure. Compared to the control, also the overall DTC values increased with the exposure period in all TBZ treated groups. In particular, the DTC values indicated the normal tissue structure of liver in basal condition (mean DTC value = 2.65 ± 0.78), a moderately damaged tissue after 48 hours of exposure (mean DTC value = 49.50 ± 1.75), a severely damaged tissue after 96 hours of exposure (mean DTC value = 90.65 ± 5.54, *** p < 0.0002) and, a severely and irreversibly damaged tissue after 192 hours of exposure to TBZ (mean DTC value = 119.60 ± 6.09, **** p < 0.0001).
Figure 4. Mean Assessment Value (MAV) (a) and Degree of Tissue Changes (DTC) (b) in the liver of Danio rerio. Graphs indicate the mean ± S.D. Significant differences among the control group and treatment groups are indicated by (***) at p < 0.0002 and (****) at p < 0.0001.
Glycogen amount showed a statistically significant difference in TBZ exposed compared to control (). In detail, the glycogen decreased after 48 and 96 hours of exposure to TBZ (***p < 0.001), reaching a drastic decline after 192 hours of exposure (****p < 0.0001).
Table I. Evaluation of the areas occupied with glycogen granules, estimation of average hepatocytes diameter (µm) and section areas positively expressing SOD in the control group and the groups of different exposure period
Compared to the control group, hepatocytes size slightly decreased after 48 hours of exposure to TBZ (). After 96 and 192 hours, the hepatocyte size further diminished, and in both treated groups, the reduction was significantly relevant (***p < 0.001).
Oxidative status
The oxidative status of both exposed and non-exposed fish was evaluated. The reactive oxygen metabolites (dROMs test) and biological antioxidant potential (BAP test) were measured in plasma. As shown in , a significant d-ROM increase could be seen in all TBZ exposed groups compared to the control reaching a maximum after 48 hours. On the contrary, no significant modifications in BAP were observed.
Immunolocalization of SOD1
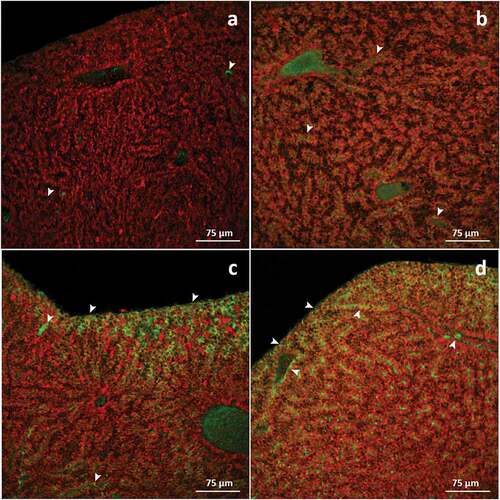
In basal conditions, it was possible to recognise very few cells in the hepatic parenchyma showed weak immunoreactivity for SOD1. Weak immunoreactivity was also visible in the vessels and sinusoids ()). After 48 hours of exposure to TBZ, a slight immunolabelling increase was observed throughout the liver parenchyma compared to the control ()). A significant increase in SOD1 expression (*p < 0.05; ) was detected after 96 hours of exposure; an intense immunopositive signal was mainly observed at the periphery of the tissue ()). After 192 hours of exposure, a marked increase in immunosignal was evident in the whole parenchyma (***p < 0.001; ), peaking in the peripheral portion of the tissue ()).
Figure 6. Confocal micrographs of Danio rerio liver. Sections are labeled with a mouse monoclonal antibody against Cu-Zn superoxide dismutase-1 (green-FITC labeled); nuclei labeled with propidium iodide (red). (a) In control specimens, no or weak signal is evident in the hepatic parenchyma (arrowhead); (b) Compared to basal conditions, after 48 hours of exposure to TBZ, a slight increase in staining intensity is observed (arrowhead). (c) After 96 hours of exposure, the immunostaining drastically increases within the parenchyma, especially at the tissue’s periphery (arrowhead). (d) The intensity of immunopositive hepatocytes further increases after 192 hours of exposure to TBZ, and immunosignal appears in the endothelial cells (arrowhead).
Discussion
Evidence for detrimental effects induced by TBZ in fish is supported by broad literature data on several specific targets of toxicity which also demonstrate that the liver is a key compartment for TBZ detoxification and accumulation. Surprisingly, a few studies have examined the effects of TBZ on the fish liver. Only two studies considered morphological endpoints furnishing a general description of histological lesions (Ferreira et al. Citation2010; Li et al. Citation2020); however, much more detail is needed to identify tissue toxicity targets. Most importantly, these reports refer to chronic trials or exposure to high TBZ concentrations.
Conversely, data on toxic effects elicited by low concentrations of water-borne pollutants are mandatory to identify the early subtle alterations and implications to the health and fitness of individuals and populations. Indeed, for TBZ and other fungicides, the more ecologically relevant exposure scenario corresponds to short-term exposure to low concentrations alternating with a period of non-exposure (Zubrod et al. Citation2019). For these purposes, we investigated for the first time the effects of short-term exposure to a very low concentration of TBZ in Danio rerio liver to fill the gap regarding TBZ toxicity in fish, and contributing to fungicides risk assessment in the aquatic environment.
In the present study, we successfully demonstrated that an environmentally realistic low concentration of TBZ induces severe histopathological and functional modifications in Danio rerio liver. Histological observations revealed that all exposed samples exhibited varying degrees of liver alterations. Furthermore, we evaluated the histological changes by applying a gradient system to make an objective comparison between the experimental groups. To our knowledge, no studies have applied histometric analysis to evaluate TBZ effects in fish liver, although the role of these tools in determining the toxic potential of xenobiotics in target tissues is well known.
We showed that the first sign of TBZ-induced changes arose after 48 hours of exposure, and both the frequency and extent of histological lesions became more intense as the exposure continued. In detail, after 48 and 96 hours, we observed moderate and severe damages of tissues, respectively. After 192 hours of exposure, histological lesions reached the highest degree, ranked as irreversible. Our results showed that hepatocyte dimensions were significantly reduced under TBZ exposure, contributing to the uneven liver appearance. Also, severe changes in the vascular system are often observed as a result of TBZ exposure. In all exposed groups, parenchymal blood vessels and sinusoids exhibited marked congestion leading to the appearance of vascular occlusion becoming more frequent after 96 hours of treatment. Moreover, a proliferation of macrophages has been seen, which migrate from the sinusoidal lumen to the hepatic parenchyma, giving rise to melano-macrophage complexes. These results clearly indicate that TBZ exposure might induce a time-dependent immune system activation.
It is known that macrophage activation, as a part of the immune response, may, in turn, lead to the increase in ROS due to the leukocyte’s respiratory burst activity (Biller-Takahashi & Urbinati Citation2014; Biller & Takahashi Citation2018). In fish, oxidative stress induction is one of the most detrimental effects ascribed to TBZ (Ferreira et al. Citation2010; Toni et al. Citation2011a, Citation2011b; Clasen et al. Citation2018; Li et al. Citation2020). ROS production is considered an early biomarker to evaluate hepatotoxic damage, and it has been reported by several authors in different fish species after exposure to pesticides (Stoyanova et al. Citation2020; Georgieva et al. Citation2021).
Here we confirm an imbalance of the overall antioxidant capacity in TBZ-exposed fish, as revealed by modulation of pro-oxidant (dROMs test) species in plasma, at all exposure times. Our results suggest that the failure to counteract the production and accumulation of ROS induced by TBZ at systemic level, may lead to organ-specific damages. In the liver, ROS formation can induce hepatocyte apoptosis and necrosis (Choi et al. Citation2010), which represent the most severe histopathological manifestations of hepatocellular toxicity (Grattagliano et al. Citation2009; Wolf & Wheeler Citation2018).
Interestingly, severe histopathological changes in zebrafish could be observed after 48 hours of exposure, when ROS production was already significantly higher than that of the control.
Mechanisms to counteract free radical damage in the organism include several defense enzymes, such as superoxide dismutase (SOD), the most potent antioxidant in the cells (Stephenie et al. Citation2020). The upregulation of SOD induced by TBZ has previously been reported in Danio rerio gills and liver after acute and chronic exposure, respectively (Macirella et al. Citation2019; Chang et al. Citation2020; Li et al. Citation2020). In this study, a significant increase in SOD immunoreactivity in the liver of TBZ-exposed animals was detected only after 96 hours of exposure. These data indicated that liver injuries induced by TBZ are not due to a depletion in antioxidant system capacity but most likely to a rapid increase in ROS generation.
The concurrent appearance of both apoptotic and necrotic events in the liver of Danio rerio exposed to TBZ resulted in an intense parenchyma dyschromia, as previously reported in the liver of other aquatic organisms after exposure to TBZ and pyrimethanil (Bernabò et al. Citation2017, Citation2020). Pale necrotic cells showed a swollen and poor cytoplasm, whereas the condensation and decrease of the diameter of nuclei were often observed in deeply colored hepatocytes indicating the onset of the apoptotic cascade (Nowak & Kingsford Citation2003; Rabitto et al. Citation2005; Narra Citation2016). The generation and increase of ROS production could be considered the main factor of hepatocyte apoptosis induction (Qiu et al. Citation2019). Li et al. (Citation2020) recently suggested that TBZ, through ROS generation, encodes for pro-apoptotic proteins that ultimately cause liver structural damage in both larvae and adults of Danio rerio.
In the present study, hepatocytes of animals exposed to TBZ showed significantly lower glycogen amounts than the control. The observed depletion was time-dependent, thus indicating enhanced energy demand under stress conditions. Our results agree with literature data showing that glycogen reduction is the most common response to pesticide exposure in fish. The glycogen depletion, due to enhanced energy demand required for the synthesis of detoxification enzymes, has been reported in several species after exposure to different classes of pesticides (Narra Citation2016; Yadavrao Citation2017; Vani et al. Citation2020).
Conclusions
Although recent developments in toxicology have provided some new insights into biochemical markers of toxicity, the first outcome of pollutants is a morphological alteration in target organs. Altogether our results provide evidence that a very low TBZ dose, easily found in natural aquatic environments, may alter the morphological and histological arrangement of the liver in zebrafish, and such alterations become irreversible after 192 hours of exposure. Histopathological changes precociously appear involving both hepatocytes and vascular components, thus disrupting homeostasis and physiology of the liver. Results from the present study suggest that a research approach involving the joined use of morphological, functional, and histometric investigation might provide a more reliable assessment of responses to environmental stressors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Altenhofen S, Nabinger DD, Wiprich MT, Pereira TCB, Bogo MR, Bonan CD. 2017. Tebuconazole alters morphological, behavioral and neurochemical parameters in larvae and adult zebrafish (Danio rerio). Chemosphere 180:483–490. DOI: 10.1016/j.chemosphere.2017.04.029.
- American Society for Testing and Materials (ASTM). 2002. Standard guide for conducting acute toxicity test with fishes, macroinvertebrates, and amphibians E 729 - 96. In: Annual book of ASTM standards. Philadelphia (PA). pp. 165–174.
- Andreu‐Sánchez O, Paraíba LC, Jonsson CM, Carrasco JM. 2011. Acute toxicity and bioconcentration of fungicide tebuconazole in zebrafish (Danio rerio). Environmental Toxicology 27:109–116. DOI: 10.1002/tox.20618.
- Avdesh A, Chen M, Martin-Iverson MT, Mondal A, Ong D, Rainey-Smith S, Taddei K, Lardelli M, Groth DM, Verdile G, Martins RN. 2012. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. The Journal of Visualized Experiments 69:e4196. DOI: 10.3791/4196.
- Bernabò I, Guardia A, Macirella R, Sesti S, Tripepi S, Brunelli E. 2020. Tissues injury and pathological changes in Hyla intermedia juveniles after chronic larval exposure to tebuconazole. Ecotoxicology and Environmental Safety 205:111367. DOI: 10.1016/j.ecoenv.2020.111367.
- Bernabò I, Guardia A, Macirella R, Tripepi S, Brunelli E. 2017. Chronic exposures to fungicide pyrimethanil: Multi-organ effects on Italian tree frog (Hyla intermedia). Scientific Reports 7:6869. DOI: 10.1038/s41598-017-07367-6.
- Biller-Takahashi JD, Urbinati EC. 2014. Fish Immunology. The modification and manipulation of the innate immune system: Brazilian studies. Anais da Academia Brasileira de Ciências 86:1484–1506. DOI: 10.1590/0001-3765201420130159.
- Biller JD, Takahashi LS. 2018. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. Anais da Academia Brasileira de Ciências 90:3403–3414. DOI: 10.1590/0001-3765201820170730.
- Bonomo MM, de Castro Sachi IT, Paulino MG, Fernandes JB, Carlos RM, Fernandes MN. 2021. Multi-biomarkers approach to access the impact of novel metal-insecticide based on flavonoid hesperidin on fish. Environmental Pollution 268:115758. DOI: 10.1016/j.envpol.2020.115758.
- Boran H, Capkin E, Altinok I, Terzi E. 2012. Assessment of acute toxicity and histopathology of the fungicide captan in rainbow trout. Experimental and Toxicologic Pathology 64:175–179. DOI: 10.1016/j.etp.2010.08.003.
- Chang Y, Mao L, Zhang L, Zhang Y, Jiang H. 2020. Combined toxicity of imidacloprid, acetochlor, and tebuconazole to zebrafish (Danio rerio): Acute toxicity and hepatotoxicity assessment. Environmental Science and Pollution Research 27:10286–10295. DOI: 10.1007/s11356-020-07653-3.
- Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu DY. 2010. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquatic Toxicology 100:151–159. DOI: 10.1016/j.aquatox.2009.12.012.
- Çilingir Yeltekin A, Oğuz A, Kankaya E, Özok N, Güneş İ. 2020. Hematological and biochemical response in the blood of Alburnus tarichi (actinopterygii: cypriniformes: cyprinidae) exposed to tebuconazole. Acta Ichthyologica et Piscatoria 50:373–379. DOI: 10.3750/aiep/02931.
- Clasen B, Loro VL, Murussi CR, Tiecher TL, Moraes B, Zanella R. 2018. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Science of the Total Environment 626:737–743. DOI: 10.1016/j.scitotenv.2018.01.154.
- de Souza RM, Seibert D, Quesada HB, de Jesus Bassetti F, Fagundes-Klen MR, Bergamasco R. 2020. Occurrence, impacts, and general aspects of pesticides in surface water: A review. Process Safety and Environmental 135:22–37. DOI: 10.1016/j.psep.2019.12.035.
- European Commission. 2020a. Pesticides. Available: https://ec.europa.eu/food/plant/pesticides_en. Accessed Mar 1 2021.
- European Commission. 2020b. Sales of pesticides in the E.U. Available: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20200603-1 Accessed Feb 2021 25.
- European Union. 2015. Implementing Regulation (E.U.) 2015/408 of 11.3.2015 on implementing Article 80 (7) of Regulation (E.C.) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and establishing a list of candidates for substitution. Official Journal of European Union L. 67:18–22.
- Ferreira D, da Motta AC, Kreutz LC, Toni C, Loro VL, Barcellos LJG. 2010. Assessment of oxidative stress in Rhamdia quelen exposed to agrichemicals. Chemosphere 79:914–921. DOI: 10.1016/j.chemosphere.2010.03.024.
- Ferreira D, Unfer TC, Rocha HC, Kreutz LC, Koakoski G, Barcellos LJG. 2012. Antioxidant activity of bee products added to water in tebuconazole-exposed fish. Neotropical Ichthyology 10:215–220. DOI: 10.1590/S1679-62252012000100021.
- Food and Agriculture Organization of the United Nations 2018. Database collection of the Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data. Accessed Feb 19 2021.
- Fustinoni S, Mercadante R, Polledri E, Rubino FM, Mandic-Rajcevic S, Vianello G, Colosio C, Moretto A. 2014. Biological monitoring of exposure to tebuconazole in winegrowers. Journal of Exposure Science and Environmental Epidemiology 24:643–649. DOI: 10.1038/jes.2014.14.
- Georgieva E, Yancheva V, Stoyanova S, Velcheva I, Iliev I, Vasileva T, Bivolarski B, Petkova E, László B, Nyeste K, Antal L. 2021. Which is more toxic? Evaluation of the short-term toxic effects of chlorpyrifos and cypermethrin on selected biomarkers in common carp (Cyprinus carpio, Linnaeus 1758). Toxics 9:125. DOI: 10.3390/toxics9060125.
- Gharaei A, Ghaffari M, Keyvanshokooh S, Akrami R. 2011. Changes in metabolic enzymes, cortisol and glucose concentrations of Beluga (Huso huso) exposed to dietary methylmercury. Fish Physiology and Biochemistry 37:485–493. DOI: 10.1007/s10695-010-9450-3.
- Grattagliano I, Bonfrate L, Diogo CV, Wang HH, Wang DQ, Portincasa P. 2009. Biochemical mechanisms in drug-induced liver injury: Certainties and doubts. World Journal of Gastroenterology 15:4865. DOI: 10.3748/wjg.15.4865.
- Hvězdová M, Kosubová P, Košíková M, Scherr KE, Šimek Z, Brodský L, Šudoma M, Škulcová L, Sáňka M, Svobodová M, Krkošková L. 2018. Currently and recently used pesticides in Central European arable soils. Science of the Total Environment 613:361–370. DOI: 10.1016/j.scitotenv.2017.09.049.
- Krewski D, Acosta JD, Andersen M, Anderson H, Bailar JC, Boekelheide K, Brent R, Charnley G, Cheung VG, Green JS, Kelsey KT, Kerkvliet NI, Li AA, McCray L, Meyer O, Patterson RD, Pennie W, Scala RA, Solomon GM, Stephens M, Yager J, Zeise L. 2010. Staff of Committee on Toxicity Testing and Assessment of Environmental Agents. Toxicity testing in the 21st century: A vision and a strategy. Journal of Toxicology and Environmental Health, Part B: Critical Reviews 13:51–138. DOI: 10.1080/10937404.2010.483176.
- La Russa D, Marrone A, Mandalà M, Macirella R, Pellegrino D. 2020. Antioxidant/Anti-Inflammatory effects of caloric restriction in an aged and obese rat model: The role of adiponectin. Biomedicines 8:532. DOI: 10.3390/biomedicines8120532.
- La Russa D, Montesano D, Pellegrino D, Frisina M, Bagetta G, Fallarino F, Amantea D. 2021. Systemic administration of sunflower oil exerts neuroprotection in a mouse model of transient focal cerebral ischaemia. Journal of Pharmacy and Pharmacology 22:rgab007. DOI: 10.1093/jpp/rgab007.
- Li S, Jiang Y, Sun Q, Coffin S, Chen L, Qiao K, Gui W, Zhu G. 2020. Tebuconazole induced oxidative stress related hepatotoxicity in adult and larval zebrafish (Danio rerio). Chemosphere 241:125129. DOI: 10.1016/j.chemosphere.2019.125129.
- Li S, Sun Q, Wu Q, Gui W, Zhu G, Schlenk D. 2019. Endocrine disrupting effects of tebuconazole on different life stages of zebrafish (Danio rerio). Environmental Pollution 249:1049–1059. DOI: 10.1016/j.envpol.2019.03.067.
- Liu N, Dong F, Xu J, Liu X, Zheng Y. 2016. Chiral bioaccumulation behavior of tebuconazole in the zebrafish (Danio rerio). Ecotoxicology and Environmental Safety 126:78–84. DOI: 10.1016/j.ecoenv.2015.12.007.
- Lutnicka H, Bojarski B, Ludwikowska A, Wrońska D, Kamińska T, Szczygieł J, Troszok A, Szambelan K, Formicki G. 2016. Hematological alterations as a response to exposure to selected fungicides in common carp (Cyprinus carpio L.). Folia Biol-Krakow 64:235–244. DOI: 10.3409/fb64_4.235.
- Lv X, Pan L, Wang J, Lu L, Yan W, Zhu Y, Xu Y, Guo M, Zhuang S. 2017. Effects of triazole fungicides on androgenic disruption and CYP3A4 enzyme activity. Environmental Pollution 222:504–512. DOI: 10.1016/j.envpol.2016.11.051.
- Macirella R, Guardia A, Pellegrino D, Bernabò I, Tronci V, Ebbesson LO, Sesti S, Tripepi S, Brunelli E. 2016. Effects of two sublethal concentrations of mercury chloride on the morphology and metallothionein activity in the liver of zebrafish (Danio rerio). International Journal of Molecular Sciences 17:361. DOI: 10.3390/ijms17030361.
- Macirella R, Tripepi M, Brunelli E. 2019. Morphological and immunohistochemical modifications in Zebrafish (Danio rerio) gills after short-term exposure to the fungicide tebuconazole. Zebrafish 16:65–76. DOI: 10.1089/zeb.2018.1638.
- Maggi F, Tang FHM, la Cecilia D, McBratney A. 2019. PEST-CHEMGRIDS, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025. Science Data 170:1–20. DOI: 10.1038/s41597-019-0169-4.
- Marcon L, Bazzoli N, Honor Mounteer A, Anjos Benjamin LD. 2015. Histological and histometric evaluation of the liver in Astyanax bimaculatus (Teleostei: Characidae), exposed to different concentrations of an organochlorine insecticide. The Anatomical Record 298:1754–1764. DOI: 10.1002/ar.23196.
- Narra MR. 2016. Single and cartel effect of pesticides on biochemical and haematological status of Clarias batrachus: A long-term monitoring. Chemosphere 144:966–974. DOI: 10.1016/j.chemosphere.2015.09.065.
- Nowak BF, Kingsford MJ. 2003. Exposure to Thiodan® results in lipofuscin accumulation in hepatocytes of the freshwater catfish Tandanus tandanus. Diseases of Aquatic Organisms 56:135–143. DOI: 10.3354/dao056135.
- Ostaszewska T, Śliwiński J, Kamaszewski M, Sysa P, Chojnacki M. 2018. Cytotoxicity of silver and copper nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Environmental Science and Pollution Research 25:908–915. DOI: 10.1007/s11356-017-0494-0.
- Othmène YB, Hamdi H, Salem IB, Annabi E, Amara I, Neffati F, Najjar MF, Abid-Essefi S. 2020. Oxidative stress, DNA damage and apoptosis induced by tebuconazole in the kidney of male Wistar rat. Chemico-Biological Interactions 330:109114. DOI: 10.1016/j.cbi.2020.109114.
- Pal S, Kokushi E, Koyama J, Uno S, Ghosh AR. 2012. Histopathological alterations in gill, liver and kidney of common carp exposed to chlorpyrifos. Journal of Environmental Science and Health, Part B 47:180–195. DOI: 10.1080/03601234.2012.632285.
- Paulo DV, Fontes FM, Flores-Lopes F. 2012. Histopathological alterations observed in the liver of Poecilia vivipara (Cyprinodontiformes: Poeciliidae) as a tool for the environmental quality assessment of the Cachoeira River, BA. Brazilian Journal of Biology 72:131–140. DOI: 10.1590/S1519-69842012000100015.
- Pereira BF, Alves RDS, Alves AL, Senhorini JA, Rocha RCGA, Scalize PH, Pitol DL, Caetano FH. 2014. Effects of biodegradable detergents in morphological parameters of liver in two neotropical fish species (Prochilodus lineatus and Astyanax altiparanae). Microscopy Research 2:39–49. DOI: 10.4236/mr.2014.22006.
- Perez-Rodriguez V, Souders CL, Tischuk C, Martyniuk CJ. 2019. Tebuconazole reduces basal oxidative respiration and promotes anxiolytic responses and hypoactivity in early-staged zebrafish (Danio rerio). Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology 217:87–97. DOI: 10.1016/j.cbpc.2018.11.017.
- Qiu L, Jia K, Huang L, Liao X, Guo X, Lu H. 2019. Hepatotoxicity of tricyclazole in zebrafish (Danio rerio). Chemosphere 232:171–179. DOI: 10.1016/j.chemosphere.2019.05.159.
- Rabitto IS, Costa JA, De Assis HS, Pelletier E, Akaishi FM, Anjos A, Randi MAF, Ribeiro CO. 2005. Effects of dietary Pb (II) and tributyltin on neotropical fish, Hoplias malabaricus: Histopathological and biochemical findings. Ecotoxicology and Environmental Safety 60:147–156. DOI: 10.1016/j.ecoenv.2004.03.002.
- Rani M, Shanker U. 2018. Removal of chlorpyrifos, thiamethoxam, and tebuconazole from water using green synthesized metal hexacyanoferrate nanoparticles. Environmental Science and Pollution Research 25:10878–10893. DOI: 10.1007/s11356-018-1346-2.
- Research and Markets. 2020. Global SDHI Fungicide Market - Forecasts from 2020 to 2025. Report ID 5215212. Available: https://www.researchandmarkets.com/reports/5215212/global-sdhi-fungicide-market-forecasts-from. Accessed March 10 2021.
- Sadauskiene I, Liekis A, Staneviciene I, Naginiene R, Ivanov L. 2020. Effects of long-term supplementation with aluminum or selenium on the activities of antioxidant enzymes in mouse brain and liver. Catalysts 10:585. DOI: 10.3390/catal10050585.
- Silva V, Mol HG, Zomer P, Tienstra M, Ritsema CJ, Geissen V. 2019. Pesticide residues in European agricultural soils - A hidden reality unfolded. Science of the Total Environment 653:1532–1545. DOI: 10.1016/j.scitotenv.2018.10.441.
- Škulcová L, Neuwirthová N, Šimek Z, Trojan M, Bielská L. 2020. Enantioselective behavior of the fungicide tebuconazole in soil. Environmental Processes 7:173–188. DOI: 10.1007/s40710-019-00409-3.
- Stephenie S, Chang YP, Gnanasekaran A, Esa NM, Gnanaraj C. 2020. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. Journal of Functional Foods 68:103917. DOI: 10.1016/j.jff.2020.103917.
- Stoyanova S, Georgieva E, Velcheva I, Iliev I, Vasileva T, Bivolarski V, Tomov S, Nyeste K, Antal L, Yancheva V. 2020. Multi-biomarker assessment in common carp (Cyprinus carpio, Linnaeus 1758) liver after acute chlorpyrifos exposure. Water 12:1837. DOI: 10.3390/w12061837.
- Šudoma M, Peštálová N, Bilkova Z, Sedláček P, Hofman J. 2021. Ageing effect on conazole fungicide bioaccumulation in arable soils. Chemosphere 262:127612. DOI: 10.1016/j.chemosphere.2020.127612.
- Tabassum H, Dawood AQ, Sharma P, Khan J, Raisuddin S, Parvez S. 2016. Multi-organ toxicological impact of fungicide propiconazole onbiochemical and histological profile of freshwater fish Channa punctata Bloch. Ecological Indicators 63:359–365. DOI: 10.1016/j.ecolind.2015.11.052.
- Tauchnitz N, Kurzius F, Rupp H, Schmidt G, Hauser B, Schrödter M, Meer R. 2020. Assessment of pesticide inputs into surface waters by agricultural and urban sources - A case study in the Querne/Weida catchment, central Germany. Environmental Pollution 267:115186. DOI: 10.1016/j.envpol.2020.115186.
- Toni C, Ferreira D, Kreutz LC, Loro VL, Barcellos LJG. 2011a. Assessment of oxidative stress and metabolic changes in common carp (Cyprinus carpio) acutely exposed to different concentrations of the fungicide tebuconazole. Chemosphere 83:579–584. DOI: 10.1016/j.chemosphere.2010.12.022.
- Toni C, Loro VL, Santi A, De Menezes CC, Cattaneo R, Clasen BE, Zanella R. 2011b. Exposure to tebuconazol in rice field and laboratory conditions induces oxidative stress in carp (Cyprinus carpio). Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology 153:128–132. DOI: 10.1016/j.cbpc.2010.09.008.
- Vani G, Veeraiah K, Kumar MV, Parveen S, Rao DP. 2020. Biochemical changes induced by cartap hydrochloride (50% S.P.), carbamate insecticide in freshwater fish Cirrhinus mrigala (Hamilton, 1822). Nature Environment and Pollution Technology 19:1821–1828. DOI: 10.46488/NEPT.2020.v19i05.005.
- Wolf JC, Wheeler JR. 2018. A critical review of histopathological findings associated with endocrine and non-endocrine hepatic toxicity in fish models. Aquatic Toxicology 197:60–78. DOI: 10.1016/j.aquatox.2018.01.013.
- Yadavrao SW. 2017. Confidor and Bavistin induced effects on total glycogen content in liver and gonads of snakeheaded fish, Channa gachua. Journal of Pharmaceutical Innovation 6:41–43.
- Youness M, Sancelme M, Combourieu B, Besse-Hoggan P. 2018. Identification of new metabolic pathways in the enantioselective fungicide tebuconazole biodegradation by Bacillus sp. 3B6. Journal of Hazardous Materials 351:160–168. DOI: 10.1016/j.jhazmat.2018.02.048.
- Zubrod JP, Bundschuh M, Arts G, Brühl CA, Imfeld G, Knäbel A, Payraudeau S, Rasmussen JJ, Rohr J, Scharmüller A, Smalling K. 2019. Fungicides: An overlooked pesticide class? Environmental Science & Technology 53:3347–3365. DOI: 10.1021/acs.est.8b04392.