Abstract
Individuals of the solitary ascidian Styela clava Herdman, 1881 have been recorded for the first time in Italian waters, in Chioggia, within the Lagoon of Venice. This finding represents the third record of the species in the Mediterranean Sea. The Lagoon of Venice is a well-known hotspot of introduction of Non-Indigenous Species (NIS) along Italian coasts. It hosts several important vectors of introduction such as commercial shipping, recreational boating, aquaculture facilities and the live seafood trade. Monitoring surveys are a crucial step for the early identification of NIS, especially those characterized by a high invasive potential such as S. clava.
Introduction
Styela clava Herdman, Citation1881 is a solitary ascidian, originally from the Northwestern Pacific, along the coasts of Japan, Korea and China (Lützen Citation1998). It was first described in Kobe (Japan) during the Challenger expedition by Herdman (Citation1881), who first reported the “remarkable” shape and the abundance of this species in Japanese seas.
Styela clava is commonly known as “clubbed tunicate”, “stalked sea squirt”, “Asian tunicate”, “leathery sea squirt” or “rough sea squirt”. These common names refer to its characteristic leathery tunic, club-shaped body or its provenance.
The individuals can measure up to 16 cm including a long stalk for attaching to the substrate; this stalk is usually about one-third of the whole length. They are characterized by a tough, wrinkled tunic; larger specimen may have a light brown body and a darker brown stalk, while smaller ones are yellow-brown, and they may grow in very dense clusters (Lützen Citation1998).
The clubbed-tunicate was first recorded in Europe, along the coasts of Plymouth by Carlisle (Citation1954), who considered it a new species, Styela mammiculata Carlisle, Citation1954. Later, Millar (Citation1960) demonstrated that S. mammiculata and S. clava were the same species.
The species was accidently introduced into England, probably attached to vessel hulls from Korea or to oysters imported from Japan during the early 1950s (Lützen Citation1998; Izquierdo-Muñoz et al. Citation2009).
Styela clava is one of the most impacting invasive alien species in the world, and it is considered a real pest (Clarke & Therriault Citation2007). Indeed, individuals of S. clava can colonize any kind of abiotic or biotic substrata, establishing populations in temperate waters worldwide. Styela clava has been reported as a major pest in oyster and mussel farms in Japan, Denmark and Canada, competing over the cultured organisms and forming dense aggregations on fishing gears, moorings and ropes (Clarke & Therriault Citation2007).
In the Mediterranean Sea, S. clava was reported for the first time within the Bassin de Thau (Sète, Marseille) in the early 2000s, where the population appeared to be expanding, on the nearby Mediterranean French coasts (Davis & Davis Citation2008), in Port Said, along Egyptian coasts in 1991 (Ghobashy & Abdel Messeih Citation1991) (even if not included in the list compiled for the region by Halim & Messeih Citation2016) and in the Sea of Marmara (Çinar Citation2016).
This study reports a new record of the highly invasive species S. clava within the Mediterranean Sea, adding a further expansion to the list of NIS (Non-Indigenous Species) ascidian species within this basin (Mastrototaro et al. Citation2019; Ragkousis et al. Citation2020; Montesanto et al. Citation2021a, Citation2021b; Orfanidis et al. Citation2021) as well as a further “alien” to the list of the several NIS recorded within the Lagoon of Venice, which represents the first Italian hotspot of species introduction (Occhipinti-Ambrogi et al. Citation2011). In addition, the Lagoon of Venice is not only a well-known area of high risk of introduction of NIS but also several NIS firstly recorded in this area have later further spread elsewhere (Marchini et al. Citation2015; Servello et al. Citation2019). We provide a detailed morphological description of the collected specimens of S. clava together with a review of its ecological characteristics as well as potential pathways of introduction and future spread within the Mediterranean basin.
Material and methods
Description of the study area
The Lagoon of Venice is the largest coastal lagoon in the Mediterranean region. It is located in the Northern Adriatic Sea (Italy) (), and it covers an area of about 550 km2, with an average depth of 1.5 m (Brambati et al. Citation2003; Semprucci et al. Citation2019). In detail, the total area of approximately 550 km2 includes approximately 390 km2 of open lagoon. This area also covers 40 km2 of channels, 70 km2 of salt marshes, and 90 km2 dedicated to aquaculture activities (Madricardo et al. Citation2019).
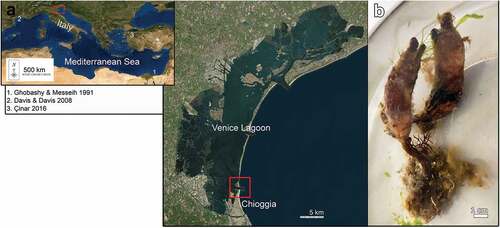
Figure 1. (a) Study area, boxes encircling the sampling site, previous occurrences of Styela clava in the Mediterranean Sea are numbered in temporal order; (b) alive specimens of S. clava collected in Lagoon of Venice.
The lagoon is characterized by a variety of shallow habitats, such as salt marshes, seagrass beds, intertidal and subtidal mudflats. These areas are crossed by a network of channels with a maximum depth of about 15 m. The channels link three inlets that connect the lagoon to the Adriatic Sea: Lido, Malamocco and Chioggia (Rova et al. Citation2019; Semprucci et al. Citation2019).
The tidal excursion is usually less than 1 m, with a salinity of 34.4–34.9‰ at high tide and 32.8–33.6‰ at low tide (Semprucci et al. Citation2019). The annual average temperature in 2008 was about 24.5°C, with the highest temperature occurring during the summer (up to 29°C) and the minimum during the winter (10°C), sometimes also reaching lower temperature in areas characterized by a minimum tidal flow (Coccioni et al. Citation2009; Bertolini et al. Citation2021).
The lagoon is affected by several human activities, such as municipal wastewater discharges, agricultural drainage, dredging, fishing activities, aquaculture activities and maritime traffics (Madricardo et al. Citation2019). Moreover, several recreational marinas and two main harbours have been built in Venice and Chioggia. In detail, the Marghera harbour is mainly devoted to commercial and international traffic, while the Chioggia one is mainly devoted to regional commercial traffic (Marchini et al. Citation2015). Furthermore, the whole lagoon hosts one of the most important areas in Europe for clam production (Marchini et al. Citation2015).
The benthic communities of the lagoon are mainly dominated by seagrass meadows represented by Cymodocea nodosa (Ucria) Asch., Zostera noltei Hornemann, 1832 and Zostera marina Linnaeus, 1753 (Solidoro et al. Citation2010) and by algal associations comprising 300 species of macroalgae (Sfriso & Curiel Citation2007). From the 1990s, some introduced species of macroalgae, such as Sargassum muticum (Yendo) Fensholt and Undaria pinnatifida (Harvey) Suringar, have replaced several autochthonous species (Curiel & Marzocchi Citation2010). Macrofauna is mostly dominated by filter-feeder species of bivalves and polychaetes, with a high biomass of invasive species such as Magallana gigas (Thunberg, 1793) and opportunistic/detritivore species of polychaetes of the genus Owenia Delle Chiaje, 1844 that are commonly found in the estuarine areas (Solidoro et al. Citation2010). As already stated, the Lagoon of Venice is the most important hotspot of NIS introduction in Italy; indeed, 50 alien species were first spotted in this area (Occhipinti-Ambrogi et al. Citation2011; Servello et al. Citation2019). Among them, the ascidians, Botrylloides violaceus Oka, 1927 (Zaniolo et al. Citation1998) and the highly invasive species Didemnum vexillum Kott, 2002 (Tagliapietra et al. Citation2012) were recorded in this area for the first time within the Mediterranean Sea (Servello et al. Citation2019).
Sampling
Several specimens of S. clava were observed, photographed and then six of them were collected during April 2021 at about 0.5 m depth on ropes and floating docks in the south-west of Chioggia, Venice lagoon (Adriatic Sea, Italy) (45°12ʹ47.9” N 12°16ʹ24.3” E) (). In the same area, other ascidian species were detected such as colonial species belonging to the genus Botryllus and Botrylloides. These observations were not the result of any systematic survey of the lagoon environment but a casual observation during sampling of other target species.
The salinity recorded was about 30 psu, while the temperature was about 14–15°C.
All collected specimens were relaxed with menthol crystals in sea-water for about 3 h until no reaction was detected and then preserved in 4% formalin solution in seawater. Individuals were observed, dissected and then treated with Mayer’s haemalum solution in order to identify the inner morphological characteristics. In detail, the dissected specimens were rinsed in distilled water overnight and then stained with Mayer’s haemalum solution for about 60 s. After being stained, the specimens were again rinsed with distilled water.
Results
Systematics
Phylum Chordata
Subphylum Tunicata
Class Ascidiacea
Order Stolidobranchia
Family Styelidae
Genus Styela
Styela clava Herdman, Citation1881
New record
2021, Chioggia, Lagoon of Venice, Adriatic Sea, North Mediterranean Sea, 0.5 m depth. Individuals preserved in 4% formalin (Code: MUZAC–6667) have been deposited in the collection of the Zoological Museum of the University of Bari.
Previous records in the Mediterranean Sea
2005, Bassin de Thau, 5 m depth (Davis & Davis Citation2008)
1991, Egyptian coasts (Port Said), depth not reported (Ghobashy & Abdel Messeih Citation1991)
2016, Sea of Marmara, 10 m depth (Çinar Citation2016)
Description
Individuals of S. clava collected from the Venice lagoon (Chioggia, Italy) range from 9 to 13 cm in size, with a very long stalk, almost half the whole-body length ()). The external appearance of the tunic is dark brown with the typical leathery consistence, wrinkled and mamillated ()). The siphons are close to each other ()) and externally marked by stripes ()).
Figure 2. Styela clava. (a) Individual of S. clava with the characteristic leathery and mammillated brown tunic and a long dark brown stalk (asterisk) and the siphons lying close to each other (arrow); (b–c) specimens without tunic showing the gonads; (b) the left side, testicular follicles constituted by small white lobes (ts) separated from the long pinkish female gland (oo), siphons marked with brown stripes (arrow); (c) right side; (d) dissected specimens without branchial sac showing gonads on both sides; (e) magnification of the gonads showing the endocarps (e), testis (ts) and ovaries (oo). Scale bars: 1 cm.
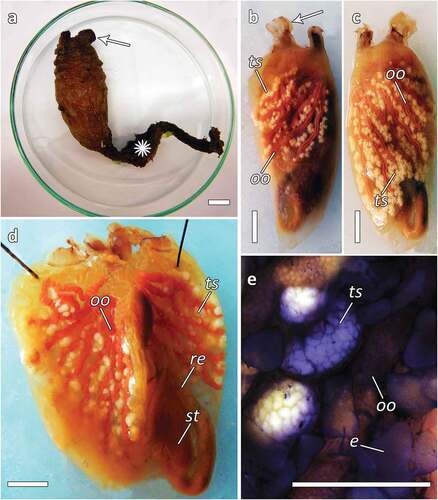
About 5–6 gonads lie on the left side, while about 6–8 gonads are placed on the right (). The testicular follicles consist of small white lobes separated from the long pinkish female gland (). The stomach is elongated and has about 40 stomach folds ()). Endocarps are visible on the body wall between the gonads ())
Figure 3. (a) Dissected specimen of S. clava showing the branchial sac with four folds per side, the oral siphon (os) and the atrial one (as), the stomach (st) and the anus opening (anus), with magnification of the anus (anus); (b) oral siphon with long thick oral tentacles (te) and smooth prebranchial area (asterisk); (c) atrial siphon without tentacles (arrow); (d) dorsal tubercle spiral-shaped; (e) branchial sac showing four folds from the dorsal lamina (dl), and 12 to 44 longitudinal vessels on the 4 branchial folds and from 4 to 20 in the interspace; (f) stomach with about 20 folds per side (arrow); (g) branchial sac with transverse vessels of two different size (asterisk points out a thicker one) (h); branchial mesh with about 5–6 stigmata, the asterisk points out the longitudinal vessel; (i) lobed anus.
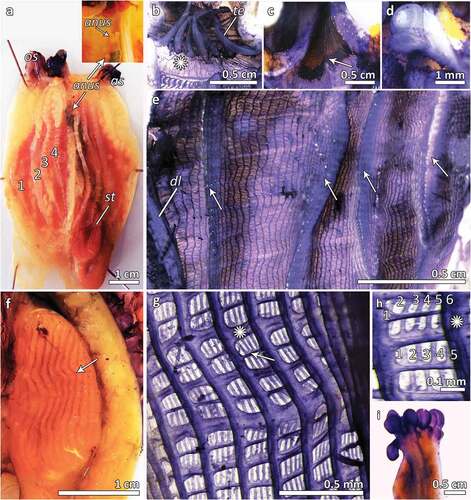
The prebranchial area is smooth ()). The oral siphon is characterized by the presence of about 30 long thick tentacles ()), while the atrial siphon does not have any tentacles ()). The dorsal tubercle has a spiral shape ()).
The branchial sac is characterized by 12 to 44 longitudinal vessels on the 4 branchial folds and from 4 to 20 in the interspaces ()), numerous transverse vessels of two orders of size (the thicker ones are about 0.1 mm, while the thinner ones are about 0.05 mm) and no parastigmatic vessels ()). Four to six stigmata per branchial mesh are counted ().
A lobed anus opens close to apertures of the siphons ()).
Remarks
Considering the macroscopic features of the species and based on the comparison with previous observations (Herdman Citation1881; Carlisle Citation1954; Millar Citation1960; Ghobashy & Abdel Messeih Citation1991; Davis & Davis Citation2008; Çinar Citation2016), it was possible to identify all the specimens collected in the Venice Lagoon as S. clava. Although this species can be “easily” recognized by its external peculiar shape and the characteristic “leathery” tunic, morphological analysis of anesthetized samples confirmed the specific identification.
Slight differences in the number of gonads of the specimens were found, with respect to previous descriptions made by other authors, who report a lower number of gonads on the right side, generally around three (Carlisle Citation1954; Millar Citation1960; Davis & Davis Citation2008) and sometimes a higher number of oral tentacles (see Millar Citation1960). Furthermore, the specimens collected from the Bassin de Thau were characterized by a slightly different orange-brown coloration (Davis & Davis Citation2008).
Despite this small morphological difference, diagnostic characteristics such as the absence of parastigmatic vessels and atrial tentacles, the number of stomach folds, the presence of endocarps and of a lobed anus allowed the identification of the specimens from the Lagoon of Venice as S. clava.
Ecology and distribution
The native range of distribution of the species S. clava is the Northwestern Pacific Ocean (Herdman Citation1881; Lützen Citation1998), but at the present time, S. clava is distributed in North America (Californian coasts), Canada (Atlantic and Pacific coasts), southern British Columbia (Pacific coast and along the Atlantic coasts of Prince Edward Island) and along Northeastern European coasts as well (from Denmark to Portugal, including Ireland) (Clarke & Therriault Citation2007).
Styela clava is a littoral species that can tolerate tidal emersion and it inhabits artificial and natural substrata down to a depth of 40 m (Lützen Citation1998; Clarke & Therriault Citation2007).
It is considered a major pest for shellfish aquaculture, smothering target species (such as bivalves) and fouling gear and equipment. Styela clava tolerates a wide range of temperature, from 2°C to 23°C (Clarke & Therriault Citation2007). It has been demonstrated that S. clava is intolerant of low salinity; the limit of a successful establishment depends on the temperature ranging from 15°C to 23°C for spawning and values of salinity between 22 and 34.5 psu (Davis & Davis Citation2008), although this species can also survive with salinity close to the known tolerance limit of the species, such as in the Sea of Marmara, where the salinity recorded was around 23 psu (Çinar Citation2016).
Styela clava, like many solitary ascidian species, is characterized by a short larval planktonic phase of about 24–48 hours at 20°C (Holmes Citation1969). This leads to a limited larval dispersal; therefore, a different dispersal vector of introduction of the species, such as the transport of adults on ship hulls or on the shells of the bivalves used in aquaculture, plays a major role in its dispersal and expansion.
Discussion
The occurrence of S. clava in the Lagoon of Venice represents the third record of this NIS in the Mediterranean Sea and the first along Italian coasts.
Styela clava is known to have a high invasion potential and its successful establishment mainly depends on the suitability of the new habitat, especially in terms of salinity and water temperature. The average temperature in the Lagoon of Venice reaches 29°C in the summer, and the salinity values range from 25 to 32 ‰ (Bertolini et al. Citation2021); these conditions are favourable for its establishment. The Mediterranean Sea mean temperature in littoral waters ranges from 11°C to 28°C with salinity between 36.2 and 39‰ (Gačić et al. Citation2013; Pastor et al. Citation2020). These conditions may represent a high risk of invasion by S. clava in further areas within the Mediterranean basin.
To date, S. clava occurrence is restricted to confined areas in the Mediterranean basin and the Black Sea (Sea of Marmara), such as the Bassin de Thau and the Lagoon of Venice, but the presence of several vectors of introduction/spread in the Lagoon of Venice may play an important role in a possible spread of the species. In fact, this area is characterized by the presence of marinas influenced by recreational boating, commercial maritime traffic and aquaculture farms. Indeed, since the larval dispersal is limited for this species, the transport of adult individuals attached to vessel hulls or on aquaculture gear are the most likely pathways of introduction and future spread of this species inside the lagoon and in further areas of the Mediterranean Sea.
The tidal excursion in the Lagoon of Venice is about 1 m (Bertolini et al. Citation2021), but the tolerance of large individuals of S. clava to air exposure has been estimated to be about 2 weeks (Hillock & Costello Citation2013), so they can easily resist being exposed to the air at low tide. Therefore, air exposure for a possible control of S. clava can be applied during ordinary cleaning of dry-docking of boats, moorings and aquaculture gears, but 2 weeks of air exposure is needed to ensure the mortality of all individuals (Hillock & Costello Citation2013).
However, since the eradication of a NIS is almost impossible, early identification and efficient monitoring surveys performed by specialised staff are needed to allow the setting up of appropriate management strategies able to control and prevent the further spread of this highly invasive species in the area. Only in this way, it will be possible to predict future spreading trends and vectors not only within the Lagoon of Venice but also throughout the Mediterranean basin.
Geolocation information
The study area is located within the Lagoon of Venice (Italy): 45°12ʹ47.9” N; 12°16ʹ24.3” E.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bertolini C, Royer E, Pastres R. 2021. Multiple evidence for climate patterns influencing ecosystem productivity across spatial gradients in the Venice Lagoon. Journal of Marine Science and Engineering 9(4):363. DOI:10.3390/jmse9040363.
- Brambati A, Carbognin L, Quaia T, Teatini P, Tosi L. 2003. The Lagoon of Venice: Geological setting, evolution and land subsidence. Episodes 26(3):264–268. DOI:10.18814/epiiugs/2003/v26i3/020.
- Carlisle DB. 1954. Styela mammiculata n.sp., a new species of ascidian from the Plymouth area. Journal of the Marine Biological Association of the United Kingdom 33(2):329–334. DOI:10.1017/S0025315400008365.
- Çinar ME. 2016. The alien ascidian Styela clava now invading the Sea of Marmara (Tunicata: Ascidiacea). ZooKeys 563:1. DOI:10.3897/zookeys.563.6836.
- Clarke CL, Therriault TW. 2007. Biological synopsis of the invasive tunicate Styela clava (Herdman 1881). Canadian Manuscript Report Fisheries and Aquatic Science 2807:1–23.
- Coccioni R, Frontalini F, Marsili A, Mana D. 2009. Benthic foraminifera and trace element distribution: A case-study from the heavily polluted lagoon of Venice (Italy). Marine Pollution Bulletin 59(8–12):257–267. DOI:10.1016/j.marpolbul.2009.08.009.
- Curiel D, Marzocchi M. 2010. Stato delle conoscenze nella laguna di Venezia di due specie aliene: Undaria pinnatifida e Sargassum muticum. Lavori Società Veneziana Scienze Naturali 35:93–106.
- Davis MH, Davis ME. 2008. First record of Styela clava (Tunicata, Ascidiacea) in the Mediterranean region. Aquatic Invasions 3(2):125–132. DOI:10.3391/ai.2008.3.2.2.
- Gačić M, Schroeder K, Civitarese G, Cosoli S, Vetrano A, Eusebi Borzelli GL. 2013. Salinity in the Sicily Channel corroborates the role of the Adriatic–Ionian bimodal oscillating system (BiOS) in shaping the decadal variability of the Mediterranean overturning circulation. Ocean Science 9(1):83–90. DOI:10.5194/os-9-83-2013.
- Ghobashy AFA, Abdel Messeih MK. 1991. Ascidians in Egyptian waters. Journal of the Egyptian-German Society of Zoology 4:313–326.
- Halim Y, Messeih MA. 2016. Aliens in Egyptian waters. A checklist of ascidians of the Suez Canal and the adjacent Mediterranean waters. The Egyptian Journal of Aquatic Research 42(4):449–457. DOI:10.1016/j.ejar.2016.08.004.
- Herdman WA. 1881. Preliminary report on the Tunicata of the challenger expedition. Cynthiidae. Proceeding of the Royal Society of Edinburgh 11(3):52–88. DOI:10.1017/S0370164600046782.
- Hillock KA, Costello MJ. 2013. Tolerance of the invasive tunicate Styela clava to air exposure. Biofouling 29(10):1181–1187. DOI:10.1080/08927014.2013.832221.
- Holmes NJ. 1969. Aspects of the biology of Styela clava Herdman. (Doctoral dissertation, Ph. D Thesis). United Kingdom: Southampton University. p. 284.
- Izquierdo-Muñoz A, Diaz Valdes M, Ramos-Esplà AA. 2009. Recent non-indigenous ascidians in the Mediterranean Sea. Aquatic Invasions 4:59–64. DOI:10.3391/ai.2009.4.1.5.
- Lützen J. 1998. Styela clava Herdman (Urochordata, Ascidiacea), a successful immigrant to North West Europe: Ecology, propagation and chronology of spread. Helgoländer Meeresuntersuchungen 52(3):383–391. DOI:10.1007/BF02908912.
- Madricardo F, Foglini F, Campiani E, Grande V, Catenacci E, Petrizzo A, Kruss A, Toso C, Trincardi F. 2019. Assessing the human footprint on the sea-floor of coastal systems: The case of the Venice Lagoon, Italy. Scientific Reports 9(1):1–13. DOI:10.1038/s41598-019-43027-7.
- Marchini A, Ferrario J, Sfriso A, Occhipinti-Ambrogi A. 2015. Current status and trends of biological invasions in the Lagoon of Venice, a hotspot of marine NIS introductions in the Mediterranean Sea. Biological Invasions 17(10):2943–2962. DOI:10.1007/s10530-015-0922-3.
- Mastrototaro F, Montesanto F, Salonna M, Grieco F, Trainito E, Gissi C. 2019. Hitch-hikers of the sea: Concurrent morphological and molecular identification of Symplegma brakenhielmi (Tunicata: Ascidiacea) in the western Mediterranean Sea. Mediterranean Marine Science 20:197–207.
- Millar RH. 1960. The identity of the ascidians Styela mammiculata Carlisle and S. clava Herdman. Journal of the Marine Biological Association of the United Kingdom 39(3):509–511. DOI:10.1017/S0025315400013503.
- Montesanto F, Chimienti G, Gissi C, Mastrototaro F. 2021a. Spread of the non-indigenous ascidian Aplidium accarense (Millar, 1953) in the Eastern Mediterranean Sea: Morphological and molecular tools for an accurate identification. Mediterranean Marine Science 22(2):246–254.
- Montesanto F, Chimienti G, Gissi C, Mastrototaro F. 2021b. Polyclinum constellatum (Tunicata, Ascidiacea), an emerging non-indigenous species of the Mediterranean Sea: Integrated taxonomy and the importance of reliable DNA barcode data. Mediterranean Marine Science 23(1):69–83.
- Occhipinti-Ambrogi A, Marchini A, Cantone G, Castelli A, Chimenz C, Cormaci M, Froglia C, Furnari G, Gambi MC, Giaccone G, Giangrande A, Gravili C, Mastrototaro F, Mazziotti C, Orsi-Relini L, Piraino S. 2011. Alien species along the Italian coasts: An overview. Biological Invasions 13(1):215–237. DOI:10.1007/s10530-010-9803-y.
- Orfanidis S, Alvito A, Azzurro E, Badreddine A, Souissi JB, Chamorro C, Crocetta F, Dalyan C, Fortič A, Galanti L, Geyran K, Ghanem R, Goruppi A, Grech D, Katsanevakis S, Madrenas E, Mastrototaro F, Montesanto F, Pavičić M, Pica D, Pola L, Pontes M, Michail Ragkousis M, Rosso A, Sánchez-Tocin L, Tierno De Figueroa JM, Tiralongo F, Tirelli V, Tsioli S, Tunçer S, Vrdoljak D, Vuletin V, Zaouali J, Zenetos A. 2021. New Alien Mediterranean biodiversity records” (March 2021). Mediterranean Marine Science 22(1):180–198.
- Pastor F, Valiente JA, Khodayar SA. 2020. Warming Mediterranean: 38 Years of increasing sea surface temperature. Remote Sensing 12:2687. DOI:10.3390/rs12172687.
- Ragkousis M, Abdelali N, Azzurro E, Badreddine A, Bariche M, Bitar G, Crocetta F, Denitto F, Digenis M, El Zrelli R, Ergenler A, Fortič A, Gerovasileiou V, Grimes S, Katsanevakis S, Koçak C, Licchelli C, Loudaros E, Mastrototaro F, Mavrič B, Mavruk S, Miliou A, Montesanto F, Ovalis P, Pontes M, Rabaoui L, Sevingel N, Spinelli A, Francesco Tiralongo F, Tsatiris A, Turan C, Vitale D, Yalgin F, Yapici S, Zenetos A. 2020. New alien Mediterranean biodiversity records (October 2020). Mediterranean Marine Science 21(3):631–652.
- Rova S, Müller F, Meire P, Pranovi F. 2019. Sustainability perspectives and spatial patterns of multiple ecosystem services in the Venice lagoon: Possible roles in the implementation of the EU water framework directive. Ecological Indicators 98:556–567. DOI:10.1016/j.ecolind.2018.11.045.
- Semprucci F, Facca C, Ferrigno F, Balsamo M, Sfriso A, Sandulli R. 2019. Biotic and abiotic factors affecting seasonal and spatial distribution of meiofauna and macrophytobenthos in transitional coastal waters. Estuarine, Coastal and Shelf Science 219:328–340. DOI:10.1016/j.ecss.2019.02.008.
- Servello G, Andaloro F, Azzurro E, Castriota L, Catra M, Chiarore A, Crocetta F, D’alessandro M, Denitto F, Froglia C, Gravili C, Langer MR, Lo Brutto S, Mastrototaro F, Petrocelli A, Pipitone C, Piraino S, Relini G, Serio D, Xentidis NJ, Zenetos A. 2019. Marine alien species in Italy: A contribution to the implementation of descriptor D2 of the marine strategy framework directive. Mediterranean Marine Science 20(1):1–48.
- Sfriso A, Curiel D. 2007. Check-list of marine seaweeds recorded in the last 20 years in Venice lagoon and a comparison with the previous records. Botanica Marina 50:22–58. DOI:10.1515/BOT.2007.004.
- Solidoro C, Bandelj V, Bernardi FA, Camatti E, Ciavatta S, Cossarini G, Facca C, Franzoi P, Libralato S, Melaku Canu D, Pastres R, Fabio Pranovi F, Raicevich S, Socal G, Sfriso A, Sigovini M, Tagliapietra D, Torricelli P. 2010. Response of Venice Lagoon ecosystem to natural and anthropogenic pressures over the last 50 years. In: Kennish MJ, Pearl HW, editors. Coastal lagoons: Critical habitats of environmental change. Vol. 19. Boca Raton, FL: CRC Press. pp. 483–511.
- Tagliapietra D, Keppel E, Sigovini M, Lambert G. 2012. First record of the colonial ascidian Didemnum vexillum Kott, 2002 in the Mediterranean: Lagoon of Venice (Italy). BioInvasions Record 1(4):247–254. DOI:10.3391/bir.2012.1.4.02.
- Zaniolo G, Manni L, Brunetti R, Burighel P. 1998. Brood pouch differentiation in Botrylloides violaceus, a viviparous ascidian (Tunicata). Invertebrate Reproduction & Development 33(1):11–23. DOI:10.1080/07924259.1998.9652338.
