Abstract
In the European Union, aquarium pets are organisms intended for closed places (e.g., pet shops, garden ponds, home aquariums). Until April 2021, regulations did not require veterinary inspection of these animals within the EU, although there is a potential risk of such organisms being released into the environment along with their symbiotes or parasites. Currently, a “disease-free” declaration is required, but no aquarium snail pathogen that needs attention in international trade has been included in the list of potential hazards. Here, we intended to check whether molluscs from the pet trade could be a source of parasites. We answered this question by using Anentome helena, a popular commodity in the ornamental pet industry, as a research model. Snail specimens were randomly collected from aquarium pet stocks imported from Bangkok (Thailand) to Warsaw (Poland) in March 2020. In total, three specimens were subjected to histological examination and 27 specimens were autopsied. Histological analysis revealed that one snail was infected with rediae (and the cercariae inside them). Our study is the first to show the presence of digenean larvae in A. helena originating from the ornamental pet industry. The spread of such “hitchhikers” in non-native areas will likely be associated with threats to environmental and public health. Therefore, it is necessary to constantly draw public attention to the possible consequences of releasing ornamental pets into the non-native environment.
Introduction
Ornamental pets are potential vectors for numerous “hitchhikers”, often pathogen and parasite causing diseases, which can be overlooked and therefore spread worldwide via the pet trade (Tamukai et al. Citation2014; Patoka et al. Citation2016a; Maciaszek et al. Citation2020). In the European Union (EU), ornamental aquatic animals are intended for closed ornamental facilities (pet shops, garden centres, garden ponds, commercial aquaria or wholesalers keeping ornamental aquatic animals). At least until April 2021, they had not required veterinary inspection within the EU, as they had no direct contact with natural waters, and they did not pose significant risks to aquaculture, human health, or wild stocks. The inspectors were not competent to monitor the origin or quantity of the pets, which were often collected from local aquarium hobbyists or were bred in the stores (Commission Regulation (EU) Citation2010; Commission Implementing Regulation (EU) Citation2020). Unfortunately, this led to the uncontrolled spread of aquarium pets (including ornamental snails) within the trade as well as a frivolous attitude and their release into the environment (Domagala et al. Citation2004; Biondo Citation2017).
According to the legal regulations provided by the Commission Implementing Regulation (EU) (Citation2020), aquatic ornamental pets in the EU are treated like other aquatic animals that are not dedicated to human consumption. Before the importation, declarations are issued for invertebrates that they are free from diseases mentioned in the animal health certificate model “aqua-entry-estab/release/other” (Model animal health certificate for the entry into the Union of aquatic animals intended for certain aquaculture establishments, for release into the wild or for other purposes, excluding human consumption). Despite the update of the regulations, still none of the freshwater mollusc diseases or pathogens is listed there. Therefore, there is a risk of various pathogens and parasites in molluscs obtained from the pet trade. The release of these host organisms into non-native environments is a potential threat not only to natural ecosystems but also to human health (Federspiel et al. Citation2020; Modrý et al. Citation2021; Rollins et al. Citation2021).
Snail-borne diseases may be dangerous helminthiasis due to their significant impact on human and animal health (Lu et al. Citation2018). Molluscs act a crucial role in the life cycles of digenean trematodes (Faltỳnková et al. Citation2008). The most attractive ornamental pets most often originate from tropical countries, including areas endemic to numerous trematosis of medical and veterinary importance (Inobaya et al. Citation2014; Toledo & Esteban Citation2016; Pratumchart et al. Citation2019; Nguyen et al. Citation2021).
In this report, we focused on Anentome helena (von dem Busch in Philippi, 1847) (Gastropoda: Nassariidae), known as the assassin snail, killer snail, or bumblebee snail (Stegeman et al. Citation2020), which commonly occurs in the lower reaches of coastal rivers, lakes, and ponds throughout Southeast Asia (CABI Citation2021). Currently, the snail is a popular commodity in the ornamental pet industry (Yanai et al. Citation2017; Hossain & Mohsin Citation2020), especially due to its curious looking shell – fusiform, prominently ribbed and spirally banded (Strong et al. Citation2017). The interest in this mollusc is intense among aquarium enthusiasts because of its voracious appetite for other snails of different species, including Helisoma sp., Melanoides sp., Physa sp., Planorbella sp., which easily breed and often become major pests in aquaria (Bogan & Hanneman Citation2013). However, it should be emphasized that A. helena is a non-selective predator and scavenger that preys on both molluscs and fish eggs as well as on crustaceans (Bogan & Hanneman Citation2013). Therefore, there is growing concern about the potential threat to native aquatic animal populations if A. helena is introduced into the natural environment (Bogan & Hanneman Citation2013; Ng et al. Citation2016a), especially that there are frequent reports of the illegal releases of aquatic pets into natural waters, where they can become invasive (Chucholl Citation2013; Ng et al. Citation2016b; Patoka et al. Citation2018; Banha et al. Citation2019). Moreover, it has not been controlled so far whether this very popular snail among aquarium enthusiasts can be a source of non-native parasites for Europe. Here, using A. helena as a research model, we report that molluscs obtained from a shipment of aquarium fauna imported from Southeast Asia to Europe could be the source of trematodes.
Material and methods
A total of 30 adults of A. helena were randomly collected from aquarium pet stocks imported from Bangkok (Thailand) to Warsaw (Poland) in March 2020. In the laboratory, snails were immediately placed individually in transparent glass beakers with a small amount (approx. 50 ml) of dechlorinated tap water and exposed to artificial light (desk lamp) for 24 h to stimulate the release of cercariae (Blankespoor & Reimink Citation1991). Then, the water was checked for cercariae presence with a stereoscopic microscope (Science ETD-101, Bresser, Rhede, Germany). Next, we looked for the presence of trematodes in the snail tissues.
The whole soft body of three randomly selected snails was fixed in Bouin’s solution for histological examination. Following the fixation, the samples were dehydrated in a graded ethanol series (Chempur, Piekary Śląskie, Poland), cleared with xylene (POCH, Gliwice, Poland), and embedded in paraffin wax (Chempur). Serial cross-sections (4–6 μm thick) were cut with a rotary microtome (Hyrax M55, Zeiss, Oberkochen, Germany). Histological slides were deparaffinized, rehydrated, and stained with Ehrlich hematoxylin (Carl Roth, Karlsruhe, Germany) for five minutes and with a 1% ethanol solution of eosin Y (Analab, Warszawa, Poland) for five minutes. Then, slides were dehydrated in 96% ethanol and twice in isopropyl alcohol (Leica, Wetzlar, Germany), cleared in Clearene (Leica), and embedded in CV Ultra (Leica). Snail tissues on the glass slides were analysed under the light microscope (Eclipse 80i, Nicon, Tokyo, Japan) in a bright field using 4, 10, 20, 100× objective magnification and photographed using a digital camera (Axio Cam MRc5, Zeiss) and image acquisition software (ZEN, Zeiss).
The remaining 27 soft bodies of snails were cut up into smaller sections, which were gently crushed between a glass slide and a coverslip (Reddy et al. Citation2004). To search for sporocysts, rediae, cercariae, and metacercariae of digenean trematodes, freshly squeezed tissues were examined under a light microscope (Primostar, Zeiss) at various objective magnifications (5, 10, 40, and 100×).
Results
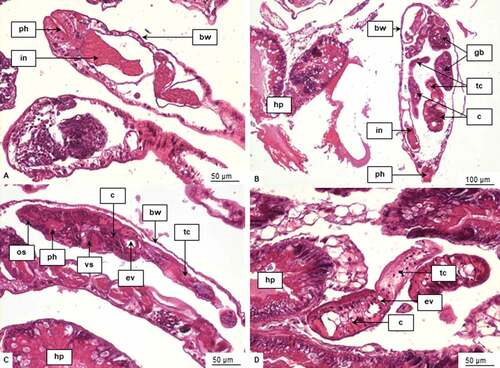
The non-invasive diagnostic method used in live snails showed no digenean cercariae in the water. In histological assessment, one of the three examined snails showed the prepatent infection of digenean larvae. More precisely, numerous rediae and cercariae inside them were observed in the hepatopancreas of the infected individual of A. helena (). Autopsy of the remaining 27 specimens revealed no developmental stages of digenean trematodes. Our research showed that digenean infection was recorded in 3.33% of all snails investigated.
Figure 1. Digenean larvae in the hepatopancreas of Anentome helena. A. Redia. B. Redia filled with cercariae and germ balls. C. Cercaria in a body of redia. D. Free cercaria between hepatopancreatic acini. Abbreviations: bw, body wall of redia; c, cercaria; ev, excretory vesicle; gb, germ balls; hp, hepatopancreas; in, intestine; os, oral sucker; ph, pharynx; tc, tail of cercaria; vs, ventral sucker.
Discussion
The reports on the presence of digenean larvae in Southeast Asia snails, including Anentome helena are scarce and insufficient (Krailas et al. Citation2012; Anucherngchai et al. Citation2016; Haruay & Piratae Citation2019; Dunghungzin & Chontananarth Citation2020, Citation2021; Wiroonpan et al. Citation2020; Yooyen Citation2020). Among the cases recorded in the native area, A. helena acts the role of the first and second intermediate host for digenean trematodes (Chantima et al. Citation2013, Citation2018; Chomchoei et al. Citation2018; Haruay & Piratae Citation2019; Butboonchoo et al. Citation2020). However, only single reports indicate that redia-born digenean species use A. helena as the first intermediate host (Wiroonpan et al. Citation2020). Here, we are not able to carry out species identification for the detected Digenea, we can only claim that it belongs to the order Echinostomida, due to the presence of rediae. Nevertheless, our research proves the possibility that imported ornamental pet snails, such as A. helena, may be a source of digenean trematodes. There is some risk to the health of the aquarium enthusiasts or the animals co-inhabiting aquariums with the introduced infected snail. Additionally, the irresponsible release of infected ornamental pets into the non-native environment may have far-reaching consequences. The basis for the pessimistic scenario is primarily the adaptability of aquatic snails introduced to non-native areas. Admittedly, A. helena is a thermophilic species with an optimal temperature range of 22 to 28°C (CABI Citation2021), and at higher latitudes it can survive in heat islands such as greenhouses and botanical garden ponds. From these anthropogenic habitats, alien snail species began the expansion to wild areas (Alexandrowicz Citation1993; Strzelec et al. Citation2006). Also, thermally polluted water bodies connected to lakes or rivers with a natural thermal regime promote the spread of non-native species (Piechocki et al. Citation2003; Domagala et al. Citation2004, Citation2007; Labecka et al. Citation2005; Maciaszek et al. Citation2020, Citation2021b). Furthermore, the global warming scenario should also be considered (Rahel & Olden Citation2008; Fenoglio et al. Citation2010).
The pet trade is a key driver of non-native aquatic species introductions (Patoka et al. Citation2020). There are numerous reports of the presence of ornamental aquatic animals in natural environments outside their native range (Weiperth et al. Citation2019; Mabrouki et al. Citation2020; Tarkan et al. Citation2021; Maciaszek et al. Citation2021a), most likely due to an intentionally release and/or as an escape from captivity (Hulme et al. Citation2008). Additionally, numerous non-native species, including molluscs, are not deliberately transported but arrive as commodity contamination (Patoka et al. Citation2016b, Citation2017; Patoka & Patoková Citation2021). It should also be emphasized that illegal or unsustainable wildlife trade is developing at a global level (Cardoso et al. Citation2021). Many of the most dangerous invaders come from the pet trade, and their invasive status is due to the disease they spread (Hatcher et al. Citation2012). New reports of pathogens or parasites in imported ornamental animals are constantly emerging, including those of veterinary and medical importance (Mehrdana et al. Citation2014; Haenen et al. Citation2020; Pace et al. Citation2020). In Europe, reports on “hitchhikers” from the aquatic pet trade have focused mainly on their presence in fish (Mehrdana et al. Citation2014; Šmiga et al. Citation2016; Haenen et al. Citation2020; Pace et al. Citation2020) and decapod crustaceans (Mrugała et al. Citation2015; Patoka et al. Citation2016a; Maciaszek et al. Citation2020, Citation2021b; Ložek et al. Citation2021). The epidemiological threat of introducing an ornamental mollusc into a non-native environment is likely to be downplayed because the main “hitchhikers” are likely to be digeneans and most often require the presence of other non-native species to complete their complex life cycle and become established. Nevertheless, Mehrdana et al. (Citation2014) emphasize that the possibility of the establishment of digeneans transported by ornamental pets to Europe should be considered a real risk. These scientists base their opinion on the detection of metacercariae of Centrocestus sp. in ornamental fish while emphasizing that the first intermediate host of this zoonotic trematode is M. tuberculata, a snail species of great interest among aquarium enthusiasts, simultaneously, its presence in European water bodies has been recorded.
Our report highlights that greater caution is needed in the procedure of international trade in ornamental animals, including aquarium molluscs. The possible spread of “hitchhikers” to non-native areas may have a cascading effect on the environment and be of public health importance. It is important to constantly draw public attention to the consequences of releasing aquarium animals into the non-native environment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alexandrowicz SW. 1993. Water snails introduced into the Botanic Garden in Cracow. Folia Malacologica 5:109–113. DOI:10.12657/folmal.005.005.
- Anucherngchai S, Tejangkura T, Chontananarth T. 2016. Epidemiological situation and molecular identification of cercarial stage in freshwater snails in Chao-Phraya basin, Central Thailand. Asian Pacific Journal of Tropical Biomedicine 6(6):539–545. DOI:10.1016/j.apjtb.2016.01.015.
- Banha F, Diniz A, Anastácio PM. 2019. Patterns and drivers of aquarium pet discharge in the wild. Ecological Indicators 106:105513. DOI:10.1016/j.ecolind.2019.105513.
- Biondo MV. 2017. Quantifying the trade in marine ornamental fishes into Switzerland and an estimation of imports from the European Union. Global Ecology and Conservation 11:95–105. DOI:10.1016/j.gecco.2017.05.006.
- Blankespoor HD, Reimink RL. 1991. The control of swimmers’ itch in Michigan: Past, present, future. Michigan Academician 24:7–23.
- Bogan AE, Hanneman EH. 2013. A carnivorous aquatic gastropod in the pet trade in North America: The next threat to freshwater gastropods. Ellipsaria 15:18–19.
- Butboonchoo P, Wongsawad C, Wongsawad P, Chai J-Y. 2020. Morphology and molecular identification of Echinostoma revolutum and Echinostoma macrorchis in freshwater snails and experimental hamsters in upper Northern Thailand. The Korean Journal of Parasitology 58(5):499–511. DOI:10.3347/kjp.2020.58.5.499.
- CABI. 2021. Clea helena. In: Invasive Species Compendium. Wallingford, UK: CAB International. Available: https://www.cabi.org/isc/datasheet/108187. Accessed Aug 2021 10.
- Cardoso P, Amponsah-Mensah K, Barreiros JP, Bouhuys J, Cheung H, Davies A, Kumschick S, Longhorn SJ, Martínez-Muñoz CA, Morcatty TQ, Peters G, Ripple WJ, Rivera-Téllez E, Stringham OC, Toomes A, Tricorache P, Fukushima CS. 2021. Scientists’ warning to humanity on illegal or unsustainable wildlife trade. Biological Conservation 263:109341. DOI:10.1016/j.biocon.2021.109341.
- Chantima K, Chai J-Y, Wongsawad C. 2013. Echinostoma revolutum: Freshwater snails as the second intermediate hosts in Chiang Mai, Thailand. The Korean Journal of Parasitology 51(2):183–189. DOI:10.3347/kjp.2013.51.2.183.
- Chantima K, Suk-Ueng K, Kampan M. 2018. Freshwater snail diversity in Mae Lao agricultural basin (Chiang Rai, Thailand) with a focus on larval trematode infections. The Korean Journal of Parasitology 56(3):247–257. DOI:10.3347/kjp.2018.56.3.247.
- Chomchoei N, Wongsawad C, Nantarat N. 2018. Investigation of cryptic diversity and occurrence of echinostome metacercariae infection in Anentome helena (von dem Busch, 1847). Asian Pacific Journal of Tropical Medicine 11(10):590–596. DOI:10.4103/1995-7645.244524.
- Chucholl C. 2013. Invaders for sale: Trade and determinants of introduction of ornamental freshwater crayfish. Biological Invasions 15(1):125–141. DOI:10.1007/s10530-012-0273-2.
- Commission Implementing Regulation (EU) 2020/2236 of 16 December 2020 laying down rules for the application of Regulations (EU) 2016/429 and (EU) 2017/625 of the European Parliament and of the Council as regards model animal health certificates for the entry into the Union and movements within the Union of consignments of aquatic animals and of certain products of animal origin from aquatic animals, official certification regarding such certificates and repealing Regulation (EC) No 1251/2008: Official Journal of the European Union L 442/410. Text with EEA relevance. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32020R2236. Accessed Aug 2021 10.
- Commission Regulation (EU) No 1143/2010 of 7 December 2010 amending Regulation (EC) No 1251/2008 as regards the period of application of the transitional provisions for certain ornamental aquatic animals intended for closed ornamental facilities. Official Journal of the European Union L 322/22. Text with EEA relevance. Available: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32010R1143. Accessed Aug 2021 10.
- Domagala J, Labecka AM, Migdalska B, Pilecka-Rapacz M. 2007. Colonisation of the channels of Międzyodrze (northwestern Poland) by Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae). Polish Journal of Natural Sciences 22:679–690. DOI:10.2478/v10020-007-0058-8.
- Domagala J, Labecka AM, Pilecka-Rapacz M, Migdalska B. 2004. Corbicula fluminea (O. F. Müller, 1774) (Bivalvia: Corbiculidae) a species new to the Polish malacofauna. Folia Malacologica 12(3):145–148. DOI:10.12657/folmal.012.011.
- Dunghungzin C, Chontananarth T. 2020. Prevalence of cercarial infections in freshwater snails and morphological and molecular identification and phylogenetic trends of trematodes. Asian Pacific Journal of Tropical Medicine 13(10):439–447. DOI:10.4103/1995-7645.291037.
- Dunghungzin C, Chontananarth T. 2021. The prevalence of cercarial infection and development of a duplex PCR for detection of the cercarial stage of Haplorchis taichui and H. pumilio in first intermediate hosts from Chai Nat province, Thailand. Acta Tropica 214:105795. DOI:10.1016/j.actatropica.2020.105795.
- Faltỳnková A, Našincová V, Kablásková L. 2008. Larval trematodes (Digenea) of planorbid snails (Gastropoda: Pulmonata) in Central Europe: A survey of species and key to their identification. Systematic Parasitology 69(3):155–178. DOI:10.1007/s11230-007-9127-1.
- Federspiel F, Skovmand S, Skarphedinsson S. 2020. Eosinophilic meningitis due to Angiostrongylus cantonensis in Europe. International Journal of Infectious Diseases 93:28–39. DOI:10.1016/j.ijid.2020.01.012.
- Fenoglio S, Bo T, Cucco M, Mercalli L, Malacarne G. 2010. Effects of global climate change on freshwater biota: A review with special emphasis on the Italian situation. Italian Journal of Zoology 77(4):374–383. DOI:10.1080/11250000903176497.
- Haenen OLM, Veldman KT, Ceccarelli D, Tafro N, Zuidema T, Mevius DJ. 2020. Potential transfer of antimicrobial resistance and zoonotic bacteria through global ornamental fish trade. Asian Fisheries Science 33:46–54.
- Haruay S, Piratae S. 2019. Situation and Cercarial Infection of Freshwater Mollusk from Sirindhorn Reservoir, Ubon Ratchathani Province, Thailand. Iranian Journal of Parasitology 14(3):421–429.
- Hatcher MJ, Dick JT, Dunn AM. 2012. Disease emergence and invasions. Functional Ecology 26(6):1275–1287. DOI:10.1111/j.1365-2435.2012.02031.x.
- Hossain MN-E-I, Mohsin ABM. 2020. Checklist of non-piscine ornamental aquatic organisms in Bangladesh. Journal of Fisheries and Life Sciences 5:26–29.
- Hulme PE, Bacher S, Kenis M, Klotz S, Kühn I, Minchin D, Nentwig W, Olenin S, Panov V, Pergl J, Pyšek P, Roques A, Sol D, Solarz W, Vilà M. 2008. Grasping at the routes of biological invasions: A framework for integrating pathways into policy. Journal of Applied Ecology 45(2):403–414. DOI:10.1111/j.1365-2664.2007.01442.x.
- Inobaya MT, Olveda RM, Chau TN, Olveda DU, Ross AG. 2014. Prevention and control of schistosomiasis: A current perspective. Research and Reports in Tropical Medicine 2014(5):65–75. DOI:10.2147/RRTM.S44274.
- Krailas D, Chotesaengsri S, Dechruksa W, Namchote S, Chuanprasit C, Veeravechsukij N, Boonmekam D, Koonchornboon T. 2012. Species diversity of aquatic mollusks and their cercarial infections; Khao Yai National Park, Thailand. The Journal of Tropical Medicine and Parasitology 35:37–47.
- Labecka AM, Domagala J, Pilecka-Rapacz M. 2005. First record of Corbicula fluminalis (O. F. Müller, 1774) (Bivalvia: Corbiculidae) in Poland. Folia Malacologica 13(1):25–27. DOI:10.12657/folmal.013.003.
- Ložek F, Patoka J, Bláha M. 2021. Another hitchhiker exposed: Diceratocephala boschmai (Platyhelminthes: Temnocephalida) found associated with ornamental crayfish Cherax spp. Knowledge & Management of Aquatic Ecosystems 422(422):25. DOI:10.1051/kmae/2021023.
- Lu X-T, Gu Q-Y, Limpanont Y, Song L-G, Wu Z-D, Okanurak K, Lv Z-Y. 2018. Snail-borne parasitic diseases: An update on global epidemiological distribution, transmission interruption and control methods. Infectious Diseases of Poverty 7(1):28. DOI:10.1186/s40249-018-0414-7.
- Mabrouki Y, Taybi AF, Bahhou J, Doadrio I. 2020. The first record of the swordtail Xiphophorus hellerii Heckel, 1848 (Poeciliidae, Actinopterygii) established in the wild from Morocco. Journal of Applied Ichthyology 36(6):795–800. DOI:10.1111/jai.14105.
- Maciaszek R, Jabłońska A, Hoitsy M, Prati S, Świderek W. 2021a. First record and DNA barcodes of non-native shrimp, Caridina babaulti (Bouvier, 1918) in Europe. The European Zoological Journal 88(1):816–823. DOI:10.1080/24750263.2021.1944337.
- Maciaszek R, Jabłońska A, Prati S, Świderek W. 2020. First report of freshwater atyid shrimp, Caridina formosae (Decapoda: Caridea) as a host of ectosymbiotic branchiobdellidan, Holtodrilus truncatus (Annelida, Citellata). Knowledge & Management of Aquatic Ecosystems 421:33. DOI:10.1051/kmae/2020027.
- Maciaszek R, Świderek W, Kaliszewicz A, Karaban K, Szpakowski B. 2021b. First report of Scutariella japonica (Matjašič, 1990), a temnocephalid epibiont from South-East Asia, found on introduced ornamental freshwater shrimp in European waters. Knowledge & Management of Aquatic Ecosystems 422:19. DOI:10.1051/kmae/2021018.
- Mehrdana F, Jensen HM, Kania PW, Buchman K. 2014. Import of exotic and zoonotic trematodes (Heterophyidae: Centrocestus sp.) in Xiphophorus maculatus: Implications for ornamental fish import control in Europe. Acta Parasitologica 59(2):276–283. DOI:10.2478/s11686-014-0237-z.
- Modrý D, Fecková B, Putnová B, Manalo SM, Otranto D. 2021. Alternative pathways in Angiostrongylus cantonensis (Metastrongyloidea: Angiostrongylidae) transmission. Parasitology 148(2):167–173. DOI:10.1017/S0031182020001857.
- Mrugała A, Kozubíková-Balcarová E, Chucholl C, Resino SC, Viljamaa-Dirks S, Vukić J, Petrusek A. 2015. Trade of ornamental crayfish in Europe as a possible introduction pathway for important crustacean diseases: Crayfish plague and white spot syndrome. Biological Invasions 17(5):1313–1326. DOI:10.1007/s10530-014-0795-x.
- Ng TH, Foon JK, Tan SK, Chan MK, Yeo DC. 2016a. First non-native establishment of the carnivorous assassin snail, Anentome helena (von dem Busch in Philippi, 1847). BioInvasions Records 5(3):143–148. DOI:10.3391/bir.2016.5.3.04.
- Ng TH, Tan SK, Wong WH, Meier R, Chan S-Y, Tan HH, Yeo DCJ. 2016b. Molluscs for sale: Assessment of freshwater gastropods and bivalves in the ornamental pet trade. PloS One 11(8):e0161130. DOI:10.1371/journal.pone.0161130.
- Nguyen HM, Van HH, Ho LT, Tatonova YV, Madsen H. 2021. Are Melanoides tuberculata and Tarebia granifera (Gastropoda, Thiaridae), suitable first intermediate hosts of Clonorchis sinensis in Vietnam? PloS Neglected Tropical Diseases 15(1):e0009093. DOI:10.1371/journal.pntd.0009093.
- Pace A, Dipineto L, Aceto S, Censullo MC, Valoroso MC, Varriale L, Rinaldi L, Menna LF, Fioretti A, Borrelli L. 2020. Diagnosis of Centrocestus formosanus infection in zebrafish (Danio rerio) in Italy: A window to a new globalization-derived invasive microorganism. Animals 10(3):456. DOI:10.3390/ani10030456.
- Patoka J, Bláha M, Devetter M, Rylková K, Čadková Z, Kalous L. 2016a. Aquarium hitchhikers: Attached commensals imported with freshwater shrimps via the pet trade. Biological Invasions 18(2):457–461. DOI:10.1007/s10530-015-1018-9.
- Patoka J, Bláha M, Kalous L, Vrabec V, Buřič M, Kouba A. 2016b. Potential pest transfer mediated by international ornamental plant trade. Scientific Reports 6(1):1–6. DOI:10.1038/srep25896.
- Patoka J, Kopecký O, Vrabec V, Kalous L. 2017. Aquarium molluscs as a case study in risk assessment of incidental freshwater fauna. Biological Invasions 19(7):2039–2046. DOI:10.1007/s10530-017-1412-6.
- Patoka J, Magalhães ALB, Kouba A, Faulkes Z, Jerikho R, Vitule JRS. 2018. Invasive aquatic pets: Failed policies increase risks of harmful invasions. Biodiversity and Conservation 27(11):3037–3046. DOI:10.1007/s10531-018-1581-3.
- Patoka J, Patoková B. 2021. Hitchhiking exotic clam: Dreissena polymorpha (Pallas, 1771) transported via the ornamental plant trade. Diversity 13(9):410. DOI:10.3390/d13090410.
- Patoka J, Prabowo RE, Petrtýl M, Reynolds JD, Kuříková P, Zámečníková-Wanma BPD, Kalous L. 2020. Marine hitchhikers: A preliminary study on invertebrates unintentionally transported via the international pet trade. NeoBiota 61:33. DOI:10.3897/neobiota.61.57682.
- Piechocki A, Wawrzyniak-Wydrowska B, Zdanowski B. 2003. Melanoides tuberculatus (O. F. Müller, 1774) (Orthogastropoda: Thiaridae), a gastropod species new for the fauna of Poland. Folia Malacologica 11(1–2):39–41. DOI:10.12657/folmal.011.004.
- Pratumchart K, Suwannatrai K, Sereewong C, Thinkhamrop K, Chaiyos J, Boonmars T, Suwannatrai AT. 2019. Ecological niche model based on maximum entropy for mapping distribution of Bithynia siamensis goniomphalos, first intermediate host snail of Opisthorchis viverrini in Thailand. Acta Tropica 193:183–191. DOI:10.1016/j.actatropica.2019.03.004.
- Rahel FJ, Olden JD. 2008. Assessing the effects of climate change on aquatic invasive species. Conservation Biology 22(3):521–533. DOI:10.1111/j.1523-1739.2008.00950.x.
- Reddy A, Ponder EL, Fried B. 2004. Effects of copper sulfate toxicity on cercariae and metacercariae of Echinostoma Caproni and Echinostoma trivolvis and on the survival of Biomphalaria glabrata snails. Journal of Parasitology 90(6):1332–1337. DOI:10.1645/GE-321R.
- Rollins RL, Cowie RH, Echaluse MV, Medeiros MC. 2021. Host snail species exhibit differential Angiostrongylus cantonensis prevalence and infection intensity across an environmental gradient. Acta Tropica 216:105824. DOI:10.1016/j.actatropica.2021.105824.
- Šmiga Ľ, Košuthová L, Koščo J, Košuth P, Pekárik L, Fečkaninová A, Lazar P. 2016. First report of Gussevia asota (Monogenea: Dactylogyridae), destructive parasite of Astronotus ocellatus (Perciformes: Cichlidae) in Europe. Biologia 71(5):547–550. DOI:10.1515/biolog-2016-0070.
- Stegeman N, Allender M, Arnold J, Bonar CJ. 2020. Aquatic invertebrates. In: Heatley JJ, Russell KE, editors. Exotic Animal Laboratory Diagnosis. Hoboken, New Jersey: John Wiley & Sons. pp. 383–408.
- Strong EE, Galindo LA, Kantor YI. 2017. Quid est Clea helena? Evidence for a previously unrecognized radiation of assassin snails (Gastropoda: Buccinoidea: Nassariidae). PeerJ 5:e3638. DOI:10.7717/peerj.3638.
- Strzelec M, Spyra A, Serafiński W. 2006. Over thirty years of Physella acuta (Draparnaud, 1805) expansion in the Upper Silesia and adjacent regions (Southern Poland). Malakologische Abhandlungen 24:49–55.
- Tamukai K, Une Y, Tominaga A, Suzuki K, Goka K. 2014. Batrachochytrium dendrobatidis prevalence and haplotypes in domestic and imported pet amphibians in Japan. Diseases of Aquatic Organisms 109(2):165–175. DOI:10.3354/dao02732.
- Tarkan AS, Tricarico E, Vilizzi L, Bilge G, Ekmekçi G, Filiz H, Giannetto D, Ilhan A, Killi N, Kirankaya SG, Koutsikos N, Kozic S, Kurtul I, Lazzaro L, Marchini A, Occhipinti-Ambrogi A, Perdikaris C, Piria M, Pompei L, Sari H, Smeti E, Stasolla G, Top N, Tsiamis K, Vardakas L, Yapici S, Yogurtcuoglu B, Copp GH. 2021. Risk of invasiveness of non-native aquatic species in the eastern Mediterranean region under current and projected climate conditions. The European Zoological Journal 88(1):1130–1143. DOI:10.1080/24750263.2021.1980624.
- Toledo R, Esteban JG. 2016. An update on human echinostomiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 110(1):37–45. DOI:10.1093/trstmh/trv099.
- Weiperth A, Gábris V, Danyik T, Farkas A, Kuříková P, Kouba A, Patoka J. 2019. Occurrence of non-native red cherry shrimp in European temperate water bodies: A case study from Hungary. Knowledge & Management of Aquatic Ecosystems 420:9. DOI:10.1051/kmae/2019002.
- Wiroonpan P, Chontananarth T, Purivirojkul W. 2020. Cercarial trematodes in freshwater snails from Bangkok, Thailand: Prevalence, morphological and molecular studies and human parasite perspective. Parasitology 148:366–383. DOI:10.1017/S0031182020002073.
- Yanai Z, Dayan T, Mienis HK, Gasith A. 2017. The pet and horticultural trades as introduction and dispersal agents of non-indigenous freshwater molluscs. Management of Biological Invasions 8(4):523–532. DOI:10.3391/mbi.2017.8.4.07.
- Yooyen T. 2020. Diversity of helminths in freshwater snails from Thaksin University, Phatthalung Province. Microscopy and Microanalysis Research–The Journal of the Microscopy Society of Thailand 33:20–23.
