Abstract
The new species Atrichopogon (Atrichopogon) tolfensis n. sp. from Tolfa Mountains (Italy, Latium, and Rome) is described and illustrated. Both sexes of the species were collected using cantharidin-baited traps from June 2020 to January 2021. The new species is an atypical member of the genus Atrichopogon.https://urn:lsid:zoobank.org:pub:3A012331-57D7-4532-B3F9-FDEE7C988FFD
Introduction
Biting midges of the genus Atrichopogon Kieffer, Citation1906 (Ceratopogonidae: Forcipomyiinae) are common in moist terrestrial habitats of all regions of the world. Adult females have functional mouthparts with toothed mandibles, and most of them feed on haemolymph of other insects as protein-rich meals are necessary for ovarian development of most biting midges (Downes Citation1958; Glukhova Citation1989; Szadziewski et al. Citation1997). Females of some species, like European A. pavidus (Winnertz) from the subgenus Atrichopogon s. str., obtain proteins feeding on pollens (Downes Citation1955; Szadziewski Citation2001). Also, females can supplement their protein-rich diet with sugar and water from nectar or honeydew. Contrarily, adult males have reduced biting mouthparts, and their diet appears to lack protein-rich meals.
Borkent and Dominiak (Citation2020) listed 513 extant valid species of Atrichopogon in the ceratopogonids world fauna. In Europe, this poorly known genus includes 44 described species grouped into five subgenera: Atrichopogon Kieffer, Citation1906, Lophomyidium Cordero, Citation1929, Meloehelea Wirth, Citation1956, Psammopogon Remm, Citation1979, and Psilokempia Enderlein, Citation1936 (Alwin-Kownacka et al. Citation2016). Meloehelea is a subgenus proposed by Wirth (Citation1956) to include species which were believed to be unique because they feed upon haemolymph of blister beetles (Coleoptera: Meloidae) and false blister beetles (Coleoptera: Oedemeridae). Beetles belonging to these two families are known to be the only producers of cantharidin, a toxic terpene involved in chemical defence and mating behaviour of several species (Carrel & Eisner Citation1974; Carrel et al. Citation1986; Dettner Citation1997). Females of some Atrichopogon species can sequester cantharidin from beetles and pass this terpene to eggs as a defence compound (Frenzel & Dettner Citation1994). Some evidences suggest that males can also sequester cantharidin in nature, but this phenomenon is still poorly studied (Frenzel & Dettner Citation1994). However, both males and females of Atrichopogon species have been repeatedly collected in cantharidin-baited traps, showing a high attraction to cantharidin and its analogues, thus being considered “canthariphilous species” (Frenzel et al. Citation1992; Hemp & Dettner Citation2001; Horiuchi et al. Citation2018).
Wirth (Citation1980) and Szadziewski et al. (Citation2007) revised the Holarctic and European species of the subgenus Meloehelea, respectively, correcting numerous misidentifications and misinterpretations and confirming previous reports that other species besides Meloehelea are attracted to meloids or cantharidin. In Europe, the following seven species of canthariphilous midges, belonging to three Atrichopogon subgenera, have been reported: (i) subgenus Meloehelea: A. lucorum (Meigen, 1818) (= A. setosipennis Kieffer, 1913) (Wirth Citation1956, Citation1980; Bologna & Havelka Citation1985; Frenzel et al. Citation1992; Szadziewski et al. Citation2007; Hashimoto and Tateno Citation2022), A. meloesugans Kieffer, 1922 (Wirth Citation1956, Citation1980; Szadziewski et al. Citation2007), A. oedemerarum Storå, 1939 (Wirth Citation1956, Citation1980; Frenzel et al. Citation1992; Szadziewski et al. Citation2007), and A. winnertzi Goetghebuer, 1922 (Szadziewski et al. Citation2007); (ii) subgenus Atrichopogon s. str.: A. brunnipes (Meigen, 1804) (Wirth Citation1980; Frenzel et al. Citation1992; Szadziewski et al. Citation2007); and (iii) subgenus Psammopogon: A. albiscapulus Kieffer, 1918 (without detailed locality records; Frenzel et al. Citation1998; Havelka and Aguilar Citation1999) and A. muelleri (Müller, 1905) (= trifasciatus auct.) (Frenzel et al. Citation1992; Szadziewski et al. Citation2007). From the list of canthariphilous species compiled by Hemp and Dettner (Citation2001), both Atrichopogon illiesi and A. (Lophomyidium) rostratus (Winnertz, 1852) should be excluded. In fact, A. illiesi has never been described and represents a typical nomen nudum (Bologna and Havelka, Citation1985), while A. rostratus has been erroneously reported by Wirth (Citation1956) due to a misidentification. The old record of the haematophagous Culicoides obsoletus (Meigen, 1818) (Bologna & Havelka Citation1985), never confirmed in other studies, should also be excluded from this list.
The species described below belongs to the subgenus Atrichopogon s. str., which is the most difficult to identify and the most neglected group within the genus. Indeed, this subgenus includes very similar species whose characters for taxonomic identification are limited and highly variable.
The purpose of this paper is to describe a new European species in the subgenus Atrichopogon s. str. which was collected with cantharidin-baited traps in central Italy.
Material and methods
All examined specimens were collected from two sites in derived pastures within oak (Quercus Linnaeus) woodlands at the western base of Tolfa Mountains: site A (Italy, Latium, Rome, Tolfa, 42.058716N, 11.941148E; 48 m a.s.l.) () and site B (Italy, Latium, Rome, Tolfa, 42.092617N, 11.974103E; 298 m a.s.l.) (). Two pairs of funnel traps were placed in each site. Paired traps consisted in one cantharidin-baited trap and one control trap, based on the protocol described in Horiuchi et al. (Citation2018). Paired traps were about 2 m distanced, and the distance between pairs was about 30 m. Traps were active for 24 h every 2 weeks (approximately) for 1 year (June 2020–June 2021). After 24 h, traps were capped with cotton wool and stored at −20°C to euthanize the specimens. Specimens were then preserved in 70% ethanol.
Specimens were mounted on microscope slides in phenol-Canada balsam following the method described by Wirth and Marston (Citation1968). The photograph of a female was taken with a LAS Montage multi-focus camera attached to a Leica stereo microscope M205A (). The materials are housed in the collection of extant invertebrates of the Department of Invertebrate Zoology and Parasitology, University of Gdańsk, Poland (CEIG), and the Zoological Museum of Roma (Museo Civico di Zoologia), Italy (MCZ). We employ the traditional terminology of Ceratopogonidae as described in Szadziewski (Citation1986).
Taxonomic accounts
Order: Diptera
Family: Ceratopogonidae Newman, Citation1834
Subfamily: Forcipomyiinae Lenz, Citation1934
Tribe: Forcipomyiini Lenz, Citation1934
Atrichopogon (Atrichopogon) tolfensis n. sp. ().
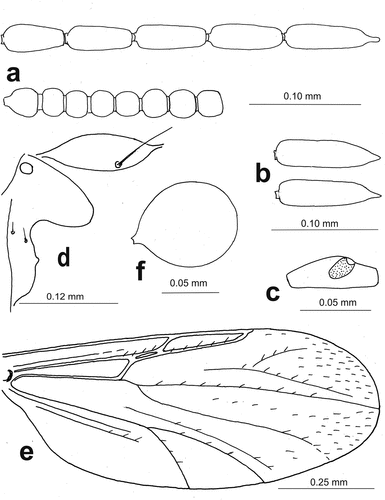
Figure 3. Atrichopogon (Atrichopogon) tolfensis n. sp., female: (a) flagellum, (b) terminal flagellomere, (c) third palpal segment, (d) paratergite and anterior anepisternum, (e) wing, and (f) seminal capsule.
Type material
Holotype: Male, site A, 29.09.2020, leg. M. Molfini (CEIG). Paratypes: 14 males and 21 females from sites A and B. Site A (11 males, 19 females): 2 males, 3 females—22.06.2020 (CEIG); 5 females—05.07.2020 (CEIG); 2 males, 5 females—04.08.2020 (CEIG); 1 male—19.08.2020 (CEIG); 1 male, 1 female—29.09.2020 (CEIG); 1 male, 1 female—29.09.2020 (MCZ); 3 females—14.10.2020 (CEIG); 1 male—28.10.2020 (CEIG); 2 males, 1 female—11.11.2020 (CEIG); 1 male—28.01.2021 (CEIG). Site B (3 males, 2 females): 1 female—19.08.2020 (CEIG); 3 males, 1 female—29.09.2020 (CEIG).
Diagnosis
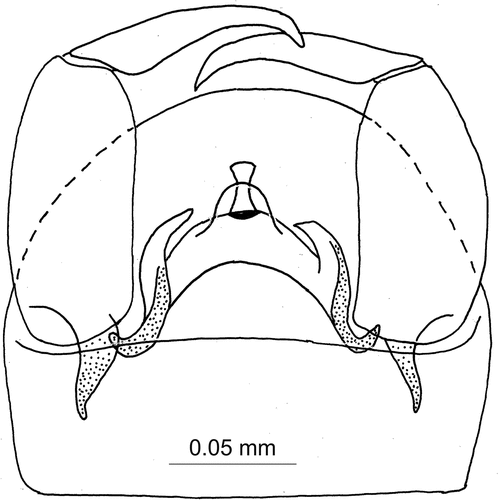
Small, dark brown biting midge with wing length 0.95–1.35 mm. Both sexes have pubescent eyes, terminal flagellomere blunt or with conical to cylindrical apical prolongation, single seta on paratergite, 0–3 setae on anterior anepisternum and four marginal bristles on scutellum; female () with single ovoid seminal capsule, wing membrane with macrotrichia in cells r2+3, m1 and few in m2; male with wing membrane bare and unique aedeagus bearing slender hourglass-like caudomedian projection with dark semi-elliptic base in ventral view ().
Description
Female () Body dark brown with yellowish legs and pale halteres; sometimes shoulders and scutellum paler (). Eye pubescent. Flagellum 0.50–0.54 mm long, antennal ratio (AR) 1.52–1.70, proximal flagellomeres 2–8 spherical ()), apex of terminal flagellomere blunt or with conical to cylindrical prolongation, not constricted at base (). Proboscis short, palpus 5-segmented, palpomere 3 slender, 0.050–0.072 mm long, with sensory pit on distal half ()). Mandible armed with distinct 17–19 teeth, largest at mid-length. Anterior anepisternum B-shaped, broadly bilobed posteriorly, bearing usually 2 (1–2) short setae at midlength ()). Paratergite ovoid with one long seta ()). Scutum covered with numerous short setae. Scutellum bearing four marginal bristles. Wing length 1.00–1.25 mm, costal ratio (CR) 0.64–67; first radial cell slit-like, second one distinct, wing membrane with macrotrichia in apical portion in cells r2+3, m1 and few in m2 ()). Legs slender, yellowish, claws with single pointed apices, empodium with simple hairs, tarsal ratio (TR) of foreleg TR(1) 3.2–3.6, of midleg TR(2) 3.1–3.4, of hind leg TR(3) 2.4–2.9. Seminal capsule single, almost spherical with short neck ()), dark brown with pale dots at neck, length with neck 0.087–0.100 mm.
Male () Similar to female, with usual sexual differences. Eyes pubescent. Flagellum with 13 separated flagellomeres, total length 0.65–0.75 mm, distal four flagellomeres elongate, plume well developed, terminal flagellomere with blunt apex or with conical to cylindrical apical prolongation without constriction at base. Third palpal segment 0.055–0.062 mm long. Anterior anepisternum bearing usually 2 (0–3) short setae at midlength. Wing length 0.95–1.35 mm, CR 0.61–0.65, wing membrane without macrotrichia. Tarsal ratio of foreleg (TR1) 3.3–3.4, of midleg TR(2) 3.2–3.4, of hind leg TR(3) 2.5–2.7. Genitalia (). Sternite 9 with straight caudal margin. Tergite 9 with rounded margin, without apicolateral projections. Gonocoxite slender, with long ventral apodemes (gonocoxal roots), dorsal apodemes not developed. Parameres absent. Aedeagus (ventral plate) arch shaped; basal arch high; aedeagal arch with two pointed lateral projections and apical ventral rounded broad expansion and dorsal hourglass-like long and slender expansion; base of hourglass expansion dark, semi-elliptic in ventral view.
Etymology
The specific name refers to the Tolfa Mountains near Rome, the type locality of the new species.
Conclusive remarks
The new species has unique aedeagus with hourglass dorsal caudomedian expansion with semi-elliptic dark base (). Such a character is unknown in all other species of the subgenus described from Europe, North Africa, the Middle East, the Caucasus, and Central Asia (Goetghebuer Citation1934; Clastrier Citation1956; Remm Citation1967, Citation1980; Szadziewski Citation1984, Citation1986, Citation2001; Szadziewski et al. Citation1996; Alwin-Kownacka et al. Citation2016).
Noteworthily, this new species represents an atypical member of the genus Atrichopogon as its terminal flagellomere 13 is blunt or without apical nipple-like prolongation, i.e. it has no constriction at base ()). Indeed, to the best of our knowledge, all adults of European species within the tribe Forcipomyiini (including two genera: Forcipomyia Meigen, 1818, and Atrichopogon) have very characteristic nipple-like prolongation on apex of terminal flagellomere. Moreover, A. tolfensis n. sp. has the anterior anepisternum bearing 2 (0–3) short setae, which is totally bare in the European congeneric species.
A. tolfensis n. sp. is the eighth on the list of canthariphilous Ceratopogonidae in Europe. The presence of males (15) and females (21) in cantharidin-baited traps evidences that both sexes are attracted to cantharidin with no significant difference: binomial test p-value >0.05 (= 0.256). Nevertheless, it should be emphasised that the attraction to cantharidin of biting midges does not demonstrate by itself interspecific interactions with cantharidin-producing species. Indeed, some species could just confuse cantharidin with similar plant or fungal molecules used in food search (Frenzel et al. Citation1992; Hashimoto & Hayashi Citation2014). Further investigations are needed to understand the canthariphilous behaviour of this species and the evolution of canthariphily in Ceratopogonidae.
Acknowledgements
We are much indebted to Dr Elżbieta Sontag of the Department of Invertebrate Zoology and Parasitology, University of Gdańsk, Poland, for taking the photo of the Atrichopogon tolfensis female presented in this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alwin-Kownacka A, Szadziewski R, Szwedo J. 2016. Biting midges of the subfamily Forcipomyiinae (Diptera: Ceratopogonidae) from the Middle East, with keys and descriptions of new species. Zootaxa 4173:351–378. DOI: 10.11646/zootaxa.4173.4.2.
- Bologna MA, Havelka P. 1985. Nuove segnalazioni di attrazione della cantaridina dei Meleoidae su coleopteri e ditteri. Bolletino dell’Associazione Romana di Entomologia 39:77–82.
- Borkent A, Dominiak P. 2020. Catalog of biting midges of the world (Diptera: Ceratopogonidae). Zootaxa 4787:1–377. DOI: 10.11646/zootaxa.4787.1.1.
- Carrel JE, Doom JP, McCormick JP. 1986. Identification of cantharidin in false blister beetles (Coleoptera, Oedemeridae) from Florida. Journal of Chemical Ecology 12:741–747. DOI: 10.1007/BF01012106.
- Carrel JE, Eisner T. 1974. Cantharidin: Potent feeding deterrent to insects. Science 183:755–757. DOI: 10.1126/science.183.4126.755.
- Clastrier J. 1956. Note sur les Cératopogonidés. I.- Quatre espèces du groupe Forcipomyia d’Algérie et de Tunisie. Archives de l’Institut Pasteur d’Algérie 34:496–512.
- Cordero EH. 1929. Contributión al studio de los dipteros del Uruguay, I. Lophomyidium uruguayense n. gen., n. sp. nueva ceratopogonina hematófaga. Anales del Museo de Historia Natural de Montevideo 3:93–108.
- Dettner K. 1997. Inter- and intraspecific transfer of toxic insect compound cantharidin. In: Dettner K, Bauer G, Völk W, editors. Vertical food web interactions. Berlin: Springer. pp. 115–145.
- Downes JA. 1955. The food habits and description of Atrichopogon pollinivorus sp. n. (Diptera: Ceratopogonidae). Transactions of the Royal Entomological Society of London 106:439–453. DOI: 10.1111/j.1365-2311.1955.tb01264.x.
- Downes JA. 1958. The feeding habits of biting flies and their significance in classification. Annual Review of Entomology 3:249–266. DOI: 10.1146/annurev.en.03.010158.001341.
- Enderlein G. 1936. Ordnung: Zweiflügler, Diptera. In: Brohmer et al., editors. Die Tierwelt Mitteleuropas 6: Insekten, Teil III. Leipzig: Quelle & Meyer. pp. 259.
- Frenzel M, Dettner K. 1994. Quantification of cantharidin in canthariphilous Ceratopogonidae (Diptera), Anthomyiidae (Diptera) and cantharidin-producing Oedemeridae (Coleoptera). Journal of Chemical Ecology 20:1795–1812. DOI: 10.1007/BF02066223.
- Frenzel M, Dettner K, Wirth D, Weibel J, Boland W. 1992. Cantharidin analogues and their attractancy for ceratopogonid flies (Diptera: Ceratopogonidae). Experiencia 48:106–111. DOI: 10.1007/BF01923620.
- Frenzel M, Havelka P, Brandl R. 1998. Morphological comparison of canthariphilic Atrichopogon (Diptera: Ceratopogonidae) from Europe and Central Africa – Implications for the evolution of canthariphily? Zoology-Analysis of Complex Systems 101(Suppl. 1):37.
- Glukhova VM. 1989. Blood-sucking midges of the genera Culicoides and Forcipomyia (Ceratopogonidae). In: Skarlato OA, editor. Fauna of the USSR, 139. Vol. 3(5a). Leningrad: Nauka. pp. 408. [ in Russian].
- Goetghebuer M. 1934. A. Die Imagines. Heleidae (Ceratopogonidae). In: Lindner E, editor. Die Fliegen der palaearktischen Region. Stuttgart: Schweizerbart’sche verlagsbuchhandlung. pp. 94.
- Hashimoto K, Hayashi F. 2014. Cantharidin world in nature. A concealed arthropod assemblage with interactions via the terpenoid cantharidin. Entomological Science 17:388–395. DOI: 10.1111/ens.12074.
- Hashimoto K, Tateno H. 2022. Holarctic canthariphilous biting midge Atrichopogon lucorum Meigen, 1818 (Diptera. Ceratopogonidae) in Japan. Oriental Insects. DOI: 10.1080/00305316.2021.2023680.
- Havelka, P, and Aguilar, M. 1999. Ceratopogonidae (33–38). In: H, S; Bahrmann, R and Stark, A, editors. Entomofauna Germanica 2. Checkliste der Dipteren Deutschlands. Studia Dipterologica, Supplement. Vol. 2. Halle/Saale: Ampyx-Verlag. p. 354.
- Hemp C, Dettner K. 2001. Compilation of canthariphilous insects. Beiträge zur Entomologie 51:231–245. DOI: 10.21248/contrib.entomol.51.1.231-245.
- Horiuchi K, Hashimoto K, Hayashi F. 2018. Cantaharidin world in air: Spatiotemporal distributions of flying cantariphilous insects in the forest interior. Entomological Science 21:306–314. DOI: 10.1111/ens.12310.
- Kieffer JJ. 1906. Diptera. Fam. Chironomidae. In: Wytsman P, editor. Genera Insectorum, Fasc. 42. Bruxelles: V. Verteneuil & L. Desmet. pp. 78.
- Lenz F. 1934. B. Die Metamorphose der Heleidae. Heleidae (Ceratopogonidae). 13a. In: Lindner E, editor. Die Fliegen der palaearktischen Region. Stuttgart: Schweizerbart’sche verlagsbuchhandlung. pp. 94.
- Newman E. 1834. Attempted division of British insects into natural orders. Entomological Magazine 2:379–431.
- Remm H. 1967. On the fauna of Ceratopogonidae (Diptera) in the Caucasus. Tartu Riikliku Ülikodi Toimetised 194:3–37. [ In Russian, English summary].
- Remm, H. 1979. Eesti NSV habesääsklaste (Diptera, Ceratopogonidae) fauna kataloog. In K. Elberg, editor. Dipteroloogilisi Uurimusi. Tartu: Eesti NSV Teaduste Akadeemia Eesti Looduseuurijate Selts. pp. 40–60. (in Estonian).
- Remm H. 1980. New species of the family Ceratopogonidae (Diptera) from the Middle Asia. Tartu Riikliku Ülikodi Toimetised 516:85–128. [ in Russian].
- Szadziewski R. 1984. Redescriptions of three species of the biting midges (Diptera, Ceratopogonidae) described by Becker from Egypt. Polskie Pismo Entomologiczne 54:183–194.
- Szadziewski R. 1986. Redescriptions and notes on some Ceratopogonidae (Diptera). Polskie Pismo Entomologiczne 56:3–103.
- Szadziewski R. 2001. European Atrichopogon of the pavidus group (Diptera: Ceratopogonidae). Polskie Pismo Entomologiczne 70:347–358.
- Szadziewski R, Dominiak P, Tothová A. 2007. European Atrichopogon biting midges of the subgenus Meloehelea (Diptera: Ceratopogonidae). Polish Journal of Entomology 76:267–284.
- Szadziewski R, Kaczorowska E, Krzywiński J. 1996. Redescriptions of some European species of Atrichopogon (Diptera: Ceratopogonidae). Polskie Pismo Entomologiczne 65:297–318.
- Szadziewski R, Krzywiński J, Giłka W. 1997. Ceratopogonidae. In: Nilsson A, editor. Aquatic insects of North Europe – A taxonomic handbook, vol. 2 Odonata, Diptera. Stenstrup: Apollo Books. pp. 243–263.
- Wirth WW. 1956. The biting midges ectoparasiting on blister beetles (Diptera: Heleidae). Proceeding of the Entomological Society of Washington 58:15–23.
- Wirth WW. 1980. A new species and corrections in the Atrichopogon midges of the subgenus Meloehelea attacking blister beetles (Diptera: Ceratopogonidae). Proceedings of the Entomological Society of Washington 82:124–139.
- Wirth WW, Marston N. 1968. A method for mounting small insects on microscope slides in balsam. Annals of the Entomological Society of America 61:783–784. DOI: 10.1093/aesa/61.3.783.