Abstract
Brown bear is a powerful, intelligent and robust beast that hides in the woods of Slovakia. Despite the bear’s intense environmental, geographical and behavior research, a detailed analysis of its bone microstructure is still absent. Therefore, our study aims to analyse the effect of age, sex and body weight on bone parameters in compact bear bone. Qualitative histological observations of compact femoral bone show a high amount of dense Haversian bone tissue in brown bears confirming that this is a mammal with high locomotory capability. Only in 8–9 month old cub lack on the subperiosteal zone lines of arested growth. The histomorphometric analysis reported weak significant alternations in the sizes of Haversian canals. On the contrary, no significant differences in the osteons size were observed among groups. The correlation between osteon and Haversian canal diameters shows that all bears together have a little correlation (r = 0.284). In particular, the greater difference is between cub bears (r = 0.268) and all adult bears together (r = 0.394). Based on the results of our work, we can state that the different locomotor behavior could influence the differences between cubs and adults.
Introduction
The brown bear (Ursus arctos) is the largest and strongest beast occurring in the territory of Slovakia (Rigg et al. Citation2011). Currently, the brown bear population in the Carpathians, which includes bears in Slovakia, Poland, Ukraine and Romania, has been estimated to comprise about 8,000 individuals. This fact makes this population the second-largest in Europe after that of the northeast (Swenson et al. Citation2000; Kaczensky et al. Citation2013). The bear range in the Western Carpathian Mountains is approximately 13,000 km2, and over 95% of Slovakia’s brown bears are found here (Janská et al., Citation2010). The brown bear is classified as a rare and endangered animal, strictly protected by national and international legislation (Rigg & Adamec Citation2007; Christiernsson Citation2018).
Brown bears are an essential part of wildlife and the subject of intensive environmental, geographical, and behavior research (Dahle et al. Citation2006; Garshelis Citation2009; Graban et al. Citation2013; Matosiuk et al. Citation2019; Stoen et al. Citation2020), which providing knowledge on their life and biology in especially in the last decades.
Studying a bear’s skeleton is particularly important from different points of view. Among them, the fact that many bear bones are very similar to those of humans, raises problems in determining the species in zooarchaeological and forensic studies (Demircioğlu et al. Citation2020). Indeed, the bear skeleton has about the same size and weight as that of humans. It could be of interest to note that, like man, bears occasionally walk with bipedal locomotion. This implies that the bones of the pelvic limb in these two species can be subjected to similar biomechanical stress (Brear et al. Citation1990). It is the reason why bear pelvic bones represent a suitable model for orthopedic studies for human clinician applications (Frӧbert et al. Citation2020).
Despite the broad multidisciplinary interest in macroscopic features of bear bones, only a few studies focus on microscopic analysis of this species and, in general, of wild mammal bones (Urbanová & Novotný Citation2005; Hillier & Bell Citation2007; Mainland et al. Citation2007; Giua et al. Citation2014). It is known that the bones of mammals are characterized by the distinctive bone appearance and the different sizes of the histological structures. The structure of bone tissue is influenced mainly by biomechanical forces operating on the bone. Other factors include, for example, the age and sex of the individual, but also pathological conditions that can affect the bones during life. These factors cause a wide range of differences, especially in the rate of Haversian canals remodeling, along with a general change in the entire microstructure (Hillier & Bell Citation2007; Brits et al. Citation2013; Kolb et al. Citation2015). Histological analyses are emerging as very useful for identifying animal species in archaeozoological and forensic studies. Many works have been carried out on bear bones to verify the effect of hibernation on the bone tissue, sometimes reporting bone histology information (Donahue et al. Citation2003; Harvey & Donahue Citation2003; McGee et al. Citation2008; McGee-Lawrence et al. Citation2009). Moreover, several studies have been directed to the bone histology of the extinct cave bear (Ursus spelaeus) to reconstruct its life history evolution (Rogoz et al. Citation2010; Veitschegger et al. Citation2018).
Therefore, our research aims to enrich knowledge about the microstructure of brown bear compact bone tissue. In addition, we compared the effect of age, sex and body weight on bone parameters, which may provide new and exciting information for other research, not only of the brown bear.
Material and methods
Bone samples
Seven femurs belonged to brown bears (Ursus arctos) from the Western Carpathians subpopulation that were killed in the regulated hunt or died due to collisions with vehicles in Slovakia. The bones were provided by the National Park Mala Fatra, Tatra National Park (TANAP), and Italzver s.r.o. Two femurs were from female bears (age range 6–11 years, mean 8.5 years, weight range 122–166 kg, mean 144 kg), two femurs were from male bears (age range 6–7 years, mean 6.5 years, weight range 120–170 kg, mean 145 kg) and three femurs were from male cubs (age range 8–24 months, mean 15 months, weight range 35–68 kg, mean 47 kg). Westwood (Citation1996) states that epiphyseal fusion occurs in bears between 6 and 8 years of age. For our study, skeletal maturity was defined as 6 years of age. Thus, two female and two male bears were skeletally mature (i.e., 6 years of age or older). No evidence of skeletal pathology in analysed individuals was detected. All applicable international, national, and/or institutional guidelines for the care and use of animals were strictly followed. All animal sample collection protocols complied with the current laws of Slovakia.
Sample processing for histology
The femurs were cleaned of muscles, ligaments, and tendons for histological analysis. The bones were sectioned in the smallest width of their diaphyses (midshaft) in order to avoid their influence on histomorphometry features. The segments were dehydrated in increasing ethanol grades (60–100%). We impregnated the dry sample with Epoxy 2000 (Cloeren, Germany) in a vacuum for approximately 30 minutes and allowed it to cure 24 hours at 22°C. After curing, we cut the samples with a diamond blade saw while cooling with water. We manually leveled the surface on abrasive powders in the presence of water on a cast iron lapping disk SiC (Silicon carbide) grain size F320, then on a glass plate SiC F600 to SiC F800. The samples were cleaned in water and ultrasound for 5 minutes. Subsequently, the samples were immersed in pure isopropyl alcohol for 10 minutes and allowed to dry at 22°C for 24 hours.
We glued the dried samples to the slide with epoxy resin, loaded them, and allowed them to cure for 24 hours at 22°C. We cut the excess sample from the slide with a diamond blade saw while cooling with water. After cutting, the sample adhered to the slide was immediately immersed in pure isopropyl alcohol for 30 minutes. The samples were again allowed to dry and mechanically lapped to a thickness of approximately 80–90 µm on SiC F800 and mono propylene glycol. The samples were cleaned with isopropyl alcohol and ultrasonically cleaned of the abrasive and dried. The slides were then placed on a hot plate (70°C) and adhered to a coverslip with Canadian balsam for 48 hours. We prepared three histological specimens for each femur. Subsequently, we examined them and the best used for the following analyses. The histological specimens were prepared at the Institute of Earth Sciences of the Slovak Academy of Sciences in Bratislava.
Qualitative histological analysis
The histological specimens of all bones were observed and photographed using a polarizated light microscope Leica DM 750P at 40× and 100× magnifications. The qualitative histological characteristics of the compact bone were determined according to the internationally accepted classification systems of Enlow and Brown (Citation1956) and Ricqlés et al. (Citation1991). The amount of each type of bone tissue and its distribution in the subperiosteal, intermediate (mesosteal) and subendosteal zones have been independently detected by several observers indicating with -, +, ++, +++, ++++, respectively, the absence (0% of the area), low presence (1–20%), low-medium presence (21–50%), high-medium presence (51–80%), high presence (81–100%).
Quantitative histological analysis
About 2,000 secondary osteons have been subjected to using Image J software (Citation2009). We measured the area, perimeter, minimum, and maximum diameters of Haversian canals and osteons. Only the secondary osteons where the cement line was well evident have been taken into account, and the bone lamellae were counted based on the position of osteocyte lacunae. Haversian canals were determined on their average according to Rämsh and Zerndt (Citation1963), Gladuhsew (Citation1964). The eccentricity of Haversian canals and secondary osteons were also evaluated and calculated considering that eccentricity (e) = square root of 1 − (b/a)2, where a is the semi-major axis, and b is the semi-minor axis. The differential eccentricity between osteons and Haversian canals has also been calculated.
Statistical analysis
The mean and standard deviation were calculated for each parameter. The Pearson’s correlation coefficient between the size of osteons and Haversian canals has been calculated, and their linear regression has been drawn in the regression plotter. Correlation coefficients were considered on the basis of the classification proposed by Hinkle et al. (Citation2003) as follows: very high positive correlation (0.90 to 1.00), high positive correlation (0.70 to 0.90), moderate positive correlation (0.50 to 0.70), low positive correlation (0.30 to 0.50), little if any correlation (0.30 to −0.30), low negative correlation (−0.30 to −0.50), moderate negative correlation (−0.50 to −0.70), high negative correlation (−0.70 to −0.90) and very high negative correlation (−0.90 to −1.00). Variances within the size of osteons and Haversian canals among individuals were analysed for statistical significance (p˂0.05) using one-way ANOVA. Statistical analyses were done using the NCSS software program.
Results
Qualitative histological analysis
Qualitative histological observations of compact femoral bone show different types of bone tissues, including non-vascular, plexiform, irregular Haversian and dense Haversian bone tissue. Non-vascular bone tissue is made by bone lamellae formed by an appositional process due to the osteogenic activity of the subendosteal and subperiosteal zone. Plexiform bone tissue is characterized by a net of vascular canals surrounded by many bone lamellae placed chaotically. Irregular and dense Haversian bone tissues show the presence of secondary osteons (Haversian systems) with Haversian canals not in order and far or parallel and close, respectively. The amount and distribution of each type of bone tissue mainly differ between adult bears and the cubs, summarized in and .
Figure 1. Microstructure of femoral bone in brown bears cubs. 1: non-vascular bone tissue, 2: dense Haversian bone tissue, 3: plexiform bone tissue with isolated secondary osteons, orange arrows: lines of arrested growth (LAGs).
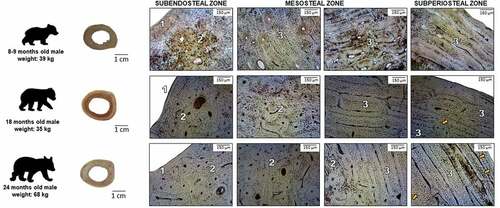
Figure 2. Microstructure of femoral bone in adult brown bears. 1: non-vascular bone tissue, 2: dense Haversian bone tissue, 3: plexiform bone tissue with isolated secondary osteons, orange arrows: lines of arrested growth (LAGs).
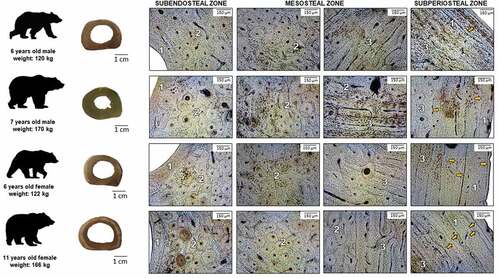
Table I. Schematic histomorphological data on the bear compact bone tissue
The subendosteal zone of the bear cubs is formed by non-vascular bone tissue, corresponding to the internal circumferential lamellae. In the middle part (mesosteal zone), we can observe plexiform bone tissue combined with scattered secondary osteons forming areas of irregular Haversian tissue. In the more peripheral part of the mesosteal zone, several osteons formed osteon banding. The subperiosteal zone is formed by plexiform bone tissue covered by non-vascular bone tissue corresponding to external circumferential lamellae.
All secondary osteons appear well formed, with a central Haversian canal surrounded by several bone lamellae and connected to each other by Volkmann’s canals. The number of the osteonal bone lamellae ranges from 3 to 8. Precisely, the more frequent number of bone lamellae in adults, both males and females, is 6 and in cubs is 4. The bigger osteon has been observed in an adult male bear, and it showed eight bone lamellae.
Histomorphometry
Histomorphometric data have been reported in , where it can be noted that osteon sizes (area, perimeter, minimum diameter and maximum diameter) do not show significant differences (p < 0.05). In contrast, the same sizes of Haversian canals (area, perimeter, minimum and maximum diameter) show a weak significative difference among groups.
Table II. Morphometrical results of brown bears compact bone tissue
The mean diameter of the Haversian canals in adult brown bears is 31.62 ± 6.71 µm and 30.45 ± 5.40 µm in cubs. Based on the classification of Haversian canals, we can consider the Haversian canals of the brown bear to be a medium width.
Eccentricity
Regarding the circularity of the secondary osteons, the eccentricity shows a higher values in the Haversian canals than in osteons. These data are reported in , where the differences expressed in percentage between the osteons and Haversian canals’ eccentricity are also shown. The Pearson’s coefficient (r), which indicates the correlation between osteon and Haversian canal diameters, shows that all bears together have a little correlation (r = 0.284). When this coefficient is evaluated for each individual, many differences can be noted (). In particular, the greater difference is that between the cub bears (r = 0.268) and all adult bears together (r = 0.394). These data are reported in , where the regression line’s variance and function are present for each individual or group of individuals. shows the scatter plots to highlight the different regression lines of the cub bears and adults.
Figure 3. Scatter plots showing the correlation between mean osteon diameter and mean Haversian canal diameter both in cubs and adult bears. Values are expressed in μm.
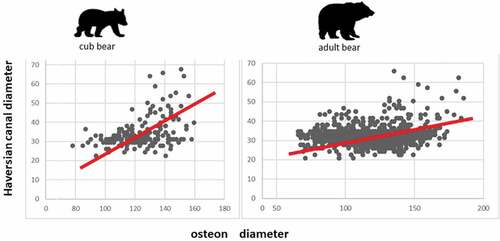
Table III. Data of the eccentricity (e) of osteons and Haversian canals in brown bears
Table IV. Statistical data on the variance of osteon diameter, Pearson’s correlation, and regression-line functions of the correlation between the diameter of osteons and Haversian canals
Lines of arrested growth (LAGs)
In adult bears, we can observe a very similar microstructure of compact bone tissue between either sex. Similarly, as in the cubs, the subendosteal and subperiosteal zones are formed with non-vascular bone tissue and plexiform bone tissue. In contrast to the cubs, in the mesosteal zone, dense Haversian tissue is the more frequent tissue present, followed by irregular Haversian bone tissue. In the subperiosteal zone of adult bears, some lines of arrested growth (LAGs) are visible. Their number ranges from (4–5) in 6 years old bears to (8–10) in the oldest bear. Similarly, we observed LAGs in cubs – three in 24-month-olds and one in 18-month-old cub. LAGs were not observed in the 8–9 month-old bear cub.
Discussion
In 1913, Foote identified in the middle part of the black bear femoral compact bone tissue the presence of plexiform bone tissue with scattered Haversian systems. In contrast, Enlow and Brown (Citation1958) noted in the mandible of Ursus americanus the presence of primary vascular reticular and dense Haversian bone tissue. In the ribs, the authors found only dense Haversian bone tissue. Correspondingly, Wojda et al. (Citation2013) state that similar to many large, rapidly growing quadrupeds, black bear compact bone consists of a mixture of primary and secondary bone. Hulsey et al. (Citation2009) also recorded osteon banding in the middle part of compact bone in black bears. According to Hillier and Bell (Citation2007), the presence of plexiform bone tissue in bear bone and its actual absence in the human bone can potentially differentiate bear bone from human bone. On the other hand, there is also the potential for misidentifying bear bone as a human bone if the distribution of Haversian bone tissue in bear bone is greater, as described by Foote (Citation1913). Especially in adult bears, the size of the secondary osteons can be comparable to humans. Interestingly, the dense Haversian tissue is present exclusively in the mesosteal zone in all bears, and it prevails in adults more than in the cubs. Moreover, the distribution of the different types of bone tissue seems to not differ between males and females (). The presence of a high amount of dense Haversian bone tissue in brown bears confirms that this is a mammal with high locomotory capability. Brown bears, despite their size, can be fast runners, capable of reaching speeds of 56 km/h. They have a great range of possibilities in different gaits comprising bipedal and quadrupedal locomotion, walking gaits in lateral sequence, oblique direction, trot and gallop (Amanat et al. Citation2020). The high amount of dense Haversian bone tissue has been described in mammals subjected to heavy biomechanical stress, such as horses and wild boars (Zedda et al. Citation2008, Citation2019, Citation2020).
These data suggest that the histomorphometric features of bone tissue depend overall on the biomechanical stress to which the limbs are subjected during locomotion. Indeed, in brown bears, the sexual dimorphism about weight shows a range from 38% to 50% in favour of males (Brown Citation1996). A previous study comparing sheep and cows weighing more than 10 times demonstrated that body mass does not affect bone histomorphometry (Zedda & Babosova Citation2021). Similarly, Joshi (Citation2006) argues that as the size and weight of an animal increases, the diameter of the Haversian canals increases relatively, but only up to 10 kg. However, the ratio of osteon diameters to Haversian canal diameters in heavier mammals remains constant from 1:4 to 1:6. That ensures efficient transport of nutrients in the bone, regardless of its size. We got a constant ratio 1: 4, which is also in line with Joshi’s results.
In our study, the size of secondary osteons does not differ significantly (see ). We hypothesize that a weak significant increase in size of Haversian canals in the cub bears, compared to adults, may be related to the bones of younger individuals needing to be better supplied with blood, as confirmed by a study by Owerkowicz and Crompton (Citation1995).
Regarding the eccentricity of the osteons and Haversian canals, we noted that the minus values are those of adult bears. In contrast, the values of osteons and Haversian canals are a bit higher in the cub bears. These data mean that adults have osteons and Haversian canals more similar to circles, whereas they are more elliptical in the cub bears. This result raises some interpretative problems because elliptical osteons are related to heavy biomechanical stress (Skedros et al. Citation2013; Zedda et al. Citation2015, Citation2017). We can assume that adults are overall subjected to heavy stress compared to young individuals. However, a key consideration can be advanced. Indeed, in mammals with elliptical osteons, the major axis is oriented towards mechanical stress. The poor eccentricity of osteons in adult bears could be related to the wide range of movements of the hindlimbs. The femur is subjected to movements of flexion, extension, rotation during the quadrupedal gait, and abduction and adduction during the bipedal lateral sequence.
This last movement seems more practiced by adult bears than by cubs. Bears differ from other large mammals because they have higher mediolateral forces in hindlimbs (Shine et al. Citation2015; Amanat et al. Citation2020). The high values of Pearson’s coefficient, indicate the rate of correlation between osteons and Haversian canals diameters, in adult bears (r = 0.394 mean value), and the lower value in the cubs (r = 0.268). It could be interpreted that the different locomotory behavior in cubs and adults above described can influence the eccentricity. A value of Pearson’s coefficient not similar to that reported for cube has been published by Skedros et al. (Citation2013) for the femur of black bear (Ursus americanus).
In recent years, a growing interest has been played to the presence of LAGs in the compact bone tissue of long bones, suggesting a key for reconstructing the life history of wild mammal species and the palaeoenvironment (Castanet et al. Citation2004; Palombo & Zedda Citation2021). According to Ray and Chinsamy (Citation2004), the LAGs indicate a cessation of growth. Chinsamy and Rubidge (Citation1993) and Horner et al. (Citation2000) state that LAGs are followed by resumption of rapid growth as shown by zones of fibrolamellar bone. Hutton (Citation1986) assumed that LAGs might be related to environmental stress or arise during hibernation (Klevezal Citation1996). In recent decades is it widely accepted that LAGs are deposited annually (Marangoni et al. Citation2009; Chinsamy-Turan Citation2012) and independently of metabolic rate and climatic background (Köhler et al. Citation2012; Kolb et al. Citation2015). Köhler et al. (Citation2012) additionally demonstrated that hormonal cues rather than environmental stresses mainly trigger the annual formation of LAGs. They are found in the modeling of bone, not the remodeling (Ray et al. Citation2009; Sander et al., Citation2016). Hinrichs (Citation2016) observed the occurrence of LAGs in the femurs of a black bear. The author states that LAGs stack on the subperiosteal zone in older animals due to the slowing of radial growth.
Conclusions
Brown bears are large mammals with high capability in locomotion. The presence and distribution of a different kind of bone tissue as the size and shape of osteons and Haversian canals reflect how the histomorphology and histomorphometry of bone tissue respond to biomechanical stress to which the bone limb is subjected. The differences between cub bears and adults in values related to the eccentricity of osteons and Haversian canals and the correlation between these have been interpreted based on the different locomotor behavior.
Authors’ contributions
R.B., M.Z. and A.B. had the original idea and conceived the study. G.C., M.K. and M.V. were responsible for bone collection, M.G. was responsible for preparation of histological sections, R.B. was responsible for photo documentation and quantitative histological analysis, R.B. and A.B. were responsible for qualitative histological analysis of bones, R.B. and M.Z. were responsible for statistical analyses and interpretation of data. All authors read and approved the final manuscript.
Acknowledgements
We thank the National park Mala Fatra, Tatra National Park (TANAP), and Italzver s.r.o. for providing the femoral bones and Fridrich Babos for his excellent technical assistance. Our thanks also go to the Institute of Earth Sciences, for help with the implementation of histological specimens.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amanat S, Mayer J, Paracha H, Ali Z, Granatosky MC. 2020. Bear locomotion. In: Vonk J, Shackelford TK, editors. encyclopedia of animal cognition and behavior, 1–6. Springer Nature Switzerland. DOI:10.1007/978-3-319-47829-6_1707-1
- Brear K, Currey JD, Pond CM. 1990. Ontogenetic changes in the mechanical properties of the femur of the polar bear Ursus maritimus. Journal of Zoology, Lond 222(1):49–58. DOI:10.1111/j.1469-7998.1990.tb04028.x.
- Brits D, Steyn M, L´Abbé EN. 2013. A histomorphological analysis of human and non-human femora. International Journal of Legal Medicine 128(2):369–377. DOI:10.1007/s00414-013-0854-3.
- Brown G. 1996. Great bear almanac. Guilford: The Lyons Press. pp. 340.
- Castanet J, Croci S, Aujard F, Perret M, Cubo J, de Margerie E. 2004. Lines of arrested growth in bone and age estimation in a small primate: Microcebus murinus. Journal of Zoology 263(1):31–39. DOI:10.1017/S0952836904004844.
- Chinsamy-Turan A. 2012. The forerunners of mammals: Radiation, histology, biology. Bloomington: Indiana University Press.
- Chinsamy A, Rubidge BS. 1993. Dicynodont (Therapsida) bone histology: Phylogenetic and physiological implications. Palaeontologia Africana 30:97–102.
- Christiernsson A 2018. Managing strictly protected species with favourable conservation status - The case of the Swedish brown bear (Ursus arctos). Available: http://www.clawsandlaws.eu/documents/bear-report-2018.pdf. Accessed Aug 2021 31.
- Dahle B, Zedrosser A, Swenson JE. 2006. Correlates with body size and mass in yearling brown bears (Ursus arctos). Journal of Zoology 269(3):273–283. DOI:10.1111/j.1469-7998.2006.00127.x.
- Demircioğlu I, Kirbaş Doğan G, Aksünger Karaavci F, Gürbüz I, Demiraslan Y. 2020. Three-dimensional modelling and morphometric investigation of computed tomography images of brown bear’s (Ursus arctos) ossa cruris (Zeugopodium). Folia Morphol 79(4):811–816. DOI:10.5603/FM.a2019.0125.
- Donahue SW, Vaughan MR, Demers LM, Donahue HJ. 2003. Bone formation is not impaired by hibernation (disuse) in black bears Ursus americanus. The Journal of Experimental Biology 206(23):4233–4239. DOI:10.1242/jeb.0067.
- Enlow DH, Brown SO. 1956. Comparative histological study of fossil and recent bone tissues. Part I. Texas Journal of Science 8:405–412.
- Enlow DH, Brown SO. 1958. A comparative histological study of fossil and recent bone tissues. Part III. Texas Journal of Science 10:187–230.
- Foote JS. 1913. A contribution to the comparative histology of the femur. Smithsonian Contrib Knowl 35(3):1–242.
- Frӧbert O, Frøbert AM, Kindberg J, Arnemo JM, Overgaardt MT. 2020. The brown bear as a translational model for sedentary lifestyle-related diseases. Journal of Internal Medicine 287(3):263–270. DOI:10.1111/joim.12983.
- Garshelis D. 2009. Family Ursidae. In: Wilson DE, Mittermeier RA, editors. Handbook of mammals of the world. volume 1. Carnivora. Barcelona, Spain: Lynx Editions. pp. 448–497.
- Giua S, Farina V, Cacchioli A, Ravanetti F, Carcupino M, Novas MM, Zedda M. 2014. Comparative histology of the femur between mouflon (Ovis aries musimon) and sheep (Ovis aries aries). Journal of Biological Research 87(2):74–77. DOI:10.4081/jbr.2014.4743.
- Gladuhsew JM. 1964. Problems of the histological investigation of the bone in forensic medicine. Sudebnomed Exp 7:23–26.
- Graban J, Kiskova J, Pepich P, Rigg R. 2013. Genetic analysis for geographic isolation comparison of brown bears living in the periphery of the western carpathians mountains with bears living in other areas. Open Journal of Genetics 3(3):174–182. DOI:10.4236/ojgen.2013.33020.
- Harvey KB, Donahue SW 2003. Analysis of bone histology, composition, and mechanical properties of black bear tibias in relation to disuse osteoporosis. Proceed. of Summer Bioengineering Conference, June 25-29, Sonesta Beach Resort in Key Biscayne, Florida
- Hillier ML, Bell LS. 2007. Differentiating human bone from animal bone: A review of histological methods. Journal of Forensic Sciences 52(2):249–263. DOI:10.1111/j.1556-4029.2006.00368.x.
- Hinkle DE, Wiersma W, Jurs SG. 2003. Rule of thumb for interpreting the size of a correlation coefficient. Applied statistic for the behavioral sciences. 5th.Boston: Houghton Mifflin:108–110
- Hinrichs J. 2016. Are lines of arrested growth in bone indicative of seasonal metabolic suppression in bears fort collins. Colorado: Colorado State University.
- Horner JR, de Ricqlés A, Padian K. 2000. Long bone histology of the hadrosaurid dinosaur Maiasaura peeblesorum: Growth dynamics and physiology based on an ontogenetic series of skeletal elements. Journal of Vertebrate Paleontology 20(1):115–129.DOI:10.1671/0272-4634(2000)020[0115:LBHOTH2.0.CO;2
- Hulsey BI, Klippel WE, Jantz LM 2009. Metacarpal and metatarsal histology of human and black bears. Abstract. Proceedings of the American Academy of Forensic Sciences. 2009 Annual Meeting, Denver CO. vol. 15, p. 303.
- Hutton JM. 1986. Age determination of living Nile crocodiles from the cortical stratification of bone. Copeia 1986(2):332–341. DOI:10.2307/1444994.
- Image J 2009. U.S. national institutes of health, Bethesda, Maryland, USA, http://rsbweb.nih.gov/ij/. Accessed Feb 2021 16.
- Janská S 2010. Brown bear vehicle collisions in Western Carpathian Mountains, Slovakia. [ Masters thesis]. Royal Veterinary College, University of London. 33 pp.
- Joshi WK 2006. What makes rats perky and dinosaurs tardy? Available online at: https://www.boloji.com/articles/2776/what-makes-rats-perky-and-dinosaurs-tardy. Accessed Apr 2021 29.
- Kaczensky P, Chapron G, von Arx M, Huber D, Andrén H, Linnell J. 2013. Status, management and distribution of large carnivores – Bear, lynx, wolf and wolverine – In Europe. Part 1, LCIE Report 72. DOI:10.13140/RG.2.2.11382.88645.
- Klevezal GA. 1996. Recording structures of mammals. Determination of age and reconstruction of life history. Rotterdam, Brookfield: A. A. Balkema.
- Köhler M, Marín-Moratalla N, Jordana X, Aanes R. 2012. Seasonal bone growth and physiology in endotherms shed light on dinosaur physiology. Nature 487(7407):358–361. DOI:10.1038/nature11264.
- Kolb C, Scheyer TM, Veitschegger K, Forasiepi AM, Amson E, van der Geer AAE, van den Hoek Ostende LW, Hayashi S, Sánchez-Villagra MR. 2015. Mammalian bone palaeohistology: A survey and new data with emphasis on Island forms. Peer J 3:1–44. DOI: 10.7717/peerj.1358.
- Mainland I, Schutkowski H, Thomson AF. 2007. Macro- and micromorphological features of lifestyle differences in pigs and wild boar. Antrophozoologica 42:89–106.
- Marangoni F, Schaefer EF, Cajade R, Tejedo M. 2009. Growth marks formation and chronology of two neotropical anuran species. Journal of Herpetology 43(3):446–450. DOI:10.1670/08-230R1.1.
- Matosiuk M, Smietana W, Czajkowska M, Paule L, Štofik J, Krajmerová D, Bashta AT, Jakimiuk S, Ratkiewicz M. 2019. Genetic differentiation and asymmetric gene flow among Carpathian brown bear (Ursus arctos) populations-Implications for conservation of transboundary populations. Ecology and Evolution 9(3):1501–1511. DOI:10.1002/ece3.4872.
- McGee-Lawrence ME, Wojda SJ, Barlow LN, Drummer TD, Castillo AB. 2009. Grizzly bears (Ursus arctos horribilis) and black bears (Ursus americanus) prevent trabecular bone loss during disuse (hibernation). Bone 45(6):1186–1191. DOI:10.1016/j.bone.2009.08.011.
- McGee ME, Maki AJ, Johnson SE, Nelson OL, Robbins CT, Donahue SW. 2008. Decreased bone turnover with balanced resorption and formation prevent cortical bone loss during disuse (hibernation) in grizzly bears (Ursus arctos horribilis). Bone 42(2):396–404. DOI:10.1016/j.bone.2007.10.010.
- Owerkowicz T, Crompton AW. 1995. Bone of contention in the evolution of endothermy. Journal Verteb Paleontol 15:47A.
- Palombo MR, Zedda M. 2021. The intriguing giant deer from the Bate cave (Crete): Could paleohistological evidence question its taxonomy and nomenclature? Integrative Zoology 17(1):54–77. DOI:10.1111/1749-4877.12533.
- Rämsch R, Zerndt B. 1963. Vergleichende Untersuchungen der Haverrschen Kanäle zwischen Menschen und Haustieren. Arch. Kriminol 131:74–87.
- Ray S, Chinsamy A. 2004. Diictodon feliceps (Therapsida, Dicynodontia): Bone histology, growth, and biomechanics. Journal of Vertebrate Palaeontology 24(1):180–194. DOI:10.1671/1914-14.
- Ray S, Mukherjee D, Bandyopadhyay S. 2009. Growth patterns of fossil vertebrates as deduced from bone microstructure: Case studies from India. Journal of Biosciences 34(5):661–672. DOI:10.1007/s12038-009-0055-x.
- Ricqlés AJ et al. 1991. Comparative microstructure of bone. In Bone 3, Bone Matrix and Bone Specific Products Hall BK. Boca Raton:CRC Press. 1–78.
- Rigg R, Adamec M. 2007. Status, ecology and management of the brown bear (Ursus arctos) in Slovakia. Liptovský Hrádok: Slovak Wildlife Society. pp. 128.
- Rigg R, Finďo S, Wechselberger M, Gorman ML, Sillero-Zubiri C, Macdonald DW. 2011. Mitiganting carnivore-livestock conflict in Europe: Lessons from Slovakia. Oryx 45(2):272–280. DOI:10.1017/S0030605310000074.
- Rogoz A, Sawlowicz Z, Socha P, Stefaniak K. 2010. Mineralization of teeth and bones of the cave bear (Ursus spelaeus) from the Biœnik Cave, Southern Poland. Mineralogia 40(1–4):65–84. DOI:10.2478/v10002-009-0003-2.
- Sander PM, Andrassay P. 2016. Lines of arrested growth and long bone histology in pleistocene large mammals from Germany: What do they tell us about dinosaur physiology? Palaeontographica Abteilung A 277(1–6):143–159. DOI:10.1127/pala/277/2006/143.
- Shine C, Penberthy S, Robbins CT, Nelson OL, McGowan CP. 2015. Grizzly bear (Ursus arctos horribilis) locomotion: Gaits and ground reaction forces. The Journal of Experimental Biology 218(Pt 19):3102. DOI:10.1242/jeb.121806.
- Skedros JG, Knight AN, Clark GC, Crowder CM, Dominguez VM, Qiu S, Mulhern DM, Donahue SW, Busse B, Hulsey BI, Zedda M, Sorenson SM. 2013. Scaling of Haversian canal surface area to secondary osteon bone volume in ribs and limb bones. American Journal of Physical Anthropology 151(2):230–244. DOI:10.1002/ajpa.22270.
- Stoen OG, Ordiz A, Elfstrom M, Hertel AG, Sahlén V, Kindberg J, Swenson JE 2020. Effects of human disturbance on brown bear behavior in bears of the world: Ecology, conservation and management publisher: Cambridge University Press, DOI:10.1017/9781108692571.019
- Swenson JE, Dahle B, Gerstl N, Zedrosser A 2000. Action plan for the conservation of the brown bear in Europe (Ursus arctos). Convention on the conservation of European wildlife and natural habitats (Bern Convention), nature and environment, No. 114, Council of Europe Publishing, Strasbourg. 68 pp.
- Urbanová P, Novotný V. 2005. Distinguishing between human and non-human bones: Histometric method for forensic anthropology. Anthropologie 43:77–85.
- Veitschegger K, Kolb C, Amson E, Scheyer TM, Sànchez-Villagra MR. 2018. Palaeohistology and life history evolution in cave bears, Ursus spelaeus sensu lato. PLoS ONE 13(11):e0206791. DOI:10.1371/journal.pone.0206791.
- Westwood S 1996. Loss of Bone mass with aging and femoral sexual dimorphism in the American black bear (Ursus americanus). [ PhD Thesis]. Provo, UT: Department of Zoology, Brigham Young University.
- Wojda SJ, Weyland DR, Gray SK, McGee-Lawrence ME, Drummer TD, Donahue SW. 2013. Black bears with longer disuse (hibernation) periods have lower femoral osteon population density and greater mineralization and intracortical porosity. Anat Rec (Hoboken) 296(8):1148–1153. DOI:10.1002/ar.22720.
- Zedda M, Babosova R. 2021. Does the osteon morphology depend on the body mass? A scaling study on macroscopic and histomorphometric differences between cow (Bos Taurus) and sheep (Ovis aries). Zoomorphology 140(1):169–181. DOI:10.1007/s00435-021-00516-6.
- Zedda M, Brits D, Giua S, Farina V. 2019. Distinguishing domestic pig femora and tibiae from wild boar through microscopic analyses. Zoomorphology 138(1):159–170. DOI:10.1007/s00435-018-0426-7.
- Zedda M, Lepore G, Biggio GP, Gadau S, Mura E, Farina V. 2015. Morphology, morphometry and spatial distribution of secondary osteons in equine femur. Anatomy Histology Embryology 44(5):328–332. DOI:10.1111/ahe.12141.
- Zedda M, Lepore G, Manca P, Chisu V, Farina V. 2008. Comparative bone histology of adult Horses (Equus caballus) and Cows (Bos Taurus). Anatomy Histology Embryology 37(6):442–445. DOI:10.1111/j.1439-0264.2008.00878.x.
- Zedda M, Palombo MR, Brits D, Carcupino M, Sathe V, Cacchioli A, Farina V. 2017. Differences in femoral morphology between sheep (Ovis aries) and goat (Capra hircus): Macroscopic and microscopic observations. Zoomorphology 136(1):145–158. DOI:10.1007/s00435-016-0329-4.
- Zedda M, Sathé V, Chakraborty P, Palombo MR, Farina V. 2020. A first comparison of bone histomorphometry in extant domestic horses (Equus caballus) and a Pleistocene Indian wild horse (Equus namadicus). Integrative Zoology 15(6):448–460. DOI:10.1111/1749-4877.12444.
