Abstract
Morphology, at both adult and larval stages is crucial for the correct identification of an insect and a better understanding of its biology and behaviour. The lack of morpho-functional information in insects is much more general in the immature stages than in adults, and major insect orders, such as Diptera are no exception. Syrphids (Diptera: Syrphidae) include various genera with aphidophagous larvae playing a key role in the control of pest insects in both natural and agricultural systems. The aphidophagous Sphaerophoria rueppellii (Wiedemann, 1830) is a syrphid widely distributed in the Palearctic Region and it is of commercial importance as a biological control agent against aphid pests. However, little is known about the fine morphology of its immature stages because it was described in 1939, when microscopy did not allow detailed studies of certain morphological features. In this work, stereomicroscope and scanning electron microscopy (SEM) were used to get a deeper and more detailed picture of the immature stage morphology of this syrphid. SEM was used to examine in detail the chaetotaxy of three larval instars, the larva/puparium posterior respiratory process (PRP), and the chorionic structure of the egg. We describe for the first time the egg, first and second larval stages, and also give a complete updated description of the third-stage larva and the puparium. The three larval instars vary from each other, especially in the number of sensillae, PRP form, colour, and body size. The thickness of both the egg chorion and puparium integument were also measured. A possible interpretation of the reasons for the variability in the number of sensillae is discussed. Illustrations and full descriptions are provided for the egg, larva, and puparium of S. rueppellii, including the head skeleton of the third larval stage.
Introduction
Syrphidae, commonly known as syrphids, hoverflies, or flower flies, is a Dipteran family consisting of more than 6000 species distributed around the world (Rotheray & Gilbert Citation2011). The adults feed on pollen and nectar, which they use, respectively, for gonad maturation and for maintaining their vigorous flight activity (Branquart & Hemptine., Citation2000; Almohamad et al. Citation2009; Amorós-Jiménez et al. Citation2012; Van Rijn & Wäckers Citation2016). Since they feed on pollen, they play an important role as pollinators in agriculture and natural areas, especially where other pollinators are absent (Pérez-Bañón et al. Citation2007; Campoy et al. Citation2020; Dunn et al. Citation2020; Pekas et al. Citation2020). Syrphid larvae differ from other fly larvae in having the two posterior breathing spiracles fused together, longitudinal grooves on the prothorax dorsally, and the anus located on the anteroventral margin of the anal segment (Miranda & Rotheray Citation2018).
The immature stages of syrphids show great adaptability, as suggested by their different feeding habits: phytophagous (aerial and subterranean parts of plants), saprophagous (bacteria or vegetal detritus feeders), or predatory (e.g., Rotheray et al. Citation2009; van Zuijen & Nishida Citation2010; Martínez-Falcón et al. Citation2012; Amorós-Jiménez et al. Citation2014; Souba-Dols et al. Citation2020). Over a third of syrphid species worldwide have predatory larvae, mainly feeding on soft-bodied Hemiptera, such as aphids (Aphididae) and psyllids (Psyllidae) (Rojo et al. Citation2003; Láska et al. Citation2006), with an even higher preference towards aphids (Almohamad et al. Citation2009). For this reason, some syrphid species are regarded as natural controllers of certain insects’ populations in nature, but they are also useful in biological and integrated pest control programmes (Rotheray & Gilbert Citation2011; Gómez-Polo et al. Citation2014; Prieto-Ruiz et al. Citation2019; Dunn et al. Citation2020; Irvin et al. Citation2021). One of these syrphid species, Sphaerophoria rueppellii (Wiedmann, 1830), is currently being commercialised as pest control agent (Amorós-Jiménez et al. Citation2012, Citation2014).
Adult stage morphology and behaviour, in general, are well known for syrphids, whilst the immature stages of many species remain unknown (Speight Citation2020). The main reasons for the lack of knowledge of the immature stages are that breeding sites are frequently unknown in nature and/or the difficulty of rearing larvae under laboratory conditions (Rotheray & Gilbert Citation1988, Citation1999). Behaviour studies on immature syrphids are usually conducted to analyse different aspects of their natural history, for example: life cycle, lifespan, feeding habits, abiotic requirements (temperature, light, and humidity) for larval development or pupation, feeding preferences, and prey availability; and these studies are often focused on species with potential as biological control agents (Láska et al. Citation2006; Amorós-Jiménez et al. Citation2012, Citation2014). However, historical descriptions of immature stages of many syrphid species lack detail due to the limitations of the observation techniques of samples in the early 20th century. For example, Bhatia (Citation1939), a classic paper on immature stage descriptions of syrphids, deals with several syrphid species of economic importance today, including S. rueppellii and Episyrphus balteatus (De Geer, 1776). Since the late 20th century, the SEM has been used for the larval description of hoverflies resulting in more detailed and accurate descriptions (Rupp Citation1989; Pérez-Bañón & Marcos García Citation1998; Scarparo et al. Citation2017; López-García et al. Citation2022). Ideally, the larvae of these species of economic importance should be studied with modern techniques (e.g., SEM) for a better understanding of their morphology and function.
The syrphid genus Sphaerophoria Le Peletier & Audinet-Serville, 1828 (Syrphidae: Syrphinae), comprises 72 species with predatory larvae widely distributed in the Holarctic, Afrotropical, Oriental, and Australasian Regions (Evenhuis & Pape Citation2021; Mengual et al. Citation2021). Of the 41 Palaearctic species, 21 occur in Europe (Thompson Citation2019; Speight Citation2020). Sphaerophoria is monophyletic (Mengual et al. Citation2021) and sister to Allograpta, as shown in phylogenetic studies based on larval morphology (Rotheray & Gilbert Citation1999) and molecular evidence (Mengual et al. Citation2008, Citation2021)
Sphaerophoria rueppellii is one of the three Sphaerophoria species with known immature stages (Bhatia Citation1939; Scott Citation1939; Dixon Citation1960: Rotheray Citation1987). The third-stage larva (L3) and puparium of this species has been studied with stereomicroscopic techniques (Bhatia Citation1939). However, some morphological details of the L3/puparium are still unknown or poorly characterised, such as the chaetotaxy. In addition, the egg and the L1/L2 larval stages are undescribed yet Sphaerophoria rueppellii is the most used syrphid as a biological control agent in Mediterranean crops (Hondelmann & Poehling Citation2007; Amorós-Jiménez et al. Citation2014; van Lenteren et al. Citation2020) and a more detailed description of all immature stages is needed. The study of the number and position of body sensillae (chaetotaxy) is especially important because sensillae are the sensitive structures that inform the larva of external conditions, both biotic and abiotic, in such a way that certain larval behaviours can be understood. With the purpose of better understanding the morpho-function of the immature stages of S. rueppellii, we describe in detail the egg, posterior respiratory process (PRP), and the three larval stages (L1, L2 and L3), including the L3 head skeleton and the number and relative positions of the sensillae on each thoracic and abdominal segment of the L3 larva.
Materials and methods
Experimental insects
The eggs, L3, and pupae of S. rueppellii used for the descriptions, were provided by BioNostrum Pest Control Company®. During the study, larvae were fed with the aphid Rhopalosiphum padi (Linnaeus, 1758) on barley plants (Hordeum vulgare L.). To compare with S. rueppellii, five eggs of E. balteaus and five puparia of Sphaerophoria scripta (Linnaeus, 1758) were used.
Preparation, observation, and imaging of the examined material
Larvae and eggs of all studied syrphid species were fixed and preserved in KAAD liquid (70% alcohol of 95%, 14% glacial acetic acid, 8% toluene, and 8% dioxane). The syrphid larvae and puparia material were cleaned on an Ultrasonic bath for 4 and 12 min, respectively. The larvae were submerged in 10% KOH for a period of 7–10 h for a deeper cleaning (Schneeberg et al. Citation2017). The head skeleton was removed from an empty puparium after a 40 min bath in KOH solution and it was examined in glycerine. Samples were brushed to remove any dirt before observation and description.
General features of the head skeleton, L3 and puparium were observed under a Leica M205 C binocular stereomicroscope. The length of the L3 (n = 15) and puparium (n = 20) was made dorsally from the tip of the prothorax to the tip of the PRP. The width and height of the larva/puparium were measured at their maxima, always in the abdomen. For the PRP, we measured the width and length of the spiracular plate and additionally its height in lateral view. Photos were produced as stacks of individual images made with a camera (Leica DFC 450) attached to a binocular stereomicroscope (Leica M205 C). Stacks were made in Leica Application Suite LAS®, v.4.12.0. The larva was drawn from a printed photo.
Scanning electron microscopy (SEM) was used for a more detailed description of the egg, L1, L2, L3 and puparium. The egg (n = 8) and larvae were dehydrated following a procedure slightly modified from Kanturski et al. (Citation2015). They were dehydrated using an ethanol/water series of two baths of 70% for 5 min each, followed by one bath of 80%, 90%, and 96% ethanol for 5 min each, and two baths of absolute ethanol for 5 min each. Dehydrated eggs/larvae were taken to a Leica EM CPD300 critical point dryer. For the eggs, L1 (n = 10) and L2 (n = 10), the length and width were measured. Three eggs were cut in half to measure the chorion thickness. The height and width measurement of the papilliform sensillae with/without setae were taken from the L3 larval stage. The puparium material (n = 5) was dehydrated following Kanturski et al. (Citation2015). Three puparia were cut in half to measure the dorsal and lateral integumental thickness. All the samples were mounted on aluminium stubs with double side adhesive carbon tape and sputter-coated in a Quorum 150 T ES Plus with a 30 nm layer. The samples were imaged with a Hitachi SU8010 FE-SEM microscope at 7 and 10 kV accelerating voltage, except for one egg photo that was done at 15 kV.
All examined material is deposited at the CEUA-CIBIO collection, University of Alicante, Spain.
Morphological terminology
The morphological terminology used for the head skeleton follows Hartley (Citation1963) and Rotheray and Gilbert (Citation2008). The egg of S. rueppellii has not been described before, so we follow the description that Chandler (Citation1968a) made for the eggs of other Sphaerophoria species. The morphological terminology used for the larva follows Láska et al. (Citation2006) and Rotheray (Citation2019). For each body segment, sensillae were numbered in the dorso-ventral direction (Rotheray Citation1991). A superscript (A2 …) is used to indicate in which body segment a sensilla is located (e.g., 1A2 – first sensilla of the second abdominal segment). A compilation of abbreviations for zoological features used in this publication is shown in .
Table I. Abbreviations used for morphological features in Sphaerophoria rueppellii immature stages descriptions
Results
Description of the immature stages of Sphaerophoria rueppellii
Egg
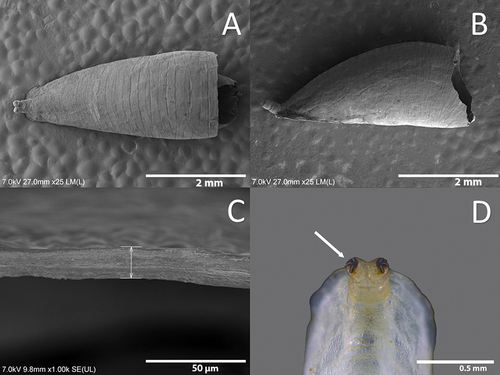
White when recently laid, yellowish darker when older. Oval, with the dorsal tip concave due to the micropyle (). The surface with an opaque pattern (). The chorionic sculpturing consists of irregular hexagonal units slightly raised with lateral branches that contact with other units (). For egg size and thickness see and .
Figure 1. Egg of Sphaerophoria rueppellii and Episyrphus balteatus. A. S. rueppellii, lateral view; B. S. rueppellii, dorsal tip (arrow, micropyle); C. S. rueppellii, chorionic sculpture; D. E. balteatus, dorsal tip; E. E. balteatus, chorionic sculpture.
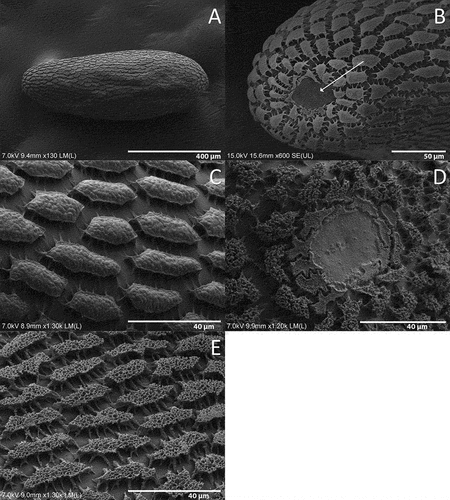
Table II. Numbers of sensilla pairs in each larval instar and body segments of Sphaerophoria rueppellii
Table III. Size of the immature stages and structures
Larva
Shared characters between all three larval instars
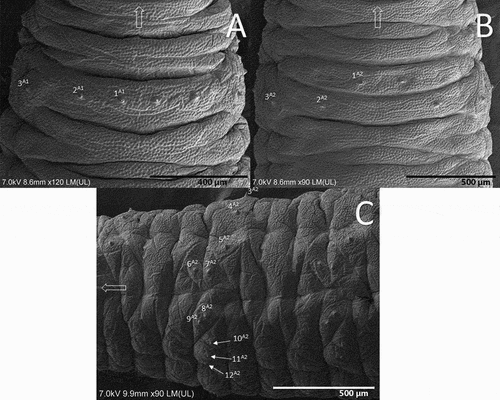
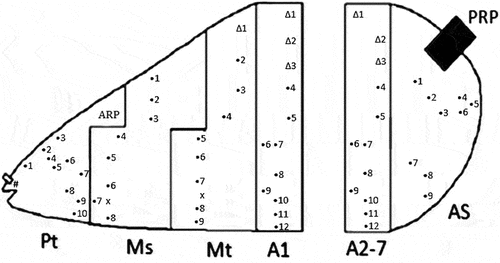
Oval in cross-section, tapering anteriorly and truncate posteriorly (). Mouthparts typical of predatory syrphid larvae adapted to piercing the prey () (Hartley Citation1963). Reduced mouth hooks (). Prothorax and mesothorax are usually retracted inside the metathorax. Prothorax with a smooth dorsal surface with a pair of ARP (). Smooth ventral surface. Seven abdominal segments plus anal segment (= eight abdominal segment) (). Pairs 1, 2, and 3 of papilliform sensillae with setae () located on the same fold on the first abdominal segment ( A); on the second to seventh abdominal segments, pair 1 of sensillae located on the anterior fold and pairs 2 and 3 located on the next fold behind (). PRP apex with a central depression. PRP without dorsal spurs.
Figure 2. Third instar (L3) of a preserved larva of Sphaerophoria rueppellii: A. Dorsal view; B. Lateral view; C. Drawing of the larva in dorsal view with indication of boundaries between abdominal segments. Yellow parts are fatty bodies (A, B).
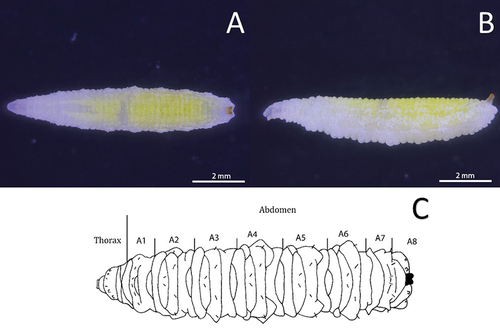
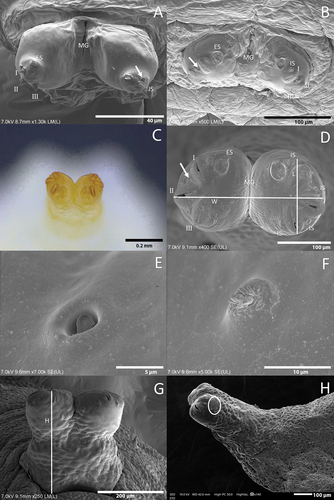
Figure 3. Head skeleton of Sphaerophoria rueppellii. A. Lateral view removed from puparium (LB, labium; LM, labrum; M, mandible; TS, lateral lips); B. Ventro-lateral view, L3 larva (arrow, anterior respiratory process; asterisk, lateral lips).
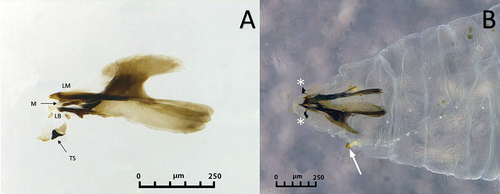
Figure 4. Third instar larva of Sphaerophoria rueppellii. A. Head dorsal surface; B. Head and thorax (arrows, lateral lips); C. Head (arrows, lateral lips; asterisk, antennomaxillary organs); D. Detail of the antennomaxillary organs.
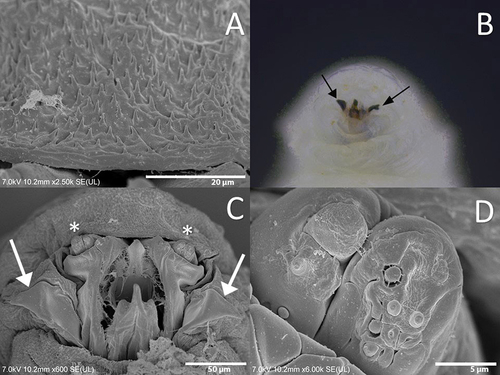
Figure 5. Thorax of the third instar larva of Sphaerophoria rueppellii. A. Dorsal surface of prothorax (Pt); B. Dorsal surface of mesothorax (Ms) (ARP, anterior respiratory process); C. Ventral surface of mesothorax (Ms) (circle, extra sensory organ); D. Dorsal surface of metathorax (Mt). Arrow indicates the head direction.
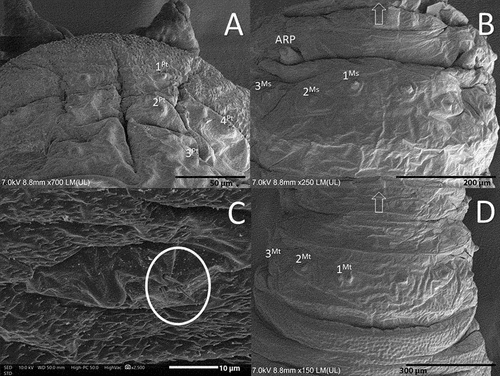
Figure 6. Abdomen of the third instar larva of Sphaerophoria rueppellii. A. Abdominal papilliform sensilla without setae; B. Abdominal papilliform sensilla with setae (BH, base height; BW, base width; SH, seta height; SW, seta width); C. Ventral surface of the second abdominal segment, with sensilla 8 − 12 (circle, locomotory organs) D. Dorsal view of the anal segment (rectangle, locomotory organs). Arrow indicates the head direction.
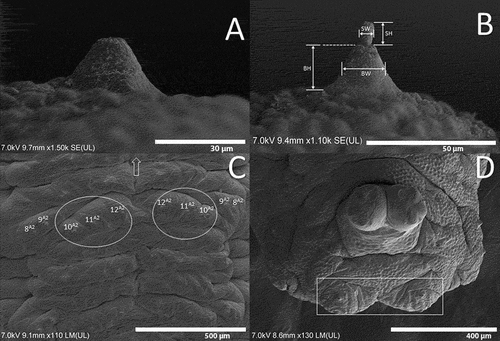
Figure 7. Third instar larva of Sphaerophoria rueppellii showing the number and relative positions of the body sensillae: P, prothorax; Ms, mesothorax; Mt, metathorax; A1, A2-7, abdominal segments; AS, anal segment; ARP, anterior respiratory process; PRP, posterior respiratory process. Symbols: Δ, sensilla with setae; •, sensilla without setae; x, extra sensory organ; #, head sensilla.
L1 larva
Colour: Live larva whitish transparent. Size: See . Thorax: On this instar, the sensillae are not visible or are very difficult to see under a stereomicroscope. Prothorax with 10 pairs of sensilla barely visible at the stereomicroscope. Positioned as follow: four pairs dorsally, three pairs laterally, and three pairs ventrally. Mesothorax with ten pairs of sensillae in the following order: one pair of sensillae with setae dorsally; six pairs of sensillae without setae laterally, and three pairs ventrally. Metathorax with eleven pairs of sensillae arranged in the following way: two pairs of sensillae with setae dorsally, six pairs of sensillae without setae laterally, and three pairs ventrally (). Abdomen: Abdominal segments with 13 pairs of papilliform sensillae and eight pairs in the anal segment (). Dorsally with three pairs of papilliform sensillae with setae, laterally with three pairs of papilliform sensillae with seta, two pairs of papilliform sensillae without setae, and two pairs of ESO. Ventrally with five pairs of papilliform sensilla without setae. The anal segment with eight papilliform sensillae without seta. PRP with an obvious separation between the two spiracular tubes compared to the L3 larva (). PRP: PRP divided by a V-shaped MG as deep as the PRP height. The carinae and the ES are located on an elevated zone on the PRP. A triangular spike appears on the surface of the ES and the carinae. One straight orifice on top of each of the three undeveloped carinae. Under SEM, one IS and one PG are observed ().
Figure 9. Posterior respiratory process (PRP) of Sphaerophoria species: A. S. rueppellii, L1 larva spiracular plated under SEM (I, II and III, carinae; ES, ecdysial scar; arrow, perispiracular gland; IS, short inter-spiracular setae; MG, median groove); B. S. rueppellii, L2 larva spiracular plated; C. S. rueppellii L3, spiracular plated under stereomicroscope; D. S. rueppellii, L3 larva, spiracular plated (W, width; L, length); E. S. rueppellii, perispiracular gland; F. S. rueppellii, short inter-spiracular setae; G. S. rueppellii, measurement of PRP (H, height); H. S. scripta (circle, position of wrinkles).
L2 larva
Colour: Live larva yellowish white. Size: See . Thorax: Prothorax with eleven pairs of sensillae barely visible under a stereomicroscope. Sensilla distributed as follows: four pairs dorsally, four pairs laterally, and three pairs ventrally. Mesothorax with 12 pairs of papilliform sensillae without setae, arranged in the following order: three pairs dorsally, five pairs laterally, and four ventrally. Metathorax with 13 pairs of papilliform sensillae without seta and one pair of ESO, arranged in the following way: three pairs of sensillae dorsally, six pairs of sensillae with an ESO between sensillae 9th and 10th laterally, and ventrally with four pairs (). Abdomen: Abdominal segments with 13 pairs of papilliform sensillae and 10 pairs in the anal segment (). Dorsally with three pairs of papilliform sensillae with setae, five pairs of sensilla without setae laterally, and five pairs ventrally. The anal segment with 10 papilliform sensillae without setae. PRP with an obvious separation between the tubes compared to L3 larva (). PRP: PRP divided by a V-shaped MG as deep as the PRP height. An ES can be present near the first carina. The straight orifices on top of each of the three developed carinae extend to the lateral side of the PRP. Under a stereomicroscope, four sensillae and a gland can be barely observed, in the following distribution: four IS, and one PG ().
L3 larva
Colour: Live larva from yellowish-green to light green, with one white line on either dorsal side (). Size: See . Head: Labrum elongated, sclerotized, and fused in the front to form a sharply pointed end. The labrum is located at the uppermost part of the head skeleton. The labium is also elongated, sclerotized, and with a sharp pointed end, but smaller in size compared to the labrum. Labium can be found under the labrum, and between them, there is a pair of mandibles. Lateral margins of the mouth with a pair of lateral lips (). The surface around the mouthparts has small spines (). Head with a pair of well-developed antennomaxillary organs (). Thorax: Prothorax with 10 pairs of sensillae barely visible at the stereomicroscope (). Positioned as follow: four pairs dorsally, three pairs laterally, and three pairs ventrally. Mesothorax with eight pairs of papilliform sensillae without setae and one pair of ESO arranged as indicated in : three pairs dorsally (), two pairs laterally, and three pairs ventrally; ESO sensillae between sensillae sixth and eighth (). Metathorax with one pair of papilliform sensillae with setae, eight papilliform sensillae without setae, and one pair of ESO sensillae (). Sensilla distributed as follows: one pair of papilliform sensilla with setae and two pairs of papilliform sensillae without setae in the dorsal surface of the metathorax (), three pairs of sensillae papilliform without setae laterally, and ventrally with three pairs of papilliform sensillae without setae; ESO between sensillae seventh and eighth (). Abdomen: Abdominal segments with 12 pairs of papilliform sensillae and anal segment with nine pairs () (). Dorsal side with three pairs of papilliform sensillae with setae. Laterally with four pairs of papilliform sensillae without setae (). The last five pairs of papilliform sensillae without setae are located on the locomotory ventral projections. Two poorly developed crochet-less locomotory projections at each segment (). In the anal segment, the locomotory projections are more prominent than in the other segments, forming a grasping bar (). The anal segment has nine pairs of papilliform sensilla without setae ( D; 7). For the size of the papilliform sensillae, with and without setae see . Posterior respiratory process (PRP): Light brown and shiny (). PRP divided by a V-shaped MG about as deep as 1/4 of the height. PRP with a smooth surface. The straight orifices on top of each of the three developed carinae extend to the lateral side of the PRP (). Each carinae is separated by a 90° angle. An ES can be present near to the first carinae. Under a stereomicroscope four sensillae and a gland can be barely observed, in the following arrangement: one PG and four IS (). Size see ().
Puparium
Teardrop-shaped, with anterior part wider and truncated, and flat ventrally (). Colour varies depending on the stages of development of the pupa, from light green to dark brown shortly before the adult emergence. The position and the size of the sensilla are the same as in the larva. Integumental thickness varies across the body, the dorsal part being the thickest () (). The PRP carinae are darker in colour than the tegument of the larva (). For size see .
Discussion
The third-stage larva of S. rueppellii was described as Sphaerophoria flavicauda (Zetterstedt, 1843) by Bhatia (Citation1939). The same author provided a rough drawing of the puparium of this species, as well as indicating its size and colour. We describe here for the first time the egg, the L1, and L2 larvae, the puparium, and the larval chaetotaxy for all three stages. Moreover, the L3 and puparium are redescribed in detail from SEM pictures and the L3 of S. rueppellii is compared with those of other congeneric species.
We found that the egg of S. rueppellii has a similar chorionic sculpture to that in S. scripta and S. interrupta (Fabricius, 1805) (as S. menthastri in Chandler Citation1968a), as expected from congeneric species. However, the SEM analysis of the egg chorion of S. rueppellii shows micro-sculpture differences between S. scripta and S. interrupta. According to Chandler (Citation1968a), the chorionic sculpturing of S. scripta has a sharp demarcation between its dorsal and ventral parts, whilst in S. interrupta there is a transition zone of at least four rows of polygonal units. In S. rueppellii, no transition/sharp demarcation has been observed between the dorsal and ventral sculpturing on the egg chorion. The egg micro-sculpture of S. rueppellii is also different from that of other syrphines also used as pest control agents (e.g., E. balteatus). The surface of the chorionic sculpturing of E. balteatus is porous () (Chandler Citation1968a), unlike those of S. rueppellii. In addition, the chorionic sculptures around the micropyle of E. balteatus are unbranched (), but in S. rueppellii are branched (). The size of the egg of E. balteatus is larger than in S. scripta, S. interrupta (Chandler Citation1968a) and S. rueppellii. A variable thickness of the egg chorion in syrphids might relate to a syrphid/prey adaptation (Dziock Citation2005). For example, a hardened egg chorion can be devoted to the protection against an attack by aphids’ (Mizuno et al. Citation1997).
The non-telescopic PRP found in Sphaerophoria is shared with all Syrphinae (Hartley Citation1961), indicating that they do not live in aquatic or semi-aquatic conditions, as the ‘long-tailed’ larvae of saprophagous genera such as Eristalis (Rotheray Citation1993). The larvae of the genus Sphaerophoria differ from those of other Syrphinae genera because the PRP is longer than wide and does not have the presence of dorsal spurs (Láska et al. Citation2013), and our results are in accordance with this. These dorsal spurs can be found on species, such as Scaeva selenitica (Meigen, 1822) (Láska et al. Citation2006) or Dioprosopa clavata (Fabricius 1794) (Lillo et al. Citation2021). In Sphaerophoria, the arrangement of the carinae in the spiracular plate differs when compared with that in genera such as Scaeva and Simosyrphus but is similar to Semiscaeva and Dioprosopa (Lillo et al. Citation2021). According to Lillo et al. (Citation2021), the carinae II and III on the genera Scaeva and Simosyrphus are parallel, compared to those in Sphaerophoria that form approximately a 90° angle between them. The numbers of IS and PG found in the Sphaerophoria PRP agree with Rotheray (Citation2019). The function of the PG is to secrete a hydrophobic material that protects the PRP from wetting and inundation (Keilin Citation1944; Rotheray Citation2019).
The identification of Sphaerophoria larvae at the species level is complicated. For example, Scott (Citation1939) stated that S. scripta and S. rueppellii could be separated only by size (length is smaller than 5 mm in S. rueppellii but greater than 5 mm in S. scripta). Nevertheless, the development and size of the larvae are influenced by food and the different developmental stage (L1, L2, and L3) (Rüzička Citation1975). Another difference is the darkness of the pale lines located on the dorsum of the larva (Bhatia Citation1939; Scott Citation1939). However, this character also varies according to the type of prey and amount of food. Other aphidophagous syrphids, such as Scaeva, also have very conspicuous white longitudinal stripes in the body (Láska et al. Citation2006). Rotheray (Citation1987) mentions that the larva of S. interrupta can be distinguished from other Sphaerophoria larvae by the PRP, which is only as long as its basal width. This apparent contradiction between Rotheray (Citation1987) and Láska et al. (Citation2013) is probably due to the fact that Rotheray (Citation1987) measured the width of the PRP basally, but Láska et al. (Citation2013) measured it at the level of the spiracular plate (apically) which is usually somewhat narrower than the PRP basally.
The sister group of Sphaerophoria is Allograpta Osten Sacken, 1875 (Mengual et al. Citation2008, Citation2021). The L3 of Allograpta exotica (Wiedemann, 1830) share various features with that of Sphaerophoria (Rotheray & Gilbert Citation1988, Citation1999; Arcaya Citation2012). However, and according to Arcaya (Citation2012), A. exotica can be distinguished from S. rueppellii by the degree of development of the PRP protuberances where the inter-spiracular setae are inserted. The results obtained in our work differ from those of Arcaya (Citation2012) since these protuberances in S. rueppellii are as developed as those in A. exotica (see figure 33 in Arcaya Citation2012). In addition, we observed a difference in the number of setae with sensillae between these two species, with A. exotica having six pairs and S. rueppellii three pairs. Another difference was found in the total number of sensillae, which is higher in S. rueppellii (120) than in A. exotica (113) ().
After the SEM examination of S. rueppellii larvae, we determined that the number of sensillae is in fact different from the number stated by Bhatia (Citation1939) (). The number of pairs of sensillae in the abdomen and anal segment of S. rueppellii is in accordance with the general number of sensillae found in the Syrphidae (Hartley Citation1961). However, Rotheray and Gilbert (Citation1999) reported up to eleven pairs of sensillae on each abdominal segment in this Diptera family. The number of sensillae found on each segment of the thorax in S. rueppellii is smaller than the number indicated by Hartley (Citation1961) and Rotheray and Gilbert (Citation1999). However, Hartley (Citation1961) mentions that the number of sensillae varies according to the genus. When comparing the number of sensillae with species of other genera such as Platycheirus chalconota (Philippi, 1865), a difference can be observed. Platycheirus chalconota has eleven pairs of sensillae on the prothorax, eight pairs on the mesothorax, nine pairs on the metathorax, eleven pairs in the abdomen, and eight pairs of sensillae on the anal segment (López-García et al. Citation2022). In contrast, S. rueppellii has 10 pairs on the prothorax, eight pairs on the mesothorax, nine pairs on the metathorax, 12 pairs in the abdomen, and nine pairs on the anal segment. This difference can be also observed in other species, such as S. selenitica which has a lower number of sensillae on the abdominal segments compared to S. rueppellii, but similar to P. chalconota (Láska et al. Citation2006; López-García et al. Citation2022).
Our results show that the number of sensillae vary according to larval instar, with L2 having the highest number in total (137), and L3 the lowest in total (120) (). The higher number of sensillae in L2 might be related to the fact that the L2 larva needs to feed and move more than L3 to meet its developmental requirements (Amorós-Jiménez et al. Citation2012). These results agree with Bargen et al. (Citation1998), who mention that the L2 responds better to contact stimuli than to odours. This could be another reason why the L2 has a higher number of mechanoreceptor sensillae. In contrast, the L3 is closer to pupation and its trophic and locomotory requirements may be reduced. However, other studies of predatory syrphids, such as A. exotica (Arcaya et al. Citation2017) or E. balteatus (Putra & Yasuda, Citation2006) found that the L2 eats less, no more than, the L3. When comparing L2 with L1 of S. rueppellii, the L2 still has a higher number of sensilla than L1 (130) (). This might be due to the fact that S. rueppellii is an aphidozoetic species, meaning that females lay eggs in or close to the aphid colony in a plant, and not on an aphid-free plant (Chandler Citation1968b; Dunn et al. Citation2020), resulting in the L1 quickly and easily locating their prey.
The abdomen is the tagma with the highest numbers of sensillae per segment, in all three larval stages, especially in the lateral area (). This might be related to the fact that the lateral margins have the most contact with the surrounding environment, thus helping them to find prey or avoid potential competitors or possible predators and parasitoids. The sensillae located ventrally are frequently covered with a wet and sticky secretion, while the larva is in motion, which gives rise to the formation of a larval track over the plant surface (pers. obs.). This may indicate that the majority of ventral sensillae are mechanoreceptors since this secretion might negatively affect the chemical recognition of these sensillae. The function of the sticky secretion of the larval tracks, is to facilitate the fixation and the mobility of the larva on different substrates, to prevent the arrival of other predators that feed on aphids, or deterrence conspecific oviposition (Almohamad et al. Citation2010a, Citation2010b; Almohamad & Hance Citation2013). Meanwhile, the anal segment is the location with the least sensillae in the three larval stages. A possible interpretation for this is that the sensillae of this last segment are particularly used by the larva to identify appropriate substrate for pupation (generally with low humidity), since this is where the grasping bar is located (Rotheray & Gilbert Citation2011). All these results need to be studied and analysed further for verification.
The presence of the ESO or “micro-sensilla” is poorly described and understood in cyclorrhaphan larvae. For this reason, we were unable to characterise them, their position and scale being the only information to give. In this case, the ESO can be observed on the L3 larva under a stereomicroscope and SEM, but on the L1 and L2 larvae, the SEM photo is necessary for precise identification. Currently, this ESO is not a useful source of taxonomic diagnostic characteristics, although very important functionally (Graham Rotheray, pers. com).
Sphaerophoria rueppellii has a sclerotized head skeleton with distinctive features of predacious syrphid larvae (Hartley Citation1961, Citation1963; Rotheray & Lyszkowski Citation2015; Rotheray Citation2016; Prieto-Ruiz et al. Citation2019; Lillo et al. Citation2021). In this sense, the labrum remains elongated and sharply pointed, and the distal ends of the labial sclerites are fused forming a sharp point in the ventral lip (Hartley, 1962). The mandibles are modified as a pair of lateral stylets with a weak connection with the slightest sclerotized tentorial bars (Hartley, Citation1963). These characters also allow morphological differentiation from the head skeleton of saprophagous syrphids (as in Merodon or in some Eumerus species), which have heavily sclerotized mandibular hooks, a sclerotized tentorial bar, and labrum not prominent (Ricarte et al. Citation2017; Souba-Dols et al. Citation2020).
The larval antennomaxillary organs consist of the ancestral antennae and maxillae, located dorsally in the prothorax (Rotheray & Gilbert Citation2011). When the sensillae register a tactile stimulus, they send a signal to the antennomaxillary organs, causing them to probe to locate the origin of the stimulus. This enables the larva to establish the nature and location of possible prey, once determined, it will then initiate prey capture (Rotheray & Gilbert Citation1999). The saliva that comes out of the mouth keeps the prey stuck to the surface of the prothorax. This will create contact with the antennomaxillary organs that causes the larva to retract the prothorax and mesothorax under the metathorax to protect itself from attacks by the prey and prevent it from escaping (Rotheray & Gilbert Citation1999). It is important to describe the antennomaxillary organs and their sensillae, as it very probably influences the predatory behaviour of each species. The locomotory projections are a very important part of the aphidophagous larvae because they hunt their prey on plants (Rotheray & Gilbert Citation1999). For this reason, the larvae have developed a grasping bar to prevent slippage, a ventral smooth surface that helps create a better attachment to the surface, and an extra fold on the ventral surface for better maneuverability (Rotheray & Gilbert Citation1999). The locomotory projections of S. rueppellii are not so prominent compared (Bhatia Citation1939) to other species, for example, of Scaeva (Láska et al. Citation2006).
The information provided by Bhatia (Citation1939), Scott (Citation1939), and Rotheray (Citation1987) to identify the Sphaerophoria puparia is very vague. According to Scott (Citation1939), S. rueppellii has a characteristic elevation of the posteroventral line, while S. scripta does not have it. This elevation was seen in both species during our study, meaning that the substrate on which it pupates affects this character of the pupa. The only difference in the puparium that we found in our study, was on the surface of the PRP, which is smooth in S. rueppellii but wrinkled in S. scripta (). The puparium of S. interrupta is inflated dorsally, uniformly pale brown, and without markings (Rotheray Citation1987), as in S. rueppellii.
In our study, we show the importance of undertaking a morphological revision of immature stages using SEM to describe all the setae present. This study with the help of the SEM enhances knowledge of the immature stages of the genus Sphaerophoria and takes the first step towards a detailed comparison with other immature stages in the future. This will help to distinguish between other predatory syrphid larvae that are not currently used as biological control agents, as S. rueppellii is the most abundant Sphaerophoria species inside Mediterranean greenhouses (Pineda & Marcos García Citation2008). The identification of Sphaerophoria larvae inside greenhouses will inform decisions on the need to carry out, or not, the release of biological control agents. Overall, there is still a lot of work to be done to be able to develop a taxonomic key to the immature stages of Sphaerophoria. One possible subject for further study is to understand the functioning of the sensillae described in this paper.
Acknowledgements
We thank Brenda Soto and Stephen Hewitt for revising the English.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Almohamad R, Hance T. 2013. Encounters with aphid predators or their residues impede searching and oviposition by the aphid parasitoid Aphidius ervi (Hymeniptera: Aphidiinae). Insect Science 21:181–188. DOI: 10.1111/1744-7917.12037.
- Almohamad R, Verheggen FJ, Francias F, Lognay G, Haubruge E. 2010b. Assessment of oviposition site quality by aphidophagous hoverflies: Reaction to conspecific larvae. Animal Behaviour 79:589–594. DOI: 10.1016/j.anbehav.2009.11.032.
- Almohamad R, Verheggen FJ, Francis F, Haubruge E. 2010a. Intraguild interactions between the predatory hoverfly Episyrphus balteatus (Diptera: Syrphidae) and the Asian ladybird, Harmonia axyridis (Coleoptera: Coccinellidae): Effect of larval tracks. European Journal of Entomology 107:41–45. DOI: 10.14411/eje.2010.004.
- Almohamad R, Verheggen FJ, Haubruge E. 2009. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): A review. Biotechnology, Agronomy and Society and Environment 13(3):467–481.
- Amorós-Jiménez R, Pineda A, Fereres A, Marcos-García MA. 2012. Prey availability and abiotic requirements of immature stages of the aphid predator Sphaerophoria rueppellii. Biological Control 63:17–24. DOI: 10.1016/j.biocontrol.2012.06.001.
- Amorós-Jiménez R, Pineda A, Fereres A, Marcos-García MA. 2014. Feeding preference of the aphidophagous hoverfly Sphaerophoria rueppellii affect the performance of its offspring. BioControl 54:427–435. DOI: 10.1007/s10526-014-9577-8.
- Arcaya E. (2012). Bionomía, diversidad y morfología preimaginal de sírfidos depredadores (Diptera: Syrphidae) en el Estado Lara, Venezuela. Importancia en el control biológico de plagas. Tesis doctoral. Centro Iberomaricano de la Biodiversidad, Instituto Universitario de Investigación, Universidad de Alicante, 79–119.
- Arcaya E, Pérez-Bañón C, Mengual X, Zubcoff-Vallejo JJ, Rojo S. 2017. Life table and predation rates of the syrphid fly Allograpta exotica, a control agent of the cowpea aphid Aphis craccivora. Biological Control 115:74–84. DOI: 10.1016/j.biocontrol.2017.09.009.
- Bargen H, Saudhof K, Poehling HM. 1998. Prey finding by larvae and adult females of Episyrphus balteatus. Entomologia Experimentalis Et Applicata 87:245–254. DOI: 10.1046/j.1570-7458.1998.00328.x.
- Bhatia MD. 1939. Biology, morphology and anatomy of aphidophagous syrphid larvae. Parasitology 31(1):78–120. DOI:10.1017/S0031182000012646.
- Branquart E, Hemptinne JL. 2000. Development of ovaries, allometry of reproductive traits and fecundity of Episyrphus balteatus (Diptera: Syrphidae). European Journal of Entomology 97:165–170. DOI: 10.14411/eje.200.031.
- ampoy A, Sáez L, Pérez-Bañón C, Rojo S. 2020. Demography and population parameters of two species of eristaline flower flies (Diptera, Syrphidae, Eristalini). Journal of Applied Entomology 144:133–143. DOI: 10.1111/jen.12717.
- Chandler AEF. 1968a. A preliminary key to the eggs of some of the commoner aphidophagous Syrphidae (Diptera) occurring in Britain. Transactions of the Royal Entomological Society of London 120(8):199–217. DOI:10.1111/j.1365-2311.1968.tb00354.x.
- Chandler AEF. 1968b. The relationship between aphid infestations and oviposition by aphidophagous Syrphidae (Diptera). Annals of Applied Biology 61:425–434. 10.111/j.1744-7348.1968.tb04544x.
- Dixon TJ. 1960. Key to and descriptions of the third instar larvae of some species of Syrphidae (Diptera) occurring in Britain. Transactions of the Royal Entomological Society of London 112:345–379. DOI: 10.1111/j.1365-2311.1960.tb00491.x.
- Dunn L, Lequerica M, Reid CR, Latty T. 2020. Dual ecosystem service of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agent. Pest Management Science 76:1973–1979. DOI: 10.1002/ps.5807.
- Dziock F. 2005. Evolution of prey specialization in aphidophagous syrphids of the genera Melastoma and Platycheirus (Diptera: Syrphidae) 1. Body size, development and prey traits. European Journal of Entomology 102:413–421. DOI: 10.14411/eje.2005.059.
- Evenhuis NL, Pape T (editors). (2021). Systema Dipterorum, Version 3.4. Available: http://diptera.org/. accessed Nov 2021 23.
- Gómez-Polo P, Traugott M, Alomar O, Castañe C, Rojo S, Agustí N. 2014. Identification of the most common predatory hoverflies of Mediterranean vegetable crops and their parasitism using multiplex PCR. Journal of Pest Science 87:371–378. DOI: 10.1007/s10340-013-0550-6.
- Hartley JC. 1961. A taxonomic account of the larvae of some British syrphidae. Proceedings of the Zoological Society of London 136:505–573. DOI: 10.1111/j.1469-7998.1961.tb05891.x.
- Hartley JC. 1963. The cephalopharyngeal apparatus of syrphid larvae and its relationship to other Diptera. Proceedings of the Zoological Society of London 33:81–87. DOI: 10.1111/j.1469-7998.1963.tb01612.x.
- Hondelmann P, Poehling HM. 2007. Diapause and overwintering of the hoverfly Episyrphus balteatus. Entomologia Experimentalis Et Applicata 124:189–200. DOI: 10.1111/j.1570-7458.2007.00568.x.
- Irvin NA, Pierce C, Hoddle MS. 2021. Evaluating the potential of flowering plants for enhancing predatory hoverflies (Syrphidae) for biological control of Diaphorina citri (Liviidae) in California. Biological Control 157. DOI: 10.1016/j.biocontrol.2021.104574.
- Kanturski M, Karcz J, Wieczirek K. 2015. Morphology of the European species of the aphid genus Eulachnus (Hemiptera: Aphididae: Lachninae) – A SEM comparative and integrative study. Micron 76:23–26. DOI: 10.1016/j.micron.2015.05.004.
- Keilin D. 1944. Respiratory systems and respiratory adaptations in larvae and pupae of Diptera. Parasitology 36(1–2):1–66. DOI:10.1017/S00331182000011975.
- Láska P, Mazánek L, Bičík V. 2013. Key to adults and larvae of the genera of European Syrphinae (Diptera, Syrphidae). Čas. Slez. Muz. Opava (A) 62:193–206. DOI: 10.2478/cszma-2013-0021.
- Láska P, Pérez-Bañón C, Mazánek L, Rojo S, Ståhls G, Marcos-García MA, Bičík V, Dušek J. 2006. Taxonomy of the genera Scaeva, Simosyrphus and Ischiodon (Diptera: Syrphdiae): Descriptions of immature stages and status of taxa. European Journal of Entomology 103:637–655. DOI: 10.14411/eje.2006.085.
- Lillo I, Pérez-Bañón C, Arcaya E, Mengual X, Rojo S. 2021. First data about the preimaginal morphology of Austroscaeva occidentalis (Shannon, 1927) and re-description of larvae and pupae of Dioprosopa clavata (Fabricius, 1794) (Diptera: Syrphidae). Austral Entomology 60(3):535–548. DOI:10.1111/aen.12549.
- López-García GP, Roig-Juñet SA, Pérez-Bañón C, Mazzitelli E, León Montoya A, Rojo S, Mengual X. 2022. Description of the third-stage larva and puparium of Platycherus (Carposcalis) chalconota (Philippi) (Diptera: Syrphidae) with new information about the trophic interactions and larval habitats. Neotropical Entomology 51:81–98. DOI: 10.1007/s13744-021-00908-9.
- Martínez-Falcón AP, Marcos-García MªA, Moreno CE, Rotheray GE. 2012. A critical role for Copestylum larvae (Diptera, Syrphidae) in the decomposition of cactus forests. Journal of Arid Environments 78:41–48. DOI: 10.1016/j.jaridenv.2011.10.010.
- Mengual X, Ståhls G, Rojos S. 2008. Molecular phylogeny of Allograpta (Diptera, Syrphidae) reveals diversity of lineages and non-monophyly of phytophagous taxa. Molecular Phylogenetics and Evolution 49(3):715–727. DOI:10.1016/j.ympev.2008.09.011.
- Mengual X, Ståhls G, Skevington JH. 2021. A life on an Island: The phylogrnetic placement of Loverignana and Afrotropical Sphaerophoria (Diptera: Syrphidae) inferred from molecular characters. Systematics and Biodiversity 19(1):22–53. DOI:10.1080/14772000.2020.1795743.
- Miranda GF, Rotheray G. 2018. Chapter 16.12 – Family Syrphidae. In: Hamada N, Thorp JH, Rogers DC, editors. Thorp and Covich`s Freshwater Invertebrates. 4th ed. UK & USA: Elsevier. pp. 779–783. DOI:10.1016/B978-0-12-804223-6.00041-X.
- Mizuno M, Itioka T, Tatematsu Y, Itô Y. 1997. Food utilization of aphidophagous hoverfly larvae (Diptera: Syrphidae, Chamaemyiidae) on herbaceous plants in an urban habitat. Ecological Research 12:239–248. DOI: 10.1007/BF02529453.
- Pekas A, De Craecker I, Boonen S, Wäckers FL, Moerkens R. 2020. One stone; two birds: Concurrent pest control and pollination services provided by aphidophagous hoverflies. Biological Control 149:104328. DOI: 10.1016/j.biocontrol.2020.104328.
- Pérez-Bañón C, Marcos García MªA. 1998. Life history and description of the immature stages of Eumerus purpurariae (Diptera: Syrphidae) developing in Opuntia maxima. European Journal of Entomology 95:373–382.
- Pérez-Bañón C, Petanidou T, Marcos-García MªA. 2007. Pollination in small Islands by occasional visitors: The case of Daucus carota subsp. Commutatus (Apiaceae) in the Columbretes archipelago, Spain. Plant Ecology 192:133–151. DOI: 10.1007/s11258-006-9233-1.
- Pineda A, Marcos García MªA. 2008. Seasonal abundance of aphidophagous hoverflies (Diptera: Syrphidae) and their population levels in and outside Mediterranean sweet pepper greenhouse. Annals of the Entomological Society of America 101(2):384–391. DOI:10.1603/0013-8746(2008)101[384:SAOAHD]2.0.CO;2.
- Prieto-Ruiz I, Garzo E, Moreno A, Dáder B, Medina P, Viñuela E, Fereres A. 2019. Supplementary UV radiation on eggplants indirectly deter Bemisia tabaci settlement without altering the predatory orientation of their biological control agents Nesidiocoris tenuis and Sphaerophoria rueppellii. Journal of Pest Science 92:1057–1070. DOI: 10.1007/s10340-019-01103-x.
- Putra NS, Yasuda N. 2006. Effects of prey species and its density on larval performance of two species of hoverfly larvae, Episyrphus balteatus de Geer and Eupeodes corollae Fabricius (Diptera: Syrphidae). Applied Entomology and Zoology 41(3):389–397. DOI:10.1303/aez.2006.389.
- Ricarte A, Souba-Dols GJ, Hauser M, Marcos-García MA. 2017. A review of the early stages and host plants of the genera Eumerus and Merodon (Diptera: Syrphidae), with a new data on four species. PLoS ONE 12(12):e0189852. DOI:10.1371/journal.pone.0189852.
- Rojo S, Gilbert F, Marcos-García MA, Nieto JM, Mier MP. 2003. A world review of predatory hoverflies (Diptera, Syrphidae: Syrphinae) and their prey. Alicante, Spain: CIBIO.
- Rotheray GE. 1987. The larvae and puparia of five species of aphidophagous syrphidae (Diptera). Entomologist’s Monthly Magazine 123:121–126.
- Rotheray GE. 1991. Larval stages of 17 rare and poorly known British hoverflies (Diptera: Syrphidae). Journal of Natural History 25:945–969. DOI: 10.1080/00222939100770621.
- Rotheray GE. 1993. Colour guide to hoverfly larvae (Diptera, Syrphidae) in Britain and Europe. Sheffield, England: Drek Whiteley.
- Rotheray GE. 2016. Improving knowledge of the cyclorrhaphan larva (Diptera). Journal of Natural History 50:2169–2198. DOI: 10.1080/00222933.2016.1180434.
- Rotheray GE. 2019. Respiration. In: Rotheray GE, editor. Ecomorphology of Cyclorrhaphan Larvae (Diptera). Vol. 4. Springer, Cham: Zoological Monographs. pp. 123–140. DOI:10.1007/978-3-319-92546_3.
- Rotheray GE, Gilbert F. 1988. The phylogeny and systematics of European predacious Syrphdiae (Diptera) based on larval and puparial stages. Zoological Journal of the Linnean Society 95:29–70. DOI: 10.1111/j.1096-3642.1989.tb02222.x.
- Rotheray GE, Gilbert F. 1999. Phylogeny of Palaearctic Syrphidae (Diptera): Evidence form larval stages. Zoological Journal of the Linnean Society 127:1–112. DOI: 10.1111/j.1096-3642.1999.tb01305.x.
- Rotheray GE, Gilbert F. 2008. Phylogenetic relationships and the larval head of the lower Cyclorrhapha (Diptera). Zoological Journal of the Linnean Society 153(2):287–323. DOI:10.1111/j.1096-3642.2008.00395.x.
- Rotheray GE, Gilbert F. 2011. The natural history of hoverflies. Wales, UK: Forrest text, Ceredigion.
- Rotheray GE, Lyszkowski R. 2015. Diverse mechanisms of feeding and movement in Cyclorrhaphan larvae (Diptera). Journal of Natural History 49:2139–2211. DOI: 10.1080/00222933.2015.1010314.
- Rotheray GE, Marcos-García MA, Hancock GE, Pérez-Bañón C, Maier CT. 2009. Neotropical Copestylum (Diptera, Syrphidae) breeding in Agavaceae and Cactaceae including seven new species. European Journal of Entomology 104(3):531–572. DOI:10.1111/j.1096-3642.2008.00503.x.
- Rupp L. 1989. The mid-European species of Volucella (Diptera, Syrphidae) as commensals and parasitoids in the nests of bees and social wasps: Studies on host finding, larval biology and mimicry. Unpublished PhD Thesis, Albert Ludwigs Universitat, Freiburg, Germany.
- Rüzička Z. 1975. The effects of various aphids as larval prey on the development of Metasyrphus corollae [Dipt.: Syrphidae]. Entomophaga 20(4):393–402. DOI:10.1007/BF02371594.
- Scarparo G, Cerretti P, Mei M, Di Giulio A. 2017. Detailed morphological descriptions of the immature stages of the ant parasite Microdon mutabilis (Diptera: Syrphidae: Microdontinae) and a discussion of its functional morphology, behaviour and host specificity. European Journal of Entomology 114:565–586. DOI: 10.14411/eje.2017.071.
- Schneeberg K, Bauernfeind R, Pohl H. 2017. Comparison of cleaning methods for delicate insect specimens for scanning electron microscopy. Microscopy Research and Technique 1–6. DOI: 10.1002/jemt.22917.
- Scott EI. 1939. An account of the developmental stages of some aphidophagous Syrphidae (Dipt.) and their parasites (Hymenopt.). Annals of Applied Biology 26(3):509–532. DOI:10.1111/j.1744-7348.1939.tb06987.x.
- Souba-Dols GJ, Ricarte A, Hauser M, Speight M, Marcos-García MA. 2020. What do Eumerus Meigen larvae feed on? New immature stages of three species (Diptera: Syrphidae) breeding in different plants. Organisms Diversity & Evolution 20:267–284. DOI: 10.1007/s13127-020-00437-0.
- Speight MCD. 2020. Species accounts of European Syrphidae, 2020. In: Speight MCD, Castella E, Sarthou JP, Vanappelghem C, editors. Syrph the Net, the database of European Syrphidae (Diptera). Vol. 104. Dublin: Syrph the Net Publications, pp. 314.
- Thompson FC. 2019 Syrphidae. Systema Dipterorum, version 2.4, 13,431 records. https://sd.zoobank.org/Nomenclator.
- van Lenteren JC, Alomar O, Ravensberg WJ, Urbaneja A. 2020. Biological control agents for control of pest in greenhouses. In: Lodovica Gullino M, Albajes R, Nicot PC, editors. Integrated pest and disease management in greenhouse crops. Plant pathology in the 21st Century. 2nd ed. Springer. pp. 409–439. DOI: 10.1007/978-3-030-22304-5_14
- Van Rijn PCJ, Wäckers FL. 2016. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. Journal of Applied Ecology 53:925–933. DOI: 10.1111/1365-2664.12605.
- van Zuijen MP, Nishida K. 2010. Life history and immature stages of Allograpta zumbadoi Thompson, a phytophagous flower fly (Diptera: Syrphidae: Syrphinae). Studia Dipterological 17:37–51.