Abstract
The raccoon dog (Nyctereutes procyonoides) is a representative of Canidae and is closely related to red fox-like canids, although the species exhibits high distinctiveness in the family. These animals were farmed for fur products, but thousands were intentionally released into the wild in western Russia. This newly established alien species in Europe has spread rapidly into many European countries. The aim of the study was to determine the origin of Polish populations by comparing the mitochondrial molecular marker cytochrome b (cytb) sequences of Polish wild and farm individuals with wild raccoon dogs from the western Russia and Asian specimens whose sequences were obtained from GenBank resources. The results of our phylogenetic analysis support the previous suggestions on the existence of two main clades clearly referring to continental and Island populations. Polish individuals were classified into a highly diverse continental group. The relationships between the haplotypes within the clade together with estimated values of genetic diversity parameters indicate that the Polish raccoon dog populations exhibit high genetic similarity to the Russian population. In combination with the history of introduction of the species to Europe, this suggests the most probable assignment of the Polish populations to the subspecies N. procyonoides ussuriensis. Our results indicate the presence of two Japanese haplotypes within the continental group, which is inconsistent with the assumed scenario of post-glacial expansion of raccoon dogs excluding gene flow between continental and Island populations.
Introduction
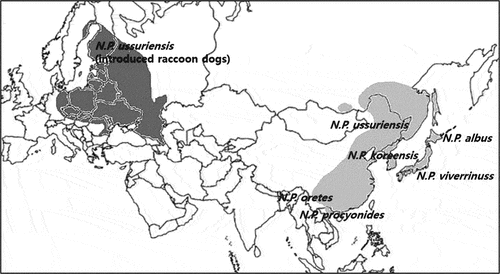
The raccoon dog Nyctereutes procyonoides (Gray 1834) is a newly established alien species with a long and complicated history of introduction and immigration in Europe. The native distribution of this species extends from northern Indochina in the Far East to the southeast corner of Russia, Mongolia, the Japanese Archipelago, East Asia, the Amur-Ussuri region in Russia, northern Vietnam, Korea, China, and Japan (Lavrov Citation1971; Kauhala & Saeki Citation2004). Currently, based on morphological and biogeographical data, six subspecies of N. procyonoides are recognized: N. p. ussuriensis in Russia, northeastern China, and Eurasia, N. p. procyonoides in Vietnam and southern China, N. p. albus and N. p. viverrinus in Japan, N. p. orestes in China, and N. p. koreensis in the Korean Peninsula (Hong et al. Citation2013, Citation2020). The introduction of raccoon dogs in Siberia and the western part of the former Soviet Union lasted from 1929 to 1955 (Kauhala & Saeki Citation2004; Ansorge et al. Citation2009) when the majority of raccoon dogs belonging to the subspecies N. p. ussuriensis were released in several localities from breeding farms and reacclimatized sites (Lavrov Citation1971; Kauhala & Saeki Citation2004; Ansorge et al. Citation2009). After introduction and adaptation in the western Russia, the raccoon dog has expanded into new areas and has spread into many western countries, including Poland (Bobrov et al. Citation2008; Ansorge et al. Citation2009; Drygala et al. Citation2010; Kauhala & Kowalczyk Citation2011). At present, N. p. ussuriensis is also abundant in Finland, Sweden, Estonia, Latvia, Lithuania, Belarus, western Russia, Ukraine, and Germany and has been reported in the Czech Republic, Slovakia, Hungary, Bulgaria, Moldova, Romania, Serbia, France, Switzerland, and Italy (Kauhala & Kowalczyk Citation2011) (). Although the precise origin of the specific introduced populations is unknown, it seems that they originate from populations inhabiting the eastern part of the former Soviet Union (Ansorge et al. Citation2009; Pitra et al. Citation2010; Korablev et al. Citation2011).
Figure 1. Geographic distribution of native (light grey) and introduced (dark grey) populations of the raccoon dog (Nyctereutes procyonoides) according to Hong et al. (Citation2020). With permission to re-publish from Springer Nature.
Most studies of the genetic diversity of raccoon dogs are based on microsatellite analysis (Hong et al. Citation2013, Citation2018; Kasperek et al. Citation2015; Drygala et al. Citation2016; Griciuvienė et al. Citation2016). In terms of the genetic diversity and structure of native populations, two major genetic clusters were found: continental (South Korean, Chinese, Russian, Finnish, and Vietnamese populations) and Island (Japanese populations) (Hong et al. Citation2013, Citation2018). As suggested by Hong et al. (Citation2018), the genetic diversity and geographic structure of raccoon dogs in East Asia have been influenced by natural barriers to gene flow. The Authors investigated the genetic diversity between raccoon dog populations at three levels: Island vs. continental, central vs. marginal within the continental group, and source vs. introduced continental populations. They indicated strong genetic differentiation and lack of gene flow between the continental and Japanese populations, which suggests that the Japanese raccoon dog might be considered as a separate species. Concerning the genetic diversity of the central vs. marginal continental populations, Chinese and Russian populations exhibited higher genetic diversity than the Vietnamese population, but similar levels to that of the Korean population. Both central populations showed lower genetic diversity than the marginal South Korean population. Considering differences between the source and introduced populations of raccoon dogs, it was found that the Finnish population showed values of genetic diversity similar to those of the Russian population, and shared genetic features with raccoon dogs within the species native range in Russian Far East and China. This suggests that the Finnish population originates from the Russian population, but is clearly distinguished from the other continental populations (Hong et al. Citation2018). These findings were also supported by another type of nuclear markers, including four autosomal genes: CHRNA1, VTN, TRSP, WT1 and ZFY. Hong et al. (Citation2020) proved that there was no genetic differentiation among the continental populations. However, significant differences were observed in the VTN and ZFY genes between the continental and Japanese raccoon dogs, implying genetic differentiation between them. In Europe, microsatellite analysis on Lithuanian raccoon dogs indicated high levels of genetic diversity within the population. The clustering analysis revealed four genetic clusters among sampled individuals, without clear separation between subpopulations. Due to the high level of genetic variation observed within the subpopulations and the low level of variation among the subpopulations, these results support the thesis on the migration and gene flow among locations (Griciuvienė et al. Citation2016). The genetic structure of the raccoon dog from north-eastern and central Europe populations also supports the claim that the extent of gene flow during the range expansion of non-native species influences the amount of genetic diversity retained in expanding populations. Three genetic clusters corresponding to Finland, Denmark, and a “central” population were distributed in the introduction areas in western Russia to northern Germany – including Poland. Strong gene flow and admixture between neighboring subpopulations have led to reduced genetic structuring and resulted in a homogenous genetic cluster (Drygala et al. Citation2016). A high level of genetic homogeneity was also found in populations from the western Russia and Polish wild animals. The authors detected clear differences in wild and captive animals through STR panels. The genetic diversity indices place the wild Polish and Russian subpopulations within a common cluster (Kasperek et al. Citation2015).
In few studies, mtDNA polymorphism, and more exactly the control region (Pitra et al. Citation2010; Korablev et al. Citation2011; Paulauskas et al. Citation2016) or the partial cytochrome b sequence (Kim et al. Citation2013), has been examined to estimate the genetic diversity and elucidate the phylogeography of invasive raccoon dogs. The mtDNA control region sequence was used to investigate the distribution of genetic variation within and among the introduced populations of the raccoon dog in Russia, Finland, and Germany (Pitra et al. Citation2010; Korablev et al. Citation2011). Genetic analysis showed a close relationship between the matrilineages of German and Finnish populations and indicated that raccoon dogs had colonized Germany from Finland along the Baltic Sea coastline (Pitra et al. Citation2010). Based on the results reported by Korablev et al. (Citation2011), it was confirmed that raccoon dogs from the introduced sites in northwestern European Russia had spread over the Baltic region through Poland to western Europe (Korablev et al. Citation2011). The patterns of molecular genetic variation in raccoon dogs from Lithuania indicated higher genetic diversity of these animals compared with those from western Europe, but lower genetic polymorphism compared with raccoon dogs introduced in the western Russia. The phylogenetic relationships among haplotypes demonstrated the presence of two major haplogroups within the introduced European populations, resulting from the introduction of raccoon dogs from two spatially isolated autochthonous populations (Paulauskas et al. Citation2016). mtDNA polymorphism in wild and farm raccoon dogs populations from Poland was previously investigated by Ślaska and Grzybowska-Szatkowska (Citation2011). The authors analyzed three mitochondrial markers: COI, COII, and cytb. Seven mitochondrial haplogroups were determined, among which three (Np1, Np2 and Np4) were found in the wild raccoon dogs and the other four (Np3, Np5, Np6 and Np7) were detected in the farm animals. The results showed fixed genetic differences at the mtDNA level between the farm and wild raccoon dogs. The authors assumed that the differences in the occurrence of particular gene haplotypes and mitochondrial haplogroups in the wild and farm raccoon dogs indicate the emergence of adaptive mutations in farm animals.
The identification of source populations and invasion routes is the first key stage in understanding biological invasion, defining the ecological characteristics of introduced populations to predict their spread and potentially direct the focus of conservation strategies. It is also a critical step for defining and testing ecological and evolutionary hypotheses and ultimately understanding the causes of the invasion success. There are sparse historical and observational data on the spread of invasive populations, but even when there is good documentary evidence, molecular genetic data can offer unique insights into the sources, routes, and mechanisms of spread (Lawson Handley et al. Citation2011). Multiple introductions of individuals originating from genetically distinct source populations, e.g. representing different subspecies, determine the heterogeneous genetic structure of an invasive species population, which may lead to their adaptation success in the new habitat (Zalewski et al. Citation2010). Identification of differences between subpopulations and genetic differentiation within a species can help to manage the species in relation to the genetic conservation aspects and its invasive potential. Tracking the dispersal and movement of individuals might also be helpful in understanding the epidemiology of zoonotic diseases and pathogen transmission (Cohen et al. Citation2013; Mullins et al. Citation2014).
The aims of the study were to: (1) determine the genetic diversity between Polish wild and farm raccoon dogs and the Russian wild population as their probable source of origin; (2) analyze phylogenetic relationships between the Polish/Russian populations and other populations with determined phylogeographic origin; (3) confirm the assignment of the Polish population to particular subspecies.
Materials and methods
The research was carried out with the approval of the II Local Ethical Committee for animal experiments in Lublin (Resolution No. 83/2009). The first group of 50 samples (whole peripheral blood collected to EDTA-containing vacuum tubes) was taken from unrelated animals kept on one farm (the relatedness of individuals was determined according to mating history in breeding books). The second group included 30 wild animals of both sexes from an area of south-eastern (SE) Poland. The samples were obtained in cooperation with the Polish Hunting Association. The material (fragments of skin with fur) was immediately stored at −4°C. The third group included 30 wild animals from western Russia, whose dried skins were purchased on an auction. Unfortunately, the exact region of their origin was not known. Conserved biological samples were taken immediately after transfer to the laboratory. DNA extraction was performed using a DNeasy Blood and Tissue Kit (QIAGEN, Hilden) according to the standard manufacturer protocol for blood and animal tissues. The purity and concentration of the isolated DNA was assessed by spectrophotometry (BioPhotometer, Eppendorf) and electrophoretic separation on 1% agarose gel containing ethidium bromide in 1xTBE buffer, with voltage 70 V for 40 minutes. The samples were visualized under UV light and archived using the ScionImage program (https://scion-image.software.informer.com).
The PCR for the investigated fragment of the cytb gene was performed using primers universal for vertebrates L14816: 5’- CCATCCAACATCTCAGCATGATGAAA −3’; H15173: 5’- CCCCTCAGAATGATATTTGTCCTCA −3’, as in Parson et al. (Citation2000). Amplification of the gene fragment (323 bp) was carried out using AmpliTaq Gold DNA Polymerase 360 (Life Technologies, Carlsbad). PCR reactions were performed in a volume of 25 µl. A single sample contained 1 x AmpliTaq Gold 360 buffer, 2.5 mM MgCl2, 1 U of AmpliTaq Gold 360 DNA Polymerase and 0.4 mM of each primer, 0.2 mM of each dNTP (Fermentas, Waltham), and 3 µl of genomic DNA. PCR was performed on a Labcycler (SensoQuest) using the following thermal profile: 10 min at 95°C prior to 35 cycles of 30 s at 95°C, 60 s at 56°C, and 60 s at 72°C, followed by an extension step of 10 min at 72°C.
Spectrophotometric evaluation of the PCR product was performed on the BioPhotometer (Eppendorf). Electrophoresis of PCR products was performed on 2% agarose gel with TBE buffer and ethidium bromide, using the Gene Ruler 100 bp DNA Ladder (Fermentas, Waltham) at a constant voltage of 70 V for 180 min. Sequencing of PCR products was performed using a BigDye®Terminator v 3.1 Cycle Sequencing Kit (Life Technologies, Carlsbad). Sequenced PCR products were purified with a DyeEx 2.0 Spin Kit (QIAGEN, Hilden) using QIAcube (QIAGEN, Hilden). Sequencing was performed on a molecular analyzer 3100-Avant Genetic Analyzer (Applied Biosystems).
Preliminary analysis of the sequencing results was carried out with DNA Baser (DNA Baser Sequence Assembler v. 3) and MEGA 7 (Kumar et al. Citation2016) software. The haplotypes were defined on the basis of SNP changes identified in the sequences of cytb. Sequence data were deposited in GenBank (accession number: MT263666-MT263669). The haplotype diversity (h), nucleotide diversity (π), and pairwise fixation index (ΦST) were calculated using the Arlequin v.3.5 program (Excoffier & Lischer Citation2010). MEGA 7 software was also used to calculate genetic distances (p-distance) within the populations and between the Polish wild and farm and the Russian wild population investigated in this study. P-distance values were also obtained for population pairs from the particular localities (countries) and between the continental and Island clades. The maximum-likelihood (ML) phylogenetic tree was obtained with Mega 7 using a Kimura 2-parameter substitution model with a bootstrap value of 5000. The analyses included additional 52 homologous raccoon dog cytb sequences with subspecies assignment and geographic origin defined at metadata, and grey wolf Canis lupus lupus sequence AM711902 downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) resources was used as the outgroup. The relationships obtained with the distance-based methods were verified using the Bayesian algorithm with MrBayes (Huelsenbeck & Ronquist Citation2001; Ronquist & Huelsenbeck Citation2003). The Bayesian analysis performed with MrBayes used four linked Markov chains per run and two simultaneous runs. Convergence and stationarity of the runs were assessed by searching for plateaus in the time series of parameter values and examining the standard deviation of split frequencies between two runs. The simulations were carried out for 1 × 106 generations, which were logged every 100 generations. The phylogram was presented in the FigTree program (http://tree.bio.ed.ac.uk/software/figtree/), although the relationships were not supported by posterior probability values. Median-joining haplotype networks were calculated using SplitsTree 4.13.1 software (Huson & Bryant Citation2006), given its recognized role in inferring phylogenetic relationships within species (Bandelt et al. Citation1999). Characteristics substitution sites in the haplotype network for the Polish/Russian populations were determined with TCS software (Clement et al. Citation2000).
Results
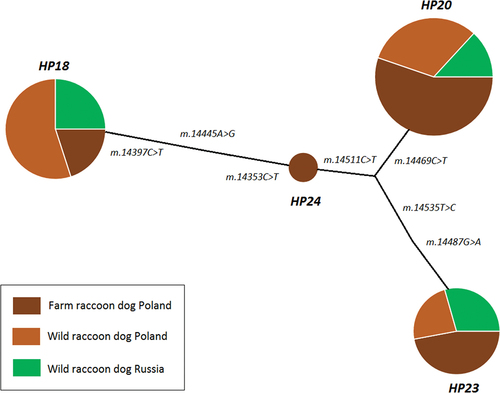
In total, we amplified 110 sequences (30 – wild individuals from the western Russia, 30 – wild animals from Poland, 50 – farm individuals from Poland). Six variable sites were found in the investigated fragment (323 bp) of cytb, and all of them were transitions. Based on the SNP changes, we identified four haplotypes. The values of haplotype diversity (h) and nucleotide diversity (π) indices for the whole sample and for the particular populations are shown in . The highest values of both parameters were estimated for the wild raccoon dogs from the Russian population, although the other two populations were characterized by similar levels of the genetic diversity indices. The pairwise ΦST values showed that both wild populations proved to be genetically homogenous, with pairwise ΦST of 0.00 (exactly −0.026). The value of ΦST between the wild and captive populations from Poland was 0.091. The pairwise ΦST value between the Polish farm individuals and the wild animals from Russia was slightly lower (0.084). All information about the numeration of the particular haplotypes used in this study with their GenBank accession numbers can be found in Supplementary data. The most frequent was haplotype HP20, which was one of the three haplotypes shared between all investigated populations from Poland and Russia. Haplotype HP24 was the least frequent, and it was private for the Polish farm raccoon dogs. It can be seen that the haplotype network () is divided into two clusters: one containing HP18 and the other including HP20, HP23, and HP24. The values of the genetic distances (p-distance) within and between the Polish and Russian populations investigated in this study are shown in . The intrapopulation distances were low, with the lowest value of 0.0064 in the Polish farm population. The genetic distances between particular pairs of populations were similar and ranged from 0.0079 between the farm and wild animals from Poland to 0.0086 between the wild populations from Poland and Russia.
Table I. Haplotype (h) and nucleotide (π) diversity values for the investigated population of wild and farm raccoon dogs (RD) from Poland and Russia; n – number of samples, H – number of haplotypes
Table II. Genetic p-distance values including standard error (SE) within and between Polish farm population and wild raccoon dogs (RD) from Poland and Russia
Figure 2. Median-joining haplotype network representing relationships between the cytb haplotypes of wild and farm raccoon dogs from Poland and Russia; the areas of the circles are proportional to the number of animals sharing each haplotype; the information next to the edges reflects the position and type of substitutions between the haplotypes.
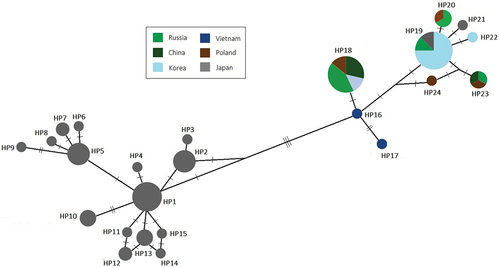
In order to investigate the relationship between the Polish populations and the other introduced and natural populations, we used GenBank sequences of a homologous fragment of cytb with determined information about the geographic origin of the investigated animals listed in Supplementary data. There is a clear division into two groups of haplotypes in the network (). As can be seen, a predominant part of haplotypes characteristic for the individuals from Japan (HP1 – HP15) is grouped together as an Island clade described in previous studies. A separate section of the haplotype network mainly consists of continental haplotypes present in Vietnam, Korea, China, Russia, and Poland. However, two Japanese haplotypes can be found in the continental group: HP19 shared between the raccoon dogs from Korea and Russia and HP21 unique to the individuals from Japan.
Figure 3. Extended median-joining haplotype network representing relationships between the cytb haplotypes of raccoon dogs from Poland and Russia described in this study and homologous sequences of individuals with determined geographical origin obtained from GenBank resources; the areas of the circles are proportional to the number of animals sharing each haplotype; haplotypes observed in the groups are marked in accordance with the legend; the number of lines between haplotypes reflects the number of mutations.
The structure of the haplogroups was also reflected in the ML tree (). Likewise, the haplotype network, the Maximum-Likelihood tree shows clear subdivision into two main clades: a homogenous Island clade containing a majority of the Japanese haplotypes and a more diverse second clade comprising mostly the continental haplotypes from Poland, Russia, China, Vietnam, and Korea with the exception of two haplotypes identified in the population from Japan. It also contains information about the determined assignment of a particular haplotype to one of the six raccoon dog subspecies. The Island clade was shared by N. p. viverrinus and N. p. albus individuals, which typically inhabit Japan, grouped within subclades. The continental clade was an admixture of N. p. procyonoides, N. p. ussuriensis, and N. p. koreensis, but they did not show such clear subdivision as the Japanese haplotypes – different subspecies were combined within common subclades. Interestingly, there were two haplotypes reported previously that were common for Russian N. p. ussuriensis and Chinese N. p. procyonoides (sequence JX099862) and for Korean N. p. koreensis and Russian N. p. ussuriensis (sequence JX099861). The two Japanese haplotypes of N. p. viverrinus present within the continental clade were closely related to the Korean and Russian individuals. The haplotypes of the Polish and Russian animals identified in this study were placed in two subclades within the main continental clade in the ML tree and showed the greatest relatedness with the neighboring Russian and Chinese haplotypes belonging to the subspecies N. p. ussuriensis and N. p. procyonoides. However, the phylogenetic relationships were ambiguous; therefore, the results were compared with the Bayesian tree topology (). The comparison also revealed the existence of the two previously mentioned major clades. The continental clade comprised four haplotypes described in this study. While tracking the topology of the tree, it can be seen that the last common ancestors for HP20 (MT263666 Poland_Russia), HP23 (MT263667 Poland_Russia), and HP24 (MT263669 Poland) were found between the mentioned haplotypes and haplotypes identified in the Russian population and those belonging to the subspecies N. p. ussuriensis. In the case of HP18 (MT263668 Poland_Russia), the closest relatedness was found between the haplotypes described in the Russian population of N. p. ussurienis and in the animals from China and Korea belonging to the subspecies N. p. procyonoides and N. p. koreensis. The results of the Bayesian analysis are consistent with those from the ML method.
Figure 4. Maximum-likelihood (ML) phylogenetic tree based on cytb sequences showing relatedness between the haplotypes of the raccoon dogs from the Polish and Russian population described in this study and homologous sequences of individuals with determined geographical origin and verified subspecies assignment obtained from GenBank; the numbers reported at nodes indicate bootstrap values (only if higher than 50%).
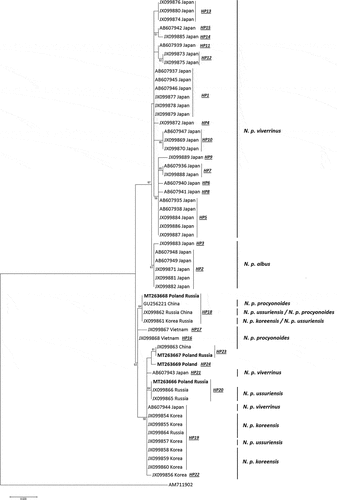
Figure 5. Bayesian phylogenetic tree based on cytb sequences showing relatedness between the haplotypes of the raccoon dogs from Polish and Russian population described in this study and homologous sequences of individuals with determined geographical origin obtained from GenBank; the relationships are not supported by posterior probability values.
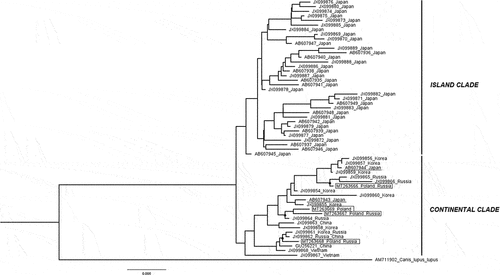
The values of the genetic distances between the populations from the different countries are presented in . The highest p-distance values were identified between the individuals from Japan and the populations from localities representing the continental clade, and they ranged from 0.0237 (Japan – Poland) to 0.0198 (Japan – Vietnam). In the continental populations, the most genetically distant was the population from Vietnam with the values of genetic distances between the other populations in a range from 0.0117 to 0.0101. The genetic distance between the raccoon dogs from Russia and Korea was the lowest and reached a value of 0.0066. The overall genetic distance value between the continental and Island clades was 0.0231 (SE = 0.0077).
Table III. Genetic p-distance values including standard error (SE) between raccoon dog populations originating from different localities belonging to Island and continental clade
Discussion
The raccoon dog, i.e. an invasive alien species, extended its range quickly after introduction, invaded many neighboring sites in Europe, and became the most numerous carnivore in many areas (Jędrzejewska & Jędrzejewski Citation1998; Sidorovich et al. Citation2000; Kauhala et al. Citation2006). The success of the raccoon dog expansion in Europe was dependent on many factors, including its wide distribution resulting from multiple introductions, great migratory ability, high reproductive capacity, plasticity of food habits, high adaptability to different climatic and environmental conditions, and the admixture of individuals from divergent matrilineages (Kauhala et al. Citation2007; Pitra et al. Citation2010; Sutor et al. Citation2010; Kowalczyk & Zalewski Citation2011).
In our study, we found high genetic homogeneity of the wild raccoon dogs from Poland and the western Russia, and the pairwise ΦST values indicated a low level of genetic diversity between the farm animals and both wild populations. Similar relationships between Polish and Russian populations of wild and farm raccoon dogs were previously reported by Kasperek et al. (Citation2015) based on microsatellite markers polymorphism and cluster analysis. The finding concerning the genetic similarity and homogeneity of introduced European populations and individuals from Russia were previously reported based on microsatellite markers (Hong et al. Citation2018) and nuclear genes (Hong et al. Citation2020). However, the panel of STR markers proposed by Kasperek et al. (Citation2015) and the genotype assignment analysis showed clear genetic differences between the wild and farm populations. Differences between Polish wild and farm raccoon dogs regarding mitochondrial haplogroups were described previously (Ślaska & Grzybowska-Szatkowska Citation2011), but similar to the findings of this study, the values of genetic diversity indices between both groups were not as high as those estimated with nuclear markers. Haplogroups determined by Ślaska and Grzybowska-Szatkowska (Citation2011) were based on concatenated haplotypes of cytb, COI, and COII. Seven mitochondrial haplogroups were determined, among which three (Np1, Np2, and Np4) were found in the wild raccoon dogs and the other four (Np3, Np5, Np6 and Np7) were detected in the farm animals. It was concluded that the occurrence of new haplogroups in the farm animals indicates the appearance of adaptive mutations. However, while considering only the cytb haplotypes, it can be seen that two haplotypes (A and B) were shared between the wild and farm populations, and one haplotype (C) was private for the farm animals. This was similar to the results of the present study, where three out of the four identified haplotypes were common for the farm and both wild populations, and a single haplotype was specific for the farm raccoon dogs. A partial sequence of the mitochondrial control region was used to describe the genetic diversity of raccoon dogs introduced in the Upper Volga basin (Korablev et al. Citation2011). The results revealed a high level of molecular genetic variation, e.g. the haplotype diversity value reached 0.95 and was higher than that calculated for the Russian population in our study. However, it should be mentioned that the control region is the most variable region of mtDNA, while the cytb gene sequence is more conserved within species. The presence of two clearly separated haplogroups that were not associated with the spatial geographic structure of the population, probably reflecting differences in the founders of the Russian population, was reported as well (Korablev et al. Citation2011). A similar situation can be observed in our results both in the initial haplotype network composed of haplotypes identified in this study and in the phylogenetic tree. In both cases, the Polish and Russian haplotypes are divided into two groups. This is in agreement with other data supporting the origin of the Polish raccoon dog population from Russian individuals (Lavrov Citation1971; Pitra et al. Citation2010; Kauhala & Kowalczyk Citation2011) and suggests that they are descendants of individuals originating from two genetically distinct founding populations and suggests that they are descendants of individuals originating from two genetically distinct founding populations and currently represent admixture of divergent matrilineages. Pitra et al. (Citation2010) made an assumption that the clades originate from the same large source population.
Genetic studies on East Asian raccoon dog populations based on microsatellite markers (Hong et al. Citation2018), nuclear genes (Hong et al. Citation2020), and the mitochondrial cytb fragment (Kim et al. Citation2013) revealed the presence of two major genetic clusters: continental – including populations from Korea, China, Russia, and Vietnam and Island – including Japanese populations. Strong genetic differentiation and lack of gene flow between continental and Japanese raccoon dogs are proposed, and several other studies suggest that the Japanese raccoon dog should be classified as a separate species due to its distinct morphological characteristics (Kauhala et al. Citation1998; Kim et al. Citation2015), different number of chromosomes (Wada & Imai Citation1991; Wada et al. Citation1991), and geographic isolation. We added to our data homologous cytb sequences from GenBank with determined geographical origin and verified subspecies assignment – according to the metadata. We used all this information to perform phylogenetic analysis based on an extended median joining haplotype network and a ML and Bayesian phylogenetic tree. These three approaches yielded consistent results indicating the presence of two haplogroups/clades representing the continental and Island populations described previously. These finding are also supported while considering the value of the genetic distance. As expected, the haplotypes of the Polish and Russian individuals from this study were placed within the continental haplotypes. Three of them, which were shared between the wild and farm raccoon dog populations, showed close relatedness to the haplotypes detected in the populations from Russia, China, and Korea. Haplotype HP24, which was private for the farm animals from Poland, was not found in any other continental population. This is probably related to selection of animals in breeding farms and association of particular haplotype variants with performance traits in farm raccoon dogs (Ślaska et al. Citation2016; Nisztuk-Pacek et al. Citation2018). The ML phylogenetic tree included information about the assignment of a particular haplotype to specific raccoon dog subspecies. We wanted to confirm the historical data related to the introduction and expansion of the raccoon dog in Europe and the subspecies assignment of the source population. In the case of the Island clade, the split into N. p. viverrinus and N. p. albus was very clear. The continental clade turned out to be much more mixed in terms of phylogeographic origin and subspecies grouping. The clade was an admixture of N. p. procyonoides, N. p. ussuriensis, and N. p. koreensis but they did not show such clear subdivision as the Japanese haplotypes – different subspecies were combined within common subclades. It shows that genetic data do not fully support the morphological and geographical determination of particular subspecies. Similar findings concerning the continental clade were reported in previous phylogeographic studies (Kim et al. Citation2013). It is interesting and should be further analyzed using additional genetic markers. The haplotypes identified in the raccoon dogs from Poland were placed within two subclades in the continental group. This may support the results reported by Paulauskas et al. (Citation2016), where the phylogenetic relationships among haplotypes demonstrated the presence of two major haplogroups within the introduced European populations, resulting from the introduction of raccoon dogs from two spatially isolated native populations. N. p. ussuriensis exhibited a high value of differentiation within the subspecies and, according to the mtDNA-based phylogeny, the European populations from Finland, Estonia, Germany, Poland, and Hungary were represented by individuals belonging to two clades (Pitra et al. Citation2010), which is in agreement with our results.
Besides the main aspects of the study connected with the Polish raccoon dog population, there was an interesting “anomaly” concerning the haplotypes identified in the Japanese raccoon dog population (Murakami & Muratou Citation2015). Many studies mentioned above have indicated that there are many fixed differences between raccoon dog populations living in the continental areas and those inhabiting the Japanese Islands, and there were even some postulates to consider these two groups of Nyctereutes procyonoides as separate species. More interesting is the fact that the two haplotypes downloaded from GenBank (AB607943 and AB607944) identified in the Japanese population, described as N. p. viverrinus and used in this study for phylogenetic analysis, were assigned to the continental group in the extended haplotype network and ML tree. Various hypotheses tested previously were focused on the number of existing glacial refugia and the intensity of gene flow between continental Asian populations and animals from the Japanese Islands (Kim et al. Citation2013). One of the proposed scenarios was that more than two refugia may have existed on the mainland of north-eastern Asia during glaciations. Korean raccoon dogs may have moved to the southern parts of the Korean Peninsula in response to the forest dynamics and formation of refugia, and this population probably underwent postglacial expansion into north-east Asia after peripheral isolation, but some raccoon dogs may have immigrated to the Japanese Islands via a land bridge formed in the Korean Strait. This hypothesis was supported by osteological data (Shikama Citation1949) indicating that Japanese raccoon dogs were osteologically similar to Korean raccoon dogs but distinct from Chinese raccoon dogs. Thus, the extant continental raccoon dog populations originated from more than two refugial populations, whereas the extant Japanese populations originated from the Korean refugial population. However, these findings were not supported in further studies. It is also difficult to evaluate the results, as the controversial haplotypes come from an unpublished study (Murakami & Muratou Citation2015). However, these findings may be considered as an added value of the study and is another question in the complicated history of raccoon dog evolution.
We confirmed the origin of the Polish raccoon dog populations as the descendants of animals released on the territory of the former Soviet Union. The Polish wild raccoon dogs and those inhabiting the western Russia proved to be genetically homogenous. Introduced European populations of Raccoon dogs from different localities, studied by other Authors, also show high genetic homogenity and similarity to individuals from Russia. All those findings support the historical data related to the introduction of Raccoon dog in Europe. Our results were also consistent with previous studies describing Russian populations belonging to two mitochondrial haplogroups. The introduced European populations, including the wild individuals from Poland, also comprise animals originating from these two matrilineages originating from large Russian source populations, as reflected by the results of this study. In general, the raccoon dog populations in Poland are typical representatives of the continental clade of the species. However, the highly mixed structure of continental clade unables the assignment of the representatives of Polish populations to particular subspecies, especially with the use of single marker approach. It is also worth to mention that based on mtDNA cytb marker, Polish farm and wild individuals share majority of haplotypes and genetic differences between them are not clearly stated.
Acknowledgements
The study was funded by the National Centre for Research and Development (NCBiR), development project no. 12-0140-10
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ansorge H, Ranyuk M, Kauhala K, Kowalczyk R, Stier N. 2009. Raccoon dog, Nyctereutes procyonoides, populations in the area of origin and in colonised regions — The epigenetic variability of an immigrant. Annales Zoologici Fennici 46(1):51–62. DOI:10.5735/086.046.0106.
- Bandelt HJ, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution 16(1):37–48. DOI:10.1093/oxfordjournals.molbev.a026036.
- Bobrov VV, Varshavskii AA, Khlyap LA. 2008. Raccoon dog Nyctereutes procyonoides (Gray, 1834). In: Drebuadze JJ, Neronov VM, editors. Alien Mammals in the Ecosystems of Russia. Moscow: KMK Scientific Press Ltd. pp. 111–117. (in Russian).
- Clement M, Posada D, Crandall KA. 2000. TCS: A computer program to estimate gene genealogies. Molecular Ecology 9(10):1657–1659. DOI:10.1046/j.1365-294x.2000.01020.x.
- Cohen TM, King R, Dolev A, Boldo A, Lichter-Peled A, Bar-Gal GK. 2013. Genetic characterization of populations of the golden jackal and the red fox in Israel. Conservation Genetics 14(1):55–63. DOI:10.1007/s10592-012-0423-1.
- Drygala F, Korablev N, Ansorge H, Fickel J, Isomursu M, Elmeros M, Kowalczyk R, Baltrunaite L, Balciauskas L, Saarma U, Schulze C, Borkenhagen P, Frantz AC. 2016. Homogenous population genetic structure of the non-native raccoon dog (Nyctereutes procyonoides) in Europe as a result of rapid population expansion. PLoS One 11(4):e0153098. DOI:10.1371/journal.pone.0153098.
- Drygala F, Zoller H, Stier N, Roth M. 2010. Dispersal of the raccoon dog Nyctereutes procyonoides into a newly invaded area in Central Europe. Wildlife Biology 16(2):150–161. DOI:10.2981/08-076.
- Excoffier L, Lischer HEIDIEL. 2010. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10(3):564–567. DOI:10.1111/j.1755-0998.2010.02847.x.
- Griciuvienė L, Paulauskas A, Radzijevskaja J, Žukauskienė J, Pūraitė I. 2016. Impact of anthropogenic pressure on the formation of population structure and genetic diversity of raccoon dog Nyctereutes procyonoides. Current Zoology 62(5):413–420. DOI:10.1093/cz/zow038.
- Hong Y, Kim KS, Kimura J, Kauhala K, Voloshina I, Goncharuk MS, Yu L, Zhang Y-P, Sashika M, Lee H, Min M-S. 2018. Genetic diversity and population structure of East Asian Raccoon dog (Nyctereutesprocyonoides): Genetic features in central and marginal populations. Zoological Science 35(3):249–259. DOI:10.2108/zs170140.
- Hong YJ, Kim K-S, Lee H, Min M-S. 2013. Population genetic study of the raccoon dog (Nyctereutes procyonoides) in South Korea using newly developed 12 microsatellite markers. Genes & Genetic Systems 88(1):69–76. DOI:10.1266/ggs.88.69.
- Hong Y, Lee H, Kim KS, Min M-S. 2020. Phylogenetic relationships between different raccoon dog (Nyctereutes procyonoides) populations based on four nuclear and Y genes. Genes & Genomics 42(9):1075–1085. DOI:10.1007/s13258-020-00972-2.
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17(8):754–755. DOI:10.1093/bioinformatics/17.8.754.
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23(2):254–267. DOI:10.1093/molbev/msj030.
- Jędrzejewska B, Jędrzejewski W. 1998. Predation in vertebrate communities: The Białowieża Primeval Forest as A Case Study. Vol. 135. Berlin-Heidelberg: Springer-Verlag. pp. 215–219.
- Kasperek K, Horecka B, Jakubczak A, Ślaska B, Gryzińska M, Bugno-Poniewierska M, Piórkowska M, Jeżewska-Witkowska G. 2015. Analysis of genetic variability in farmed and wild populations of Raccoon dog (Nyctereutes Procyonoides) using microsatellite sequences. Annals of Animal Science 15(4):889–901. DOI:10.1515/aoas-2015-0048.
- Kauhala K, Holmala K, Lammers W, Schregel J. 2006. Home ranges and densities of medium-sized carnivores in south-east Finland, with special reference to rabies spread. Acta Theriologica 51(1):1–13. DOI:10.1007/BF03192650.
- Kauhala K, Holmala K, Schregel J. 2007. Seasonal activity patterns and movements of the raccoon dog, a vector of diseases and parasites, in southern Finland. Mammalian Biology 72(6):342–353. DOI:10.1016/j.mambio.2006.10.006.
- Kauhala K, Kowalczyk R. 2011. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: History of colonization, features behind its success, and threats to native fauna. Current Zoology 57(5):584–598. DOI:10.1093/czoolo/57.5.584.
- Kauhala K, Saeki M. 2004. Finnish and Japanese raccoon dogs: On the road to speciation? In: Macdonald DW, Sillero-Zubiri C, editors. Biology and conservation of wild canids, 217–226. Oxford: Oxford University Press.
- Kauhala K, Viranta S, Kishimoto M, Helle E, Obara I. 1998. Skull and tooth morphology of Finnish and Japanese raccoon dogs. Annales Zoologici Fennici 35:1–16.
- Kim S-I, Oshida T, Lee H, Min M-S, Kimura J. 2015. Evolutionary and biogeographical implications of variation in skull morphology of raccoon dogs (Nyctereutes procyonoides, Mammalia: Carnivora). Biological Journal of the Linnean Society 116(4):856–872. DOI:10.1111/bij.12629.
- Kim S, Park S, Lee H, Oshida T, Kimura J, Kim Y, Nguyen S, Sashika M, Min M. 2013. Phylogeography of K orean raccoon dogs: Implications of peripheral isolation of a forest mammal in East Asia. Journal of Zoology 290(3):225–235. DOI:10.1111/jzo.12031.
- Korablev NP, Korablev MP, Rozhnov VV, Korablev PN. 2011. Polymorphism of the mitochondrial DNA control region in the population of raccoon dog (Nyctereutes procyonoides Gray, 1834) introduced into the Upper Volga basin. Russian Journal of Genetics 47(10):1378–1385. DOI:10.1134/S1022795411100103.
- Kowalczyk R, Zalewski A. 2011. Adaptation to cold and predation—shelter use by invasive raccoon dogs Nyctereutes procyonoides in Białowieża Primeval Forest (Poland). European Journal of Wildlife Research 57(1):133–142. DOI:10.1007/s10344-010-0406-9.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870–1874. DOI:10.1093/molbev/msw054.
- Lavrov NP. 1971. Results of raccoon dog introductions in different parts of the Soviet Union. Trudy Kafedry Biologii MGZPI 29:101–160. (In Russian).
- Lawson Handley L-J, Estoup A, Evans DM, Thomas CE, Lombaert E, Facon B, Aebi A, Roy HE. 2011. Ecological genetics of invasive alien species. BioControl 56(4):409–428. DOI:10.1007/s10526-011-9386-2.
- Mullins J, McDevitt AD, Kowalczyk R, Ruczyńska I, Górny M, Wójcik JM. 2014. The influence of habitat structure on genetic differentiation in red fox populations in north-eastern Poland. Acta Theriologica 59(3):367–376. DOI:10.1007/s13364-014-0180-2.
- Murakami M, Muratou Y. 2015. Population genetic structure of Japanese raccoon dogs by mitochondrial DNA polymorphism. Unpublished
- Nisztuk-Pacek S, Śląska B, Zięba G, Rozempolska-Rucińska I. 2018. Two mitochondrial genes are associated with performance traits in farmed raccoon dogs (Nyctereutes procyonoides). Czech Journal of Animal Science 63(No. 3):110–118. DOI:10.17221/2/2017-CJAS.
- Parson W, Pegoraro K, Niederstätter H, Föger M, Steinlechner M. 2000. Species identification by means of the cytochrome b gene. International Journal of Legal Medicine 114(1–2):23–28. DOI: 10.1007/s004140000134. PMID: 11197623.
- Paulauskas A, Griciuvienė L, Radzijevskaja J, Gedminas V. 2016. Genetic characterization of the raccoon dog (Nyctereutes procyonoides), an alien species in the Baltic region. Turkish JOURNAL OF ZOOLOGY 40:933–943. DOI: 10.3906/zoo-1502-34.
- Pitra C, Schwarz S, Fickel J. 2010. Going west-invasion genetics of the alien raccoon dog Nyctereutes procyonoides in Europe. European Journal of Wildlife Research 56(2):117–129. DOI:10.1007/s10344-009-0283-2.
- Ronquist F, Huelsenbeck JP. 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574. DOI:10.1093/bioinformatics/btg180.
- Shikama T. 1949. The Kuzuü, Ossuaries. Geological and palaeontological studies of the limestone fissure deposits, in Kuzuü, Totigi Prefecture. Science Reports of Tohoku University 2(23):1–209.
- Sidorovich VE, Polozov AG, Lauzhiel GO, Krasko DA. 2000. Dietary overlap among generalist carnivores in relation to the impact of the introduced raccoon dog Nyctereutes procyonoides on native predators in northern Belarus. Zeitschrift für Säugetierkunde 65:271–285.
- Ślaska B, Grzybowska-Szatkowska L. 2011. Analysis of the mitochondrial haplogroups of farm and wild-living raccoon dogs in Poland. Mitochondrial DNA 22(4):105–110. DOI:10.3109/19401736.2011.624603.
- Ślaska B, Zięba G, Rozempolska-Rucińska I, Jeżewska-Witkowska G, Nisztuk S, Horecka B, Zoń A. 2016. Mitochondrial DNA haplotypes are associated with performance traits in Raccoon dogs. Animal Science Papers and Reports 34:293–302.
- Sutor A, Kauhala K, Ansorge H. 2010. Diet of the raccoon dog Nyctereutes procyonoides: A canid with an opportunistic foraging strategy. Acta Theriologica 55(2):165–176. DOI:10.4098/j.at.0001-7051.035.2009.
- Wada MY, Imai HT. 1991. On the robertsonian polymorphism found in the Japanese raccoon dog (Nyctereutes procyonoides viverrinus). The Japanese Journal of Genetics 66(1):1–11. DOI:10.1266/jjg.66.1.
- Wada MY, Lim Y, Wurster-Hill DH. 1991. Banded karyotype of a wild-caught male Korean raccoon dog, Nyctereutes procyonoides koreensis. Genome 34(2):302–306. DOI:10.1139/g91-049.
- Zalewski A, Michalska-Parda A, Bartoszewicz M, Kozakiewicz M, Brzeziński M. 2010. Multiple introductions determine the genetic structure of an invasive species population: American mink Neovison vison in Poland. Biological Conservation 143(6):1355–1363. DOI:10.1016/j.biocon.2010.03.009.
