Abstract
Environmental responsibility is becoming part of the social profile of modern society on an international scale. The analysis of ecosystems in the Baltic Sea presents an example of the use and functioning of ecological systems under increased anthropogenic pressure globally. Wintering and feeding swans, i.e. birds wintering in large urban agglomerations, are particularly useful bioindicators of the degrees of environmental pollution. The aim of the study was to assess the element concentrations in the soil of the birds’ habitat and compare these results with metal contents in birds’ feathers and oxidative stress data [diene conjugates (DC) and middle-mass molecules (MM), the activity of superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx), and the total antioxidant status (TAS)] in a wintering population of the mute swan (Cygnus olor) living in northern Poland (southern Baltic Sea). Soil samples collected from bird habitats in Słupsk, Gdynia, and Sopot. These areas differ in the levels of anthropogenic pressure (urban agglomerations, recreational activity, and tourism). The analysis showed significant differences in the Al, Si, Ti, Mn, Fe, Cu, Zn, Zr, Rh, and Ru levels between the soil from Słupsk and both Gdynia and Sopot areas and in the Rh and Ru content between all studied areas. Our results indicated high dependence on the localization, age, and sex of the birds, which were assessed by the level of DC in the blood. The study showed a connection between the lipid peroxidation and antioxidant system functioning, high MM values, and a decreased level of the TAS in adult males from Słupsk, compared to juvenile males from the same area. The functioning of the antioxidant system reflected in the activity of antioxidative enzymes and TAS values was as follows: GR > SOD > CAT > GPx and TAS.
Introduction
Environmental responsibility is becoming part of the social profile of modern society on an international scale. The relationship between global environmental problems and impacts on wildlife proves the trinity of the elements of sustainable development, as confirmed by the worldwide focus of the action program on assessment of the risks of disruption of animal and plant food chains, animal habitats, climate change, overexploitation of wildlife, and pollution. The analysis of ecosystems in the Baltic Sea is an example of the use and functioning of ecological systems under increased anthropogenic pressure (HELCOM Citation2018; Shahabi-Ghahfarokhi et al., Citation2021).
The Baltic Sea, one of the world’s largest bodies of brackish water, is an ecologically unique ecosystem (HELCOM Citation2007). The geographical position of the sea and specific oceanographic characteristics (i.e., shallow waters, low salinity, hindered water exchange with the North Sea, large riverine discharge) cause its high sensitivity. Moreover, with high sensitivity, the catchment area of the Baltic Sea (four times larger than the sea) is densely populated (about 85 million people) and highly industrialized (c.a. 15% of global production). Currently, the Baltic Sea drainage basin covers about 20% of the European continent. Thus, the Baltic Sea is exposed to great anthropogenic pressure. The sea has one of the longest histories of contamination in the world (HELCOM Citation2018). The Baltic Sea has been exposed to extensive use of hazardous substances from the beginning of the industrialization of the region in the late 19th century. The unsustainable settlement in the catchment of the Baltic Sea region has turned this waterbody into one of the most polluted seas and dead zones in the world (HELCOM Citation2018; Shahabi-Ghahfarokhi et al., Citation2021).
One of the pollutant groups is heavy metals, i.e. a toxic class of inorganic pollutants. They can be toxic (even at low concentrations) to humans and animals and are incorporated into the seafood web through water, food, and sediments. Additionally, heavy metals can accumulate in marine food .
Table I. Concentrations of heavy metals in living organisms of the Baltic Sea
The main factors contributing to heavy metal inputs include soil properties, industrial activity, high population density, exploitation of minerals and other natural resources, application of fertilizers in agricultural areas, and atmospheric deposition from local and distant emission sources. The input of heavy metals to the Baltic Sea is mainly waterborne via rivers and atmospheric deposition and via direct point sources. In 2014, the total amounts of heavy metals discharged into the Baltic Sea were 23 t Cd, 4.8 t Hg, and 443 t Pb. In the case of cadmium and lead, the riverine load was about 60% of the total input, whereas atmospheric deposition discharged about 40% of Cd and Pb. More than 70% and about 25% of mercury was discharged via atmospheric deposition and rivers, respectively. In all cases, the direct point sources make the smallest contribution to the total inputs (4% of mercury inputs, <1% of cadmium and lead) (HELCOM Citation2018). The largest load of heavy metals reaches the Baltic Sea from the Polish territory (about 57–98%) (Szefer et al. Citation2009). In 2014, the total amounts of heavy metals discharged from Poland into the Baltic Sea were Cd 23 t/year, Hg 4.8 t/year, and Pb 443 t/year (HELCOM Citation2018).
In the case of the Baltic Sea, bottom sediments (internal supply) can also be a source of heavy metals. In water systems, metals tend to accumulate in sediments, and they may be released with changing conditions in the bottom layer (such as changes in pH, temperature, and oxygen concentration). On the one hand, the geophysical properties of the Baltic Sea sediments (clay materials) favor the accumulation of pollutants, and on the other hand, the deteriorating environmental conditions cause the release of pollutants accumulated over the years from the sediments .
Table II. Concentrations of heavy metals (in µg/g d.w.) in surface sediments from the Gulf of Gdańsk and from the Polish part of the Baltic Sea (Szefer et al. Citation2009)
The biomonitoring of elements in living organisms is carried out all over the world (Karimi et al. Citation2016). Birds are susceptible to environmental changes, which make them particularly important as indicators of environmental contamination, including metal concentrations (Tkachenko & Kurhaluk Citation2012; Kurhaluk et al., Citation2021; Tkachenko et al., Citation2021). The mute swan [Cygnus olor Gmelin (Anatidae)] as a common water bird of lowland freshwaters and coastal shallows is an effective model system of environmental contamination.
In the middle of the 20th century, mute swans wintered in Poland in small numbers and only on the Baltic coast. Gradually, their number and range increased. Currently, the species winters on all available unfrozen water reservoirs in Poland (from the Baltic coast to the south). More than 70% of birds wintering in Poland are native specimens. The number of wintering birds depends on the character of winter and changes in the behavior of birds from Poland and areas located east and north. Many of these birds show strong attachment to permanent wintering grounds, while others change them from year to year and even during the winter (Wieloch & Remisiewicz Citation2001). Large concentrations of swans occur within urban areas, where they readily benefit from feeding by humans.
Mute swans live only in places where the environment is not severely transformed and birds can find rich feeding grounds that can assure their survival. Regional-scale analysis of the causes of changes in the distribution and population size of the species provides much valuable information on the threats to this species. Therefore, mute swans are a good object for indication research, which helps acquire knowledge of the environment and dependencies and shapes an active attitude towards the surrounding environment (Meissner et al. Citation2020). Active participation in protection of the species corroborates the need and effectiveness of human activities in this field. It may be concluded that environmental transformations favorable for the mute swan in Europe coincided with anthropopressure and synanthropization of this species. The changes in the population dynamics, number, fecundity, and mortality were the results of some processes, among which the impact of environmental pollutants and changes in chemical element transfer are the most significant (Grúz et al. Citation2015).
As a result of anthropogenic transformations, the environment changes, which can lead to the development of oxidative stress and affect the redox processes in animals that live and are totally dependent on this environment (Salmón et al. Citation2018). The presence of toxins in the environment and water, atmospheric pollution from exhaust gases, and pesticides are preconditions for the activation of the formation of toxic forms of oxygen or disturbances in the functioning of the antioxidant system in living organisms that inhabit the environment (Mudway et al. Citation2020). The accumulation of pollutants or toxic active substances that interfere with the redox reactions of the body and tissues can have a detrimental effect on macromolecules, cell membranes, and DNA of living organisms (Vizuete et al. Citation2019). The presence of toxic substances changes the biological properties of membranes, enzymes, and receptors, disrupts cell functioning, and leads to cell death and, at the level of the organism, to the development of pathological conditions (Møller et al. Citation2020). Therefore, investigations of oxidative stress biomarkers in long-lived wild animals facilitate detection of such disturbances in anthropogenically modified environments (McCleery et al. Citation2008). This is important given the similarity of the mechanisms of induction of lipid peroxidation and antioxidant defenses at all levels of functioning of living systems that use oxygen for life processes. Some wild swans are known to have an average life span of 12 years in the wild. Much older individuals have also been officially recorded (Johnsgard Citation2016).
Redox or nitrosative stress is the result of accumulation of free radicals or reactive oxygen and nitrogen forms that exceeds the organism’s ability to defend itself (Paithankar et al. Citation2021). In addition to direct damage to biological molecules and tissues, oxidative stress can also activate transcription factors, e.g. nuclear factor-kB (NF- kB), which induces signal cascades, cytokine release, and inflammation (Møller et al. Citation2020). Antioxidants are part of a large group of endogenous enzymes and compounds that protect against oxidative stress by inhibiting the formation of reactive molecules, binding, neutralizing, and removing free radicals, slowing oxidative chain reactions, chelating active metals, and repairing damage to biological molecules. The ability to prevent and cope with oxidative stress depends on the functioning of endogenous and exogenous antioxidant defense systems, the study of which in wild-living organisms under multilevel anthropogenic pressures is an important issue in assessing environmental conditions (Vizuete et al. Citation2019).
In a number of pathological conditions (toxemia, shock, renal failure) and in many diseases, elevated concentrations of middle-mass molecules (MM) are found in the blood plasma (Winchester & Audia Citation2006). These compounds most often play the role of non-specific markers of intoxication (Clark & Winchester Citation2003), which can be detected in the bodies of wild birds functioning in anthropogenically modified environments. It should be noted that the study of this important intoxication indicator in wintering swans is poorly represented in the literature. The literature suggests that MM can cause not only endogenous intoxication syndrome but also microcirculatory disorders, renal disorders, immune response suppression, decreased enzyme activity, impaired transport of sodium and potassium ions through membranes, inhibition of phagocytosis, and hemoglobin synthesis processes (Cobo et al. Citation2018). Undoubtedly, research of such processes is advisable. It has been shown that normally up to 95% of all MM are eliminated by glomerular filtration; hence, the relationship between the level of peroxidation and the accumulation of MM is exacerbated by a decrease in renal function and a reduction of the body’s defense reactions. In addition, MM have immunosuppressive, cytotoxic, neuro- and psychotropic properties, and their overall intoxicating effect is evident (Wolley et al. Citation2018). Therefore, the study of the relationships between accumulation of metals, levels of oxidative stress biomarkers, and intoxication markers in birds of different ages and sexes inhabiting different areas of the Pomeranian region (southern part of the Baltic Sea) contributes to evaluation of the functioning of the environment and humans.
Based on the assumption that ecophysiological responses affecting the status of mute swan populations are based on adaptive mechanisms in the human-altered trophic chain of the population (elevated heavy metal concentrations, disturbed elemental balance), we aimed to assess the levels of chemical elements in the soil of the birds’ habitat and compare these results with metal contents in birds’ feathers and oxidative stress biomarkers (diene conjugates and middle-mass molecules, the activity of superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase, and the total antioxidant status). We also considered whether the location of the habitats of these birds as well as the age and sex of individuals influenced the bioaccumulation of elements.
Materials and methods
The experiments were conducted following the Guidelines of the European Commission and the current laws in Poland and approved by the General Directorate for Environmental protection (Permission DOP-oz. 6401.03.278.2012.km) issued for Natalia Kurhaluk, Halyna Tkachenko, and Maria Wieloch. All animal experiments complied with ARRIVE guidelines and were conducted in accordance with the Animals (Scientific Procedures) Act 1986 and related guidelines, EU Directive 2010/63/EU for animal experiments.
Study areas
The study was conducted in Słupsk and the Gulf of Gdańsk on the southern Baltic coast on the municipal beaches of two neighboring cities of northern Poland, i.e. Sopot and Gdynia .
Figure 1. Study areas. Wintering population of the mute swan (Cygnus olor) in Słupsk, Gdynia, and Sopot (northern Poland).
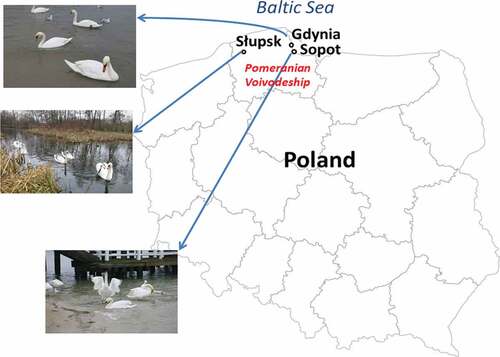
The area of Słupsk encompasses about 43 km2 and about 90 thousand inhabitants. About 50% of the urban area is built-up, and the remaining 50% is covered by woodlands, including communal forests (12%), arable lands, allotment gardens, surface waters (8%), and urban green lands (3%). Słupsk is situated in a Moraine Valley formed in the valley of the meandering Słupia River. The system of the city’s hydrographic network, consisting of four floodplain terraces, constitutes a retention system in the city’s area and is an element of the Słupia basin. In the case of Słupsk, the hydrographic system is of paramount importance in shaping the functional structure of the city. In the city, apart from the almost 12-kilometer stretch of the river, there are over 40 small water reservoirs (Jarosiewicz et al. Citation2014).
The Gulf of Gdańsk is one of the largest bays of the Baltic Sea. The Gulf is delimited by the Hel Peninsula in the north, and its western part consists of shallow waters of the Bay of Puck. To the east of Gdańsk, there are the three main outlets of the River Vistula, the longest river in Poland. The Gulf of Gdańsk exchanges water with the Vistula Lagoon through the Baltiysk Strait. The major ports and coastal cities are Gdańsk, Gdynia, Sopot, Puck, Kaliningrad, and Primorsk. The coastal zone of the Gulf of Gdańsk is urbanized, densely populated, and heavily industrialized. It is also a place of high touristic attractiveness. In the summer season, up to 1 million tourists come to Gdynia’s beaches every year. Thus, the Gulf is exposed to pollution and is regarded as one of the pollution “hot spots” of the Baltic Sea. The River Vistula is the main source of pollutants, including heavy metals. Annually, the riverine heavy metal load discharged into the Gulf of Gdańsk amounts to about 10 tons of cadmium, 90 tons of lead, and almost 1 ton of mercury. The concentration of heavy metals in sediments from the Gulf of Gdańsk exceeds the natural geochemical background and is the highest in the Polish maritime zone (Zaborska et al. Citation2016).
In Słupsk, birds were caught in municipal Park lakes where 12–15 of mute swans stay during their non-breeding period, while the municipal beaches of Sopot and Gdynia were habitats of more than one hundred birds (Meissner et al. Citation2020).
Characteristics of groups
The data were collected between October and November in 2012–2013. Each bird was sampled only once. The mute swans were sexed by cloacal examination (Brown & Brown Citation2002) and their age was assessed based on the plumage characteristics and color of the bill (Baker Citation2016). Since the majority of the birds had been ringed previously as part of other projects, their history, i.e. the date and age at ringing, was known (data provided by the Polish Bird Ringing Centre).
We analyzed two age groups: juvenile birds (before the third year of life) and adults (older than three years), as the mute swan starts to breed not earlier than in the third or fourth year of life (Coleman & Coleman Citation2002). The group in Słupsk included 10 male juveniles, 12 male adults, 10 female juveniles, and 9 female adults. In Gdynia, 12 male juveniles, 18 male adults, 14 female juveniles, and 12 female adults were investigated. The group of birds from Sopot comprised 10 male juveniles, 17 male adults, 14 female juveniles, and 13 female adults. In total, samples of feathers and blood were collected from 151 birds.
All necessary biometric measurements were taken according to a methodology or standard procedures used in bird ringing stations and developed for studies of mute swans (Mathiasson Citation2005). The birds were in good physiological condition, their body mass was 7.32–11.23 kg (females) and 7.92–11.96 kg (males), and no individual exhibited pathological conditions and diseases. A detailed description of the procedures carried out to evaluate the health and condition of the birds under study, their age and sex, as well as blood sampling for further biochemical tests to determine antioxidant status, have already been described by the authors in paper (Kurhaluk & Tkachenko Citation2021).
Feathers and soil samples
Contour feathers (2–3) were taken from the rump without disturbing the birds. The sampled feathers were first washed three times with tap water, splashed with distilled water, and then cleaned in acetone (Battaglia et al. Citation2003) to ensure that there was no external pollutant (Goede & Bruin Citation1984). Next, the samples were dried in an oven for 48 h at 30°C. The feathers were fragmented into small pieces and the samples were weighed in grams and used for analysis.
In Słupsk, 12 soil samples (each sample was analyzed in three replicates) were taken from Lake Park, where the mute swan population lives. In Sopot and Gdynia, 12 soil samples (analysis of each sample was carried out in three replications) were collected in a coastal area near the Baltic Sea where the birds receive food and water (100 m in width, 100 m in length of the coastal zone). The soil samples were collected from topsoil at the depth of 3–5 cm using a soil sampler. In each of the three sites (Słupsk, Sopot, and Gdynia), four sub-sites were established at the perimeter of the area, and three soil samples were collected from each of the four sub-sites. Twelve samples, i.e. one from each site, were transferred into polyethylene bags. All sampled materials were picked up with a plastic shovel and stored in plastic bags to avoid manual contamination. Then, they were taken to the laboratory for further analysis. The samples were air-dried for one week, ground to pass through a 200-mesh sieve, transferred to polyethylene bags, and left until the analysis. Each soil sample was analyzed in three series. Between different reads, the soil sample was thoroughly mixed within the same bag. The results of three reads were averaged.
Determination of element concentrations
The soil samples were collected at a depth of 3–5 cm. Then, the samples were aggregated and air-dried before storage and analysis. Each soil and feather sample were analyzed in three series. Between different reads, the soil sample was thoroughly mixed within the same bag. The results of three reads were averaged.
The concentrations of chemical elements were determined in the feather and soil samples with an X-Ray fluorescence (XRF) analyzer at the Department of Physics, Pomeranian University in Słupsk (Poland). The XRF analyzer (model Sci Sps X-200 from Sci Sps, Inc.) was used for the determination of the concentrations of chemical elements in the samples. The analyzer is designed to study the content of elements in different samples such as soil, alloys, precious metals, and some others.
The XRF (X-Ray Fluorescence) analyzer generates an X-ray beam that can be used for irradiating the sample. The interaction of the X-ray quanta with the analyzed sample causes characteristic X-ray emission from chemical elements present in the sample. The analyses were conducted with a Rh target (50 kV, 600 μA) and polycapillary optics providing a spot size of 25 μm. The X-ray fluorescence signal was collected by two XFlash silicon drift detectors. They provide a high spectral resolution of 135 eV measured on the full width at half maximum, FWHM, at 5.95Mn K-alpha line. The detectors register the spectra of Roentgen fluorescence, or X-ray fluorescence, containing information about the presence of chemical elements and their concentrations. Commonly, the K and L series of X-ray fluorescence is used for identification of chemical elements, as they yield the best results. The detectors have an active area of 30 mm2 placed at 45° to the X-ray beam. The analyses were carried out under vacuum (20 mbar), using a sampling step of 20 μm and 10 ms dwell time. The apparatus is factory-calibrated with 37 standard elements including all measurable pathfinders. The X-ray fluorescence hyperspectral data were processed using PyMca 5.1.3 (Solé et al. Citation2007) and Datamuncher (Alfeld & Janssens Citation2015) software. The device software uses either standard methods such as basic parameters for the spectra of the given elements (the method used in our measurements) or user-generated empirical calibration curves to relate the X-ray spectrum to the element concentrations.
QA/QC for chemical analyses
This instrument was manufactured and calibrated according to SciAps Inc. manufacturing and calibration procedures (Certificate number: 181,227-X200-00847, SciAps, Inc. and Certificate of Registration Nr USA/QMS/00035/8338 of the Quality Management System of SCIAPS, Inc.). The calibration was verified and confirmed by analyzing various certified reference materials produced by Analytical Reference Materials International (ARMI), Rocky Mountain Reference Materials (RMRM), National Institute of Standards and Technology (NIST) and/or other certified reference materials. All used calibration materials are traceable and within their expiration date.
Biochemical analysis
Diene conjugates
The diene conjugate content was determined in plasma using a heptane-isopropanol mixture in an acidic medium (pH 2.0) as described elsewhere (Kamyshnikov Citation2004). After settling and stratification of the mixture in the heptane layer, the content of primary LPO products (diene conjugates) was determined by the degree of absorption of lipid extract in monochromatic light flux in the ultraviolet region of the spectrum (233 nm) and expressed in nmol per mL.
Middle-mass molecules
The concentration of middle-mass molecules in blood serum was determined spectrophotometrically as proposed by Kamyshnikov (Citation2004) and expressed in units of optical density per L (U∙L−1).
Superoxide dismutase activity assay
The Randox kit method (RANSOD, Cat. N SD 125, Randox Laboratories Limited, UK) was used for the determination of superoxide dismutase activity as proposed by Woolliams et al. (Citation1983) and Suttle and McMurray (Citation1983). The principle of the method is as follows: xanthine and xanthine oxidase (XOD) generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride to form a red formazan dye. The results were calculated and presented in U per mL.
Catalase activity assay
Catalase activity was determined with the method proposed Koroliuk et al. (Citation1988) by measuring the decrease in H2O2 in the reaction mixture. One unit of CAT activity was defined as the amount of the enzyme required for decomposition of 1 μmol H2O2 per min per mL.
Glutathione reductase activity assay
The Glut Red Assay Kit (RX Monza, GR 2368, Randox Laboratories Limited, UK) was used for determination of glutathione reductase activity in whole blood. This method of determination of glutathione reductase activity (GR) assay followed the principle adopted in the methodology described by Goldberg and Spooner (Citation1983) and Melissinos et al. (Citation1981) with our modification. The Glut Red Assay method Kit was based on colorimetric measurement of the reduced glutathione level at 340 nm on the Rx Monza analyzer. The initial absorbance results were presented in U per mL.
Glutathione peroxidase activity assay
The activity of glutathione peroxidase (GPx) was measured with the standard method in whole blood using a Ransel Glutathione Peroxidase Assay Kit (RX Monza, RS 504, Randox Laboratories Limited, UK). This method is based on GPx catalysis of the oxidation of glutathione by cumene hydroperoxide. All procedures followed the methodology proposed by Paglia and Valentine (Citation1967) and Kraus and Ganther (Citation1980) with our modification. The decrease in absorbance at 340 nm was measured on the Rx Monza analyzer in a 1 cm cuvette light path at +37°C and presented in U per mL.
Total antioxidant status assay
Total Antioxidant Status (TAS) assay kit was used (Randox, Cat. N NX 2332, Randox Laboratories Limited, UK) for these assays. TAS was approximated using a 2,2’-Azino-di-[3-ethylbenzthiazoline sulphonate] assay. The method was based on the absorbance of the ABTS+ radical cation, as proposed by Miller et al. (Citation1993). The assay principle adopted in this methodology consisted in incubation of ABTS (2,2’-Azino-di-[3-ethylbenzthiazoline sulphonate]) with peroxidase (metmyoglobin) and H2O2 to produce the radical cation ABTS+. The results were calculated and presented in μmol per ml. Steady flow chart presented in .
Statistical analysis
The basic statistical analysis (significance of regression slopes, analysis of variance for significance) was carried out using the STATISTICA 13.3 package (TIBCO Software Inc.). The data were tested for homogeneity of variance using Levene’s test of equality of error variances. Normality was checked with the use of the Kolmogorov-Smirnov test.
The results were expressed as mean ± S.D. Significant differences among the means were measured using a multiple range test at min. P < 0.05. Data not having a normal distribution were log-transformed. Student t-tests with 95% confidence intervals (α = 0.05) were applied to determine the significance of differences between element concentrations in the types of regions and the significance of differences in the element level in the soil, feathers of from the different regions and oxidative stress data. The arithmetic means of the concentrations of elements were estimated using three-way ANOVA. The use of multivariate significance tests of the main effects (type of the environment, age, sex, and their combined effects) allowed the determination of statistically significant relationships for all three values.
We used the coefficients of multiple correlation analysis (R), the coefficient of determination (R2), and its corrected form reduced by random errors (R2 adjusted) in the data analysis for the description of the full model. We used the SS test to describe the share of all analyzed parameters and significance was assessed with the F test (Stanisz Citation2006, 2007).
Results
Metals in soils
As shown in , basic statistical analysis (analysis of variance for significance between the localities) of the metal content in the soils in the areas studied (Słupsk, Gdynia, and Sopot) different significantly, with statistical significance (p = 0.000) for the following metals: Al, Si, Ti, Mn, Fe, Cu, Zn, Zr, Rh, and Ru. This allows classification of these localities as areas with different levels of contamination.
Table III. Analysis of variance for significance of the concentrations of metals in soil samples collected from the different areas (Słupsk, Gdynia, and Sopot) of the Pomeranian region, northern Poland
In the soil samples (g/kg) from Słupsk, compared to the values in Gdynia and Sopot, the levels of Al, Si, Ti, Mn, Fe, Cu, and Zn were statistically significantly different (p = 0.000). The results of the analysis are shown in . The levels of Rh and Ru differed statistically significantly in the three studied areas. The level of Zr was statistically significantly different only in the samples from Gdynia and Sopot. It was noted that our study does not confirm the differences in the Ni, Pb, and Pd levels. Therefore, the analysis of metal contents in the soil from the mute swan habitats showed different results. Therefore, we suggested that there were alterations in the bioaccumulation of the metals in the organism of birds caused by migration processes.
Table IV. Mean concentrations of elements (mg/kg) ± standard deviations in soil samples collected from the Słupsk, Sopot and Gdynia (Pomeranian region, northern Poland) habitat of the mute swan populations
Metals in feathers
Based on the hypothesis that the analyzed wintering population of the mute swan can change under the influence of such main factors as the habitat, age, and sex, we determined metal concentrations based on the analysis of feathers of these birds. The use of multivariate significance tests of the main effects (type of environment, age, sex, and their combined effects) helped to determine statistically significant relationships for all three values .
Table V. Multivariate significance tests and effective hypothesis decomposition for the element contents in the feathers of mute swans of different ages and sex collected from three different habitats in the Pomeranian region, northern Poland
MANOVA with sigma-restricted parameterization of the effective hypothesis decomposition performed with the three-way method was used for the analysis of the metal contents in the feathers in the wintering mute swan population. Our data indicated the highest significant dependencies of such main effects as the environment (F = 44.73, p = 0.000) and its combination with the other main factors in the statistical model, i.e. age and sex (F = 32.20, p = 0.000 and F = 23.12, p = 0.000, respectively). In decreasing order, the influence of the other main factors according to our statistical model is as follows: age (F = 28.0, p = 0.000) and sex (F = 25.96, p = 0.000), sex and age, and habitat and sex. Thus, the influence of the habitat on the distribution of metals in the feathers of birds is the most pronounced, although the age and sex of birds are equally important. Therefore, we divided our next studies on the metal content in the feathers into these three main groups of dependencies according to the results of this statistical analysis (Stanisz Citation2006, 2007).
Habitats
The next stage of our research was to determine the metal levels in the feathers of birds living in different areas of Pomeranian regions. The contents of the elements in mute swan feathers were ambiguous. The analysis of variance (ANOVA) performed to assess the main effect of the habitat on the content of elements in the feathers of the mute swan from Słupsk, Gdynia, and Sopot revealed statistically significant relationships only in the case of Al (F = 4.81, p = 0.000). The results of the variance analysis are shown in .
Table VI. Analysis of variance of the impact of the main effects on the element contents in the feathers of mute swans living in the different localizations (Słupsk, Gdynia, and Sopot)
Age
We found a statistically significant effect of age on the bioaccumulation of certain metals in the feathers from the different-aged birds. As shown in , the metal content in the feathers in the juvenils (below 3-year-old) was statistically significant for Al (F = 3.83, p = 0.004) and Ru (F = 2.62, p = 0.030). In the group of adult birds (over 3-year-old), the trend was maintained for Al and was significant for Cu as well. Thus, our data show age-specific accumulation of Al, regardless of the age of the birds, which may show differences in the accumulation of the metals depending on the habitat and during the development of birds.
Table VII. Analysis of variance of the impact of the main effects on the element contents in mute swans of different ages (juveniles and adults)
Sex
To verify the hypothesis of the impact of sex on the accumulation of metals in the feathers of mute swans, we decided to evaluate these assumptions by analysis of variance of the impact of the main effects on the accumulation of metals in the feathers of male and female mute swans. These dependencies are shown in . The results of the MANOVA test presented in this table confirmed the influence of the selected metals on the sex of the birds. Statistically significant effects were observed in the case of Cu (F = 2.43, p = 0.042) in the male mute swans and in the case of (F = 5.78, p = 0.000) and Ru (F = 2.58, p = 0.033) in the female group Thus, the data presented for the three factors in the statistical analysis, i.e. the locations, age, and sex of the mute swans, for all the three separately presented forms of influence showed a predominance of accumulation of such metals as Al and Ru.
Table VIII. Analysis of variance of the impact of the main effects on the element contents in mute swans of different sex (males and females)
Interdependencies between the habitat, age, and sex
The mean concentrations of elements (mg/kg) in swan’s feathers collected from the cities along the metal pollution gradient using the three main factors: habitats, age, and sex simultaneously are presented in . The birds in Słupsk differed in the Al content, while different Ru values were determined in the group from Gdynia. The birds in Sopot differed statistically significantly in the Ru and Cu levels.
Table IX. Mean concentrations of elements (mg/kg) ± standard deviations in the feathers of mute swans of different ages (Juveniles, n = 10; adults, n = 12) and sex (males, n = 10, females, n = 9) living in Sopot, Gdynia and Słupsk (Pomeranian region, northern Poland)
We used the SS test in three-way ANOVA for the whole model vs. residual SS of the metal level in the birds’ feathers. The multiple correlation coefficient R, the coefficient of determination R2, and adjusted R2 were used for the analysis of all independent processes in the holistic model. These tests allowed us to formulate the following conclusions on the role of each element analyzed in an integral model. The whole model showed the following dependencies: Al – 26.6%, Rh – 2.3%, Cu – 6.2%, Ru – 3.4%, and Fe – 2.3%. Thus, the levels of Al, Cu, and Ru in birds indicated the effective accumulation of the metals in the wintering population of the mute swan living in northern Poland.
Biochemical analysis
Lipid peroxidation
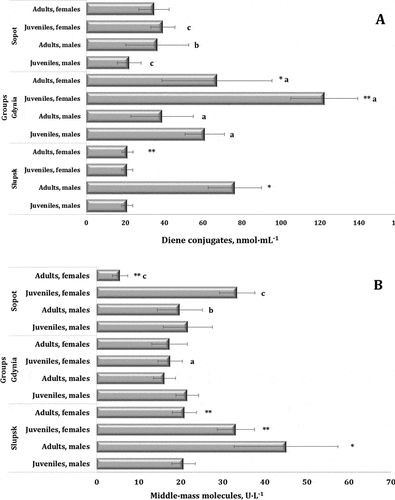
The primary products of peroxide oxidation of lipids (LPO) are lipid hydroperoxides consisting of diene conjugates (DC) accounting for a certain percentage, and these data are shown in . The LPO process develops further via a branched-out mechanism. The statistically significant dependencies in the MANOVA variance analysis regarding the age, sex, and habitat of the swans according to the level of diene conjugates shown in our study confirmed this concept (F11.139 = 61.99, p = 0.000). It should be noted that the level of diene conjugates differed significantly depending on the age in the same habitat, which can be seen in the juvenile and adult male swans, and between the male and female birds from Słupsk. We also confirmed the trend of different values of diene conjugates depending on the age and sex in the birds from the Gdynia, where we observed the highest levels of DC among the three investigated regions (60.86 ± 10.06 nmol∙mL−1 for juvenile males and 122.27 ± 17.16 nmol∙mL−1 for juvenile females). This may indicate pressure on the antioxidant defense system in young developing organisms in anthropopressure conditions, as no similar changes were found in the same groups in the other regions.
Figure 3. Levels of diene conjugates (A, nmol∙mL−1) and middle-mass molecules (B, U∙L−1) in the blood of mute swans of different ages and sexes inhabiting Słupsk, Sopot, and Gdynia (northern Poland).
Middle-mass molecules (MM)
It should be noted that the level of MM also differed significantly between the swans relative to the sex, age, and habitat. This is confirmed by the results of the MANOVA variance analysis (F11.139 = 50.80, p = 0.000) for the studied parameters presented in . We showed a maximum level of MM in the birds from Słupsk (45.08 ± 12.34 U∙L−1) and a minimum value in the adult females from Sopot (5.60 ± 1.79 U∙L−1).
Antioxidant enzymes
SOD activity is the most frequently used informative and specific test characterizing the intensity of superoxide anion radical generation and deactivation in tissues. We investigated the activity of this enzyme for the three investigated localisations depending on the sex and age of the birds. We showed a statistically significant difference in the level of SOD activity by the ANOVA variance test (F11.139 = 14.73, p = 0.000). SOD activity is shown in . We showed a maximum level of SOD activity in the adult males from Słupsk (793.15 ± 178.05 U∙mL−1) and a minimum value in the adult females from Sopot (318.22 ± 79.58 U∙mL−1).
Figure 4. Activities of superoxide dismutase (A, U∙mL−1) and catalase (B, μmol H2O2∙min−1∙mL−1) in the blood of mute swans of different ages and sexes inhabiting Słupsk, Gdynia, and Sopot (northern Poland).
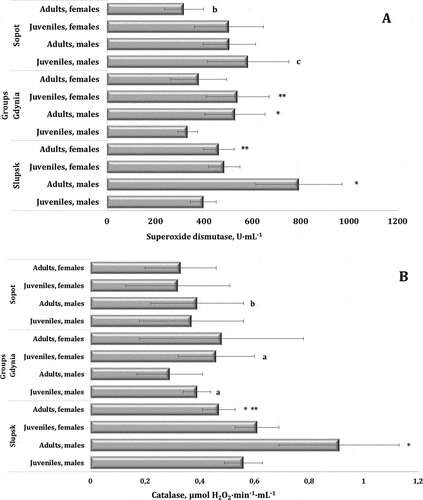
SOD catalyzes the superoxide anion radical dismutation reaction. The reaction produces hydrogen peroxide, which can inactivate SOD; therefore, the enzyme always “works” together with catalase, which quickly and effectively deactivates hydrogen peroxide to a completely neutral compound. The determination of the enzymatic system of antioxidant defense involving analysis of catalase (CAT) activity in the blood of wintering mute swans was the next stage of our study . The statistical variability assessed using the ANOVA test was similar to the SOD activity distribution variability test (F11.139 = 12.98, p = 0.000). The adult males from Gdynia had the lowest level of CAT activity among the studied birds, whereas the same age/sex group from Słupsk had the highest activity of this enzyme.
Since the glutathione system plays an important role in maintaining the balance of production and elimination of ROS, we included analyses of glutathione-dependent enzymes, i.e. glutathione reductase (GR) and glutathione peroxidase (GPx), in our studies of swan’s blood samples. Importantly, the central metabolite of this defense system,i.e. reduced glutathione, has its own antioxidant activity and acts as a cofactor of antioxidant enzymes. This is an informative biomarker for assessing the level of peroxidase responses in animals. GR and GPx activities in swan’s blood samples depending on the habitat, age, and sex are shown in . The results of the ANOVA test was F11.139 = 31.61 (p = 0.000) for GR activity and statistically significant but lower for GPx activity (F11.139 = 8.06, p = 0.000).
Figure 5. Activities of glutathione reductase (A, U∙mL−1) and glutathione peroxidase (B, U∙mL−1) in the blood of mute swans of different ages and sexes inhabiting Słupsk, Gdynia, and Sopot (northern Poland, Pomeranian region).
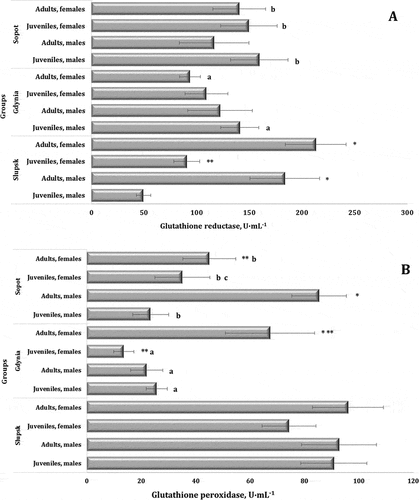
The GR activity in the birds from Słupsk was significantly higher in the adult individuals than in the juvenile ones, and this trend was maintained in the results in the group of males of different ages and females. In the birds from Gdynia and Sopot, we observed no similar trends. We observed the maximum GR activity in the adult males (183.79 ± 33.11 U∙mL−1) and in the adult females (213.03 ± 28.91 U∙mL−1) from Słupsk; the minimum values of GR activity were observed in the juvenile males from Słupsk (49.63 ± 6.65 U∙mL−1). The trend in GPx activity showed a statistically increasing trend with age in most cases, which can be demonstrated by the example of the females of different ages (juveniles, adults) from Gdynia. The lowest value of GPx activity was observed in the juvenile females from Gdynia. The highest level of GPx activity was found in the adult birds from Słupsk, with values ranging from 92.66 ± 13.79 U∙mL−1 to 95.96 ± 13.02 U∙mL−1, and in the adult males from Sopot with values of 85.34 ± 10.07 U∙mL−1.
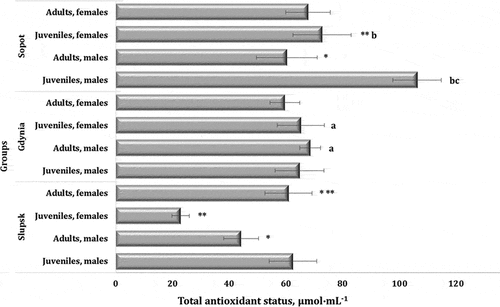
The study of total antioxidant status (TAS) showed different values of this parameter depending on the age and sex of the birds and on the location of the mute swan populations. These data are shown in . A significant decrease in the TAS value was shown in the juveniles and adults of male mute swans between Słupsk and Sopot. It is possible that these effects of environmental stress interact together with the increasing age of birds and cause a decrease in the TAS value of the wild-living organisms analyzed in our study.
Figure 6. Level of total antioxidant status (TAS, μmol∙mL−1) in the blood of mute swans of different ages and sexes inhabiting Słupsk, Gdynia, and Sopot (northern Poland, Pomeranian region).
Summarizing, the analysis of metal content in the soil, the dependence of their levels in the feathers of birds, and the functioning of the antioxidant defense system in the blood of mute swans showed the dependence of these parameters on the birds’ habitat, age, and sex. Statistical analysis of the complete MANOVA model in the current study on the functioning of birds in different anthropogenically modified environments, taking into account their sex and age, was performed using the sum of squares (SS-test) and data residual values. All biomarkers of antioxidant defenses and lipid peroxidation, considering the values of MM, were analyzed, which allowed us to present these dependencies as shown below. Noteworthy, in the case of DC, we obtained the maximum values of the coefficient of determination R2 both in the direct and adjusted form R2 = 81.7% (F = 61.98, p = 0.000). The MM level was also significant and was R2 = 78.5% (F = 50.80, p = 0.000), which may indicate the high value of the chosen parameter for evaluation of efficient functioning of body systems in this bird species for biomonitoring purposes. The activity of antioxidative enzymes and the TAS values were as follows: GR (68.18%) > SOD (50.17%) > CAT (46.76%) > GPx (34.11%), and TAS (11.22%).
Discussion
The present study assessed the effects of the habitat, age, and sex on the accumulation of chemical elements in the feathers and compared these results with oxidative stress biomarkers (diene conjugates and middle-mass molecules, activity of superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase, and the total antioxidant status) in the blood of the mute swan wintering population living in northern Poland. The relevance of our study was that we used bioindicators with a long lifespan, such as the mute swans in our study, is a promising solution for improving the wildlife living conditions in such environments with pronounced anthropogenic pressure as the Baltic Sea ecosystems. We measured the concentrations of metals in the soil from the three habitats of these birds in northern Poland. Therefore, our analysis of ecosystems in the Baltic Sea provides an example of how ecological systems are used and function under increasing anthropogenic pressure on a global scale. Also, we assessed the impact of selected metals on their accumulation in birds’ feathers and oxidative stress biomarkers, i.e. diene conjugates and middle-mass molecules, activity of superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase, and the total antioxidant status in their blood samples. Importantly, the impact of the metals exerted on living organisms depends on their type, form, duration, and intensity of the exposure and on the interactions with other xenobiotics. The important finding of the present study is the confirmation of the dependence of the environmental pollution level with the level of metals in the body of wild birds, which can determine the functional state of their organism. In order to identify the impact of such factors as age and sex in the bioaccumulative mechanisms of metals, we also performed the analysis in both young and older animals. Equally important in our studies was the factor of the swans’ sex.
There are several new findings in our study. Firstly, the contents of Al, Si, Ti, Mn, Fe, Cu, Zn, Zr, Rh, and Ru differed significantly in the soils of the studied areas from northern Poland (Słupsk, Gdynia, and Sopot). In Słupsk, the anthropogenic impact on the waterfowl was predominantly related to the content of Al and, to a lesser extent, Rh and Ru. In turn, increased levels of Rh and Ru were determined in the soil from Gdynia and Sopot, which are characterized by a high level of anthropogenic pressure associated with their Baltic Sea beaches. Secondly, our results indicate high-level aluminum-dependent interactions in the birds – up to 30% of the statistical environmental model. Thirdly, the accumulation of metals in the feathers of the mute swans was strongly dependent on the habitats, sex, and age of the birds. The typical breeding system of these birds involves lifelong monogamous pairs which vigorously defend large breeding territories, sometimes killing intruding swans that are unable to escape. However, in some unusual circumstances (superabundant food coupled with limited nesting sites), mute swans may nest colonially. The population of this species increased dramatically over the last decades in Western Europe, leading to concerns about its potential impact on aquatic ecosystems (Day et al. Citation2003). Indeed, the consequences of the swan impact on the environment remain poorly investigated, although the mute swan population largely depends on a high level of anthropogenic pressure in aquatic systems, especially on the beaches of the Baltic Sea, i.e. Gdynia and Sopot.
Mute swans often are used as effective bioindicators of environmental pollution (Beyer & Day Citation2004). In the study conducted by Grúz et al. (Citation2015), blood and feathers of these birds were analyzed. Monitoring of the heavy metal burden in mute swans from the Keszthely Bay of Lake Balaton (Hungary) (especially arsenic, cadmium, chromium, copper, mercury, and lead) was measured in contour (body) feathers. It was detected that copper and lead were present at the highest level, and other metals were mostly under the detection limit (Grúz et al. Citation2015).
Waterfowl are especially sensitive to toxic exposure in aquatic ecosystems identified as areas with a high risk of pollution, mainly due to hunting and fishing pressures (Perrins et al. Citation2003). There are many suggestions that they are ideal indicators of metal pollution because they occupy a relatively high trophic position in the aquatic food chains, have a fast metabolic rate and a long lifespan, and are quite common and widespread (Meissner et al. Citation2020). Salah-Eldein et al. (Citation2012) used wild birds (Little tern, Little grebe, Moorhen) as indicators of Cd, Zn, Pb, and Cu in Egypt (Lake Manzala). Guo et al. (Citation2001) studied the level and distribution of mercury in feathers of birds and found that the distributions of Hg in different types of feathers and different parts for the same feather vary. As shown by Liu et al. (Citation2015), cadmium may reduce the reproduction and growth performance of birds. Ingestion of even trace quantities of Cd can affect the physiology and health of migratory bird species. The constant proportion between metal concentrations in feathers and body burden may be observed for some metals and species, as shown by Mansouri and Hoshyari (Citation2012).
The current study of the impact of the sex indicated a statistically significantly higher content of Al and Ru in the juveniles, and only in the case of Al in the adult birds. Dietary organically complexed Al, probably in synergy with other contaminants, may be absorbed easily and can interfere with important metabolic processes in mammals and birds. The mechanism of Al action is not known, although it is noted that Al competes with cations in biological systems, especially magnesium, cadmium, zinc, and iron (Seńczuk Citation2006; Briffa et al. Citation2020). Al can interfere with biochemical reactions as well as cell functions that are important in mineralization processes. Al binds also irreversibly to cell nucleus components. The lungs, central nervous system, and bone seem to be the target of aluminum poisoning (Briffa et al. Citation2020). Particular attention should be paid to the statistically high values of correlations between the parameters of oxidative stress and Al in the juvenile birds: DC – Al (r = −0.48, p = 0.000), MM – Al (r = 0.26, p = 0.026), GPx – Al (r = 0.26, p = 0.029), Zn – Al (r = −0.71, p = 0.000), Rh – Al (r = −0.64, p = 0.000), and Ru – Al (r = −0.46, p = 0.000).
The presence of Al in birds producing defective eggshells was demonstrated in bone marrow tissue of humeri of pied flycatchers (Ficedula hypoleuca) (Nyholm Citation1981). Studies conducted in humans and animals show that aluminum influences heme biosynthesis by changing the activity of enzymes. Increased levels of Al in the brain were found in patients with Alzheimer’s disease. Aluminum is not eliminated from the brain and may accumulate over the years (Seńczuk Citation2006). Many neurodegenerative disorders are associated with comparable neurofibrillary pathological changes, thus showing that abnormal neuronal function caused by aluminum might involve changes in the cytoskeleton protein function in the affected cells (Briffa et al. Citation2020).
Our data on the role of the selected elements analyzed in an integrated model demonstrated the important role in Al, Rh, and Ru accumulation. The concentration of platinum-group metals (PGM) (i.e. Ir, Os, Pd, Pt, Rh, Ru) in the environment has increased significantly during the last 20 years. The increase was caused by e.g. the production of cars with catalytic converters and industrial process catalysts (Gagnon et al. Citation2006; Wichmann et al. Citation2007), metal production, and medical applications. For example, the global demand for Rh increased from 8 tons in 1985 to about 26 tons in 2005 (Rauch & Morrison Citation2008). PGMs, like other heavy metals, represent a bioavailable form, which may result in uptake and eventually toxic effects on living organisms. Recent results from cellular studies show that PGMs are related to respiratory sensitization, allergic reactions, dermatitis, urticaria, damage to epithelial lung cells, asthma, lymphocyte proliferation, cytokine release, and possibly cancer (Gagnon et al. Citation2006).
It has been noted that urban areas are usually connected with a wide spectrum of pollution. Our studies showed that the concentrations of aluminum in both sexes were higher in the birds living in Słupsk, whereas the concentrations of Ru and Rh were higher in the feathers of swans from Sopot and Gdynia. This allows us to infer that even if the main sources of Al pollution are centralized in both types of urbanized areas, some metals reach higher concentrations in swans living on the beaches of the Baltic Sea. We cannot exclude the possibility of early migration among some birds; hence, the image of the local pollution can be disturbed by the exposure of birds in previous staging areas.
Fourthly, we used biochemical assays to study the initial stages of lipid peroxidation processes (diene conjugates) and the antioxidant defenses in the blood of swans in order to evaluate the efficiency of functioning of the antioxidant defense system. The importance finding of our study is the confirmation of the high dependence between the biomarkers of the initial stages of lipid peroxidation measured as the DC level in the blood of swans and the habitat, age, and sex of the birds. The high levels of coefficient of determination in the statistical analysis strongly confirmed our hypothesis. This part of the analysis is presented in the Results section.
We performed a correlation analysis of the metal levels and biomarkers of oxidative stress and antioxidant defense system taking into account three criteria: the habitat, age, and sex of the birds. We showed statistically significant correlations of the analyzed parameters for the three study sites. The following dependencies were determined for the site in Słupsk: DC – MM (r = 0.86, p = 0.000), DC – SOD (r = 0.92, p = 0.000), DC – CAT (r = 0.88, p = 0.000), MM – TAS (r = −0.38, p = 0.013), GPx – TAS (r = −0.73, p = 0.000), Zn – Rh (r = −0.75, p = 0.000), and Al – Rh (r = −0.51, p = 0.001). For the Gdynia site, these dependencies were as follows: Zn – Al (r = −0.62, p = 0.000), Rh – Al (r = −0.65, p = 0.000), and Ru – Al (r = −0.27, p = 0.000). The following dependencies were found for the Sopot area: MM – SOD (r = 0.56, p = 0.000), MM – Al (r = 0.32, p = 0.018), Rh – Al (r = −0.55, p = 0.000), Ru – Al (r = −0.28, p = 0.038), and Zn – Rh (r = 0.83, p = 0.000). The findings of the statistical relationships between the levels of metals and oxidative stress biomarkers depending on the habitat underline the important role of these factors for the functioning of the bird organism in a particular habitat.
Using the birds’ age as a main factor in the statistical analysis, the highest significant correlations presented in this statistical model in the adult bird group were as follows: MM – DC (r = 0.47, p = 0.000), MM – SOD (r = 0.82, p = 0.000), MM – CAT (r = 0.73, p = 0.000), and MM – GR (r = 0.34, p = 0.002). These correlations confirm the high dependence of endogenous intoxication processes that develop with age in older animals. When we analyzed the sex-dependent relations, high statistically significant interactions in the following relationships were observed: MM – DC (r = 0.60, p = 0.000), MM – SOD (r = 0.83, p = 0.000), Rh – Al (r = −0.75, p = 0.000), and Rh – Zn (r = 0.78, p = 0.000) in the male group and Rh – Zn (r = 0.83, p = 0.000), Cu – Zn (r = 0.56, p = 0.000), Rh – Cu (r = 0.57, p = 0.000), and GPx – DC (r = −0.63, p = 0.000) in the female group. Thus, the formation of the antioxidant defense and metal-induced bioaccumulative mechanisms determined in our analysis showed a multilevel profile of their functioning, which we were able to show using the age, sex, and location of the birds.
The literature suggests that lipid peroxidation (LPO) is a chain-free radical process (Münzel & Daiber Citation2018). Unsaturated lipids or free fatty acids, which are part of the phospholipids of biological membranes, are most easily oxidized in this way. Therefore, the rate of lipid peroxidation primarily affects the function of membranes and induces the development of pathological changes in these membranes. The initiation of LPO by free radicals results in the development of a chain reaction resulting in the formation of lipid molecules with two conjugated double bonds (diene conjugates) with an absorption maximum at 233 nm (Tangvarasittichai & Tangvarasittichai Citation2018). The generation of peroxide radicals in this process by interaction with new fatty acid molecules leads to the formation of lipid hydroperoxides ROOH and another lipid radical R.
Literature data show a connection between endogenous intoxication against lipid peroxidation reactions and disturbances in the antioxidant system functioning (Ung et al. Citation2017). Our studies confirm this concept, as high levels of MM and a decreased TAS level were found in the swan population, as shown in the adult males from Slupsk compared to the juvenile males from the same area . In this case, the MM value may be an indicator of the severity of disorders and the efficiency of the antioxidant defenses. Researchers link this to the accumulation of proteolytic enzymes in the damaged organs themselves or in the blood, as well as catabolic processes and intensification of reactions in endogenous intoxication accompanying the development of cell destruction. At the same time, the accumulation of MM is not only a marker of endogenous detoxification at a high autorepression level but also aggravates the pathological process, affecting the activity of all organs and systems.
The main feature of MM shown in a number of works, which consists in their biological activity, is associated with the aggravation of metabolic disorders that cause their synthesis, forming a kind of vicious circle (Macías et al. Citation2018; Nlandu et al. Citation2019). Considering the oxidative stress biomarkers and antioxidant defenses analyzed in the present study, we believe that one of the consequences of the toxic action of xenobiotics in the body of the wintering swans from the northern Poland population was the formation of free radicals that interact with lipids and proteins and damage cell membrane structures. Particular attention should be paid to the fact that we obtained statistically higher values of correlative dependencies in the adult birds in comparison with the juveniles only for the MM value, namely MM – DC (r = −0.47, p = 0.000), MM – SOD (r = 0.82, p = 0.000), MM – CAT (r = 0.73, p = 0.000), MM – TAS (r = −0.58, p = 0.000).
The increased formation of free radicals, which we assessed by the level of DC, leading to the activation of lipid peroxidation and the formation of MM, was presented in the current study and in our previous study (Kurhaluk & Tkachenko Citation2021). However, the relationship between the initiation of oxidative stress and the MM level, as shown convincingly in this study, was assessed for the first time, although it was previously presented by various authors in a number of exclusively clinical works (Clark & Winchester Citation2003; Winchester & Audia Citation2006; Cobo et al. Citation2018; Wolley et al. Citation2018).
Our study had several limitations. An important limitation was the small number of animals in accordance with the Permission Commission Decision for the environmental study. Coastal areas of the Baltic Sea may be considered as areas of environmental risk with different pollutant impacts. These areas are identified based on population density, degree of industrialization and employment in industry, the work of the port and maritime industries, water consumption, amount of sewage discharged, emission of gaseous and particulate pollutants, land use structure, area of degraded land, and amount of industrial waste production, etc. All of these parameters affect the Baltic Sea ecosystem in different ways.
New strategies for elucidation of the functioning of the living environment at different levels are needed to enrich the existing knowledge in order to protect the environment through interdisciplinary, techno-economic, and sustainable approaches. This knowledge based on bioindicators with a long lifespan, such as the mute swans in our study, is a promising solution for improving the wildlife living conditions in such environments with pronounced anthropogenic pressure as the Baltic Sea ecosystems. Further studies are required to evaluate the risk factors for anthropopressure of the Baltic Sea with a large number of different bioindicator species.
Conclusions
Our current study focused on determination of the concentrations of chemical elements in the soil of Słupsk, Gdynia, and Sopot in northern Poland and feathers of wintering populations of the mute swan (Cygnus olor) living in these areas using the X-Ray fluorescence method. In this study, we examined three issues associated with the metal concentrations in soil and feathers of mute swans living in areas with differing levels of anthropogenic pressure (urban agglomerations, recreational sea beach activity, and tourism). The analysis of variance showed significant differences in the levels of Al, Si, Ti, Mn, Fe, Cu, Zn, Zr, Rh, and Ro between the soil samples from Słupsk and both Gdynia and Sopot, and in the Rh and Ro content between all studied areas. This allowed us to classify those areas as locations with different levels of chemical contamination.
Based on the hypothesis that the analyzed wintering population of swans can change under the influence of such main factors as habitats, age, and sex, we used a MANOVA analysis to detect factors influencing the accumulation and distribution of elements in feathers. Our data indicated the highest significant dependencies of such main effects as the habitats and their combination with the other main factors, i.e. age and sex. In decreasing order, the influence of the other main factors according to our statistical model was as follows: age and sex, sex and age, and habitats and sex. The Al and Ru contents in the feathers in juveniles were statistically significant. In the adult group, the trend was maintained for Al and Cu as well. Thus, our data revealed age-specific accumulation of Al, regardless of the age of the birds, which may indicate differences in the geochemistry processes of metal accumulation depending on the habitats and during the development of birds. In our study, statistically significant results were obtained in the case of the Cu content in the male group of swans and in the case of Al and Ru in the female group.
Our results indicated high dependence of the biomarkers of the initial stages of lipid peroxidation in swans on the habitat, age, and sex of the birds, which we assessed by the level of diene conjugates in blood samples. The study showed a connection between the biomarkers of endogenous intoxication, lipid peroxidation reactions, and antioxidant defense functioning reflected by high levels of middle molecules and a decreased level of the total antioxidant status in the wintering mute swan population, as shown in the adult males from Slupsk compared to the juvenile males from the same area. The functioning of the activity of antioxidative enzymes and TAS value was as follows: GR > SOD > CAT > GPx > TAS.
Author contribution
idea, design of the work, critical revision: NK, HT;
analysis, interpretation of data: NK, HT, TH, AW, VT, PK;
drafting the manuscript: NK, HT, AJ.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alfeld M, Janssens K. 2015. Strategies for processing mega-pixel X-ray fluorescence hyperspectral data: A case study on a version of caravaggio’s painting supper at emmaus. Journal of Analytical Atomic Spectrometry 30(3):777–789. DOI:10.1039/C4JA00387J.
- Baker J. 2016. Identification of European non-Passerines. Thetford: British Trust for Ornithology.
- Battaglia A, Ghidini S, Campanini G, Spaggiarri R. 2003. Heavy metal contamination in little owl (Athene noayua) and common buzzard (Buteo buteo) from northern Italy. Ecotoxicol Environ Saf 60:126–131. DOI:10.1016/j.ecoenv.2003.12.019.
- Beyer WN, Day D. 2004. Role of manganese oxides in the exposure of mute swans (Cygnus olor) to Pb and other elements in the Chesapeake Bay, USA. Environmental Pollution 129(2):229–235. DOI:10.1016/j.envpol.2003.10.026.
- Briffa J, Sinagra E, Blundell R. 2020. Heavy metal pollution in the environment and their toxicological effect of human. Heliyon 6(9):1–26. DOI:10.1016/j.heliyon.2020.e04691.
- Brown A, Brown L. 2002. The Accuracy of Sexing Mute Swan Cygnets by Cloacal Examination. Waterbirds: The International Journal of Waterbird Biology 25:352–354. http://www.jstor.org/stable/1522375
- Clark WR, Winchester JF. 2003. Middle molecules and small-molecular-weight proteins in ESRD: Properties and strategies for their removal. Advances in Renal Replacement Therapy 10(4):270–278. DOI:10.1053/j.arrt.2003.11.004.
- Cobo G, Lindholm B, Stenvinkel P. 2018. Chronic inflammation in end-stage renal disease and dialysis. Nephrology Dialysis Transplantation 33(Suppl. 3):iii35–iii40. DOI:10.1093/ndt/gfy175.
- Coleman JT, Coleman AE. 2002. A preliminary analysis of mute swan biometrics in relation to sex, region and breeding status. Waterbirds: The International Journal of Waterbird Biology 25:340–345. http://www.jstor.org/stable/1522373
- Day DD, Beyer WN, Hoffman DJ, Morton A, Sileo L, Audet DJ, Ottinger MA. 2003. Toxicity of lead-contaminated sediment to mute swans. Archives of Environmental Contamination and Toxicology 44(4):510–522. DOI:10.1007/s00244-002-1140-4.
- Fialkowski W, Rainbow PS, Smith BD, Zmudzinski L. 2003. Seasonal variation in trace metal concentrations in three talitrid amphipods from the Gulf of Gdansk, Poland. Journal of Experimental Marine Biology and Ecology 288(1):81–93. DOI:10.1016/S0022-0981(02)00594-4.
- Gagnon ZE, Newkirk C, Hicks S. 2006. Impact of platinum group metals on the environment: A toxicological, genotoxic and analytical chemistry study. Journal of Environmental Science and Health, Part A 41(3):397–414. DOI:10.1080/10934520500423592.
- Goede AA, Bruin MD. 1984. The use of bird feather parts as a monitor for metal pollution. Environmental Pollution Series B, Chemical and Physical 8(4):281–298. DOI:10.1016/0143-148X(84)90028-4.
- Goldberg DM, Spooner RJ. 1983. Assay of glutathione reductase. Bergmeyen HV, editor. Methods of enzymatic analysis. 3rd ed. Deerfiled Beach: Verlog Chemie. Vol. 3 258–265.
- Grúz A, Szemerédy G, Kormos É, Budai P, Majoros S, Tompai E, Lehel J. 2015. Monitoring of heavy metal burden in mute swan (Cygnus olor). Environmental Science and Pollution Research 22(20):15903–15909. DOI:10.1007/s11356-015-4809-8.
- Guo DL, Zhou MS, Xi YY. 2001. Preliminary studies on the level and distribution of mercury in feathers of birds. Acta Zoologica Sinica 47:139–149.
- HELCOM, 2007. Heavy metal pollution to the Baltic Sea in 2004. HELCOM Baltic Sea Environment Proceedings No. 108, Baltic Marine Environment Protection Commission – Helsinki Commission, Helsinki, Finland, pp. 33. http://www.helcom.fi
- HELCOM, 2010. Hazardous substances in the Baltic Sea – An integrated thematic assessment of hazardous substances in the Baltic Sea, Environment Proceedings No. 120B, Baltic Marine Environment Protection Commission – Helsinki Commission, Helsinki, Finland, pp 119. http://www.helcom.fi
- HELCOM, 2018. Inputs of hazardous substances to the Baltic Sea. Environment Proceedings No. 162, Baltic Marine Environment Protection Commission – Helsinki Commission, Helsinki, Finland, http://www.helcom.fi
- Jarosiewicz A, Radawiec B, Hetmański T. 2014. Influence of water chemistry and habitat parameters on the abundance of pond-breeding amphibians. Polish Journal of Environmental Studies 2:349–355.
- Johnsgard PA. 2016. Swans: Their biology and natural history. zea e-books. book 38. Lincoln, Nebraska: Zea Books. http://digitalcommons.unl.edu/zeabook/38
- Kamyshnikov VS. 2004. Handbook of clinical and biochemical investigations and laboratory diagnostics. Moscow: Medpress-inform.
- Karimi MH, Hassanpour M, Pourkhabbaz AR, Błaszczyk M, Paluch J, Binkowski ŁJ. 2016. Trace element concentrations in feathers of five Anseriformes in the south of the Caspian Sea, Iran. Environmental Monitoring and Assessment 188(1):22. DOI:10.1007/s10661-015-5015-3.
- Koroliuk MA, Ivanova LI, Maiorova IG, Tokarev VE. 1988. Metod opredeleniia aktivnosti katalazy [A method of determining catalase activity]. Laboratornoe delo(1):16–19. Russian
- Kraus RJ, Ganther HE. 1980. Reaction of cyanide with glutathione peroxidase. Biochemical and Biophysical Research Communications 96(3):1116–1122. DOI:10.1016/0006-291x(80)90067-4.
- Krzymiński W, ed. 2017. Environmental assessment of Polish Baltic Marine areas based on data monitoring with the year 2017, on the background of 2007-2016. Warszawa [in Polish]: Inspection of Environmental Protection – Environmental Monitoring Library.
- Kurhaluk N, Tkachenko H. 2021. Habitat-, age-, and sex-related alterations in oxidative stress biomarkers in the blood of mute swans (Cygnus olor) inhabiting Pomeranian coastal areas (Northern Poland). Environmental Science and Pollution Research International 29(18):27070–27083. DOI:10.1007/s11356-021-18393-3.
- Kurhaluk N, Tkachenko H, Hetmański T, Włodarkiewicz A, Tomin V. 2021. Profile of heavy metals and antioxidant defense in the muscle tissues of pigeons (Columba livia f. urbana) from anthropogenically transformed areas in the Pomeranian region (Northern Poland). Archives of Environmental Contamination and Toxicology 80(3):601–614. DOI:10.1007/s00244-021-00825-3.
- Liu J, Liang J, Yuan X, Zeng G, Yuan Y, Wu H, Huang X, Liu J, Hua S, Li F, Li X. 2015. An integrated model for assessing heavy metal exposure risk to migratory birds in wetland ecosystem: A case study in Dongting Lake Wetland, China. Chemosphere 135:14–19. DOI:10.1016/j.chemosphere.2015.03.053.
- Macías N, Vega A, Abad S, Aragoncillo I, García-Prieto AM, Santos A, Torres E, Luño J. 2018. Middle molecule elimination in expanded haemodialysis: Only convective transport? Clinical Kidney Journal 12(3):447–455. DOI:10.1093/ckj/sfy097.
- Mansouri B, Hoshyari E. 2012. Nickel concentration in two bird species from hara biosphere reserve of southern Iran. Chinese Birds 3(1):54–59. DOI:10.5122/cbirds.2012.0007.
- Mathiasson S. 2005. Biometrics and structures of the mute swan, Cygnus olor – Parameters and technique used in a Swedish project. Gothenburg, Sweden: Göteborgs Naturhistoriska Museum Årstryck. pp. 77–86.
- McCleery RH, Perrins CM, Sheldon BC, Charmantier A. 2008. Age-specific reproduction in a long-lived species: The combined effects of senescence and individual quality. Proceedings. Biological Sciences 275(1637):963–970. DOI:10.1098/rspb.2007.1418.
- Meer W, Binkowski ŁJ, Barker J, Hahn A, Trzeciak M. 2020. Relationship between blood lead levels and physiological stress in mute swans (Cygnus olor) in municipal beaches of the southern Baltic. Science of the Total Environment 710:136292. DOI:10.1016/j.scitotenv.2019.136292.
- Meissner W, Binkowski ŁJ, Barker J, Hahn A, Trzeciak M. 2020. Relationship between blood lead levels and physiological stress in mute swans (Cygnus olor) in municipal beaches of the southern Baltic. Science of the Total Environment 710:136292. DOI:10.1016/j.scitotenv.2019.136292
- Melissinos KG, Delidou AZ, Varsou AG, Begietti SS, Drivas GJ. 1981. Serum and erythrocyte glutathione reductase activity in chronic renal failure. Nephron 28(2):76–79. DOI:10.1159/000182115.
- Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. 1993. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical Science 84(4):407–412. DOI:10.1042/cs0840407.
- Møller P, Scholten RH, Roursgaard M, Krais AM. 2020. Inflammation, oxidative stress and genotoxicity responses to biodiesel emissions in cultured mammalian cells and animals. Critical Reviews in Toxicology 50(5):383–401. DOI:10.1080/10408444.2020.1762541.
- Mudway IS, Kelly FJ, Holgate ST. 2020. Oxidative stress in air pollution research. Free Radical Biology and Medicine 151:2–6. DOI:10.1016/j.freeradbiomed.2020.04.031.
- Münzel T, Daiber A. 2018. Environmental stressors and their impact on health and disease with focus on oxidative stress. Antioxidants & Redox Signaling 28(9):735–740. DOI:10.1089/ars.2017.7488.
- Nlandu Y, Padden M, Seidowsky A, Hamaz S, Vilaine É, Cheddani L, Essig M, Massy ZA. 2019. Toxines urémiques de moyen poids moléculaire: Un véritable regain d’intérêt [Middle-molecule uremic toxins: A renewed interest]. Néphrologie & Thérapeutique 15(2):82–90. French DOI:10.1016/j.nephro.2018.09.003.
- Nyholm NE. 1981. Evidence of involvement of aluminum in causation of defective formation of eggshells and of impaired breeding in wild passerine birds. Environmental Research 26(2):363–371. DOI:10.1016/0013-9351(81)90212-7.
- Paglia DE, Valentine WN. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine 70(1):158–169.
- Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK. 2021. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 262:128350. DOI:10.1016/j.chemosphere.2020.128350.
- Perrins CM, Cousquer G, Waine J. 2003. A survey of blood lead levels in Mute Swans Cygnus olor. Avian Pathology 32(2):205–212. DOI:10.1080/0307946021000071597.
- Protasowicki M, Dural M, Jaremek J. 2008. Trace metals in the shells of blue mussels (Mytilus edulis) from the Poland coast of Baltic sea. Environmental Monitoring and Assessment 141(1–3):329–337. DOI:10.1007/s10661-007-9899-4.
- Rauch S, Morrison GM. 2008. The environmental relevance of the platinum group elements. Elements 4(4):259–263. DOI:10.2113/GSELEMENTS.4.4.259.
- Salah-Eldein AM, Gamal-Eldein MA, Lamiaa IM. 2012. Resident wild birds as bio-indicator for some heavy metals pollution in Lake Manzala. Suez Canal Veterinary Medicine Journal XVII(1):109–121.
- Salmón P, Watson H, Nord A, Isaksson C. 2018. Effects of the Urban Environment on Oxidative Stress in Early Life: Insights from a Cross-fostering Experiment. Integrative and Comparative Biology 58(5):986–994. DOI:10.1093/icb/icy099.
- Seńczuk W, ed. 2006. Contemporary toxicology. PZWL, Warszawa [in Polish]: PZWL Medical Publishing.
- Shahabi-Ghahfarokhi S, Josefsson S, Apler A, Kalbitz K, Åström M, Ketzer M. 2021. Baltic Sea sediments record anthropogenic loads of Cd, Pb, and Zn. Environmental Science and Pollution Research 28(5):6162–6175. DOI:10.1007/s11356-020-10735-x.
- Solé VA, Papillon E, Cotte M, Walter P, Susini J. 2007. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochimica Acta Part B: Atomic Spectroscopy 62(1):63–68. DOI:10.1016/j.sab.2006.12.002.
- Stanisz A. 2006, 2007. An accessible course of statistics with the use of STATISTICA PL on examples from medicine (Przystępny kurs statystyki z zastosowaniem STATISTICA PL na przykładach z medycyny). 3rd ed. Vol. 1-3 Krakow (In Polish): StatSoft Polska.
- Suttle NF, McMurray CH. 1983. Use of erythrocyte copper: zinc superoxide dismutase activity and hair or fleece copper concentrations in the diagnosis of hypocuprosis in ruminants. Research in veterinary science 35(1):47–52. DOI:10.1016/S0034-5288(18)32201-X.
- Szefer P, Glasby GP, Geldon J, Renner RM, Björn E, Snell J, Frech W, Warzocha J. 2009. Heavy-metal pollution of sediments from the Polish exclusive economic zone, southern Baltic Sea. Environmental Geology 57(4):847–862. DOI:10.1007/s00254-008-1364-3.
- Tangvarasittichai O, Tangvarasittichai S. 2018. Oxidative stress, ocular disease and diabetes retinopathy. Current Pharmaceutical Design 24(40):4726–4741. DOI:10.2174/1381612825666190115121531.
- Tkachenko H, Kurhaluk N. 2012. Pollution-induced oxidative stress and biochemical parameter alterations in the blood of white stork nestlings Ciconia ciconia from regions with different degrees of contamination in Poland. Journal of Environmental Monitoring 14(12):3182–3191. DOI:10.1039/c2em30391d.
- Tkachenko H, Kurhaluk N, Hetmański T, Włodarkiewicz A, Tomin V. 2021. Changes in energetic metabolism and lysosomal destruction in the skeletal muscle and cardiac tissues of pigeons (Columba livia f. Urbana) from urban areas of the northern Pomeranian region (Poland). Ecotoxicology 30(6):1170–1185. DOI:10.1007/s10646-021-02423-4.
- Ung L, Pattamatta U, Carnt N, Wilkinson-Berka JL, Liew G, White AJR. 2017. Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Clinical Science 131(24):2865–2883. DOI:10.1042/CS20171246.
- Vizuete J, Pérez-López M, Míguez-Santiyán MP, Hernández-Moreno D. 2019. Mercury (Hg), Lead (Pb), Cadmium (Cd), Selenium (Se), and Arsenic (As) in Liver, Kidney, and Feathers of Gulls: A Review. Reviews of Environmental Contamination and Toxicology 247:85–146. DOI:10.1007/398_2018_16.
- Wichmann H, Anquandah GA, Schmidt C, Zachmann D, Bahadir MA. 2007. Increase of platinum group element concentrations in soils and airborne dust in an urban area in Germany. Science of the Total Environment 388(1–3):121–127. DOI:10.1016/j.scitotenv.2007.07.064.
- Wieloch M, Remisiewicz M. 2001. Changes in wintering area of the Mute Swan Cygnus olor. In: Švažas S, Meissner W, Serebryakov V, Kozulin A, Grishanov G. (red.) Changes of wintering sites of Waterfowl in Central and Eastern Europe, Vilnius. 94–103.
- Winchester JF, Audia PF. 2006. Extracorporeal strategies for the removal of middle molecules. Seminars in Dialysis 19(2):110–114. DOI:10.1111/j.1525-139X.2006.00135.x.
- Wolley M, Jardine M, Hutchison CA. 2018. Exploring the clinical relevance of providing increased removal of large middle molecules. Clinical Journal of the American Society of Nephrology 13(5):805–814. DOI:10.2215/CJN.10110917.
- Woolliams JA, Wiener G, Anderson PH, McMurray CH. 1983. Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Research in veterinary science 34(3):253–256. DOI:10.1016/S0034-5288(18)32219-7.
- Zaborska A, Kosakowska A, Bełdowski J, Bełdowska M, Szubska M, Walkusz-Miotk J, Żak J, Ciechanowicz A, Wdowiak M. 2016. The distribution of heavy metals and 137 Cs in the central part of the Polish maritime zone (Baltic Sea) – The area selected for wind farm acquisition. Estuarine, Coastal and Shelf Science 198:471–481. DOI:10.1016/j.ecss.2016.12.007.