Abstract
Echiniscoididae are the only family of the clade Echiniscoidea residing in marine habitats. Their characteristic feature is the multiplication of claws on legs, commonly regarded as an adaptation to unstable tidal environment. Although DNA barcoding data indicated the presence of numerous unnamed candidate species within Echiniscoides about a decade ago, only recently have new species been formally described. In this paper, we present new genetic, morphological and morphometric data and establish five Echiniscoides species found in samples acquired from various tropical regions: Echiniscoides basalticus sp. nov. (Mauritius, Indian Ocean), Echiniscoides bufocephalus sp. nov. (Qatar, Indian Ocean), Echiniscoides lichenophilus sp. nov. (Dominican Republic, Atlantic Ocean), Echiniscoides musa sp. nov. (Brazil, Atlantic Ocean), and Echiniscoides trichosus sp. nov. (Rapa Nui, Pacific Ocean). We discuss the taxonomic importance of cuticular sculpturing in Echiniscoididae. Finally, we elevate all subspecies of Echiniscoides sigismundi to species level. The key to all echiniscoidids is provided.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:63154710-C3BA-402A-A11B-C211DAE59CAB
Introduction
Tardigrades inhabit a variety of environments, ranging from mountain peaks (Kristensen Citation1987) to bathyal zone in oceans (Bussau Citation1992). Marine tardigrades are usually adapted to specific lifestyles, such as dwelling on the surface of bottom sediments (Jørgensen & Kristensen Citation2001), parasitising holothurians as epibionts (Kristensen Citation1980), or clinging to thecae of barnacles in the tidal zone (Kristensen & Hallas Citation1980). The last type of lifestyle, entailing commensal and/or parasitic relationships with adult barnacles, characterises the family Echiniscoididae Kristensen & Hallas, Citation1980. Its type species, Echiniscoides sigismundi (M. Schultze, Citation1865), represents one of the first described tardigrade species (Ramazzotti & Maucci Citation1982). Plate (Citation1889) divided Echiniscus C.A.S. Schultze Citation1840 into two subgenera: Echiniscus and Echiniscoides, using the number of claws per leg as a discriminative criterion. Echiniscus was defined as grouping species with two (in the larval stage) or four claws (in later life stages), whereas species with seven to nine claws per leg were accommodated in Echiniscoides, at the same time underlying the crucial autapomorphy of echiniscoidid tardigrades: the unprecedented multiplication of claws.
No important contributions to the classification of Echiniscoides were published since the revision of Plate (Citation1889) until Renaud-Mornant (Citation1976) described E. sigismundi polynesiensis from the waters of Mo’orea in the French Polynesia. This paper, together with the fundamental revision of the Echiniscoides variability presented in Kristensen and Hallas (Citation1980), somehow consolidated a trend of establishing many new echiniscoidid taxa as subspecies of E. sigismundi, which was in accordance with the prevailing view of that period, assuming cosmopolitan distributions of most meiofaunal taxa (Cerca et al. Citation2018). Kristensen and Hallas (Citation1980) introduced the claw formula and type of cuticular sculpturing as decisive taxonomic traits. Furthermore, they indicated some similarities between Echiniscoididae and Coronarctidae, such as the shape of secondary clavae (Renaud-Mornant Citation1987). Later on, Hallas and Kristensen (Citation1982) proposed the length of cirrus E as a useful criterion in the internal classification of Echiniscoides. The echiniscoidid resemblance to Coronarctidae was raised again by D’Addabbo Gallo et al. (Citation1992), who stressed that the annulation of cirrus E has a plesiomorphic nature and, consequently, should not be used is phylogenetic inference.
In a series of important papers, Faurby et al. (Citation2011, Citation2012) demonstrated that populations of Echiniscoides collected worldwide are strongly geographically differentiated and the main process responsible for diversification of multiple evolutionary lineages within Echiniscoididae was ecological speciation. Faurby and Barber (Citation2015) corroborated the utility of intertidal Echiniscoides populations in phylogeography and augmented the evidence for numerous undescribed species within the genus (Clausen et al. Citation2014). Perry and Miller (Citation2015) described the first and the only species to date with a flexible posterior portion of the buccal tube, E. wyethi. Finally, the most recent revisions (Møbjerg et al. Citation2016, Citation2020) divided Echiniscoididae into Echiniscoidinae and Isoechiniscoidinae on the basis of strong ecomorphological disparities: the claw number and isonychy (7–13 anisonych vs usually 6 isonych), pillars in the epicuticle (absent vs present), the length of sensory appendages (comparatively short vs long), and inhabited niche (barnacles, algae and lichens in tidal zone vs interstitial species, respectively); the anal system was additionally proposed as a next taxonomic trait worth detailed documentation in the case of Echiniscoididae, and the genus Neoechiniscoides established for species with well-developed lateral anal lobes. Last but not least, Møbjerg et al. (Citation2020) instantiated the family Anisonychidae, pointing out to a long-questioned affinity between Anisonyches and Echiniscoides (Grimaldi de Zio et al. Citation1987; Fujimoto et al. Citation2017).
In this paper, we aim at unravelling diversity of Echiniscoides populations sampled in tropical waters. We provide integrative descriptions of five new species, discuss the variability and utility of cuticular sculpturing in echiniscoidid and echiniscoidean systematics, and update the taxonomic key to the genus (Hallas & Kristensen Citation1982) and its relatives. We express hopes that the studies, which triggered molecular research on Echiniscoides (Faurby et al. Citation2011, Citation2012) and uncovered unparalleled genetic diversity within the genus, will be followed by integrative data, including both an influx of further DNA barcodes and detailed morphological observations.
Material and methods
Sampling and comparative material
Summary of Echiniscoides specimens processed in different analyses is presented in . Samples of barnacles and lichens were first completely dried, and then re-soaked in distilled water. Isolated animals were either mounted on slides or preserved in 95% ethanol for sequencing and scanning microscopy. Furthermore, we examined representatives of E. sigismundi groenlandicus Kristensen & Hallas, Citation1980 (7♀♀ and 1♂, collected from barnacles in Qeqertarsuaq, Greenland), E. hoepneri Kristensen & Hallas, Citation1980 (9♀♀ and 2♂♂, collected from barnacles in Iceland; Faurby et al. Citation2011), and E. sigismundi hispaniensis Kristensen & Hallas, Citation1980 (5♀♀ and 2♂♂, collected from barnacles at the entrance to the lagoon in Praia da Barra, Central Portugal at 3rd April 2009; temperature: 16.5°C, salinity = 35.5‰).
Table I. Collecting site locations, characteristics and collecting dates for new species. Abbreviations: j – juvenile, l – larva, u – sex unidentified. The geographic reference system: WGS84.
Microscopy and imaging
When present, alive specimens were observed and photographed (using objectives up to ×400 magnification). A fraction of specimens was later mounted in glycerol and Hoyer’s medium (also in the polyvinyl-lactophenol in the case of E. trichosus sp. nov.) on permanent slides. Mounted exemplars were photographed using a DP20 camera on an Olympus BX51 compound microscope with differential interference contrast (DIC) optics, and Olympus BX53 light microscope with phase contrast (PCM), associated with an Olympus DP74 digital camera. Preparations for scanning electron microscopy (SEM) were performed according to Hygum et al. (Citation2016). Individuals were photographed and subsequently stained with osmium tetroxide (OsO4). The specimens were carefully rinsed in distilled water and then dehydrated in a graded series of ethanol and acetone prior to critical point drying. Afterwards, the dry specimens were mounted on aluminium stubs and coated with platinum/palladium alloy or gold and analysed with a JEOL JSM-6335 F Field Emission and Versa 3D DualBeam SEM. All light microscopy figures were assembled in Corel Photo-Paint X8. For deep structures that could not be fully focused on a single PCM photograph, a series of images were taken every ca. 0.1 μm of vertical focusing and then assembled manually in Corel Photo-Paint into a single deep-focus image. General phenotype drawings were prepared based on in vivo photographs of selected individuals and assembled photographs of holotypes.
Morphometry and terminology
Structures were measured only when oriented properly and not broken or deformed, according to the schemes from Kristensen and Hallas (Citation1980). Echiniscoidid claw formula presents claw numbers on subsequent legs (Kristensen & Hallas Citation1980). Body length was measured from the anterior to the posterior end of the body, excluding the hind legs. General heterotardigrade terminology follows Fontoura et al. (Citation2017).
Genotyping and phylogenetics
A Chelex® 100 resin (Bio-Rad) extraction method was used in DNA extraction (Casquet et al. Citation2012; Stec et al. Citation2020). Four DNA fragments were sequenced: the small ribosome subunit 18S rRNA, the large ribosome subunit 28S rRNA, the internal transcribed spacer ITS-1 (ITS-2 did not amplify in all species), and the cytochrome oxidase subunit I COI. Various 28S rRNA primers were used in literature to amplify Echiniscoides gene fragments (Møbjerg et al. Citation2016, Citation2020); thus, we used all of them to obtain different regions of this marker. All fragments were amplified and sequenced according to the protocols described in Stec et al. (Citation2020); primers and original references for specific PCR programmes are listed in Supplementary Material 1. GenBank accession numbers for all newly sequenced species are provided in . All echiniscoidid COI sequences available in GenBank were aligned with Echiniscus testudo (Doyère, Citation1840) as an outgroup, using the ClustalW Multiple Alignment tool (Thompson et al. Citation1994) implemented in BioEdit (Hall Citation1997). We deliberately did not concatenate COI and 28S rRNA sequences since the latter represent various DNA regions and restricting the dataset to echiniscoidid taxa with both available markers would greatly diminish it. There are no available ITS sequences for echiniscoidids, and 18S sequences are accessible for <5% of taxa with COI. That is why we performed a monolocus reconstruction. The aligned fragment (543 bp, Supplementary Material 2) was edited and checked manually in BioEdit. Uncorrected pairwise distances (Supplementary Material 3) were calculated in MEGA7 (Kumar et al. Citation2016). Using PartitionFinder version 2.1.1 (Lanfear et al. Citation2017) with applied Bayesian Information Criterion (BIC) and greedy algorithm (Lanfear et al. Citation2012), the best substitution model and partitioning scheme was chosen for posterior phylogenetic analysis. As the best-fit partitioning scheme, PartitionFinder suggested one partition characterised by TIM+I+G model. Bayesian inference (BI) marginal posterior probabilities were first calculated using MrBayes v.3.2 (Ronquist & Huelsenbeck Citation2003). Random starting trees were used, and the analysis was run for ten million generations, sampling the Markov chain every 1000 generations. An average standard deviation of split frequencies of <0.01 was used as a guide to ensure that the two independent analyses had converged. Tracer v1.3 (Rambaut et al. Citation2014) was then used to ensure Markov chains had reached stationarity and to determine the correct “burn-in” for the analysis, i.e. the first 10% of generations. The Effective Sample Size values were greater than 200 and the consensus tree (visualised in FigTree v.1.4.3 available from https://tree.bio.ed.ac.uk/software/figtree) was obtained after summarising the resulting topologies and discarding the “burn-in”.
Table II. GenBank accession numbers for the Echiniscoides spp. sequenced in this work.
Results
Phylogeny
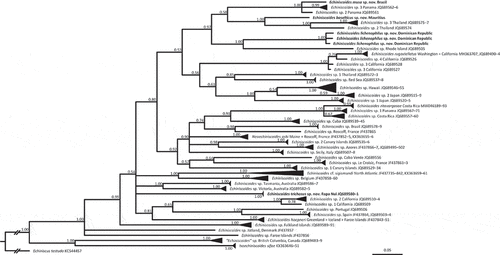
Overall, the family tree based on COI was well-resolved, with a clear distinction between Isoechiniscoidinae and Echiniscoidinae (). Neoechiniscoides was deeply embedded within various Echiniscoides species. As concerns the new species, E. basalticus sp. nov. was sister to a clade of undescribed Thai species (JQ689574, JQ689575–7; p-distance = 11.5–14.5%); E. lichenophilus sp. nov. was sister to an undescribed Echiniscoides from North-Western Atlantic (JQ689505; p-distance = 17.0–17.5%); E. musa sp. nov. stayed in the tritomy with two undescribed species from Panama (JQ689561 and JQ689562–6; p-distance = 4.8–6.4%); and E. trichosus sp. nov. (JQ689580–1) had unresolved relationships, staying in polytomy with numerous distantly related species ().
Taxonomic account
Phylum: Tardigrada Doyère, Citation1840
Class: Heterotardigrada Marcus, Citation1927
Order: Echiniscoidea Richters, Citation1926
Family: Echiniscoididae Kristensen & Hallas, Citation1980 (amended by Møbjerg et al. Citation2020)
Subfamily: Echiniscoidinae Kristensen & Hallas, Citation1980 (amended by Møbjerg et al. Citation2020)
General remark: Given the remarkable interspecific variability in dorsal sculpturing among Echiniscoides spp. (see Discussion), we elevate all subspecies of E. sigismundi to species level: E. galliensis Kristensen & Hallas, Citation1980 stat. nov., E. groenlandicus Kristensen & Hallas, Citation1980 stat. nov., E. hispaniensis Kristensen & Hallas, Citation1980 stat. nov., E. mediterranicus Kristensen & Hallas, Citation1980 stat. nov., E. polynesiensis Renaud-Mornant, Citation1976 stat. nov., E. porphyrae Grimaldi de Zio et al., Citation2000 stat. nov., E. verrucariae Grimaldi de Zio et al., Citation2000 stat. nov., which is in accordance with recent suggestions (Møbjerg et al. Citation2020; Bartels et al. Citation2021).
Species: Echiniscoides basalticus sp. nov.
,
Table III. Measurements [in µm] of selected morphological structures of individuals of Echiniscoides basalticus sp. nov. (type series) mounted in Hoyer’s medium. Abbreviations: N – number of females/structures measured, Range refers to the smallest and the largest structure among all measured specimens; SD – standard deviation.
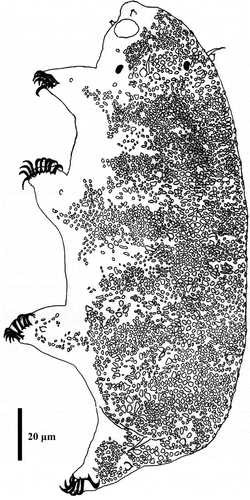
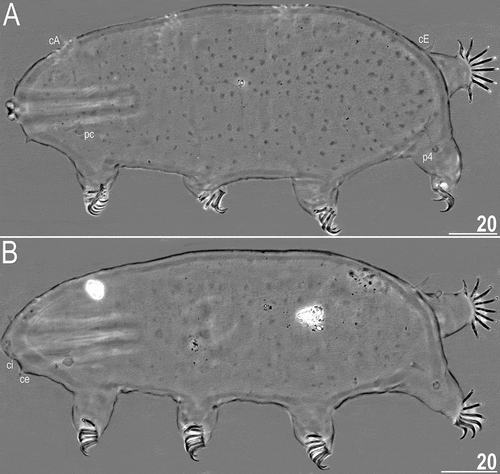
Figure 2. Habitus of Echiniscoides basalticus sp. nov. (PCM): A. holotypic female, B. allotypic male. List of abbreviations (identical for all figures): cA – cirrus A, ce – cirrus externus, cE – cirrus E, ci – cirrus internus, p1–4 – sense organs on legs I–IV, pc – primary clava, sc – secondary clava (cephalic papilla). Scale bars in μm.
http://www.zoobank.org/urn:lsid:zoobank.org:act:C11697D2-F7FD-46E8-9F1F-9ED7A9C4DC33
Type material
Holotype (♀, slide MAU.01.01), allotype (♂, slide MAU.01.06), and four paratypes (3♀♀ and one juvenile, slides MAU.01.02–5). Collected by R.M. Kristensen.
Type locality
20°00ʹ30”S, 57°40ʹ47”E: Indian Ocean, Mauritius, Rivière du Rempart, Grand Gaube.
Etymology
The name underlines the substrate (magmatic rock), which contained a barnacle population inhabited by the new species. An adjective in nominative singular.
Description
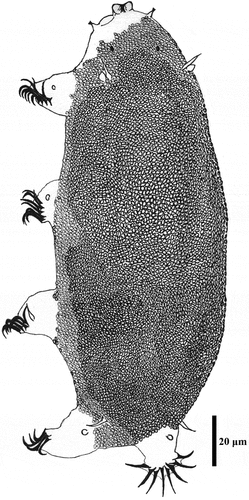
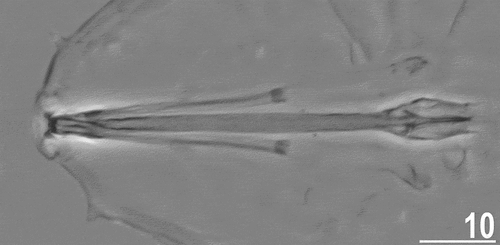
Adult females. Body cylindrical and plump (). Small black eyes present (). Cirri interni and externi conical, of similar length and with weakly tufted tips. Median cephalic cirrus absent. Lateral cirri A and E short, with smooth base and pointed flagellum (). Papillary primary clavae and lenticular secondary clavae present (). Subterminal mouth without mouth plates. Buccal tube 40.9 μm long in the holotype (buccal tube length/body length ratio = 0.22), rigid and wide (2.6 μm). Large stylet furcae dissolved in Hoyer’s medium; stylet supports absent. Pharynx with poorly sclerotised placoids ().
Dorsum sculptured with irregular polygonal tubercles (∼1.0–2.0 μm in diameter) that are poorly elevated above the remaining cuticle and faint in PCM. The sculpturing covers only distal part of the head, but does not reach its proximal part, legs (it vanishes at the limb bases) and venter (). All legs with sensory organs: poorly marked and weakly protruding papillae on legs I and II; thin and spine-like on legs III; large papillae on legs IV, differing from primary clavae by the lack of distal pointing tip.
Anisonych claws; claw formula 10,9,9,8 in three females (including the holotype), and 9,9,9,9 in one female. Gonopore hexapartite, rosette-shaped. Anus trilobed, with two lateral lobes and a short posterior lobe, lacking additional lobes.
Adult male. Body slenderer and shorter than in females (). Claw formula 9,8,8,8. Gonopore oval and weakly protruding. No other differences with regard to females.
Juvenile. Body shorter than in sexually mature individuals. Claw formula 9,9,9,8. Gonopore lacking.
Differential diagnosis
Considering the overall claw variability, we assign the claw formula 9/10,9,9,8 to E. basalticus sp. nov. There are nine species of Echiniscoides with strongly sculptured dorsal cuticle, but they differ from E. basalticus sp. nov...
E. bruni D’Addabbo Gallo et al., Citation1992, by 10,10,10,10 adult claw formula (8,7,7,8 in larvae = first instar juveniles) and long spine-like sensory organs on legs I.
E. costaricensis Bartels & Fontoura, Citation2021, by 7,7,7,6 adult claw formula (5,5,5,5 in larvae) and the conspicuousness of the dorsal sculpture (more elevated granules).
E. galliensis Kristensen & Hallas, Citation1980, by 10,9,10,9 adult claw formula and the morphology of dorsal tubercles seen in light microscope (circular, each with a small central region of discontinuous lines).
E. hispaniensis Kristensen & Hallas, Citation1980, by 7,7,7,6 adult claw formula and the morphology of dorsal tubercles seen in light microscope (arranged like roofing tiles).
E. polynesiensis Renaud-Mornant, Citation1976, by the morphology of dorsal tubercles seen in light microscope (each with an elevation in the centre) and claw shape (central claws bent at a right angle).
E. porphyrae de Zio Grimaldi et al., Citation2000, by the dorsal sculpturing (minute punctuations that form discontinuous belts interrupted by smooth patches) and non-tufted tips of cephalic cirri.
E. ritavargasae Bartels et al., Citation2021, by long spine-like sensory organs on legs II and much larger anus-gonopore distance (22.4–35.6 μm).
E. rugostellatus Perry et al., Citation2018, by the conspicuousness of the dorsal sculpture (more evident granules and dorsal discs that are probably muscle attachment points) and sensory organs I–II unobservable in PCM.
E. verrucariae de Zio Grimaldi et al., Citation2000, by 9,10,9,9 adult claw formula, the dorsal sculpturing (minute punctuations), and non-tufted tips of cephalic cirri.
Species: Echiniscoides bufocephalus sp. nov.
,
Table IV. Measurements [in µm] of selected morphological structures of adult females of Echiniscoides bufocephalus sp. nov. (type series) mounted in Hoyer’s medium. Abbreviations: N – number of specimens/structures measured, Range refers to the smallest and the largest structure among all measured specimens; SD – standard deviation.
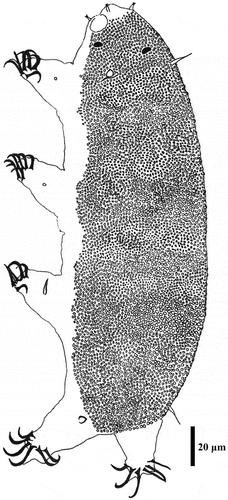
Figure 5. Habitus of the holotypic female of Echiniscoides bufocephalus sp. nov. (PCM). Scale bar in μm.

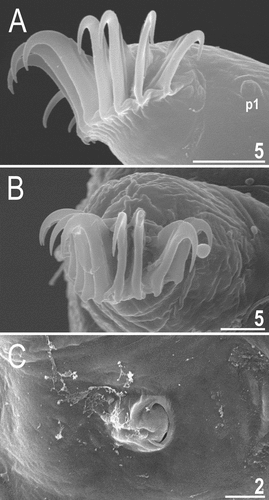
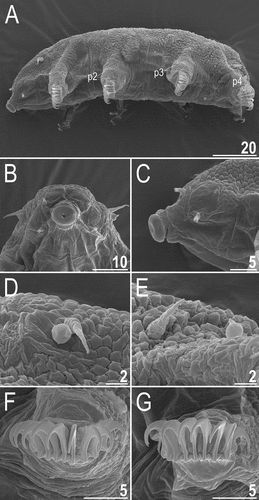
Figure 7. Detailed morphology of Echiniscoides bufocephalus sp. nov.: A. specimen in toto (SEM), B. head in close-up (dorsal view, SEM), C. head in close-up (ventral view, SEM), D. buccal apparatus (PCM). Scale bars in μm.
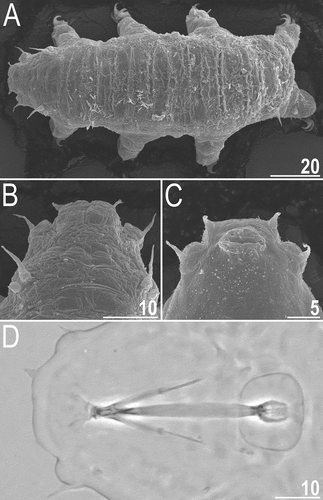
Figure 8. Cephalic and trunk appendages of Echiniscoides bufocephalus sp. nov.: A. peribuccal cirri and secondary clava (PCM), B. cirrus internus in close-up (SEM), C. cirrus A and primary clava (SEM), D. sensory organ on leg II (SEM), E. sensory organ on leg III (PCM), F. cirrus E (SEM). Scale bars in μm.
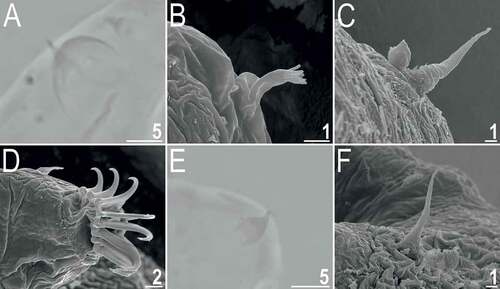
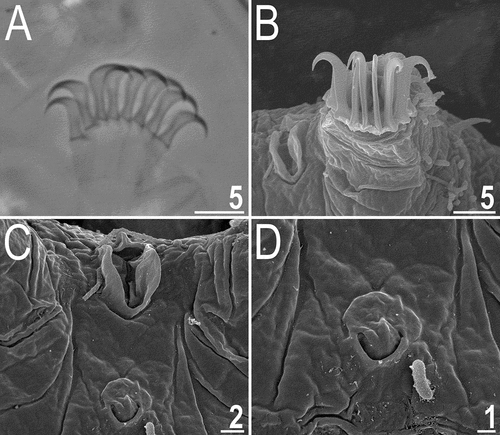
Figure 9. Detailed morphology of Echiniscoides bufocephalus sp. nov.: A. claws I (PCM), B. claws IV (SEM), C. anus and male gonopore (SEM), D. male gonopore in close-up (SEM). Scale bars in μm.
http://www.zoobank.org/urn:lsid:zoobank.org:act:B3FE43AA-ACA8-4B37-AC1A-842BFF625315
Type material
Holotype (♀, slide HT-MA.21), and 28 paratypes (19♀♀, four juveniles, one larva and four specimens of unidentified sex, slides HT-MA.01–21). Collected by R.M. Kristensen.
Type locality
25°59ʹ17”N, 51°01ʹ25”E: Indian Ocean, Persian Gulf, Qatar, Al-Zubarah.
Etymology
From Latin Bufo = toad + Ancient Greek Κέφαλος (kephalos) = head, meaning “toad-headed”, which refers to the unique shape of the cephalic body portion in the new species. An adjective in nominative singular.
Description
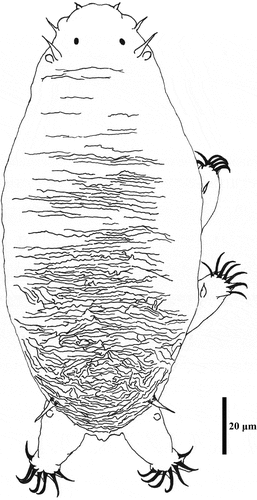
Adult females. Body cylindrical and plump (). Small black eyes present (), dissolving in Hoyer’s medium. Cirri interni short, conical, with weakly tufted tips, and clearly separated from secondary clavae (cephalic papillae); cirri externi elongated and spine-like, embedded in the lateral side of secondary clavae (). Median cephalic cirrus absent. Lateral cirri A and E moderately long and robust, with annulated base and pointed flagellum (, 8C, 8F). Papillary primary clavae and large, tumescent secondary clavae present (). Anteriormost body portion rectangular and well-delimited from protruding secondary clavae (). Subterminal mouth without mouth plates. Buccal tube 30.3 μm long in the holotype (buccal tube length/body length ratio = 0.20), rigid and wide (3.4 μm). Large stylet furcae dissolved in Hoyer’s medium; stylet supports absent. Pharynx with poorly sclerotised placoids ().
Dorsum sculptured with strong irregular wrinkles that are perpendicular to the main body axis, best-delineated in the caudal body portion at the level of legs III (). Legs and venter smooth. All legs with sensory organs: poorly marked and weakly protruding papillae on legs I; thin and spine-like on legs II (), more robust and spine-like on legs III (); large papillae on legs IV (), identical in shape to primary clavae.
Anisonych claws (); claw formula 8,8,7,7, rarely some females have asymmetrically developed seven or nine claws on legs I–II and eight or six claws on legs III–IV. Gonopore hexapartite, rosette-shaped. Anus trilobed, with two lateral lobes and a short posterior lobe, lacking additional lobes.
Adult males. Found only among specimens processed for SEM. Qualitatively alike females, beside of the circular gonopore with a semicircular slit ().
Juveniles. In a single juvenile (in the simplex stage) positioned conveniently for measurements, the body length = 151 μm, and body width = 69 μm. Cephalic appendages: cirrus internus 3.3 μm, secondary clava 2.5 μm high and 5.6 μm wide, cirrus externus 7.2 μm, primary clava 3.6 μm; cirrus A 9.8 μm. Trunk appendages lengths: p1 non-identifiable, p2 4.5 μm, p3 5.7 μm, p4 2.0 μm; cirrus E 8.6 μm. Claw formula 8,9,7,7; heights of branches: 5.7–6.9 μm. Gonopore lacking.
Larva. In a single found larva, the body length = 94 μm, and body width = 40 μm. Buccal apparatus: buccal canal 24.3 μm, pharynx length 8.7 μm, placoid 3.2 μm. Cephalic appendages: cirrus internus 3.1 μm, secondary clava 3.1 μm high and 4.9 μm wide, cirrus externus 4.4 μm, primary clava 2.8 μm; cirrus A 6.3 μm. Trunk appendages lengths: p1 1.1 μm, p2 3.0 μm, p3 4.7 μm, p4 1.9 μm; cirrus E 6.9 μm. Claw formula 8,7,7,7; heights of branches: 4.3–5.4 μm. Gonopore and anus lacking.
Differential diagnosis
There are few Echiniscoides species with spine-like sensory organs on legs II, and only one of them, E. ritavargasae, exhibits sculptured dorsal cuticle, but it can be easily told apart from E. bufocephalus sp. nov. based on the type of sculpturing (circular tubercles instead of strong irregular wrinkling), adult claw formula 9,9,9,9, short, tufted cirri externi (2.5–5.2 μm in E. ritavargasae vs 5.6–8.2 μm in E. bufocephalus sp. nov.) clearly separated from secondary clavae, and much larger distance between anus and gonopore (22.4–35.6 μm in E. ritavargasae vs 10.7–16.1 μm in E. bufocephalus sp. nov.).
Echiniscoides lichenophilus sp. nov.
,
Table V. Measurements [in µm] of selected morphological structures of adult females of Echiniscoides lichenophilus sp. nov. (type series) mounted in Hoyer’s medium. Abbreviations: N – number of specimens/structures measured, Range refers to the smallest and the largest structure among all measured specimens; SD – standard deviation.
Table VI. Measurements [in µm] of selected morphological structures of adult males of Echiniscoides lichenophilus sp. nov. (type series) mounted in Hoyer’s medium. Abbreviations: N – number of specimens/structures measured, Range refers to the smallest and the largest structure among all measured specimens; SD – standard deviation.
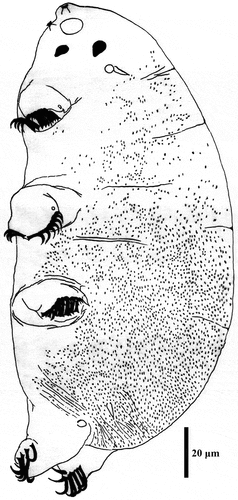
Figure 10. Habitus of the holotypic female of Echiniscoides lichenophilus sp. nov. (PCM). Scale bar in μm.
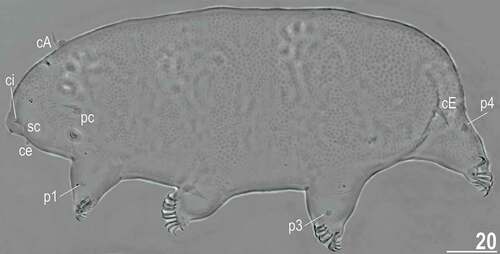
Figure 12. Detailed morphology of Echiniscoides lichenophilus sp. nov. (DIC): A. buccal apparatus, B. caudal body portion with cirri E, C. claws II and sense organ on leg II. Scale bars in μm.
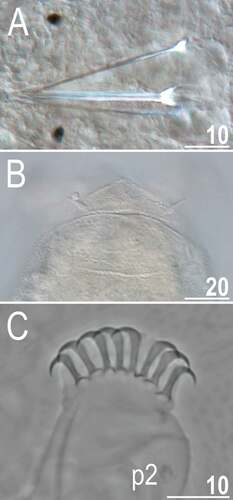
Figure 13. Detailed morphology of Echiniscoides lichenophilus sp. nov. (SEM): A. specimen in toto, B. cephalic body portion with peribuccal cirri in frontal view, C. cephalic body portion with peribuccal cirri in lateral view, D. cirrus A and primary clava, E. cirrus E and sense organ on leg IV, F. claws II, G. claws III. Scale bars in μm.
http://www.zoobank.org/urn:lsid:zoobank.org:act:97830353-CE65-4F9E-AD3A-7F80F9F4B448
Type material
Holotype (♀, slide DR.01.03), allotype (♂, slide DR.01.31), and 98 paratypes (35♀♀, 35♂♂, and 28 juveniles, slides DR.01.01–39). Collected by R.M. Kristensen.
Type locality
18°20ʹ54”N, 68°49ʹ54”W: Atlantic Ocean, Antilles, Hispaniola, Bayahíbe, La Roca Beach.
Etymology
From Ancient Greek λειχήν (leíkhō = to lick, liverwort) + φῐ́λος (phílos = love) = “loving lichens”. The name alludes to the substrate in which the new species was found. An adjective in nominative singular.
Description
Adult females. Body cylindrical (, 13A). Large black eyes present (). Cirri interni and externi conical, of similar length and with weakly tufted tips (). Median cephalic cirrus absent. Lateral cirri A and E short (the latter slightly longer), with smooth base and pointed flagellum (, , ). Papillary primary clavae (, ) and lenticular secondary clavae present (, ). Subterminal mouth without mouth plates. Buccal tube 41.5 μm long in the holotype (buccal tube length/body length ratio = 0.16), rigid and wide (2.1 μm). Large stylet furcae; stylet supports absent. Pharynx with poorly sclerotised placoids ().
Dorsum strongly sculptured with irregular polygonal tubercles (∼0.5–2.0 μm in diameter) that are well-elevated above the remaining cuticle and easily identifiable under various microscopes (, , ). The sculpturing covers head and extends towards distal parts of legs (, ), but venter is smooth. All legs with sensory organs: poorly marked and weakly protruding papillae on legs I and II; evident papillae on legs III; large papillae on legs IV of the shape analogous to primary clavae (, ).
Anisonych claws (); claw numbers particularly variable and instable – claw formula 8/12,8/12,8/10,8/9, lacking a dominant configuration. Gonopore hexapartite, rosette-shaped. Anus trilobed, with two lateral lobes and a short posterior lobe, lacking additional lobes.
Adult males. Body slenderer and shorter than in females. Claw formula 8/10,8/10,8/9,7/8; in general, males characterised by the body size comparable to that of females have 1–2 claws less per limb. Gonopore oval and weakly protruding. No other differences with regard to females.
Juveniles. Body shorter than in sexually mature females, but overlapping with males. Claw formula 9,9,9,8. Gonopore lacking.
Differential diagnosis
There is only one species of Echiniscoides exhibiting papilliform sensory organs on legs III – E. travei Bellido & Bertrand, Citation1981, but it has smooth cuticle, its representatives attain much larger body size (up to 420 μm), and sensory organs on legs I are not identifiable under PCM.
Echiniscoides musa sp. nov.
,
Table VII. Measurements [in µm] of selected morphological structures of adults of Echiniscoides musa sp. nov. (type series) mounted in Hoyer’s medium. Abbreviations: SD – standard deviation.
Figure 14. Detailed morphology of Echiniscoides musa sp. nov. (PCM): A. holotypic female, B. anterolateral body portion of paratypic female, C. buccal apparatus, D. claws II. Scale bars in μm.
http://www.zoobank.org/urn:lsid:zoobank.org:act:C16D5AE5-2BAB-4620-8D7B-C2E558C466E6
Type material
Holotype (♀, slide B1), allotype (♂, slide B2), and one paratype (♀, slide B3). Collected by R.M. Kristensen.
Type locality
25°25ʹ19”S, 48°24ʹ26”W: Atlantic Ocean, Baía de Paranaguá, Ilha das Bananas.
Etymology
Musa L. is the generic name of banana. As the new species was found at shores of Banana Island, we created an eponym for it. A noun in apposition.
Description
Adult females. Body cylindrical and elongated (). Large black eyes present (). Cirri interni and externi conical, of similar length and with tufted tips. Median cephalic cirrus absent. Lateral cirri A and E short and of similar length, with smooth base and pointed flagellum (). Papillary primary clavae and lenticular secondary clavae present (). Subterminal mouth without mouth plates. The holotype is in the simplex stage.
Dorsum strongly sculptured with irregular polygonal tubercles (∼1.0–2.2 μm in diameter) that are well-elevated above the remaining cuticle and easily identifiable under PCM (). The sculpturing covers head, but dorsolateralmost body portions, legs and ventral side are smooth (). All legs with sensory organs: poorly marked and weakly protruding papillae on legs I and II; thin and spine-like on legs III; large papillae on legs IV of the shape analogous to primary clavae ().
Anisonych claws (); claw formula 7,6,7,6. Gonopore hexapartite, rosette-shaped. Anus trilobed, with two lateral lobes and a short posterior lobe, lacking additional lobes.
Adult male. Buccal tube 36.5 μm long in the allotype (buccal tube length/body length ratio = 0.18), rigid and wide (2.2 μm). Large stylet furcae; stylet supports absent. Pharynx with poorly sclerotised placoids (). Claw formula 7,7,6,6. Gonopore oval and weakly protruding. Sexual dimorphism weakly marked.
Juveniles. Not found.
Differential diagnosis
With the adult claw formula 7,6/7,6/7,6, E. musa sp. nov. is similar to only two other species, but it is easily distinguishable from:
E. costaricensis, because the latter exhibits higher conspicuousness of the dorsal sculpture (more elevated granules), which covers legs, and the annulated bases of cirri A and E.
E. hispaniensis, because the latter has different dorsal tubercles when seen in light microscope (arranged like roofing tiles) and sensory organs on legs I–II not identifiable in PCM.
Echiniscoides trichosus sp. nov.
,
Table VIII. Measurements [in µm] of selected morphological structures of adult females of Echiniscoides trichosus sp. nov. (type series) mounted in Hoyer’s medium. Abbreviations: N – number of specimens/structures measured, Range refers to the smallest and the largest structure among all measured specimens; SD – standard deviation.
Figure 16. Habitus of the holotypic female of Echiniscoides trichosus sp. nov. (PCM). Scale bar in μm.
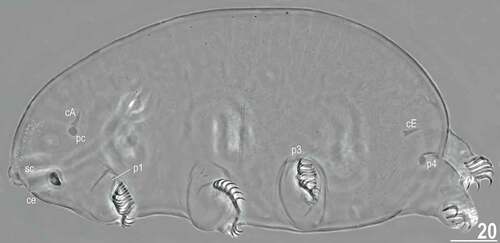
Figure 18. Detailed morphology of Echiniscoides trichosus sp. nov. (all but A in SEM): A. specimen in toto (DIC), B. dorsal sculpturing in the form of microtrichia, C. cephalic body portion with peribuccal cirri in frontal view, D. cephalic body portion with peribuccal cirri in lateral view, E. anterolateral body portion of paratypic female. Scale bars in μm.
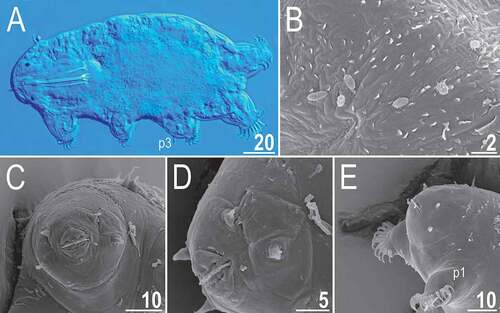
Figure 19. Dorsal sculpturing of Echiniscoides trichosus sp. nov. in close-up (SEM). Scale bars in μm.
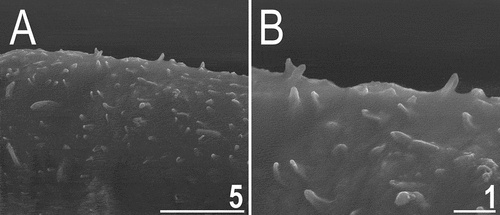
Figure 20. Detailed morphology of Echiniscoides trichosus sp. nov. (all but D in SEM): A. specimen in toto in dorsal view, B–C. specimens in toto in lateroventral view, D. claws III and sense organ on leg III (PCM), E. claws III, F. claws IV. Scale bars in μm.
http://www.zoobank.org/urn:lsid:zoobank.org:act:CE33291E-E920-483D-8358-8713AB385533
Type material
Holotype (♀, slide RN.01.07), allotype (♂, slide RN.01.04), and 15 paratypes (13♀♀ and 2♂♂, slides RN.01.01–14). Collected by R.M. Kristensen.
Type locality
27°04ʹ21”S, 109°19ʹ28”W: Pacific Ocean, Rapa Nui (Easter Island), Anakena Beach.
Etymology
From Ancient Greek θρίξ (thríx = hair), referring to the peculiar dorsal sculpturing that reminds of hair. An adjective in nominative singular.
Description
Adult females. Body plump (, , ). Large black eyes present (). Cirri interni and externi conical, of similar length and with weakly tufted tips (). Median cephalic cirrus absent. Lateral cirri A and E short and of similar length, with smooth base and pointed flagellum (). Papillary primary clavae and lenticular secondary clavae present (, ). Subterminal mouth without mouth plates. Buccal tube 53.9 μm long in the holotype (buccal tube length/body length ratio = 0.29), rigid and wide (2.7 μm). Large stylet furcae; stylet supports absent. Pharynx with poorly sclerotised placoids ().
Dorsum seems finely punctuated under PCM (), but it consists of unique, minute cuticle protrusions called here microtrichia (). They are well-identifiable under SEM (, ), and their density is highest in the caudal body portion, gradually becoming less numerous towards head (). The sculpturing does not cover head (), legs and ventral body side. All legs with sensory organs: poorly marked and weakly protruding papillae on legs I and II (, ); thin and spine-like on legs III (, , ); large papillae on legs IV of the shape analogous to primary clavae ().
Anisonych claws (); claw formula 11,11/13,11/13,10; single female exhibits 12 claws on the first pair of legs, and the most common claw configuration is 11,11,11,10. Gonopore hexapartite, rosette-shaped. Anus trilobed, with two lateral lobes and a short posterior lobe, lacking additional lobes.
Adult males. Body of similar size and shape to that of females. Gonopore oval and weakly protruding. Sexual dimorphism weakly marked.
Juveniles. Not found.
Differential diagnosis
With the adult claw formula 11,11/13,11/13,10, E. trichosus sp. nov. is similar to only two other species, but it is easily distinguishable from:
E. mediterranicus Kristensen & Hallas, Citation1980, because the latter exhibits smooth or wrinkled cuticle, and sensory organs on legs I–II are not identifiable under PCM.
E. travei, because the latter has papillary sensory organs on legs III, smooth cuticle, much larger body size (up to 420 μm), and sensory organs on legs I are not identifiable under PCM.
Echiniscoides hispaniensis Kristensen & Hallas, Citation1980
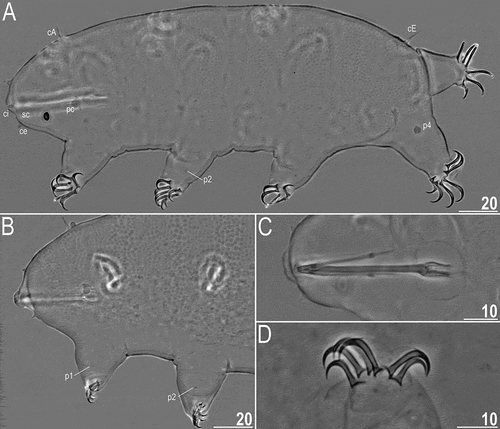
Figure 21. Habitus of Echiniscoides hispaniensis Kristensen & Hallas, Citation1980 stat. nov. (SEM): A. female in lateral view, B. female in anterolateral view, C. male in ventral view, D. female in ventral view. Scale bars in μm.
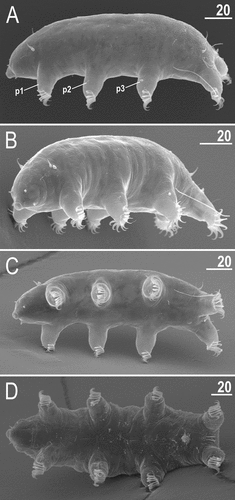
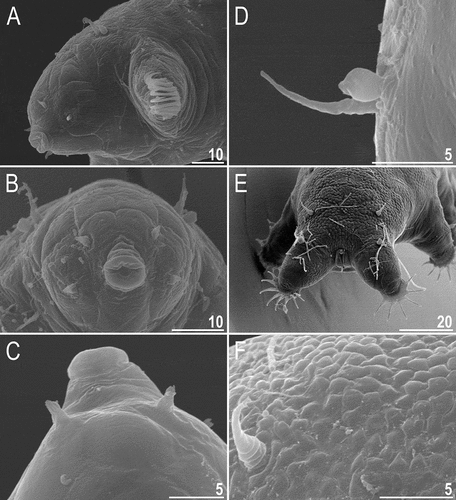
Figure 22. Detailed morphology of Echiniscoides hispaniensis (SEM): A. cephalic body portion with peribuccal cirri in lateral view, B. cephalic body portion with peribuccal cirri in frontal view, C. peribuccal cirri and secondary clava in close-up, D. cirrus A and primary clava, E. caudal body portion, F. cirrus E and dorsal sculpturing in close-up. Scale bars in μm.
New material from Atlantic Ocean
5♀♀ and 2♂♂ collected from barnacles nearby the lagoon Ria de Aveiro in Central Portugal. Collected by R.M. Kristensen.
Updated shortened description
Adult females. Body plump (). Cirri interni and externi conical, of similar length and with weakly tufted tips (). Median cephalic cirrus absent. Lateral cirri A and E short and of similar length, with annulated base and pointed flagellum (). Papillary primary clavae and lenticular secondary clavae present (). Subterminal mouth without mouth plates.
Dorsum with flat epicuticular granules () in SEM that become poorly elevated in the proximal body part (). The sculpturing does not cover lateralmost sides of the dorsum, legs and ventral body side (). All legs with sensory organs: poorly marked and weakly protruding papillae on legs I and II (); thin and spine-like on legs III (); large papillae on legs IV of the shape analogous to primary clavae.
Isonych claws (); claw formula 7,7,7,6. Gonopore hexapartite, rosette-shaped (). Anus trilobed, with two lateral lobes and a short posterior lobe, lacking additional lobes; it is placed dorsally rather than ventrally ().
Adult males. Body elongated (), but qualitatively like in females beside of the circular gonopore ().
Discussion
Cuticular sculpturing in Echiniscoidea
The utility of the cuticular ultrastructure as a systematic criterion has been demonstrated several times for both Heterotardigrada and Eutardigrada. In the latter, the most striking example is that of the Murrayidae, which share the plesiomorphic presence of pillars with Heterotardigrada (Kristensen Citation1982; Guidetti et al. Citation2000), whereas the remaining Macrobiotoidea are characterised by the apomorphic lack of pillars in cuticle. In contrast to the great majority of eutardigrades with a smooth cuticle, heterotardigrade cuticle typically contains pillars (Greven Citation1975; Kristensen & Neuhaus Citation1999) that may be particularly elongated, e.g. in the genera Raiarctus (Jørgensen et al. Citation2014) and Rhomboarctus (Hansen et al. Citation2003).
Dorsum is typically sculptured in representatives of Echiniscoidea: it consists of both endocuticular pillars and epicuticular granules/tubercles in Carphaniidae, Oreellidae and Isoechiniscoidinae (Binda & Kristensen Citation1986; Dastych et al. Citation1998; Møbjerg et al. Citation2016). Echiniscoidinae lack pillars (Møbjerg et al. Citation2016) and sometimes their cuticle is completely smooth (Kristensen & Hallas Citation1980; Chang & Rho Citation1998), whereas sculpturing may be variously developed in Echiniscidae, frequently forming richly ornamented patterns (e.g. Kristensen Citation1987; Guil et al. Citation2013; Gąsiorek et al. Citation2019). The interspecific variability mirrored in dorsal sculpturing of Echiniscoididae is so pronounced (e.g. compare E. sigismundi () with Neoechiniscoides horningi (Miller & Kristensen, Citation1999) and an undescribed species from Pacific Ocean ()) that, after four decades since the first echiniscoidid revision (Kristensen & Hallas Citation1980), we decided to elevate all subspecies of E. sigismundi to species level. This move is supported by significant disparities in claw numbers, cuticle morphotypes and geographic isolation of these taxa as well (only E. groenlandicus + E. sigismundi and E. porphyrae + E. verrucariae were found in the same localities). Some former subspecies of E. sigismundi require SEM examinations to uncover the three-dimensional structure of cuticular tubercles, as we documented that the cuticle looks different in the scanning compared to the light microscopy for E. hispaniensis. This would be relevant in the case of E. galliensis, in which a central circle of discontinuous lines visible in light microscopy supposedly represents an irregular wrinkling at the surface of tubercles, or E. groenlandicus that exhibits punctuations variable in size and shape (Kristensen & Hallas Citation1980).
Figure 24. Cuticular morphotypes within Echiniscoides (SEM): smooth cuticle – A–B. Echiniscoides sigismundi from Julebæk Strand (Sjælland, neotype locality), rugose cuticle – C. an undescribed Echiniscoides species from Los Angeles. Scale bars in μm.
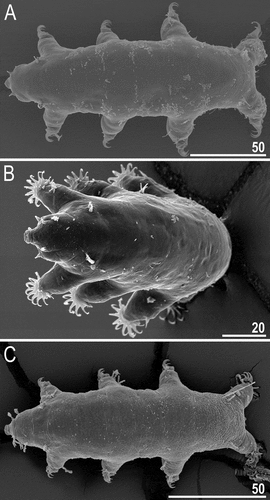
The division of the family Echiniscoididae into two subfamilies based on several morphological criteria, including the development of cuticular pillars, is firmly supported in our COI-based phylogeny (), and further corroborates the usefulness of pillars in tardigrade classification (Møbjerg et al. Citation2018). In the future, when the splitting of Echiniscoides into new genera advances, it will be intriguing to investigate whether main sculpturing morphotypes (e.g. punctuations vs large polygons) correspond with molecularly reconstructed clades and could be introduced into putative diagnoses of newly erected echiniscoidid taxa.
Taxonomic key to Echiniscoididae
Summary of ecomorphological traits pertaining solely to Echiniscoides is shown in . The key presented below deals with traits observable in sexually mature (adult) specimens of all Echiniscoididae, and substitutes the narrow key from Kristensen and Hallas (Citation1980). Given the wide variability in claw formula in some species, we used that trait as a last resort in certain cases.
Table IX. Main characteristics of described species of Echiniscoides.
1. Typically six isonych claws, pillars in the cuticle, long cirri E; primarily interstitial, truly marine species .................... (Isoechiniscoidinae) 2
–. Six to thirteen (most often seven to ten) anisonych or isonych claws, lacking pillars in the cuticle, short cirri E; usually inhabiting barnacles, algae and lichens in tidal zones (Echiniscoidinae) 3
2(1). Females much larger than males (prominent sexual dimorphism), single dorsal mouth plate, lacking mid-dorsal plates in the caudal body portion, hexapartite female gonopore without swellings ...................... Isoechiniscoides higginsi
–. Females similar to males in body size (small sexual dimorphism), two dorsal mouth plates, a pair of mid-dorsal plates located in the caudal body portion, hexapartite female gonopore with ring-like swellings, usually present in marine Heterotardigrada ............... Isoechiniscoides sifae
3(1). The anal system comprising the paired additional lobes (wings) associated with the lateral lobes of the anus and the posterior lobe (Neoechiniscoides) 4
–. The anal system comprising three lobes: two lateral and one posterior .....................................................................................(Echiniscoides) 6
4(3). Subcephalic cuticle strongly sculptured 5
–. Subcephalic cuticle smooth Neoechiniscoides horningi
5(4). Two paired and a single unpaired muscle attachment sites devoid of polygonal sculpture present, peribuccal cirri terminate in a single hair-like structure, three annulated oral rings present Neoechiniscoides aski
–. Muscle attachment sites absent (caudal sculpturing continuous), peribuccal cirri terminate in small bundle of eight or nine villi, two dorsal mouth plates ............... Neoechiniscoides pollocki
6(3). Papilliform sensory organs on legs III 7
–. Spine-like (elongated) sensory organs on legs III 8
7(6). Strongly sculptured cuticle (polygonal tubercles), sensory organs on legs I identifiable under PCM as minute papillae Echiniscoides lichenophilus
–. Smooth cuticle, sensory organs on legs I not identifiable under PCM Echiniscoides travei
8(6). Spine-like (elongated) sensory organs on legs II 9
–. Papilliform sensory organs on legs II or organs not identifiable under PCM 12
9(8). Cuticle sculptured 10
–. Cuticle smooth 11
10(9). Cuticle sculptured intensely in the caudal body portion (circular tubercles), adult claw formula 9,9,9,9, cirri externi short, tufted, and clearly separated from secondary clavae Echiniscoides ritavargasae
–. Cuticle strongly and irregularly wrinkled perpendicularly to the body axis, adult claw formula 8,8,7,7, cirri externi elongated, spine-like, and attached to lateral side of secondary clavae Echiniscoides bufocephalus
11(9). Buccal tube flexible, calcium carbonate encrustations present in the placoids, living in the crevices between plates of the carapace of Semibalanus balanoides Echiniscoides wyethi
–. Buccal tube rigid, calcium carbonate encrustations absent in the placoids, living in the basement membrane or within the brood chamber (marsupium) of Semibalanus balanoides Echiniscoides hoepneri
12(8). Cuticle smooth 13
–. Cuticle sculptured 15
13(12). Adult claw formula 11,12,11,10 Echiniscoides mediterranicus
–. Adults typically with less than 10 claws per leg 14
14(13). Adult claw formula 9,9,9,8; stylets rigid Echiniscoides sigismundi
–. Adult claw formula 8,8,8,7; flexible stylets tapering with wide basal portion to sharp apical point ...................... Echiniscoides andamanensis
15(12). Cuticle punctuated in light microscope 16
–. Cuticle strongly sculptured (tubercles usually in the form of large polygons, more rarely circular granules) 19
16(15). Adult claw formula 11,11/13,11/13,10 Echiniscoides trichosus
–. Adults typically with less than 10 claws per leg 17
17(16). Cuticle covered with minute granules of uniform size 18
–. Cuticle covered with variable punctuations: minute granules (points), slightly larger hemispherical granules, and minute irregular polygons .............. Echiniscoides groenlandicus
18(17). Evident punctuated sculpturing with smooth discs corresponding with the muscle attachment points .......... Echiniscoides porphyrae
–. Faint punctuated sculpturing lined on thin and parallel, irregularly arranged cuticular wrinkles ..................................................... ..............................Echiniscoides verrucariae
19(15). Spine-like (elongated) sensory organs on legs I ................................... Echiniscoides bruni
–. Papilliform sensory organs on legs I or organs not identifiable under PCM 20
20(19). Cuticular tubercles peculiarly arranged like roofing tiles under PCM Echiniscoides hispaniensis
–. Cuticular tubercles not overlapping under PCM 21
21(20). Cuticular tubercles looks like circles inside which a smaller circle of discontinuous lines can be observed in light microscope Echiniscoides galliensis
–. Cuticular tubercles in the form of polygons or circles without the discontinuous lines identifiable in light microscope 22
22(21). Adults with 6–7 claws per leg 23
–. Adults with 8–10 claws per leg 24
23(22). Cuticular tubercles (mostly polygonal, some circular in shape) elevated and conspicuous in light microscope, sculpturing covers legs, annulated bases of cirri A and E Echiniscoides costaricensis
–. Cuticular tubercles only polygonal, poorly elevated and faint in light microscope, sculpturing does not cover legs, smooth bases of cirri A and E Echiniscoides musa
24(22). Cuticular tubercles with an elevation in the centre ......................... Echiniscoides polynesiensis
–. Cuticular tubercles without an elevation in the centre 25
25(24). Poorly marked cuticular granules and no dorsal discs, sensory organs I–II identifiable in PCM as minute papillae ..................... Echiniscoides basalticus
–. Conspicuous cuticular granules and regularly arranged dorsal discs = muscle attachment points, sensory organs I–II unidentifiable in PCM Echiniscoides rugostellatus
Supporting information
Supplementary Material 1. List of primers and PCR programmes.
Supplementary Material 2. COI alignment used in phylogenetic inference.
Supplementary Material 3. COI p-distances.
Supplemental Material
Download MS Excel (173.7 KB)Supplemental Material
Download (175.5 KB)Supplemental Material
Download MS Word (34.8 KB)Acknowledgements
We are grateful to Maikon Di Domenico (Federal University of Parana, Centre for Marine Studies, Pontal do Paraná, Brazil) for the support during sampling in Brazil. The study was supported by the Preludium programme (grant no. 2019/33/N/NZ8/02777 to PG) funded by the Polish National Science Centre. PG’s internships in Copenhagen were financed from the sources of the Carlsberg Foundation (CF16-0238) and the project WIN – Supporting Interdisciplinary Character of PhD Programme in Biology. RMK received funding from the Carlsberg Foundation (2009_01_0053, CF2012_01_0123 and CF16-0238) and the Danish Natural Science Research Council (FNU272-08-0576).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2022.2079737.
Additional information
Funding
References
- Bartels PJ, Fontoura P, Nelson DR, Orozco-Cubero S, Mioduchowska M, Gawlak M, Kaczmarek Ł, Cortés J. 2021. A trans-isthmus survey of marine tardigrades from Costa Rica (Central America) with descriptions of seven new species. Marine Biology Research 17(2):120–166. DOI:10.1080/17451000.2021.1901936.
- Bellido A, Bertrand M. 1981. Echiniscus travei n. sp., un tardigrade marin des iles Kerguelen (Heterotardigrada). Bulletin du Muséum National d’Histoire Naturelle 3(3):789–798.
- Binda MG, Kristensen RM. 1986. Notes on the genus Oreella (Oreellidae) and the systematic position of Carphania fluviatilis Binda, 1978 (Carphanidae fam. nov., Heterotardigrada). Animalia 13(1–3):9–20.
- Bussau C. 1992. New deep-sea Tardigrada (Arthrotardigrada, Halechiniscidae) from a manganese nodule area of the eastern South Pacific. Zoologica Scripta 21(1):79–91. DOI:10.1111/j.1463-6409.1992.tb00311.x.
- Casquet JT, Thebaud C, Gillespie RG. 2012. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Molecular Ecology Resources 12(1):136–141. DOI:10.1111/j.1755-0998.2011.03073.x.
- Cerca J, Purschke G, Struck TH. 2018. Marine connectivity dynamics: Clarifying cosmopolitan distributions of marine interstitial invertebrates and the meiofauna paradox. Marine Biology 165(8):123. DOI:10.1007/s00227-018-3383-2.
- Chang CY, Rho HS. 1998. Three new tardigrade species associated with barnacles from the Thai coast of Andaman Sea. Korean Journal of Biological Sciences 2(3):323–331. DOI:10.1080/12265071.1998.9647426.
- Clausen LKB, Andersen KN, Hygum TL, Jørgensen A, Møbjerg N. 2014. First record of cysts in the tidal tardigrade Echiniscoides sigismundi. Helgoland Marine Research 68(4):531–537. DOI:10.1007/s10152-014-0409-0.
- D’Addabbo Gallo M, de Zio Grimaldi S, Morone de Lucia RM, Troccoli A. 1992. Halechiniscidae and Echiniscoididae from the Western Mediterranean Sea (Tardigrada: Heterotardigrada). Cahiers de Biologie Marine 33:299–318.
- Dastych H, McInnes SJ, Claxton SK. 1998. Oreella mollis Murray, 1910 (Tardigrada): A redescription and revision of Oreella. Mitteilungen aus den Hamburgischen Zoologischen Museum und Institut 95:89–113.
- de Zio Grimaldi S, Gallo D’Addabbo M, Pietanza R. 2000. Two new sub-Antarctic Echiniscoididae from Marion Island (Heterotardigrada, Echiniscoidea). Italian Journal of Zoology 67(2):221–228. DOI:10.1080/11250000009356315.
- Doyère M. 1840. Mémoire sur les tardigrades. Annales Des Sciences Naturelles, Paris 14:269–362.
- Faurby S, Jørgensen A, Kristensen RM, Funch P. 2011. Phylogeography of North Atlantic intertidal tardigrades: Refugia, cryptic speciation and the history of the Mid-Atlantic Islands. Journal of Biogeography 38(8):1613–1624. DOI:10.1111/j.1365-2699.2011.02533.x.
- Faurby S, Barber P. 2015. Extreme population subdivision despite high colonization ability: Contrasting regional patterns in intertidal tardigrades from the west coast of North America. Journal of Biogeography 42(6):1006–1017. DOI:10.1111/jbi.12500.
- Faurby S, Jørgensen A, Kristensen RM, Funch P. 2012. Distribution and speciation in marine tidal tardigrades: A test of the roles of climate and geographic isolation. Journal of Biogeography 39(9):1596–1607. DOI:10.1111/j.1365-2699.2012.02720.x.
- Fontoura P, Bartels PJ, Jørgensen A, Kristensen RM, Hansen JG. 2017. A dichotomous key to the genera of the marine heterotardigrades (Tardigrada). Zootaxa 4294(1):1–45. DOI:10.11646/zootaxa.4294.1.1.
- Fujimoto S, Jørgensen A, Hansen JG. 2017. A molecular approach to arthrotardigrade phylogeny (Heterotardigrada, Tardigrada). Zoologica Scripta 46(4):496–505. DOI:10.1111/zsc.12221.
- Gąsiorek P, Morek W, Stec D, Michalczyk Ł. 2019. Untangling the Echiniscus Gordian knot: Paraphyly of the “arctomys group” (Heterotardigrada: Echiniscidae). Cladistics 35(6):633–653. DOI:10.1111/cla.12377.
- Greven H. 1975. New results and considerations regarding the fine structure of the cuticle in tardigrades. In: Higgins RP, ed. International Symposium on Tardigrades, 1974. Memorie dell’Istituto Italiano di Idrobiologia 32(Suppl.):113–131.
- Grimaldi de Zio S, D’Addabbo Gallo M, Morone de Lucia MR, Daddabbo L. 1987. Marine Arthrotardigrada and Echiniscoidea (Tardigrada, Heterotardigrada) from the Indian Ocean. Bollettino di Zoologia 54(4):347–357. DOI:10.1080/11250008709355608.
- Guidetti R, Rebecchi L, Bertolani R. 2000. Cuticle structure and systematics of the Macrobiotidae (Tardigrada, Eutardigrada). Acta Zoologica 81(1):27–36. DOI:10.1046/j.1463-6395.2000.00034.x.
- Guil N, Jørgensen A, Giribet G, Kristensen RM. 2013. Congruence between molecular phylogeny and cuticular design in Echiniscoidea (Tardigrada, Heterotardigrada). Zoological Journal of the Linnean Society 169(4):713–736. DOI:10.1111/zoj12090.
- Hall TA. 1997. BIOEDIT: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95–98.
- Hallas TE, Kristensen RM. 1982. Two new species of the tidal genus Echiniscoides from Rhode Island, U.S.A. (Echiniscoididae, Heterotardigrada). In: Nelson DR, editor. Proceedings of the Third International Symposium on the Tardigrada, August 3–6, 1980. Johnson City, Tennessee, USA. Johnson City: East Tennessee State University Press. pp. 179–192.
- Hansen JG, D’Addabbo Gallo M, de Zio Grimaldi S. 2003. A comparison of morphological characters within the genus Rhomboarctus (Tardigrada: Heterotardigrada) with the description of two new species. Zoologischer Anzeiger 242(1):83–96. DOI:10.1078/0044-5231-00089.
- Hygum TL, Clausen LKB, Halberg KA, Jørgensen A, Møbjerg N. 2016. Tun formation is not a prerequisite for desiccation tolerance in the marine tidal tardigrade Echiniscoides sigismundi. Zoological Journal of the Linnean Society 178(4):907–911. DOI:10.1111/zoj.12444.
- Jørgensen A, Kristensen RM. 2001. A new tanarctid arthrotardigrade with buoyant bodies. Zoologischer Anzeiger 240(3–4):425–439. DOI:10.1078/0044-5231-00051.
- Jørgensen A, Boesgaard TM, Møbjerg N, Kristensen RM. 2014. The tardigrade fauna of Australian marine caves: With descriptions of nine new species of Arthrotardigrada. Zootaxa 3802(4):401–443. DOI:10.11646/zootaxa.3802.4.1.
- Kristensen RM. 1980. Zur Biologie des marinen Heterotardigraden Tetrakentron synaptae. Helgoländer Meeresuntersuchungen 34(2):165–177. DOI:10.1007/BF01984038.
- Kristensen RM. 1982. New aberrant eutardigrades from homothermic springs on Disko Island, West Greenland. In: Nelson DR, editor. Proceedings of the Third International Symposium on the Tardigrada, August 3–6, 1980. Johnson City, Tennessee, USA. Johnson City: East Tennessee State University Press. pp. 203–220.
- Kristensen RM. 1987. Generic revision of the Echiniscidae (Heterotardigrada), with a discussion of the origin of the family. In: Bertolani R, editor. Biology of tardigrades. Selected symposia and monographs U.Z.I. Mucchi, Modena. Vol. 1. pp. 261–335.
- Kristensen RM, Hallas TE. 1980. The tidal genus Echiniscoides and its variability, with erection of Echiniscoididae fam. n. (Tardigrada). Zoologica Scripta 9(1–4):113–127. DOI:10.1111/j.1463-6409.1980.tb00657.x.
- Kristensen RM, Neuhaus B. 1999. The ultrastructure of the tardigrade cuticle with special attention to marine species. Zoologischer Anzeiger 238(3–4):261–281.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870–1874. DOI:10.1093/molbev/msw054.
- Lanfear R, Calcott B, Ho SY, Guindon S. 2012. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29(6):1695–1701. DOI:10.1093/molbev/mss020.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2017. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34(3):772–773. DOI:10.1093/molbev/msw260.
- Marcus E. 1927. Zur Anatomie und Ökologie mariner Tardigraden. Zoologische Jahrbücher Abteilung Für Systematik 53:487–558.
- Miller WR, Kristensen RM. 1999. Tardigrades of the Australian Antarctic: A new species of the marine genus Echiniscoides from Macquarie Island, Subantarctica. Zoologischer Anzeiger 238(3–4):289–294.
- Møbjerg N, Kristensen RM, Jørgensen A. 2016. Data from new taxa infer Isoechiniscoides gen. nov. and increase the phylogenetic and evolutionary understanding of echiniscoidid tardigrades (Echiniscoidea: Tardigrada). Zoological Journal of the Linnean Society 178(4):804–814. DOI:10.1111/zoj.12500.
- Møbjerg N, Jørgensen A, Kristensen RM, Neves RC. 2018. Morphology and functional anatomy. In: Schill RO, editor. Water Bears: The Biology of Tardigrades, Zoological Monographs 2, Chapter 2. Cham: Springer. pp. 57–94. DOI:10.1007/978-3-319-95702-9_2.
- Møbjerg N, Jørgensen A, Kristensen RM. 2020. Ongoing revision of Echiniscoididae (Heterotardigrada: Echiniscoidea), with the description of a new interstitial species and genus with unique anal structures. Zoological Journal of the Linnean Society 188(3):663–680. DOI:10.1093/zoolinnean/zlz122.
- Perry ES, Miller WR. 2015. Echiniscoides wyethi, a new marine tardigrade from Maine, U.S.A. (Heterotardigrada: Echiniscoidea: Echiniscoididae). Proceedings of the Biological Society of Washington 128(1):103–110. DOI:10.2988/0006-324X-128.1.103.
- Perry ES, Rawson P, Ameral NJ, Miller WR, Miller JD. 2018. Echiniscoides rugostellatus a new marine tardigrade from Washington, U.S.A. (Heterotardigrada: Echiniscoidea: Echiniscoididae: Echiniscoidinae). Proceedings of the Biological Society of Washington 131(1):182–193. DOI:10.2988/18-00004.
- Plate LH. 1889. Beiträge zur Naturgeschichte der Tardigraden. Zoologische Jahrbücher Abteilung für Anatomie und Ontogenie der Tiere 3:487–550. DOI:10.5962/bhl.part.1265.
- Ramazzotti G, Maucci W. 1982. A history of tardigrade taxonomy. In: Nelson DR, editor. Proceedings of the Third International Symposium on the Tardigrada, August 3–6, 1980. Johnson City, Tennessee, USA. Johnson City: East Tennessee State University Press. pp. 11–30.
- Rambaut A, Suchard MA, Xie D, Drummond AJ 2014. Tracer v1.6. 2014. Available at https://beast.bio.ed.ac.uk/Tracer. Accessed Aug 2021 10.
- Renaud-Mornant J. 1976. Tardigrades marins de Polynesie. Cahiers du Pacifique 19:289–297.
- Renaud-Mornant J. 1987. Bathyal and abyssal Coronarctidae (Tardigrada), descriptions of new species and phylogenetical significance. In: Bertolani R, editor. Biology of tardigrades. selected symposia and monographs U.Z.I. Mucchi, Modena. Vol. 1. pp. 229–252.
- Richters F. 1926. Tardigrada. In: Kükenthal W, Krumbach T, editors. Handbuch der Zoologie. Berlin and Leipzig: Walter de Gruyter & Co. Vol. 3, pp. 58–61.
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574. DOI:10.1093/bioinformatics/btg180.
- Schultze CAS. 1840. Echiniscus Bellermanni, Animal Crustaceum, Macrobioto Hufelandii Affine. Berlin: Apud G. Reimer. pp. 1–8.
- Schultze M. 1865. Echiniscus sigismundi. Archiv für mikroskopische Anatomie 1(1):428. DOI:10.1007/BF02961427.
- Stec D, Kristensen RM, Michalczyk Ł. 2020. An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger 286:117–134. DOI:10.1016/j.jcz.2020.03.007.
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Resources 22(22):4673–4680. DOI:10.1093/nar/22.22.4673.