Abstract
The genus Centroptilum Eaton, 1869 is widely distributed in the West Palaearctic region, but poorly diversified as it previously only encompassed two valid species, C. luteolum (Müller, 1776) and C. pirinense Ikonomov, 1962. The discovery of a new congener, C. volodymyri Martynov, Godunko and Palatov, sp. nov., is therefore remarkable; the new species is described based on larvae collected in Georgia, Turkey and Iran in 2012–2019. This new species can be distinguished by the structure of the cuticle with flattened, stretched shallow, triangular and semilunar-shaped corrugations; scales and their bases densely scattered on body surface; features of the mouth parts, including the presence of an expanded laterally labrum, in combination with segment III of maxillary palp and superlinguae of hypopharynx rounded apically, without any pointed projections at the tip; numerous small teeth scattered proximally in two rows along inner margin of the pretarsal claw; the shape of denticulation along the posterior margin of the abdominal terga, and the number of prominent posterolateral spines on tergum VII; and the shape of gills II–VII, with a distinctly concave posterior margin. Together with a morphological description, data on the distribution and mitochondrial cytochrome c oxidase subunit I gene (COI) barcode sequences of the species are provided. The species delimitation of C. volodymyri Martynov, Godunko and Palatov, sp. nov. and C. luteolum is tested using COI barcode sequences. The data obtained show that the new species can be distinctly recognised as a separate lineage. The habitat preferences associated with the different parts of the area of C. volodymyri Martynov, Godunko and Palatov, sp. nov. are described and compared. Distribution of C. volodymyri Martynov, Godunko and Palatov, sp. nov. confirms the connections between Caucasian and Asian fauna of Ephemeroptera, as already demonstrated in various families and genera. Short notes on the distribution of mayfly species common to the Caucasus, north-western Iran and Central Asia are presented. http://www.zoobank.org/urn:lsid:zoobank.org:act:1D2B7557-0DEC-4ACD-8CA3-A6B50FCC4A51.
Introduction
The mayfly fauna of the Caucasus is still largely unexplored, although considerable progress has been achieved recently. Important taxonomic studies, checklists and critical reviews of literature data were published in the last decade (e.g. Kluge et al. Citation2013; Godunko et al. Citation2015; Martynov et al. Citation2015, Citation2016; Salur et al. Citation2016; Martynov & Godunko Citation2017; Bojková et al. Citation2018; Gabelashvili et al. Citation2018; Hrivniak et al. Citation2018, Citation2020). The family Baetidae Leach, 1815 represents an important component of mayfly diversity; however, it remains incompletely known in the Caucasus and adjacent regions, especially when considering taxa from lentic habitats and low altitudes. They include mainly species belonging to the subfamily Cloeoninae Kazlauskas, Citation1972. A new species from this group, Procloeon (Pseudocentroptilum) caspicum Sroka, 2018, was recently described from Iran based on larvae (Bojková et al. Citation2018). Other genera of this subfamily, mainly Cloeon Leach, 1815 and Centroptilum Eaton, 1869, remain unexplored or their published records are doubtful. Importantly, the taxonomy of these genera is not fully resolved and a critical review of Cloeoninae is necessary. This study is hence focused on the genus Centroptilum, the concept of which has greatly evolved since its original description by Eaton (Citation1871).
During more than a century, it conventionally encompassed all species of Cloeoninae with hind wings from all over the world (including Nearctic, Afrotropical, Oriental and Australasian regions). The concept was circumscribed step by step, for instance by excluding all African species (Gillies Citation1990). Kazlauskas (Citation1972) and Keffermüller and Sowa (Citation1975, Citation1984) listed the distinguishing characters for the C. luteolum species group, including the structure of larval mouthparts and cerci, the shape of the gills and the surface of abdominal terga, and the shape of the hind wing and forceps-base projection in male imago. Jacob (Citation1973, Citation1991) recognised the polyphyletic origin of the species grouped into Centroptilum sensu Eaton (Citation1871) and divided them into C. luteolum and the C. stenopteryx complex. McCafferty and Waltz (Citation1990) discussed diagnostic characters for Centroptilum, including Nearctic representatives originally described within this genus, Cloeon and Neocloeon Traver, 1932. Neocloeon was considered a junior synonym of Centroptilum. Kluge and Novikova (Citation1992a, Citation1992b) summarised previous data and proposed additional diagnostic characters for genera and subgenera within Cloeoninae. Because these authors applied a broader generic concept, several taxa traditionally recognised as genera of Cloeoninae were subordinated as subgenera to Cloeon. The presence/absence of hind wings was no longer considered a reliable character to separate genera; Centroptilum was distinguished from Procloeon Bengtsson, 1915 by the absence of spines on the lateral margin of tergites VII to IX and the absence of greatly enlarged spines on the outer margin of cerci, and from Anafroptilum Kluge, Citation2012 by the presence of a patella-tibial suture on fore legs of larvae (Kluge Citation2012, Citation2016). Finally, Jacobus and Wiersema (Citation2014) reinstated the genus Neocloeon and transferred seven Nearctic species originally described in Centroptilum or Cloeon to the genus Anafroptilum.
Currently, the distribution of Centroptilum s.s. is limited to the Palearctic region only, encompassing two species, the widely distributed C. luteolum (Müller, 1776) and the poorly known C. pirinense Ikonomov, Citation1962. The latter was originally described from the Balkans and only its larval stage is known. Keffermüller and Sowa (Citation1984) considered C. pirinense to be closely related to C. luteolum. Kluge and Novikova (Citation1992a, Citation1992b) did not cite this species in the revision of the systematics of the subfamily Cloeoninae. Bauernfeind and Soldán (Citation2012) reviewed the distinguishing characters, distribution and ecology of its larvae. They pointed out its possible synonymy with C. luteolum and considered it a species inquirenda. Jacobus and Wiersema (Citation2014) considered it a valid Eurasian species in the discussion of species belonging to Centroptilum s.s. They noted the need for additional studies of C. pirinense, and also mentioned a single larva identified as C. pirinense by R. D. Waltz, that was partly mounted on the slide and deposited in the collection of the Purdue University Entomological Research Collection (USA). The specimen was labelled as collected in Turkey, without an exact locality – not even an indication of whether it was collected in the European or Asian part of the country.
This study aims to describe a new species of Centroptilum, C. volodymyri Martynov, Godunko and Palatov, sp. nov., based on material collected during field trips to Turkey, Georgia and Iran in 2012–2019; to discuss in detail morphological differences of the new species from C. luteolum (Müller, 1776) and C. pirinense Ikonomov, Citation1962; and to briefly discuss affinities of Baetidae faunas of the Caucasus and adjacent regions.
Material and methods
Sampling of material, preservation and morphological study
The present contribution is based on the larval material collected by A. V. Martynov, D. Palatov and J. Bojková in 2012–2019, in several regions of Georgia, Turkey and Iran. Administrative divisions and geographic coordinates of localities are taken from Google Earth (http:// earth.google.com). All specimens were collected by kick sampling in different freshwater habitats and preserved in the field in 96% ethanol.
The holotype (from Georgia) is preserved in ethanol; paratypes from Georgia are preserved in ethanol (one larva), mounted on slides in Canada balsam (three larvae) (see below for the inventory numbers (IN)), preserved on scanning electron microscopy (SEM) stubs for taking SEM photos (one larva), and preserved as the external skeleton only in alcohol (two larvae – used for molecular study); paratypes from Turkey are preserved on a slide (one larva) and in ethanol (48 larvae).
The main part of the type material is housed in several research institutions: the holotype and four paratypes (all from Georgia, three in slides) are deposited in the collection of the National Museum of Natural History, NASU, Kyiv [NMNH NASU]; one paratype from Turkey deposited in the Laboratory of Synecology of the A. N. Severtsov Institute of Ecology and Evolution, RAS, Moscow [IEE]; 44 paratypes from Turkey (one mounted on a slide) are stored in the collection of the Institute of Entomology [IE], BC CAS, České Budějovice; and six paratypes (two from Georgia and four from Turkey) are deposited in the Museum of Zoology Lausanne, Switzerland [MZL].
Also, some non-type material of Centroptilum volodymyri Martynov, Godunko and Palatov, sp. nov. from Iran was used in the current study: 19 larvae deposited in IEE; one larva in NMNH NASU; four larvae in MZL; and nine larvae in the Department of Botany and Zoology of Masaryk University, Brno [MU].
The inventory numbers of samples from the type locality (Georgia) deposited in the NMNH NASU are: Grg24Censp/1 and Grg24Censp/2 for vials with larvae in alcohol; and 648, 661 and 662 for larvae mounted on slides.
For morphological analysis using SEM, larval mouthparts, legs and parts of the cuticle, and eggs dissected from a female larva were obtained from C. volodymyri sp. nov. (from type locality) and C. luteolum (Russian Federation (RF), Moscow Region, Naro-Fominsky District, Turussa River near Kryukovo village, 55.465°N, 36.537°E, 15 August 2017, Palatov D. M. leg.). All parts were subsequently dehydrated through a stepwise immersion in ethanol, dried by critical point drying (Leica EM CPD300), and mounted on SEM stubs. The mounted material of C. volodymyri sp. nov. from Georgia was coated with a 5 nm Au/Pd layer (Leica EM ACE200) and subsequently examined and photographed with a Zeiss EVO LS 15 SEM at the State Museum of Natural History Stuttgart, Germany [SMNS]. The material of C. luteolum was analysed using Vega3 Tescan in the Paleontological Museum of the Paleontological Institute, Russian Academy of Sciences, Moscow [PIN]. All the photographs were subsequently sharpened and adjusted for contrast and tonality in Adobe Photoshop™ CS5 and CS6.
Macrophotographs of the larval habitus of C. volodymyri sp. nov. (material from Georgia) were taken with a Canon PowerShotA 640 camera and Zeiss Stemi 2000 binocular microscope in the I. I. Schmalhausen Institute of Zoology, NASU, Kyiv. Photo stacks were processed with Helicon Focus Pro 6.4.1 to obtain combined photographs with extended depth of field, and subsequently enhanced with Adobe Photoshop™ CS3. The material was examined using Olympus SZX7 and Leica M205 C stereomicroscopes. Line drawings were made using an Olympus BX41 microscope equipped with a drawing tube.
Comparative material of Centroptilum luteolum (Müller, 1776)
GEORGIA: 18 larvae, Adjara, territory of Kobulety town, Kintrishi River, 41.804°N, 41.776°E, 4–5 June 2013, Martynov A. V. leg. – IN Grg6Cenlut; two larvae, Adjara, territory of Kobulety town, Kintrishi River (near estuary) and Kinkishi River, 41.804°N, 41.782°E, 17 April 2016, Martynov A. V. leg. – IN Grg23Cenlut. UKRAINE: five larvae, Donetsk Region, Shahtarsk district, vicinity of Chystiakove (= Torez) town, stream in bayrak, 14 May 2011, Martynov A. V. leg. – IN Don332; 25 larvae, Donetsk Region, Bakhmut district, vicinity of Debaltseve town, Bulavina River, 48.315°N, 38.438°E, 2 May 2010–29 April 2011, Martynov A. V. leg. – IN Don360, Don397, Don399, Don403, Don411, Don495, Don496, Don503; 13 larvae, Luhansk Region, Antratsyt District, vicinity of Khrustal’nyi village, Khryshtaleva River, 48.182°N, 38.877°E, 30 April 2012, Martynov A. V. leg. – IN Lug 94; one larva, Rivne Region, vicinity of Poznan’ village, Stvyha River, 51.591°N, 27.478°E, h – 141 m a.s.l., 5 July 2017, Martynov A. V. leg. – IN Riv4Cenlut; 16 larvae, Chernivtsi Region, vicinity of Berehomet urban-type settlement, Stebnyk River, 13 May 2008, Martynov V. V. – IN Chrt25; four larvae, Chernivtsi Region, vicinity of Berehomet urban-type settlement, slow-flowing reservoir in valley of Stebnyk River, 12 May 2008, Martynov V. V. – IN Chrt29.
RUSSIAN FEDERATION: one larva, W Siberia, Yamalo-Nenets Autonomous Okrug, Tyumen Region, Priuralskii District, Chtchouchia River, 67.334°N, 68.432°E, 5 July 2006, Palatov D. M. leg.; 12 larvae, Murmansk Region, Kandalakshkii District, Virma River, 67.169°N, 32.240°E, 24 July 2015, Palatov D. M. leg.; eight larvae, Moscow Region, Stupino City-district, small stream (tributary of Bunchikha River near Pesochnaya village), 54.979°N, 38.204°E, 14 September 2017, Palatov D. M. leg.; one larva, Krasnodarskii Krai [Province], Tuapsinskii District, Psebe River, 44.287°N, 38.910°E, 29 March 2012, Palatov D. M. leg.
AZERBAIJAN: 19 larvae, Talysh Mts., Lenkaran District, small stream (the tributary of the Vesharyu River near the Dashtatuk village), 38.695°N, 48.757°E, 7 April 2011, Palatov D. M. leg.
TURKEY: 10 larvae, Kastamonu il [Province], Daday District, small stream 1 km southwest of Gekeren Keyu village, 41.450°N, 33.129°E, 15 April 2010, Palatov D. M. leg.
SWITZERLAND: 25 larvae, Vaud, Vallée de Joux, Le Chenit, La Burtignière, Orbe stream, 46.562°N, 6.170°E, 23 August 2001, Wagner A. leg.
Terminology
The terminology and abbreviations proposed by Godunko et al. (Citation2015) for the subgenus Rhodobaetis Jacob, 2003 are used to describe body setation of larvae. Their applicability was confirmed also for the genus Nigrobaetis Novikova and Kluge, 1987 (Godunko et al. Citation2018). The larval characters used to distinguish the the genus Centroptilum from its congeners were taken from and adapted based on references cited in the Remarks to Table II.
DNA extraction, polymerase chain reaction amplification and sequencing, and DNA sequence analysis
Total genomic DNA from XY specimens was extracted using the BioSprint 96 extraction robot (Qiagen Inc., Hilden, Germany), following the supplier’s instructions. The non-destructive protocol described in Vuataz et al. (Citation2011), which enables post-extraction morphological study of specimens, was implemented. We then amplified a 658-bp fragment at the 5’ end of the mitochondrial cytochrome c oxidase subunit I gene (COI), corresponding to the standard animal barcode region, using the HCO2198 and LCO1490 primers (Folmer et al. Citation1994). Polymerase chain reaction (PCR) was conducted in a volume of 30 μL, consisting of 9 μL of template DNA, 1. 5 μL (10 μM) of each primer, 0.24 μL (25 mM) of dNTP solution (Promega), 6 μL of 10X buffer (Promega) containing 7.5 mM of MgCl2, 3 μL (25 mM) of MgCl2, 1.5 U of Taq polymerase (Promega), and 8.46 μL of sterile ddH2O. Optimised PCR conditions included initial denaturation at 95°C for 5 min, 38 cycles of denaturation at 95°C for 40s, annealing at 50°C for 40s, and extension at 72°C for 40s, with final extension at 72°C for 7 min. Purification and automated sequencing were carried out in Microsynth (Balgach, Switzerland).
In order to provide a comprehensive taxon sampling for the species delimitation, we analysed COI sequences from 62 specimens. We included all obtained sequences (three for C. volodymyri and three for C. luteolum; ) and added sequences from related taxa published in the GenBank database (https://www.ncbi.nlm.nih.gov/) as well as unpublished sequences from the platform FREDIE (Freshwater Diversity in Europe: https://www.fredie.eu/). In total, 43 sequences of C. luteolum, one sequence of C. victoriae McDunnough, 1938 and the outgroup sequences of Cloeon dipterum (Linnaeus, 1761), Cloeon simile Eaton, 1870, Procloeon pennulatum (Eaton, 1870) and P. bifidum (Bengtsson, 1912) were used for the analysis. Sequences were edited and aligned using Geneious Prime v. 2019.0.4 (Biomatters Ltd.). Evolutionary analyses were conducted in MEGA X (Kumar et al. Citation2018; Stecher et al. Citation2020; http://www.megasoftware.net). We applied the GTR+G + I model of molecular evolution as it produces the lowest Bayesian information criterion (BIC) scores and is therefore considered to describe the substitution pattern the best.
Table I. Codes and origin of new sequences used in molecular study. For each specimen, the specimen catalogue number (GBIF code), the sample information (country, locality, coordinates and date of sampling), the GenBank accession number of the COI sequence, and nomenclature of genetic sequences (GenSeq; follows Chakrabarty et al. Citation2013) are provided.
Tree topology was reconstructed with MEGA X using the maximum likelihood method based on the Tamura–Nei model (Tamura & Nei Citation1993). The tree with the highest log likelihood is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches (bootstrap with 1000 replicates). For grouping sequences, the Automatic Barcode Gap Discovery (ABGD) method was performed on the webserver (http://wwwabi.snv.jussieu.fr/public/abgd/abgdweb.html; 09.09.2021) using as input the multiple sequence alignment obtained below, default values for the prior intraspecific divergences, relative gap width and distance distribution, and a Jukes–Cantor (JC69) distance matrix. The genetic variability between groups of sequences obtained above was calculated using Kimura two-parameter distances (K2P, Kimura Citation1980), with MEGA X (http://www.megasoftware.net).
Results and discussion
Taxonomy
Centroptilum volodymyri Martynov, Godunko and Palatov, sp. nov.
(; , , , , , , , , , , , ))
Table II. Morphological characters of larvae that distinguish Centroptilum volodymyri sp. nov. from C. luteolum (Müller, 1776) and C. pirinense Ikonomov, Citation1962. Distinct differential characters are in bold.
Table III. Genetic distances (COI) between sequenced species of Centroptilum, Cloeon and Procloeon and within them, using Kimura two-parameter.
Figure 1. Larva of Centroptilum volodymyri sp. nov., Georgia, holotype. (a, b), Body in lateral view. Scale bars: 1 mm.

Figure 2. Larva of Centroptilum volodymyri sp. nov., Iran. (a) Body in dorsal view; (b) body in lateral view; (c) head and part of thorax in lateral view. Scale bars: 1 mm.

Figure 3. Larvae of Centroptilum volodymyri sp. nov., paratype (a, c, e) and C. luteolum (Müller, 1776) (b, d, f). (a, b) Scapus and pedicellus; (c) flagellum, basal part; (d) flagellum, central part; (e, f) surface of frons. Scale bars: a, c, d = 20 μm; b = 30 μm; e = 10 μm; f = 100 μm.
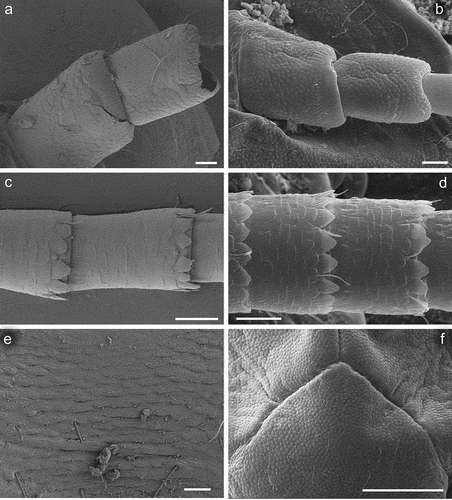
Figure 4. Larvae of Centroptilum volodymyri sp. nov., paratype (a–e) and C. luteolum (Müller, 1776) (f). (a) Labrum; (b) galea-lacinia; (c, d) mandibles, left (c) and right (d); (e, f) maxillary palp. Scale bars: a, b, e, f = 30 μm; c, d = 100 μm.
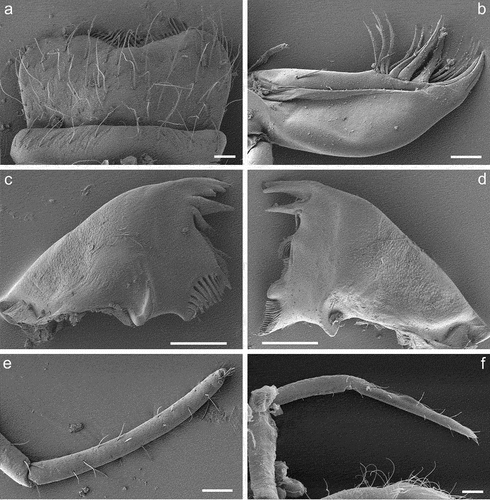
Figure 5. Larvae of Centroptilum volodymyri sp. nov., paratypes. (a, b) Labrum, ventral (a) and dorsal (b) view; (c) hypopharynx (left side – dorsal view; right side – ventral view); (d, e) glossa and paraglossa, dorsal (d) and ventral (e) view; (f) maxilla; (g, h) labial palp, ventral (g) and dorsal (h) view.
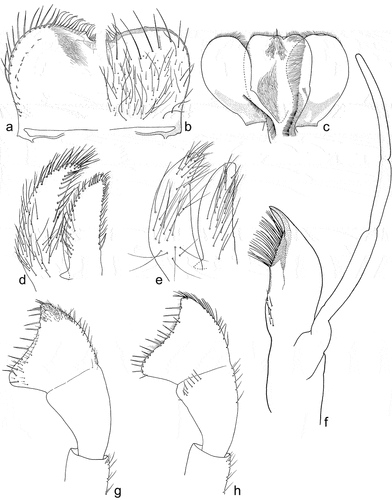
Figure 6. Larvae of Centroptilum volodymyri sp. nov., paratype (a, c, e) and C. luteolum (Müller, 1776) (b, d, f). (a, b) Pronotum, dorsal surface; (c, d) hind femur, dorsal surface; (e, f) hind tibia, dorsal surface. Scale bars: a–c, e, f = 30 μm; d = 10 μm.
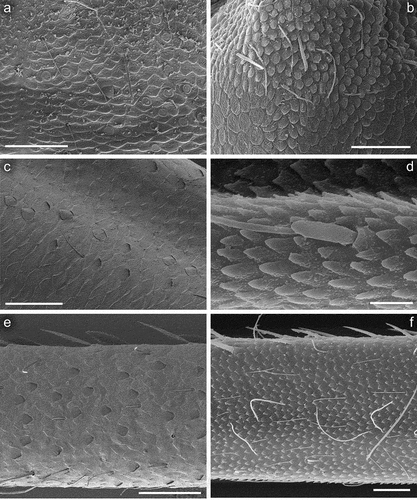
Figure 7. Larvae of Centroptilum volodymyri sp. nov., paratype (a, c, e, g) and C. luteolum (Müller, 1776) (b, d, f). a, b = hind pretarsal claw (apex of claw not visible in (a)); (c, d) denticles of hind pretarsal claw; (e) surface of sternum IV; (f) surface and posterior margin of sternum VI; (g) surface and posterior margin of sternum VII. Scale bars: a = 50 μm; b = 100 μm; c–e, g = 30 μm; f = 10 μm.
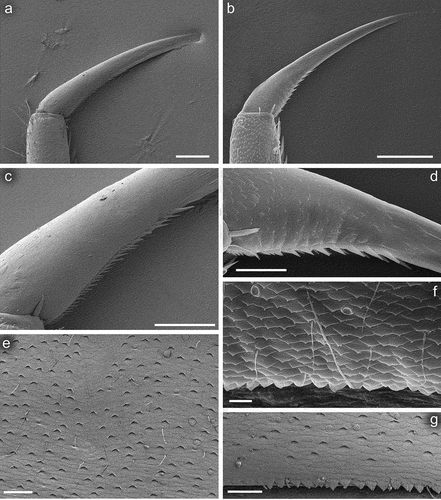
Figure 8. Larvae of Centroptilum volodymyri sp. nov., paratypes. (a) Fore leg; (b) middle leg; (c) hind leg.
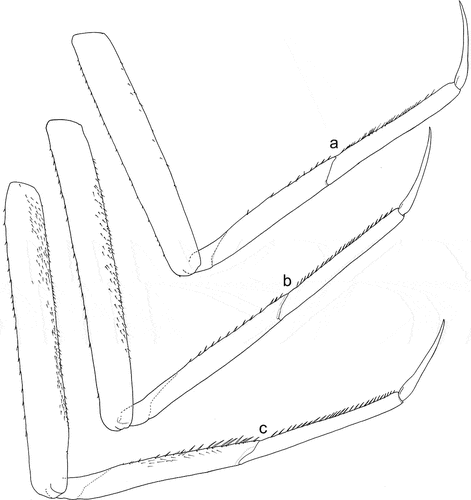
Figure 9. Larvae of Centroptilum volodymyri sp. nov., paratype (a, c, e, g) and C. luteolum (Müller, 1776) (b, d, f, h). Surfaces and posterior margins of terga: (a, b) tergum II; (c, d) tergum IV; (e, f) tergum VI; (g, h) tergum VIII. Scale bars: a, b = 20 μm; c–h = 30 μm.
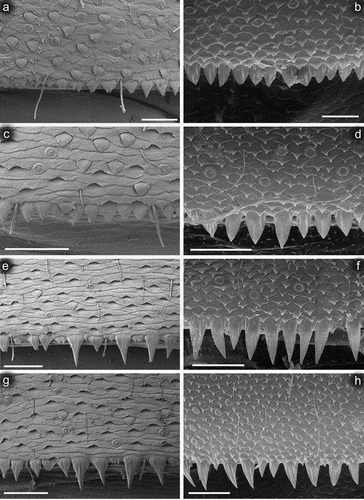
Figure 10. Larvae of Centroptilum volodymyri sp. nov., paratypes. (a) Gill I; (b) gill II; (c) gill III; (d) gill IV; (e) gill V; (f) gill VI; (g) gill VII; (h) posterolateral part of tergum VII; (i) paraproct plate.
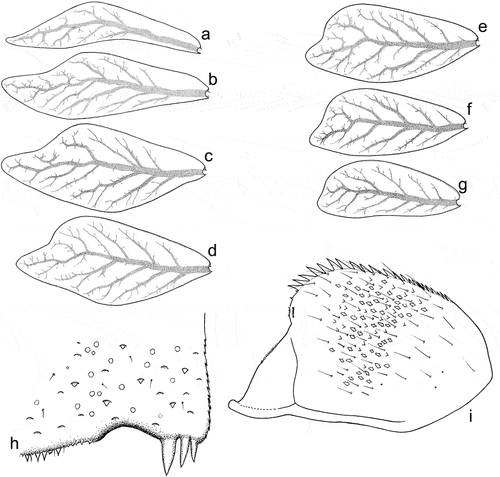
Figure 11. Larvae of Centroptilum volodymyri sp. nov., paratype (a, c, e) and C. luteolum (Müller, 1776) (b, d, f). (a, b) Apical part of paraproct; (c) surface of paraproct; (d) tergum X and caudal filaments; (e) caudal filaments, basal part; (f) caudal filament, medial part. Scale bars: a, b, e, f = 30 μm; c = 20 μm; d = 100 μm.
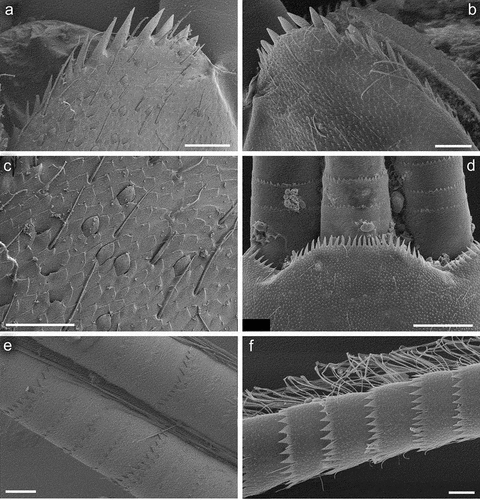
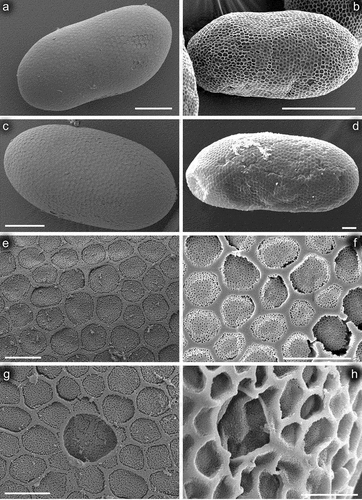
Figure 12. Eggs of Centroptilum volodymyri sp. nov., from paratype (a, c, e, g) and C. luteolum (Müller, 1776) (b, d, f, h); provided by Nicolás Ubero-Pascal). (a–d) General view of egg; (e, f) chorionic surface; (g, h) micropyle. Scale bars: a, c = 30 μm; b = 80 μm; d = 20 μm; e, g = 5 μm; f = 10 μm; h = 5 μm.
http://www.zoobank.org/urn: lsid:zoobank.org:act:1D2B7557-0DEC-4ACD-8CA3-A6B50FCC4A51
Centroptilum sp.: Martynov et al. Citation2016: 170 [distribution in Georgia (Kintrishi River valley), with remark on belonging to undescribed new species]
Centroptilum sp.: Bojková et al. Citation2018: 91, 96, tables 1, 2 [distribution in Iran within Zayanderud (based on Mahboobi Soofiani et al. Citation2012) and Gilan (two localities listed also in present contribution) provinces]
? Centroptilum sp.: Mahboobi Soofiani et al. Citation2012: 137 [Zayandeh-Roud River in the Zayanderud Province; this record should be verified]
Diagnosis [based on larvae]
Larvae of C. volodymyri sp. nov. can be distinguished from the two other Western Palearctic representatives of the genus Centroptilum, namely C. luteolum and C. pirinense, by a combination of the following diagnostic characters: (1) the cuticle with flattened, stretched shallow triangular and semilunar-shaped corrugations, and relatively numerous scales and their bases covering the body surface; (2) labrum expanded laterally, with the U-shaped incurvation on the anterior margin; (3) apically rounded shape of the segment III of maxillary palps; (4) apically rounded shape of superlinguae of hypopharynx without a projection at the tip; (5) pretarsal claw with more than 60 small teeth in basal half of the claw, these teeth arranged into two rows; (6) the posterior margin of terga I–IV(V) with robust equilateral triangle-shaped spines scattered along the segment (equilateral spines sparse on terga (V)VI–X); (7) tergum VII near gill base with 3–4 relatively prominent posterolateral spines and sometimes several small spines; (8) gills II–VII with distinctly concave posterior margin.
Description
Based on specimens from the type locality only.
Mature larva
Body length 9.5–10.2 mm (male larvae slightly smaller than female larvae), length of cerci 5.5–5.8 mm (approx. 0.5× body length); length of paracercus 3.0–3.3 mm.
General body colouration intensively brown/dark brown and blackish; base of fore wing pads, lateral and ventral side of meso- and metathorax darker, yellow to brownish black and black. Abdominal segments paler than thorax; legs and cerci lightest ().
Head. Colour yellowish brown to light brown, with markedly darker area between ocelli, diffused light brown maculation on frons and vertex; clypeus and genae slightly darker, brown. Larval compound eyes brown. Antennae unicolor, light brown, distinctly longer than head and thorax. Frontal suture V-shaped. Head cuticle flattened, without pronounced corrugation. Surface of two first antennal segments and frons covered with sparse B and Hr setae ()); clypeus additionally with two groups of long setae grouped on both sides of body axis.
Labrum expanded laterally, approximately 1.8–2.0× broader than long; median notch flattened; anterior margin concave, with U-shaped median incurvation relatively deep; anterior margin laterally from medial notch relatively symmetrically rounded. Dorsal surface of labrum covered with dense long and short Hr not grouped in rows and sparse B (); ventral surface with row of submarginal short setae ()).
Superlinguae of hypopharynx rounded apically, without any projections ()).
Mandible incisors clearly divided into two groups, deeply separated throughout their length. Left mandibular incisor groups terminated by 4 + 2 stout denticles; left prostheca terminated by a group of slender setae (2–3 setae larger than other ones). Right mandibular incisor groups terminated by 3 + 2 stout denticles; right prostheca is stick-like ()).
Maxillae with long slender canines and dentisetae; 1st dentiseta is simple, 2nd and 3rd dentisetae are bifid; dentisetae not pressed, and well separated from canines. Maxillary palps 3-segmented: segment I distinctly wider than segment II; segment III distinctly longer than segment II, rounded apically, without stout setae apically of surface; surface of maxillary palps covered with sparse Hr and FT () and 5(f)).
Labial palps 3-segmented. Segment III distinctly expanding apically, nearly trapezoidal with rounded outer angles; inner margin distinctly concave apically; posterolateral and posteromedian corners of different shape; stout setae along of apical margin and numerous Hr scattered on surface. Segment II distinctly wider than base of segment III; ventral side of segments I and II with scattered Hr; dorsal side of segment II with 6–10 elongated setae near to apicointernal margin ()).
Glossae nearly as broad as paraglossae ()). Paraglossae slightly longer; dorsal surface with single row of elongated stout setae along inner margin; ventral surface with fine long setae scattered over entire surface (apical setae shorter). Glossae with irregular row of submarginal short stout setae along outer and inner margins; denser setation shaped by fine long setae on dorsal side. Basal part of glossae and paraglossae with numerous fine long setae.
Thorax. Pronotum narrow, approximately 3.0–3.5× longer than broad. Pronotum with two yellowish to light brown maculae centrally; two spreading maculae of same colour on darker background laterally [yellow to yellowish brown, with occasional dirty yellow diffuse spots laterally in specimens from Iran]. Mesonotum with elongated brown strips on light brown background; coxae intensively brown to black brown [slightly paler in specimens from Iran]. Metanotum with spreading yellowish spot centrally, yellowish brown to dark brown laterally. Thoracic pleura slightly darker than terga ()).
Hind wing pads well developed. Thoraxal cuticle formed by flattened, stretched shallow triangular and semilunar-shaped corrugations. Surface of pronotum covered with Hr, B, micropores [chloride cells], sparse SC-et [6–8 μm in length] and SCS-et ()).
Legs relatively pale, whitish yellow to dirty brown [paler in specimens from Iran]; all leg segments slightly darker distally ()). Legs slender; femora parallel-sided; patella-tibial suture developed on all legs ()). Fore leg coxae pale, whitish yellow to intensively brown; coxae of middle and hind legs darker, intensively brown to dark brown. Trochanters with diffused brownish spot. Brownish elongated diffused macula along outer margin of all femora; tibiae yellowish to light brown; tarsi of the same colour as tibiae, occasionally slightly darker distally; claws yellowish brown. Cuticle of the legs formed by flattened equilateral triangle- and semilunar-shaped corrugations. Surface of femora with SC-et, SCS-et, Hr, B setae; the same types of scales and setae, with dominance of SCS-et distributed of surface of tibiae and tarsi ()). Numerous short, stout, pointed setae along outer margin of femora; numerous longer pointed setae along inner margin of tibiae and tarsi; outer margin of tibiae and tarsi covered with Hr and occasional B setae. Pretarsal claw length less than or equal to 1/2 of tarsus length ()); more than 60 small teeth arranged into two rows distributed in basal half to 2/3 of claw length ()).
Abdomen. Terga dark, yellowish brown to intensively brown ()); terga I with two broad spots centrally, and two smaller spots anterolaterally. Terga II–V with uniform colour pattern, viz. a small pale spot centrally near anterior margin of segment; large brownish diffused spot centrally, with paler maculae near posterior margin of segment; additionally, yellowish-brown to light brown elongated strips surrounding pale maculae. Tergum VI darkest, with colour pattern similar to those on previous segments, except of more diffused light spot anteriorly, and presence of two pale dots and two oblique strokes centrally. Terga VII–VIII with colour pattern similar to those on terga II–V (occasionally without small pale spot anteriorly), or uniformly light brown. Tergum IX uniformly light brown to brown without conspicuous pattern. Tergum X pale, yellow to light brown, with light transversal macula anteriorly, two unclear maculae centrally near anterior margin of segment and two brown small spots laterally. Anterolateral area of terga I–IX pale, yellowish brown.
Abdominal sterna predominantly uniformly coloured, whitish yellow to light brown, without distinct pattern. Occasionally sterna with contrasting pattern: sternum I whitish yellow, with dark brown spots anterolaterally; sterna II–V (VI) with diffused triangular brownish spot on yellowish background; sterna VII–VIII darker than previous segments, light brown to brown, with longitudinal pale band and two yellowish spots centrally; sterna VIII–IX darkest, intensively brown, with diffused paler maculation centrally and laterally; sternum X slightly paler than two previous segments, light brown.
Surface of terga with cuticle formed by flattened equilateral triangle- and semilunar-shaped corrugations; terga I–IV covered with micropores, Hr and B, dominant SC-et and more sparse SCS-et; terga V–X with the same type of micropores, Hr and B, sparse SC-et and dominant SCS-et ()). Posterior margin of terga I–IV (V) with robust equilateral triangle-shaped spines, alternating with sparse shorter ones and B setae; posterior margin of terga VI–X (occasionally tergum V) with thin and robust isosceles and equilateral triangle-shaped spines, alternating with shorter ones and sparse B setae. Tergum VII near gill bases with 3–4 relatively prominent posterolateral spines, and sometimes with several additional small spines; terga VIII–IX with 2–4 relatively prominent posterolateral spines, and sometimes with several additional small spines ()).
Cuticle of sterna shaped by flattened undulating corrugations covered with micropores, elongated Hr and B setae, sparse SC-et and dominant SCS-et ()). Posterior margin of sterna with robust equilateral triangle-shaped spines alternating with occasional short B setae.
Gills whitish yellow, with well visible tracheisation, light brown to intensively brown coloured. All gills asymmetrical, rounded apically; gill I distinctly sinuous, narrow, lanceolate; gills II–VII with posterior margin distinctly concave ()).
Paraproct plate with 13–15 pointed strong teeth along inner margin; ventral surface of paraproct plate covered with leaf-shaped SC and their SCS grouped mainly centrally, elongated Hr and B setae ()).
Cerci and paracercus slightly paler than body, light brown, without coloured rings. Posterior margin of caudal filament segments with combination of robust marginal spines, occasional Hr and B setae; submarginal area with solitary SC-et ()). Cerci (inner margin) and paracercus (both margins) up to the apex bear well-developed primary swimming setae, except last one or two segments. Outer margin of cerci with relatively short Hr and B setae.
Egg (taken from larva)
Oval; 130–140 µm long, 65–70 µm wide. Chorionic surface shaped by thin, flat reticulated ridges forming irregular polygonal mesh; cells (2.5–4.2 µm in diameter) of this mesh mostly with rounded margins; no knob-terminated coiled thread inside of chorionic cells ()). One, occasionally two oval micropyles in equatorial area, 5.5–5.8 µm long and 4.8–5.0 µm wide; no micropylar rim surrounding micropyle.
Imago and subimago
Unknown.
Type material
Holotype
Female mature larva, GEORGIA, Autonomous Republic of Adjara, Kobuleti District, vicinity of Chakhati village, valley of the Kintrishi River, small lentic water bodies, 41.762°N, 41.978°E, ′325 m a.s.l., 18 April 2016, Martynov A. V. leg. (inventory number in NMNH NASU collection: Grg24Censp/1).
Paratypes
Six larvae, GEORGIA, same locality and date as holotype (three larvae in slides 648, 661, 662; one larva in ethanol deposited in NMNH NASU with inventory number Grg24Censp/2);
49 larvae, TURKEY, Rize il [Province], Fındıklı Region, Kaçkar Mountains, Büyük [Çağlayan] stream, 41.236°N, 41.270°E, 337 m a.s.l., 18 August 2012, Palatov D. M. leg.;
Non-type material
10 larvae, IRAN Guilan Province, unnamed small brook (left tributary of Shalmanrud River), SW of Amlash town, 37.046°N, 50.095°E, 185 m a.s.l., 21 May 2016, Bojková J., Soldán T., Imanpour Namin J. leg.;
One larva, IRAN, ibid., SW of Amlash town, 37.037°N, 50.083°E, 287 m a.s.l., 21 May 2016, Bojková J., Soldán T., Imanpour Namin J., leg.;
22 larvae, IRAN, Golestan Province, unnamed small brook (right tributary of Rude-Tengen River), S of Qarangi-ye Jangal town, 37.529°N, 55.792°E, 604 m a.s.l., 23 June 2019, Palatov D. M. leg.
Etymology
The species is named in honour of Volodymyr Martynov, senior son of the first author, for his field assistance in mayfly sampling during a series of field trips.
Distribution and habitat
In Georgia, the larvae of C. volodymyri sp. nov. were collected in small pools, where small brooks flowed in, in the valley of the Kintrishi River at 325 m a.s.l. ()). They were shaded by the ancient colchic forests (chestnut, hornbeam and box trees). Pools were about 0.3 m deep, with bed substrate composed of silt and detritus, without macrophytes. No other mayflies were found there.
Figure 13. Habitats of Centroptilum volodymyri sp. nov. in Georgia, Iran and Turkey. (a, b) Small lentic waterbody, Georgia, Adjara, type locality (April 2016; photos by A. Martynov); (c, d) small brook, left tributary of Shalmanrud River, Guilan Province, Iran (May 2016; photos by J. Bojková); (e, f) small stream, Rize Province, Turkey (August 2012; photos by D. Palatov).

Figure 14. Map of Centroptilum volodymyri sp. nov. distribution. Red star – type locality; green pentagrams - non-type localities.
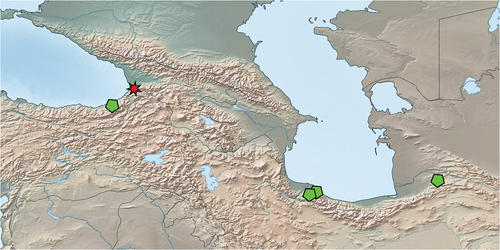
In Iran, the larvae were collected in two small brooks (1–3 m wide) flowing in steep terrain below 300 m a.s.l. on the northern slopes of the Alborz Mts., close to the Caspian Sea coastal area in Guilan Province () and ). They were shaded by humid deciduous forest and shallow, with an average depth of 0.05–0.15 m (max. 0.3 m in pools). They were characterised by alternating riffles and pools, where Centroptilum larvae were collected. Coarse bed substratum in riffles alternated with finer substrates formed by coarse and fine gravel in pools. Bedrock and boulders were sparsely covered with mosses. Water was relatively cold (16°C) and clear, although one brook was slightly turbid. The mayfly taxocene consisted of the following species: C. luteolum (dominant), Baetis (Rhodobaetis) cf. gadeai Thomas, 1999, Nigrobaetis (Alainites) muticus (Linnaeus, 1758) (all Baetidae), Electrogena pseudaffinis (Braasch, 1980) (Heptageniidae), and Caenis macrura Stephens, 1835 (Caenidae).
In Turkey, larvae were collected in a small stream (1.5–2.5 m width; 0.1–0.2 m depth) flowing in the forest at 337 m a.s.l. ()). Its bed substrate was composed of pebbles, detritus and silted stones and the flow velocity was 0.3–0.6 m.s−1. The mayfly taxocene consisted of the following taxa: Electrogena sp. (Heptageniidae) (dominant), Habroleptoides cf. confusa Sartori and Jacob, 1986 (Leptophlebiidae), B. (R.) cf. gadeai, and N. (A.) muticus (Baetidae).
Affinities
We attribute C. volodymyri sp. nov. to the genus Centroptilum based on larval and egg characters depicted by Jacob (Citation1991), Kluge and Novikova (Citation1992a, Citation1992b), and Bauernfeind and Soldán (Citation2012). The attribution is confirmed by the presence of (1) epicranial suture met at an obtuse angle; (2) mandibular incisors deeply separated into two groups from their base; (3) segment III of maxillary palp not shortened, longer than segment II; (4) narrow pronotum, approximately 3.0–3.5 × longer than its width [4.0 times according to Kluge and Novikova (Citation1992a, Citation1992b)]; (5) pretarsal claws with two rows of small teeth; (6) simple gills with pinnate tracheisation [according to Bauernfeind and Soldán (Citation2012), in Centroptilum simple gills acutely pointed apically and almost symmetric, except for the very narrow gill I]; (7) abdominal segments almost without strong posterolateral setation, except for tergum VII covered by 1–3 relatively prominent spines [no strong posterolateral setation in Centroptilum according to Bauernfeind and Soldán (Citation2012)]; (8) surface of abdominal terga with flattened, non-pointed corrugation and micropores [pointed chagrin structure in the form of minute spines, and circular sensillae according to Bauernfeind and Soldán (Citation2012)]; (9) posterior margin of terga with robust spines alternating with shorter ones [the same for some terga of C. luteolum (see )) and C. pirinense (according to Ikonomov Citation1962); the posterior margin of abdominal terga in Centroptilum with spines of subequal length according to Bauernfeind and Soldán (Citation2012)]; (10) no long setation on last two caudal segments [basal and apical 1/5 part of caudal filaments are without setation according to Bauernfeind and Soldán (Citation2012)]; and (11) chorionic surface of eggs with dense uniformly reticulated ridges, forming irregular polygonal cells [“meshes” according to Bauernfeind and Soldán (Citation2012)].
Centroptilum volodymyri sp. nov. occupies a relatively independent position within the genus due to characteristics of the cuticle, setation of the posterolateral margin of tergum VII and the gill shape. At the larval stage, it clearly differs from the Western Palearctic representatives C. luteolum and C. pirinense by numerous scales and scale bases densely scattered on the body surface, some features of the mouthparts (particularly the shape of the labrum and superlinguae of the hypopharynx, and the proportions and setation of maxillary and labial palps; see also ), and dense denticulation of claws. Minor differences are found in the size of eggs and their cells within the chorionic mesh. The structure of the chorionic surface is thus very similar to that of C. luteolum (it was not described for C. pirinense). A detailed comparative overview of the distinguishing characters for the delimitation of the three species discussed above is given in .
Specimens of C. volodymyri sp. nov. collected in the Caucasus and Iran show some variability in colouration and morphology. Caucasian specimens are distinctly darker: their pleural sclerites and particularly anterior part of mesonotum and fore wing pads are markedly brown to dark brown. In contrast, Iranian specimens are distinctly paler, with body whitish yellow to brown. While Caucasian specimens had brown abdominal tergum and sternum of segment IX, Iranian specimens show a whitish-yellow central part of the tergum IX lined by two brownish oblique strips ()) and small light brown smudges on the sternum IX. Some minor differences between Caucasian and Iranian populations can be recognised in the structure of mouthparts and arrangement of scales and setae along the posterior margin of flagellar segments. Iranian specimens had a more elongated labrum width/length ratio (1.6–1.7, compared to 1.8–2.0 in Caucasian specimens). Some Iranian specimens have fewer setae (fewer than six) on the dorsal side of segment II of the labial palp than the Caucasian specimens (6–8 setae). The setae under the maxillary crown are arranged in a nearly distinct row in specimens from the Caucasus, while they are mostly scattered in some specimens from Iran. The specimens from Georgia and Turkey have bluntly pointed or apically rounded scales on posterior margins of flagellar segments, while two or three pointed apices are found on each marginal scale in specimens examined from Iran. Nevertheless, these differences are not consistent in all specimens and characters, and we consider the specimens from Caucasus, Turkey and Iran to be conspecific, belonging to C. volodymyri sp. nov.
Molecular results
The molecular reconstruction highly supported C. volodymyri sp. nov. as a monophyletic clade, with a bootstrap support of 99% (). Georgian and Iranian sequences are separated by genetic distances (13%, ) that are generally considered higher than intraspecific. Centroptilum volodymyri sp. nov. is recovered as the sister species of C. luteolum; the genetic distances between C. volodymyri sp. nov. and the two clades of C. luteolum are higher than 25%. Centroptilum luteolum is composed of two monophyletic lineages (clade 1 and clade 2 in and ); both are highly supported as monophyletic and may represent distinct species (K2P distances higher than 20%).
Discussion
Integrative approaches clearly confirm the validity of the new species C. volodymyri sp. nov. This species is closely related to the type species of Centroptilum, C. luteolum, both morphologically and genetically. The two species can be morphologically distinguished by the shape and length of the maxillary palp, the length and denticulation of the claws and the shape of the gills. High molecular distances strengthen the validity of the new species. At present, three populations of C. volodymyri are known, from Georgia, Turkey and Iran. As these three countries represent the limit of distribution of C. luteolum, these two species of Centroptilum are partially sympatric. The molecular distances between the Georgian and Iranian population of C. volodymyri sp. nov. are rather high and may suggest the presence of two cryptic species. We refrain from considering the two as separate species as no morphological characters support this division. The high molecular distance could also be due the geographical gap between the populations and the absence of gene flow between them. The discovery of additional populations between them might challenge the present interpretation and reduce the average intraspecific distances.
In addition to C. luteolum and C. pirinense known from the West Palaearctic region, Bauernfeind and Soldán (Citation2012) considered three extralimital Palaearctic species of Centroptilum. Two of them are poorly known species described based on adults, namely C. chinense You and Gui, 1995 based on a male imago from China and C. rotundum Takahashi, 1929 based on a female imago from Japan. Comparing the species described here with these taxa is impossible because the imago is unknown in C. volodymyri sp. nov. The third species, C. kazlauskasi Kluge, Citation1983, distributed in Mongolia, and the Far East and the Republic of Buryatia in the Russian Federation, was designated as the type species of the genus Anafroptilum Kluge, 2011. The genus is established for non-African taxa, initially attributed to Centroptilum (Kluge Citation2012). In contrast to Centroptilum (incl. C. volodymyri sp. nov. as well as the closely related Nearctic genus Neocloeon), the representatives of Anafroptilum are characterised by the absence of the patella-tibial suture in larvae and adults of both sexes on the fore legs, and therefore are considered to belong to a completely separate lineage from Centroptilum.
There are some other taxa nominally placed within Centroptilum and distributed in the Palearctic Region. Centroptilum hungaricum Pongrácz, Citation1913 was described as the female imago from the Trenčianska župa [Trencsén County in the former Kingdom of Hungary; now Western Slovakia] (Pongrácz Citation1913). Centroptilum obtusum Navás, Citation1915 from Spain is known from the old material only (Navás Citation1915), but Alba-Tercedor and Peters (Citation1985) reported the absence of the types of this species in the collection of Longinos Navás at the Museo de Zoologia del Ayuntamiento (Barcelona, Spain). These two taxa are mentioned as dubious species (for additional information and respective references see Jacobus & Wiersema Citation2014). Additionally, C. striatum Bengtsson, 1904 is considered a nomen nudum (see Bauernfeind & Soldán Citation2012) and the generic placement of C. lituratum (Pictet, 1843) is considered unclear, because the taxon was described from adult material from Switzerland and the type material is lost (Keffermüller & Sowa Citation1984). The comparison of the presently discovered species with the taxa referred to above is impossible, again because the imaginal stage of C. volodymyri sp. nov. is unknown.
Summarised data on the diversity of the subfamily Cloeoninae of Iran were published by Bojková et al. (Citation2018): five species of this subfamily were reported, belonging to Cloeon, Centroptilum and Procloeon. Regarding biogeographic patterns of Iranian Cloeoninae, the dominant part of the species exhibits a broad Palearctic and Holarctic distribution. This is in clear contrast to the distribution of C. volodymyri sp. nov., which is limited to Northern Middle East, Southern Caucasus and Eastern Anatolia. A single species of Procloeon (P. caspicus) was recorded from Northwest Iran, close to the border with Azerbaijan (Bojková et al. Citation2018).
The fauna of Caucasian Cloeoninae is more diverse. Altogether, 11 species belonging to three genera were recorded. While Centroptilum luteolum is distributed throughout the region (see Cherchesova Citation2004; Palatov Citation2013; Martynov et al. Citation2016; Hrivniak et al. Citation2018), the taxon listed as Centroptilum sp. from the Kintrishi River (AR Adzharia, Georgia) corresponds to the new species described here (see the list of synonymies and Martynov et al. Citation2016). The species of Cloeon are a typical component of mayfly taxocenes of Caucasian river basins. However, in comparison to the widely distributed C. dipterum, three other species were only reported from a restricted number of regions of the Caucasus: Cloeon simile Eaton, 1870 listed for Azerbaijan, Georgia (AR Abkhazia) and the Russian part of the Caucasus (Sokolova Citation1937; Kasymov Citation1972; Puthz Citation1978); Cloeon cognatum Stephens, 1835 reported by Cherchesova (Citation2004) for the Northern Caucasus within North Ossetia-Alania, Kabardino-Balkaria and Karachay-Cherkessia; and Cloeon inscriptum Bengtsson, 1914 known only from a single doubtful record labelled “material from Caucasus”, without additional precision (see Silina Citation1994). The Caucasian fauna of the genus Procloeon (Procloeon) Bengtsson, 1915 is represented by P. bifidum (Bengtsson, 1912) (the records from Krasnodarskii krai only; see Palatov Citation2013) and P. heterophyllum Kluge and Novikova, Citation1992a (endemic to the Caucasus, known from the type locality only; see Kluge Citation1997). There are three species assigned to the subgenus Pseudocentroptilum Bogoescu, 1947, within the Caucasus: P. pulchrum (Eaton, 1885) and P. unguiculatum Tshernova, 1941, occurring in water-flows in Georgia and the Russian Federation (see Palatov Citation2013; Martynov et al. Citation2016), and P. pennulatum (Eaton, 1870) reported from Armenia and Azerbaijan (Palatov & Sokolova Citation2016; Hrivniak et al. Citation2018).
The literature published up to now contains no records of Cloeoninae from the Pontic Mountains within Turkey. Fragmentary information about the distribution of P. pulchrum and C. dipterum was published by Kazancı (Citation1984) from a neighbouring region in the Ardahan il.
The species composition of Cloeoninae of Turkey, Iran and Central Asia remains only partially established. For now, only Cloeon dipterum (Linnaeus, 1761) is known to be common throughout these three territories (Tshernova Citation1930; Brodsky Citation1930; Demoulin Citation1964; Kazancı & Türkmen Citation2012; Martynov et al. Citation2016; Hrivniak et al. Citation2018; Bojková et al. Citation2018; authors’ original data). The species is characterised by wide ecological plasticity. Cloeon dipterum inhabits brackish water bodies of deserts and semi-deserts (Hassell et al. Citation2006; authors’ original data). However, it is highly probable that Central Asian limnophilic Cloeoninae, such as Cloeon tadjikistanicum Brodsky, 1950, will be found in Iran. Cloeon tadjikistanicum was recorded in Pyandzh River, on the border between Tajikistan and Afghanistan. One more species, Centroptilum rubidum Kimmins, Citation1950, was described from the border of Iran and Afghanistan (Kimmins Citation1950). Also, most likely Cloeon karachinensis Ali, Citation1970 (described from Western Pakistan) will be recorded within Sistan and Baluchestan Province. Pseudocentroptilum unguiculatum (Tshernova, 1941), common within Central Asia and occurring from Eastern Uzbekistan to Mongolia (Kluge & Novikova Citation1992a, Citation1992b), also has not yet been recorded from Iran.
Generally, in our opinion, the description of a new valid species of the restricted genus Centroptilum s.s., the distribution of which falls within several regions (Southern Caucasus, Eastern Anatolia and Albors Mts), should be of great interest for future investigation of relations and origins of the region’s mayfly faunas.
Acknowledgements
We are grateful to Nicolás Ubero-Pascal (Universidad de Murcia, Spain) for SEM photos of eggs of C. luteolum and valuable comments on their structure. We are indebted to Arnold H. Staniczek and Milan Pallmann (SMNS) for macro- and SEM photos of Centroptilum species, and provision of Cloeoninae comparative material. We highly appreciate the meaningful insights and reviews made by André Wagner (Switzerland) and Carlo Belfiore (Italy), which significantly improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alba-Tercedor J, Peters WL. 1985. Types and additional specimens of Ephemeroptera studied by Longinos Navás in the Museo de Zoologia del Ayuntamiento, Barcelona, Spain. Aquatic Insects 7(4):215–227. DOI: 10.1080/01650428509361222.
- Ali SR. 1970. Certain mayflies (Order: Ephemeroptera) of west Pakistan. Pakistan Journal of Science V 22(3–4):119–124.
- Bauernfeind E, Soldán T. 2012. The mayflies of Europe. Ollerup: Apollo Books. pp. 1–781.
- Bojková J, Sroka P, Soldán T, Namin JI, Staniczek AH, Polášek M, Hrivniak L, Abdoli A, Godunko RJ. 2018. Initial commented checklist of Iranian mayflies, with new area records and description of Procloeon caspicum sp. n. (Insecta, Ephemeroptera, Baetidae). ZooKeys 749:87–123. DOI: 10.3897/zookeys.749.24104.
- Brodsky K. 1930. Zur Kenntnis der mittelasiatischen Ephemeropteren I. (Imagines). Zoologische Jahrbücher. Abteilung für Systematik 59:681–720.
- Chakrabarty P, Warren M, Page LM, Baldwin CC. 2013. GenSeq: An updated nomenclature and ranking for genetic sequences from type and non-type sources. ZooKeys 346:29–41. DOI: 10.3897/zookeys.346.5753.
- Cherchesova SK. 2004. Aquatic insects (Ephemeroptera, Plecoptera, Trichoptera) of rivers of the Northern Osetiya. Moscow: Izdatel’stvo Moskovskoi Sel’skokhozyaystvennoi Akademii imeni K.A. Timiryazeva (MSKhA). pp. 1–238. [ in Russian].
- Demoulin G. 1964. Mission HG Amsel en Afghanistan (1956) Ephemeroptera. Bulletin del’Institut Royal des Sciences Naturelles de Belgique Entomologie 100:351–363.
- Eaton AE. 1871. A monograph on the Ephemeridae. Transactions of the Entomological Society of London 1871:1–164.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5):294–299.
- Gabelashvili S, Mumladze L, Bikashvili A, Sroka P, Godunko RJ, Japoshvili B. 2018. The first annotated checklist of mayflies (Ephemeroptera: Insecta) of Georgia with new distribution data and a new record for the country. Turkish Journal of Zoology 42:252–262. DOI: 10.3906/zoo-1709-4.
- Gillies MT. 1990. A revision of the African species of Centroptilum Eaton (Baetidae, Ephemeroptera). Aquatic Insects 12(2):97–128. DOI: 10.1080/01650429009361395.
- Godunko RJ, Palatov DM, Martynov AV. 2015. Mayflies of the Caucasus Mountains. III. A new representative of the subgenus Rhodobaetis Jacob, 2003 (Baetidae: Baetis) from the South-Western Caucasus. Zootaxa 3948(2):182–202. DOI: 10.11646/zootaxa.3948.2.2.
- Godunko RJ, Martynov AV, Gattolliat J-L. 2018. Redescription of Nigrobaetis rhithralis (Soldán & Thomas, 1983) (Ephemeroptera: Baetidae). Zootaxa 4462:41–72. DOI: 10.11646/zootaxa.4462.1.2.
- Hassell KL, Kefford BJ, Nugegoda D. 2006. Sub-lethal and chronic salinity tolerances of three freshwater insects: Cloeon sp. and Centroptilum sp. (Ephemeroptera: Baetidae) and Chironomus sp. (Diptera: Chironomidae). Journal of Experimental Biology 209(20):4024–4032. DOI: 10.1242/jeb.02457.
- Hrivniak L, Sroka P, Godunko RJ, Palatov D, Polášek M, Manko P, Oboňa J. 2018. Diversity of Armenian mayflies (Ephemeroptera) with the description of a new species of the genus Ecdyonurus (Heptageniidae). Zootaxa 4500(2):195–221. DOI: 10.11646/zootaxa.4500.2.3.
- Hrivniak L, Sroka P, Bojková J, Godunko RJ, Namin JI, Bagheri S, Nejat F, Abdoli A, Staniczek AH. 2020. Diversity and distribution of Epeorus (Caucasiron) (Ephemeroptera, Heptageniidae) in Iran, with descriptions of three new species. ZooKeys 947:71–102. DOI: 10.3897/zookeys.947.51259.
- Ikonomov P [Икономов П]. 1962. Baëtidae (Ephemeroptera) на Македониjа. Faculté des Sciences Naturelles de l’Úniversité de Skopje. Biologie 13:83–140. [ in Macedonian].
- Jacob U. 1973. Ein Centroptilum des stenopteryx-Komplexes aus dem mitteleuropäischen Flachland (Baetidae, Ephemeroptera). Reichenbachia 14(21):163–170.
- Jacob U. 1991. Ephemeroptera: Zur Systematik der europäischen Baetidae auf Gattungsebene. Verhandlungen der Westdeutschen Entomologischen Tagung 1990:271–290.
- Jacobus LM, Wiersema NA. 2014. The genera Anafroptilum Kluge, 2011 and Neocloeon Traver, 1932, reinstated status, in North America, with remarks about the global composition of Centroptilum Eaton, 1869 (Ephemeroptera: Baetidae). Zootaxa 3814(3):385–391. DOI: 10.11646/zootaxa.3814.3.5.
- Kaltenbach T, Gattolliat J-L. 2017. New species of Indocloeon Müller-Liebenau from South-East Asia (Ephemeroptera, Baetidae). ZooKeys 723:43–60. DOI: 10.3897/zookeys.723.20578.
- Kasymov AG. 1972. Freshwater fauna of the Caucasus. Baku: Izdatel’stvo Elm. pp. 287. [ in Russian].
- Kazancı N. 1984. New Ephemeroptera (Insecta) records from Turkey. Aquatic Insects 6(4):253–258. DOI: 10.1080/01650428409361191.
- Kazancı N, Türkmen G. 2012. The checklist of Ephemeroptera (Insecta) species of Turkey. Review of Hydrobiology 5(2):143–156.
- Kazlauskas RS. 1972. Neues über das System der Eintagsfliegen der Familie Baetidae (Ephemeroptera). Proceedings: XIII International Congress of Entomology (2–9 August 1968), Moscow 3:337–338.
- Keffermüller M, Sowa R. 1975. Les espèces du groupe Centroptilum pulchrum Eaton (Ephemeroptera, Baetidae) en Pologne [Gatunki z grupy Centroptilum pulchrum Eaton (Ephemeroptera, Baetidae) w Polsce]. Polskie Pismo Entomologiczne 45:479–486.
- Keffermüller M, Sowa R. 1984. Survey of Central European species of the genera Centroptilum Eaton and Pseudocentroptilum Bogoescu (Ephemeroptera, Baetidae) [Przegląd środkowoeuropejskich gatunków z rodzajów Centroptilum Eaton i Pseudocentroptilum Bogoescu (Ephemeroptera, Baetidae)]. Polskie Pismo Entomologiczne 54:309–340.
- Kimmins DE. 1950. The 3rd Danish expedition to Central Asia. Zoological results 4. Odonata, Ephemeroptera and Neuroptera (Insecta) from Afghanistan. Videnskabelige Meddelelser Dansk Naturhistorisk Forening 112:237–241.
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16:111–120. DOI: 10.1007/BF01731581.
- Kluge NJ [Клюге НЮ]. 1983. New and little known mayflies of the family Baetidae (Ephemeroptera) from Primorye Territiry [Новые и малоизвестные поденки сем. Baetidae (Ephemeroptera) из Приморья]. Entomologicheskoe Obozrenie 61(1):65–79.
- Kluge NJ, Novikova EA. 1992a. Revision of Palearctic genera and subgenera of mayflies in the subfamily Cloeoninae (Ephemeroptera, Baetidae) with descriptions of new species from the USSR. Entomological Review 71(9):4–13. [In Russian.]
- Kluge NJ, Novikova EA. 1992b. Revision of Palearctic genera and subgenera of mayflies in the subfamily Cloeoninae (Ephemeroptera, Baetidae) with descriptions of new species from the USSR. Entomological Review 71:29–54.
- Kluge NJ [Клюге НЮ]. 1997. The order Ephemeroptera [Отряд поденки Ephemeroptera]. In: Tsalolikhin SJ, editor. [Цалолихин СЯ (ред.)]. Key to freshwater invertebrates of Russia and adjacent lands [Определитель пресноводных беспозвоночных России и сопредельных территорий]. Vol. 3. St-Petersburg: Zoological institute RAS. pp. 176–220.
- Kluge NJ. 2012. Non-African representatives of the plesiomorphon Protopatellata (Ephemeroptera: Baetidae). Russian Entomological Journal 20(4):361–376. DOI: 10.15298/rusentj.20.4.02.
- Kluge NJ, Godunko RJ, Apanaskevich DA. 2013. Mayflies of the Caucasus Mountains. II. Description of the first representative of the subgenus Helvetoraeticus Bauernfeind & Soldán, 2012 (Heptageniidae: Ecdyonurus). Zootaxa 3608(1):51–66. DOI: 10.11646/zootaxa.3666.4.5.
- Kluge NJ. 2016. Redescription of the genus Cheleocloeon Wuillot & Gillies 1993 (Ephemeroptera: Baetidae) with descriptions of three new species from Zambia and Uganda. Zootaxa 4067(2):135–167. DOI: 10.11646/zootaxa.4067.2.2.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35:1547–1549. DOI: 10.1093/molbev/msy096.
- Mahboobi Soofiani N, Hatami R, Hemami MR, Ebrahimi E. 2012. Effects of trout farm effluent on water quality and the macrobenthic invertebrate community of the Zayandeh-roud River, Iran. North American Journal of Aquaculture 74:132–141. DOI: 10.1080/15222055.2012.672367.
- Martynov AV, Palatov DM, Godunko RJ. 2015. The larvae of West Palaearctic Eurylophella Tiensuu, 1935 (Ephemeroptera: Ephemerellidae), with description of a new species from Georgia. Zootaxa 3904(1):123–143. DOI: 10.11646/zootaxa.3904.1.8.
- Martynov AV, Godunko RJ, Palatov DM. 2016. Kintrishi state nature reserve – A hotspot for mayfly (Insecta: Ephemeroptera) diversity in Adjara (Georgia). Zoosymposia 11:168–173. DOI: 10.11646/zoosymposia.11.1.18.
- Martynov AV, Godunko RJ. 2017. Mayflies of the Caucasus Mountains. IV. New species of the genus Nigrobaetis Novikova & Kluge, 1987 (Ephemeroptera, Baetidae) from Georgia. Zootaxa 4231(1):70–84. DOI: 10.11646/zootaxa.4231.1.4.
- McCafferty WP, Waltz RD. 1990. Revisionary synopsis of the Baetidae (Ephemeroptera) of North and Middle America. Transactions of the American Entomological Society 116(4):769–799.
- Navás L. 1915. Notas entomologicas 2. Ser. 12. Excursiones por Cataluña. Julio de 1914. Boletín de la Sociedad Aragonesa de Ciencias Naturales 14:27–59, 67–80.
- Palatov DM. 2013. Novye dannye o faune i rasprostranenii podenok semeistva Baetidae (Ephemeroptera) na territorii Kavkaza i Zakavkaz’ja [New data on the fauna and distribution of mayfly family Baetidae (Ephemeroptera) of the Caucasus and Transcaucasia]. In: Prokin AA, Petrov PN, Zhavoronkova OD, Tuzovskij PV editors. Hydroentomology in Russia and adjacent countries: Materials of the fifth all-Russia symposium on amphibiotic and aquatic insects. Yaroslavl’: Izdatel’stvo Filigran. pp. 107–114. [ in Russian, with English summary].
- Palatov DM, Sokolova AM. 2016. Mayflies (Ephemeroptera) of the Talish Mountains (Azer-baijan). Problemy vodnoy entomologii Rossii i sopredel’nykh stran. Vladikavkaz: Izdatel’stvo Severo- Osetinskogo Gosudarstvennogo Universiteta im. KL Khetagurova. pp. 83–87. [ in Russian].
- Pongrácz S. 1913. Újabb adatok Magyarország Neuroptera-faunájához. Rovartani Lapok 20:175–186.
- Puthz V. 1978. Ephemeroptera. In: Illies J, editor. Limnofauna Europaea. 2nd ed. Stuttgart: Gustav Fischer. pp. 256–263.
- Salur A, Darilmaz MC, Bauernfeind E. 2016. An annotated catalogue of the mayfly fauna of Turkey (Insecta, Ephemeroptera). ZooKeys 620:67–118. DOI: 10.3897/zookeys.620.9405.
- Silina AE. 1994. Ecological peculiarities of the sympatric species of mayflies, Cloeon dipterum L. and C. inscriptum Btss. (Ephemeroptera, Baetidae). Entomological Review 73(7):43–49.
- Sokolova MF [Соколова МФ]. 1937. To the hydrobiological characteristics of the surface water bodies of Abkhazia [К гидробиологической характеристике водоемов Абхазии]. Trudy Tropicheskogo Instituta Narkomzdrava Abkhazskoy ASSR 3:59–78. [ in Russian].
- Stecher G, Tamura K, Kumar S. 2020. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Molecular Biology and Evolution 37(4):1237–1239. DOI: 10.1093/molbev/msz312.
- Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10:512–526. DOI: 10.1093/oxfordjournals.molbev.a040023.
- Tshernova OA. 1930. Beiträge zur Kenntnis der paläarktischen Ephemeropteren. Zoologischer Anzeiger 92:214–218.
- Ubero-Pascal NA, Fortuño JM, Puig MA. 2005. New application of air-drying techniques for studying Ephemeroptera and Plecoptera eggs by scanning electron microscopy. Microscopy Research and Technique 68:264–271. DOI: 10.1002/jemt.20248.
- Ubero-Pascal NA, Puig MA. 2007. Microscopy and egg morphology of mayflies. In: Méndez-Vilas, and Díaz J, editors. Modern research and educational topics in microscopy. Badajoz: Formatex. pp. 326–335.
- Vuataz L, Sartori M, Wagner A, Monaghan MT. 2011. Toward a DNA taxonomy of Alpine Rhithrogena (Ephemeroptera: Heptageniidae) using a mixed Yule-coalescent analysis of mitochondrial and nuclear DNA. PLoS ONE 6(5):1–11. DOI: 10.1371/journal.pone.0019728.