Abstract
Recent studies suggest that the lithological properties of hard substrates play an important role in influencing the diversity and structure of marine assemblages involving macroalgae, sessile organisms and vagile animals like heterobranchs gastropods and fishes. The present study aims at exploring whether the influence of different substrates (limestone vs granite) could drive the occurrence of different vagile groups (crustacean decapods, echinoderms and fishes). The study was carried out at the “Tavolara-Punta Coda Cavallo” Marine Protected Area (NE Sardinia, Mediterranean Sea) where substrates of different lithology, namely granites and limestones, occur on a local spatial scale. The diversity and the abundance of 16 vagile species (four crustaceans, seven echinoderms and five fishes) were investigated by the analysis of photographs (2352 images) collected in 20 sites (10 limestones and 10 granites), between 30 and 50 m depth. Statistical analyses showed a clear-cut separation between the assemblages assessed on the two lithological substrate types, with assemblages on granites that were tightly grouped and those on limestones more dispersed. The total species richness did not significantly vary between limestones and granites. Galathea strigosa and Marthasterias glacialis were exclusively recorded on granites, while Palinurus elephas, Scyllarides latus, Arbacia lixula, Ophidiaster ophidianus and Serranus scriba were exclusively found on limestones. The observed patterns could be partially explained by multiple factors related to lithology: i) the different availability of preferred food sources, potentially influenced by substrate type; ii) the site geomorphology, that could provide different dens and refuges (in terms of quantity and types of shelters) suitable for different species; iii) the colour of different substrates enhancing the mimicry of different species according to their livery and camouflage ability. Our and literature data suggest that the substrate type in rocky reefs could interact with other environmental factors (i.e., light intensity related to depth) in shaping the structure of rocky-reef assemblages.
Introduction
Structure and diversity of hard-bottom benthic assemblages are known to be shaped by a wide variety of bio-ecological processes (e.g., prey/predator relationships, competition/cooperation) (Dayton Citation1971; Jones et al. Citation1994) as well as chemical-physical factors (e.g., depth, water column variables, light intensity, rugosity, pH) that may act at different spatial and temporal scales (Gaston Citation2000).
Among the abiotic factors, the substrate features can play a primary role in affecting early colonization stages and development of the algal canopy and sessile zoobenthos, which in turn determine a wide variety of seascapes (McCoy et al. Citation1991; Cerrano et al. Citation1999; Bavestrello et al. Citation2000; Diez et al. Citation2003). Although it is not easy to disentangle the relative and/or interacting effects of geomorphology, surface texture at macro and microscale, and chemical composition of the rocks (Coombes et al. Citation2013, Citation2015), it is nowadays clear that the rocky substrate type is one of the main drivers in determining the diversity and the structure of associated assemblages. Besides the evidence reported for ophiolitic rocks (Bavestrello et al. Citation2018), most of the available data concern the differences between assemblages associated with limestones and granites (Guidetti et al. Citation2002, Citation2004; Schiaparelli et al. Citation2003; Canessa et al. Citation2020a, Citation2020b, Citation2021a, Citation2021b). Thanks to the presence of the limestones of the Tavolara Island and the granites of the surrounding Molara Island and Molarotto islet, the Tavolara-Punta Coda Cavallo Marine Protected Area (TPCCMPA) (North-eastern Sardinia, western Mediterranean Sea) is the ideal setting to investigate the effect of lithology on the composition and structure of associated assemblages. Literature data show that, in this area, the most impressive difference between the two substrate types (namely, limestone and granite) is due to the encrusting coralline algae. On limestone these algae build a consistent coralligenous basal bioherm that have a key role in the stabilization of the surface and in the increasing of 3D complexity. On the contrary, on granites, algal bioherms are only occasionally present (Canessa et al. Citation2020a).
Focusing on epibenthic species, the large, massive and erected sponges such as Sarcotragus foetidus Schmidt, 1862, Axinella polypoides (Schmidt, 1862) and Axinella spp. characterize the granitic seascape. The gorgonian Eunicella cavolini (Koch, 1887) is far more common on limestone than on granite, while Paramuricea clavata (Risso, 1826) is mainly found on granite rocks (Canessa et al. Citation2020a, Citation2021a).
Finally, some attempts were done to test if differences in the assemblages settled on the two substrate types scaled also up to higher trophic levels in the food web. Distribution patterns of fish assemblages at shallow rocky reefs (around 5 m depth) are reported to significantly differ according to the substrate type. Labrids of the genus Symphodus and the serranid Serranus scriba (Linnaeus, 1758) tend to be more abundant on granite, while Serranus cabrilla (Linnaeus, 1758), the blennid Parablennius rouxi (Cocco, 1833), the gobid Gobius incognitus Kovačić & Šanda, 2016 and the labrid Thalassoma pavo (Linnaeus, 1758) showed greater densities on limestone. Also, the distribution of echinoids was investigated, without recording significant differences between the two substrates (Guidetti et al. Citation2002, Citation2004).
A recent study examined the diversity and the abundance of stenophagous predators like heterobranch gastropods associated with granite and limestone, suggesting that their distribution patterns reflect the variety and availability of the preferred food items, in their turn affected by the substrate features (Canessa et al. Citation2021b).
The present study is aimed at exploring, through an observational-correlative approach (sensu Underwood Citation1997), if the lithology of the substrate can drive the diversity and abundance of different groups of vagile organisms thriving in circalittoral rocky reefs, exploiting a wide variety of food items and shelter features. In particular, we have investigated more in depth the distribution patterns of filter feeders like ophiuroids and crinoids, grazers like sea urchins, active predators of benthic organisms like starfishes and crustaceans and vagile predators such as fishes.
Materials and methods
Sampling procedure
Twenty sites were chosen based on their comparable depths within the TPCCMPA. Ten sites were characterised by limestone cliffs and outcrops, located along all the eastern coast of Tavolara Island (Occhio di Dio (OdD), Tegghja Liscia (TL), Cala Cicale (CC), Grottone (Gr), Papa Shoal 1 (P1), Papa Shoal 2 (P2); Archetto (Ar), Picchi della Mandria (PdM)) Papa Point (PP), Punta Timone (PT). The other ten sites were granitic outcrops located in the middle of the Tavolara Channel (NEW 01 (N1), Mandria Shoal (N26), NEW 27 (N27), NEW 34 (N34), Angelo Shoal (N35), Lulù Shoal (N36), NEW 38 (N38), NEW41 (N41), NEW 118 (N118), Pinnacolo (N151) (; ).
Figure 1. A, Location of the Tavolara-P.ta Coda Cavallo Marine Protected Area (MPA). B, the position of the investigated limestone (white spots) and granite (grey spots) sites. White spots: limestone sites (Archetto (Ar); Cala Cicale (CC); Occhio di Dio (OdD); Papa Shoal 1 (P1); Papa Shoal 2 (P2); Papa Point (PP); Punta Timone (PT); Grottone (Gr); Picchi della Mandria (PdM); Tegghja Liscia (TL)). Grey spots: granite sites (NEW 01 (N1), Mandria Shoal (N26), NEW 27 (N27), NEW 34 (N34), Angelo Shoal (N35), Lulù Shoal (N36), NEW 38 (N38), NEW41 (N41), NEW 118 (N118), Pinnacolo (N151)). The colored polygons are the boundaries of the zones with different levels of protection. Red, fully protected (no entry-no take) zones; yellow and green (partially protected) buffer zones. WGS84 reference coordinate systems.
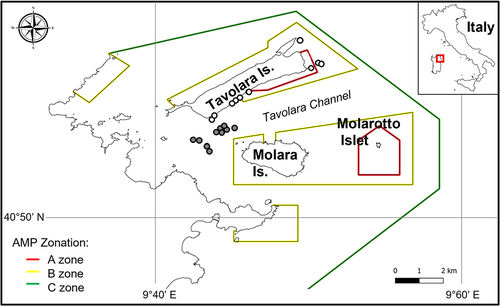
Table I. Position and depth range of the investigated sites within the Tavolara – P. ta Coda Cavallo Marine Protected Area, with the relative number dives, photos and species found and average specific richness found on the two substrates. the geographical coordinates can be consulted on the AMP GIS platform.
We examined photographs making part of the personal collection of one of us (ET) taken at these sites by SCUBA diving between 2016 and 2019 in the 30–50 m depth range. A total of 2352 images (942 and 1410 from limestones and granites, respectively) were selected and the vagile species in each image were classified and counted. Photographs were obtained by a Sony A6000 camera, 24 megapixels, 2 Inon S2000 flashes, colour temperature 5000° K; Sony 16–50 lens; Sea & Sea MDX-a6000 underwater case with flat porthole with Wide Wet Lens Nauticam WWL1. The images correspond to wide-angle close-ups taken to make it possible for the identification of the organisms at the species level. Photographs were analysed by Photoshop CS6 at maximum screen resolution using the short-cut cmd 1. Each original image, measuring 50 × 34 cm at 300 dpi, was examined three times larger at 72. This methodology was illustrated in Canessa et al. (Citation2021b). Abundance data were obtained for crustaceans, echinoderms and fishes. Concerning the latter group, the species taken into account in this study were chosen on the base either of their remarkable conservation relevance (e.g., the dusky grouper Epinephelus marginatus and the brown meagre Sciaena umbra) or for the previously available data collected at this study area (Guidetti & Cattaneo-Vietti Citation2002; Guidetti et al. Citation2004).
For the analysis, only the species present in at least three sites out of 20 were considered. Species abundance, expressed as the number of specimens normalized on the number of photos collected per site, was calculated (). The feeding strategies and food items of the considered species were summarized from the available literature ().
Table II. List of species found in the photographic samples, specimen abundance per site normalised on the number of available photos, average abundance ± SE on granites (GA) limestones (LA) and on the whole dataset (TA).
Table III. Feeding strategies and food items according to literature of each investigated species.
Statistical analyses
Putative differences in the abundance patterns of the whole vagile assemblages and single groups associated with limestones and granites were investigated by means of multivariate methods. Data were appropriately transformed (cubic root) to reduce the influence of dramatically abundant and gregarious taxa (e.g., Periclimenes scriptus) in the analyses.
The non-metric Multi-Dimensional Scaling (nMDS) plot was used to possibly visually represent clustering patterns between the assemblages associated with the two considered lithologies using the species abundance dataset. PERMutational ANalysis Of VAriance (PERMANOVA) was performed to test for putative differences attributable to the factor “Substrate” (two levels, fixed) and “Site” (20 levels, random and nested in each level of “Substrate”) (Anderson Citation2005). The significant PERMANOVA result about the “Substrate” factor was further analysed with PERutational analysis of Multivariate DISPersions (PERMDISP) to determine the difference in dispersion (variance) among a priori groups, considering the mean distance of samples from centroids as measure of the heterogeneity of assemblages (Euclidean Distance similarity Index measure, permutation = 9999) (Anderson Citation2006) (). Finally, the SIMPER (SIMilarity PERcentage) routine was conducted to assess species contribution to the dissimilarity between groups (here the two substrate types), both for the whole assemblages and single groups (Bray–Curtis similarity Index measure, permutation = 9999) (Clarke Citation1993) (). All statistical analyses were performed using PRIMER-e 7 with PERMANOVA+ Add On package.
Table IV. PERMANOVA and PERMDISP analysis performed on the species abundance matrix, showing the significative effect of the factor “Substrate” (Fixed, two levels) and “Site” (twenty levels, random) reported in bold. Data cubic-root transformed. Bray-Curtis index and Euclidean Distance Index used for resemblance matrix calculation, respectively, for PERMANOVA and PERMDISP; permutation N: 9999.
Table V. SIMilarity PERcentage (SIMPER) routine for species contributions to the dissimilarity between limestone and granite, performed overall (ALL) and on single taxonomic group (Echinoderms, Crustaceans and Fishes) abundance dataset. Permutation N: 9999.
Results
On the whole, 16 species, four crustaceans, seven echinoderms and five fishes, occurred in at least three sites. Total species richness did not significantly vary between limestones and granites (14 vs 11 species, respectively), with two species exclusive of granites (Galathea strigosa and Marthasterias glacialis) and five of limestones (Palinurus elephas, Scyllarides latus, Arbacia lixula, Ophidiaster ophidianus, Serranus scriba). The species richness per site ranged from 0 to 11 (5.9 ± 1.1, mean ± SE) and from 3 to 8 (5.7 ± 0.5, mean ± SE) on limestone and granites, respectively (, 2).
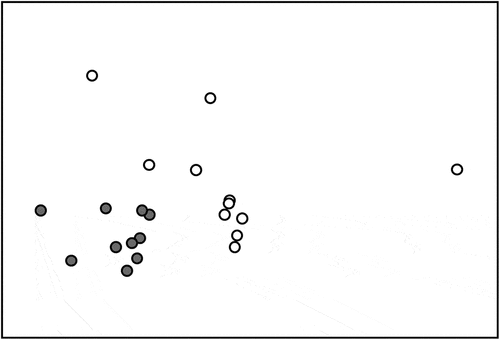
The nMDS plots performed on the abundance data showed a clear-cut separation between the vagile assemblages associated with the two substrate types, with assemblages associated with granites that were tightly grouped, whereas those associated with limestone were more dispersed (). PERMANOVA showed a significant effect of the factor “Substrate” () besides a significant variability at the scale of “Site”. PERMDISP detected a significant difference in dispersion between the two substrates (P = 0.0127) (). Also, the SIMPER analysis highlighted a far lower average similarity among limestones than among granites ().
Figure 2. Non-metric Multi-Dimensional Scaling (MDS) plot, performed on the whole abundance matrix, showing the clustering between limestone (white dots) and granite (grey dots) sites. Brey-Curtis similarity index, Shepard plot stress: 0.07.
Considering the whole assemblages of vagile species, granites were mainly characterised by Periclimenes scriptus (Risso, 1822), while Serranus cabrilla, Epinephelus marginatus (Lowe, 1834) and Sciaena umbra Linnaeus, 1758 were the most representative species on limestones (; ).
Figure 3. Average percent abundance ± SE of species recorded in more than three sites, divided in Crustaceans (A), Echinoderms (B) and Fishes (C), according to the substrate type. Data normalized on the number of photos and cubic-root transformed. Grey bars, granite-selected species; White bars, limestone-selected species.
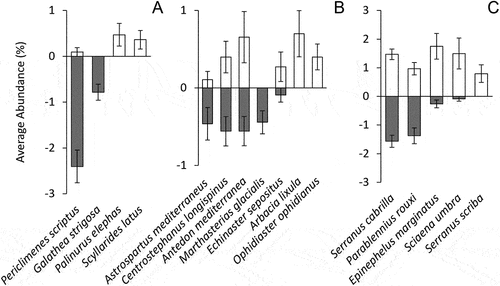
Considering the single taxonomic groups, the two substrates hosted two fairly different assemblages, as showed by values of average dissimilarity between groups (). Among crustaceans, P. scriptus and Galathea strigosa (Linnaeus, 1761) were found only in granites, while Palinurus elephas (Fabricius, 1787) and Scyllarides latus (Latreille, 1803) were recorded only on limestones (, ). This scenario revealed the dramatic difference in occurrence and abundance among species associated with the two substrates (99.53%).
This pattern was mirrored by echinoderms (89.85% average dissimilarity between granite and limestone): two species (Arbacia lixula (Linnaeus, 1758) and Ophidiaster ophidianus (Lamarck, 1816)) were exclusively found on limestones, Marthasterias glacialis (Linnaeus, 1758) was recorded only on granites, Astrospartus mediterraneus (Risso, 1826) was mostly associated to granite, while Antedon mediterranea (Lamarck, 1826), Centrostephanus longispinus (Philippi, 1845) and Echinaster sepositus (Retzius, 1783) were ubiquitous (, ).
Finally, the average dissimilarity between granite and limestone in occurrence and abundance about fishes was slightly lower if compared with other groups (77.33%): Serranus scriba occurred only in limestone sites while no species were exclusively found on granites. E. marginatus and S. umbra were strongly associated with limestone, while the other recorded fishes showed similar abundance on both substrates (, ).
Discussion
Recent studies conducted in the TPCCMPA provided evidence about the importance of the lithology of the hard substrates in structuring benthic assemblages through the selection of macroalgae and sessile zoobenthos under different environmental conditions (Canessa et al. Citation2020a, b, Citation2021a). Moreover, in this study area, it was strongly suggested that specialised vagile predators, like heterobranchs, are associated with different substrates, generally according to their benthic prey occurrence (Canessa et al. Citation2021b).
In the present study, it was explored whether the lithological nature of the substrate can drive diversity and abundance of other vagile groups, composed of species that exploit a wide variety of food items and shelter features.
At the assemblage level, our data revealed a different dispersion pattern but also a clear separation according to substrate lithology, due to the exclusive or preferential occurrence and different relative abundance of several species making part of the assemblages associated with granite or limestone.
The sea urchins Arbacia lixula is one of the most common echinoids on shallow subtidal rocky habitats in the Mediterranean and is considered a key species capable of modifying macroalgae assemblages (Benedetti-Cecchi et al. Citation1998; Sala et al. Citation1998; Bulleri et al. Citation1999; Boudouresque & Verlaque Citation2001; Guidetti Citation2006). In the Tavolara area, around 5 m depth, this species did not show significant differences related to rock lithology (Guidetti et al. Citation2004). On the contrary, at 30–50 m depth, this sea urchin was recorded only on limestones. This pattern is more than likely related to the paucity of macroalgae on granitic substrates at deeper stands, while on limestone coralline algae are abundant (Canessa et al. Citation2020a). Literature data about gut contents report that encrusting coralline algae are a primary food source for A. lixula (Chiantore et al. Citation2008; Privitera et al. Citation2008) (). The other sea urchin recorded in this study, Centrostephanus longispinus, is characterised by a wide feeding plasticity (the diet includes benthic invertebrates such as bryozoans, tunicates and sponges; ), was equally distributed and abundant on both substrate types.
In this study, three species of starfishes were considered, and their distribution resulted almost in agreement with their known food preferences. Starfishes are generally classified as obligate herbivores, omnivores and carnivores, depending on the species (Coleman Citation2017). Field studies and stable isotope analyses indicated that crustose coralline algae and Peyssonnelia spp. are an essential part of the diet of Ophidiaster ophidianus (Trapani et al. Citation2017), while the keratose sponge Ircinia variabilis resulted in its second most frequent food item (Di Trapani et al. Citation2020) (). The record of this starfish almost exclusively on limestones is, therefore, in agreement with the distribution of its preferred food items. The opportunistic Echinaster sepositus, exploiting a wide variety of food sources (mainly coralline algae and sponges), was equally recorded on both substrates (Vasserot Citation1961; Sarà & Vacelet Citation1973; Garcia_Raso et al. Citation1992; Villamor and Becerro (Citation2010). The large predator and scavenger Marthasterias glacialis was recorded only in granite sites. The observed affinity of this species for granitic substrates is hardly explained just based on its feeding strategies (Verling et al. Citation2003) (). In any case, due to the low number of recorded specimens, the observed distribution could include some stochastic components.
The two filter feeder species Astrospartus mediterraneus and Antedon mediterranea displayed different distribution patterns in relation to the substrate type. A. mediterraneus was virtually exclusively recorded on granite sites, while A. mediterranea was equally abundant on limestones and granites (). Even though the SIMPER analysis indicates that A. mediterranea is more associated with limestones, this output is biased by its extremely high abundance at the P2 site (). A. mediterraneus is a typical acrophylic species associated with erected organisms, such as gorgonians and erected sponges that, in the abovementioned site, were mainly found on granitic substrates (Canessa et al. Citation2020a, Citation2021a).
The most impressive differences in distribution patterns according to substrates concern the four species of studied decapod crustaceans. The shrimp Periclimenes scriptus was typically found in large groups associated with the purple gorgonian Paramuricea clavata (Ledoyer Citation1968; Manconi & Mori Citation1992) () that was mainly recorded in association with granite substrates. Remarkably, the shrimp was never found on the gorgonians settled on limestone. A possible explanation for this pattern may be the higher number of colonies of P. clavata affected by local diseases at limestone sites P1 and P2 (Huete‐Stauffer et al. Citation2011). P. scriptus essentially feeds on the mucus of octocorals (Manconi & Mori Citation1992), whose production is strongly reduced by the stress due to periodical diseases (Mistri & Ceccherelli Citation1994).
The biology and ecology of the genus Galathea are poorly studied but it is generally accepted that species belonging to this genus obtain food by detrital deposit feeding and scavenging (De Grave & Turner Citation1997) (). These feeding strategies, however, are hardly attributable to the observed affinity of G. strigosa for granitic substrates. Nevertheless, the quality of the sediment in limestone and granite sites is different: the wide presence of corallinales on limestone drives the structure of the detritus that is mostly coarse, while the sediments that surround granite outcrops are fine and often silty (ET personal observation). Moreover, G. strigosa prefers horizontal crevices, where it stands both on the vault and on the bottom (ET personal observation): this kind of crevices is typical of granite cracks.
On the contrary, the spiny lobster Palinurus elephas and the slipper lobster Scyllarides latus were recorded only in limestone sites. This evidence can be explained by two possible factors related to food preferences and substrate morphology. The food sources of these two large decapods mainly include bivalves, sea urchins, small crustaceans and coralline algae (Goñi & Latrouite Citation2005) () that are typically recorded in limestone sites and are scarce or absent on granitic substrates (Canessa et al. Citation2020a). On the other hand, also the 3D complexity of the substrate can be invoked to explain a differential presence in the two different lithological situations. Lobsters prefer dens with small, multiple openings to those with larger entrances (Spanier & Almog-Shtayer Citation1992; Spanier & Lavalli Citation2006). Limestones sites, due to the presence of coralligenous bioherms, are characterized by a greater variety of structures, ravines, overhangs and crevices, which are likely to provide more suitable shelters. Differences in the distribution of juveniles of P. elephas related to the rocky substrate were studied by Diaz et al. (Citation2001). These Authors observed a more intense settlement on limestones in comparison with metamorphic and siliceous rocks. This selection was explained by the presence on the limestone of empty holes of the date mussel Lithophaga lithophaga (Linnaeus, 1758) providing daytime refuge for young specimens. Similar patterns in the use of empty holes of endolithic molluscs on limestone were observed for juvenile sea urchins (Guidetti Citation2011) and some small cryptobenthic fishes (Parravicini et al. Citation2008).
Two out of the five fish species examined in this study, Parablennius rouxi and Serranus cabrilla, displayed similar distribution patterns on limestone and granite. The remaining three fishes, namely Epinephelus marginatus, Sciaena umbra and S. scriba, were clearly more associated with limestone.
Many and non-mutually exclusive plausible factors (considering those intrinsically related to lithology) could explain the distribution patterns of fish observed in this study, directly or indirectly related to substrate type: i) the colour of the rocky background related to the mimetic ability of fish; ii) the different architectural structures offering shelters for juvenile and adult fish (from small holes to boulders, forming larger shelters or small caves); iii) different food sources associated to different rock types (e.g., vagile invertebrates and juvenile fish comprising most preys of the fish species studied here).
The abundance of E. marginatus and S. umbra in Mediterranean rocky reefs is known to be negatively influenced by the intensity of fishing (Di Franco et al. Citation2009; Guidetti & Micheli Citation2011) and positively influenced by the shelter availability (www.fishbase.org). In the study area, the rocky substrate 3D structure is often more complex on limestone rocky substrates, due to accumulations of boulders collapsing from the emerged cliffs. Such boulders’ accumulations form a rocky habitat rich in shelters and crevices that could support higher abundances of these two species compared to granitic rocks. This scenario is, first of all, in agreement with the observations carried out by Desiderà et al. (Citation2021), who reported higher abundances of E. marginatus on limestone. Also, Guidetti et al. (Citation2004), reported that the densities of other shelter-related fish species, namely Chromis chromis and Apogon imberbis, were higher on limestones than granites. C. chromis is a planktivorous fish swimming and feeding in the water column daytime but searching for shelter close to the sea bottom during the night, while A. imberbis is well known to be a sciaphylic fish, thriving in rocky reefs rich in crevices and caves (Bussotti et al. Citation2015).
In this study, P. rouxi was recorded as equally partitioned between limestone and granite substrates at relatively deep stands (around 30–50 m depth), while in a previous survey Guidetti et al. (Citation2004) recorded higher abundances on limestone in shallow rocky reefs. These authors suggested that the observed distribution pattern could be the result of the camouflage of this small light-colored blennid fish over bare limestones (Guidetti Citation2006). Moreover, in this habitat empty holes of endolithic molluscs offer an additional opportunity for shelter (Parravicini et al. Citation2008). At deeper stands, “light” rocky substrates void of vegetation and endolithic molluscs are fairly rare, and this situation could explain the absence of differences in the abundance of this species between limestone and granite.
Previous studies conducted in the same area (Guidetti & Cattaneo-Vietti Citation2002; Guidetti et al. Citation2004) but at shallow stands (< 10 m) reported that S. scriba and S. cabrilla were more abundant on granite and limestone, respectively. These Authors suggested that substrate colour could again account for differences in the distribution patterns of these two territorial fishes associated with granite or limestone rocks. As already stated, limestones at shallow stands are often lighter than granites, and S. cabrilla could be more abundant due to mimicry offered by its paler livery. At deeper stands, limestone rocks are covered by well-developed coralligenous formations, which could nullify such a “background-camouflage” advantage for S. cabrilla on shallow limestones.
In a different lithological situation, i.e., the granite vs schist rocks of the Asinara Island (Sardinia, Italy), the fish community showed different species occurrence/abundance on the two substrates (Pais et al. Citation2004). For example, S. cabrilla was more abundant on schists than on granites at 24–30 m depth. For other species, like E. marginatus, P. rouxi, S. scriba, S. umbra, occurrence did not vary according to the substrate.
Generally, our data about several taxa of vagile fauna, characterized by different feeding strategies and shelter preferences, suggest that the substrate features may influence the circalittoral rocky-reef assemblages in different ways, concerning multiple trophic levels. Such an influence can be explicated by the differential presence of the preferred food source, that in turn, is directly conditioned by the type of substrate. Secondly, also the geomorphology (i.e., 3D architectural complexity) of sites could play an important role, providing different dens and refuges suitable for different species. Field and experimental studies have demonstrated how substate morphology, particularly the presence of holes and galleries, can affect fish species richness and abundance patterns (Risk Citation1972; Luckhurst & Luckhurst Citation1978; Gratwicke & Speight Citation2005; Rogers et al. Citation2014). Finally, the colour of the substrates can enhance the mimicry of different species according to their livery. In our case, the different rugosity and bioconditioning (covering, etching) of the two substrates, deriving from different chemical properties and aptitude to support the development of coralligenous bioconstructions, is likely to create an intricate pattern of interactions that probably drives the vagile fauna assemblages (Aguilera et al. Citation2014).
Our data suggest that the substrate works probably in synergy with other environmental factors (i.e., light intensity changing with depth) in shaping the community structure (McGuinness Citation1989; Hadfield & Paul Citation2001; Bavestrello et al. Citation2018). This is clearly shown by the sea urchin A. lixula that only down to 30 m depth displays different distribution patterns related to lithology, but not in shallow waters where the algal canopy is present both on granites and limestones (Guidetti et al. Citation2004). The small serranid S. cabrilla, on the contrary, showed a different distribution related to lithology in infralittoral but not in circalittoral substrates (Guidetti & Cattaneo-Vietti Citation2002; Guidetti et al. Citation2004).
In conclusion, this study reported for the first time a possible interaction between lithology and “depth” (actually, the factors changing with depth) in influencing circalittoral rocky-reef assemblages compared to literature data about infralittoral assemblages. The interaction between substrate lithology and other environmental factors and processes changing with depth (e.g., light intensity), spatial scales (latitude related to climate change) and levels of human disturbance (exploitation, pollution, etc.) surely represent an interesting field for future observational and experimental studies to carry on in the field and mesocosms.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aguilera MA, Broitman BR, Thiel M. 2014. Spatial variability in community composition on a granite breakwater versus natural rocky shores: Lack of microhabitats suppresses intertidal biodiversity. Marine Pollution Bulletin 87(1–2):257–268. DOI:10.1016/j.marpolbul.2014.07.046.
- Anderson MJ. 2005. Permutational multivariate analysis of variance. Department of Statistics, University of Auckland Auckland 26:32–46.
- Anderson MJ. 2006. Distance‐based tests for homogeneity of multivariate dispersions. Biometrics 62(1):245–253. DOI:10.1111/j.1541-0420.2005.00440.x.
- Andrew NL, Byrne M. 2007. Ecology of Centrostephanus. In: Developments in aquaculture and fisheries science. Vol. 37. Elsevier. pp.191–204
- Bavestrello G, Bianchi CN, Calcinai B, Cattaneo-Vietti R, Cerrano C, Morri C, Puce S, Sara M. 2000. Bio-mineralogy as a structuring factor for marine epibenthic communities. Marine Ecology Progress Series 193:241–249. DOI:10.3354/meps193241.
- Bavestrello G, Bo M, Betti F, Canessa M, Gaggero L, Rindi F, Cattaneo-Vietti R. 2018. Differences in the composition of shallow-water marine benthic communities associated with two ophiolitic rock substrata. Estuarine, Coastal and Shelf Science 200:71–80. DOI:10.1016/j.ecss.2017.10.009.
- Benedetti-Cecchi L, Bulleri F, Cinelli F. 1998. Density dependent foraging of sea urchins in shallow subtidal reefs on the west coast of Italy (western Mediterranean). Marine Ecology Progress Series 163:203–211. DOI:10.3354/meps163203.
- Boudouresque CF, Verlaque M. 2001. Ecology of Paracentrotus lividus. Developments in Aquaculture and Fisheries Science 32:177–216.
- Bulleri F, Benedetti-Cecchi L, Cinelli F. 1999. Grazing by the sea urchins Arbacia lixula L. and Paracentrotus lividus Lam. in the Northwest Mediterranean. Journal of Experimental Marine Biology and Ecology 241(1):81–95. DOI:10.1016/S0022-0981(99)00073-8.
- Bussotti S, Di Franco A, Francour P, Guidetti P. 2015. Fish assemblages of Mediterranean marine caves. PloS one 10(4):e0122632. DOI:10.1371/journal.pone.0122632.
- Canessa M, Bavestrello G, Bo M, Trainito E, Panzalis P, Navone A, Caragnano A, Betti F, Cattaneo-Vietti R. 2020a. Coralligenous assemblages differ between limestone and granite: A case study at the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Mediterranean Sea). Regional Studies in Marine Science 35:101159. DOI:10.1016/j.rsma.2020.101159.
- Canessa M, Bavestrello G, Trainito E, Navone A, Cattaneo-Vietti R. 2020b. Lithology could affect benthic communities living below boulders. Journal of the Marine Biological Association of the United Kingdom 100(6):879–888. DOI:10.1017/S0025315420000818.
- Canessa M, Bavestrello G, Trainito E, Bianchi CN, Morri C, Navone A, Cattaneo-Vietti R. 2021a. A large and erected sponge assemblage on granite outcrops in a Mediterranean Marine Protected Area (NE Sardinia). Regional Studies in Marine Science 44:101734. DOI:10.1016/j.rsma.2021.101734.
- Canessa M, Bavestrello G, Cattaneo-Vietti R, Furfaro G, Doneddu M, Navone A, Trainito E. 2021b. Rocky substrate affects benthic heterobranch assemblages and prey/predator relationships. Estuarine, Coastal and Shelf Science 261:107568. DOI:10.1016/j.ecss.2021.107568.
- Cerrano C, Arillo A, Bavestrello G, Benatti U, Calcinai B, Cattaneo‐Vietti R, Cortesogno L, Gaggero L, Giovine M, Puce S, Sarà M. 1999. Organism–quartz interactions in structuring benthic communities: Towards a marine bio‐mineralogy? Ecology Letters 2(1):1–3. DOI:10.1046/j.1461-0248.1999.00041.x.
- Chiantore M, Vielmini I, Privitera D, Mangialajo L, Cattaneo-Vietti R. 2008. Habitat effects on the population structure of Paracentrotus lividus and Arbacia lixula. Chemistry and Ecology 24(S1):145–157. DOI:10.1080/02757540801965423.
- Clarke KR. 1993. Non‐parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18(1):117–143. DOI:10.1111/j.1442-9993.1993.tb00438.x.
- Coleman RA. 2017. Filling in the grazing puzzle, A synthesis of herbivory in starfish. Oceanography and Marine Biology Annual Review 55:1–34.
- Coombes MA, Naylor LA, Viles HA, Thompson RC. 2013. Bioprotection and disturbance: Seaweed, microclimatic stability and conditions for mechanical weathering in the intertidal zone. Geomorphology 202:4–14. DOI:10.1016/j.geomorph.2012.09.014.
- Coombes MA, La Marca EC, Naylor LA, Thompson RC. 2015. Getting into the groove: Opportunities to enhance the ecological value of hard coastal infrastructure using fine-scale surface textures. Ecological Engineering 77:314–323. DOI:10.1016/j.ecoleng.2015.01.032.
- Dayton PK. 1971. Competition, disturbance, and community organization: The provision and subsequent utilization of space in a rocky intertidal community. Ecological Monographs 41(4):351–389. DOI:10.2307/1948498.
- De Grave S, Turner JR. 1997. Activity rhythms of the squat lobsters, Galathea squamifera and G. strigosa (Crustacea: Decapoda: Anomura) in south-west Ireland. Journal of the Marine Biological Association of the United Kingdom 77(1):273–276. DOI:10.1017/S002531540003397X.
- Desiderà E, Trainito E, Navone A, Blandin R, Magnani L, Panzalis P, Mazzoldi C, Guidetti P. 2021. Using complementary visual approaches to investigate residency, site fidelity and movement patterns of the dusky grouper (Epinephelus marginatus) in a Mediterranean marine protected area. Marine Biology 168(7):1–16. DOI:10.1007/s00227-021-03917-9.
- Di Franco A, Bussotti S, Navone A, Panzalis P, Guidetti P. 2009. Evaluating effects of total and partial restrictions to fishing on Mediterranean rocky-reef fish assemblages. Marine Ecology Progress Series 387:275–285. DOI:10.3354/meps08051.
- Di Trapani F, Agnetta D, Bonaviri C, Badalamenti F, Gianguzza P. 2020. Unveiling the diet of the thermophilic starfish Ophidiaster ophidianus (Echinodermata: Asteroidea) combining visual observation and stable isotopes analysis. Marine Biology 167:1–11. DOI:10.1007/s00227-020-03704-y.
- Díaz D, Marí M, Abelló P, Demestre M. 2001. Settlement and juvenile habitat of the European spiny lobster Palinurus elephas (Crustacea: Decapoda: Palinuridae) in the western Mediterranean Sea. Scientia Marina 65(4):347–356. DOI:10.3989/scimar.2001.65n4347.
- Dıez I, Santolaria A, Gorostiaga JM. 2003. The relationship of environmental factors to the structure and distribution of subtidal seaweed vegetation of the western Basque coast (N Spain). Estuarine, Coastal and Shelf Science 56(5–6):1041–1054. DOI:10.1016/S0272-7714(02)00301-3.
- Frid CLJ. 1992. Foraging behaviour of the spiny starfish Marthasterias glacialis in Lough Ine, Co. Cork. Marine and Freshwater Behaviour and Physiology 19(4):227–239. DOI:10.1080/10236249209378811.
- Garcia_Raso E, Luque AA, Templado J, Salas C, Hergueta E, Moreno D, Calvo M. 1992. Fauna y Flora marina del Parque Natural de Cabo de Gata-Nijar (Ed. Universidad de Málaga, Universidad autónoma de Madrid y Museo Nacional de Ciencias Naturales, Madrid). Junta de Andalucia Madrid.
- Gaston KJ. 2000. Global patterns in biodiversity. Nature 405(6783):220–227. DOI:10.1038/35012228.
- Goñi R, Reñones O, Quetglas A. 2001. Dynamics of a protected Western Mediterranean population of the European spiny lobster Palinurus elephas (Fabricius, 1787) assessed by trap surveys. Marine and Freshwater Research 52(8):1577–1587. DOI:10.1071/MF01208.
- Goñi R, Latrouite D. 2005. Review of the biology, ecology and fisheries of Palinurus spp. species of European waters: Palinurus elephas (Fabricius, 1787) and Palinurus mauritanicus (Gruvel, 1911). Cahiers de Biologie Marine 46(2):127–142.
- Gratwicke B, Speight MR. 2005. Effects of habitat complexity on Caribbean marine fish assemblages. Marine Ecology Progress Series 292:301–310. DOI:10.3354/meps292301.
- Guidetti P, Bianchi CN, La Mesa G, Modena M, Morri C, Sara G, Vacchi M. 2002. Abundance and size structure of Thalassoma pavo (Pisces: Labridae) in the western Mediterranean Sea: Variability at different spatial scales. Journal of the Marine Biological Association of the United Kingdom 82(3):495–500. DOI:10.1017/S0025315402005775.
- Guidetti P, Cattaneo-Vietti R. 2002. Can mineralogical features influence distribution patterns of fish? A case study in shallow Mediterranean rocky reefs. Journal of the Marine Biological Association of the United Kingdom 82:1043–1044. DOI:10.1017/S0025315402006653.
- Guidetti P, Bianchi CN, Chiantore M, Schiaparelli S, Morri C, Cattaneo-Vietti R. 2004. Living on the rocks: Substrate mineralogy and the structure of subtidal rocky substrate communities in the Mediterranean Sea. Marine Ecology Progress Series 274:57–68. DOI:10.3354/meps274057.
- Guidetti P. 2006. Estimating body size of sea urchins, Paracentrotus lividus and Arbacia lixula, from stomach contents of Diplodus sargus, a Mediterranean predatory fish. Journal of Applied Ichthyology 22(1):91–93. DOI:10.1111/j.1439-0426.2006.00612.x.
- Guidetti P. 2011. The destructive date-mussel fishery and the persistence of barrens in Mediterranean rocky reefs. Marine Pollution Bulletin 62(4):691–695. DOI:10.1016/j.marpolbul.2011.01.029.
- Guidetti P, Micheli F. 2011. Ancient art serving marine conservation. Frontiers in Ecology and the Environment 9(7):374–375. DOI:10.1890/11.WB.019.
- Hadfield MG, Paul VJ. 2001. Natural chemical cues for settlement and metamorphosis of marine invertebrate larvae. Marine Chemical Ecology 13:431–461.
- Huete‐Stauffer C, Vielmini I, Palma M, Navone A, Panzalis P, Vezzulli L, Misic C, Cerrano C. 2011. Paramuricea clavata (Anthozoa, Octocorallia) loss in the Marine Protected Area of Tavolara (Sardinia, Italy) due to a mass mortality event. Marine Ecology 32:107–116. DOI:10.1111/j.1439-0485.2011.00429.x.
- Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers. In: Ecosystem management. New York NY: Springer. DOI:10.1007/978-1-4612-4018-1_14.
- Ledoyer M. 1968. Ecologie de la faune vagile des biotopes méditerranéens accessibles en scaphandre autonome (Région de Marseille principalement) IV.-Synthèse de l’étude écologique. Recueil des Travaux de la Station marine d’Endoume 60(44):125–295.
- Leonard AB. 1989. Functional response in Antedon mediterranea (Lamarck)(Echinodermata: Crinoidea): The interaction of prey concentration and current velocity on a passive suspension-feeder. Journal of Experimental Marine Biology and Ecology 127(1):81–103. DOI:10.1016/0022-0981(89)90210-4.
- Luckhurst BE, Luckhurst K. 1978. Analysis of the influence of substrate variables on coral reef fish communities. Marine Biology 49(4):317–323. DOI:10.1007/BF00455026.
- Manconi R, Mori M. 1992. Caridean shrimps (Decapoda) found among Corallium rubrum (L., 1758). Crustaceana 62(1):105–110. DOI:10.1163/156854092X00109.
- McCoy ED, Bell SS, Mushinsky HR. 1991. Habitat structure: Synthesis and perspectives. In: Bell SS, McCoy ED, Mushinsky HR, editors. Habitat structure. Population and community biology series. Vol. 8. Dordrecht: Springer. DOI:10.1007/978-94-011-3076-9_21.
- McGuinness KA. 1989. Effects of some natural and artificial substrata on sessile marine organisms at Galeta Reef, Panama. Marine Ecology Progress Series Oldendorf 52(2):201–208. DOI:10.3354/meps052201.
- Mistri M, Ceccherelli VU. 1994. Growth and secondary production of the Mediterranean gorgonian Paramuricea clavata. Marine Ecology-Progress Series 103: 291-291. DOI:10.3354/meps103291.
- Pais A, Azzurro E, Chessa LA. 2004. Distribution patterns of coastal fish assemblages associated with different rocky substrates in Asinara Island National Park (Sardinia, Italy). Italian Journal of Zoology 71(4):309–316. DOI:10.1080/11250000409356588.
- Parravicini V, Donato M, Morri C, Villa E, Bianchi CN. 2008. Date mussel harvesting favours some blennioids. Journal of Fish Biology 73:2371–2379. DOI:10.1111/j.1095-8649.2008.02085.x.
- Penney AJ, Griffiths CL. 1984. Prey selection and the impact of the starfish Marthasterias glacialis (L.) and other predators on the mussel Choromytilus meridionalis (Krauss). Journal of Experimental Marine Biology and Ecology 75(1):19–36. DOI:10.1016/0022-0981(84)90021-2.
- Privitera D, Chiantore M, Mangialajo L, Glavic N, Kozul W, Cattaneo-Vietti R. 2008. Inter-and intra-specific competition between Paracentrotus lividus and Arbacia lixula in resource-limited barren areas. Journal of Sea Research 60(3):184–192. DOI:10.1016/j.seares.2008.07.001.
- Risk MJ. 1972. Intertidal substrate rugosity and species diversity. ProQuest Dissertations Publishing.
- Rogers A, Blanchard JL, Mumby PJ. 2014. Vulnerability of coral reef fisheries to a loss of structural complexity. Current Biology 24(9):1000–1005. DOI:10.1016/j.cub.2014.03.026.
- Romeo T, Florio G, Lentini F, Castriota L, Falautano M, Consoli P, Pelusi P, Greco S. 2004. Morphometric aspects of Scyllarides latus. Mediterranean Marine Science 5(2):65–71. DOI:10.12681/mms.204.
- Rost Martins H. 1985. Biological studies of the exploited stock of the Mediterranean locust lobster Scyllarides latus (Latreille, 1803)(Decapoda: Scyllaridae) in the Azores. Journal of Crustacean Biology 5(2):294–305. DOI:10.2307/1547877.
- Sala E, Boudouresque CF, Harmelin-Vivien ML. 1998. Fishing, trophic cascades, and the structure of algal assemblages: Evaluation of an old but untested paradigm. Oikos 82:425–439. DOI:10.2307/3546364.
- Sarà M, Vacelet J. 1973. Écologie des Démosponges. In: Grasse PP, editor. Traité de Zoologie Vol. 3. Spongiaires. Paris: Masson et Cie. pp. 462–576.
- Schiaparelli S, Guidetti P, Cattaneo-Vietti R. 2003. Can mineralogical features affect the distribution patterns of sessile gastropods? The Vermetidae case in the Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom 83(6):1267–1268. DOI:10.1017/S0025315403008622.
- Spanier E, Almog-Shtayer G. 1992. Shelter preferences in the Mediterranean slipper lobster: Effects of physical properties. Journal of Experimental Marine Biology and Ecology 164:103–116. DOI:10.1016/0022-0981(92)90139-2.
- Spanier E, Lavalli KL. 2006. Scyllarides species. In: Lobsters: Biology, management, aquaculture and fisheries. Oxford, UK: Blackwell Publishing. pp. 462–499.
- Trapani D, Bonaviri C, Costa V, Badalamenti F, Gianguzza P 2017. Feeding behaviour of Ophidiaster ophidianus (Lmk.) (Asteroidea) in Mediterranean rocky reefs. In “La ricerca ecologica in un mondo che cambia” 28 Congresso Società Italiana di Ecologia, Napoli.
- Underwood AJ. 1997. Experiments in Ecology: Their logic design and interpretation using analysis of variance. Cambridge: University Press.
- Vasserot J. 1961. Caractère hautement spécialisé du régime alimentaire chez les astérides Echinaster sepositus et Henricia sanguinolenta, prédateurs de spongiaires. Bulletin de la Société zoologique de France 86:796–809.
- Verling E, Crook AC, Barnes DK, Harrison SS. 2003. Structural dynamics of a sea-star (Marthasterias glacialis) population. Journal of the Marine Biological Association of the United Kingdom 83(3):583–592. DOI:10.1017/S0025315403007513h.
- Villamor A, Becerro MA. 2010. Matching spatial distributions of the sea star Echinaster sepositus and crustose coralline algae in shallow rocky Mediterranean communities. Marine Biology 157(10):2241–2251. DOI:10.1007/s00227-010-1489-2.
- Zibrowius H. 1978. Nouvelles observations de l’ophiure gorgenocephale Astrospartus mediterraneus sur la côte méditerraneenne de France. Bibliographie annotee et repartition. Vol. 4. France: Travaux scientifiques du Parc national de Port-Cros. pp. 157–169.
