Abstract
We hereby provide the first documented data on the occurrence of a viable Procambarus virginalis population in a nature reserve in Poland and the steps that were taken to prevent further introductions of the species in the country. To date, Poland represents the most north-eastward distribution area in Europe, where the species occurs in ecosystems with natural water temperature regimes. The ecological plasticity of P. virginalis and its obligate parthenogenetic reproduction make this crayfish an exceptional invader. The crayfish may have detrimental effects on the native invertebrates, amphibians, and fish, and alter the state of the entire ecosystem. Therefore, we investigated the presence of the species in the nature reserve of Pojezierze Łęczyńskie Landscape Park after a suspected P. virginalis individual was found by a local citizen. The nature reserve also includes an area designated to protect native amphibians and turtles. Our study revealed the presence of a thriving population of P. virginalis in the protected area and clear indications of its impact on native and invasive species, both in aquatic and terrestrial environments. If no action is taken, the species will likely expand to other water bodies across the country. An extensive sensibilization campaign allowed us to find additional invaded areas and significantly contribute to the effective ban of this and other invasive crayfish species from private and commercial online trade. Raising public awareness and banning invasive crayfish species trade is essential for successfully detecting and preventing further introductions.
Introduction
Biological invasions have severely affected global biodiversity, particularly over the course of the last centuries (Catford et al. Citation2012; Gallardo et al. Citation2016; Pyšek et al. Citation2020). Human activities primarily mediate the introduction of species outside their native ranges, and the number of translocated species is globally increasing (Savini et al. Citation2010; Early et al. Citation2016; Seebens et al. Citation2017). This often results in the establishment of non-native and potentially invasive species. Aquatic ecosystems are particularly vulnerable and are often invaded by multiple alien species that interact with native biota and alter the ecosystem through additive and multiplying effects (Hudina et al. Citation2011; Jackson et al. Citation2015). Moreover, widespread habitat degradation and climate warming are likely to enhance the adverse impact of invasive species (MacDougall & Turkington Citation2005; Walther et al. Citation2009).
Invasive crayfish, besides their biological traits, such as rapid growth, fecundity, and tolerance to environmental stress, are among the most destructive of invasive species, with their impact affecting multiple ecological levels of invaded ecosystems (Lodge et al. Citation2012; Twardochleb et al. Citation2013; Lipták Citation2019). Decline and extinction of native crayfish through direct and indirect competition, and transmission of the crayfish plague, a disease caused by the oomycete Aphanomyces astaci, are among the most alarming effects of non-native crayfish introductions (Lodge et al. Citation2012; Twardochleb et al. Citation2013; Svoboda et al. Citation2017; Ungureanu et al. Citation2020). Crayfish are generalist omnivores; thus, they may severely affect primary and secondary production (Perry et al. Citation2000). Moreover, they are ecosystem engineers. Through their grazing and burrowing activity, they increase the rate of leaf-litter breakdown, nutrient cycling, and coarse sediments transport, which coupled with a reduction of benthic algae and macrophyte cover could alter the state of the ecosystem from clear to phytoplankton dominated turbid water (Bobeldyk & Lamberti Citation2008; Matsuzaki et al. Citation2009; Johnson et al. Citation2011; Twardochleb et al. Citation2013). Crayfish predation on native invertebrates, fish, and amphibians in concomitance with habitat alteration often causes a decline in diversity and abundances of native species (Kats & Ferrer Citation2003; Correia & Anastácio Citation2008; Twardochleb et al. Citation2013). Invasive crayfish are often carrier of pathogens such as A. astaci which is lethal for native species (Svoboda et al. Citation2017), or the chytrid fungus Batrachochytrium dendrobatidis, which is pathogenic for amphibians (Oficialdegui et al. Citation2019) as well as other non-native epibionts including branchiobdellidans, temnocephalans and ostracods (Ohtaka et al. Citation2005; Aguilar-Alberola et al. Citation2012; Du Preez & Smit Citation2013). Hence, non-native crayfish are a major threat to freshwater biodiversity and ecosystem functioning (Twardochleb et al. Citation2013; Gallardo et al. Citation2016; Pyšek et al. Citation2020).
In Europe, invasive crayfish are spreading at alarming rates. There are numerous non-native crayfish species with established populations in central and western Europe and others may follow in the near future (Kouba et al. Citation2014; Weiperth et al. Citation2017; Collas Citation2019; Haubrock et al. Citation2021). Among them, the marbled crayfish Procambarus virginalis Lyko, 2017 formerly known as Procambarus fallax (Hagen, 1870) f. virginalis, is the only decapod crustacean that reproduces by obligate parthenogenesis (Scholtz et al. Citation2003; Kozák et al. Citation2015). Nowadays, this crayfish species is well established also outside Europe, in countries such as Madagascar (Andriantsoa et al. Citation2019) and Japan (Usio et al. Citation2017). The settling of P. virginalis in the wild results from intentional or unintentional releases (Patoka et al. Citation2017; Hossain et al. Citation2018). This North American species is one of the most common ornamental crayfish in the pet trade (Patoka et al. Citation2014; Faulkes Citation2015; Gutekunst et al. Citation2021). Despite being listed as an invasive alien species, P. virginalis is still present in aquarium trade within the European Union (EU), which banned the species in 2016. Similarly, the invasive Procambarus clarkii, is regularly sold as a popular ornamental species in European pet shops or via Internet (Patoka et al. Citation2014), including Poland (Maciaszek et al. Citation2019). Likewise, these species remain broadly available in neighboring non-EU countries such as Ukraine and Russia, where the trade of these decapods has not been yet restricted by law (Kotovska et al. Citation2016; Vodovsky et al. Citation2017). The EU Regulation 1143/2014 on invasive alien species requires the notification of the mentioned species for early detection and/or eradication. However, no information had been received by the Notification System of European Alien Species Information Network (EASIN Notsys), and no official monitoring or eradication has been performed to date in Poland, although both Procambarus species have been already detected. The invasive P. virginalis was first reported in Szczecin (north-west Poland) in 2009. Afterward, other observations followed, mostly of single individuals (Śmietana et al. Citation2018). The first breeding population was observed in 2019 in the semi-natural pond of Morskie Oko park in Warsaw (Mazurska et al. Citation2019), where thousands of crayfish have already been caught under Łowca Obcych/Alien Hunter initiative (Maciaszek Citation2022). Fortunately, this semi-natural pond is well isolated from other water bodies and to date no overland dispersion of the species has been observed.
The invasive P. virginalis is one of major concerns due to its rapid spread, dominance over native and some invasive crayfish species, predation on native invertebrates and vertebrates, the spread of A. astaci, and habitat alterations through grazing and burrowing activity (Lipták Citation2019; Hossain et al. Citation2020; Kouba et al. Citation2021). Moreover, P. clarkii is a more efficient amphibian predator than European native crayfish species (Gherardi et al. Citation2001). It could potentially transmit B. dendrobatidis and exclude amphibians from potentially suitable reproductive areas as observed in the Iberic peninsula (Cruz et al. Citation2006; Oficialdegui et al. Citation2019). For instance, P. clarkii was responsible for the disappearance of over half of the amphibian species in a nature reserve in Portugal (Cruz et al. Citation2008) and the local extinction of tadpole in northern Italy, including the threatened Rana latastei (Boulenger, 1879) (Ficetola et al. Citation2011). Thus, we can expect similar impacts in the case of P. virginalis invasion.
In this study, we investigated the presence of P. virginalis in the Pojezierze Łęczyńskie Landscape Park (Poland), after being notified by a local citizen who found the suspected crayfish in the proximity of the Nature Reserve of Ostoja Parczewska. This nature reserve also includes an area designated for the protection of the European fire-bellied toad, Bombina bombina (Linnaeus, 1761), an amphibian whose population have declined across the continent (De Cahsan et al. Citation2019), as well as the European pond turtle, Emys orbicularis (Linnaeus, 1758). The presence of native Astacus astacus (Linnaeus, 1758), the noble crayfish has also been reported in a nearby area. Our investigation aimed to assess if P. virginalis established the first viable population in a Polish nature protected area and its potential impact on native species. Moreover, we share the action taken following our findings as an example that could be implemented elsewhere to prevent further introductions of invasive crayfish species.
Material and methods
Locality
Pojezierze Łęczyńskie Landscape Park is located in eastern Poland and is a part of Łęczyńsko-Włodawskie Lakeland. It is characterized by a relatively flat topography with lakes differing in water purity, shape, trophy, and vegetation type. Bogs and ponds often surround the lakes. On the east side, the park borders the Polesie National Park, which is also protected under the Ramsar Convention. This territory is undoubtedly one of the most valuable natural areas in Poland and is also known for its aquaculture ponds.
Miejskie Lake (N 51.5077, E 22.8847) is a natural shallow lake with a surface area of 49 ha and a depth of ca. 2 m, located near Ostrów Lubelski city in the eastern part of Pojezierze Łęczyńskie Landscape Park. The bottom of the lake is silty and sandy. The lake has no inflow of surface waters. However, it is connected with the neighboring Kleszczów Lake via amelioration ditches. It is surrounded by wet meadows and wetlands with overgrown trees and a pine forest. Miejskie Lake is protected for ecological use and is a known place for recreational activities, including fishing.
Kleszczów Lake (N 51.5213, E 22.8932) is a natural, mesotrophic lake with a surface area of 54 ha and a depth of ca. 3 m, also situated in Pojezierze Łęczyńskie Landscape Park. The bottom of the lake is silty and sandy. Wetlands and pine forests surround it. The lake and its immediate surroundings are included in the Special Area of Conservation Ostoja Parczewska PLH 060013 and the Special Protection Area of Lasy Parczewskie PLB 060006. This Natura 2000 habitat zone also includes an area designated for the protection of B. bombina as well as E. orbicularis. Same as Miejskie Lake, this ecosystem is protected for ecological use. On the southern part, Kleszczów Lake is connected with the Miejskie Lake, while in the northern part is connected with the Bobrówka river via amelioration ditches. The Bobrówka river is a tributary of the Tyśmienica river, which is a part of the Vistula river basin.
The melioration ditches connecting both lakes and the Kleszczów Lake with the Bobrówka river seem to be neglected remains of past melioration works. They are often dry or covered by swamp vegetation and tree branches, and only in the aftermath of heavy rainfall water fill them up. Amphibians, mainly frogs and toads, widely inhabit these ditches.
Sampling sites designation
Following observations carried out by local citizens in June and July 2020, we investigated the area to assess the possible origin and migration pathways for P. virginalis on July 28–29, 2020. All crayfish observed by locals have been found in holiday plots located at the Miejskie Lake. In each case, they were found after heavy rainfalls that occurred in the second part of June and the beginning of July 2020. Most crayfish were found dead or alive on plot lawns and local roads, mainly near the forest. Initial consultations with local anglers confirmed the presence of the species and revealed that no other crayfish than P. virginalis had been deliberately introduced in Miejskie Lake. According to local anglers, P. virginalis was introduced there approximately three years earlier as a food source for native predatory fish that had been overfished and competitively displaced by the invasive brown bullhead, Ameiurus nebulosus (Lesueur, 1819). The population was then kept by anglers in good condition by immediate releasing caught crayfish to the lake. Locals also mentioned the potential occurrence of the spiny-cheek crayfish Faxonius limosus (Rafinesque, 1817) and an unidentified crayfish (most likely the native A. astacus or the narrow-clawed crayfish Pontastacus leptodactylus (Eschscholtz, 1823) known from neighboring water bodies in the region) in both lakes as well as Bobrówka river.
Sampling was conducted in Ostrów Lubelski (7 sampling sites) and Warsaw (1 sampling site) (). The sampling sites in Ostrów Lubelski were designated on the basis of reports from the local community and included lakes and ditches (, ). We sampled along the shoreline of Miejskie (1) and Kleszczów (6) lakes using transects of 100 m in length and 4 m in width (half open water and half occupied by littoral vegetation). Other transects of 200 m in length and 2 m in width were used in wet (3, 5, 7) and dry (2, 4) ditches. Individuals collected in Warsaw were used as the reference for morphological and genetic analyses.
Figure 1. Sampling locations where Procambarus virginalis was detected by local citizens prior to our survey (red squares) in Ostrów Lubelski. Highlighted water bodies represent lakes and ponds (light blue areas), rivers (dark blue lines), and amelioration ditches (light blue lines).
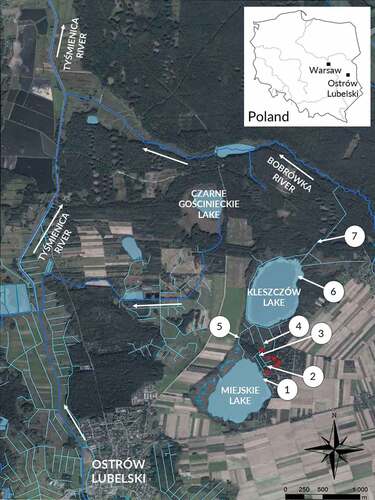
Table I. Sampling sites examined for crayfish presence.
Crayfish detection and identification
Live crayfish were caught in water using hydrobiological nets or were gathered by hand during day and night time on August 1–2, 2020. On land, they were tracked following dead crayfish bodies as well as indications of their activities, like burrows or migration channels. When found, they were hand-gathered or excavated from the burrows. All collected specimens were then measured using total and carapax lengths (Kozák et al. Citation2015). Further evaluated parameters included incidence of pre- and post-molting individuals and egg-carrying females. Species identity was confirmed by combining morphological (Kozák et al. Citation2015) and molecular methods.
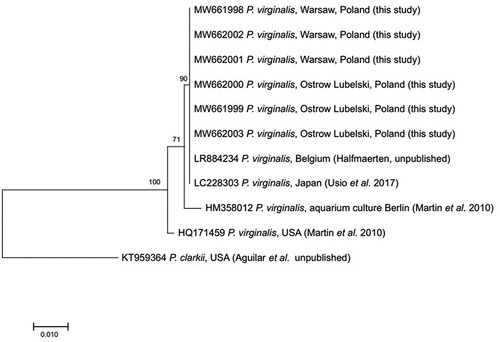
The DNA was isolated from the muscle tissue of six P. virginalis collected in Ostrów Lubelski and Warsaw, using the Chelex method (Casquet et al. Citation2012). The COI (cytochrome oxidase I) fragment was amplified with the primer pair LCO JJ and HCO JJ (Astrin & Stüben Citation2008) in PCR reaction following the protocol by Hou et al. (Citation2007). The PCR products were purified with exonuclease I (Exo I) and alkaline phosphatase (FastAP) and then sent for sequencing to Macrogen Inc., Europe. Obtained sequences were blasted against GenBank records (Altschul et al. Citation1990). The sequences were aligned and trimmed to the same length of 624 bp using Geneious 10.0.2 software (Kearse et al. Citation2012). Obtained sequences were deposited in the BOLD Systems (Ratnasingham & Hebert Citation2007), as well as in GenBank (Benson et al. Citation2005) with accession numbers MW661998, MW662001, MW662002 (P. virginalis from Warsaw) and MW661999, MW662000, MW662003 (P. virginalis from Ostrów Lubelski). The COI haplotypes were designated in DnaSPv5 software (Librado & Rozas Citation2009). The phylogenetic tree based on the HKY model (Hasegawa et al. Citation1985) was constructed with the Maximum Likelihood (ML) method in MEGA X software (Kumar et al. Citation2018). The validity of nodes was estimated with the bootstrap test (1000 replicates) (Felsenstein Citation1985). To supplement the tree following sequences were taken from GenBank collection: P. virginalis of the accession number LR884234, deposited by Halfmaerten (unpublished), P. virginalis (acc. no. HM358012 and HQ171459) deposited by Martin et al. (Citation2010), P. virginalis (acc. no. LC228303) deposited by Usio et al. (Citation2017), and P. clarkii (acc. no. KT959364) deposited by Aguilar et al. (unpublished).
Statistical analysis
The total and carapax length of P. virginalis collected at different sites were expressed as mean ± SD (mm). The significance of differences between these groups was assessed using the analysis of variance (ANOVA, post hoc tests). All statistical calculations were performed using IBM SPSS Statistics 27.
Results
A total of 715 crayfish were collected in Ostrów Lubelski sites, of which 714 were identified as P. virginalis and a single individual as F. limosus (Figure S1). All specimens were females. The vast majority of P. virginalis (84.90%) were collected in a wet ditch (), while the remaining individuals were collected in a dry ditch (13.01%) as well as along the shoreline of Lake Kleszczów (1.96%) and Lake Miejskie (0.13%). The only specimen of F. limosus was collected along the shoreline of Lake Kleszczów. No native crayfish has been captured or noticed. All crayfish collected in Warsaw were morphologically identified as P. virginalis.
Figure 2. Locations in Ostrów Lubelski where live specimens of Procambarus virginalis were detected: Miejskie Lake – 1 (a), Kleszczów Lake – 6 (b), wet ditch – 3 (c), dry ditch – 2 (d).

All barcoded specimens represented only one haplotype, which was also common with the haplotype reported from Belgium and Japan (). BOLD assigned investigated specimens to only one, non-unique Barcode Index Number (BIN) ACG1580 (dx.doi.org/10.5883/BOLD:ACG1580), together with other representatives of the species deriving from private data deposited in this database.
Figure 3. Maximum Likelihood phylogenetic tree including sequences obtained from collected Procambarus virginalis and GenBank stocked sequences.
In the wet ditch, crayfish were mostly observed underwater, in puddles, and migration channels (). Single individuals were also observed on land in the proximity of the ditch. At least one specimen was found in each migration channel and burrow. On one occasion, 276 individuals were caught from a single puddle of ca. 2 m2 surface area and 15 cm depth. A total of 237 individuals were collected in another puddle built in an abandoned fox burrow and connected to an underwater lair system comprising four burrows of maximum length of 70 cm built rather horizontally. Increased activity of crayfish in the ditch was observed 3 hours before and to 6 hours after sunset. Only several crayfish were found freshly dead, most likely due to unsuccessful molting.
Figure 4. Indications of Procambarus virginalis in the terrestrial and littoral zone: intact dead specimens found on forest roads (a); dead crayfish in freshly dried ditch (b); crayfish burrows: in compacted forest soil (c), in moist sand soil (d), in moist clay (e), in leaves litter (f); used crayfish migration channels connecting puddles in wet the ditch (g, h).

The vast majority of crayfish collected in the dry ditch was extracted from burrows. Here, burrows were present in higher numbers compared to the wet ditch. These were often inhabited by a single individual (33.3%); however, in few instances, burrows hosted up to 17 crayfish (). Only a few individuals were observed outside the burrows, some of which were dead. Increased activity (crayfish moving on the ground), in this case, was observed 3 hours after sunset.
Table II. The number of crayfish individuals inhabiting burrows in wet and dry ditches.
In both lakes, all crayfish were caught individually in open water. No specimens were observed in the water depth exceeding 1.5 m. Increased crayfish activity was observed three hours before and after sunset and ceased immediately after A. nebulosus juveniles came closer to the shore. In all examined areas, no evidence of predation on crayfish by native or non-native predators was observed. In contrast to the lakes, no amphibians or mollusks were observed in the ditches. Also, no sign of crayfish presence (e.g. burrows) was observed in water or on land, in lakes. All detected dead crayfish presumably died of natural causes, as the bodies were found mostly intact.
The majority of collected P. virginalis () specimens had a total length of 80–84 mm (25.9%). However, individuals collected in the lakes were significantly bigger (87.2 mm) than those collected from the ditches (81.9 mm and 78.0 mm). The average carapax length of crayfish collected from the lake and dry ditch (Tab. S1) was similar (40.2–41.4 mm), while the significantly smaller individuals (37.7 mm), were collected from the wet ditch. No individuals below 30 mm were found. The total length of a single caught F. limosus was 112 mm, while its carapax length was 55 mm.
Table III. Size classes and number of collected Procambarus virginalis individuals (n), their proportion (%), average total lenght (AVG) in mm ± SD.
Most of the specimens (97.2%) had all ten pairs of pereiopods. Their claws were usually intact, the number of individuals missing one and both claws were 55 (7.7%) and 7 (0.9%), respectively. In contrast to crayfish collected in ditches, those from lakes did not have missing pereiopods (). The specimen of F. limosus had all pereiopods. Only 13.4% of the P. virginalis specimens recently underwent molting and were found primarily in wet ditches (99.0%). Two females (0.3%) in the wet ditch and two (2.2%) in the dry ditch incubated eggs. In contrast to the wet ditch, those in the dry ditch incubated a far lower amount of eggs. No females with attached crayfish juveniles were found.
Table IV. The presence of pereiopods, moulting and egg-carrying individuals.
Discussion
We hereby provide the first documented data on the occurrence of viable P. virginalis population in a nature-protected area in Poland and the steps that were taken to detect and prevent further introduction of the species in the country. The new site is located approximately 150 km from the nearest stable population of P. virginalis of Morskie Oko park in Warsaw. The new localization in Ostrów Lubelski represents the most north-eastward distribution area of the species in European waters with a natural water temperature regime. Despite originating from warmer climates, experimental studies indicate that P. virginalis is cold tolerant (Veselý et al. Citation2015; Haubrock et al. Citation2019) thus a future north-eastward expansion can be expected. To date, the closest wild populations in temperate waters outside Polish borders are those found in the Czech Republic, Germany, Slovakia, and Ukraine (Maiakovska et al. Citation2021). The only confirmed established population of P. virginalis located further north-east is known from heated waters of the Narva power plant in Estonia (approx. 900 km; Ercoli et al. Citation2019).
The high density and size variation of P. virginalis individuals collected is a clear indication that the species is well established both in wet and dry habitats. As P. virginalis was found primarily in ditches, it is plausible that the crayfish initially introduced to Miejskie Lake used the ditches to invade the neighboring Lake Kleszczów. However, interference of anglers or the local community focused on expanding the range of the species cannot be excluded. Its migration to other water bodies and ditches could have also arisen from a need to find more favorable places and escape predation from A. nebulosus. The exclusive presence of ovigerous females in ditches may also be an indication of predator avoidance. Several crayfish species are known to undergo local adaptations in their behavior and resource use in response to predator pressure (Reisinger et al. Citation2020; Wood & Moore Citation2020); hence, a trade-off between resource availability and predation risk can be expected. Unfortunately, according to local knowledge, these ditches are also the preferred habitat for amphibians, including the threatened B. bombina.
The introduction of P. virginalis in Pojezierze Łęczyńskie Landscape Park might have significantly impacted both native crayfish and amphibian communities. Procambarus virginalis is a known carrier of the crayfish plague pathogen A. astaci which is one of the main driver of declines and local extinctions of native European crayfish populations (Keller et al. Citation2014; Mrugała et al. Citation2015) and likely can also transmit the chytrid fungus B. dendrobatidis which is pathogenic for amphibians (Oficialdegui et al. Citation2019). The two native crayfish species A. astacus and P. leptodactylus, which are threatened by the crayfish plague, were not recorded in the study area. Likewise, amphibians were only found in ditches where the crayfish was absent. Although pathogens may have been partly responsible for the local disappearance of native fauna, other factors such as predation and competition could have potentially played an important role (Kats & Ferrer Citation2003; Cruz et al. Citation2006; Oficialdegui et al. Citation2019)
Procambarus virginalis, like P. clarkii, is a polytrophic consumer, which can efficiently predate a wide variety of invertebrates and vertebrates, including amphibians (Veselý et al. Citation2021). Following the introduction of P. virginalis in the Pojezierze Łęczyńskie Landscape Park, the local community confirmed a remarkable shift in frog croaking activity, with the forest becoming increasingly quiet. Accordingly, in areas invaded by P. clarkii, exclusion or even local extinction of amphibians have been observed (Cruz et al. Citation2006, Citation2008; Ficetola et al. Citation2011). Unfortunately, no previous amphibian survey has been conducted in the same area. Therefore, we cannot quantify the decline caused by the marbled crayfish. Furthermore, predation pressure by high crayfish density might explain the absence of mollusks in the ditches.
The absence of native species and the presence of only one individual of F. limosus might be the result of P. virginalis dominance in antagonistic interactions: faster growth, earlier maturation, more frequent reproduction and higher fecundity (Kouba et al. Citation2021). Native crayfish are commonly outcompeted by both invasive species. However, P. virginalis is able to outcompete other invasive crayfish including F. limosus (Linzmaier et al. Citation2018). Compared to F. limosus, P. virginalis is a more recent invader in European waters, thus it is more likely that native species might have suffered from two subsequent invasions, the first perpetrated by F. limosus and the second by P. virginalis, in concomitance with anthropogenic disturbance. However, due to the lack of prior information, we cannot assert with certainty the correct sequence of events.
Besides negatively impacting the native fauna of P. virginalis might cause further damages to the environment with its burrowing activity. The extent of burrowing observed in this study suggests that this crayfish can hamper water retention and stability of banks, and alter sediment dynamics. Such damages might result in severe economic costs as seen for P. clarkii (Souty-Grosset et al. Citation2016; Kouba et al. Citation2022). Moreover, the ability to burrow may reduce exposure to predators and provide shelter during periods of droughts as well as winters enhancing overall invasion success (Kouba et al. Citation2016).
If no action is taken, a further spread of the species is to be expected, as the population in Pojezierze Łęczyńskie Landscape Park is already out of control. In such a scenario, it is plausible that P. virginalis will share the invasion corridors used by F. limosus and colonize most of the Polish waterbodies. Therefore, organized actions, including early detection and eradication as well as education of local communities, are necessary as we cannot truly stop the spread of the species.
We hereby present the steps taken to prevent the further spread of P. virginalis as an example that can be implemented elsewhere to contrast current and future introductions of non-native crayfish species. Firstly, we removed all the crayfish we could get ahold of in the ditches while conducting our study. After our finding, we immediately redacted reports of the species for all regional and national nature conservation institutions, city governments, and angler associations. We provided identification keys for the species that were subsequently distributed via local media following an official statement of the Polesie National Park. Soon after, information about the invasion of P. virginalis was also broadcasted throughout the country. Workshops with live specimens presentations were organized for all the entities involved in nature conservation and for local citizens. Education on native and invasive crayfish species was introduced in local schools. Identification keys for all six species known from Polish waters have been provided in educational nature boards located at the lakes. Thanks to these actions, P. virginalis became one of the most recognizable invasive alien species in the country. Tourists notified another breeding population of P. virginalis on August 22, 2020, in Białka Lake (51.5337, 23.0157), ca. 8 km west from Kleszczów Lake and ca. 7 km from the borders of Polesie National Park. Information on the occurrence of P. virginalis in Pojezierze Łęczyńskie Landscape Park and Białka Lake was published in EASIN Notsys database and becoming the first record of the species from Poland. Moreover, our actions played a significant role in banning P. virginalis, P. clarkii, and other crayfish listed as invasive species in the European Union from private and commercial online trade since December 2020, where they were still present. Banning the trade of invasive species, coupled with increased awareness of the threats posed by non-native crayfish species, is a fundamental step to prevent further introductions.
To sum up, P. virginalis is well adapted to withstand climatic conditions of temperate areas and is likely to expand its distribution area further. Its presence in the Pojezierze Łęczyńskie Landscape Park may have impacted native and invasive species both in aquatic and terrestrial environments. Sensibilization of public opinion and local knowledge is essential for the early detection and eradication of invasive species.
EASIN Detection: After acceptance of this paper, it will be attached to detection details of “Procambarus fallax f. virginalis - Poland #1” in EASIN database:
https://easin.jrc.ec.europa.eu/notsys/PUB/Detections/Details/5a6035f9-e131-4343-b3b6-632bc4e4b480.
Supplemental Material
Download JPEG Image (140.3 KB)Supplemental Material
Download MS Word (14.1 KB)Acknowledgements
We would like to thank Rafał Antoniszewski, Nadia Chlebicka, Bartłomiej Gorzkowski, Damian Gorzkowski, Beata Grzegrzółka, Joanna Hatylak, Karol Hatylak, Katarzyna Karpińska, Dominik Olak, Piotr Maciaszek, Dorota Marcinek, Christian Schabinger and Wojciech Solarz for their valuable help in collecting crayfish and identification of new sites.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2022.2095046
Additional information
Funding
References
- Aguilar-Alberola JA, Mesquita-Joanes F, López S, Mestre A, Casanova JC, Rueda J, Ribas A. 2012. An invaded invader: High prevalence of entocytherid ostracods on the red swamp crayfish Procambarus clarkii (Girard, 1852) in the Eastern Iberian Peninsula. Hydrobiologia 688:63–73. DOI: 10.1007/s10750-011-0660-1.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215:403–410. DOI: 10.1016/S0022-2836(05)80360-2.
- Andriantsoa R, Tönges S, Panteleit J, Theissinger K, Carneiro VC, Rasamy J, Lyko F. 2019. Ecological plasticity and commercial impact of invasive marbled crayfish populations in Madagascar. BMC Ecology 19:8. DOI: 10.1186/s12898-019-0224-1.
- Astrin JJ, Stüben PE. 2008. Phylogeny in cryptic weevils: Molecules, morphology and new genera of western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebrate Systematics 22:503–522. DOI: 10.1071/IS07057.
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. 2005. GenBank. Nucleic Acids Research 33:D34–D38. DOI: 10.1093/nar/gki063.
- Bobeldyk AM, Lamberti GA. 2008. A decade after invasion: Evaluating the continuing effects of rusty crayfish on a Michigan river. Journal of Great Lakes Research 34:265–275. DOI: 10.3394/0380-1330(2008)34[265:ADAIET]2.0.CO;2.
- Casquet J, Thebaud C, Gillespie RG. 2012. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol‐stored spiders. Molecular Ecology Resources 12:136–141. DOI: 10.1111/j.1755-0998.2011.03073.x.
- Catford JA, Vesk PA, Richardson DM, Pyšek P. 2012. Quantifying levels of biological invasion: Towards the objective classification of invaded and invasible ecosystems. Global Change Biology 18:44–62. DOI: 10.1111/j.1365-2486.2011.02549.x.
- Collas M. 2019. Premier signalement de l’écrevisse à taches rouges Faxonius rusticus, Girard 1852 en Europe, France (department de L’aveyron). Available: http://especes-exotiques-envahissantes.fr. Accessed March 2020 16.
- Correia AM, Anastácio PM. 2008. Shifts in aquatic macroinvertebrate biodiversity associated with the presence and size of an alien crayfish. Ecological Research 23:729–734. DOI: 10.1007/s11284-007-0433-5.
- Cruz MJ, Rebelo R, Crespo EG. 2006. Effects of an introduced crayfish, Procambarus clarkii, on the distribution of south-western Iberian amphibians in their breeding habitats. Ecography 29:329–338. DOI: 10.1111/j.2006.0906-7590.04333.x.
- Cruz MJ, Segurado P, Sousa M, Rebelo R. 2008. Collapse of the amphibian community of the Paul do Boquilobo natural reserve (central Portugal) after the arrival of the exotic American crayfish Procambarus clarkii. Herpetological Journal 18:197–204.
- De Cahsan B, Westbury MV, Drews H, Tiedemann R. 2019. The complete mitochondrial genome of a European fire-bellied toad (Bombina bombina) from Germany. Mitochondrial DNA Part B 4:498–500. DOI: 10.1080/23802359.2018.1547143.
- Du Preez L, Smit N. 2013. Double blow: Alien crayfish infected with invasive temnocephalan in South African waters. South African Journal of Science 109:01–04. DOI: 10.1590/sajs.2013/20130109.
- Early R, Bradley BA, Dukes JS, Lawler JJ, Olden JD, Blumenthal DM, Gonzalez P, Grosholz ED, Ibañez I, Miller LP, Sorte CJB, Tatem AJ. 2016. Global threats from invasive alien species in the twenty-first century and national response capacities. Nature Communications 7:12485. DOI: 10.1038/ncomms12485.
- Ercoli F, Kaldre K, Paaver T, Gross R. 2019. First record of an established marbled crayfish Procambarus virginalis (Lyko, 2017) population in Estonia. BioInvasion Reords 8:675–683. DOI: 10.3391/bir.2019.8.3.25.
- Faulkes Z. 2015. Marmorkrebs (Procambarus fallax f. virginalis) are the most popular crayfish in the North American pet trade. Knowledge and Management of Aquatic Ecosystems 416:20. DOI: 10.1051/kmae/2015016.
- Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39:783–791. DOI: 10.1111/j.1558-5646.1985.tb00420.x.
- Ficetola GF, Siesa ME, Manenti R, Bottoni L, Bernardi FD, Padoa‐Schioppa E. 2011. Early assessment of the impact of alien species: Differential consequences of an invasive crayfish on adult and larval amphibians. Diversity and Distributions 17:1141–1151. DOI: 10.1111/j.1472-4642.2011.00797.x.
- Gallardo B, Clavero M, Sánchez MI, Vilà M. 2016. Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology 22:151–163. DOI: 10.1111/gcb.13004.
- Gherardi F, Renai B, Corti C. 2001. Crayfish predation on tadpoles: A comparison between a native (Austropotamobius pallipes) and an alien species (Procambarus clarkii). Bulletin Français de la Pêche et de la Pisciculture 361:659–668. DOI: 10.1051/kmae:2001011.
- Gutekunst J, Maiakovska O, Hanna K, Provataris P, Horn H, Wolf S, Skelton CE, Dorn NJ, Lyko F. 2021. Phylogeographic reconstruction of the marbled crayfish origin. Communications Biology 4:1–6. DOI: 10.1038/s42003-021-02609-w.
- Hasegawa M, Kishino H, Yano T. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution 22:160–174. DOI: 10.1007/BF02101694.
- Haubrock PJ, Kubec J, Veselý L, Buřič M, Tricarico E, Kouba A. 2019. Water temperature as a hindrance, but not limiting factor for the survival of warm water invasive crayfish introduced in cold periods. Journal of Great Lakes Research 45:788–794. DOI: 10.1016/j.jglr.2019.05.006.
- Haubrock PJ, Oficialdegui FJ, Zeng Y, Patoka J, Yeo DC, Kouba A. 2021. The redclaw crayfish: A prominent aquaculture species with invasive potential in tropical and subtropical biodiversity hotspots. Reviews in Aquaculture 13:1488–1530. DOI: 10.1111/raq.12531.
- Hossain MS, Patoka J, Kouba A, Buřič M. 2018. Clonal crayfish as biological model: A review on marbled crayfish. Biologia 73:841–855. DOI: 10.2478/s11756-018-0098-2.
- Hossain MS, Guo W, Martens A, Adámek Z, Kouba A, Buřič M. 2020. Potential of marbled crayfish Procambarus virginalis to supplant invasive Faxonius immunis. Aquatic Ecology 54:45–56. DOI: 10.1007/s10452-019-09725-0.
- Hou Z, Fu J, Li S. 2007. A molecular phylogeny of the genus Gammarus (Crustacea: Amphipoda) based on mitochondrial and nuclear gene sequences. Molecular Phylogenetics and Evolution 45:596–611. DOI: 10.1016/j.ympev.2007.06.006.
- Hudina S, Galić N, Roessink I, Hock K. 2011. Competitive interactions between co-occurring invaders: Identifying asymmetries between two invasive crayfish species. Biological Invasions 13:1791–1803. DOI: 10.1007/s10530-010-9933-2.
- Jackson MC, Ruiz-Navarro A, Britton JR. 2015. Population density modifies the ecological impacts of invasive species. Oikos 124:880–887. DOI: 10.1111/oik.01661.
- Johnson MF, Rice SP, Reid I. 2011. Increase in coarse sediment transport associated with disturbance of gravel river beds by signal crayfish (Pacifastacus leniusculus). Earth Surface Processes and Landforms 36:1680–1692. DOI: 10.1002/esp.2192.
- Kats LB, Ferrer RP. 2003. Alien predators and amphibian declines: Review of two decades of science and the transition to conservation. Diversity and Distributions 9:99–110. DOI: 10.1046/j.1472-4642.2003.00013.x.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. DOI: 10.1093/bioinformatics/bts199.
- Keller NS, Pfeiffer M, Roessink I, Schulz R, Schrimpf A. 2014. First evidence of crayfish plague agent in populations of the marbled crayfish (Procambarus fallax forma virginalis). Knowledge and Management of Aquatic Ecosystems 15.
- Kotovska G, Khrystenko D, Patoka J, Kouba A. 2016. East European crayfish stocks at risk: Arrival of non-indigenous crayfish species. Knowledge and Management of Aquatic Ecosystems 37.
- Kouba A, Petrusek A, Kozák P. 2014. Continental-wide distribution of crayfish species in Europe: Update and maps. Knowledge and Management of Aquatic Ecosystems 413:5. DOI: 10.1051/kmae/2014007.
- Kouba A, Tíkal J, Císař P, Veselý L, Fořt M, Příborský J, Patoka J, Buřič M. 2016. The significance of droughts for hyporheic dwellers: Evidence from freshwater crayfish. Scientific Reports 6:1–7. DOI: 10.1038/srep26569.
- Kouba A, Lipták B, Kubec J, Bláha M, Veselý L, Haubrock PJ, Oficialdegui FJ, Niksirat H, Patoka J, Buřič M. 2021. Survival, growth, and reproduction: Comparison of marbled crayfish with four prominent crayfish invaders. Biology 10:422. DOI: 10.3390/biology10050422.
- Kouba A, Oficialdegui FJ, Cuthbert RN, Kourantidou M, South J, Tricarico E, Gozlan RE, Courchamp F, Haubrock PJ. 2022. Identifying economic costs and knowledge gaps of invasive aquatic crustaceans. Science of the Total Environment 813:152325. DOI: 10.1016/j.scitotenv.2021.152325.
- Kozák P, Ďuriš Z, Petrusek A, Buřič M, Horká I, Kouba A, Kozubíková-Balcarová E, Policar T, and Němečková K. 2015. Crayfish biology and culture, University of South Bohemia in České Budějovice. České Budějovice: Faculty of Fisheries and Protection of Waters.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35:1547–1549. DOI: 10.1093/molbev/msy096.
- Librado P, Rozas J. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. DOI: 10.1093/bioinformatics/btp187.
- Linzmaier SM, Goebel LS, Ruland F, Jeschke JM. 2018. Behavioral differences in an over-invasion scenario: Marbled vs. spiny-cheek crayfish. Ecosphere 9:e02385. DOI: 10.1002/ecs2.2385.
- Lipták B. 2019. Trophic role of marbled crayfish in a lentic freshwater ecosystem. AI 14:299–309. DOI: 10.3391/ai.2019.14.2.09.
- Lodge DM, Deines A, Gherardi F, Yeo DCJ, Arcella T, Baldridge AK, Barnes MA, Chadderton WL, Feder JL, Gantz CA, Howard GW, Jerde CL, Peters BW, Peters JA, Sargent LW, Turner CR, Wittmann ME, Zeng Y. 2012. Global introductions of crayfishes: Evaluating the impact of species invasions on ecosystem services. Annual Review of Ecology, Evolution, and Systematics 43:449–472. DOI: 10.1146/annurev-ecolsys-111511-103919.
- MacDougall AS, Turkington R. 2005. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86:42–55. DOI: 10.1890/04-0669.
- Maciaszek R, Bonk M, Strużyński W. 2019. New records of the invasive red swamp crayfish Procambarus clarkii (Girard, 1852) (Decapoda: Cambaridae) from Poland. Knowledge and Management of Aquatic Ecosystems 420:39. DOI: 10.1051/kmae/2019033.
- Maciaszek R. 2022. Łowca Obcych – polish database of aquatic alien species. Available: https://lowcaobcych.pl. Accessed Apr 2022 01.
- Maiakovska O, Andriantsoa R, Tönges S, Legrand C, Gutekunst J, Hanna K, Pârvulescu L, Novitsky R, Weiperth A, Sciberras A, Deidun A, Ercoli F, Kouba A, Lyko F. 2021. Genome analysis of the monoclonal marbled crayfish reveals genetic separation over a short evolutionary timescale. Communications Biology 4:74. DOI: 10.1038/s42003-020-01588-8.
- Martin P, Dorn NJ, Kawai T, van der Heiden C, and Scholtz G. 2010. The enigmatic Marmorkrebs (marbled crayfish) is the parthenogenetic form of Procambarus fallax (Hagen, 1870). In: Arntzen JW, editor. CTOZ. Vol. 79. Leiden: Brill. pp. 107–118.
- Matsuzaki SS, Usio N, Takamura N, Washitani I. 2009. Contrasting impacts of invasive engineers on freshwater ecosystems: An experiment and meta-analysis. Oecologia 158:673–686. DOI: 10.1007/s00442-008-1180-1.
- Mazurska K, Baranowski A, Ceryngier P, Cygańska A, Fuszara M, Hayatli F, Jarska A, Kaźmierska A, Koperski P, Ptaszyński M, Romanowski J, Rozwałka R, Sikorski P, Skłodowski M, Strużyński W, Zięba G. 2019. Raport z inwentaryzacji bioblitz przeprowadzonej na terenie parku Morskie Oko w Warszawie. Zarząd Zieleni m. st. Warszawy. http://www.zzw.waw.pl. Accessed April 2022 01.
- Mrugała A, Kozubíková-Balcarová E, Chucholl C, Cabanillas Resino S, Viljamaa-Dirks S, Vukić J, Petrusek A. 2015. Trade of ornamental crayfish in Europe as a possible introduction pathway for important crustacean diseases: Crayfish plague and white spot syndrome. Biological Invasions 17:1313–1326. DOI: 10.1007/s10530-014-0795-x.
- Oficialdegui FJ, Sánchez MI, Monsalve-Carcaño C, Boyero L, Bosch J. 2019. The invasive red swamp crayfish (Procambarus clarkii) increases infection of the amphibian chytrid fungus (Batrachochytrium dendrobatidis). Biological Invasions 21:3221–3231. DOI: 10.1007/s10530-019-02041-6.
- Ohtaka A, Gelder SR, Kawai T, Saito K, Nakata K, Nishino M. 2005. New records and distributions of two North American branchiobdellidan species (Annelida: Clitellata) from introduced signal crayfish, Pacifastacus leniusculus, in Japan. Biological Invasions 7:149–156. DOI: 10.1007/s10530-004-8146-y.
- Patoka J, Kalous L, Kopecký O. 2014. Risk assessment of the crayfish pet trade based on data from the Czech Republic. Biological Invasions 16:2489–2494. DOI: 10.1007/s10530-014-0682-5.
- Patoka J, Bláha M, Kalous L, Kouba A. 2017. Irresponsible vendors: Non‐native, invasive and threatened animals offered for garden pond stocking. Aquatic Conservation: Marine and Freshwater Ecosystems 27:692–697. DOI: 10.1002/aqc.2719.
- Perry WL, Lodge DM, Lamberti GA. 2000. Crayfish (Orconectes rusticus) impacts on zebra mussel (Dreissena polymorpha) recruitment, other macroinvertebrates and algal biomass in a lake-outlet stream. The American Midland Naturalist 144:308–316. DOI: 10.1674/0003-0031(2000)144[0308:CORIOZ]2.0.CO;2.
- Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, Kleunen M, Vilà M, Wingfield MJ, Richardson DM. 2020. Scientists’ warning on invasive alien species. Biological Reviews 95:1511–1534. DOI: 10.1111/brv.12627.
- Ratnasingham S, Hebert PDN. 2007. bold: The barcode of life data system (barcodinglife.org). Molecular Ecology Notes 7:355–364. DOI: 10.1111/j.1471-8286.2007.01678.x.
- Reisinger LS, Glon MG, Pintor LM. 2020. Divergence in foraging and predator avoidance behavior across the geographic range of native and non-native crayfish. Hydrobiologia 847:803–818. DOI: 10.1007/s10750-019-04139-3.
- Savini D, Occhipinti-Ambrogi A, Marchini A, Tricarico E, Gherardi F, Olenin S, Gollasch S. 2010. The top 27 animal alien species introduced into Europe for aquaculture and related activities: The top 27 animal alien species introduced into Europe. Journal of Applied Ichthyology 26:1–7. DOI: 10.1111/j.1439-0426.2010.01503.x.
- Scholtz G, Braband A, Tolley L, Reimann A, Mittmann B, Lukhaup C, Steuerwald F, Vogt G. 2003. Parthenogenesis in an outsider crayfish. Nature 421: 806–806. DOI: 10.1038/421806a.
- Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S, Pyšek P, Winter M, Arianoutsou M, Bacher S, Blasius B, Brundu G, Capinha C, Celesti-Grapow L, Dawson W, Dullinger S, Fuentes N, Jäger H, Kartesz J, Kenis M, Kreft H, Kühn I, Lenzner B, Liebhold A, Mosena A, Moser D, Nishino M, Pearman D, Pergl J, Rabitsch W, Rojas-Sandoval J, Roques A, Rorke S, Rossinelli S, Roy HE, Scalera R, Schindler S, Štajerová K, Tokarska-Guzik B, van Kleunen M, Walker K, Weigelt P, Yamanaka T, Essl F. 2017. No saturation in the accumulation of alien species worldwide. Nature Communications 8:14435. DOI: 10.1038/ncomms14435.
- Śmietana P, Bonk M, Solarz W. 2018. Karta informacyjna gatunku – Rak marmurkowy. Generalna Dyrekcja Ochrony Środowiska. www.projekty.gdos.gov.pl/igo-procambarus-fallax. Accessed Apr 2022 01.
- Souty-Grosset C, Anastacio PM, Aquiloni L, Banha F, Choquer J, Chucholl C, Tricarico E. 2016. The red swamp crayfish Procambarus clarkii in Europe: Impacts on aquatic ecosystems and human well-being. Limnologica 58:78–93. DOI: 10.1016/j.limno.2016.03.003.
- Svoboda J, Mrugała A, Kozubíková-Balcarová E, Petrusek A. 2017. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: A review. Journal of Fish Diseases 40:127–140. DOI: 10.1111/jfd.12472.
- Twardochleb LA, Olden JD, Larson ER. 2013. A global meta-analysis of the ecological impacts of non-native crayfish. Freshwater Science 32:1367–1382. DOI: 10.1899/12-203.1.
- Ungureanu E, Mojžišová M, Tangerman M, Ion MC, Parvulescu L, Petrusek A. 2020. The spatial distribution of Aphanomyces astaci genotypes across Europe: Introducing the first data from Ukraine. Freshwater Crayfish 25:77–87. DOI: 10.5869/fc.2020.v25-1.077.
- Usio N, Azuma N, Sasaki S, Oka T, Inoue M. 2017. New record of Marmorkrebs from western Japan and its potential threats to freshwater ecosystems. Cancer 26:5–11.
- Veselý L, Buřič M, Kouba A. 2015. Hardy exotics species in temperate zone: Can “warm water” crayfish invaders establish regardless of low temperatures? Scientific Reports 5:1–7. DOI: 10.1038/srep16340.
- Veselý L, Ruokonen TJ, Weiperth A, Kubec J, Szajbert B, Guo W, Ercoli F, Bláha M, Buřič M, Hämäläinen H, Kouba A. 2021. Trophic niches of three sympatric invasive crayfish of EU concern. Hydrobiologia 848:727–737. DOI: 10.1007/s10750-020-04479-5.
- Vodovsky N, Patoka J, Kouba A. 2017. Ecosystem of Caspian Sea threatened by pet-traded non-indigenous crayfish. Biological Invasions 19:2207–2217. DOI: 10.1007/s10530-017-1433-1.
- Walther G-R, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H. 2009. Alien species in a warmer world: Risks and opportunities. Trends in Ecology and Evolution 24:686–693. DOI: 10.1016/j.tree.2009.06.008.
- Weiperth A, Gál B, Kuříková P, Bláha M, Kouba A, Patoka J. 2017. Cambarellus patzcuarensis in Hungary: The first dwarf crayfish established outside of North America. Biologia 72:1529–1532. DOI: 10.1515/biolog-2017-0159.
- Wood TC, Moore PA. 2020. Fine-tuned responses to chemical landscapes: Crayfish use predator odors to assess threats based on relative size ratios. Ecosphere 11:e03188. DOI: 10.1002/ecs2.3188.
