Abstract
Nearly ubiquitous and usually speciose in most aquatic habitats, the meiofaunal-sized gastrotrichs are recognized as an important component of marine and freshwater ecosystems. The common observations that gastrotrichs feed on bacteria, microalgae and biodetritus strongly imply that they play a relevant role in linking the microbial loop to the higher trophic levels. Which are the organisms that in turn prey on gastrotrichs is, however, a substantially unexplored question. Inspecting meiofauna samples collected from shallow sites of the Tyrrhenian coast, we had the chance to spot a wild case of a macrodasyidan gastrotrich predated by a dileptid ciliate. This case is documented here with a set of in-vivo photos, jointly with an unequivocal taxonomic identification of the preyed gastrotrich with Paraturbanella teissieri and a tentative identification of the predator ciliate with Pseudomonilicaryon marinus.
Introduction
Gastrotrichs, commonly referred to as hairybellies or hairybacks, form a phylum of microscopic (0.05_3.0 mm in length), benthic invertebrates living in every aquatic ecosystem. Over 860 accepted species are traditionally subdivided between Macrodasyida, distributed in marine and brackish waters, and Chaetonotida, colonizers of marine and freshwater waters (Todaro et al. Citation2019a, Citation2022; Kieneke & Todaro Citation2021). Although pervasively distributed (Balsamo et al. Citation2014; Kieneke & Schmidt-Rhaesa Citation2015), it is probably in the marine sandy environment that gastrotrich communities thrive more abundantly and diversified (Leasi et al. Citation2018; Curini-Galletti et al. Citation2020). In the interstitial habitats, gastrotrichs may reach densities up to 105 individuals/m2 and, in general, represent the meiofaunal taxon which is third in abundance only to nematodes and harpacticoid copepods (Hummon Citation1976). However, cases in which they rank first in numerical dominance appear to be all but rare (Coull et al. Citation1985; Hochberg Citation1999). An instructive example of the high species diversity that is intrinsic to marine gastrotrich communities is provided by the compendium of the Italian marine gastrotrichs (Todaro et al. Citation2001). Over a total of 256 locations sampled, it was calculated a mean of 8.5 species/location with peaks of 29 synchronous (littoral + sublittoral) and 25 syntopic (sublittoral) species counted in sites of the beach of Ischia Porto.
Considering the large interest that gastrotrichs are increasingly rising in reason of their abundance, morphological diversity, reproductive biology and phylogeny (Todaro & Rocha Citation2004; Todaro et al. Citation2012, Citation2019b; Hochberg et al. Citation2014; Martinéz et al., Citation2019, Citation2020; Araujo & Hochberg Citation2021), it stands odd how little we known about their ecological role in marine ecosystems in particular. Essentially observing their gut lumen, usually filled with biodetritus, bacteria and microalgae, gastrotrichs have been proposed to constitute a link between the microbial loop and the higher trophic levels (Balsamo & Todaro Citation2002; Kieneke & Schmidt-Rhaesa Citation2015; Balsamo et al. Citation2020). This “link” hypothesis is here supported by a rare in vivo observation, related to a survey of meiofauna from the Tyrrhenian coast, that the consumer macrodasyidan gastrotrichs may in turn be a substantial food source for their ecological counterparts such as the predatory dileptid ciliates.
Material and methods
The material of this study was isolated from one of several samples of sandy-bottom surface collected, on June 3, 2009, from the sublittoral zone of Castiglione della Pescaia, Tuscany, Italy (42°45’ N; 10°51’ E). The samples were drawn by hand at a depth of about 0.5 m using 500-ml jars, which were placed in a thermostatic container and brought to the laboratory at the University of Modena and Reggio Emilia to be inspected on the next day for their gastrotrich community. Extraction was carried out by means of the narcotization/decantation technique using a 7% magnesium chloride solution as a narcotic (e.g., Todaro et al. Citation1992). The fauna-containing supernatant was successively poured directly into 5-cm in diameter Petri dishes and scanned under a Wild M8 dissecting microscope for gastrotrichs. Single specimens were thereafter picked out with a hand-held micropipette, mounted on glass slides, and observed in vivo with Nomarski differential interference contrast optics using a Nikon Eclise 90i microscope. Photomicrograph recording was conducted with a DS-Fi1 Nikon digital camera, and measurements were carried out with the Nikon NIS-F v 4.0 software.
Results and discussion
Previous surveys of gastrotrichs from the sublittoral zone of Castiglione della Pescaia have resulted in the identification of a total of 17 (12 macrodasyidan and 5 chaetonotid) species (Todaro et al. Citation2001). Three of them, namely Dactylopodola mesotyphle Hummon et al., Citation1998, Tetranchyroderma papii Gerlach, Citation1953, and Paraturbanella teissieri Swedmark, Citation1954, were found to be largely represented in the samples of this study, collected on June 3, 2009. Among all the specimens preliminarily recognized as P. teissieri, one was spotted to be not fully conformed with the behaviour and standard morphology of the taxon. A trained eye could not fail to notice a reduced motility and, in particular, a rather eccentric shape of the terminal body region. It looked tapered and roundish, instead of bilobed with each lobe provided medially of adhesive tubes as is the case in all species of Paraturbanella (Luporini et al. Citation1971; Dal Zotto et al. Citation2018; Todaro et al. Citation2019c).
To inspect more closely whether this eccentric behaviour and shape reflected some wounding, a case of intraspecific polymorphism, or even a new taxon (see the recent case of Chimaeradasys species; Kieneke & Todaro Citation2021), the odd specimen was mounted on a glass slide to be observed and photographed at increasing magnifications.
As shown in the set of pictures in , the odd specimen soon appeared to be a P. teissieri individual trapped for most of the body extension inside a sort of pouch-like structure delimited by a thin, pliable and “hairy” surface. Only the anteriormost body region down to the protruding accessory adhesive organs appeared to be still free, most likely because these lateral organs represented a mechanical, “spiny” obstacle to a whole encapsulation.
Figure 1. Differential interference contrast photomicrographs documenting the predation of Paraturbanella teissieri (Gastrotricha) by the dileptid Pseudomonilicaryon marinum (Ciliophora). (A, B) P. teissieri engulfed by P. marinum as seen at different focal planes; dotted lines trace the predator cytostome and the bilobed caudum of the engulfed prey; arrows indicate one of the two lateral adhesive organs of the prey; the insert highlights the predator “hairy” surface. (C, D) regurgitation of the prey; the predator cytostome (arrow) and its proboscis (arrowhead) are indicated. (E, F) the definitive separation between prey and predator; (E) P. teissieri appears completely immotile while P. marinum begins to regain its normal shape; (F) P. teissieri showing partially digested caudal lobes (arrow) and an uninjured anterior region as denoted (insert) by still well-shaped anterior adhesive tubes (arrow) and pristine tubes of the accessory adhesive organ (arrowhead). (G, H) the predator freed from the prey showing a fully functional cell body, in which the proboscis tip includes an agglomerate (arrow) of dark granules and the trunk accommodates some contractile and digestive vacuoles (asterisks). See text for further details.
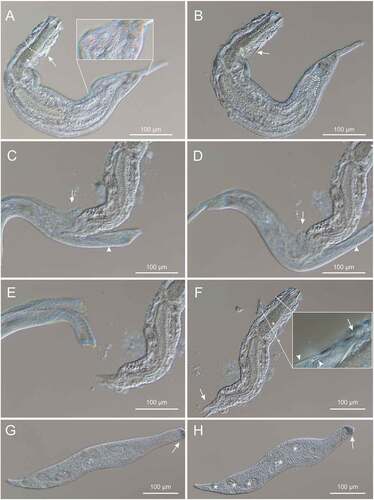
After about 10 minutes of observations, the “hairy pouch” disclosed its nature of predatory organism by suddenly expelling out the no-longer motile, paralysed P. teissieri specimen (body size, 480 × 90 μm), which was observed to suffer a partial degeneration (digestion) of the adhesive tubes on the body rear, and to accommodate a not well-differentiated reproductive apparatus suggestive of a subadult stage.
Once get rid of the prey and re-acquired the proper (not deformed) cell body morphology, the predatory organism (dimensions, 530 × 60 μm) could promptly be identified with a raptorial dileptid ciliate, which was still fully capable of swimming back and forth propelled by the beating of cilia evenly distributed (in “holotrichous” fashion) all over the body surface. A long flexible proboscis of about 130 μm (extended circa one/third the body length) anterior to a large bulged cytostome and a sub-oval trunk tapering with a conic tail represented unequivocal dileptid-specific diagnostic traits.
Yet adequate for a taxonomic recognition with a dileptid ciliate, these traits were clearly insufficient for a solid identification at the species level. This identification would have required to be supported by detailed observations (barred by the practical and accidental availability of a single and largely threatened individual) on, at least, the nuclear apparatus and the patterns of the somatic and oral ciliature.
However, for a tentative identification at the species level, it came of help the exhaustive “Monograph of the Dileptids” by Vďačný and Foissner (Citation2012), which lists and illustrates the 66 species and subspecies that are officially recognized as members of the family Dileptidae. The great majority of these species (representative of six genera) live exclusively in terrestrial/semi-terrestrial and freshwater biotopes. Only six, namely Apotrachelius multinucleatus (Vďačný, Al-Rasheid & Foissner, Citation2012), Dileptus estuarinus (Dragesco Citation1960), Pseudomonilicaryon marinum (Kahl, Citation1933) (possibly distinct between P. marinum marinum and P. marinum minimum), P. massutti (Kahl, Citation1933), Rimaleptus lacazei (Gourret & Roesser, Citation1886) and R. tirjakovae (Vďačný & Foissner, Citation2008), have been reported from brackish waters, the sea and/or saline soil. Compared with these ecologically related species, the dileptid specimen here at issue came out to be more reliably identifiable with P. marinum (Kahl, Citation1933). First, it is equivalent to P. marinum in body dimensions and shape, and bears in common an unusual accumulation of dark granules at the tip of the proboscis. Second, P. marinum is the unique marine Pseudomonilicaryon species dwelling in European coastal sites, having been isolated from sandy sediments of the Kiel Bay in Germany (Kahl Citation1933; Bock Citation1952a, Citation1952b; Telesh et al. Citation2008), the mesopsammon of Roscoff in France (Dragesco Citation1960, Citation1963), the sublittoral of the Biscay Bay in Spain (Fernandez-Leborans & Novillo Citation1993), and the Divichinskiy estuary in Russia (Agamaliev & Aliev Citation1983).
Future studies are required to confirm or disprove our identification. Beside the taxonomical issue, the presence of this type of protists along the Italian coastline assumes also faunistic and biogeographic relevance, especially considering the ample geographic distance from previous records of P. marinus, and also that no marine raptorial dipleptid species phylogenetically close to Pseudomonilicaryon (e.g. Dileptus and Rimaleptus) are listed in the most recent checklist of Italian marine and brackish Protozoa (Banchetti et al. Citation2008).
Conclusions
Gastrotrichs have been reported to be a food source for a variety of other benthic organisms (Glockling Citation1997; Balsamo et al. Citation2014; Kieneke & Schmidt-Rhaesa Citation2015). However, observations accounting for a gastrotrich preyed in nature are mostly unvouchered and anecdotal. The only reliable report of this predation comes from Bovee and Cordell (Citation1971), who finely described, under laboratory conditions, the engulfment and complete digestion of specimens of the freshwater species, Chaetonotus vulgaris Brunson, Citation1950, by the heliozoon Actinophrys sol Ehrenberg, Citation1830, also providing temporal details and nicely drafted drawings.
The case reported here of P. teissieri preyed by a dileptid ciliate provides the first documented evidence of a marine macrodasyidan gastrotrich eaten in nature by another organism, a ciliate protist.
Dileptids are well-known voracious predators on a wide spectrum of micro- and macroscopic invertebrates, including cnidarians (Hydra), turbellarians (Planaria, Stenostomum), rotifers (Brachionus), nematodes (Cephalobus), branchiobdellids (Nais), pond snails (Physa), and naupliar stages of copepods (Brown & Jenkins Citation1962; Fenchel Citation1996; Vďačný et al. Citation2011; Vďačný & Foissner Citation2012). Like other raptorial ciliates, such as Didinium, Coleps, Homalozoon and Litonotus, they harpoon and paralyze the prey by means of an array of cell cortex-anchored, membrane-bound ejectable organelles (Verni & Gualtieri Citation1997). These organelles, collectively designated as extrusomes (Rosati & Modeo Citation2003), are rich in lytic enzymes (in the first place, acid phosphatase) and noxious compounds (mostly derived from various biogenetic precursors of the primary metabolism) that have raised strong interest from an applied perspective for their cytotoxic activity on a variety of cell systems (Buonanno & Ortenzi Citation2018). The amazing capacity shown by P. marinum to paralyze and engulf P. teissieri, a sturdy prey with a large body size, provides further evidence of the remarkably powerful cytotoxic effects of dileptid extrusomes and stimulates research to elucidate the molecular basis of these effects.
Acknowledgements
We would like to dedicate this work to the late friend and colleague, Prof. Paolo Tongiorgi, with whom we shared fascination and research interest in little creatures, and warmly thank Prof. Peter Vďačný (University of Bratislava) for valuable advice on dileptid taxonomy. This study was partially supported by a grant ‘Far attrezzature 2021’ to M.A.T. from the University of Modena e Reggio Emilia, Italy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Agamaliev FG, Aliev AR. 1983. Benthic infusoria from the Divichinskiy estuary of the Caspian Sea. Hydrobiological Journal 18(years 1982/1983):20–24.
- Araujo TQ, Hochberg R. 2021. Marine Gastrotricha of the Azores: Updated checklist from São Miguel island. Açoreana 11(Supl.):57–77.
- Balsamo M, Todaro MA. 2002. Gastrotricha. In: Rundle SD, Robertson AI, Schmid-Araya JM, editors. Freshwater Meiofauna Biology and Ecology. Leiden, Netherlands: Backhuys Publisher. pp. 45–61.
- Balsamo M, Grilli P, Guidi L, d’Hondt J-L. 2014. Gastrotricha - Biology, ecology and systematics. Families Dasydytidae, Dichaeturidae, Neogosseidae, Proichthydiidae. In: H.j.f D, editor. Identification guides to the plankton and benthos of inland waters. Vol. 24. Weikersheim, Germany: Backhuys. pp. 1–187.
- Balsamo M, Artois T, Smith JPS, Todaro MA, Guidi L, Leander BS, Van Steenkiste NWL. 2020. The curious and neglected soft-bodied meiofauna: Rouphozoa (Gastrotricha and Platyhelminthes). Hydrobiologia 847(12):2613–2644. DOI:10.1007/s10750-020-04287-x.
- Banchetti R, Gradoni L, and Dini F. 2008. Protozoa. In: Relini G, La Posta S, editors. La checklist della fauna marina italiana. Vol. 15. Genova: Biologia Marina Mediterranea. pp. 1–41. suppl
- Bock KJ. 1952a. Über einige holo- und spirotriche Ciliaten aus den marinen Sandgebieten der Kieler Bucht. Zoologischer Anzeiger 149:107–115.
- Bock KJ. 1952b. Zur Ökologie der Ciliaten des marinen Sandgrundes der Kieler Bucht I. Kieler Meeresforsch 9:77–89.
- Bovee EC, Cordell DL. 1971. Feeding on gastrotrichs by the heliozoon Actinophrys sol. Transactions of the American Microscopical Society 90(3):365–369. DOI:10.2307/3225197.
- Brown HP, Jenkins MM. 1962. A Protozoon (Dileptus; Ciliata) Predatory upon Metazoa. Science 136(3517):710–711. DOI:10.1126/science.136.3517.710.a.
- Brunson RB. 1950. An introduction to the taxonomy of Gastrotricha with a study of eighteen species from Michigan. Transactions of the American Microscopical Society 69:325–352.
- Buonanno F, Ortenzi C. 2018. Predator-prey interactions in ciliated protists. In: Najjari A, Cherif A, Sghaier H, H.i O, editors. In: Extremophilic microbes and metabolites – Diversity, bioprespectin and biotechnological applications. Londra, United Kingdom: InTech Open Editor. pp. 1–30 152 pp.
- Coull BC. 1985. Long-term variability of estuarine meiobenthos: An 11-year study. Marine Ecology Progress Series 24:205–218. DOI:10.3354/meps024205.
- Curini-Galletti M, Artois T, Di Domenico M, Fontaneto D, Jondelius U, Jorger KM, Leasi F, Martínez A, Norenburg JL, Sterrer W, Todaro MA. 2020. Contribution of soft-bodied meiofaunal taxa to Italian marine biodiversity. European Zoological Journal 87(1):369–384. DOI:10.1080/24750263.2020.1786607.
- Dal Zotto M, Leasi F, Todaro MA. 2018. A new species of Turbanellidae (Gastrotricha, Macrodasyida) from Jamaica, with a key to species of Paraturbanella. Zookeys 734:105–119. DOI:10.3897/zookeys.734.23023.
- Dragesco J. 1960. Ciliés mésopsammiques littoraux. Systématique, morphologie, écologie. Travaux de la Station Biologique de Roscoff 122:1–356.
- Dragesco J. 1963. Révision du genre Dileptus, Dujardin 1871 (Ciliata Holotricha) (systématique, cytologie, biologie). Bulletin Biologique de la France et de la Belgique 97:103–145.
- Ehrenberg, CG. 1830. Organisation, Systematik, und geographisches Verhältnis der Infusionstierchen. Berlin: Druckerei der Königlichen akademie der wissenschaften. pp. 1–108.
- Fenchel T. 1996. The ecology of marine microbenthos IV. Structure and function of the benthic ecosystem, its chemical and physical factors and the microfauna communities with special reference to the ciliated protozoa. Ophelia 6(1):1–182. DOI:10.1080/00785326.1969.10409647.
- Fernández-Leborans G, Novillo A. 1993. Sublittoral protistan communities of the shores of the Sea of Cantabria (Bay of Biscay). Internationale Revue der gesamten Hydrobiologie und Hydrographie 78(2):201–218. DOI:10.1002/iroh.19930780205.
- Gerlach SA. 1953. Gastrotrichen aus dem Kuestengrundwasser des Mittelmeeres. Zoologischer Anzeiger 150:203–211.
- Glockling SL. 1997. Zoophagus cornus: A new species from Japan. Mycological Research 101(10):1179–1182. DOI:10.1017/S095375629700378X.
- Gourret P, and Roeser P. 1886. Protozoaires du vieux-port de Marseille. Archives de Zoologie Expérimentale et Générale 4:443–534.
- Hochberg R. 1999. Spatiotemporal size-class distribution of Turbanella mustela (Gastrotricha: Macrodasyida) on a northern California beach and its effect on tidal suspension. Pacific Science 53:50–60.
- Hochberg R, Atherton S, Kieneke A. 2014. Marine Gastrotricha of Little Cayman Island with the description of one new species and an initial assessment of meiofaunal diversity. Marine Biodiversity 44(1):89–113. DOI:10.1007/s12526-013-0186-z.
- Hummon WD. 1976. Seasonal changes in secondary production, faunal similarity and biological accommodation, related to stability among the Gastrotricha of two semi-enclosed Scottish beaches. In: Persoone G, Jaspers E, editors. Proc. of the 10th European Symposium on Marine Biology. Vol. 2. Wetteren, Belgium: Universa Press Population Dynamics. pp. 309–336.
- Hummon WD, Todaro MA, Tongiorgi P, and Balsamo M. 1998. Italian marine Gastrotricha: V. Four new and one redescribed species of Macrodasyida in the Dactylopodolidae and Thaumastodermatidae. Italian Journal of Zoology 65:109–119. DOI: 10.1080/11250009809386731
- Kahl A. 1933. Ciliata libera et ectocommensalia. In: Grimpe G, & Wagler E editors. Die Tierwelt der Nord- und Ostsee 23, Teil II. c3. Leipzig: Akademische Verlagsgesellschaft. pp. 29–146.
- Kieneke A, Schmidt-Rhaesa A. 2015. Gastrotricha and Gnathifera. In: Schmidt-Rhaesa A, editor. Handbook of Zoology: Gastrotricha, Cycloneuralia and Gnathifera. Vol. 3. Berlin/Boston: Walter de Gruyter GmbH. pp. 1–135.
- Kieneke A, Todaro MA. 2021. Discovery of two ‘chimeric’ Gastrotricha and their systematic placement based on an integrative approach. Zoological Journal of the Linnean Society 192(3):710–735. DOI:10.1093/zoolinnean/zlaa117.
- Leasi F, Sevigny JL, Laflamme EM, Artois T, Curini-Galletti M, Jesus Navarrete A, Di Domenico M, Goetz F, Hall JA, Hochberg R, Jörger KM, Jondelius U, Todaro MA, Wirshing HH, Norenburg JL, Thomas WK. 2018. Biodiversity estimates and ecological interpretations of meiofaunal communities are biased by the taxonomic approach. Communications Biology 1:1–12. DOI:10.1038/s42003-017-0002-6.
- Luporini P, Magagnini G, Tongiorgi P. 1971. Contribution a la connaissance des gastrotriches des côtes de Toscane. Cahiers de Biologie Marine 12:433–455.
- Martínez A, Di Domenico M, Leasi F, Curini-Galletti M, Todaro MA, Dal Zotto M, Gobert S, Artois T, Norenburg J, Jörger KM, Núñez J, Fontaneto D, Worsaae K. 2019. Patterns of diversity and endemism of soft-bodied meiofauna in an oceanic island, Lanzarote, Canary Islands. Marine Biodiversity 49(5):2033–2055. DOI:10.1007/s12526-019-01007-0.
- Martínez A, Eckert EM, Artois T, Careddu G, Casu M, Curini-Galletti M, Gazale V, Gobert S, Ivanenko VN, Jondelius U, Marzano M, Pesole G, Zanello A, Todaro MA, Fontaneto D. 2020. Human access impacts biodiversity of microscopic animals in sandy beaches. Communications Biology 3(1):175. DOI:10.1038/s42003-020-0912-6.
- Rosati G, Modeo L. 2003. Extrusomes in ciliates: Diversification, distribution, and phylogenetic implications. The Journal of Eukaryotic Microbiology 50(6):383–402. DOI:10.1111/j.1550-7408.2003.tb00260.x.
- Swedmark B. 1954. Description de Paraturbanella teissieri, n.sp. (Gastrotriche Macrodasyoide). Bulletin de la Société Zoologique de France 79:46–49.
- Telesh I, Postel L, Heerkloss R, Mironova E, Skarlato S. 2008. Zooplankton of the open Baltic Sea: Atlas. Meereswissenschaftliche Berichte Warnemünde 73:1–251.
- Todaro MA, Balsamo M, Tongiorgi P. 1992. Marine gastrotrichs from the Tuscan Archipelago (Tyrrhenian Sea): I. Macrodasyida, with description of three new species. Bollettino di Zoologia 59(4):471–485. DOI:10.1080/11250009209386709.
- Todaro MA, Hummon WD, Balsamo M, Fregni E, Tongiorgi P. 2001. Inventario dei Gastrotrichi marini italiani: Una checklist annotata. Atti della Società Toscana di Scienze Naturali Memorie, Serie B 107:75–137.
- Todaro MA, Rocha CEF. 2004. Diversity and distribution of marine Gastrotricha along the northern beaches of the state of São Paulo (Brazil), with description of a new species of Macrodasys (Macrodasyida, Macrodasyidae). Journal of Natural History 38(13):1605–1634. DOI:10.1080/0022293031000156169.
- Todaro MA, Guidi L, Ferraguti M, Balsamo M. 2012. A fresh look at Dinodasys mirabilis (Gastrotricha, Macrodasyida), with focus on the reproductive apparatus and sperm ultrastructure. Zoomorphology 131(2):115–125. DOI:10.1007/s00435-012-0147-2.
- Todaro MA, Dal Zotto M, Cesaretti A. 2019a. Marine gastrotrichs from Lanzarote, with a description of a phylogenetically relevant species of Urodasys (Gastrotricha, Macrodasyida). Marine Biodiversity 49(5):2109–2123. DOI:10.1007/s12526-017-0747-7.
- Todaro MA, Dal Zotto M, Kånneby T, Hochberg R. 2019b. Integrated data analysis allows the establishment of a new, cosmopolitan genus of marine Macrodasyida (Gastrotricha). Scientific Reports 9(1):7989. DOI:10.1038/s41598-019-43977-y.
- Todaro MA, Sibaja-Cordero JA, Barquero JD, Barquero JD, Barquero JD, Dal Zotto M, Dal Zotto M. 2019c. An introduction to the study of Gastrotricha, with a taxonomic key to families and genera of the group. Diversity 11(7):117. DOI:10.3390/d11070117.
- Todaro A, d’Hondt J-L, Hummon W. 2022. World Gastrotricha Database. Gastrotricha. Available: http://www.marinespecies.org/aphia.php?p=taxdetails&id=2078. Accessed March 2022 21
- Vďačný P, and Foissner W. 2008. Morphology, conjugation, and postconjugational reorganization of Dileptus tirjakovae n. sp. (Ciliophora, Haptoria). Journal of Eukaryotic Microbiology 55:436–447.
- Vďačný P, Orsi W, Bourland WA, Shimano S, Epstein SS, Foerb W. 2011. Morphological and molecular phylogeny of dileptid and tracheliid ciliates: Resolution at the base of the class Litostomatea (Ciliophora, Rhynchostomatia). European Journal of Protistology 47(4):295–313. DOI:10.1016/j.ejop.2011.04.006.
- Vďačný P, Foer W. 2012. Monograph of the Dileptids (Protista, Ciliophora, Rhynchostomatia). Denisia 31:1–529.
- Verni F, Gualtieri P. 1997. Feeding Behaviour in Ciliated Protists. Micron 28(6):487–504. DOI:10.1016/S0968-4328(97)00028-0.
