Abstract
The COVID-19 pandemic, which modified levels of visitor attendance to urban forests and green spaces, provided a unique opportunity to measure the effects of human presence on wild animals and their activity. The aim of our study was to describe how the daily activity pattern of roe deer (i.e. proportion of day- and night-time observations) changed in response to visitor frequency in urban forests of Warsaw in four periods of 2020 (the year the COVID-19 pandemic was announced) compared to the same periods in 2019 (control year). In total, 662 observations of roe deer were recorded by camera traps in both years. The frequency of roe deer observations in urban forests of Warsaw in day- and night-time did not differ between March 2019 and 2020 (period before lockdown, no changes in the number of visitors). Next, between 1 and 20 April 2020 (national lockdown; urban forests were closed for public) the activity of roe deer increased during the day-time. Between 21 April and 30 June in both 2019 and 2020 the frequency of roe deer observations in day and night-time did not differ (green areas were reopened, remote learning). Finally, in July–August 2020 (summer holidays, tourist traffic in forests elevated all day round) the activity of roe deer visibly increased during the night-time. Our research shows how their activity rapidly shifted toward day-time when forests were closed for visitors, and toward night-time when the number of visitors was higher in urban forests after the lockdown. To summarize, roe deer in urban forests showed high ecological plasticity to human presence and rapidly adjusted to changes in the number of visitors in urban forest during the COVID-19 pandemic.
1. Introduction
The rapid and widespread changes in human activities caused by COVID-19 pandemic had an impact on the environment and wildlife. During lockdown (also called “anthropause”, Rutz et al. Citation2020; or “COVID-19 quietus”, Montgomery et al. Citation2021), reductions in air pollution (Venter et al. Citation2020; Briz-Redón et al. Citation2021), noise (Lecocq et al. Citation2020) and the volume of road traffic were recorded (Rupani et al. Citation2020; Driessen Citation2021). Such changes caused, for example, an increase in bird detectability due to lower noise (Gordo et al. Citation2021) and a smaller number of wildlife–vehicle collisions due to lower traffic volume (Bíl et al. Citation2021; Driessen Citation2021; Łopucki et al. Citation2021; Pokorny et al. Citation2021; Shilling et al. Citation2021). Moreover, this new situation might have changed animal behavior (e.g. Bates et al. Citation2020; Manenti et al. Citation2020; Rutz et al. Citation2020; Zellmer et al. Citation2020; Montgomery et al. Citation2021); an increase in species richness in temporarily less-disturbed habitats (Manenti et al. Citation2020), the arrival of new animal species to towns and an increased frequency of animal observations in urban environments (Rutz et al. Citation2020; Zellmer et al. Citation2020; Silva-Rodríguez et al. Citation2021) were reported.
Roe deer is known for its plasticity (Ciach, Fröhlich Citation2019; Bonnot et al. Citation2020), which allows it to inhabit urban areas such as Warsaw, where its presence has been confirmed since the 1970s (Jasińska et al. Citation2021). Daily activity of roe deer Capreolus capreuolus in natural conditions is crepuscular, with two peaks – during dawn and dusk (Pagon et al. Citation2013; Oberosler et al. Citation2017; Bonnot et al. Citation2020). To deal with anthropogenic stressors, roe deer may shift their activity and become more nocturnal (Bonnot et al. Citation2013) or maintain their natural, crepuscular, rhythms (Wevers et al. Citation2020). Specific conditions during the COVID-19 pandemic could have also affected roe deer activity.
In Poland, an epidemic emergency was announced on 14 March 2020. After that day new restrictions were gradually introduced, and finally between 1 and 20 April, forests, parks, public boulevards and beaches were closed for visitors, and controlled by police to prevent trespassing. Therefore, the number of visitors in urban forests must have been dramatically lower than in 1–20 April 2019. After lockdown, remote learning was continued in April, May and June, so that visitor traffic could concentrate mostly in afternoon and evening hours. During summer holidays the number of visitors was probably greatly elevated as the school break started, but people traveled about 30% less than in previous years (Statistics Poland Citation2021). Indeed, the demand for access to outdoor green spaces increased during the lockdown, resulting in an increasing number of visitors in urban green areas in the post-lockdown period (Geng et al. Citation2020).
Anthropause offered a unique opportunity to show roe deer reactions to changes in the number of visitors in urban forests in Warsaw. We compared roe deer activity in urban forests in the spring and summer months of 2019 and 2020, describing changes in the roe deer daily activity patterns before, during and after the COVID-19 lockdown period of 2020 compared to the same periods of 2019, and in the light of pandemic-caused changes in the number of visitors in urban forests. We assumed that the activity of roe deer would be (1) similarly distributed between day- and night-time in March 2019 and 2020 (before the lockdown, when no apparent differences in visitor numbers were expected); (2) more diurnal during the lockdown compared to the same period in 2019, in response to the dramatic decrease in visitors to urban forests; and (3) more nocturnal during post-lockdown and summer holiday periods (compared to 2019), because of increased forest penetration by city inhabitants, being driven by restrictions on social gathering, and limited access to indoor recreational places (Geng et al. Citation2020), as well as limited holiday travels (Statistics Poland Citation2021).
2. Materials and methods
2.1. Study area
Warsaw (52°13′47″N, 21°00′42″E) is the capital and the largest (517 km2) and most populous (3437 inhabitants/km2) city of Poland (Statistics Poland Citation2019). It is situated at an altitude of 113 m above sea level in a temperate zone, with an annual rainfall of about 500 mm and an average temperature of 7.7°C (Citationclimate-data.org). The Vistula River runs throughout Warsaw and divides the city into two parts. Warsaw is characterized by a high proportion of green areas (Luniak et al. Citation1997; Statistics Poland Citation2019): urban forests, parks, botanical and zoological gardens, squares, cemeteries, allotments, home gardens, residential and roadside vegetation, and natural riparian forests (Nature 2000 protected). Urban forests constitute ca. 15% of the city area, which makes Warsaw one of the cities with the highest proportion of this type of land cover. Urban forests are located mainly in peripheral districts of the city. Many species of mammals have been recorded in urban forests of Warsaw, including small rodents (Gryz et al. Citation2017), red squirrel (Sciurus vulgaris: Krauze-Gryz et al. Citation2021), medium-sized carnivores (e.g. red fox – Vulpes vulpes: Jackowiak et al. Citation2021; badger – Meles meles, martens – Martes spp.: KDJ unpublished data) or large ungulates (e.g. wild boar – Sus scrofa, moose – Alces alces, red deer – Cervus elaphus: KDJ unpublished data). These green spaces are commonly used by humans for recreational activities and leisure.
2.2. Methods
To determine the activity patterns of roe deer, we used camera traps set haphazardly in selected urban forests in the spring and summer of 2019 and 2020. Camera traps were located in forest complexes in three parts of Warsaw, north (Henryków and Dąbrówka Forest Park, Bródno Forest, Młociny Forest), southeast (Sobieski Forest incl. Jan III Sobieski Nature Reserve, Olszynka Grochowska Nature Reserve, Kawęczyn Nature Reserve), and southwest (Morysin Nature Reserve, Kabaty Nature Reserve). The forest complexes differed in size (21–918 ha) (Appendix, ). During the exposition period, the camera traps were regularly inspected at 1–1.5 month intervals. During the inspection the batteries and memory cards were changed. The traps were located far from established routes to avoid possible theft of or damage to the devices.
In both 2019 and 2020, we used several types of camera traps (Reconyx HyperFire: PC90, PC800, PC850, PC900; Ltl Acorn 6210 MC; Browning Spec Ops Advantage). The same types of camera traps were set in each forest complex in both years of research. The camera traps were set in trees, 30 cm above the ground, and we did not use any attractant to lure animals. Camera traps were set in 25 (2019) and 29 (2020) locations (in all forests in both 2019 and 2020) in February and disassembled in late summer/early autumn (2503 camera trap-days were collected for 2019 and 2315 camera trap-days for 2020) (Appendix, ). In photo analysis, we recorded each roe deer appearing in the images without distinguishing between individuals. A new observation was considered if a minimum of 15 minutes elapsed between subsequent photos (see Popescu et al. Citation2014; Jasińska et al. Citation2021). This rule was abandoned only when an animal in the photo was clearly different in age, sex, body condition and antler development, indicating that the animal in the photo was not the same as the previously registered one. A group of several individuals appearing in one picture was also recorded as a single observation. Camera traps recorded the date of the observation, and the time (24 h record) in Central European Time (CET).
We analyzed activity of roe deer (defined as the number of observations) during two parts of a day (day-time and night-time) in the March–August period of 2019 and 2020. We used the time of sunrise and sunset for Warsaw in the years 2019–2020 (Citationdateandtime.info) converted to CET and defined day-time as the time between sunrise and sunset, and night-time as the time between sunset and sunrise.
Referring to pandemic changes in the number of visitors (Geng et al. Citation2020) in urban forests of Warsaw, four periods were distinguished: (1) before lockdown (March 2020), (2) lockdown (1–20 April 2020), (3) post-lockdown (21 April–30 June 2020), and (4) summer holidays (July–August 2020) (see Introduction).
To present daily activity of roe deer in four periods of 2019 and 2020, we used the kernel density function in R (v. 4.1.2, R Core Team Citation2021). Differences in the number of diurnal and nocturnal observations of roe deer in each of the four periods in 2019 and 2020 were evaluated using Chi2 test performed in PAST4.03 software (Hammer et al. Citation2001).
3. Results and discussion
In total, 662 observations of roe deer were recorded in urban forests of Warsaw during March–August in the years 2019 and 2020 (337 in 2019 and 325 in 2020). There were no significant differences in the number of observations between 2019 and 2020 (Chi2 test with Yates correction; χ2 = 0.22, df = 1, p > .641). We found no differences in the number of roe deer records during day-time and night-time in March of 2019 (50 and 22 observations, respectively) compared to March 2020, before lockdown (32 and 13 observations, respectively) (Chi2 test; χ2 = 0.04, df = 1, p > .848) ()). Activity patterns of roe deer differed in 1–20 April of 2019 vs 2020 (national lockdown, Chi2 test; χ2 = 5.77, df = 1, p < .0163). During lockdown the activity of roe deer was higher in the day-time (31 of 35 observations) compared to 2019 (33 of 56 observations) ()). For most of April 2020, all green spaces in Poland were closed to visitors. Although in April of both 2019 and 2020 the number of roe deer observations in urban forests of Warsaw was higher in the day-time, the share of daytime roe deer observations in 2020 exceeded 80%. Probably, as human visitation to urban forests plummeted (and human disturbances decreased), roe deer shifted its activity toward day-time. Indeed, the lack of human activity during anthropause released animals from a landscape of fear, which resulted in diurnal activity of some species (Manenti et al. Citation2020). Moreover, animal behavior reactions to anthropause were rapid (Gordo et al. Citation2021). There were no significant differences in the number of roe deer recorded during day-time and night-time in the post-lockdown period (87 and 36 observations, respectively) compared to 21 April–30 June 2019 (77 and 25 observations, respectively) (Chi2 test; χ2 = 0.64, df = 1, p > .424) ()). Eventually the lockdown ended, and urban forests and other green spaces were re-opened, but simultaneously the school and academic year continued. In this period, in both 2019 and 2020, the highest activity of roe deer was recorded in the morning. Presumably human visitation to forests (even if elevated in this post-lockdown time; Geng et al. Citation2020), which were naturally concentrated in the period after school and work-time, did not affect roe deer presence as much.
Figure 1. Activity pattern of roe deer in urban forests of Warsaw (a) in March 2019 and 2020, (b) 1–20 April in 2019 and 2020 (national lockdown), (c) 21 April–30 June in 2019 and 2020 (post-lockdown), and (d) July–August in 2019 and 2020 (summer holiday). Gray shaded areas represent night-time (from midnight until sunrise and from sunset to midnight).
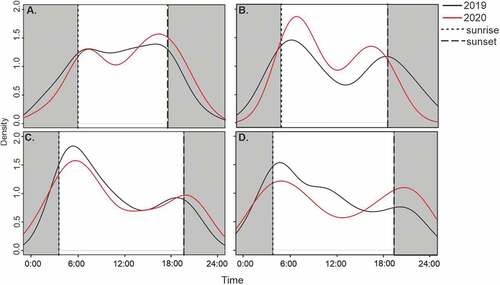
Roe deer were more active in the night-time during the summer holidays in 2020 (58 of 122 observations) compared to night-time during July–August 2019 (34 of 107 observations) (Chi2 test; χ2 = 5.90, df = 1, p < .0152) ()). Increased human activity in green areas was presumably associated with behavioral fear responses in animals (Putman et al. Citation2017), which may lead to activity shifts. Although in natural conditions the activity of roe deer was found to be higher after dusk in summer (Pagon et al. Citation2013), in our case, before the pandemic more observations of roe deer were registered in the morning than around sunset. During summer 2020, a clear drop in the frequency of roe deer observations was seen from late morning to afternoon. The end of school and the summer vacation allowed humans to visit forests at any time of day. Also, holiday travels were less frequent in 2020 than in previous years (Statistics Poland Citation2021), which probably resulted in an elevated rate of visitation to local urban forests. The observed two peaks of roe deer activity, i.e. around sunrise and sunset, and a higher overall number of roe deer observations in night-time might be explained by an avoidance of high human disturbance during the day but may also reflect the natural concentration of activity at night (Pagon et al. Citation2013).
Since the COVID-19 pandemic started, many papers have been published showing how this new situation changed animal behavior (e.g. Bates et al. Citation2020; Manenti et al. Citation2020; Rutz et al. Citation2020; Zellmer et al. Citation2020; Montgomery et al. Citation2021). For example, the pandemic pushed urban species into more natural habitats, which could have resulted in competition with other species and caused potentially significant changes to animal assemblage structure (Gilby et al. Citation2021). Interestingly, the effect of the COVID-19 pandemic may differ for different species, also in urban areas, where different behavioral responses of species may reflect their evolutionary history in cohabiting the same habitats (Vardi et al. Citation2021). We showed how the roe deer rapidly shifted its activity toward day-time when forests were closed for visitors, and, in turn, toward night-time when the number of visitors was higher in urban forests after the lockdown. This suggests high plasticity of the species but also points to the need to study the response of other species, differently adapted to human presence.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bates AE, Primack RB, Moraga P, Duarte CM. 2020. COVID-19 pandemic and associated lockdown as a “Global Human Confinement Experiment” to investigate biodiversity conservation. Biological Conservation 248:108665. DOI: 10.1016/j.biocon.2020.108665.
- Bíl M, Andrášik R, Cícha V, Arnon A, Kruuse M, Langbein J, Náhlik A, Niemi M, Pokorny B, Colino-Rabanal VJ, Rolandsen CM. 2021. COVID-19 related travel restrictions prevented numerous wildlife deaths on roads: A comparative analysis of results from 11 countries. Biological Conservation 256:109076. DOI: 10.1016/j.biocon.2021.109076.
- Bonnot N, Morellet N, Verheyden H. 2013. Habitat use under predation risk: Hunting, roads and human dwellings influence the spatial behavior of roe deer. European Journal of Wildlife Research 59:185–193. DOI: 10.1007/s10344-012-0665-8.
- Bonnot NC, Couriot O, Berger A, Cagnacci F, Ciuti S, de Groeve JE, Gehr B, Heurich M, Kjellander P, Kröschel M, Morellet N. 2020. Fear of the dark? Contrasting impacts of humans versus lynx on diel activity of roe deer across Europe. Journal of Animal Ecology 89:132–145. DOI: 10.1111/1365-2656.13161.
- Briz-Redón Á, Belenguer-Sapiña C, Serrano-Aroca Á. 2021. Changes in air pollution during COVID-19 lockdown in Spain: A multi-city study. Journal of Environmental Sciences 101:16–26. DOI: 10.1016/j.jes.2020.07.029.
- Ciach M, Fröhlich A. 2019. Ungulates in the city: Light pollution and open habitats predict the probability of roe deer occurring in an urban environment. Urban Ecosystems 22:513–523. DOI: 10.1007/s11252-019-00840-2.
- climate-data.org. Available: https://pl.climate-data.org/europa/polska/masovian-voivodeship/warszawa-4560/. Accessed Oct 2020 29.
- dateandtime.info. Available: https://dateandtime.info/pl/citysunrisesunset.php?id=756135&month=8&year=2019. Accessed Oct 2020 29.
- Driessen MM. 2021. COVID-19 restrictions provide a brief respite from the wildlife roadkill toll. Biological Conservation 256:109012. DOI: 10.1016/j.biocon.2021.109012.
- Geng DC, Innes J, Wu W, Wang G. 2020. Impacts of COVID-19 pandemic on urban park visitation: A global analysis. Journal of Forestry Research 32:553–567. DOI: 10.1007/s11676-020-01249-w.
- Gilby BL, Henderson CJ, Olds AD, Ballantyne JA, Bingham EL, Elliott BB, Jones TR, Kimber O, Mosman JD, Schlacher TA. 2021. Potentially negative ecological consequences of animal redistribution on beaches during COVID-19 lockdown. Biological Conservation 253:108926. DOI: 10.1016/j.biocon.2020.108926.
- Gordo O, Brotons L, Herrando S, Gargallo G. 2021. Rapid behavioural response of urban birds to COVID-19 lockdown. Proceedings of the Royal Society B 288(1946):20202513. DOI: 10.1098/rspb.2020.2513.
- Gryz J, Lesiński G, Krauze-Gryz D, Stolarz P. 2017. Woodland reserves within an urban agglomeration as important refuges for small mammals. Folia Forestalia Polonica Series A-Forestry 59:3–13. DOI: 10.1515/ffp-2017-0001.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:4.
- Jackowiak M, Gryz J, Jasińska K, Brach M, Bolibok L, Kowal P, Krauze-Gryz D. 2021. Colonization of Warsaw by the red fox Vulpes vulpes in the years 1976–2019. Scientific Reports 11:13931. DOI: 10.1038/s41598-021-92844-2.
- Jasińska KD, Jackowiak M, Gryz J, Bijak S, Szyc K, Krauze-Gryz D. 2021. Habitat-related differences in winter presence and spring–summer activity of roe deer in Warsaw. Forests 12(8):970. DOI: 10.3390/f12080970.
- Krauze-Gryz D, Gryz J, Brach M. 2021. Spatial organization, behaviour and feeding habits of red squirrels: Differences between an urban park and an urban forest. Journal of Zoology 315(1):69–78. DOI: 10.1111/jzo.12905.
- Lecocq T, Hicks SP, van Noten K, van Wijk K, Koelemeijer P, de Plaen RSM, Massin F, Hillers G, Anthony RE, Apoloner MT, Arroyo-Solórzano M. 2020. Global quieting of high frequency seismic noise due to COVID-19 pandemic lockdown measures. Science 369:1338–1343. DOI: 10.1126/science.abd2438.
- Łopucki R, Kitowski I, Perlińska-Teresiak M, Klich D. 2021. How is wildlife affected by the COVID-19 pandemic? Lockdown effect on the road mortality of hedgehogs. Animals 11(3):868. DOI: 10.3390/ani11030868.
- Luniak M, Kozłowski P, Nowicki W. 1997. Magpie Pica pica in Warsaw—Abundance, distribution and changes in its population. Acta Ornithologica 32:77–86.
- Manenti R, Mori E, di Canio V, Mercurio S, Picone M, Caffi M, Brambilla M, Ficetola GF, Rubolini D. 2020. The good, the bad and the ugly of COVID-19 lockdown effects on wildlife conservation: Insights from the first European locked down country. Biological Conservation 249:108728. DOI: 10.1016/j.biocon.2020.108728.
- Montgomery RA, Raupp J, Parkhurst M. 2021. Animal behavioral responses to the COVID-19 quietus. Trends in Ecology & Evolution 36(3):184–186. DOI: 10.1016/j.tree.2020.12.008.
- Oberosler V, Groff C, Iemma A, Pedrini P, Rovero F. 2017. The influence of human disturbance on occupancy and activity patterns of mammals in the Italian Alps from systematic camera trapping. Mammalian Biology 87:50–61. DOI: 10.1016/j.mambio.2017.05.005.
- Pagon N, Grignolio S, Pipia A, Bongi P, Bertolucci C, Apollonio M. 2013. Seasonal variation of activity patterns in roe deer in a temperate forested area. Chronobiology International 30:772–785. DOI: 10.3109/07420528.2013.765887.
- Pokorny B, Cerri J, Bužan E. 2021. Roadkill in a time of pandemic: The analysis of wildlife-vehicle collisions reveals the differential impact of COVID-19 lockdown over mammal assemblages. DOI: 10.32942/osf.io/p3zft.
- Popescu VD, de Valpine P, Sweitzer RA. 2014. Testing the consistency of wildlife data types before combining them: The case of camera traps and telemetry. Ecology and Evolution 4(7):933–943. DOI: 10.1002/ece3.997.
- Putman BJ, Drury JP, Blumstein DT, Pauly GB. 2017. Fear no colors? Observer clothing color influences lizard escape behavior. PLOS ONE 12:e0182146. DOI: 10.1371/journal.pone.0182146.
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for 374 Statistical Computing.
- Rupani PF, Nilashi M, Abumalloh RA, Asadi A, Samad S, Wang S. 2020. Coronavirus pandemic (COVID-19) and its natural environmental impacts. International Journal of Environmental Science and Technology 17:4655–4666. DOI: 10.1007/s13762-020-02910-x.
- Rutz C, Loretto MC, Bates AE, Davidson SC, Duarte CM, Jetz W, Johnson M, Kato A, Kays R, Mueller T, Primack RB. 2020. COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nature Ecology & Evolution 4(9):1156–1159. DOI: 10.1038/s41559-020-1237-z.
- Shilling F, Nguyen T, Saleh M, Kyaw MK, Tapia K, Trujillo G, Bejarano M, Waetjen D, Peterson J, Kalisz G, Sejour R. 2021. A reprieve from US wildlife mortality on roads during the COVID-19 pandemic. Biological Conservation 256:109013. DOI: 10.1016/j.biocon.2021.109013.
- Silva-Rodríguez EA, Gálvez N, Swan GJF, Cusack JJ, Moreira-Arce D. 2021. Urban wildlife in times of COVID-19: What can we infer from novel carnivore records in urban areas? Science of the Total Environment 765:142713. DOI: 10.1016/j.scitotenv.2020.142713.
- Statistics Poland. 2019. Statistical yearbook of Warsaw. Warsaw, Poland: Zakład Wydawnictw Statystycznych.
- Statistics Poland. 2021. Tourism in 2020. Warszawa, Rzeszów: Statistical Office in Rzeszów.
- Vardi R, Berger-Tal O, Roll U. 2021. iNaturalist insights illuminate COVID-19 effects on large mammals in urban centers. Biological Conservation 254:108953. DOI: 10.1016/j.biocon.2021.108953.
- Venter ZS, Aunan K, Chowdhury S, Lelieveld J. 2020. COVID-19 lockdowns cause global air pollution declines. Proceedings of the National Academy of Sciences 117:18984–18990. DOI: 10.1073/pnas.2006853117.
- Wevers J, Fattebert J, Casaer J, Artois T, Beenaerts N. 2020. Trading fear for food in the Anthropocene: How ungulates cope with human disturbance in a multi-use, suburban ecosystem. Science of the Total Environment 741:140369. DOI: 10.1016/j.scitotenv.2020.140369.
- Zellmer AJ, Wood EM, Surasinghe T, Putman BJ, Pauly GB, Magle SB, Lewis JS, Kay CA, Fidino M. 2020. What can we learn from wildlife sightings during the COVID-19 global shutdown? Ecosphere 11(8):e03215. DOI: 10.1002/ecs2.3215.
Appendix
Table A1. Characterization of urban forests of Warsaw where the camera traps were distributed in the years 2019–2020.
