Abstract
Climate change and extreme weather events may be seen as an opportunity to help understand the mechanisms by which birds adjust their breeding parameters to environmental variability. In the breeding season of 2020, several factors, such as a prolonged drought period, a relatively warm winter with no snow cover, and a cold spring (the coldest May in Poland since 1991) affected many bird populations in Poland. The great tit Parus major tended to produce much smaller clutches, much fewer fledglings, and had very low breeding success, increasing with the progress of the breeding season in 2020, in contrast to the more typical breeding season characterised by average weather parameters in 2018. In light of the results obtained, we agree with the view that the phenotypic plasticity of the breeding parameters of the studied birds, such as phenology, may be insufficient to adjust to the upcoming extreme weather events. Additionally, further examining of the impact of extreme weather events on the breeding biology of animals is an important goal for future studies.
Introduction
The influence of climate and weather variation on reproductive parameters has gained increasing attention nowadays because of the effects of global climate change (Martin Citation2001; Goodenough et al. Citation2010; Chamberlain and Pearce-Higgins Citation2013; Glądalski et al. Citation2016; Matter and Rolland Citation2017; van de Pol et al. Citation2017; Fox et al. Citation2019; Radchuk et al. Citation2019). There is still not enough information about how climate change and extreme weather events may influence breeding traits and reproduction parameters in animals (Charmantier & Gienapp Citation2014; van de Pol et al. Citation2017; Wingfield et al. Citation2017; Glądalski et al. Citation2020a, Citationb). In general, it has been suggested that unusual or extreme weather events may be treated as natural experiments that could help understand the effects of climate change and various weather parameters on the breeding biology of animals, including birds (Pipoly et al. Citation2013; Bailey and van de Pol Citation2016; Marrot et al. Citation2017).
Variation in ambient temperature and precipitation before and during the breeding season are well known to have a significant impact on the breeding parameters of an adult bird and survival of the offspring (Perrins and McCleery Citation1989; Perrins Citation1991; Monaghan Citation2007; Bründl et al. Citation2020; Corregidor-Castro and Jones Citation2021; Skwarska et al. Citation2022). Extreme weather episodes may disturb life history strategies of birds and various species and could have complex effects on fitness (Chamberlain & Pearce-Higgins Citation2013; Whitehouse et al. Citation2013). For insectivorous passerines that feed caterpillars to their nestlings (considered as the most suitable kind of food for nestlings), temperatures in early spring are a potential clue for future seasonal prey availability as a crucial driver of breeding success (Visser et al. Citation1998; Glądalski et al. Citation2014, Citation2016, Citation2018; Tomás Citation2015; Diez-Mendez et al. Citation2021). Therefore, impact of extreme weather events on breeding parameters of organisms may be an important goal for research (Tomás Citation2015).
In the climatic conditions of Poland, adult winter moths emerge from their pupae in late autumn (November), mate, and lay eggs on the branches of the host tree species (Szujecki Citation1987). During the following spring (April and May), the emerging caterpillars from the hatched eggs feed on fresh foliage (van Asch & Visser Citation2007; van Asch et al. Citation2013). Low temperatures during regular winter conditions cause wintering insects and eggs to enter diapause, where lowered metabolism allows them to survive a long period without food (Lees Citation1956; Chechłacz Citation1997). A warm winter period may cause insects to become prematurely active and eggs to develop when they normally would be dormant. This activity uses stored fats to survive harsh winter conditions until spring and without any access to food, these active insects can starve to death before their plant food becomes available – as a result, it may reduce later, spring caterpillar abundance needed by birds (Neven Citation2000; Sinclair Citation2015). In addition, winter moths during pupal development in spring are temperature sensitive, and it was shown by Peterson and Nilssen (Citation1998) that low temperature may increase their mortality. Drought may both enhance insect mortality and affect tree leafing phenology and through this caterpillar phenology and abundance. In general, it is assumed that yearly changing weather conditions may to a high degree affect the phenology and demographic parameters of local populations of moths (Skagen and Adams Citation2012, Schöll and Hille Citation2020). Robinet and Roques (Citation2010) have also suggested that the effects of extreme weather and climate change on the development and dispersal of insects may be much more complex than a simple linear response to changes in temperature.
Patterns found in comparative productivity of forest and urban populations of tits show that forest birds tend to lay later, produce larger clutch sizes, and more fledglings than their conspecifics from urban areas (Massa et al. Citation2004; Chamberlain et al. Citation2009; Seress and Liker Citation2015; Glądalski et al. Citation2017). Earlier laying dates in urban areas are probably caused by higher urban temperatures, tree species composition, or better adult condition during winter due to human-provided food resources (Haggard Citation1990; Eeva et al. Citation2000; Robb et al. Citation2008). Variation in breeding success may result from differences in characteristics of territory quality (Martin Citation1987; Nager and van Noordwijk Citation1992), food abundance (Cresswell & McCleery Citation2003; Nadolski et al. Citation2021) or habitat structure (Cowie & Hinsley Citation1987).
The Earth is experiencing extensive changes in both temporal and spatial patterns of climate variables, such as temperature, precipitation, and wind. Due to climate change in the past 20 years, Europe experiences an increased frequency of droughts (Liu et al. Citation2020). Since the summer of 2018 a large part of central Europe (including Poland, Pińskwar et al. Citation2020) has experienced a next record-breaking, extreme drought, caused by the increase in temperatures that was accompanied by concurrent significant reduction in precipitation. The intensity and duration of this drought are unprecedented in the last 250 years (Hari et al. Citation2020). Hari et al. (Citation2020) have also suggested that this rainfall deficit should be treated as an extreme weather event based on global warming. In Poland, the drought started in the summer of 2018 and continued during the warm winter (2019/2020) and the dry but cold spring of 2020 (Pińskwar et al. Citation2020). These climate and weather changes have widespread effects in ecosystems and are seen to affect the breeding biology of organisms (Radchuk et al. Citation2019; Salinas et al. Citation2019; Barras et al. Citation2021).
Our present study is based on the co-occurrence of an extreme, prolonged multi-seasonal period of drought, a warm winter (with no snow cover and the average winter air temperature was 3.9°C higher than the average for this period for Poland, see Ustrnul et al. Citation2021), and a cold spring (it was the coldest May in Poland since 1991; Ustrnul et al. Citation2021) in 2020 versus the occurrence of typical weather, treated as a natural experiment influencing reproductive performance of great tits. This situation may be seen as a consequence of climate change and a progressive trend for climate in central Poland to become drier and with extreme weather events (droughts, downpours, tornadoes, and floods) becoming more and more frequent (Podstawczyńska Citation2010; Tylkowski Citation2017; Hari et al. Citation2020; Kasprzak and Salamon Citation2020; Liu et al. Citation2020). In this paper, we report the response of breeding parameters (laying date/hatching date, clutch size, fledgling number, and breeding success) of the great tit to unusual weather conditions in spring 2020, in contrast to a typical spring such as the one of 2018. We predict that due to drought conditions, warm winter, and low temperatures during the breeding season of 2020 great tits should have lower clutch size, fledgling number, and breeding success in comparison to breeding parameters 2018.
Methods
This study was carried out in 2018 and 2020 in two floristically and structurally contrasting study areas, about 10 km apart; a mature mixed deciduous forest site (51°50′ N; 19°29′ E) and an urban parkland site (51°45′ N; 19°24′ E). Both study areas are located around the city of Łódź in central Poland. The forest study site is around 150 ha area (with about 300 nest boxes) in the interior part of the Łagiewniki Forest. The entire Łagiewniki Forest is a deciduous forest of considerable size, in total about 1,250 ha, neighbouring with the suburbia of Łódź. The dominant tree species in the forest study area are two oak species: Quercus petraea and Quercus robur (constituting more than 50% of all trees), also with the hornbeam Carpinus betulus, maples Acer spp., birches Betula pendula, and limes Tilia spp. being much less numerous species (Nadolski et al. Citation2021).
The urban parkland study site (c.a. 67 ha, with around 200 nest boxes), consisting of the Botanic and Zoological Gardens (both in Łódź), is located in the western part of the city. The vegetation of the Botanic Garden was mainly formed artificially for plant exposition, and, therefore, the tree cover is patchy with a large number of flower beds and lawns. Tree patches are a mixture of various coniferous and deciduous trees and shrubs, containing a large number of exotic/foreign species. Among the most frequent deciduous and native species are hornbeam Carpinus betulus, birches Betula pendula, limes Tilia spp., beech Fagus sylvatica, oaks Quercus spp., poplars Populus spp. and maples Acer spp. The most numerous exotic/foreign deciduous tree species are chestnut trees Aesculus spp., plane trees Platanus spp., silver lime Tilia tomentosa, oaks Quercus spp., and maples Acer spp. In addition, in the Botanic Garden there are more than 50 species of other, less numerous foreign deciduous trees (Glądalski et al., Citation2020b, Glądalski et al. Citation2021).
The main weather-related peculiarity of the breeding season in 2020 was that it started during a prolonged drought lasting from 2018, and especially severely from the summer of 2019. Winter 2019/2020 was relatively warm, with mean monthly temperatures of January and February higher than 0°C (), and with no snow cover. First, substantial rainfall occurred as late as the second half of May 2020, while the first half of spring was not only dry but also relatively cold – May of 2020 in Poland was the coldest in 29 years (, Ustrnul et al. Citation2021). Winter 2019/2020 was the warmest winter since the beginning of our research project on the breeding biology of secondary hole-nesting birds that occupied nest boxes near Łódź in 1999 (). By contrast, there was much more precipitation during winter 2017/2018, with some snowfall and frosty February 2018. The spring of 2018 was characterized by mild temperatures and regular, moderate rainfall (Glądalski, personal observations, ). Although the deficit of precipitation has occurred in Central Europe for more than 20 years, thus influencing terrestrial ecosystems, it differed among regions of Europe as well as from year to year and, especially, from spring to spring (Liu et al. Citation2020; Pińskwar et al. Citation2020). In our study area, a severe deficit of precipitation started in the summer of 2018, after the breeding season of tits. Because the breeding season of 2018 occurred in mild weather conditions despite a general long-term trend, we decided to consider this season as a kind of benchmark.
Table I. Summary of pre-breeding and breeding period weather conditions: mean temperature (°C) for January and February (2018 and 2020) for the urban parkland (Lublinek) and the forest study area (Dobra-Nowiny), the warmth sum (°C) for January and February (the sum of the daily maximum temperatures during the period) for 2018 and 2020 for both study areas, the sum of precipitation (mm) for March and April (2018 and 2020) for both study areas, and the mean temperature and the warmth sum for May for 2018 and 2020 for both study areas.
Figure 1. Mean temperatures (°C) of January and February (1999–2020) and the warmth sum (°C) for January and February (the sum of the daily maximum temperatures during the period) for the period 1999–2020, based on the data from the Lublinek meteorological Station.
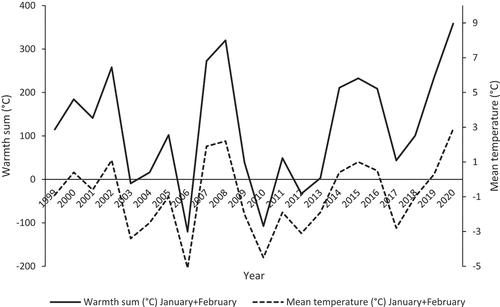
We obtained data on local temperatures and precipitation for Łódź from Łódź-Lublinek Meteorological Station (for the urban parkland study site) and Dobra-Nowiny Meteorological Station (for the forest study site; using the automatic weather station of Department of Meteorology and Climatology, University of Lodz). As indicators of the general thermal conditions for the two study sites with nest boxes, we used warmth sums of the daily maximum temperatures between 1 and 31 May in both years (Perrins & McCleery Citation1989; Glądalski et al. Citation2014, Citation2016).
Overall, the central Poland area to which Łódź belongs has tended to become drier and drier over the last decades, with some irregular variation in precipitation and mean temperatures occurring (Podstawczyńska Citation2010; Pińskwar et al. Citation2020; Jaagus et al. Citation2021). The case of the Botanic Garden in Łódź shows that this tendency leads to substantial ecological consequences. Over the last couple of years, we have observed in the Botanic Garden an indication of increasing drought, likely accompanying climate warming. Prolonged drought has been recorded not only in the spring-summer season but also during autumn and winter. We have found a negative impact of drought and heat waves on native trees. This effect is especially clear in the case of c. 130-years-old lime trees (Tilia sp.) of different origin that were planted in line in the central part of the Garden. The tree line contains native species Tilia cordata and Tilia platyphyllos, as well as south-European Tilia tomentosa. In the spring-summer seasons 2017–2019, the native trees showed evident symptoms of drought-related bad condition, while Tilia tomentosa was prospering very well. From 2017 on, we have been recording an increasing frequency of dying birches Betula pendula and from 2019 on also dying spruces of different species (Picea). In the case of spruces, dying was especially frequent in 2020. On the other hand, at the same time, some exotic trees coming from warmer areas have started to prosper better and better, some of which have even started to flower for the first time. Examples of such well-prospering species include Catalpa bignonioides, Ailanthus altissima, Parrotia persica, Paulownia tomentosa (Mańkowska, personal observations).
At the beginning of the breeding seasons, the study areas were visited every day to record laying date, clutch size, hatching day and the number of nestlings. In tits, the female tends to lay one egg per day (Perrins Citation1979). Only the first clutches of great tits were analyzed. A total of 249 first clutches (56 in 2018 in the forest, 53 in 2018 in the parkland, 77 in 2020 in the forest, 63 in 2020 in the parkland), 2164 of eggs (563 in 2018 in the forest, 455 in 2018 in the parkland, 653 in 2020 in the forest, 493 in 2020 in the parkland) and 1810 of nestlings (497 in 2018 in the forest, 390 in 2018 in the parkland, 537 in 2020 in the forest, 386 in 2020 in the parkland) of great tits were studied in 2018 and 2020. All the nest boxes in both study areas were visited after the end of the breeding season (July) to determine the number of fledglings.
We assumed that in the climatic conditions of central Poland the minimum temperatures in spring are more limiting to breeding great tits than the mean or maximum temperatures. We decided to characterize thermal conditions of critical stages of nesting using simple indicators based on minimum daily temperature. Taking into account different studies on ecological sensitivity of females, eggs and nestlings during incubation and brooding as well as the development of nestling endothermic abilities (Shilov Citation1973; Mainwaring & Hartley Citation2016; Marques-Santos & Dingemanse Citation2020; Broggi et al. Citation2022), we consider the egg stage of uninterrupted incubation of eggs and the nestling stage that requires most brooding before achieving physiological endothermy as most sensitive to low temperatures. We assumed that each of these most sensitive stages lasts about 10 days. Therefore, using the weather data from the Lublinek and Dobra-Nowiny meteorological stations, for every brood, we calculated three site-specific hatching-date-adjusted indicators of minimum daily temperature to characterize the pre-hatching stage (incubation, based on temperatures from the last 10 days before hatching), post-hatching stage (10 days after hatching), and both these stages pooled (20 days).
Differences in the brood-adjusted temperature indicators and basic breeding characteristics (mean laying date, hatching date, clutch size, and number of fledglings) between the years and study sites as factors were tested using general linear models with the identity link function (Gaussian error structure) – factorial models including the factor interactions (Crowley Citation2002).
Breeding success, treated as a binomial proportion-dependent variable, the number of fledglings in relation to clutch size in individual broods as unit records, was initially modelled using generalized linear models, assuming binomial error structure and applying the logit link function, so that breeding success was expressed as natural log of the odds of fledged eggs to unfledged eggs (Crawley Citation2002; Heck et al. Citation2012). In exactly the same way, we modelled hatching success (the number of hatchlings in relation to clutch size) and fledging success (the number of fledglings in relation to the number of hatchlings). In all these cases, initial models included the year and site factors and their interaction. To obtain the final models, non-significant interactions were removed, whereas significant ones were retained (Crawley Citation2002).
Keeping the same error structure and assumptions, we computed a generalized linear model of breeding success with respect to the year and site factors, but including also the date of hatching as a quantitative explanatory variable (number of days from 1 March). The initial model included these explanatory variables as well as their three-way and two-way interactions. Then, we backward removed non-significant interactions (Crawley Citation2002). To interpret the remaining significant interactions, we computed two sub-models, separately for 2018 and 2020, with the site factor and the date of hatching as independent variables, and further, sub-sub-models of the lowest level, separately for the years and study sites, with the date of hatching as the only independent variable.
All statistical computations were performed using IBM SPSS v. 22 software (IBM SPSS Statistics 22 Citation2013).
Results
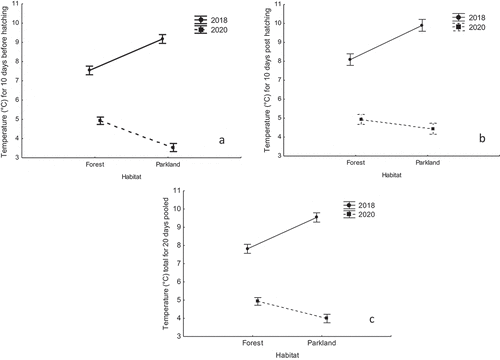
The minimum temperature indicators adjusted to individual brood phenology show that the breeding stage of great tits, including the pre-hatching and nestling-raising stages, took place under substantially warmer conditions in 2018 (good breeding season) than in 2020 (bad breeding season) (, ). A similar pattern of variation in these adjusted indicators was found for 10 days before hatching (), 10 days from hatching () and the pooled 20-day indicator () (), with some slight differences between the urban park and forest study sites.
Table II. Summary of three separate general linear models examining differences in the brood-adjusted minimum temperature (°C) for 10 days before hatching (incubation), 10 days from hatching and total of 20 days pooled between both study sites and years – 2018 vs. 2020 (significant p-values in the model are in bold). Each model included a particular adjusted temperature indicator as the response variable assuming Gaussian error structure in relation to year and site factors and their interaction.
Figure 2. Minimum values of individual great tit brood adjusted temperature (°C) for 10 days before hatching (A), for 10 days after hatching (B) and total for 20 days pooled (C) in the forest and in the urban parkland study areas - 2018 vs. 2020 (data shown as mean ± SE).
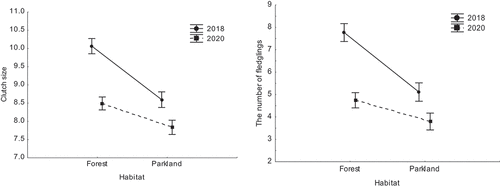
The laying and hatching dates were not significantly different between 2018 and 2020 in the forest study site, while they were on average later in 2018 (days from the first March: 53.04 ± 1.73 SE and 73.41 ± 1.71 SE) than in 2020 (48.71 ± 1.43 SE and 68.51 ± 1.35 SE) in the urban park site (, ). Both clutch sizes and numbers of fledglings were much higher in 2018 (forest clutch size 10.05 ± 0.24 SE and no. of fledglings 7.75 ± 0.42 SE; parkland clutch size 8.59 ± 0.21 SE and no. of fledglings 5.09 ± 0.42 SE) than in 2020 (forest clutch size 8.48 ± 0.16 SE and no. of fledglings 4.73 ± 0.33 SE; parkland clutch size 7.83 ± 0.19 SE and no. of fledglings 3.78 ± 0.36 SE), with this effect being stronger in the forest study site than in the urban park site (, ). Both clutch sizes and numbers of fledglings were also consistently lower in the urban park than in the forest site (, ).
Table III. Summary of two separate general linear models examining differences in the mean laying dates (days from 1 March) and mean hatching dates between both study sites and years – 2018 vs. 2020 (significant p-values in the model are in bold). In both cases, the date was modelled as the response variable (Gaussian error structure) in relation to year and site factors and their interaction.
Table IV. Summary of two general linear models examining differences in clutch size and the number of fledglings between both study sites and years – 2018 vs. 2020 (significant p-values in the model are in bold). In both cases, the response variable (clutch size or the number of fledglings) was modelled assuming Gaussian error structure in relation to year and site factors and their interaction.
Figure 3. Mean laying and hatching dates (days from 1 March) in the forest and in the urban parkland study areas – 2018 vs. 2020 (data shown as mean ± SE).
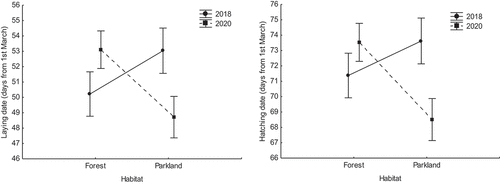
Figure 4. Mean clutch size and number of fledglings in the forest and in the urban parkland study areas - 2018 vs. 2020 (data shown as mean ± SE).
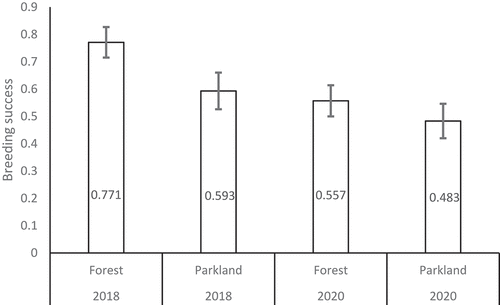
In general, breeding success, measured as a proportion of the number of fledglings to clutch size in individual broods, was higher in 2018 than in 2020 at both the study sites (generalized linear model, binomial error structure, logit link function, with year and site factors only: Year*Site interaction Wald χ21 = 8.08, p = 0.004) (). Differences in hatching success and fledging success between 2018 and 2020 and between the forest and park sites show a similar pattern. In both the study sites hatching success was higher in 2018 (0.88 ± 0.011 SE and 0.86 ± 0.014 SE, respectively) than in 2020 (0.82 ± 0.014 SE and 0.78 ± 0.017 SE, respectively) (generalized linear model, binomial error structure, logit link function: Year effect Wald χ21 = 17.21, p < 0.001; Site effect χ21 = 4.24, p = 0.040). In the case of fledging success, the difference between 2018 and 2020 represented the same tendency but was relatively higher in the forest site (0.87 ± 0.015 SE in 2018 and 0.67 ± 0.020 SE in 2020) than in the park site (0.69 ± 0.023 SE in 2018 and 0.62 ± 0.025 SE in 2020) (generalized linear model, binomial error structure, logit link function: Year*Site interaction effect χ21 = 15.61, p < 0.001; Year effect Wald χ21 = 48.47, p < 0.001; Site effect χ21 = 37.02, p < 0.001).
Figure 5. Breeding success (proportion of fledglings produced per egg laid) in the forest and in the urban parkland study areas - 2018 vs. 2020 (data shown as mean ± SE).
The further analysis including the hatching date of individual broods found that breeding success of individual broods was linked to both year and site factors and to the date of hatching as a quantitative independent variable in a complex way, as shown by two significant two-way interactions (). The final sub-model for 2018 revealed a significant difference in breeding success between the study sites (). On the other hand, there was a significant interaction between the study site and hatching date for 2020 (). Therefore, we computed sub-sub-models for the study sites and years separately. This analysis confirmed that there was no significant relationship between breeding success and hatching date in neither site in 2018, while in both the urban park site and the forest site the hatching date significantly influenced breeding success (). In both sites breeding success increased with later hatching dates, with the effect being stronger in the forest site (b = 0.068 ± 0.012(SE)) than in the urban parkland site (b = 0.027 ± 0.012(SE)).
Table V. Summary of the generalized linear model of breeding success (the number of fledglings in relation to clutch size, assuming binomial error structure) of great tits in relation to year (2018 vs. 2020) and site (urban park vs. forest) as factors and in relation to the year-centered date of hatching (hatch date) as a covariate. The logit link function was applied, so that breeding success was expressed as natural log of the odds of hatched eggs to unhatched egg. The main model included the response variable in relation to year and site factors and the date of hatching (quantitative variable), and their interactions.
Discussion
In this study, we investigated differences in laying and hatching date, clutch size, fledgling number, and breeding success in two breeding seasons different in terms of weather conditions, 2018 and 2020, in two structurally and floristically contrasting study areas, an urban parkland and a deciduous forest. In general, the great tit breeding season in 2020 proceeded under unfavorable weather conditions and can be classified as a bad season, using the terminology proposed by Järvinen and Väisänen (Citation1984). The winter prior to the 2018 breeding season was rather typical, with temperatures that did not stand out and with the mean temperature in Łódź near or below zero, but during the winter of 2020 the temperatures were much higher and remained widely and continuously above 0°C. In addition, the extreme drought that started in the summer of 2018 lasted through the breeding season 2020 (until late May of 2020, Pińskwar et al. Citation2020). However, during the breeding season, temperature indicators adjusted to individual brood phenology showed that the breeding period of great tits, including the pre-hatching and nestling raising stages, occurred under substantially cooler conditions in 2020 than in 2018. May 2020 in Poland was the coldest in 29 years (with a negative anomaly of −2.3°C (reference period 1981–2010, see Ustrnul et al. Citation2021). Interestingly, both contrasting years did not differ in the nesting phenology of great tits, with the phenology differing only between the study areas. In 2020, the adjusted temperatures (for 10 days before and post hatching and 20 days pooled, , ) were lower in the parkland study area than in the forest study area, and in 2018 it was colder in the forest study site than in the parkland study site. Great tits tended to lay/hatch earlier in the forest in 2018, while in 2020 they tended to lay/hatch earlier in the parkland (, ). Great tits tended to produce much smaller clutches, much fewer fledglings, and had much lower breeding success in 2020 than in 2018 (). Furthermore, clutch size, the number of fledglings and breeding success of great tits were lower in the urban parkland study area than in the forest study area in both studied years. What should be emphasized is the fact that, even when lower temperatures produced lower breeding success and the brood-adjusted temperatures remained higher in the forest compared to the parkland, the breeding success for the forest fell almost double than the parkland did (forest: 0.77 to 0.56; parkland: 0.59 to 0.49). This suggests that i) urban habitats have an impact in breeding success; ii) extreme events have a bigger toll in natural habitats.
We suggest that two main factors caused this large difference in the breeding parameters of great tits between 2018 and 2020. First, severe low temperature in spring 2020 could have synergistically negatively affected already malnourished nestlings. Secondly, long-term drought and warm winter 2019/2020 caused a large decrease in the amount of tit food and it should be emphasized that caterpillar abundance was catastrophically low in 2020 (all the authors, personal observations at both study areas). It is also important to emphasize that the clutch size (in the forest in 2020 mean 8.5 vs earlier lowest value in 2005 mean 9.2 and in the parkland in 2020 mean 7.3 vs earlier lowest value in 2009 mean 8.8) and the number of fledglings (in the forest in 2020 mean 5.8 vs earlier lowest value in 2005 mean 7.3 and in the parkland in 2020 mean 4.9 vs earlier lowest value in 8.8) in 2020 had the lowest values recorded in the history of our long-term study of great tits in both areas (in 2020 in the forest mean see Wawrzyniak et al. Citation2015, Citation2020).
Low ambient temperatures during the breeding season probably affected reproductive parameters of great tits at different levels. The cold and dry spring could limit the access to snail shells necessary for egg production (Perrins Citation1996; Rosin Citation2007; Bańbura et al. Citation2010, Citation2018). On the one hand, ambient temperature during incubation and nestling stages may significantly affect breeding performance directly (by e.g., physiological stress) and/or indirectly, by food shortage or other ecological conditions (Martin Citation1987; Nord and Giroud Citation2020). Low temperatures increase nestling energy demands and, at the same time, enforce females to brood nestlings in the cavity to about the time the nestlings establish endothermy (about 8–9 days old). During that time, the nestlings are especially vulnerable to suboptimal thermal conditions, which results in negative effects of longer absences of foraging females from nests (Mertens Citation1977; Perrins Citation1979; Bradbury et al. Citation2003). Low temperatures may also limit nestling condition, as colder nest microclimates require a higher investment in thermoregulation, at the expense of processes such as the development of the immune system or growth (Rodríguez & Barba Citation2016a, Citation2016b). In addition, the activity of arthropods tends to increase with higher temperatures (Kacelnik Citation1979; Szujecki Citation1987). Low temperatures during the nestling stage likely affect and reduce insect activity and thus the availability of food for nestlings, which may affect their survival and condition (Avery & Krebs Citation1984). We have previously shown in our long-term study on great tits (Wawrzyniak et al. Citation2020) that fledging success and breeding success were significantly positively correlated with mean daily temperatures in May. Recently, Marques-Santos and Dingemanse (Citation2020) have shown for great tits in Germany that lower temperatures during the nestling phase could negatively affect nestling mass and survival. In that study, nestlings between 6 and 9 days old were the most vulnerable to this form of variation in weather parameters. In addition, fledging success was higher whenever minimum temperatures were relatively high when the nestlings were 6–23 days old. Marques-Santos and Dingemanse (Citation2020) also suggested that weather conditions during crucial moments of nestling life may be important determinants of reproductive success. This could also mean that extreme climatic and weather events occurring within years could have serious consequences for reproductive traits and overall fitness in tits and many other species.
The course of reproduction of the great tit in spring 2020 was influenced by a mixture of factors that, indeed, also influenced the availability of food. As a result of warm winter and cool spring, adult birds preparing for breeding were probably exposed to nutritional deficiencies in terms of preferred insects (Nadolski et al. Citation2021). In general, authors who study the phenology of tit reproduction only analyze the conditions prevailing in the spring, in the pre-egg and egg-laying phases. This is justified but may not fully reflect the complex relationship between broadly understood weather conditions and herbivorous insects that are the main food for the nestlings – in other words, the problem is understudied. Food abundance, especially caterpillar abundance, may vary over the years, but may also depend on weather anomalies occurring in critical phases of the life cycle of insects (Visser et al. Citation2006; Salis et al. Citation2016; Nadolski et al. Citation2021). Winter weather conditions may be an important, yet unexplored factor. The dormancy, egg development, and diapause of both adult and larval insects depend on the temperature. Therefore, the temperature during the dormant period, which overlaps with the effect of prolonged drought, may cause premature development or awakening from diapause (Salis et al. Citation2016). This should result in increased mortality and very low insect/caterpillar abundance in spring that we observed.
Due to harsh weather and food conditions, in the 2020 breeding season of our study population of great tits, there was significant brood mortality in all phases, from eggs to fledgling. Under normal conditions, tit breeding success is negatively correlated with time, so that broods that start earlier are more successful than those that start later (Lack Citation1954; Perrins Citation1970; Glądalski et al. Citation2017; Wawrzyniak et al. Citation2020). However, in the specific conditions of spring 2020, when significant mortality occurred, breeding success increased over time during the season. This was due to the fact that from mid-May the temperature increased significantly and there was rainfall, which influenced vegetation and, consequently, increased the availability of insects (all the authors, personal observations). Therefore, breeding success increased over the course of the season, as measured by the date of hatching. In both environments, broods that achieved any success were on average 4 to 5 days later than broods with zero success.
In general, studies at multiple locations in Europe have shown that nest box populations of tit species in urban habitats produce smaller clutches and fewer fledglings than do birds in non-urban habitats (Solonen Citation2001; Seress and Liker Citation2015; Wawrzyniak et al. Citation2015; Glądalski et al. Citation2017; Seress et al. Citation2020, Citation2020). The main reason for that difference, which is usually suggested, is the variation in prey availability, habitat quality, and the habitat structure. Amininasab et al. (Citation2016) showed that the vegetation structure surrounding nest areas may be seen as a reliable index of prey availability for insectivorous birds. The habitat characteristics of our study areas confirm these assumptions because the mean total number of trees per nest box plot is 3 times greater in the forest than in the parkland (forest – 130.7 trees/nest box (with larger numbers of Quercus spp., and those tree species are the main source of prey abundance for insectivorous birds) and parkland - 43.5 trees/nest box, Glądalski et al. Citation2017). A few studies have simultaneously investigated the availability of natural food (abundance of specific prey items) and the breeding success of urban tits, and their results suggest a clear link between the scarcity of arthropods and the decreased breeding success in those birds (Seress et al. Citation2018). Seress et al. (Citation2018) have found that the biomass of leaf-eating caterpillars was approximately 8.5–24 times higher in their forest area than in urban study areas. Nadolski et al. (Citation2021) have shown on long-term data (16 years) that our study areas also differ in the abundance of caterpillars and the average peak mass of caterpillar frass fall has always been greater in the forest area than in the urban parkland area, but to a different degree in different years, from a factor of about 1.5 up to 6.5. Nadolski et al. (Citation2021) have also found that the annual average peak mass of caterpillar frass fall was a significant predictor of the annual mean number of fledgling tits per brood in both study areas. Our present study confirms all the results discussed.
In light of our results and other studies, we agree with the view that the phenotypic plasticity of the breeding parameters of the studied birds may be insufficient to adjust to the upcoming extreme weather events and further studies are needed. In general, we agree with Radchuk et al. (Citation2019) that long-term weather trends and extreme weather events could and should be treated as natural experiments. They may explain and in some cases give the rare opportunity to study events and processes that would be impossible or at least very difficult to explore in a different way (van de Pol et al. Citation2017; Glądalski et al. Citation2018). In light of a large amount of evidence collected on climate change and extreme weather events, the future weather will with high probability become more unpredictable and unstable (NAS Citation2016; Stott Citation2016; Otto et al. Citation2018; Trouet et al. Citation2018; NOAA Citation2021). As a consequence, we may have, whether we like it or not, a lot of opportunities to gaze and examine natural experiments of similar type on various organisms in the coming decades.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures using animals were authorized under permits from the University of Lodz.
Acknowledgements
We would like to thank E. Wróblewska, A. Jaksa, M. Winsche, and J. Białek for their help and consent in conducting research in the areas under their administration. We are very grateful to two anonymous referees for critical comments and constructive suggestions on the previous draft of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amininasab SM, Vedder O, Schut E, de Jong B, Magrath MJL, Korsten P, Komdeur J. 2016. Influence of fine scale habitat structure on nest-site occupancy, laying date and clutch size in blue tits Cyanistes caeruleus. Acta Oecologica 70:37–44. DOI:10.1016/j.actao.2015.11.006.
- Avery MI, Krebs JR. 1984. Temperature and foraging success of great tits Parus major hunting for spiders. Ibis 126:33–38. DOI: 10.1111/j.1474-919X.1984.tb03661.x.
- Bailey LD, van de Pol M. 2016. Tackling extremes: Challenges for ecological and evolutionary research on extreme climatic events. Journal of Animal Ecology 85:85–96. DOI:10.1111/1365-2656.12451.
- Bańbura M, Sulikowska-Drozd A, Kaliński A, Skwarska J, Wawrzyniak J, Kruk A, Zieliński P, Bańbura J. 2010. Egg size variation in blue tits Cyanistes caeruleus and great tits Parus major in relation to habitat differences in snail abundance. Acta Ornithologica 45:121–129. DOI:10.3161/000164510X551264.
- Bańbura M, Glądalski M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2018. A consistent long-lasting pattern of spatial variation in egg size and shape in blue tits (Cyanistes caeruleus). Frontiers in Zoology 15:1–10. DOI:10.1186/s12983-018-0279-4.
- Barras AG, Niffenegger CA, Candolfi I, Hunziker YA, Arlettaz R. 2021. Nestling diet and parental food provisioning in a declining mountain passerine reveal high sensitivity to climate change. Journal of Avian Biology 52:1–12. DOI: 10.1111/jav.02649.
- Bradbury RB, Wilson JD, Moorcraft D, Morris AJ, Perkins AJ. 2003. Habitat and weather are weak correlates of nestling condition and growth rates of four UK farmland passerines. Ibis 145:295–306. DOI:10.1046/j.1474-919X.2003.00142.x.
- Broggi J, Hohtola E, Koivula K, Rytkonen S, Nilsson JA. 2022. Prehatching temperatures drive inter- annual cohort differences in great tit metabolism. Oecologia 198:619–627. DOI:10.1007/s00442-022-05126-7.
- Bründl AC, Sallé L, Lejeune LA, Sorato E, Thiney AC, Chaine AS, Russell AF. 2020. Elevational gradients as a model for understanding associations among temperature, breeding phenology and success. Frontiers in Ecology and Evolution 8:563377. DOI:10.3389/fevo.2020.563377.
- Chamberlain DE, Cannon AR, Toms MP, Leech DI, Hatchwell BJ, Gaston KJ. 2009. Avian productivity in urban landscapes: A review and meta-analysis. Ibis 151:1–18. DOI:10.1111/j.1474-919X.2008.00899.x.
- Chamberlain D, Pearce-Higgins J. 2013. Impacts of climate change on upland birds: Complex interactions, compensatory mechanisms and the need for long-term data. Ibis 155:451–455. DOI:10.1111/ibi.12070.
- Charmantier A, Gienapp P. 2014. Climate change and timing of avian breeding and migration: Evolutionary versus plastic changes. Evolutionary Applications 7:15–28. DOI:10.1111/eva.12126.
- Chechłacz M. 1997. Rola czynników środowiskowych, neuroendokrynalnych i biochemicznych w regulacji diapauzy owadów. Kosmos 46:221–230 (in Polish, with English summary).
- Corregidor-Castro A, Jones OR. 2021. The effect of nest temperature on growth and survival in juvenile great tits Parus major. Ecology and Evolution 11:1–8. DOI:10.1002/ece3.7565.
- Cowie RJ, Hinsley SA. 1987. Breeding success of blue tits and great tits in suburban gardens. Ardea 75:81–90.
- Crawley MJ. 2002. Statistical computing: An introduction to data analysis using S-Plus. Chichester: Wiley.
- Cresswell W, McCleery R. 2003. How great tits maintain synchronization of their hatch date with food supply in response to long–term variability in temperature. Journal of Animal Ecology 72:356–366. DOI:10.1046/j.1365-2656.2003.00701.x.
- Diez-Méndez D, Sanz JJ, Barba E. 2021. Impacts of ambient temperature and clutch size on incubation behaviour onset in a female-only incubator songbird. Ibis 163:1056–1071. DOI:10.1111/ibi.12937.
- Eeva T, Veistola S, Lehikoinen E. 2000. Timing of breeding in subarctic passerines in relation to food availability. Canadian Journal of Zoology 78:67–78. DOI:10.1139/z99-182.
- Fox RJ, Donelson JM, Schunter C, Ravasi T, Gaitán-Espitia JD. 2019. Beyond buying time: The role of plasticity in phenotypic adaptation to rapid environmental change. Philosophical Transactions of the Royal Society B 374:20180174. DOI: 10.1098/rstb.2018.0174.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2014. Extreme weather event in spring 2013 delayed breeding time of great tit and blue tit. International Journal of Biometeorology 58:2169–2173. DOI: 10.1007/s00484-014-0816-6.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2016. Effects of extreme thermal conditions on plasticity in breeding phenology and double-bloodedness of great tits and blue tits in central Poland in 2013 and 2014. International Journal of Biometeorology 60:1795–1800. DOI:10.1007/s00484-016-1152-9.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Bańbura J. 2017. Differences in the breeding success of blue tits (Cyanistes caeruleus) between a forest and an urban area: Along-term study. Acta Ornithologica 52:59–68. DOI:10.3161/00016454AO2017.52.1.006.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2018. Hatching delays in great tits and blue tits in response to an extreme cold spell: A long-term study. International Journal of Biometeorology 62:1437–1445.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2020a. Extreme temperature drop alters hatching delay, reproductive success and physiological condition in great tits. International Journal of Biometeorology 64:623–629. DOI:10.1007/s00484-019-01851-6.
- Glądalski M, Mainwaring MC, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Bańbura J, Hartley IR. 2020b. Consequences of hatching deviations for breeding success: A long-term study on blue tits Cyanistes caeruleus. European Zoological Journal 87:385–394. DOI: 10.1080/24750263.2020.1787532.
- Glądalski M, Wolski GJ, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Bańbura J. 2021. Differences in use of bryophyte species in tit nests between two contrasting habitats: An urban park and a forest. European Zoological Journal 88:807–815. DOI: 10.1080/24750263.2021.1947397.
- Goodenough AE, Hart AG, Stafford R. 2010. Is adjustment of breeding phenology keeping pace with the need for change? Linking observed response in woodland birds to changes in temperature and selection pressure. Climate Change 102:687–697. DOI:10.1007/s10584-010-9932-4.
- Haggard WH. 1990. Urban weather. International Journal of Environmental Studies 36:73–82. DOI:10.1080/00207239008710584.
- Hari V, Rakovec O, Markonis Y, Hanel M, Kumar R. 2020. Increased future occurrences of the exceptional 2018–2019 Central European drought under global warming. Scientific Reports 10:12207. DOI: 10.1038/s41598-020-68872-9.
- Heck RH, Thomas SL, Tabata LN. 2012. Multilevel modelling of categorical outcomes using IBM SPSS. New York: Routledge.
- IBM SPSS Statistics. 22. 2013. SPSS for windows release 22.0. Armonk: IBM Corporation.
- Jaagus J, Aasa A, Aniskevich B, Bojariu B, Briede R, Danilovich A, Castro I, Dumitrescu FD, A LM et al. 2021. Long-term changes in drought indices in eastern and central Europe. International Journal of Climatology 1–25. DOI: 10.1002/joc.7241.
- Järvinen A, Väisänen RA. 1984. Reproduction of pied flycatchers (Ficedula hypoleuca) in good and bad breeding seasons in a northern marginal area. Auk 101:439–450. DOI:10.1093/auk/101.3.439.
- Kacelnik A. 1979. The foraging efficiency of great tits (Parus major) in relation to light intensity. Animal Behaviour 27:237–241. DOI:10.1016/0003-3472(79)90143-X.
- Kasprzak M, Salamon A. 2020. Dry Poland. https://uni.wroc.pl/en/dry-poland/. Accessed July 2021 14.
- Lack D. 1954. The natural regulation of animal numbers. Oxford: Oxford University Press.
- Lees AD. 1956. The physiology and biochemistry of diapause. Annual Review of Entomology 1:1–16. DOI:10.1146/annurev.en.01.010156.000245.
- Liu X, He B, Guo L, Huang L, Chen D. 2020. Similarities and differences in the mechanisms causing the European summer heatwaves in 2003, 2010, and 2018. Earth’s Future 7:e2019EF001386. DOI: 10.1029/2019EF001386.
- Mainwaring MC, Hartley IR. 2016. Local weather conditions have complex effects on the growth of blue tit nestlings. Journal of Thermal Biology 60:12–19. DOI:10.1016/j.jtherbio.2016.05.005.
- Marques-Santos F, Dingemanse NJ. 2020. Weather effects on nestling survival of great tits vary according to the developmental stage. Journal of Avian Biology 51:e02421. DOI: 10.1111/jav.02421.
- Marrot P, Garant D, Charmantier A. 2017. Multiple extreme climatic events strengthen selection for earlier breeding in a wild passerine. Philosophical Transactions of the Royal Society B 372:20160372. DOI: 10.1098/rstb.2016.0372.
- Martin TE. 1987. Food as a limit on breeding birds: A life-history perspective. Annual Review of Ecology, Evolution, and Systematics 18:453–487. DOI:10.1146/annurev.es.18.110187.002321.
- Martin TE. 2001. Abiotic vs. biotic influences on habitat selection of coexisting species: Climate change impacts? Ecology 82:175–188. DOI:10.1890/0012-9658(2001)082[0175:AVBIOH]2.0.CO;2.
- Massa B, Lo Valvo F, Margagliotta B, Lo Valvo M. 2004. Adaptive plasticity of blue tits (Parus caeruleus) and great tits (Parus major) breeding in natural and semi-natural insular habitats. Italian Journal of Zoology 71:209–217. DOI: 10.1080/11250000409356574.
- Matter SF, Roland J. 2017. Climate and extreme weather independently affect population growth, but neither is a consistently good predictor. Ecosphere 8:e01816. DOI:10.1002/ecs2.1816.
- Mertens JAL. 1977. Thermal conditions for successful breeding in great tits (Parus major L.). Oecologia 28:1–29. DOI: 10.1007/BF00346834.
- Monaghan P. 2007. Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B 363:1635–1645. DOI:10.1098/rstb.2007.0011.
- Nadolski J, Marciniak B, Loga B, Michalski M, Bańbura J. 2021. Long-term variation in the timing and height of annual peak abundance of caterpillars in tree canopies: Some effects on a breeding songbird. Ecological Indicators 121:107–120. DOI: 10.1016/j.ecolind.2020.107120.
- Nager RG, Van Noordwijk AJ. 1992. Energetic limitation in the egg-laying period of great tits. Philosophical Transactions of the Royal Society B 249:259–263.
- NAS. 2016. Attribution of extreme weather events in the context of climate change. Washington, DC: National Academies Press.
- Neven LG. 2000. Physiological responses of insects to heat. Postharvest Biology and Technology 21:103–111. DOI:10.1016/S0925-5214(00)00169-1.
- NOAA. 2021. NOAA National centers for environmental information (NCEI) U.S. Billion-dollar weather and climate disasters. Available online: https://www.ncdc.noaa.gov/billions/. Accessed July 2021 14.
- Nord A, Giroud S. 2020. Lifelong effects of thermal challenges during development in Birds and Mammals. Frontiers Physiology 11:419. DOI: 10.3389/fphys.2020.00419.
- Otto FEL, Philip S, Kew S, Li S, King A, Cullen H. 2018. Attributing high-impact extreme events across timescales—a case study of four different types of events. Climate Change 149:399–412. DOI: 10.1007/s10584-018-2258-3.
- Perrins CM. 1970. The timing of birds’ breeding seasons. Ibis 112:242–255. DOI:10.1111/j.1474-919X.1970.tb00096.x.
- Perrins CM. 1979. British Tits. Glasgow: Collins.
- Perrins CM, McCleery RH. 1989. Laying dates and clutch size in the great tit. Wilson Bulletin 101:236–253.
- Perrins CM. 1991. Tits and their caterpillar food supply. Ibis 133:49–54. DOI:10.1111/j.1474-919X.1991.tb07668.x.
- Perrins CM. 1996. Eggs, egg formation and the timing of breeding. Ibis 138:2–15. DOI:10.1111/j.1474-919X.1996.tb04308.x.
- Peterson NA, Nilssen AC. 1998. Late autumn eclosion in the winter moth Operophtera brumata: Compromise of selective forces in life-cycle timing. Ecological Entomology 23:417–426. DOI:10.1046/j.1365-2311.1998.00155.x.
- Pińskwar I, Choryński A, Kundzewicz ZW. 2020. Severe drought in the spring of 2020 in Poland - more of the same? Agronomy 10:1646. DOI: 10.3390/agronomy10111646.
- Pipoly I, Bókony V, Seress G, Szabó K, Liker A. 2013. Effects of extreme weather on reproductive success in a temperate-breeding songbird. PLoS One 8:e80033. DOI:10.1371/journal.pone.0080033.
- Podstawczyńska A. 2010. Temperatura powietrza i opady atmosferyczne w regionie łódzkim w ostatnim stuleciu. In: Twardy J, Żurek S, Forysiak J, editors. Torfowisko Żabieniec: Warunki naturalne i zapis zmian paleoekologicznych w jego osadach. Poznań: Bogucki Wydawnictwo Naukowe. pp. 63–74.
- Radchuk V, Reed T, Teplitsky R, van de Pol M, Charmantier A et al. 2019. Adaptive responses of animals to climate change are most likely insufficient. Nature Communications 10:3109. DOI: 10.1038/s41467-019-10924-4.
- Robb GN, McDonald RA, Chamberlain DE, Reynolds SJ, Harrison THE, Bearhop S. 2008. Winter feeding of birds increases productivity in the subsequent breeding season. Biology Letters 4:220–223. DOI:10.1098/rsbl.2007.0622.
- Robinet C, Roques A. 2010. Direct impacts of recent climate warming on insect populations. Integrative Zoology 5:132–142. DOI:10.1111/j.1749-4877.2010.00196.x.
- Rodríguez S, Barba E. 2016a. Effects of cool nest microclimates on nestling development: An experimental study with Mediterranean great tits Parus major. Ardeola 63:251–260. DOI: 10.13157/arla.63.2.2016.ra2.
- Rodríguez S, Barba E. 2016b. Nestling growth is impaired by heat stress: An experimental study in a Mediterranean great tit population. Zoology Studies 55:e40. DOI:10.6620/ZS.2016.55-40.
- Rosin ZM. 2007. Gospodarka wapniem u ptaków wróblowych w okresie lęgowym. Wiadomości Ekologiczne 53:55–87 in polish.
- Salinas S, Irvine SE, Schertzing CL, Golden SQ, Munch SB. 2019. Trait variation in extreme thermal environments under constant and fluctuating temperatures. Philosophical Transactions of the Royal Society B 374:20180177. DOI: 10.1098/rstb.2018.0177.
- Salis L, Lof M, van Asch M, Visser ME. 2016. Modeling winter moth Operophtera brumata egg phenology: Nonlinear effects of temperature and developmental rate. Oikos 125:1772–1781. DOI:10.1111/oik.03257.
- Schöll E, M Hille SM. 2020. Heavy and persistent rainfall leads to brood reduction and nest failure in a passerine bird. Journal of Avian Biology 51:e02418. DOI:10.1111/jav.02418.
- Seress G, Liker A. 2015. Habitat urbanization and its effects on birds. Acta Zoologica Academiae Scientiarum Hungaricae 61:373–408. DOI:10.17109/AZH.61.4.373.2015.
- Seress G, Hammer T, Bókony V, Vincze E, Preiszner B, Pipoly I, Sinkovics E, Evans KL, Liker A. 2018. Impact of urbanization on abundance and phenology of caterpillars and consequences for breeding in an insectivorous bird. Ecological Applications 28:1143–1156. DOI:10.1002/eap.1730.
- Seress G, Sándor K, Evans KL, Liker A. 2020. Food availability limits avian reproduction in the city: An experimental study on great tits Parus major. Journal of Animal Ecology 89:1570–1580. DOI:10.1111/1365-2656.13211.
- Shilov IA. 1973. Heat regulation in Birds. New Delhi: Amerind Publishing Corporation.
- Sinclair BJ. 2015. Linking energetics and overwintering in temperate insects. Journal of Thermal Biology 54:5–11. DOI:10.1016/j.jtherbio.2014.07.007.
- Skagen SK, and Adams AA. 2012. Weather effects on avian breeding performance and implications of climate change. Ecological Applications 22:1131–1145. DOI:10.1890/11-0291.1.
- Skwarska J, Podstawczyńska A, Bańbura M, Glądalski M, Kaliński A, Markowski M, Wawrzyniak J, Zieliński P, Bańbura J. 2022. Effects of ambient temperature during the nestling stage on a stress indicator in nestling pied flycatchers Ficedula hypoleuca. International Journal of Biometeorology 66:139–148. DOI:10.1007/s00484-021-02199-6.
- Solonen T. 2001. Breeding of the great tit and blue tit in urban and rural habitats in southern Finland. Ornis Fennica 78:49–60.
- Stott P. 2016. How climate change affects extreme weather events. Science 352:1517–1518. DOI:10.1126/science.aaf7271.
- Szujecki A. 1987. Ecology of forest insects. Dordrecht: Dr W. Junk Publishers.
- Tomás G. 2015. Hatching date vs laying date: What should we look at to study avian optimal timing of reproduction? Journal of Avian Biology 46:107–112. DOI:10.1111/jav.00499.
- Trouet V, Babst F, Meko M. 2018. Recent enhanced high-summer North Atlantic Jet variability emerges from three-century context. Nature Communications 9:180. DOI:10.1038/s41467-017-02699-3.
- Tylkowski J. 2017. Extreme weather events in Poland in the 21st century. Journal of Education, Health and Sport 7:40–51. DOI:10.5281/zenodo.1000972.
- Ustrnul Z, Wypych A, Jakusik E, Biernacik D, Czekierda D, Chodubska A 2021. Klimat Polski 2020. Instytut Meteorologii i Gospodarki Wodnej, Państwowy Instytut Badawczy (in Polish).
- van Asch M, Visser ME. 2007. Phenology of forest caterpillars and their host trees: The importance of synchrony. Annual Review of Entomology 52:37–55. DOI:10.1146/annurev.ento.52.110405.091418.
- van Asch M, Salis L, Holleman LJM, van Lith B, Visser ME. 2013. Evolutionary response of the egg hatching date of a herbivorous insect under climate change. Nature Climate Change 3:244–248. DOI:10.1038/nclimate1717.
- van de Pol M, Jenouvrier S, Cornelinnen JHC, and Visser ME 2017. Behavioural, ecological and evolutionary responses to extreme climatic events: Challenges and directions. Proceedings of the Royal Society B 372:20160134. DOI:10.1098/rstb.2016.0134.
- Visser ME, van Noordwijk Aj, Tinbergen JM, and Lessells CM 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proceedings of the Royal Society B 265:1867–1870. DOI:10.1098/rspb.1998.0514.
- Visser ME, Holleman LJM, Gienapp P. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147:167–172. DOI:10.1007/s00442-005-0299-6.
- Wawrzyniak J, Kaliński A, Glądalski M, Bańbura M, Markowski M, Skwarska J, Zieliński P, Cyżewska I, Bańbura J. 2015. Long-term variation in laying date and clutch size of the great tit Parus major in Central Poland: A comparison between urban parkland and deciduous forest. Ardeola 62:311–322. DOI:10.13157/arla.62.2.2015.311.
- Wawrzyniak J, Glądalski M, Kaliński A, Bańbura M, Markowski M, Skwarska J, Zieliński P, Bańbura J. 2020. Differences in the breeding performance of great tits Parus major between a forest and an urban area: A long term study on first clutches. European Zoological Journal 87:294–309. DOI: 10.1080/24750263.2020.1766125.
- Whitehouse MJ, Harrison NM, Mackenzie J, Hinsley SA. 2013. Preferred habitat of breeding birds may be compromised by climate change: Unexpected effects of an exceptionally cold, wet spring. PLoS One 8:e75536. DOI:10.1371/journal.pone.0075536.
- Wingfield JC, Pérez JH, Krause JS, Word KR, González-Gómez PL, Lisovski S, Chmura HE. 2017. How birds cope physiologically and behaviourally with extreme climatic events. Philosophical Transactions of the Royal Society B 372:20160140. DOI:10.1098/rstb.2016.0140.
