Abstract
Water is crucial for birds, especially during hot weather. However, the availability of water, and its use by birds in modern anthropogenic habitats, is far from understood, especially outside arid regions. Here, we analyze a large nationwide dataset collected in the temperate zone and present an overview of small water resources used by birds in urban and rural habitats in Poland. We investigated the proportion of birds using free-standing water, preferences for various water sources, and factors and threats influencing drinking and bathing behaviour. Birds using water resources are represented by various taxonomic and ecological groups. Species composition differed slightly due to environmental conditions in the vicinity of the water resource and the background species composition. In total 51 species were observed using water, representing 64% of the 80 species recorded in the vicinity. The probability of water usage was positively related to temperature, which further emphasizes the importance of water under future climate-warming scenarios. We show that small water resources, including those provided by people, were less likely to be used by birds than resources resembling natural waters (puddles, ponds, fountains). This novel finding may have particular importance for avian conservation planning, including appropriate behaviour for nature lovers (providing water sources and reducing stress to birds due to predation risk). Finally, we assessed potential threats to bathing and drinking birds, such as moving cars, risk of drowning, and the presence of predators. Any kind of surface water is currently beneficial for wild birds inhabiting human modified landscapes. During heatwaves and droughts access to water can be crucial for many birds. Unfortunately, such extreme events are predicted to become more frequent and more severe under climate change. Therefore, we would encourage further research in the use by birds of free-standing water, similar to the many studies of birdfeeders in winter, and to consider the maintenance of diverse sources of accessible water in environmental management.
1. Introduction
Factors limiting the presence and numbers of birds are the interactions between intrinsic, species-specific attributes, such as behaviour and demography, and external environmental influences, such as competition, predation, food and water supply (Newton Citation1998; Bicudo et al. Citation2021). Food limitation is particularly often discussed because it can influence life history traits, population sizes, and community structure (Martin Citation1987). Recent studies, both observational and experimental, show that access to artificially provided food in bird-feeders can change bird behaviour such as escape distance, level of neophobia, avoiding dangerous places, but most of all it can change the structure of local bird assemblages (e.g. Galbraith et al. Citation2015; Goławski et al. Citation2015, Citation2019; Møller et al. Citation2015; Tryjanowski et al. Citation2015a, Citation2015b, Citation2016).
In contrast, access to water can also be a limiting factor, especially in hot summers. However, very little is known about the influence of watering places on animals, except the situation in deserts or other dry environments (Webster & Weathers Citation2000; Bicudo et al. Citation2021). Water sources seem to play an important role in the life of birds and all other wildlife, nevertheless only a few studies have been published on birds and also on arboreal folivores, bats and some other animals, (e.g. Okahisa et al. Citation2015; Ancillotto et al. Citation2019; Mella et al. Citation2019; Nystrom & Bennett Citation2019). On the other hand, it has been reported that more birds can be attracted where both watering and feeding are provided (Miller et al. Citation2015; Pustkowiak et al. Citation2021), and the phenomenon has been known for more than a century (Woods Citation1911), but surprisingly has not been studied in detail.
Birds usually need water for two major reasons, that is, for drinking and bathing, and each of those activities are of great importance in the life of birds and also to keep feathers in good condition (e.g. Brilot & Bateson Citation2012; Okahisa et al. Citation2015). Water is very important for birds because of their high metabolic rate and it plays a key role in avian thermoregulation, especially during hot weather and in the driest conditions when the need for drinking increases (Abdu et al. Citation2018a; Smit et al. Citation2019).
This is more obvious in deserts where access to water is limited. Arid-zone birds are very sensitive to increasing air temperatures and heatwaves, which could broadly influence their water balance, daily activity, or geographic distribution (Albright et al. Citation2017). Birds from deserts show several adaptations to inhabiting such habitats (Dean & Williams Citation2004), and also have reduced basal and field metabolic rates compared with species from mesic areas (Tieleman & Williams Citation2000). In such areas, free-standing water is especially important during hot days (Smit et al. Citation2019).
It is expected that access to water for animals might be particularly important in the face of heatwaves and progressive global warming (e.g. Houghton Citation2005; Albright et al. Citation2017; Pille & Säumel Citation2021). The increase of temperature influences water loss and raises demand immediately (Qiu et al. Citation2019), therefore birds need to expend more energy and time to obtain water. It seems that the most accessible sources, especially to birds living far from rivers and lakes, may be ubiquitous puddles after rain that persist for some time on concrete or asphalt, water dripping from gutters, a fountain in a city park, or a deliberately provided container of water. Such water resources could also include ponds which also contribute to local biodiversity (Hassall Citation2014; Pille & Säumel Citation2021; Pustkowiak et al. Citation2021).
Here, we studied the influence of water sources on birds in summer, the warmest time of the year in temperate Europe, when birds especially need water (McKechnie & Wolf Citation2010). We believe that water influences species composition and numbers of birds in both urban and rural habits, but we also expect some differences in both of these areas in relation to the accessibility of the water sources and the background biodiversity of avifauna. Therefore, in the current study, we wanted to identify which bird species used small water sources in summer, and which water sources were available for birds. Furthermore, we hypothesised that the types of water source may also play an important role in relation to the number of birds and to species diversity (Tu et al. Citation2020; Pustkowiak et al. Citation2021).
Finally, we examined which factors could affect the use of the watering places, for example, the distance to the nearest rivers and waterbodies, and temperature. During observations of bathing and drinking birds we tried to identify potential threats to birds, with reference to bird-feeders whose use can be modified by factors, such as predator presence (Tvardíková & Fuchs Citation2011; Tryjanowski et al. Citation2016) or faeces of other birds and microbiological contamination (Cleary et al. Citation2016; Tryjanowski et al. Citation2020; Schaper et al. Citation2021).
2. Material and methods
2.1. Study sites
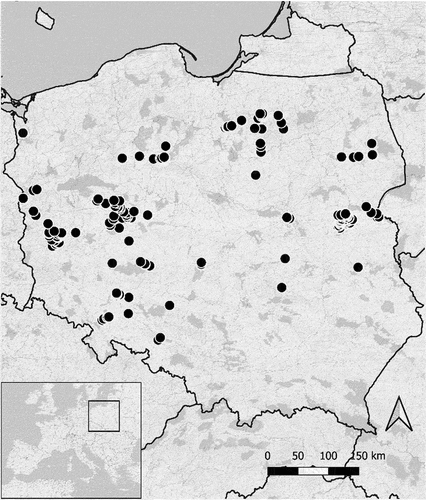
Data were collected at small water resources located across Poland (). A total of 928 such sites were visited in summer (early-June – mid-September) 2019, with the average value (± SD) for a day of observation, estimated 24 July 2019 ± 15 (min 01 July 2019, max 04 September 2019).
2.2. Data collection
We undertook two types of counts: a background census, and records of birds, which used the water resource. Firstly, the observer searched for water resources and when found recorded the birds using the water resource (examples in ), and all birds in the background during 5-min counts. During the counts, we collected the following environmental parameters: date, hour, temperature, distance to nearest water body or river. Additionally, at 506 randomly selected water resources (rural: n = 236; urban: n = 270) we tried to identify visible, potential threats to drinking and bathing birds.
Figure 2. Examples of water sources used by various birds during summer in Poland. 1 – blue tit Cyanistes caeruleus; 2 – hooded crow Corvus cornix and feral pigeon Columba livia f. domestica; 3 – jackdaw Corvus monedula; 4 – magpie Pica pica; 5 – green woodpecker Picus viridis; 6 – starling Sturnus vulgaris, tree sparrow Passer montanus and house sparrow Passer domesticus; 7 – blackbird Turdus merula; 8 – white stork Ciconia ciconia. Photographs by: P. Czechowski (1), B. Dulisz (8), A. Goławski (2, 6), P. Indykiewicz (4), P. Tryjanowski (7), A. Wuczyński (3, 5).

Threats were then divided into seven categories: 1. presence of domestic cats Felis catus; 2. presence of a dog Canis familiaris; 3. presence of sparrowhawk Accipiter nisus - a predator frequently spotted in urban areas; 4. high drowning probability due to abrupt edges around the water; 5. fast moving cars; 6. visible chemical contamination, and an additional category – 7. water places where no threats were identified.
2.3. Environmental variables
The environment within 1,000 m radius of each water resource was obtained using Quantum GIS software version 3.14.16 on Corine land cover 2018 data. The variables were computed as the proportion of the total area in the following categories: arable (excluding pasture), pasture, amenity grass (all green areas in urban areas), forests (forest and semi-natural areas), industrial (industrial, commercial, transport, mines, construction sites), urban continuous (continuous urban fabric), urban discontinuous (discontinuous urban fabric) and water bodies presence (yes or no). During the survey, we also categorized the habitat as rural or urban.
We distinguished three different types of water sources. The first category was water containers and we included in this category: buckets, taps, cups, pots, different kinds of bowls (plastic, metal), water dripping from fire hydrants, plastic containers, and water bowls for birds or dogs. The second category was of long-lasting water sources including fountains, garden ponds, fire ponds, small pools, and barrels. The third category was the short-lasting water source of puddles.
2.4. Species presence/absence
For the analysis of species presence/absence (using only those species that were present in more than 19 sites) in relation to environmental variables and around water sources we used Canonical Correspondence Analysis (CCA) in the “vegan” package (Oksanen Citation2011) in R software (R Development Core Team Citation2018). We selected the final CCA model by forward stepwise model building using permutation tests (F-statistics with Monte Carlo Permutation Tests based on 20,000 replicates).
The significance of axes was performed by testing separately each constrained axis (all previous constrained axes were used as conditions). We tested each environmental variable by an F-statistic with a Monte Carlo Permutation Test based on 20,000 replicates as separate significance tests for each marginal term in a model containing all other terms.
2.5. Proportion of birds using water sources
In this analysis, we reduced the number of Corine land cover environmental variables by using Principal Components Analysis (PCA). The first principal component (PC1; explained 29% of variance) was positively correlated with the arable variable (r = 0.79, p < 0.001) and negatively with the urban discontinuous (r = −0.81, p < 0.001) and urban continuous categories (r = −0.42, p < 0.001). Thus, the first principal component is a good predictor of the gradient from urban to rural areas (hereafter PC1 will be called urban-rural gradient). To analyse the proportion of the number of individuals to the total number of birds of that species (background birds plus birds by the water sources) in relation to features of the water sources we used generalised linear models with a binomial error distribution (lme4 package in R (Bates Citation2010)).
Because different species differed in their affinity to the water sources, we controlled this variability by adding species as a random effect. We also added the site of the water source as a random effect because many different species visited one water source or were present in the background. Lastly, we added observer as a random effect because different observers were used in different regions in Poland.
As explanatory variables we took continuous variables (hour, day of year, distance to other water, temperature, urban-rural gradient) and categorical variables (type of water source: water container, long-lasting, short-lasting), presence/absence of other water bodies). Before finally including the continuous variables we checked for and found no issue with multicollinearity (for all variables Variation Inflation Factor [VIF] < 1.2).
To test the significance of the effect, we used a chi-square test comparing the full model to a reduced one where the target variable has been dropped (using drop1() function in R software). We made multiple comparisons using Tukey’s post-hoc test in the package emmeans (Lenth Citation2020).
2.6. Threat to birds
The relationship between the type of habitat (urban/rural) and the type of threat to birds (seven categories, including that of no visible threats to birds) and the occurrence of species (56 species) was carried out using Correspondence Analysis in the Statistica 13 package (TIBCO Software Inc Citation2017).
3. Results
The mean (±SD) temperature during observation was 22°C ± 4.3 (min 11°C, max 34°C). The mean (±SD) observation time was 1:00 PM ± 3:28 (min 5:06 AM, max 9:15 PM).
In total, we observed birds at 928 locations; 507 water containers, 196 long-lasting and 225 short-lasting water sources.
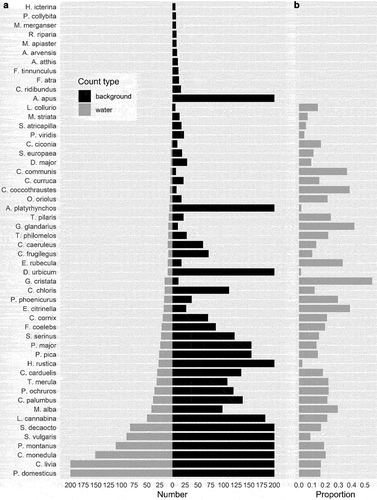
A total of 2,335 birds were seen using the water sources for drinking and/or bathing and 13,932 birds in the background. A total of 51 species were observed using the water sources ( and Table S1). The most numerous species were house sparrow Passer domesticus, feral pigeon Columba livia, jackdaw Corvus monedula, tree sparrow Passer montanus, starling Sturnus vulgaris, and collared dove Streptopelia decaocto ( and Table S1).
Figure 3. Total number of individuals counted using water (grey) and in the background (black) (a). For clarity, the horizontal axis is truncated at 200 and species with less than 6 individuals omitted; for exact numbers of individuals see Table S1 in the supplementary materials. Panel (b) shows the proportion of individuals using water sources to the total number of individuals (background plus birds by water source) of a given species.
3.1. Model of species presence/absence
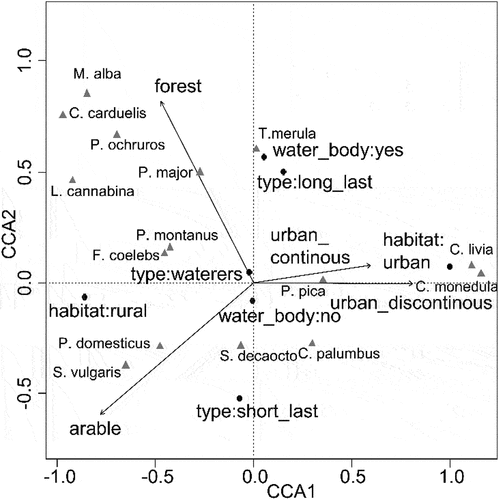
The final model of bird presence at water sources included seven significant (all p < 0.007) explanatory variables: habitat (urban or rural), forest, arable, type of water source (type), urban continuous, urban discontinuous and water body presence (water). Some variables were excluded: pasture, amenity grass and industrial, because non-significant influence on studied system (all p > 0.05). All canonical variables explained 7.5% of the total variance (first axis 5.0% and second 1.0%).
Permutation tests showed that both axes were significant (p < 0.001) and the ordination diagram and the first axis indicates the gradient from rural to urban areas (). The second axis shows the gradient from the short-lasting water sources which were often found in arable areas to long-lasting water sources which were found more often in areas where more trees were present.
3.2. Proportion of birds using water sources
In the model of the proportion of birds in the vicinity that used small local water bodies, the following variables were not significant: distance to water body (LRT = 1.371, df = 1, p = 0.242), hour (LRT = 0.005, df = 1, p = 0.942), day of year (LRT = 2.644, df = 1, p = 0.104), water body presence (LRT = 1.008, df = 1, p = 0.315), urban-rural gradient (LRT = 0.333, df = 1, p = 0.564). We also checked the quadratic effects of distance to water body, hour, day of year, and temperature and none were significant (respective p values: 0.063, 0.488, 0.081, 0.879). Our analysis revealed a significant linear effect of temperature (LRT = 4.239, df = 1, p = 0.040) – during warmer summer days the use of water sources was higher (β = 0.027 ± 0.013; parameters are tested using z-statistics).
Moreover, the type of water source was significant (LRT = 40.122, df = 2, p < 0.001). The predicted proportion of the birds using water containers was 0.038 ± 0.009 and was significantly lower than the proportion in short-lasting (0.060 ± 0.015) and in long-lasting water sources (0.058 ± 0.014) (Tukey post-hoc test: p values: 0.001, 0.003 respectively). There was no significant difference between short- and long-lasting water sources (p = 0.954). Estimated parameters are presented in .
Table I. The estimated parameters of environmental variables on the probability (proportion) of birds using water sources. Please note that the model parameters were tested by z-statistics.
3.3. Threats
The presence of fast moving cars was the most common threat to birds using water sources (n = 93), because many water sources used by birds were simply temporary puddles in roads. Birds were also threatened by the presence of cats (n = 87), potential drowning (n = 64), presence of a dog (n = 40), a sparrowhawk (n = 20) and finally by visible chemical contamination (n = 14). In 188 cases (37.1%), we did not identify any threats to bathing and/or drinking birds.
The Canonical Correspondence Analysis ordination () clearly separates the urban environment (Axis 1: positive coefficients) from the rural environment (Axis 1: negative coefficients). The ordination indicates more frequent observation of species using the water resources in urban habitats (e.g. rook Corvus frugilegus, great tit Parus major, feral pigeon Columba livia f. urbana, hooded crow Corvus cornix) and in rural areas (e.g. barn swallow Hirundo rustica, pied wagtail Motacilla alba, goldfinch Carduelis carduelis, linnet Linaria cannabina, Eurasian jay Garrulus glandarius, white stork Ciconia ciconia). Axis 1 is also partly related to the types of threat, which differ especially in the urban environment (). On the other hand, Axis 2 is associated with the type of threat, which clearly separates the presence of the sparrowhawk from the other threats in the urban environment (). A closely located point to this is the position of hooded crow, which is sufficiently large not to be threatened by this bird of prey. In the rural environment, the CCA ordination separates the no threat category, which is associated with the presence of species with a low synanthropy level (e.g. white stork, Eurasian jay, hoopoe Upupa epops, reed bunting Emberiza schoeniclus, red crossbill Loxia curvirostra).
Figure 5. Canonical correspondence analysis ordination diagram of the bird species in relation to habitat (urban = u/rural = r), and threats. The codes for species names are shown in Table S1.
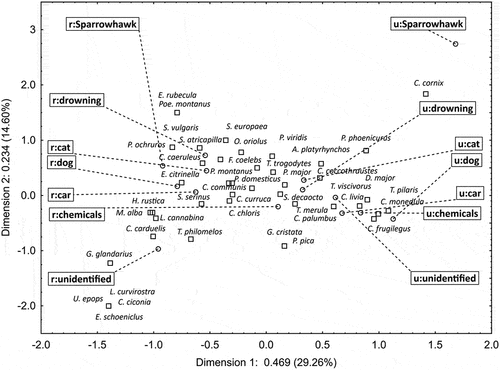
The total inertia for the tested model was 1.699 (χ2 = 866.49; df = 585; p = 0.0001). For our interpretation, two axes were used, of which axis 1 explained 29.26% of the inertia (eigenvalue = 0.469) and axis 2–14.60% (eigenvalue = 0.234).
4. Discussion
Using a large dataset collected at a national scale, we have presented, for the first time, an overview of small water sources used by birds in human modified landscapes during the summer in the northern temperate zone. We investigated the proportion of birds using free-standing water in rural and urban habitats, the types of water sources preferably used by birds, the environmental factors influencing this behaviour, and the threats to birds associated with the use of water sources. With such a broad approach we aimed to provide both baseline information on this behaviour, but also to highlight implications seen from a broader biological and conservation perspective.
However, before discussing the results in more detail it is worth stating the potential limitations of the study. Firstly, our observations were restricted to the summer season (mid June – mid September), whereas water resources could be more critical earlier in the year when feeding young (higher energy expenditures). In temperate Europe, air temperatures can also be very high in May, and a different picture could emerge (more species) if observations were made during this period. Moreover, most observations were made during the early afternoon, when air temperatures are highest, but this does not mean the use of the water resources would peak at this time since birds tend to restrict their activity at this time of the day (particularly outside the breeding season). We may even expect most intensive use of water sources in the early hours, particularly in summer (see Fisher et al. Citation1972; Lee et al. Citation2017). Five minute counts, although widely used for bird abundance and presence, especially in cities (Heezik & Seddon Citation2017) has some limitations, because there is a tendency to detect the most common species or, in our particular case, those which use water resources more frequently. Birds were also not individually marked, so we cannot determine how many individuals of a species used particular water resources. On the other hand, we included data from a huge number of water resources located across the entire country to avoid potential pseudoreplication.
Therefore, we recommend that future studies increase the focus on the detection of usage patterns and highlight the role of water sources, especially for breeding individuals.
Our data confirm that most birds regularly need to drink water, even if this behaviour can rarely be observed in many species. As much as 64% of the 80 bird species from the study areas were observed using the water sources. As a result, the species composition included birds from various taxonomic and ecological groups. Common species and birds habituated to human settlements had the largest frequency but there were also forest species (robin, Eurasian jay, golden oriole, red crossbill), farmland birds (yellowhammer Emberiza citrinella, whitethroat Curruca communis, hoopoe), water birds (mallard Anas platyrhynchos, herring gull Larus argentatus), birds eating moist food (thrushes, corvids), and granivores relying on a relatively dry diet (finches, sparrows, pigeons). It was noticeable that water birds occurred infrequently, but this may be due to access to water in their natural aquatic habitats. Surprisingly, the species with the highest proportion at water (0.56) was crested lark Galerida cristata. This is a species adapted to extremely dry, desert-steppe environments (Tieleman et al. Citation1999, Citation2003).
Temperature was the only environmental factor significantly related to the probability of water source use by birds. The relationship between ambient temperature and water use has long been addressed in ornithological studies (Temple Citation2001; Lee et al. Citation2017). However, most data come from arid zones, where temperature poses a substantial threat to birds (Williams & Tieleman Citation2001; Albright et al. Citation2017). Our data show that even in temperate areas the temperature is also of primary importance for the water balance, daily activity, and survival of birds. This is especially true in the hot and dry season, for small-bodied species particularly vulnerable to dehydration (Smit et al. Citation2016), and for young birds due to their low thermoregulatory efficiency (Sturkie Citation1965). Indeed, in our data the majority were small-bodied species, so these groups were likely to affect the overall results. In any case, our findings suggest that the projected increases in the severity, duration, and frequency of heatwaves (Guerreiro et al. Citation2018), which in relation to global warming are already not uncommon in temperate areas, are likely to intensify the challenges for birds (Smit et al. Citation2016).
A most interesting and novel outcome of our study are the birds’ preferences for the type of water source. Small sources, including water containers intentionally provided by people for wild birds and domestic animals, were less likely to be chosen than other sources, such as puddles or backyard ponds and fountains, resembling natural waters. This result looks intuitive but has not been empirically confirmed before. A straightforward explanation for the less willing use of small water sources is simply their smaller size and amount of water. However, there are other size-related features, such as potentially warmer and dirtier water, both likely to affect birds’ behaviour (cf. Davies Citation1982; Shellenbarger et al. Citation2008). In addition, intentional water containers may be associated with a greater risk of predation. Our observations show that it is not uncommon, especially in cities, to provide the water containers and feeders for cats and dogs in proximity (see ), which can cause obvious stress to the birds. There are indications that predation may have a greater impact on bird behaviour than environmental conditions, such as temperature. For example, predation risk has been attributed to highly synchronized drinking patterns of double-banded sandgrouse Pterocles bicinctus (Berry et al. Citation2001). Similarly, Molokwu et al. (Citation2010) suggested that the risk of predation had a stronger influence on foraging bird behaviours than high temperatures within a west African Savannah. In our data, the importance of predation risk is expressed by the remote position of the sparrowhawk urban threat in the ordination space (). The only species with a high probability of using the water sources in urban habitats despite the sparrowhawk presence was the hooded crow, that is, a species – due to its large size – not threatened by this bird of prey.
The less willing use of water containers does not diminish their importance for birds. Our observations indicate that they too were regularly used by birds both in rural and urban habitats, particularly as they are the most common source of surface water for birds in anthropogenic environments. This is especially true in urban habitats where free-standing water is generally scarce, and the large sources are limited to city centres (fountains) or are only short-lived (puddles after rain). This means that many small birds reliant on a dry diet, can be dependent on water provided by humans during the hot season (Krausman et al. Citation2006; Cleary et al. Citation2016; Abdu et al. Citation2018a, Citation2018b).
Modern anthropogenic habitats influence a high avian diversity (Lepczyk et al. Citation2008; Matuoka et al. Citation2020). However, during hot periods water availability and dehydration risk may be an important determinant of individual behaviour, species distribution and community composition. We now show this is also true in the European temperate zone. Our findings supplement a remarkably small literature on this topic from temperate regions (e.g. Williams & Koenig Citation1980)). Consequently, we recommend further research on the use of free-standing water by birds in other-than-arid regions, especially under future climate-warming scenarios (McKechnie et al. Citation2012, Citation2021; Dilling et al. Citation2019).
Our findings provide a clear message for environmental management and conservation. Admittedly, providing water has been a contentious and highly debated issue (Smit et al. Citation2019; Pille & Säumel Citation2021), similar to the debate on additional bird feeding. However, it is and probably will continue to be widely practiced, therefore it is appropriate to consider some recommendations. Our data indicate that any kind of surface water is beneficial for wild birds, especially in hot weather and with limited water availability. In addition to offering water to birds, shaded sites should also be promoted (Abdu et al. Citation2018b; McKechnie et al. Citation2021), as well as locating water containers where predation risk is reduced, for example, far from domestic pets. It should also be considered whether to maintain diverse sources of accessible water in standard administrative conservation planning. At present, the issue of water supply is almost invariably ignored and, paradoxically, is implemented only by some nature-loving inhabitants of towns and villages.
Authors’ contributions
Design and methodology: PT.
Data collection: PT, ŁJ, PCz, BD, AG, PI, ZK, CM, JN, MP, JS, AW.
Data analysis: PT, ŁJ, TS.
Writing original draft: PT, JN, AW, ŁJ, CM, ZK, TS.
Review and editing: all authors.
Compliance with ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Ethics approval consent to participate
All the fieldwork was done according to the ethical standards recommended by those institutions.
Geolocation information
Zachodniopomorskie, Szczecin, Kujawsko-pomorskie, Bydgoszcz, Warmińsko-Mazurskie, Olsztyn, Lubuskie, Zielona Góra, Wielkopolskie, Poznań, Podlaskie, Białystok, Mazowieckie, Siedlce, Dolnośląskie, Wrocław, Opolskie, Żywocice, Lubelskie, Lublin, Małopolskie, Kraków, Poland, Central Europe
Supplemental Material
Download MS Word (40 KB)Acknowledgements
We would like to thank owners of gardens and yards for allowing us to undertake research on their properties. We are also grateful to G. Krogulec, M. Tracz and M. Broniszewska for their help in fieldwork. Two anonymous referees provided very useful comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data analyses in this study are available from the corresponding author upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2022.2101699
Additional information
Funding
References
- Abdu S, Lee ATK, Cunningham SJ. 2018a. The presence of artificial water points structures an arid-zone avian community over small spatial scales. Ostrich 89:339–346. DOI: 10.2989/00306525.2018.1509904.
- Abdu S, McKechnie AE, Lee ATK, Cunningham SJ. 2018b. Can providing shade at water points help Kalahari birds beat the heat? Journal of Arid Environments 152:21–27. DOI: 10.1016/j.jaridenv.2018.01.018.
- Albright TP, Mutiibwa D, Gerson AR, Smith EK, Talbot WA, O’Neill JJ, McKechnie AE, Wolf BO. 2017. Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proceedings of the National Academy of Sciences of the United States of America 114(9):2283–2288. DOI: 10.1073/pnas.1613625114.
- Ancillotto L, Bosso L, Salinas-Ramos VB, Russo D. 2019. The importance of ponds for the conservation of bats in urban landscapes. Landscape and Urban Planning 190:103607. DOI: 10.1016/j.landurbplan.2019.103607.
- Bates D. 2010. lme4: Mixed-effects modeling with R. New York: Springer. Prepublication version. Available: http://lme4.r-forge.r-project.org/book/
- Berry HH, Fox VE, Berry PE. 2001. Synchrony of drinking in double-banded sandgrouse, Pterocles bicinctus, at Etosha National Park, Namibia. Ostrich 72(1–2):109–113. DOI: 10.2989/00306520109485294.
- Bicudo JEPW, Buttemer WA, Chapell MA, Pearson JT, Bech C. 2021. Ecological and environmental physiology of birds. Oxford, UK: Oxford University Press.
- Brilot BO, Bateson M. 2012. Water bathing alters threat perception in starlings. Biology Letters 8(3):379–381. DOI: 10.1098/rsbl.2011.1200.
- Cleary GP, Coleman BR, Davis A, Jones DN, Miller KK, Parsons H. 2016. Keeping it clean: Bird bath hygiene in urban and rural areas. Journal of Urban Ecology 2(1):1–4. DOI: 10.1093/jue/juw005.
- Davies SJJF. 1982. Behavioural adaptations of birds to environments where evaporation is high and water is in short supply. Comparative Biochemistry and Physiology 71(4):557–566. DOI: 10.1016/0300-9629(82)90204-3.
- Dean WRJ, Williams JB. 2004. Adaptations of birds for life in deserts with particular reference to Larks (ALAUDIDAE). Transactions of the Royal Society of South Africa 59(2):79–91. DOI: :10.1080/00359190409519166.
- Dilling L, Daly ME, Kenney DA, Klein R, Miller K, Ray AJ, Travis WR, Wilhelmi O. 2019. Drought in urban water systems: Learning lessons for climate adaptive capacity. Climate Risk Management 23:32–42. DOI: :10.1016/j.crm.2018.11.001.
- Fisher CD, Lindgren E, Dawson WR. 1972. Drinking patterns and behavior of Australian desert birds in relation to their ecology and abundance. Condor 74(2):111–136. DOI: :10.2307/1366276.
- Galbraith JA, Beggs JR, Jones DN, Stanley MC. 2015. Supplementary feeding restructures urban bird communities. Proceedings of the National Academy of Sciences of the United States of America 112(20):E2648–E2657. DOI: :10.1073/pnas.1501489112.
- Goławski A, Polakowski M, Filimowski P, Stępniewska K, Stępniewski K, Kiljan G, Kilon D. 2015. Factors influencing the fat load variation in three wintering bird species under stable food access conditions. Journal of Ethology 33(3):205–211. DOI: :10.1007/s10164-015-0433-9.
- Goławski A, Polakowski M, Filimowski P, Stępniewski K, Stępniewska K, Kiljan G, Kilon D, Pietkiewicz M, Sztwiertnia H, Cichocka A, Kosicki JZ. 2019. Does the sex and age of birds and the size of human settlements affect recapturing of the Great Tit (Parus major) at bird feeders? Behavioural Processes 162:162–166. DOI: :10.1016/j.beproc.2019.03.007.
- Guerreiro SB, Dawson RJ, Kilsby C, Lewis E, Ford A. 2018. Future heat-waves, droughts and floods in 571 European cities. Environmental Research Letters 13(3):034009. DOI: 10.1088/1748-9326/aaaad3.
- Hassall C. 2014. The ecology and biodiversity of urban ponds. Wiley Interdisciplinary Reviews: Water 1(2):187–206. DOI: 10.1002/wat2.1014.
- Heezik YV, Seddon PJ. 2017. Counting birds in urban areas: A review of methods for the estimation of abundance. In: Murgui E, Hedblom M, editors. Ecology and conservation of birds in urban environments. Cham: Springer. pp. 185–207.
- Houghton J. 2005. Global warming. Reports on Progress in Physics 68(6):1343–1403. DOI: 10.1088/0034-4885/68/6/R02.
- Krausman PR, Rosenstock SS, Cain JW III. 2006. Developed waters for wildlife: Science, perception, values, and controversy. Wildlife Society Bulletin 34(3):563–569. DOI: 10.2193/0091-7648(2006)34[563:DWFWSP]2.0.CO;2.
- Lee ATK, Wright D, Barnard P. 2017. Hot bird drinking patterns: Drivers of water visitation in a fynbos bird community. African Journal of Ecology 55(4):541–553. DOI: 10.1111/aje.12384.
- Lenth R. 2020. Emmeans: Estimated marginal means, aka least-squares means. R package, version 1.4.5. Available: https://CRAN.R-project.org/package=emmeans.
- Lepczyk CA, Flather CH, Radeloff VC, Pidgeon AM, Hammer RB, Liu J. 2008. Human impacts on regional avian diversity and abundance. Conservation Biology 22(2):405–416. DOI: 10.1111/j.1523-1739.2008.00881.x.
- Martin TE. 1987. Food as a limit on breeding birds: A life-history perspective. Annual Review of Ecology and Systematics 18(1):453–487. DOI: 10.1146/annurev.es.18.110187.002321.
- Matuoka MA, Benchimol M, de Almeida-Rocha JM, Morante-Filho JC. 2020. Effects of anthropogenic disturbances on bird functional diversity: A global meta-analysis. Ecological Indicators 116:106471. DOI: 10.1016/j.ecolind.2020.106471.
- McKechnie AE, Wolf BO. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biology Letters 6(2):253–256. DOI: 10.1098/rsbl.2009.0702.
- McKechnie AE, Hockey PAR, Wolf BO. 2012. Feeling the heat: Australian landbirds and climate change. Emu 112(2):1–7. DOI: 10.1071/MUv112n2_ED.
- McKechnie AE, Rushworth IA, Myburgh F, Cunningham SJ. 2021. Mortality among birds and bats during an extreme heat event in eastern South Africa. Austral Ecology 46(4):687–691. DOI: 10.1111/aec.13025.
- Mella VSA, McArthur C, Krockenberger MB, Frend R, Crowther MS. 2019. Needing a drink: Rainfall and temperature drive the use of free water by a threatened arboreal folivore. PLoS ONE 14(5):1–16. DOI: 10.1371/journal.pone.0216964.
- Miller KK, Blaszczynski VN, Weston MA. 2015. Feeding wild birds in gardens: A test of water versus food. Ecological Management and Restoration 16(2):156–158. DOI: 10.1111/emr.12157.
- Møller AP, Tryjanowski P, Díaz M, Kwieciński Z, Indykiewicz P, Mitrus C, Goławski A, Polakowski M. 2015. Urban habitats and feeders both contribute to flight initiation distance reduction in birds. Behavioral Ecology 26(3):861–865. DOI: 10.1093/beheco/arv024.
- Molokwu MN, Nilsson JÅ, Ottosson U, Olsson O. 2010. Effects of season, water and predation risk on patch use by birds on the African Savannah. Oecologia 164(3):637–645. DOI: 10.1007/s00442-010-1781-3.
- Newton I. 1998. Population limitation in birds. San Diego, London, Boston, New York, Sydney, Tokyo, Toronto: Academic Press.
- Nystrom GS, Bennett VJ. 2019. The importance of residential swimming pools as an urban water source for bats. Journal of Mammalogy 100(2):394–400. DOI: :10.1093/jmammal/gyz020.
- Okahisa Y, Nakahara T, Sato NJ, Theuerkauf J, Ueda K. 2015. Puddle use by New Caledonian rainforest birds. Ornithological Science 14(1):41–45. DOI: 10.2326/osj.14.41.
- Oksanen J. 2011. Multivariate analysis of ecological communities in R: Vegan tutorial. Available: http://cc.oulu.fi/~jarioksa/popular.html.
- Pille L, Säumel I. 2021. The water-sensitive city meets biodiversity: Habitat services of rain water management measures in highly urbanized landscapes. Ecology and Society 26(2):23. DOI: 10.5751/ES-12386-260223.
- Pustkowiak S, Kwieciński Z, Lenda M, Żmihorski M, Rosin ZM, Tryjanowski P, Skórka P. 2021. Small things are important: The value of singular point elements for birds in agricultural landscapes. Biological Reviews 96(4):1386–1403. DOI: 10.1111/brv.12707.
- Qiu J, Shen Z, Leng G, Xie H, Hou X, Wei G. 2019. Impacts of climate change on watershed systems and potential adaptation through BMPs in a drinking water source area. Journal of Hydrology 573:123–135. DOI: 10.1016/j.jhydrol.2019.03.074.
- R Development Core Team. 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Schaper L, Hutton P, McGraw KJ. 2021. Bird-feeder cleaning lowers disease severity in rural but not urban birds. Scientific Reports 11(1):1–8. DOI: 10.1038/s41598-021-92117-y.
- Shellenbarger GG, Athearn ND, Takekawa JY, Boehm AB. 2008. Fecal indicator bacteria and Salmonella in ponds managed as bird habitat, San Francisco Bay, California, USA. Water Research 42(12):2921–2930. DOI: 10.1016/j.watres.2008.03.006.
- Smit B, Zietsman G, Martin RO, Cunningham SJ, McKechnie AE, Hockey PAR. 2016. Behavioural responses to heat in desert birds: Implications for predicting vulnerability to climate warming. Climate Change Responses 3(1):1–14. DOI: 10.1186/s40665-016-0023-2.
- Smit B, Woodborne S, Wolf BO, McKechnie AE. 2019. Differences in the use of surface water resources by desert birds are revealed using isotopic tracers. Auk 136(1):1–13. DOI: 10.1093/auk/uky005.
- Sturkie PD. 1965. Avian physiology. Ithaca, New York: Cornell University Press.
- Temple SA. 2001. Individuals, populations, and communities: The ecology of birds. In: Podulka S, Rohrbaugh JR, Bonney R, editors. Handbook of bird biology. Ithaca, NY: The Cornell Lab of Ornithology. pp. 1–134.
- TIBCO Software Inc. 2017. Statistica (Data analysis software system).
- Tieleman BI, Williams JB, Michaeli G, Pinshow B. 1999. The role of the nasal passages in the water economy of Crested Larks and Desert Larks. Physiological and Biochemical Zoology 72(2):219–226. DOI: 10.1086/316658.
- Tieleman BI, Williams JB. 2000. The adjustment of avian metabolic rates and water fluxes to desert environments. Physiological and Biochemical Zoology 73(4):461–479. DOI: 10.1086/317740.
- Tieleman BI, Williams JB, Bloomer P. 2003. Adaptation of metabolism and evaporative water loss along an aridity gradient. Proceedings of the Royal Society B: Biological Sciences 270(1511):207–214. DOI: 10.1098/rspb.2002.2205.
- Tryjanowski P, Skórka P, Sparks TH, Biaduń W, Brauze T, Hetmański T, Martyka R, Indykiewicz P, Myczko Ł, Kunysz P, Kawa P, Czyż S, Czechowski P, Polakowski M, Zduniak P, Jerzak L, Janiszewski T, Goławski A, Duduś L, Nowakowski JJ, Wuczyński A, Wysocki D. 2015a. Urban and rural habitats differ in number and type of bird feeders and in bird species consuming supplementary food. Environmental Science and Pollution Research 22:15097–15103.
- Tryjanowski P, Sparks TH, Biaduń W, Brauze T, Hetmański T, Martyka R, Skórka P, Indykiewicz P, Myczko Ł, Kunysz P, Kawa P, Czyz S, Czechowski P, Polakowski M, Zduniak P, Jerzak L, Janiszewski T, Goławski A, Dudu L, Nowakowski JJ, Wuczyński A, Wysocki D. 2015b. Winter bird assemblages in rural and urban environments: A national survey. PLoS ONE 10(6):1–25. DOI: 10.1371/journal.pone.0130299.
- Tryjanowski P, Møller AP, Morelli F, Biaduń W, Brauze T, Ciach M, Czechowski P, Czyz S, Dulisz B, Golawski A, Hetmański T, Indykiewicz P, Mitrus C, Myczko Ł, Nowakowski JJ, Polakowski M, Takacs V, Wysocki D, Zduniak P. 2016. Urbanization affects neophilia and risk-taking at bird-feeders. Scientific Reports 6(1):1–7. DOI: 10.1038/srep28575.
- Tryjanowski P, Nowakowski JJ, Indykiewicz P, Andrzejewska M, Śpica D, Sandecki R, Mitrus C, Goławski A, Dulisz B, Dziarska J, Janiszewski T, Minias P, Świtek S, Tobolka M, Włodarczyk R, Szczepańska B, Klawe JJ. 2020. Campylobacter in wintering great tits Parus major in Poland. Environmental Science and Pollution Research 27(7):7570–7577. DOI: 10.1007/s11356-019-07502-y.
- Tu HM, Fan MW, Ko JCJ. 2020. Different habitat types affect bird richness and evenness. Scientific Reports 10(1):1221. DOI: 10.1038/s41598-020-58202-4.
- Tvardíková K, Fuchs R. 2011. Do birds behave according to dynamic risk assessment theory? A feeder experiment. Behavioral Ecology and Sociobiology 65(4):727–733. DOI: 10.1007/s00265-010-1075-0.
- Webster MD, Weathers WW. 2000. Seasonal changes in energy and water use by verdins, Auriparus flaviceps. Journal of Experimental Biology 203(21):3333–3344. DOI: 10.1242/jeb.203.21.3333.
- Williams PL, Koenig WD. 1980. Water dependence of birds in a temperate Oak Woodland. The Auk 97(2):339–350. DOI: 10.1093/auk/97.2.339.
- Williams JB, and Tieleman BI. 2001. Physiological ecology and behavior of desert birds. In: Nolan JV, Thompson CF, editors. Current ornithology. Vol. 16. Boston, MA: Springer. pp. 299–353.
- Woods C. 1911. The drought and the birds. Nature 87(2182):247–248. DOI: 10.1038/087247d0.