Abstract
Quantitative studies on population dynamics and life history traits of key species are useful to predict changes in the structure and organization of biological communities. In this context, Hermodice carunculata (Pallas, 1766) is a selective scavenger/carnivore polychaete species (known as a fireworm) that, in recent years, has exhibited a northern expansion along the whole basin, including the Italian coasts, and an increasing abundance in its southern areas. Here we report ecological data and fireworm abundances from two shallow stations of the Salento peninsula in the Ionian Sea (Lecce, Italy), characterized by different hydrodynamic exposure levels and structural communities. The observed densities by visual census in the years 2019–2021 (up to 18 ind./15 m2) document a striking shift compared to the past anecdotal reports of the rare occurrence of fireworms along the studied area. Additionally, their abundance seems to be driven by the substrate coverage; in fact, a higher density of fireworms was observed where the biodiversity is richer. Results from this study will serve as a baseline reference for future investigation of the invasiveness potential of a species that can act as a biological marker of ocean warming.
Introduction
Biogeographical species distribution is changing at an accelerating rate due to global warming, a major threat to world biodiversity, with consequences for the structure and organization of biological communities (Chen et al. Citation2011; Pecl et al. Citation2017). In this regard, the Mediterranean Sea is considered a double “hotspot”, both for marine biodiversity and for climate change (Giorgi Citation2006; Coll et al. Citation2010). Although new endemic species are continually being discovered (i.e. Furfaro & Mariottini Citation2020; Furfaro et al. Citation2021), ocean warming is affecting the Mediterranean the most among the European seas, with an unceasing arrival of warm-water species from the Suez Canal favoured by intense shipping (Zenetos et al. Citation2012; Galil et al. Citation2014; Tsiamis et al. Citation2020), with corollary negative effects on native diversity.
Long-term predictions also suggest more frequent, intense, and longer marine heat waves (MHW) in the Mediterranean region, with a further rising of the sea surface temperature by 2100 (Darmaraki et al. Citation2019). The geographic distribution of a species depends mainly on their ecophysiological tolerance, the outcome of interspecific interactions, and dispersal constraints (Peterson et al. Citation2011). The increase of temperature, other than favouring the spread of thermophilic alien species, can also increase the ecological success of species that may become invasive in their native habitats, thus being classified as “native invaders” (Carey et al. Citation2012). This is the case for the Mediterranean fireworm Hermodice carunculata (Pallas, 1766), a thermophilic amphinomid polychaete mostly living in the infralittoral upper zone of rocky bottom coastal habitats (Righi et al. Citation2020). Hermodice carunculata is widely distributed across the subtropical Atlantic coasts, the Red Sea and, so far, in the central Mediterranean coastlines (Fishelson Citation1971; Ahrens et al. Citation2013; Righi et al. Citation2020). Recently, the genetic structure of fireworm populations across the Atlantic Ocean and the Mediterranean Sea has been investigated, disclosing high connectivity between populations of the two regions (Ahrens et al. Citation2013; Righi et al. Citation2019). From a morphological point of view, this species is characterized by a remarkable degree of intraspecific variability that could reflect in some cases a high ecological plasticity: for instance, the number of branchial filaments increases when dissolved oxygen is lower (Ahrens et al. Citation2013; Grimes et al. Citation2020; Lucey et al. Citation2020). Recently, in the Mediterranean Sea H. carunculata seems to have expanded its distribution towards the Italian peninsula, with an increasing northward distribution (Krzelj et al. 2020; Righi et al. Citation2020), and a simultaneous increase in abundance of its southern populations. This gradual northward spread into the Mediterranean Sea seems to be kept in check by a critical environmental constraint, as H. carunculata larval development is blocked at seawater temperature < 22°C (Toso et al. Citation2020). This ontogenetic bottleneck makes this worm a powerful bioindicator of ocean warming (Righi et al. Citation2020; Toso et al. Citation2020).
Hermodice carunculata is known to prey on several other invertebrates as well as on small fishes or even on large fishes entangled in fishermen’s nets, causing considerable economic impacts (Celona & Comparetto Citation2010; Simonini et al. Citation2018; Krželj et al. Citation2020). Adult worms can reach large size (up to 45 g in weight) and, due to their highly motile behaviour, the occurrence of high-density populations may lead to overall significant impacts in the benthic food web as well as to shifts in the composition and functioning of hard-bottom coastal communities (Krželj et al. Citation2020). These motivations call for attention to H. carunculata distribution and abundance as a native invader of the Mediterranean Sea and to the possible consequences for Mediterranean rocky bottom communities.
To date, reports on the expansion of fireworm population come from citizen science (i.e. records from fishermen, snorkelers and scuba divers along the Southern Mediterranean coasts) and from scientific reports; however, the available observations focused only on presence/absence of worms at depths from around 0.5 m to 20 m (Simonini et al. Citation2017, Citation2021; Krželj et al. Citation2020; Righi et al. Citation2020).
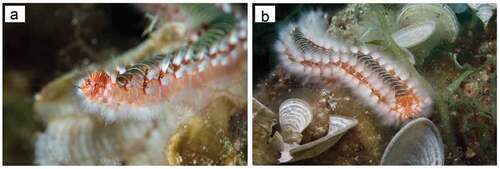
Here new data are provided on the seasonal abundance of H. carunculata () from Santa Caterina di Nardò, in the Salento peninsula (Italy, Ionian Sea) () over the last two years (2019–2021) as a baseline study for future monitoring and management strategies. Further, these data are compared to and discussed in light of previous observations made by two of us (AG and SP) at the same coastal site, nearly 30 years ago. Finally, we gathered new data on the feeding ecology of H. carunculata, from both in situ and laboratory observations of prey selection, as well as by examining the undigested material present in the faeces of the stored specimens.
Materials and methods
Field study
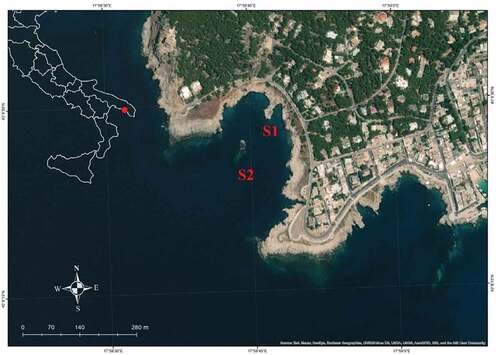
The study was carried out in a shallow subtidal rocky bottom area off Santa Caterina di Nardò – Lecce, Italy (Ionian Sea) 40°08ʹ26.9”N, 17°58ʹ44.1”E, where two stations were selected: Station S1, on the coastline facing the “Chiapparo” rock, and Station S2, located around the rock called “Chiapparo” ().
A vertical slope that extends along the entire coastline to a maximum depth of 6–8 m delimits Station S1 ()); its bottom is made of small stones that in spring/summer are partially covered by filamentous algae. This station is characterized by a lower biodiversity compared to S2 (). In fact, sea urchin barrens with few macroalgae (mostly composed of brown algae) and sponges such as Chondrilla nucula Schmidt, 1862 and Chondrosia reniformis (Nardo, 1847) are the main components of S1 sessile biodiversity. Some small animals can be found under the stones, such as small Ophiuridae, Terebellidae, blennies, ascidians, and large quantities of small and common gastropods. Station S1 is affected by higher human pressure (especially in summer season; authors’ personal observations) with respect to Station S2, probably because of its position closer to the coastline. In fact, at the bottom of its vertical slope can be found the remains of marine shells ranging from mussels and oysters to sea urchins and crustaceans (i.e. utilized as bait or raw food).
Figure 3. Characterization of stations S1 and S2. (a, c, e) station S1 and (b, d, f) station S2 at an ever-increasing magnification level. Credits: Michele Solca.

Station S2 ()) is characterized by a small rock 60 m from the coastline and by a maximum depth of 20 m. The area is entirely characterized by a rocky bottom, and the shore is also characterized by the presence of little caves developing to a depth of 6–10 m. The rocky bottom shows the typical infralittoral algal assemblages composed by red, green and brown algae such as Codium bursa (Agardh, 1817), Halimeda tuna (J.Ellis & Solander) J.V. Lamouroux, 1816, Padina pavonica (Linnaeus) Thivy, 1960, Taonia atomaria (Woodward) J.Agardh, 1848, Udotea petiolata (Turra) Børgesen, 1925 and various Corallinaceae. The sessile invertebrates are mainly sponges: Chondrosia reniformis, Haliclona fulva (Topsent, 1893), Penares helleri (Schmidt, 1864), Sarcotragus spinosulus (Schmidt, 1862) with Dromia personata (Linnaeus 1758) as its associated decapod, and the protected Spongia officinalis (Linnaeus, 1759). Among Cnidaria, the madreporaria Cladocora caespitosa (Linnaeus, 1767), Clavularia crassa (Milne Edwards, 1848) and Pennaria disticha Goldfuss, 1820 and the stoloniferous Phyllangia mouchezii (Lacaze-uthiers, 1897) can also be observed. Large and visible sessile polychaetes included sabellids such as Branchiomma luctuosum (Grube 1870), Sabella spallanzanii (Gmelin, 1791) and Protula tubularia (Montagu, 1803). Echinodermata such as Arbacia lixula (Linnaeus, 1758), Coscinasterias tenuispina (Lamarck, 1816), Paracentrotus lividus (Lamarck, 1816), Echinaster sepositus (Retzius, 1783), Marthasterias glacialis (Linnaeus, 1758), Ophidiaster ophidianus (Lamarck, 1816) and Sphaerechinus granularis (Lamarck, 1816) are also abundant. Finally, the presence of several species of Gastropoda molluscs (Heterobranchia) inhabiting station S2, such as Aplysia fasciata Poiret, 1789, A. punctata and the alien congeneric A. dactylomela Rang, 1828, Elysia timida (Risso, 1818), Thuridilla hopei (Vérany, 1853) and Umbraculum mediterraneum (Lamarck, 1819), and the nudibranchs Cratena peregrina (Gmelin, 1791), Edmundsella pedata (Montagu, 1816), Felimare picta (Philippi, 1836) and Flabellina affinis (Gmelin, 1791), is quite important since it reflects the high diversity characterizing this habitat. In fact, these marine sea slugs usually have a very specialized diet with species that feed only on one or a very few sessile species (Furfaro et al. Citation2017; Canessa et al. Citation2021; Trainito et al. Citation2021).
These sites are both characterized by exposure to hydrodynamic forces from the fourth quadrant (from west to south), but S1 is more sheltered than S2 (). The relative position of the two stations within the bay creates different faunal compositions, with a more diverse well-structured benthic community in S2 with respect to S1 (); authors’ personal observations).
Sampling was carried out by scuba diving at 8–10 m depth range, in July and October 2019, February, June and October 2020 and February and April 2021. Fireworm density was assessed by visual census using linear transects of 10 m length × 1.5 m width (15 m2) and three replicates at each site, randomly selected at each sampling time at each station. The results are expressed as the mean of the three repetitions for each station. The total time of each dive was 45 minutes. The sea temperature was recorded during each dive by an Aladin A2 diving computer. Occasionally, water temperature records were confirmed by simultaneous measurements by a HYDROLAB HL7 multiparametric probe. To test for significant differences between and within groups of data, one-way analyses of variance (ANOVAs) were employed.
Laboratory analysis
Twenty specimens of fireworms (i.e. adult size > 15 cm), collected in June 2020, were kept starved – in a tank of 100 L of seawater (filtered on 20 μm mesh) gently aerated at a constant temperature of 22°C (environmental temperature) – for 2 days in order to collect from the tank bottom (using a large glass pipette) all material undigested and excreted through the fireworm faeces. The undigested material and the seawater were filtered through a 20 μm mesh net and the resulted filtered debris was conserved in 60% ethanol until microscopic sorting using a Nikon SMZ25 stereomicroscope fitted with NIS-Elements imaging software.
After this first step, all 20 specimens were moved to a larger aquarium (200 L) filled with seawater, that was set to a temperature of 22°C, a salinity of 35–38‰ and 12 LTD (12-h light-dark). The water was never changed, but only refilled to regulate the salinity. This aquarium was set up with sand and rocks collected from the studied area, and with various specimens: the teleosteans Lepadogaster purpurea (Bonnatterre, 1788) and Mycrolipophyrys nigriceps (Vinciguerra, 1883) and the mussel Mytilus galloprovincialis (Lamarck, 1819). Additionally, in the same aquarium we introduced three specimens of the sea urchin Paracentrotus lividus (Lamarck, 1816) (medium size) for a total period of 3 months. Fireworms were fed ad libitum once a month using frozen fish. During the feeding time (i.e. every 30 days) the fireworms were kept in a small jar for a total of 1 hour to avoid contamination of the aquarium with organic matter. The food was given beginning 30 days after the start of the observation in the aquarium.
Result
Density and seasonal variability of fireworm population
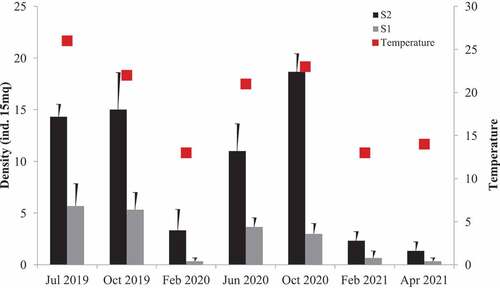
Results from in situ observations are shown in and revealed a spatial-temporal change in fireworms population. The highest density for S2 was detected beginning in the July–October period, with a peak of 18.6 ± 1.7 (standard deviation [SD]) ind./15 m2 (in October 2020), and the lowest was in February–April at 1.3 ± 0.8 (SD) ind./15 m2 (April 2021), with water temperature (recorded during the visual census dives in the two higher and lower density founded) ranged from 22°C (October) to 13°C (February/April). Site S1 followed the same density trend as S2, showing the highest value in summer/autumn, of 3.6 ± 0.8 (SD) ind./15 m2, and the lowest in winter/spring, of 0.3 ± 0.3 (SD) ind./15 m2. The differences between the two sites and among times were statistically significant GMAV ANOVA test site (F = 5.77, P < .01), test time (F = 9.5, P < .00001), with a significant increase of specimens in site S2 with respect to S1.
Trophic behaviour
During the in situ visual census over the different seasons, some fireworms were observed actively preying on small sessile invertebrate taxa (such as bryozoans and small-sized polychaetes) but most of them were found browsing the substrate and the algal canopy in search for food, without evidence of captured prey. Conversely, dense aggregations of worms were recorded around dead and wounded organisms, such as sea slug Aplysia dactylomela, holothurians (Holothuria sp.) and sea urchins, but with no evidence that the worms were responsible for their death. Fireworm aggregations were also observed on dead fishes and on pieces of chicken leg used as bait by fishermen ()).
Figure 5. (a) Hermodice carunculata eating a dead Aplysia specimen; (b) H. carunculata eating a dead sea urchin; (c) H. carunculata eating a piece of chicken used as bait. Credits: Andrea Toso (a, b) and Michele Solca (c).

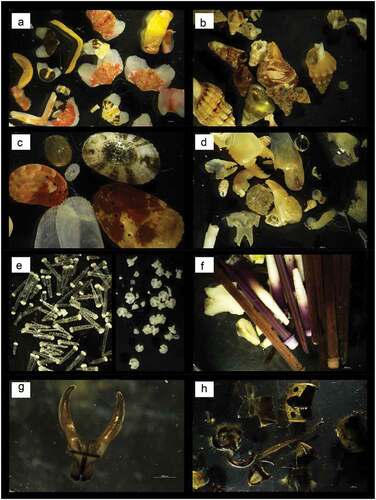
Laboratory observations, under a Nikon SMZ25 microscope, of undigested material (faeces) of fireworms showed a high concentration of undigested material from small sessile and vagile invertebrates such as echinoderms, crustaceans, molluscs, annelids and insects. In particular, most of the fragments were identified as Mollusca, represented by chitons, bivalves and gastropods ()), followed by high numbers of Crustacea ()) and Echinodermata, with many skeletal elements of ophiurids and sea urchins like Arbacia lixula, Paracentrotus lividus and Sphaerechinus granularis ()). For the phylum Annelida, only one partial maxillary system of a polychaete (probably Lumbrineridae; )) was found. The class Insecta is represented by maggots probably used by fishermen as bait, and by the remains of the body of a hornet ()). However, in the laboratory, we observed no interaction between large-sized H. carunculata and the sea urchin P. lividus over 3 months of coexistence in the same aquarium.
Figure 6. (a) Plates of chitons; (b) gastropod (Mollusca) with a hermit crab visible inside one of the shells; (c) various gastropods together with the valves of a bivalve; (d) parts of the skeleton characteristic of crustaceans; (e) skeletal elements typical of the ophiures; (f) skeleton and spines of sea urchins; (g) partial maxillary system of a polychaete (probably Lumbrineridae); (h) chitinous fragments of a hornet.
Discussion
Investigations carried out on Hermodice carunculata in the Salento peninsula revealed that the density of the fireworm population is markedly increasing compared to 30-year-old anecdotal reports from the same locality. Previous observations on the presence of fireworms go back to the year 1990 when, at site S2, only a few, scattered individual fireworms were present: 0–1 specimens was reported as the average number detected by scuba divers during an average dive time of 45 min (AG & SP personal observations).
Recently, Simonini et al. (Citation2021) provided updated data about the density of fireworms in the studied area, reporting that the density is constant at depths between 0.5 and 18 m and ranges between 0.2 and 1 ind. m2. The selection of a specific depth is important in investigating potential preferred areas and habitats.
Nowadays, a larger number of specimens can be rapidly detected at the same site (S2), in the range of 200–400 specimens recorded during the 45 minutes diving time. Interestingly, we observed a significant increase of specimens where the local benthic biodiversity is high, as in the case of site S2, with respect to site S1 where sea urchin barrens are dominant and the number of fireworms is lower.
Even if this comparison is only qualitative, it is meaningful and evidences the dramatic change in the density of this species in the studied area. In this scenario, we can hypothesize that H. carunculata is taking advantage of the climate change-dependent increase of water temperature in the Mediterranean Sea in the last few decades (Pastor et al. Citation2018). The rise of temperature might be key to supporting an increasing larval recruitment and successful metamorphosis at latitudes where 30 years ago the H. carunculata life cycle was not always completed. If this hypothesis was confirmed, fireworms might be considered a conspicuous bioindicator of the “meridionalization” of the Mediterranean Sea (Riera et al. Citation1995; Toso et al. Citation2020).
Assessing the density of fireworms in the field was not an easy task: the small size of the specimens, individuals hiding under rocks, frequent patchy aggregations of worms, and their high mobility made each counting session a time-consuming effort. In fact, studies using the visual census technique on the seasonal distribution of this species were never performed before, and the only data nowadays available are obtained using traps or mesh bags (Wolf et al. Citation2014; Simonini et al. Citation2021). The use of mesh bags for collecting data was started by Wolf et al. (Citation2014), who utilized this device to collect fireworms in a coral reef ecosystem. Subsequently, Simonini et al. (Citation2021) performed a similar experiment along the Apulian coast utilizing a specific trap filled with bait (waste bin) covered with holes. This kind of approach is very useful to collect worms and to provide data on their presence/absence with relatively less effort. However, this approach cannot be used to assess the density of fireworms in a specific area due to the fireworms’ ability to escape from the holes of the traps after eating the bait and due to the impossibility to identify the exact area of the capture. Future studies are desirable to create a specific and effective trap for fireworms; in fact, this could produce an indirect secondary advantage for fishermen who are affected, nowadays, by worms’ attacks on their daily catch. The species H. carunculata is known as a long-living iteroparous form, reproducing in summer (Toso et al. Citation2020): however, small juveniles were never found in nature. The smallest size observed was about 5 cm in length and 0.5 g, thus suggesting the existence of some kind of ontogenetic migration of juveniles in habitats different from those where adult specimens live.
Overall, these findings highlight a marked ecological change boosting the success of H. carunculata along the Salento peninsula coastline, seemingly driven by the rise in temperature of the upper water layers in the Mediterranean Sea. As a corollary, the outbreak of a dense population of fireworms might represent a severe threat to the composition of shallow hard-bottom communities, potentially leading to a shift in the benthic assemblages and food webs. However, knowledge on the ecological impact of H. carunculata is still scant. The warm-water metabolic affinity of the worms is confirmed by the inter-seasonal variability of the Santa Caterina H. carunculata population, with significantly higher densities observed in the warmer months compared to the colder period, which is associated with a sharp decrease in worm density. The increase of fireworms during the summer season has a negative effect also on human health, especially on swimmers and divers, due to the stinging bristles visible along the worm’s body that have an irritating and stinging action.
Fireworms thrive in biodiversity-rich host communities: at S2 (with higher biodiversity, lower barren conditions, and a high percentage of substrate covered by algae and sessile organisms), the density of fireworms is higher with respect to S1, where extensive sea urchin barrens drive a manifest decrease of sessile biodiversity, and there is a significant reduction in the number of fireworm specimens per square metre.
Interestingly, over the course of our investigations, predation on vagile invertebrates, such as sea urchins, was not observed in the field. Conversely, dense aggregations of worms around dead and wounded organisms were detected, suggesting a scavenger feeding habit (Schulze et al. Citation2017). We speculate that H. carunculata fireworms can opportunistically shift their feeding strategy, adopting an energetically cost-effective scavenging strategy whenever decaying organic matter is available and consequently attacking dying or injured organisms in nature. By contrast, laboratory investigations of interspecific interactions with different invertebrates – in particular, with sea urchins – showed no interaction between fireworms and sea urchins over 3 months of coexistence in the same aquarium. This is in contrast with previous findings (Simonini et al. Citation2017), when fireworms in captivity were shown to prey on sea urchins. To explain this contrasting evidence of predation on sea urchins, different hypotheses can be proposed: (a) heterogeneous laboratory conditions can differently impact on the fireworm feeding behaviour. Starved worm specimens might display aggressive behaviour against sea urchins in the absence of alternative food sources. In our experiment, the sea urchins and worms were jointly living in an aquarium in which fish flesh was offered at regular times (i.e. once a month; period of starvation: 30 days), where all worms behaved as scavengers. Alternatively, (b) the physiological condition of the sea urchin may be a driver of the predatory behaviour of the worms, either by detection of unhealthy sea urchins or by the chemical recognition of gonad maturation in sea urchins, leading to predation on ripe urchins only. These are working hypotheses that will soon be tested experimentally.
Conclusion
In this study we evaluated the density of fireworms by underwater visual census. We observed a striking increase in density compared to anecdotical information dating back 30 years ago; additionally, the detection of a changing inter-seasonal density reflects the warm-water affinity and ecophysiological optima of fireworms. Indeed, water temperature clearly regulates the abundance of H. carunculata, with warmer waters boasting high population densities and sexual reproduction (Schulze et al. Citation2017; Righi et al. Citation2019, Citation2020; Toso et al. Citation2020). In a Mediterranean Sea warming scenario, it is foreseeable that H. carunculata population abundances will be on the rise, expanding fireworms’ distribution towards northern latitudes across the Mediterranean Sea, with the potential to become locally invasive, as in the Santa Caterina di Nardò hotspot and in many other locations around the Salento peninsula.
So far, clear evidence of predators or competitors of H. carunculata in the Mediterranean Sea has not been reported. Therefore, its expansion and demographic outbreak might result in rapid and substantial changes in the structure and functioning of benthic communities. However, at present there is no evidence of dramatic structural changes and biodiversity loss in the benthic communities related to H. carunculata outbreaks. In any case, changes in species composition of the benthic assemblages or even the eradication of single species due to fireworm outbreaks cannot be ruled out, but also can never be proved. Specific studies are still missing to rule out the hypothesis that fireworms may act as keystone, biodiversity-depressing species (Fanelli et al. Citation1999; Piraino et al. Citation2002). This study highlights the importance of monitoring sentinel species, such as H. carunculata, to understand the effect of global warming in the Mediterranean Sea.
Acknowledgements
The authors thank the professional photographer Michele Solca for kindly providing the underwater pictures of Hermodice carunculata and of the Stations S1 and S2 reported in this paper. The authors thank the two anonymous reviewers who helped improve the article with their helpful suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahrens JB, Borda E, Barroso R, Paiva PC, Campbell AM, Wolf A, Nugues MM, Rouse GW, Schulze A. 2013. The curious case of Hermodice carunculata(Annelida: Amphinomidae): Evidence for genetic homogeneity throughout the Atlantic Ocean and adjacent basin. Molecular Ecology 22(8):2280–2291. DOI:10.1111/mec.12263.
- Canessa M, Bavestrello G, Cattaneo Vietti R, Furfaro G, Doneddu M, Navone A, Trainito E. 2021. Rocky substrate affects benthic heterobranch assemblages and prey/predator relationships. Estuarine, Coastal and Shelf Science 261:31. DOI: 10.1016/j.ecss.2021.107568.
- Carey MP, Sanderson BL, Barnes KA, Olden JD. 2012. Native invaders-challenges for science, management, policy, and society. Frontiers in Ecology and the Environment 10(7):373381. DOI:10.1890/110060.
- Celona A, Comparetto G. 2010. Scientific and Research Center, Republic of Slovenia. Prime osservazioni sulla predazione opportunistica del” vermocane” Hermodice carunculata (Pallas, 1766), ai danni della piccola pesca artigianale nelle acque di Lampedusa (is. Pelagie). Annales: Series Historia Naturalis 20(1):15–20.
- Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science. DOI: 10.1126/science.1206432.
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F et al. 2010. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PloS One 5(8):e11842. DOI: 10.1371/journal.pone.0011842.
- Darmaraki S, Somot S, Sevault F, Nabat P, Cabos Narvaez WD, Cavicchia L, Djurdjevic V, Li L, Sannino G, Sein DV. 2019. Future evolution of Marine Heatwaves in the Mediterranean Sea. Climate Dynamics 53(3–4):1371–1392. DOI:10.1007/s00382-019-04661-z.
- Fanelli G, Piraino S, Esposito L, Boero F. 1999. Opposite roles in sea urchins and starfishes in marine benthic communities. In: Candia Carnevali MD, Bonasoro F, editors. Echinoderm research 1998. Rotterdam: Balkema. pp. 453–457.
- Fishelson L. 1971. Ecology and distribution of the benthic fauna in the shallow waters of the Red Sea. Marine Biology: International Journal on Life in Oceans and Coastal Waters 10(2):113–133. DOI:10.1007/BF00354828.
- Furfaro G, Mariottini P. 2020. A new Dondice Marcus Er. 1958 (Gastropoda: Nudibranchia) from the Mediterranean Sea reveals interesting insights into the phylogenetic history of a group of Facelinidae taxa. Zootaxa 4731(1):001–022. DOI:10.11646/zootaxa.4731.1.1.
- Furfaro G, Salvi D, Trainito E, Vitale F, Mariottini P. 2021. When morphology does not match phylogeny: The puzzling case of two sibling nudibranchs (Gastropoda). Zoologica Scripta 50:1–16. DOI: 10.1111/zsc.12484.
- Furfaro G, Trainito E, De Lorenzi F, Fantin M, Doneddu M. 2017. Tritonia nilsodhneri Marcus Ev., 1983 (Gastropoda, Heterobranchia, Tritoniidae): First records for the Adriatic Sea and new data on ecology and distribution of Mediterranean populations. Acta Adriatica 58(2):261–270. DOI: 10.32582/aa.58.2.6.
- Galil B, Boero F, Campbell ML, Carlton JT, Cook E, Fraschetti S, Gollasch S, Hewitt CL, Jelmert A, Macpherson E, Marchini A, McKenzie C, Minchin D, Occhipinti-Ambrogi A, Ojaveer H, Olenin S, Piraino S, Ruiz GM. 2014. ‘Double trouble’: The expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biological Invasions 17(4):973–976. DOI: 10.1007/s10530-014-0778-y.
- Giorgi F. 2006. Climate change hot-spots. Geophysical Research Letters 33(8). DOI: 10.1029/2006GL025734.
- Grimes CJ, Paiva PC, Petersen LH, Schulze A. 2020. Rapid plastic responses to chronic hypoxia in the bearded fireworm, Hermodice carunculata (Annelida: Amphinomidae). Marine Biology 167(9):1–10. DOI: 10.1007/s00227-020-03756-0.
- Krželj M, Cerrano C, Gioia Di Camillo C. 2020. Enhancing diversity knowledge through Marine citizen science and social platforms: The case of Hermodice carunculata (Annelida, Polychaeta). Diversity 12(8):311. DOI: 10.3390/d12080311.
- Lucey NM, Collins M, Collin R. 2020. Oxygen‐mediated plasticity confers hypoxia tolerance in a corallivorous polychaete. Ecology and Evolution 10(3):1145–1157. DOI: 10.1002/ece3.5929.
- Pastor F, Valiente JA, Palau JL. 2018. Sea surface temperature in the Mediterranean: Trends and spatial patterns (1982–2016). Pure and Applied Geophysics 175(11):4017–z. DOI: 10.1007/s00024-017-1739-z.
- Pecl GT, Araújo MB, Bell JD, Blanchard J, Bonebrake TC, Chen IC, Clark TD, Colwell RK, Danielsen F, Evengård B, Falconi L, Ferrier S, Frusher S, Garcia RA, Griffis RB, Hobday AJ, Janion-Scheepers C, Jarzyna MA, Jennings S, Williams SE. 2017. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. In Science 355(6332). DOI: 10.1126/science.aai9214.
- Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M, Araújo MB. 2011. Ecological niches and geographic distributions (MPB-49). In: Ecological niches and geographic distributions (MPB-49). Princeton University Press. DOI:10.23943/princeton/9780691136868.001.0001.
- Piraino S, Fanelli G, Boero F. 2002. Variability of species’ roles in marine communities: Change of paradigms for conservation priorities. Marine Biology 140(5):1067–1074. DOI: 10.1007/s00227-001-0769-2.
- Riera F, Grau AM, Pastor E, Pou S. 1995. La Mèditerranèe: Variabilités climatiques, environnement et biodiversité Faunistical and demographical observations in balearic ichthyofauna. Meridionalization or subtropicalization phenomena. Act Coll Sci OKEANOS Montpellier. pp. 213–220.
- Righi S, Maletti I, Maltagliati F, Castelli A, Barbieri M, Fai S, Prevedelli D, Simonini R. 2019. Morphometric and molecular characterization of an expanding Ionian population of the fireworm Hermodice carunculata (Annelida). Journal of the Marine Biological Association of the United Kingdom 1–9. DOI: 10.1017/S002531541900064X.
- Righi S, Prevedelli D, Simonini R. 2020. Ecology, distribution and expansion of a Mediterranean native invader, the fireworm Hermodice carunculata (Annelida). Mediterranean Marine Science. DOI:10.12681/MMS.23117.
- Schulze A, Grimes CJ, Rudek TE. 2017. Tough, armed and omnivorous: Hermodice carunculata (Annelida: Amphinomidae) is prepared for ecological challenges. Journal of the Marine Biological Association of the United Kingdom 97(5):1075–1080. DOI:10.1017/S0025315417000091.
- Simonini R, Maletti I, Righi S, Fai S, Prevedelli D. 2018. Laboratory observations on predator–prey interactions between the bearded fireworm (Hermodice carunculata) and Mediterranean benthic invertebrates. Marine and Freshwater Behaviour and Physiology 51(3):145–158. DOI:10.1080/10236244.2018.1502031.
- Simonini R, Righi SS, Maletti I, Fai S, Prevedelli D. 2017. Bearded versus thorny: The fireworm Hermodice carunculata (anellida) preys on the seaurchin Paracentrotus lividus. Ecology 98(10):2730–2732. DOI: 10.1002/ecy.1919.
- Simonini R, Righi S, Zanetti F, Fai S, Prevedelli D. 2021. Development and catch efficiency of an attracting device to collect and monitor the invasive fireworm Hermodice carunculata in the Mediterranean Sea. Mediterranean Marine Science 22(3):706–714.
- Toso A, Boulamail S, Lago N, Pierri C, Piraino S, Giangrande A. 2020. First description of early developmental stages of the native invasive fireworm Hermodice carunculata (Annelida, Amphinomidae): A cue to the warming of the Mediterranean Sea. Mediterranean Marine Science. DOI: 10.12681/mms.22043.
- Trainito E, Fantin M, Manganelli E, Furfaro G. 2021. What are you doing here? Investigating on an unexpected association in shallow Mediterranean dark caves sheds light on the diet of Marionia blainvillea (Mollusca, Gastropoda, Nudibranchia). Turkish Journal of Zoology 45(7):550–556. DOI: 10.3906/zoo-2104-43.
- Tsiamis K, Gervasini E, D’ Amico F, Deriu I, Katsanevakis S, Crocetta F, Zenetos A, Arianoutsou M, Backeljau T, Bariche M, Bazos I, Bertaccini A, Brundu G, Carrete M, Çinar ME, Curto G, Faasse M, Justine JL, Király G, Langer MR, Levitt Y, Panov VE, Piraino S, Rabitsch W, Roques A, Scalera R, Shenkar N, Sîrbu I, Tricarico E, Vannini A, Vøllestad LA, Zikos A, Cardoso AC. 2020. Management of Biological Invasions, The EASIN Editorial Board: Quality assurance, exchange and sharing of alien species information in Europe. Management of Biological invasions 7(4):321–328.
- Wolf AT, Nugues MM, Wild C 2014. Distribution, food preference, and trophic position of the corallivorous fireworm Hermodice carunculata in a Caribbean coral reef. Coral Reefs 33(4):1153–1163. DOI:10.1007/s00338-014-1184-8.
- Zenetos A, Gofas S, Morri C, Rosso A, Violanti D, García Raso JE, Çinar ME, AlmogiLabin A, Ates AS, Azzurro E, Ballesteros E, Bianchi CN, Bilecenoglu M, Gambi MC, Giangrande A, Gravili C, Hyams-Kaphzan O, Karachle PK, Katsanevakis S, Verlaque M. 2012. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterranean Marine Science 13:328–352.