Abstract
The study provides a series of distinctive morphological features of the auditory ossicles alongside comparative morphometric data, bringing facts in respect to morphology and some morpho-functional elements of the auditory ossicles in this little-studied species. The most relevant features noted are evident conical shape of muscular process of malleus and triangular aspect of the handle of malleus. For the incus, a short body of the bone and the direct continuation is mentioned, with no clear distinction as an individualized piece for the lenticular process. As for the stapes, the clear profiling of the muscular tubercle for the stapedial muscle and elliptic shape of the foot of the stapes is noted. A series of comparative measurements and indices are also calculated in the attempt of profiling differences from the domestic couterspecies- sheep and goat.
Introduction
Taxonomically, the roe deer belongs to the Artiodactyla order, subfamily Capreolinae, genus Capreolus, species Capreolus capreolus, with a series of subspecies that may sum up to 55 (Mennecart et al. Citation2017). The distinct external morphological features of the roe deer are the relatively long hindlimbs compared to the forelimbs, long neck, short trunk, large auricles and short tail. These represent an adaptative change to the specific habitat they live in nowadays (Sempéré et al. Citation1996) represented by sylvatic areas with dense vegetation (Jeppesen Citation1987).
Specialty literature regarding roe deer focuses on reproduction, genetic differentiation, behavior, and a series of financial-related aspects and parasitology (Lorenzini et al. Citation2002; Roviani Citation2014; Tóth et al. Citation2016; Čurlík et al. Citation2019). Last, but not least, veterinary anatomy approaches several aspects of roe deer morphology such as morphological aspects of the digestive (Konjević et al. Citation2012; Wang et al. Citation2005), cardiovascular (Frackowiak et al. Citation2007; Düzler et al. Citation2015), or some peripheral nervous system features (Kabak & Onuk Citation2010; Düzler et al. Citation2015). With regard to sense organs, literature mentions the importance of olfaction in roe deer with more than 300 million olfactory receptors at the level of the olfactory mucosa as opposed to the approximately 30 million receptors described in humans (Sempéré et al. Citation1996). Another sensory organ, comprising a series of structures that, complexly embryological, originate from the neural crests, endodermal, mesodermal, and ectodermal layers, is the ear (Maier et al. Citation2018), while a series of studies follow the embryonal development of the middle ear or different morphological aspects of the internal ear in roe deer (Mennecart et al. Citation2017).
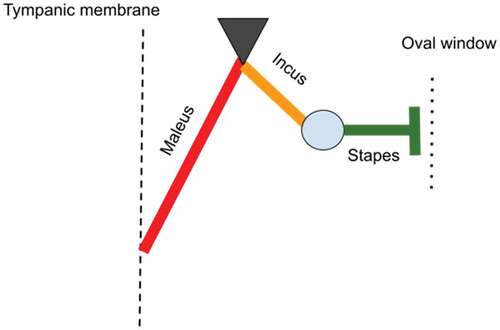
Anatomically, the ear consists of three distinctive sectors: the external ear (auris externa), the middle ear (auris media) and the internal ear (auris interna). Placed in the dorsal part of the tympanic cavity (cavum tympany), within the temporal bone, the petrosal part (os temporale, pars petrosa), the assembly of the auditory ossicles (ossicula auditus) plays an important role in the sound transmission from the tympanic membrane (membrana tympany) to the internal ear (vestibular fenestra – fenestra vestibuli) (Martonos et al. Citation2019; Pfaff et al. Citation2019). For ruminants, some studies focused on the morphology of the middle ear or ossicular structures such as the ones in cows, sheep, or goats (Costeur Loic et al. Citation2016; Péus et al. Citation2017, Citation2020; Simaei et al. Citation2017).
Given the importance of hearing in wild and game animals, the present study aims to provide a series of morphological and morphometrical data concerning the auditory ossicles that might be helpful for researchers and clinicians. Some attention is paid to some mechanical and physical elements of the anatomy of the middle ear, specifically pointing at explanatory elements regarding the involvement and correlations of the metrical features of the ear ossicles to the specificity of sound transmission for this wild species, given the importance of this so-called “transitional type” of the ear, situated between the freely mobile and the malleus-incus microtype (Péus et al. Citation2020).
Material and methods
The biological material was represented by six roe deer crania originating from the collection of comparative specimens included in the Faculty of Veterinary Medicine Cluj-Napoca, Romania Anatomy Department’s reserve. A number of six malleus pieces, six incus and eight tsapes were recovered in decent or good state of preservation.
To facilitate access to the tympanic cavity, a series of fine incisions were made around the bony auditory canal with a fine electric circular saw. A bone rongeur was used to create a small window to allow the approach of the middle ear cavity and the acoustic bone assembly. Once the ossicles visualized, they were carefully extracted one by one with the help of anatomical tweezers. Particular care was given to the elements on the surface of the ossicles.
Once ossiclesextracted, they were gently cleansed and prepared for in-depth macroscopical analysis. Based on the collected digital images (Olympos MTX stereomicroscope and camera) we re-created an enhanced graphical model that fits the basic didactical morphology approach using a graphical digital tablet (Wacom Intuos Art & Touch Small) (Wacom Corporation Intl) for the graphical interface and the Corel Painter Essentials 5 suite (Corel Tm) for supplementary image-processing actions.
For measurements of the ossicles, a scaled surface was used (millimetric paper or ruler) while further processing (measuring, data collection) was done using ImageJ software after the proper calibration procedure (Willett & Johnson Citationn.d.).
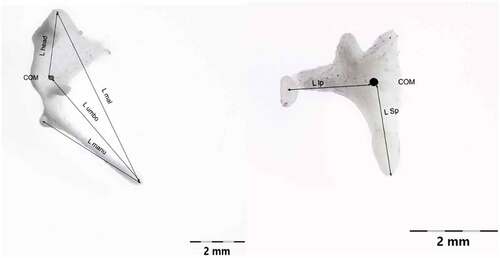
Measurements on the auditory ossicles were made in accordance with data suggested by a series of authors (Kurtul et al. Citation2003; Wang & Gan Citation2016; Gürbüz et al. Citation2019; Péus et al. Citation2020) with some adaptations, as some of the reference points suggested by the available literature were not applicable for our specimens. Also, some other sets of measurements (Péus et al. Citation2020) were used for the evaluation of the morpho-physical aspects of the comparative part, focusing on the landmarks established in a functional approach, such as the main functional axis of the maleo-incal complex that works mainly in a rotational type of movement (Mason & Farr Citation2012). A set of measurements point us to a landmark that is usually not established morphologically but through CT investigations and 3D volume reconstruction procedures, the establishment of the principal axes and the moments of inertia allowed the establishment of the C.O.M (Center of mass) (Péus et al. Citation2020). Our approach did not use this kind of investigation, but due to the high resemblance to the sheep model that sources illustrated and cited, a similar point was established by using the three principal axes suggested (Péus et al. Citation2020) (). These measurements differ slightly from the classically approached measurements () as some reference points are different (). Others, such as the total length of the malleus, are quite similar, as reference points are similar. To evaluate similarly the available data for the assessment of the lever ratio in the maleo-incal complex, data available or reprocessed for sheep (Péus et al. Citation2017), goat (with basic measurements available) (Martonos et al. Citation2021) and roe-deer are compared only, as these three species are very similar in their general and systemic anatomy, with the difference given by their living habitat.
Table I. Supplementary measurements of the auditory ossicles in roe deer (Péus et al. Citation2020).
Table II. Basic measurements of the auditory ossicles in roe deer.
Results
After the removal of the posterior part of the mastoid process, the assembly of the three ossicles was exposed, allowing observations on the three ossicles that play an important role in the transmission of the vibrations towards the internal ear.
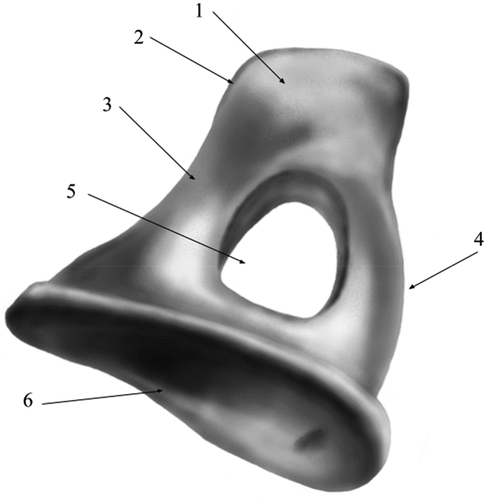
The largest of the ossicles () - the malleus (Malleus) is the laterally placed one, connected to the smaller incus (Incus) and then, the most medially placed, the stapes (Stapes).
The Malleus () consists of a head (Caput mallei) connected by a neck (Collum mallei) to the handle (Manubrium mallei). The head of the malleus in Capreolus capreolus is oval-shaped, slightly obliquely placed medio-laterally, cranio-caudally. At the level of this segment, on its dorso-caudal border, an articular surface (Facies articularis) with a relatively circular shape and somehow divided into two distinctive, uneven surfaces. The angulation between the two surfaces was measured to be approximately 135 degrees (n = 7 measurements). This articular surface is specific for the articulation with the body of incudis (that shows the negative of this surface). The neck of the malleus is well profiled, obliquely placed in ventro-lateral and caudal direction, and appears slightly flattened, with an overall curly appearance. In its proximal part, the dorsal border is convex while in the distal part it appears slightly concave. The neck has three processes: the anterior process (Processus rostralis), the muscular process (Processus muscularis), and the lateral process. The anterior process extends from the ventro-lateral part of the neck in ventro-cranial direction.
Figure 1. Malleus-native specimen.
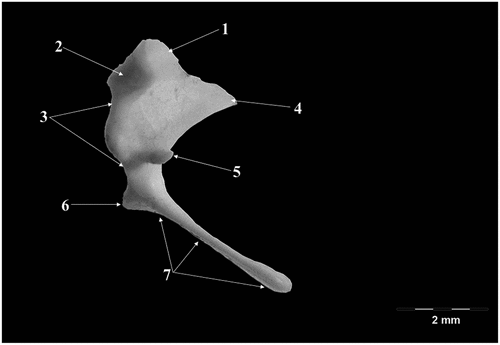
Figure 2. Malleus- graphical representation.
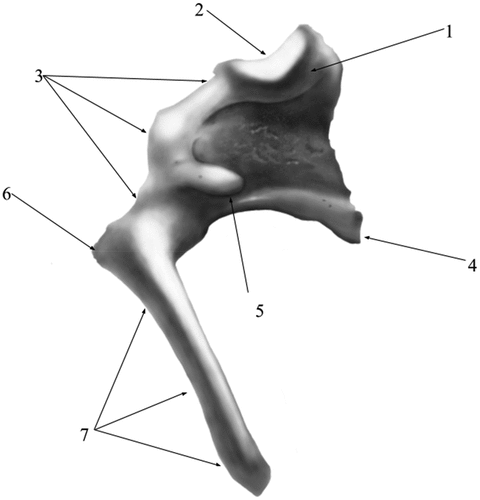
In the area between the anterior process, the ventral surface of the proximal neck part, and the ventral surface of the caput, in all studied specimens there was a thin ossicular lamina that extended in a pointed direction, overpassing the overall shape of the ossicle, conferring the overall shape of a triangular pennant.
The muscular process detaches from the medial surface of the neck, approximately at the same level as the anterior process is detaching. Its free part has a conical shape, and it serves as an insertion point for the tensor muscle of the tympanic membrane (M. tensor tympani).
The lateral process detaches from the lateral surface of the neck, close to the terminal part of this segment, in fact marking the passage to the manubrium (next segment of the malleus). In the studied specimens, this process has a triangular shape and can be easily identified through the tympanic ring.
The last segment the handle of malleus (Manubrium mallei) continues ventrally to the neck. It has an overall tri-faceted process that terminates flattened, pointed, almost rounded distally. Its length exceeds 50% out of the total length of the malleus ().
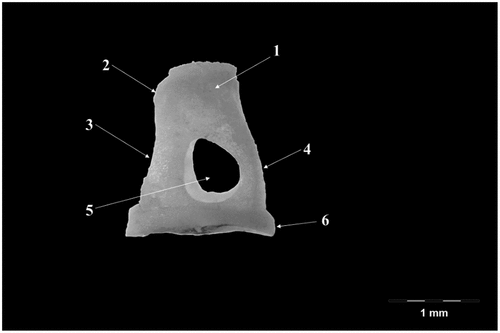
The incus (Incus) (), smaller than the malleus (), has a body (Corpus incudis) and two crura (Crus breve, Crus longum), the body being the most developed segment of this ossicle. Overall, the ossicle has the shape of a human bicuspid tooth. The body possesses a visible articular process- Processus articularis- on its cranial aspect with a saddle-like aspect, slightly excavated, as the negative of the surface described in malleus. The two facing surfaces are placed at an approximate angle of 60–70 degrees. The crura of the incus detach from the aboral part of the body of the ossicle. The short crus (Crus breve), conical and thicker at its base points in dorso-medial, slightly oblique direction. The long crus (Crus longum) detaches from the ventral border of the body to bend medially to continue with the lenticular process (Processus lenticularis). This process is cylindrically shaped, quite distinctive at the tip of the crus, with an oval articular surface for the junction with the head of stapes
Figure 3. Incus-native specimen.
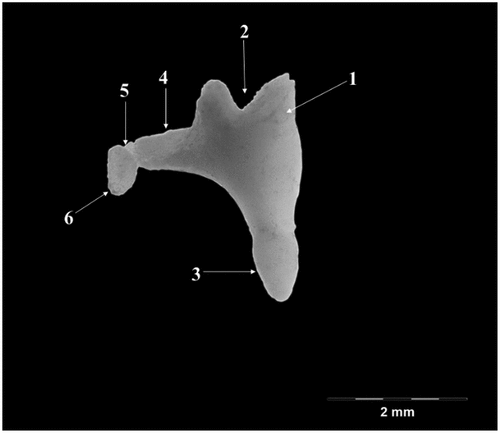
Figure 4. Incus-graphical representation.
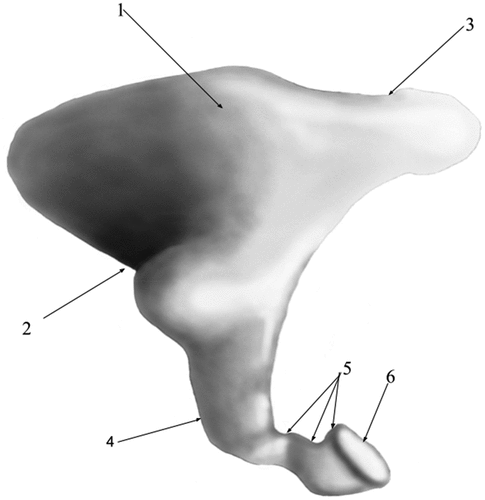
The stapes (Stapes) () is the innermost and the smallest of the ossicular assembly (), located in between the incus and the oval window. Almost triangular, with an oblique, ventro-medial and cranial disposition, it is composed of a head (Caput stapedis), dorsally placed, a base (Basis stapedis), ventro-medial and cranially placed linked by two crurae- the anterior crus (Crus rostrale) and the posterior crus (Crus caudale). The head of the stapes seems quite reduced, with a hemispherical articular surface for the lenticulate bone. Its caudal border bears a reduced tuberosity that serves as an insertion point for the stapedial muscle (m.stapedius)- the muscular process. Arising from the neck, the two crura, cylindrical and slightly divergent connect to the basal part of the bone. The crura are slightly different as far as their size is concerned, the posterior one being thicker than the anterior one. The caudal border of the posterior crus seems concave while the anterior border is straight. The anterior crus, much thinner, presents a posterior border that is slightly concave while its anterior border is convex. This makes the anterior crus as being somehow curved as appearance, while the posterior one seems quite straight. The ventral border of the head with the corresponding crural margins delimit the almost round intercrural space. The base (footplate) of the stapes is elliptically shaped, with a medial surface that serves as attachment point to the oval window.
Figure 5. Stapes-native specimen.
Discussion
Gross morphological features of the auditory ossicles
Generally, scientific literature that deals with the middle ear anatomy, more likely the ossicular chain anatomy, focuses on several rodents such as the rat and the Guinea pig, where several similarities with human anatomy were established (Albuquerque et al. Citation2009). Other species are also targeted in another series of studies such as the rat, mouse, gerbil, chinchilla that might be used as experimental species, with a series of pros and cons for this usage (Bergin et al. Citation2013).
For roe deer (Capreolus capreolus), the ossicular chain comprises the standard set of ossicles: the malleus, incus and the stapes. Some literature reports state the existence of the fourth ossicle (the lenticular bone) as a distinctive component as described in the donkey (Nazih Citation2017). Other ruminants we found as cited sources point to the cow (Costeur et al. Citation2016), sheep (Simaei et al. Citation2017; Péus et al. Citation2020), and buffalo (Nourinezhad et al. Citation2021), where the anatomical description of the ossicles is available in the same general manner. In studies published for other species (the naked mole-rat, Guinea pig, paca, and chinchilla), the authors mention a maleo-incal complex, resulted from the fusion of the maleus and incus in a single piece (Martins et al. Citation2015; Mason et al. Citation2016; Guan et al. Citation2019; Martonos et al. Citation2019).
The morphological aspects in case of the malleus in Capreolus capreolus are similar to those described in buffalo (Nourinezhad et al. Citation2021), donkeys (Nazih Citation2017), mice (Mohammadpour Citation2010) and even humans (Pracy et al. Citation1998).
The oval aspect of the head of malleus was reported also in sheep (Simaei et al. Citation2017) and swine (Pracy et al. Citation1998) while in cavimorphs, moles and bovids (Argyle & Mason Citation2008; Simaei et al. Citation2017) these features are slightly different.
The neck of the malleus shows the existence of three visible processes, opposite to the situation found in bovids and buffalo, where this segment is difficult to pinpoint (Nourinezhad et al. Citation2021). The three processes are noticeable in donkey (Nazih Citation2017; Gürbüz et al. Citation2019), rabbit (Kurtul et al. Citation2003), pig, and humans (Pracy et al. Citation1998). For the roe deer specimens, a worth-mentioning aspect refers to the muscular process, which is quite evident and conically shaped, somehow similar to the situation described in buffalo (Nourinezhad et al. Citation2021), while in the donkey, a reduced degree of development of this process is noted (Gürbüz et al. Citation2019).
The shape of the handle of malleus on a cross-section appears different from the situation found in other ruminants – buffalo, cow and sheep (Nourinezhad et al. Citation2021) where this profile seems more quadrilateral shaped
The incus follows mostly the classical features of the bi-radicular molar, with a slightly shorter appearance at the level of the body of the incus when compared to the cow- (Nourinezhad et al. Citation2021). In regards to the lenticular process, one must mention that this piece continues directly the long crus of the incus in medio-lateral direction. Similar facts were reported for goat ossicles (Martonos et al. Citation2021), dromedary camel (Nourinezhad et al. Citation2021), rabbit (Kurtul et al. Citation2003), rat (Li et al. Citation2015) and chinchilla (Martonos et al. Citation2019). This individualized piece of ossicle was also reported in some donkey individuals (Nazih Citation2017) and its absence was cited for sheep fetuses (Simaei et al. Citation2017)
The stapes is the smallest of the auditory ossicles. It is trapezoidal-shaped, similar to the features of the ossicle described in sheep (Nourinezhad et al. Citation2021), wolf (Gürbüz et al. Citation2019) and rabbit (Kurtul et al. Citation2003) while in other studied species, the ossicle is more like a rectangular piece. Such a note is made in the case of the buffalo (Nourinezhad et al. Citation2021), the cow (Costeur et al. Citation2016) and the pig (Pracy et al. Citation1998). Presence of the muscular process (that serves for the insertion of the stapedial muscle) was also noted for other species like the donkey, bovids, rabbit, and wolf (Kurtul et al. Citation2003; Costeur et al. Citation2016; Nazih Citation2017; Simaei et al. Citation2017; Gürbüz et al. Citation2019) while in humans and sheep fetuses it seems like the structure is just a small rough surface (Isaacson Citation2014; Simaei et al. Citation2017). The footplate of the stapes varies much as far as its shape is concerned from species to species. For the roe-deer, we have observed an elliptic shape, somehow similar to the bactrian camel (Bai et al. Citation2009; Nourinezhad et al. Citation2021), goat (Martonos et al. Citation2021), and chinchilla (Martonos et al. Citation2019). In sheep, this segment is described as almost squared (Nourinezhad et al. Citation2021).
The auditory ossicles: morpho-functional correlations
The middle ear, in its morphology, can be regarded as a pressure amplifier. The ossicular arrangement, its joints, ligaments, and muscles do nothing but change the efficiency of the sound transmission (Guelke & Keen Citation1952; Huttenbrink Citation1992; Chhan et al. Citation2016) and generate mechanical advantage, from the tympanic membrane to the oval window towards the cochlear system, depending on many factors such as friction between ear ossicles, masses of the eardrum and ossicular rigidity and flexibility of the membranes, ligaments, muscles and air density (Remy Werner Citation2003; Uziel et al. Citation1983; Pujol Citation2010) (). The malleus and the incus can be regarded as a type 1 lever that has a counter-clock rotational movement (sometimes named also swing (Guelke & Keen Citation1952)) as the tympanic membrane moves inward, pressing then against the internal crus of the stapes onto the oval window, with a cited ratio up to 19:1 effective tympanum to oval window (Guelke & Keen Citation1952; Lynch et al. Citation1998; Rémy Pujol Citation1989; Rosowski Citation1994) (). Same joint might be regarded as a saddle-like synovial joint that further on articulate with the stapes, intermediated by the lenticular bone that plays another significant role by being attached to the long process of incus by a mobile membrane that seems to be at least as mobile as the one that links to the stapes (Mason & Farr Citation2012). Another term used for the description of this assembly is “hinge-like rotational motion” (Péus et al. Citation2020) with leveraging capabilities. There is a direct relation between the surface area of the tympanic membrane, the surface of the footplate of the stapes, and the lever system of the middle ear, with a cited ratio between the two arms of the lever of aprox 1.3 (Uziel et al. Citation1983). By adopting this lever system to the actual measurements, mainly the ones that point to the elements forming the two arms of the lever, we can establish comparative data among related species that have available data (maleo-incal complex and stapes). More than that, some researchers suggest a complex calculation for the expected gain by this hinge-like rotation by multiplying the area ratio between the tympanic membrane (pars tensa) and oval window by the lever ratio of the malleo-incal complex.
The compared ratios are among the L manu-L mal, L head-L umbo (for malleus) and the incus the L sp-L lp ratio (). As suggested by the comparative figures available for the afford-mentioned species, calculated based on the two distinctive sets of measurements, some interesting facts arose here. The simple calculations of the total lengths of the malleus vs incus () show some higher ratio for the goat ossicles, as the ones for sheep and roe deer are not so distant. Values for the length of the long crus of incus (lever arm in the mentioned assembly) as opposed to the length of the stapes are showing higher values for goat, while the pair sheep-roe deer is similar. The malleus vs stapes total lengths are similar in all three compared sets of specimens.
Table III. Comparative measurements and ratios of the auditory ossicles in sheep, goat and roe-deer.
In terms of the measurements used in the assessment of some ratios based on the data suggested by Peus (Péus et al. Citation2020), an interesting series of facts are revealed. Although the Center of Mass (C.O.M) point was estimated in our approach for the available goat and roe deer samples, the data is slightly different for the measurement series. The different ratios () are pointing to a much clearer differentiation of ratios in the case of the roe-deer data from the ones calculated for sheep and goats. This separation of figures may be a better indicator for the accuracy of the measurements taken into consideration in direct relation to the functional aspects that point to the mechanics of hearing in these species, especially to the lever ratios mentioned in many investigations (Guelke & Keen Citation1952; Péus et al. Citation2020). Special attention is drawn towards the ratio of length of malleus and the length of the long crus of incus that shows a much smaller ratio for roe deer, as a possible differentiation that might be the result of another pattern in the transmission of vibrations along the complex system of the middle ear (Mason & Farr Citation2012; Péus et al. Citation2020).
Table IV. Comparative values and ratios for the auditory ossicles in sheep, goat and roe deer based on the functional measurements suggested by Peus 2020.
Conclusions
The present study, to the best of our knowledge, brings into light some new elements of the middle ear anatomy for a species relatively little studied. This makes the main purpose of the study: the complete morphological description of the ear ossicles, alongside some morphometrical data. All these, combined, with series of graphical elements are meant to serve as a useful didactical tool in the study of the comparative morphology of the middle ear and to point to new directions in this study such as the complex investigation of the morphology of the middle ear by means of CT scanning and interpretation of the inertial properties of the ossicular assembly in correlation to classical morphometrical data.
Author contributions
All authors contributed to the study concept and design. Material preparation, data collection and analysis were performed by Martonos Cristian and Gudea Alexandru. The first draft of the manuscript was written by Martonos Cristian and Gudea Alexandru. All authors commented on previous versions of the manuscript and read and approved the last version of this manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author, Gudea Alexandru upon reasonable request.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. It was approved by the Committee of Bioethics and Research Ethics from the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Romania (188/04.12.2019).
Consent to participate
This study did not involve human subjects or usage of biological material from humans. As the biological material was represented by game specimens placed in an osteological collection, consent from the owner was not necessary.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Albuquerque AAS, Rossato M, De Oliveira JAA, Hyppolito MA. 2009. Conhecimento da anatomia da orelha de cobaias e ratos e sua aplicação na pesquisa otológica básica. Brazilian Journal of Otorhinolaryngology 75(1):43–49. DOI: 10.1016/S1808-8694(15)30830-2.
- Argyle EC, Mason MJ. 2008. Middle ear structures of octodon degus (Rodentia: Octodontidae), in comparison with those of subterranean caviomorphs. Journal of Mammalogy 89(6):1447–1455. DOI: 10.1644/07-mamm-a-401.1.
- Bai Z, Wang H, Yuan G, ye W, He J, Wang J. 2009. A functional anatomy of the external and middle ear of the bactrian camel (Camelus bactrianus). Journal of Camel Practice and Research 16:115–120.
- Bergin M, Vlajkovic S, Bird P, Thorne P. 2013. Systematic review of animal models of middle ear surgery. World Journal of Otorhinolaryngology 3(3):71–88. DOI: 10.5319/wjo.v3.i3.71.
- Chhan D, Bowers P, McKinnon ML, Rosowski JJ. 2016. Middle-ear and inner-ear contribution to bone conduction in chinchilla: The development of Carhart’s notch. Hearing Research 340:144–152. DOI: 10.1016/j.heares.2016.02.015.
- Costeur L, Mennecarta B, Muller B, Schulzb G. 2016. Middle ear bones of a mid-gestation ruminant foetus extracted from X-ray computed tomography. In: Developments in X-Ray Tomography X (Vol. 9967). SPIE. DOI:10.1117/12.2238119.
- Čurlík J, Konjević D, Bujanić M, Sabol Z, Martinković F, Sindičić M. 2019. The first description of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in roe deer from Croatia. Helminthologia (Poland) 56(3):252–255. DOI: 10.2478/helm-2019-0015.
- Düzler A, Kabak M, Onuk B, Atalar K. 2015. Arterial supply of the cranial cervical ganglion in Roe Deer (Capreolus capreolus). Pakistan Journal of Zoology 47(2): 435–440.
- Frackowiak H, Jasiczak K, Pluta K, Godynicki S. 2007. Coronary arteries of the roe deer (Capreolus capreolus; Linnaeus 1758) heart. Polish Journal of Veterinary Sciences 10(2):105–108. http://www.ingentaconnect.com/content/docdel/art1073772716.
- Guan M, Zhang J, Jia Y, Cao X, Lou X, Li Y, Gao X. 2019. Middle ear structure and transcanal approach appropriate for middle ear surgery in rabbits. Experimental and Therapeutic Medicine 17(2):1248–1255. DOI: 10.3892/etm.2018.7064.
- Guelke R, Keen JA. 1952. A study of the movements of the auditory ossicles under stroboscopic illumination. The Journal of Physiology 116(2):175. DOI: 10.1113/JPHYSIOL.1952.SP004698.
- Gürbüz İ, Demiraslan Y, Orhun Dayan M, Aslan K. 2019. Morphometric and macroanatomic examination of auditory ossicles in male wolves (Canis lupus). Folia Morphologica (Poland) 78(3):600–605. DOI: 10.5603/FM.a2019.0011.
- Huttenbrink KB. 1992. The mechanics and the function of the middle-ear. Part 1: Normal ossicular chain and middle-ear muscles. Laryngo- Rhino- Otologie 71(11):545–551.
- Isaacson G. 2014. Endoscopic anatomy of the pediatric middle ear. Otolaryngology-Head and Neck Surgery 150(1):6–15. DOI: 10.1177/0194599813509589.
- Jeppesen JL. 1987. The disturbing effects of orienteering and hunting on roe deer (Capreolus capreolus). Danish Review of Game Biology 13(3): 1–24. Available: https://www.amazon.com/disturbing-effects-orienteering-Capreolus-capreolus/dp/B0007BHZUA. Accessed May 2021 30.
- Kabak M, Onuk B. 2010. Macro anatomical investigations of the cranial cervical ganglion in roe deer (Capreolus capreolus). Ankara Üniversitesi Veteriner Fakültesi Dergisi 57(1): 1–6.
- Konjević D, Jelenko I, Severin K, Njemirovskij V, Poličnik H, Pokorny B et al. 2012. Toward a reduction in tooth number: The case of P1 in roe deer from Slovenia. Italian Journal of Zoology 79(3):395–401. DOI: 10.1080/11250003.2011.654271.
- Kurtul I, Cevik A, Bozkurt EU, Dursun N. 2003. A detailed subgross morphometric study on the auditory ossicles of the new Zealand rabbit. Journal of Veterinary Medicine Series C: Anatomia Histologia Embryologia 32(4):249–252. DOI: 10.1046/j.1439-0264.2003.00483.x.
- Li P, Gao K, Ding D, Salvi R. 2015. Characteristic anatomical structures of rat temporal bone. Journal of Otology 10(3):118–124. DOI: 10.1016/j.joto.2015.11.002.
- Lorenzini R, Lovari S, Masseti M. 2002. The rediscovery of the Italian roe deer: Genetic differentiation and management implications. Italian Journal of Zoology 69(4):367–379. DOI: 10.1080/11250000209356482.
- Lynch TJ, Peake WT, Rosowski JJ. 1998. Measurements of the acoustic input impedance of cat ears: 10 Hz to 20 kHz. The Journal of the Acoustical Society of America 96(4):2184. DOI: 10.1121/1.410160.
- Maier W, Tröscher A, Ruf I. 2018. The anterior process of the malleus in extant Lagomorpha (Mammalia). Journal of Morphology 279(1):132–146. DOI: 10.1002/jmor.20759.
- Martins LL, Almeida-Silva I, Rossato M, Murashima AAB, Hyppolito MA, Machado MRF. 2015. Macroscopic description of the external and middle ear of paca (Cuniculus Paca Linnaeus, 1766). Pesquisa Veterinaria Brasileira 35(6):583–589. DOI: 10.1590/S0100-736X2015000600017.
- Martonos C, Damian A, Gudea AI, Bud I, Stan GF. 2019. Morphological and morphometrical study of the auditory ossicles in chinchilla. Journal of Veterinary Medicine Series C: Anatomia Histologia Embryologia 48(4):340–345. DOI: 10.1111/ahe.12446.
- Martonos C, Gudea A, Damian A, Lăcătuș R, Purdoiu R, Cocan D, Stan FG. 2021. Morphological and morphometrical aspects of the auditory ossicles in goat (Capra hircus). Journal of Veterinary Medicine Series C: Anatomia Histologia Embryologia 50(1):184–191. DOI: 10.1111/ahe.12617.
- Mason MJ, Cornwall HL, Smith ESJ. 2016. Ear structures of the naked mole-rat, heterocephalus glaber, and its relatives (Rodentia: Bathyergidae). PloS one 11(12):e0167079. DOI: 10.1371/journal.pone.0167079.
- Mason MJ, Farr M. 2012. Flexibility within the middle ears of vertebrates. The Journal of Laryngology and Otology 127:1–13. DOI: 10.1017/S0022215112002496.
- Mennecart B, DeMiguel D, Bibi F, Rössner GE, Métais G, Neenan JM et al. 2017. Bony labyrinth morphology clarifies the origin and evolution of deer. Scientific Reports 7(1):13176. DOI: 10.1038/s41598-017-12848-9.
- Mohammadpour AA. 2010. Morphological study of auditory ossicles in the mouse. Journal of Applied Animal Research 37(2):269–271. DOI: 10.1080/09712119.2010.9707139.
- Nazih AM. 2017. Anatomical study on the middle ear of donkey (Equus acinus). International Journal of Advanced Research in Biological Sciences (IJARBS) 4(8):110–121. DOI: 10.22192/ijarbs.2017.04.08.016.
- Nourinezhad J, Abedini M, Shamsi MM, Dabbaghi A, Janeczek M. 2021. Evaluation of the middle ear in water buffaloes (Bubalus bubalis) by gross anatomy and cone-beam computed tomography. Folia Morphologica 80(1):177–185. DOI: 10.5603/FM.A2020.0036.
- Péus D, Dobrev I, Pfiffner F, Sim JH. 2020. Comparison of sheep and human middle-ear ossicles: Anatomy and inertial properties. Journal of Comparative Physiology A 206(5):683–700. DOI: 10.1007/s00359-020-01430-w.
- Péus D, Dobrev I, Prochazka L, Thoele K, Dalbert A, Boss A et al. 2017. Sheep as a large animal ear model: Middle-ear ossicular velocities and intracochlear sound pressure. Hearing Research 351. DOI: 10.1016/j.heares.2017.06.002.
- Pfaff C, Schultz JA, Schellhorn R.2019. August 1. The vertebrate middle and inner ear: A short overview. Journal of Morphology. John Wiley and Sons Inc 280(8): 1098–1105. DOI: 10.1002/jmor.20880.
- Pracy JP, White A, Mustafa Y, Smith D, Perry ME. 1998. The comparative anatomy of the pig middle ear cavity: A model for middle ear inflammation in the human? Journal of Anatomy 192(3):359–368. DOI: 10.1046/J.1469-7580.1998.19230359.X.
- Pujol R. 1989. Anatomie et physiologie de la cochlée. Archives of Physiology and Biochemistry 97(4):51–78. DOI: 10.3109/13813458909105536.
- Pujol R. 2010. Imaging the cochlea: Milestones of the last decades. Audiological Medicine 8(2):45–49. DOI: 10.3109/1651386X.2010.494077.
- Rosowski JJ. 1994. Outer and middle ears. Comparative Hearing:Mammals 1:172–247. DOI: 10.1007/978-1-4612-2700-7_6.
- Roviani D. 2014. A lethal interaction between two female roe deer. Hystrix, the Italian Journal of Mammalogy 25(2):117–118. DOI: 10.4404/hystrix-25.2-10239.
- Sempéré AJ, Sokolov VE, Danilkin AA. 1996. Capreolus capreolus. Mammalian Species 538(538):1–9. DOI: 10.2307/3504309.
- Simaei N, Soltanalinejad F, Najafi G, Shalizar Jalali A. 2017. Anatomical and morphometrical study of middle ear ossicles in 2 to 3-month-old makouei sheep fetuses. Veterinary Research Forum : an International Quarterly Journal 8(3): 237–241. Available: http://www.ncbi.nlm.nih.gov/pubmed/29085612 Accessed March 2019 2
- Tóth B, Schally G, Bleier N, Lehoczki R, Csányi S. 2016. First description of spatial and temporal patterns of river crossings by European roe deer Capreolus capreolus (Mammalia: Cervidae): Characteristics and possible reasons. Italian Journal of Zoology 83(3):423–433. DOI: 10.1080/11250003.2016.1184332.
- Uziel A, Marot M, Pujol R. 1983. The Gunn rat: An experimental model for central deafness. Acta Otolaryngol 95(5–6):651–656. DOI: 10.3109/00016488309139458.
- Wang X, Gan RZ. 2016. 3D finite element model of the chinchilla ear for characterizing middle ear functions. Biomechanics and Modeling in Mechanobiology 15(5):1263–1277. DOI: 10.1007/s10237-016-0758-5.
- Wang L, Hong M, Xiao X, Ma J. 2005. Morphology and food particles distribution of the digestive tract of roe deer during winter. Acta Theriologica Sinica 25:361–366.
- Werner Y. 2003. Mechanical leverage in the middle ear of the American bullfrog, Rana catesbeiana. Hearing Research 175(1–2):54–65. DOI: 10.1016/S0378-5955(02)00709-8.
- Willett M, Johnson P. n.d. An introduction to image analysis using ImageJ. Available: https://www.southampton.ac.uk/~assets/doc/ImageAnalysis.pdf. Accessed July 2022 14