Abstract
We report the first isolation of a widely distributed free-living soil amoeba Leptomyxa (Amoebozoa, Leptomyxida) from endozoic conditions. The amoebae were detected after 14 days following inoculation of the intestine of the earthworm Lumbricus terrestris on an agar plate with Escherichia coli. The earthworm was collected from Calcaric Fluvisol (pH 7) in the Upper Váh region, north-west Slovakia. Observed amoebae were uninucleate, polypodial with typically branched pseudopodia, and morphologically resembled Leptomyxa australiensis. This study enlarges the range of amphizoic tendency in leptomyxid amoebae and indicates their interactions with Oligochaeta.
Introduction
Free-living amoebae (FLA) able to reproduce and occur in environmental in addition to endozoic conditions are termed amphizoic amoebae. Some species of FLA, such as Acanthamoeba spp., Balamuthia mandrillaris, Neagleria fowleri and Sappinia pedata, have caused numerous cases of severe infections in humans and in animals, frequently with fatal consequences (e.g. Foreman et al. Citation2004; Schuster & Visvesvara Citation2004; Qvarnstrom et al. Citation2009; Moussa et al. Citation2015; Siddiqui et al. Citation2016; Cope et al. Citation2020). Information about the isolation of other freshwater and soil amoebae from endozoic conditions is not so extensive, but their pathogenic potential was proved by findings of symptomatic and asyptomatic infections in animals, particularly in fishes (Dyková et al. Citation1997, Citation2005).
A recent comprehensive phylogenetic and morphological analysis of the order Leptomyxida Pussard and Pons, 1976 has led to the reclassification of several species along with the description of new species of the genera Leptomyxa Goodey, 1915; Rhizamoeba Page, 1972; and Flabellula Schaeffer, 1926 (Smirnov et al. Citation2017). At present, the family Leptomyxidae Pussard and Pons, 1976 includes 10 valid species of the genus Lepotomyxa Goodey, 1915 (Siemensma Citation1987; Del Valle et al. Citation2017; Smirnov et al. Citation2017; Smirnov Citation2018; Glotova et al. Citation2021), which have been isolated from river and lake sediments (Mrva Citation2003; Smirnov et al. Citation2009), moss (Matis & Mrva Citation1998), leaf litter (Mrva & Matis Citation2000), permafrost-affected soils (Shmakova et al. Citation2013), and plant roots (Ramirez et al. Citation2010; Smirnov et al. Citation2017). Species of this genus usually feed on bacteria, but fungi, yeasts and small amoebae have also been observed to be a food source (Del Valle et al. Citation2017; Smirnov et al. Citation2017).
Although it is known that FLA are an important part of the microbial community of intestinal contents in earthworms (Monroy et al. Citation2008) and may play a significant role in their nutrition (Bonkowski & Schaefer Citation1997), the data on the presence of FLA in internal organs of earthworms is scarce. Some experiments with artificial introduction of amoebae into the digestive tract of Lumbricus terrestris and Eisenia fetida have been performed (Rouelle Citation1983). However, only a single record of a free-living amoeba, Thecamoeba quadrilineata, isolated from an earthworm intestine was reported (Borovičková et al. Citation2019).
In this study, we present the first isolation of amoebae of the genus Leptomyxa from the intestine of the common earthworm Lumbricus terrestris and, at the same time, the first isolation of this genus from endozoic conditions.
Materials and methods
Individuals of Lumbricus terrestris Linnaeus, 1758 were examined for the presence of amoebae in their internal organs. Ten specimens of the earthworm were collected from Calcaric Fluvisol (Šály Citation2000) (pH 7) in the Upper Váh region, north-west Slovakia (49°11´30”N, 18°30´17”E) and stored in a glass vessel filled with the soil (up to ¾ of the volume), in a refrigerator at 6°C. The day after collection, an autopsy in sterile conditions with repeatedly sterilised dissecting instruments was performed. Before the autopsy, the surface of each annelid was sterilised with ethanol. The anterior and posterior end of each annelid was pinned in a dissecting dish; a cut into the medial part of the ventral side from posterior to anterior end followed. The seminal vesicles, intestine and all its contents were removed, smeared with a sterile needle on an agar surface and cultivated.
The organs of L. terrestris were inoculated separately in Petri dishes with 2% non-nutrient agar (NNA) and Escherichia coli providing a food source for amoebae. After this, 50 µL of Modified Neff’s amoeba saline (AS) solution was added (Page Citation1991). The cultures were obtained only from the intestine and were incubated at laboratory room temperature (22°C) and under undirected light.
The cultures were examined on a daily basis. Observation and documentation of isolated amoebae were performed with a Leica DM 2500 light microscope and Canon EOS 70D camera equipment. Morphology and locomotion of trophozoites were noted using the method of hanging drops, which provided appropriate conditions for amoeba movement (Page Citation1991). Cell shape, anterior and posterior end of trophozoites, shape and number of pseudopodia, hyaloplasm, type of nucleus and localisation and number of vacuoles were noted. The length (L), breadth (B), L/B ratio and size of hyaloplasm and organelles were measured in AxioVision Release 4.6.3.0 (Carl Zeiss, Jena, Germany) software. For identification, the works of Page (Citation1983, Citation1991), Smirnov and Goodkov (Citation1999), Smirnov and Brown (Citation2004), Smirnov et al. (Citation2009, Citation2011, Citation2017), Del Valle et al. (Citation2017), Smirnov (Citation2018) were used.
Results and discussion
A leptomyxid amoeba culture was obtained from a single specimen of L. terrestris, from its intestine which showed normal anatomy. The amoebae were detected on an agar surface after 14 days of cultivation. Initial mixed cultures of leptomyxid amoebae and acanthamoebae were massively contaminated by unidentified hyphae of moulds originating from the earthworm intestinal contents. Therefore, attempts to purify and isolate the amoebae were performed. However, the establishment of monoxenic cultures was not successful. The moulds persisted and successively destroyed the cultures after several days in each established subculture.
We identified isolated amoebae as members of the genus Leptomyxa Goodey, 1915. The amoebae were polypodial, with branch-extended pseudopodia and slow locomotion (), or formed an expanded, flattened irregular form (). Monopodial forms were not observed. The length of the cells ranged from 47.0 to 80.2 µm (average 65.4 µm), the breadth from 5.9 to 63.0 µm (average 39.0 µm). The pseudopodia frequently branched on their anterior end and individual branches extended from 12.5 to 29.7 µm. The shape of pseudopodia varied from expanded and shorter with almost digitiform outline, to narrow and long which formed a branched trophozoite shape (). Pseudopodia never formed anastomoses. A hyaline zone was situated on their rounded apical end, with maximum size of 3.9 µm. The posterior end of the cells formed a mass from which pseudopodia radiated. Although no uroidal filaments were noted, the uroidal part of the cells was occasionally covered with attached debris. During locomotion, not all the pseudopodia adhered well to the glass surface; some protruded towards the water column and adhered afterwards. Adhesion of trophozoites on a cover glass was observed after 90 minutes in a wet chamber.
Figure 1. Light microscopy of Leptomyxa sp. trophozoites from the intestine of Lumbricus terrestris during locomotion in vivo. a–c, Polypodial trophozoites with branch-extended pseudopodia and with vesicular nucleus in (b) and (c) (arrowheads) and contractile vacuoles in (a) and (b) (arrows). d, Expanded, flattened irregular form in slow locomotion. Scale bars = 10 µm.
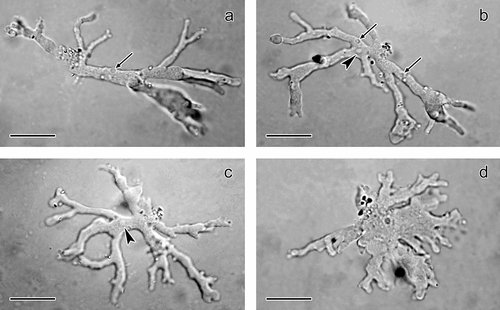
Several small contractile vacuoles, which did not exceed 2.5 µm, were located in various parts of the cell cytoplasm (). The size of the single vesicular nucleus reached about 3.2 µm (). Multinucleate cells were not detected. No cytoplasmic inclusions were present. Typical cysts belonging to the species of the genus Leptomyxa were not observed. Monoxenisation and purification of mixed cultures was not successful due to the high abundance of acanthamoebae.
On the basis of the overall morphology, and in comparison with the data in publications on leptomyxid amoebae, the amoebae were identified as Leptomyxa sp. Observed trophozoites were polypodial with typically branched pseudopodia, or formed an expanded, flattened irregular form. Monopodial forms were not observed. Generally, species of the genus Leptomyxa form polypodial, branched to monopodial cells (Smirnov et al. Citation2017) and therefore they are classified as branched and temporarily also to the monotactic morphotype (Smirnov & Brown Citation2004). The morphology of the present amoebae clearly differed from that of polypodial species from the order Euamoebida including the genera Amoeba, Chaos, Polychaos, Parachaos and Deuteramoeba which bear several cylindrical or sub-cylindrical pseudopodia that never take on an expanded or flattened irregular form (Smirnov et al. Citation2011). Therefore, these genera were excluded from the identification.
As the cytoplasm of the present amoebae contained a single nucleus, exclusively multinucleate Leptomyxa species can be excluded from the identification. From the other described species, the present amoeba is morphologically most similar to the soil species Leptomyxa australiensis. According to published descriptions, this species reaches 30–180 µm in size and more than 95% of the cells are uninucleate (Page Citation1991; Matis & Mrva Citation1998). The size of the present amoebae cultivated from the intestine of the earthworm was within the size range of L. australiensis. Similarity was noted also in the shape of the polypodial or flattened irregular forms. However, a detailed comparison revealed several differences. In our trophozoites we did not observe the formation of a monopodial form, which is typical for L. australiensis, and also the adhesive uroidal filaments were not detected. However, this may also be the result of poor adherence of the cells to the glass surface.
Among other uninucleate Leptomyxa species the present amoeba resembles, to a certain extent, three other species that take on flattened and/or polypodial forms. Leptomyxa neglecta (Smirnov et al. Citation2009) differs in the polypodial flattened form, which possesses distinctly shorter pseudopodia that narrow towards the tips and consist mostly of the hyaloplasm. The polypodial form of the species L. valladaresi, depicted in micrographs and a video sequence by Del Valle et al. (Citation2017), differs in having a lower number of pseudopodia which are also shorter than in the present amoeba. Moreover, both of the abovementioned species are typically found with monopodial limax-shaped locomotive forms, which are rather frequent during the locomotion. In this sense we can almost certainly exclude the species L. ambigua due to its dominant monopodial locomotive form (Smirnov Citation2018).
Findings of amoebae in invertebrates are not numerous. The presence of amoebae has been reported in crustaceans (Mullen et al. Citation2005; Nowak et al. Citation2010) and sea urchins (Dyková et al. Citation2007) using molecular methods. Patsyuk (Citation2017) isolated FLA from molluscs. Moreover, Mortazavi et al. (Citation2010) experimentally infected locust Locusta migratoria with Acanthamoeba spp. and observed the death of individuals up to 17 days after infection. Amoebae were present in the muscles, brain lesions, fat tissue and hemolymph of locusts.
The intestine of the earthworms is well studied, and conditions such as water content, organic compounds and pH were proved to change and to be different in comparison with the soil (Horn et al. Citation2003). Information has been published about bacteria (Singleton et al. Citation2003; Meier et al. Citation2018) and the effect of digestive enzymes on ciliates (Piearce & Phillips Citation1980). There is evidence that the transit through the intestine of earthworms could have a positive effect on the density of FLA (Monroy et al. Citation2008). However, except for a single record of Thecamoeba quadrilineata (Borovičková et al. Citation2019), amoebae in the digestive tract of Lumbricus terrestris have not been studied so far. Experimental inoculation of trophozoites of Acanthamoeba, Saccamoeba and Thecamoeba into Lumbricus terrestris and Eisenia foetida intestine was performed, but intestine section revealed only Acanthamoeba cysts three hours after inoculation (Rouelle Citation1983).
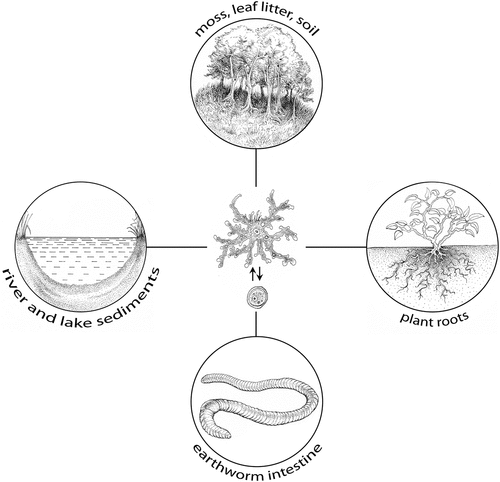
In we summarise the present knowledge about a relatively broad spectrum of habitats from which Leptomyxa amoebae were isolated. Although Leptomyxa amoebae have been isolated from natural habitats such as cold permafrost-affected soils (Shmakova et al. Citation2013), the survival of these amoebae in diverse environments and at various temperature levels is not well clarified. Ramirez et al. (Citation2010) successfully tested the survival of Leptomyxa sp. isolated from the root of water hyacinth Eichhornia crassipes (now Pontederia crassipes), even at 30°C. Although a wide range of thermotolerance is considered an important precondition for pathogenicity potential, the pathogenicity of Leptomyxa spp. has not been studied yet and none of the described species has been detected as a pathogen. However, within the order Leptomyxida, the genus Leptomyxa is related to the genus Flabellula Schaeffer, 1926, also detected from soil habitats (Esteban et al. Citation2006). Some strains of Flabellula have also been isolated from fish tissues (Dyková et al. Citation2008). At present we may assume that although species of the genus Leptomyxa do not represent a risk for man or homeothermic animals, the poikilothermic vertebrates and invertebrates may host them under certain conditions. The finding of Leptomyxa sp. in the earthworm intestine opens further questions. The majority of Leptomyxa species form cysts and we can also suppose these form in the present species; however, in this case they were not detected. Detection of Leptomyxa sp. in the earthworm gut may be explained not only by the ability of trophozoites to survive in endozoic conditions, but also by a passage of amoeba cysts through the earthworm intestine.
Figure 2. Known habitats of Leptomyxa spp. The life cycle of Leptomyxa spp. illustrated in the centre of the figure comprises trophozoite and cyst stages.
In conclusion, the present work enlarges our knowledge on the habitat spectrum of leptomyxid amoebae. Furthermore, it shows that an amphizoic tendency in FLA is common and we can expect further species isolated from endozoic conditions from various invertebrate and vertebrate taxa. Considering the available information, this is the first isolation of Leptomyxa sp. from the intestine of Lumbricus terrestris and also the first isolation of amoebae of the genus Leptomyxa from endozoic conditions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bonkowski M, Schaefer M. 1997. Interactions between earthworms and soil Protozoa: A trophic component in the soil food web. Soil Biology & Biochemistry 29(3–4):499–502. DOI: 10.1016/S0038-0717(96)00107-1.
- Borovičková T, Mrva M, Garajová M. 2019. Thecamoeba quadrilineata (Amoebozoa, Lobosa) as a new member of amphizoic amoebae—first isolation from endozoic conditions. Parasitology Research 118(3):1019–1023. DOI: 10.1007/s00436-019-06207-y.
- Cope JR, Ali IK, Visvesvara GS. 2020. Pathogenic and opportunistic free-living ameba infections. In: Ryan ET, Hill DR, Solomon T, Aronson NE, Endy TP, editors. Hunter’s tropical medicine and emerging infectious disease. 10th ed. London: Elsevier Inc. pp. 814–820. DOI: 10.1016/C2016-0-01879-X.
- Del Valle AOF, Lorenzo-Morales J, Maciver SK. 2017. Leptomyxa valladaresi n. sp. (Amoebozoa, Tubulinea, Leptomyxida), from Mount Teide, Tenerife, Spain. Experimental Parasitology 183:85–91. DOI: 10.1016/j.exppara.2017.09.017.
- Dyková I, Fiala I, Pecková H, Dvořáková H. 2008. Phylogeny of Flabellulidae (Amoebozoa: Leptomyxida) inferred from SSU rDNA sequences of the type strain of Flabellula citata Schaeffer, 1926 and newly isolated strains of marine amoebae. Folia Parasitologica 55(4):256–264. DOI: 10.14411/fp.2008.033.
- Dyková I, Macháčková B, Pecková H. 1997. Amoebae isolated from organs of farmed tilapias, Oreochromis niloticus. Folia Parasitologica 44:81–90.
- Dyková I, Nowak B, Pecková H, Fiala I, Crosbie P, Dvořáková H. 2007. Phylogeny of Neoparamoeba strains isolated from marine fish and invertebrates as inferred from SSU rDNA sequences. Diseases of Aquatic Organisms 74:57–65. DOI: 10.3354/dao074057.
- Dyková I, Pindová Z, Fiala I, Dvořáková H, Macháčková B. 2005. Fish-isolated strains of Hartmannella vermiformis page, 1967: Morphology, phylogeny and molecular diagnosis of the species in tissue lesions. Folia Parasitologica 52(4):295–303. DOI: 10.14411/fp.2005.040.
- Esteban GF, Clarke KJ, Olmo JL, Finlay BJ. 2006. Soil protozoa – An intensive study of population dynamics and community structure in an upland grassland. Applied Soil Ecology 33(2):137–151. DOI: 10.1016/j.apsoil.2005.07.011.
- Foreman O, Sykes J, Ball L, Yang N, De Cock H. 2004. Disseminated infection with Balamuthia mandrillaris in a dog. Veterinary Pathology 41(5):506–510. DOI: 10.1354/vp.41-5-506.
- Glotova AA, Loiko SV, Istigichev GI, Kulemzina AI, Abakumov EV, Lapidus AL, Smirnov AV. 2021. Description of Leptomyxa silvatica n. sp. (Amoebozoa, Tubulinea, Leptomyxida), a new soil amoeba species from Chernevaya taiga soil of West Siberia, Russia. Protistology 15:312–320. DOI: 10.21685/1680-0826-2021-15-4-7.
- Horn MA, Schramm A, Drake HL. 2003. The earthworm gut: An ideal habitat for ingested N 2 O-producing microorganisms. Applied and Environmental Microbiology 69(3):1662–1669. DOI: 10.1128/AEM.69.3.1662-1669.2003.
- Matis D, Mrva M. 1998. Nové meňavkovce (Rhizopoda) pre faunu Slovenska. Folia faunistica Slovaca 3:1–7.
- Meier AB, Hunger S, Drake HL. 2018. Differential engagement of fermentative taxa in gut contents of the earthworm Lumbricus terrestris. Applied and Environmental Microbiology 84(5):e01851–17. DOI: 10.1128/AEM.01851-17.
- Monroy F, Aira M, Domínguez J. 2008. Changes in density of nematodes, protozoa and total coliforms after transit through the gut of four epigeic earthworms (Oligochaeta). Applied Soil Ecology 39(2):127–132. DOI: 10.1016/j.apsoil.2007.11.011.
- Mortazavi PN, Goldsworthy G, Kirk R, Khan NA. 2010. Acanthamoeba produces disseminated infection in locusts and traverses the locust blood-brain barrier to invade the central nervous system. BMC Microbiology 10(1):186–194. DOI: 10.1186/1471-2180-10-186.
- Moussa M, Tissot O, Guerlotté J, De Jonckheere JF, Talarmin A. 2015. Soil is the origin for the presence of Naegleria fowleri in the thermal recreational waters. Parasitology Research 114(1):311–315. DOI: 10.1007/s00436-014-4197-x.
- Mrva M. 2003. Notes on active gymnamoebae (Rhizopoda, Gymnamoebia) in Turiec river. Folia faunistica Slovaca 8:23–26.
- Mrva M, Matis M. 2000. Meňavkovce (Rhizopoda) v hrabanke niektorých lokalít dubovo-hrabových lesov Malých Karpát (Západné Slovensko). Folia faunistica Slovaca 5:1–9.
- Mullen TE, Nevis KR, O´Kelly CJ, Gast RJ, Frasca S. 2005. Nuclear small-subunit ribosomal RNA gene-based characterization, molecular phylogeny and PCR detection of the Neoparamoeba from western Long Island Sound lobster. Journal of Shellfish Research 24:719–731. DOI: 10.2983/0730-8000(2005)24[719:NSRRGC]2.0.CO;2.
- Nowak BF, Bryan J, Jones SRM. 2010. Do salmon lice, Lepeophtheirus salmonis, have a role in the epidemiology of amoebic gill disease caused by Neoparamoeba perurans? Journal of Fish Diseases 33(8):683–687. DOI: 10.1111/j.1365-2761.2010.01158.x.
- Page FC. 1983. Marine gymnamoebae. Cambridge, UK: Institute of Terrestrial Ecology. pp. 54.
- Page FC. 1991. Nackte Rhizopoda. In: Page FC, Siemensma FJ, editors. Nackte Rhizopoda und Heliozoea. Stuttgart–New York: Gustav Fischer Verlag. pp. 1–170.
- Patsyuk M. 2017. Parasitic amoebae found in water bodies of Ukraine. Experimental Parasitology 183:81–84. DOI: 10.1016/j.exppara.2017.10.010.
- Piearce TG, Phillips MJ. 1980. Fate of ciliates in the earthworm gut: An in vitro study. Microbial Ecology 5(4):313–319. DOI: 10.1007/BF02020338.
- Qvarnstrom Y, da Silva AJ, Schuster FL, Gelman BB, Visvesvara GS. 2009. Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis. Journal of Infectious Diseases 199(8):1139–1142. DOI: 10.1086/597473.
- Ramirez E, Robles E, Martinez B. 2010. Free-living amoebae isolated from water-hyacinth root (Eichhornia crassipes). Experimental Parasitology 126(1):42–44. DOI: 10.1016/j.exppara.2010.01.020.
- Rouelle J. 1983. Introduction of amoebae and Rhizobium japonicum into the gut of Eisenia foetida (Sav.) and Lumbricus terrestris L. In: Satchell J, editor. Earthworm ecology: From Darwin to vermiculture. Dordrecht, Netherlands: Springer. pp. 375–381.
- Šály R. 2000. Morphogenetic soil classification system of Slovakia. Bratislava, Slovakia: VÚPaOP. pp. 76.
- Schuster FL, Visvesvara GS. 2004. Free-living amoebae as opportunistic and nonopportunistic pathogens of humans and animals. International Journal for Parasitology 34(9):1001–1027. DOI: 10.1016/j.ijpara.2004.06.004.
- Shmakova LA, Fedorov-Davydov DG, Rivkina EM. 2013. The amoeboid protists of cryogenic soils in the Kolyma Lowland. Soil Biology 46:1211–1218. DOI: 10.1134/S1064229314010116.
- Siddiqui R, Ali IKM, Cope JR, Khan NA. 2016. Biology and pathogenesis of Naegleria fowleri. Acta Tropica 164:375–394. DOI: 10.1016/j.actatropica.2016.09.009.
- Siemensma FJ. 1987. De Nederlandse Naaktamoeben (Rhizopoda, Gymnamoebia). Hoogwoud, Netherlands: Koninklijke Nederlandse Natuurhistorische Vereniging. pp. 136.
- Singleton DR, Hendrix PF, Coleman DC, Whitman WB. 2003. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae; Oligochaeta). Soil Biology & Biochemistry 35(12):1547–1555. DOI: 10.1016/S0038-0717(03)00244-X.
- Smirnov A. 2018. Fine structure of Leptomyxa ambigua n. sp. CCAP 1546/2 strain, formerly known as “Rhizamoeba flabellata” (Amoebozoa Tubulinea, Leptomyxida). European Journal of Protistology 62:95–100. DOI: 10.1016/j.ejop.2017.12.001.
- Smirnov AV, Brown S. 2004. Guide to the methods of study and identification of soil gymnamoebae. Protistology 3:148–190.
- Smirnov AV, Chao E, Nassonova ES, Cavalier-Smith T. 2011. A revised classification of naked lobose amoebae (Amoebozoa: Lobosa). Protist 162(4):545–570. DOI: 10.1016/j.protis.2011.04.004.
- Smirnov AV, Goodkov AV. 1999. An illustrated list of basic morphotypes of Gymnamoebia (Rhizopoda, Lobosea). Protistology 1:20–29.
- Smirnov A, Nassonova E, Fahrni J, Pawlowski J. 2009. Rhizamoeba neglecta n. sp. (Amoebozoa, Tubulinea) from the bottom sediments of freshwater Lake Leshevoe (Valamo Island, North-Western Russia), with notes on the phylogeny of the order Leptomyxida. European Journal of Protistology 45(4):251–259. DOI: 10.1016/j.ejop.2009.04.002.
- Smirnov A, Nassonova E, Geisen S, Bonkowski M, Kudryavtsev A, Berney C, Glotova A, Bondarensko N, Dyková I, Mrva M, Pawlowski J. 2017. Phylogeny and systematics of Leptomyxid amoebae (Amoebozoa, Tubulinea, Leptomyxida). Protist 168(2):220–252. DOI: 10.1016/j.protis.2016.10.006.
