?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The black stork Ciconia nigra is a rare species of bird that inhabits old forests near wetlands. The early 21st century has brought a regress of its population in north-eastern Poland. We verified the assumption that an important reason for the observed changes was the colonization of the study area by white-tailed eagle Haliaeetus albicilla, because we observed a correlation between decreasing numbers of the black stork and increasing numbers of the eagle. Based on the long-term monitoring, we analyzed changes in the numbers and distribution of the black storks’ nests and compared the colonization of the study area by the white-tailed eagle in the most extensive forest complex in Poland. We found 42 occupied territories of storks and no eagles in 1989 within the study area. In 2019, there were just 23 stork pairs, but the population of eagles increased to 13. We found a correlation between the rate of decline of the black storks population and the increase in the white-tailed eagles. In the first half of study period, storks’ nests have been distributed randomly, after which they became clustered. The pattern of nests distribution was influenced by the increase in the population of the eagle over time, with this ensuring the aggregation of the nests of storks in places more distant from the nests of eagles. Similarly, the nearest neighbor distance of storks’ nests was dependent on distances from the nests of eagles and the shares of territory accounted for by forests. We surmise that changes in the population size of storks were induced mainly by the growth of population of eagles. Storks avoided occupying nests less than 4.6 km from the eagles’ nests. Our study suggests that the determining role can be played by fear of the predator. Overall, it must be concluded that the protection of top predators can affect other co-occurring species in unexpected ways.
Higlights
In the Augustów Forest (north-eastern Poland), the number of the black stork Ciconia nigra over 30 years has strongly declined and the distribution of its occupied nests has changed from almost solitary to clustered.
The colonization of the study area by the white-tailed eagle Haliaeetus albicilla was a plausible reason for the observed changes.
Storks avoided occupying nests less than 4.6 km from the white-tailed eagles’ nests, which can be defined as a safety distance. The determining role can be played by the fear of the predator more than by direct predation.
Introduction
The black stork Ciconia nigra (BS) is a relatively rare species of migratory bird that inhabits old forest stands near wetlands and areas of shallow water. Its nests are placed on large, old trees, but shallow streams and other bodies of water are used as the feeding habitat. The range of the BS extends to temperate regions of Europe and the western part of Asia (Cramp & Simmons Citation1980), with the population across the European range being estimated at merely 9,800–13,900 pairs (BirdLife International Citation2021). In Poland, the BS is a widespread but not abundant species, present across almost the whole country. According to Tomiałojć and Stawarczyk (Citation2003), this was a species present in Poland for which the trend in the second half of the 20th century was upward, to the extent that the breeding range, as well as the population size, grew. While there had been perhaps 540 pairs in the country as of 1966, by 2000, the figure was 1100–1200 breeding pairs. By the beginning of the 2010s, the Polish population of the BS was estimated at 1400–1600 breeding pairs (Zieliński et al. Citation2011), while the figure for 2013–2018 was even as high as 1300–1900 (Chodkiewicz et al. Citation2019). However, there is evidence that the last 10 years have brought a regress of the population in Poland, with the decrease being possibly even as great as 30% (Chylarecki et al. Citation2018).
In the stronghold area of north-eastern Poland, a local decrease in BS population numbers has been documented in particular (Pugacewicz Citation2015; K. Henel, P. Mirski, and D. Zawadzka, unpubl. data). Further, it is worth noting that long-term downward trends also seem to characterize populations of the BS in the Baltic States (Treinys et al. Citation2009; Strazds et al. Citation2017; Konovalov et al. Citation2019; Väli et al. Citation2021) as well as in Belarus (Dmitrenok & Pakul Citation2018) and Ukraine (Horban & Bumar Citation2006; Bokotej & Dziubenko Citation2012). In turn, the population is stable in the Czech Republic (Pojer & Kameníková Citation2018). The leading causes include adverse changes in habitat conditions, primarily forest disturbance and human impact, and climate-mediated habitat degradation (Rosenwald & Lõhmus Citation2003). Certainly, populations of BSs might be affected negatively by intensive logging and where ages of final tree-cutting are lowered (Treinys et al. Citation2016; Banaś et al. Citation2019). However, an Estonian study shows how changes in the forest structure do not impact the decline in the population of the BS (Lõhmus et al. Citation2005). Meanwhile, the population numbers can be reduced due to the lowering of water tables, given the effect on food availability for nestlings (Pugacewicz Citation2015; Dmitrenok & Pakul Citation2018). Further insight has been that while the number of nests occupied has declined, breeding parameters did not change over a long-term study (Treinys et al. Citation2009). Another factor limiting the numbers of the BS might be connected with a lack of partners close to the limits of the range (Konovalov et al. Citation2019). The reproduction rate in the species can, in turn, be hit by the contamination of the eggs (Strazds Citation2015). In particular parts of the range, individual factors may have different importance.
An important factor not yet fully explored may be the adverse effects of top predators on the BS population. The goshawk (Accipiter gentilis) predation on the BS chicks in nests has been documented by camera traps in Belarus, Latvia (Dmitrenok & Pakul Citation2018; Strazds & Ose Citation2018), and Poland (D. Anderwald, unpubl. data). Still, we have only scarce information on a few cases. The recent decades have seen many species of birds of prey present in Europe rebuild their ranges and populations. Specific interactions among large-sized birds – above all predators – might be an important factor shaping their numbers and spatial distribution. Predators’ presence in the vicinity of their nests may generate areas of more intensive predation, which potential prey avoid (Rebollo et al. Citation2017). Another effect that has been poorly recognized so far in birds is the so-called landscape of fear, where the mere presence of an effective predator modifies the behavior of potential prey species (Lyly et al. Citation2015; Bleicher Citation2017). Non-lethal impacts of predators may alter the activity, space use and other behavioral responses of its prey (Salo et al. Citation2008).
In Central Europe, the white-tailed eagle (Haliaeetus albicilla) (WtE) is the largest avian predator living in temperate forests. In the 20th century, the European WtE population declined dramatically due to human persecution. However, the last decades did bring a population recovery and even further expansion of the WtEs (Treinys et al. Citation2011; Heuck et al. Citation2017). The above-mentioned broader trends are exemplified by Poland, where the 1994 population of 223 breeding pairs of the WtE is compared with the 2008 population of some 767 pairs (Zawadzka et al. Citation2009). The Polish population was estimated at 1400 pairs in 2017 (Chylarecki et al. Citation2018).
As a dominant top predator, the WtE can impact directly and indirectly other co-occurring bird species using persecution, predation, or competition, as well as generation of the landscape of fear. The BS can be adversely affected by competition over habitat against the WtE on the one hand and by the persecution by it on the other. The study from the Polesia region of Belarus noted that BS accounted for 12,6% of the WtE’s diet (Yurko Citation2016), which indicates the direct intense predation pressure exerted by the WtE at the local level at the very least. Reports from Latvia and Poland documented single cases of the direct and indirect impact of the WtE on the BS’s broods. This raptor species has destroyed both eggs and juveniles of BS. In response to its visits to BS’ nests, the storks changed nesting sites also when no direct harm has not been done (Strazds et al. Citation2017; Anderwald unpubl. data). According to Dmitrenok and Pakul (Citation2018), the WtE was responsible for the most significant reduction to the local population BS in Belarus.
The WtE and the BS are listed in Annex I to the Directive 2009/147/EC of the European Parliament and the Council of 30 November 2009 on the conservation of wild birds while enjoying strict protection under Polish law. Since 1984, the nests of both species have been provided further protection by establishing protection zones around nesting trees. Most of the analyzed nest in the Augustów Forest (AF) was in protection zones, which saved the potential nest habitats. This paper reports on a long-term study of the population trend and distribution of the BS in the largest forest complex of NE Poland. We analyzed available historical and recently collected data on the numbers and distributions of occupied nests of the BS over 30 years in the AF. Our observations led us to hypothesize that one of the important reasons for changes observed in BS population and nests distribution was the expansion in the population of the WtE, which has taken place in the region under study. More specifically, we test if: 1) the increase of the WtE’s breeding pairs in the area was sufficient to affect how nests of the BS were distributed, and 2) the distribution of nests of the BS depends on both the habitat conditions and the impact of the WtE.
Material and methods
Study area
The Polish part of the AF is in the country’s northeast (at 23°15ʹE, 53°54ʹN). It extends over some 1400 km2. The area is relatively flat, with elevations between 135 and 190 m a.s.l. The climate is rather cold, with a mean annual temperature of 6.5°C. The forest cover here is around 90%, while lakes account for a further 6% of the area. Tree stands are dominated by Scots pine Pinus sylvestris (78%), Norway spruce Picea abies (8%), black alder Alnus glutinosa (9%), silver birch Betula verrucosa (5%), and pedunculate oak Quercus robur (1%). The average age of tree stands here is 65 years but stands older than 100 years old account for about 15% of the overall forest area. Among the forest site types, mesic pine forest accounts for almost 40% of the area, while a further 27% comes in the form of mesic mixed/coniferous forest. Some 7% of the forest area is, in turn, the wet forest. There are more than 100 lakes in the AF, including 13 with an area of more than 1 km2. The largest lake has an area of almost 22 km2. Most of the lakes lie in the northern and central parts of the study area.
Wet forest sites are located mainly in the southern part of the AF, whereas mesic pine forest is present around the lakes in its northern part. The AF is included within Europe’s Natura 2000 network – as Special Protection Area for Birds PLB200002 “Puszcza Augustowska”. Nevertheless, most of the area comprises commercial stands managed by six Forest Districts of Poland’s State Forests National Forest Holding. The Lake Wigry National Park, covering some 150 km2, is located in the north-western part of the AF () (Sokołowski Citation2010; Zawadzki et al. Citation2020).
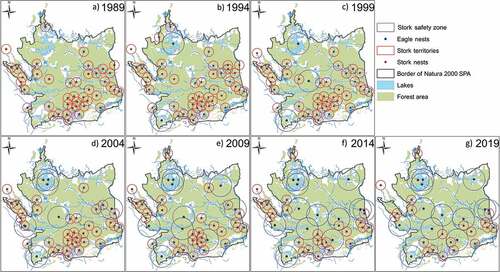
Figure 1. Distributional changes noted for the nests of the black stork and the white-tailed eagle in the period from 1989 through to 2019 (red points – black storks’ nests; red circles – black storks’ territories, constructed as a two-kilometer buffer; blue points – white-tailed eagles’ nests; blue circles – white-tailed eagles’ territories and safety distances for black storks; green – forests; blue – lakes and rivers).
Data collection and analysis
We analyzed data from 1989 to 2019 concerning active, occupied nests of the BS or its nesting places if the exact locality of the nest was unknown (two cases). All known nests were monitored throughout the study period, from finding the particular nest until it disappeared or disintegrated. All data on new and old occupied nests were collected. About 80% of the BS’s nests distribution and years of occupancy data came from the author’s direct field surveys. The rest (localisations and years of occupancy and abandonment) were reconstructed based on the basic information obtained from direct interviews with foresters (employees of the State Forests) and nature conservation services (employees of government agencies). In all the cases during the last 30 years, we have verified data about the BS’s nests locations, which we had obtained in the field. It was not possible to confirm the presence of a BS nest in all the locations identified by the collaborators. In the analyses, we used only data on nests about which we had complete information.
For about 5% of nesting sites, the probable location of the nests was determined based on criteria used in The Atlas of Breeding Birds in Poland. Probable nesting sites were determined based on regular observations of a pair of adult birds (in early April) near or in the old-growth forest; a pair of birds flying down into an old stand of trees suitable for nesting (Sikora et al. Citation2007). Observations of single birds in the study area or unverified information have all been rejected. GPS coordinates of nests were plotted on numerical forest maps. All known nests were controlled each year during the study, mainly in April or the beginning of May.
During the whole study period, we also searched nests of WtE. Each year, its nests were monitored, from finding a nest until it disappeared or disintegrated. We successively found new building nests or breeding activity centers of the WtE in the AF, starting with the detection of the first one in 1991 (Zawadzka & Zawadzki Citation1995), which was achieved by a regular, intense penetration of stands older than 100 years and located in the vicinity of big lakes, particularly in areas of frequent observations of WtEs (Zawadzka et al. Citation2006, Citation2017). We also used all information that came directly from foresters. Most of the detected nests of WtE were building within 1–3 years before they were found.
We analyzed changes in the numbers and distributions of active (occupied) nests or nesting places based on a breakdown into six 5-years-long periods. In each case, we estimated the nearest neighbor distances (NNDs) present among the nests of the BS and WtE and the minimum distances separating each nest of a BS from the nest of a WtE to determine changes in its distribution in the study area. For the analysis, we used only territories with known nests.
We found different types of nests’ occupancy of BS during this long study period. First – territory with a known nest used incessantly, sometimes for even 30 years. Second – two territories with all-year standing water – big beaver Castor fiber pools, with lots of observations, where the nest was remaining. Third type - a nest found during our studies; fourth –a territory with a known nest, which was abandoned during the study time. A territory was classified as active when we had either data about a nest used by the BS or numerous observations indicating its occupation. The territory was considered abandoned after the nest disintegrated or showed no signs of rebuilding for five consecutive years ().
We also addressed the issue of differences between the BS’s territories in terms of the habitat changes around the nests. We wanted to see if there were any habitat differences over the course and what was the impact of the habitat conditions during the study period. For this purpose, we associated each nest of a BS with a buffer zone of a radius of 2000 m, based on the half of the BSs’ Nearest Neighbor Distance (NND) during our study (about 4 km). Although BSs use much larger areas during the breeding season (Tucker and Heath (Citation1994); Jiguet and Villarubias (Citation2004)), we only analyzed the spatial distribution of nests. In each buffer, we collected data on the percentage shares of the area accounted for by forest and waters (FC [forest cover] and WA [water area], respectively, in line with digital mapping). We further measured the lengths of all the watercourses (rivers, streams, and channels) – LR. The historical maps show that the environmental factors listed above have not substantially changed in 30 years. The area of forest cover remains unchanged owing to the rules of forest management in the State Forests, which is why there was no interference such as land drainage or deepening in the course of streams (the Polish Water, unpubl. data).
By using forest site types’ maps, we also assigned the categories of mesic, wet, or boggy, with WS being the indicator revealing the share of the area included in the wet or boggy categories. No continuous variability was obtained for the proportion of forest cover and water surface (in the buffer of nests). In the case of forest cover, three groups of observations were obtained with values around 50%, 70%, and 90%. In the case of water surfaces, open water surfaces did not occur at all or in a negligible proportion of about 1–3%. The breakdown of variables into defined classes was as follows: forest cover <60%, 61–80% or 81–100%; water area 0%, >1%. Share of boggy or wet forest and distances separating the nests of BSs from the nearest nest of a WtE and the lengths of watercourses in that they were continuous variables. This spatial and mapping work was done with the ArcMap 10.8.1 system, while the data source was Poland’s Forest Data Bank, run by the State Forests (BDL 2020, htps://www.bdl.lasy.gov.pl/portal/mapy-en).
Statistical analysis
To determine the impact of habitat features and the influence of the WtE on the density of the occurrence of the BS’s nests, we performed an analysis using a linear mixed model for repeated measures observations. We used a hybrid model because it allows us to include a time factor instead of creating a series of time snapshots hindering the overall analysis. We did so for the entire study period and then for the periods during which the nests of BSs showed either a random or a clustered distribution (i.e., the 1989–1999 and 2004–2019 periods, respectively). We divided the data into two periods, considering the non-uniform influence exerted by the presence of WtEs over the study period. For this reason, we used the aggregation index after Clark and Evans (Citation1954) to assess the spatial pattern characterizing the locations of nests of BSs across the AF (clarkevans tests in spatstat, R). We also used logistic regression for repeated measurements (glmer in lme4, R) to examine the dependence between distance from black stork’s nests to the open water and BS’s nests occupancy and also the impact of the WtE’s colonization on the BS’s settling or abandonment of territories, as well as, ultimately, to check the potential presence of the so-called “safety distance” applying to BSs with respect to the WtE’s nests.
In analyses, we used data on the existing nests of the BS, checking to specify the active or the abandoned status in the context of each successive inventory. In each case, the distance separating the nest of a BS from the nearest nest of a WtE was measured with reference to GPS coordinates. This step was followed by the overall assessment of the relative impact of the WtE’s presence as opposed to the features of the habitat with regard to both the density and the location of the population of the BS. The dependent variable was thus the NND, i.e., the distance separating each nest of a BS from the closest nest of that same species. The explanatory variables were: (i) the distance separating each nest of the BS from WtE’s nest (DBSN); (ii) forest cover (FC); (iii) water area (WA); (iv) wet forest share (WS); and (v) lengths of watercourses (LR). The year served as a random variable. The influence of all the variables listed above on the NND was assessed using a linear mixed model (lme in nlme, R) for repeated measures observations, as follows:
where NNDij is the distance from a BS’s nest i to the nearest-neighbor BS’s nest in year j; α0 is the overall mean; α1 is the effect of the space separating nests of the BS from the nest of the WtE; α2 is the fixed effect of the forest; α3 is the fixed effect of water areas; α4 is the fixed effect of the share of bog and wet forest sites; α5 is the effect of the length of watercourses measure; α6 is the random effect of the year, and εij is the overall error.
The statistical analyses involving these variables and parameters were carried out using the R (version 4.0.3) statistical software (R Core Team Citation2020).
Table I. Measurement results describing distances between the black stork’s and the white-tailed eagle’s territories in the analyzed periods.
Results
Population dynamic
In 1989, the number of territories of the BS in the AF known to be occupied was 42 ()). Two years later, the nesting of the WtE was first recorded, with the next in 1993 ()). Subsequent periods of the study saw a decline in the number of breeding pairs of BS to 35 by 2004 and 22 by 2019. The population change noted for the WtE was seven pairs by 2004 and 13 by 2014 (), ).
Figure 2. Changes in the numbers of breeding pairs of the black stork and the white-tailed eagle (columns, eagle), and in the near neighbor distances characterizing nests of both studied species (lines, stork).
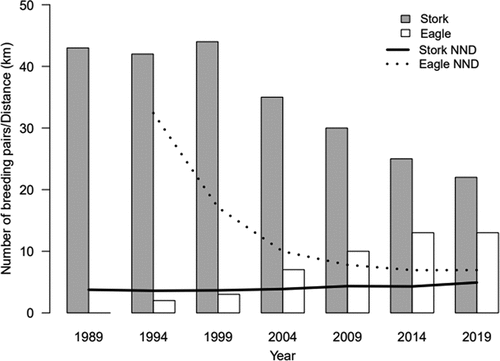
The colonization of the AF by the WtE was paralleled by a reduction in NNDs between the occupied nests of the species from 32.4 km in 1994 to just 8.1 km in 2019 (). In contrast, the NND for the nests of the BS’s changed only slightly (from 3.62 in 1989 to 4.04 km in 2019), where the calculations take no account of isolated “edge” pairs. The average rate of the decline in the population of BSs was 0.7 pairs/year, while the average rate of population increase characterizing WtEs was 0.43 pairs/year. Decrease among BSs and increase in growth among WtEs correlated strongly together (r = −0.969, p = 0.0003).
Spatial pattern to the locations of nests of the black storks
The 30-years-long period brought a marked change in the spatial distributions of territories noted for the two species under scrutiny ()). It was typical of WtEs to locate their nests in the old forest close to large lakes, while BSs showed a preference for moist patches of tree stands. The appearance of new nests of WtEs resulted in changes in the location of the active, occupied nests of BSs. The relationship between the NND and the distance separating the nests of BSs and WtEs was found to change over time as the population of the latter species increased (). During the first 10-years-long period (for as long as the WtE remained relatively rare), there was, nevertheless, a decline in the population density noted for the BS. The BS remained an abundant species, but its territories disappeared, and nests ceased to be occupied from places as far as 5 km away from the nests of the WtE ()).
Figure 3. The density of the occurrence of the nests of black storks (NND – nearest neighbor distance) as set against distances to the nests of white-tailed eagles at successive dates throughout the period 1994–2019 (a, b – random distribution; c-f – clustered distribution).
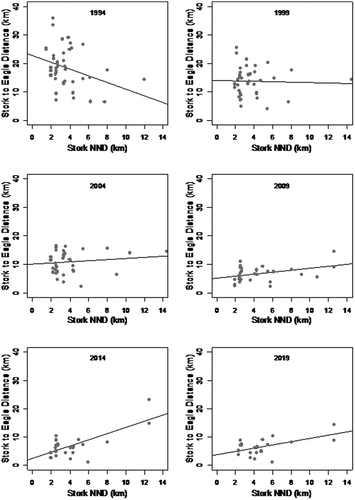
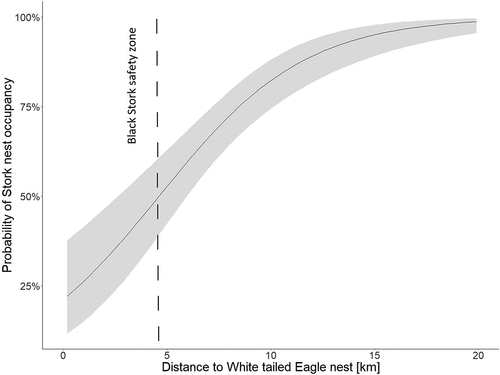
Figure 4. Impact of distance to lakes on the black stork’s nests occupancy throughout the period 2004–2019 – period of clustered storks’ distribution.
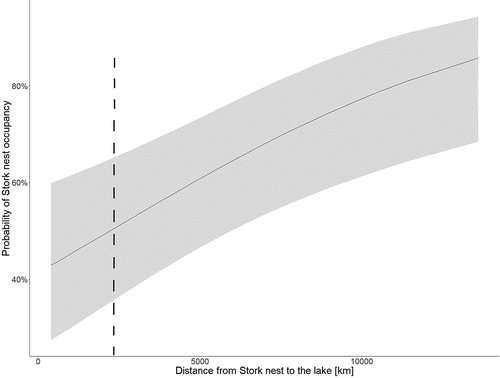
The period post-2000 coincided with a more tangible increase in the numbers of WtEs; the NNDs between BSs’ nests decreased in line with the closer “approach” of the nests of WtEs. The Clark-Evans index analysis of the locations of BSs’ nests shows these to have been distributed randomly through to 2004, after which they became clustered. The pattern of the distribution of nests was influenced by the increase in the population of the WtE over time, with this ensuring the aggregation of the nests of BSs in places more distant from the nests of WtEs. In practice, this denoted locations further from the open water, close to which the WtEs build their nests most often (). Logistic mixed model for period post-2000 (consumed data about nest occupancy in 5-year periods, distance from nest to the lake and time component) was as follows:
and showed that the distance to water was an important parameter for predicting of Stork’s nests occupancy (Z = 3.477, p = 0.0005), . This relation was not significant in previous (1989–2004) period (Z = 0.898, p = 0.369).
The effect was for the density of the occurrence of the BS’s population to remain relatively high, even as there was a decline in the overall numbers of breeding pairs of the BS, as well as an increase in the proximity of the territories of WtEs ()). Over the next 10 years (2009–2019), the nests of BSs that were more distant from their neighbors disappeared rapidly (), )).
The impact of habitat
Throughout the study period, distances between the nests of BSs were under the significant influence of the presence of the forest cover (F = 31.67, p < 0.0001) and water area (F = 4.29, p = 0.04). In contrast, no effect on distances between nests could be found in either the presence of WtEs nests (F = 0.95, p = 0.33) or wet forest area (F = 0.04, p = 0.85) or rivers length (F = 0.53, p = 0.47). The average distance between the known nests of BSs in the study area was 5.62 km, where the forest cover was the lowest but significantly smaller under the circumstances of high forest covers (70% or 90% classes). Differences between areas with and without open water were not significant ().
Table II. Impact of features of the habitat and of white-tailed eagles on near neighbor distances in 1989–2019, during the whole study time.
When it came to the period through which the nests of BSs presented a random distribution, the sole significant effect of distances between the nests was due to the forest cover (F = 16.70, p < 0.0001). No significance could be demonstrated for other traits (WA F = 1.63, p = 0.20; WS F = 0.099, p = 0.75; LR F = 0.096, p = 0.76), and these included the influence that WtEs were able to exert (F = 0.024, p = 0.876). Over the period, the average distance between the nests of BSs was 5.41 km when it came to the areas of the lowest forest cover (<60%), but significantly smaller where the forest cover was steadily higher, i.e., reduced by about 2.5 km and average distances were about 2.9 km. The effect of the remaining explanatory variables did not achieve significance ().
Table III. Impact of features of the habitat and of white-tailed eagles on nearest neighbor distances in 1989–2004, when nests exhibited random distribution.
Then, in turn, where the analysis turns to the period during which the nests of BSs had already assumed a clustered distribution, we found that the nests of BSs were separated by gaps dependent on the distance from the nests of WtEs, but also on the buffer-zone share of forest (). The distances separating BSs’ nests from that of WtEs were found to correlate positively with the NND. This means that the NND among nests was higher where distances from the nests of WtEs were greater, with the nests distributed at the highest density among BSs nesting in relatively close proximity to WtEs. The factors of the area of water (F = 2.527, p = 0.11), wet forest sites (F = 0.079, p = 0.78), and length of watercourses (F = 0.002, p = 0.97) were not found to be significant. However, the values for the NND were lower where the share of the forest cover within the territories of the BS was greater. Thus, during the period in question, buffer-zone areas with a percentage of the forest below 60% were associated with an NND of about 4.1 km. When the forests accounted for more than 60% of an area, the NND was about 1.6 km (). Average distances between the nests of BSs were significantly lower where the forest cover was greater (F = 8.26, p = 0.0005). Distances from BS’s to WtE’s nests were significant (F = 16.88, p < 0.0001). NNDs of BSs were bigger by about 0.2 km with every kilometer closer to the WtE’s nest ().
Figure 5. Relationships among distances to white-tailed eagles and the presence of forest cover on black storks’ near neighbor distance – NND(FC 50. 70. 90 percentage of forest cover).
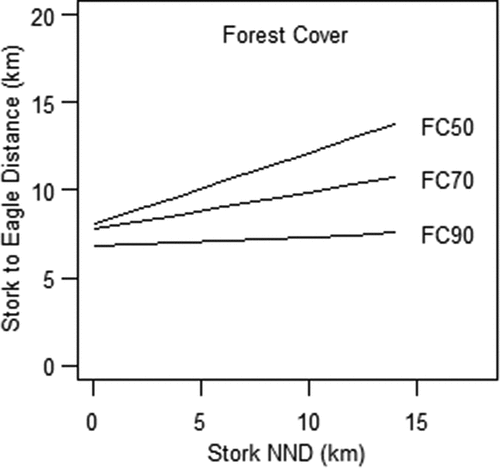
Table IV. Impact of features of the habitat and of white-tailed eagles on near neighbor distances in 2004–2019, when nests exhibited a clustered distribution.
Interspecies relations
To determine the nature of any dependent relationship between distances separating established nests of WtEs from established nests of BSs (and hence to identify any potential “safety distances”), the logit model elaborated for the parameterized logistic regression was as follows:
where: EN is the probability of nest occupancy by a BS, and DBSN is the distance from a BS’s nest to a WtE’s nest.
In essence, this model showed that, over the recent 30-year period, BSs came to abandon their nests and territories any closer than 4.6 km from the nests of WtEs (). Almost all territories of BSs present at shorter distances were abandoned (z = 6.369, p < 0.0001). The abandonment occurred in the first year (about 40% of nests) or between the second and the fourth year after the WtE’s settlement. This indicates the de facto generation by WtEs of nest-specific “stork non-safety zones”. Furthermore, each new settlement of a pair of the WtE resulted in BSs leaving the surrounding area. Occupation of nests by BSs was more stable in places located at greater distances from WtEs.
Discussion
The relationship between the black stork and the white-tailed eagle
The goal of our study was to document and explain the transformation in the population of the BSs in the AF and indicate the causes of it. The development of the population of the WtE was one of the essential factors that influenced the population number and distribution of BS in AF. We also documented, to some extent, the changes in the habitats. The term “transformation” seems reasonable, given how, within 30 years, WtEs have essentially retreated from over 50% of the territories once occupied and, indeed, from about 40% of the entire area of the AF. As WtEs have taken over habitats most suitable for them, BSs have been retreated to areas beyond the main areas of the territory of WtEs. Most nests of the latter are, in practice, located in the northern part of the studied forest complex, i.e., in the vicinity of the lakes that are present there. This leaves the southern part with a negligible share of lakes and a large share of wet forests, and it is here that the nests of BSs are now located.
It proved possible to note a robust correlation between the rates at which the population of the BS declined and the population of the WtE increased. In subsequent pentads of the study, there has been a shortening of the NND between the nests of WtEs by as much as 80%, while the NND between the nests of BSs increased steadily and by 20% overall. As WtEs colonized the AF in its majority, it was typical of BSs to leave their nests unoccupied in successive periods, i.e., those placed in zones around WtEs’ nests. This was confirmed in a logistic model showing how BSs disappear following the appearance of WtEs in some areas. In essence, then, the development of the population of the WtE was sufficient to halve the population of the BS present in the study area, and it happened over just 30 recent years.
In almost the whole of NE Europe within the breeding range of both species under scrutiny, the decline in numbers of the BS is signaled, parallel to the development of the WtE’s population. The population trends of these large bird species are opposite (Rosenwald & Lõhmus Citation2003; Lõhmus et al. Citation2005; Treinys et al. Citation2009, Citation2011; Zawadzka et al. Citation2009; Pugacewicz Citation2015; Strazds et al. Citation2017). Changes in the location and separation of the nests of BSs on the one hand, and those of WtEs on the other, reflect the latter’s appearance and the effects they can exert. A negative impact of the WtE on the BS (as manifested by the latter abandoning nests once an area has been settled by the former), as in our study, was also reported in Latvia, where the WtE has contributed to the reduction of the available range of the BS by pushing it out of former breeding sites (Strazds et al. Citation2017). Recordings from camera traps in Latvia and Poland have documented the destruction of the BS’s broods (both eggs and juveniles) by the WtE (Strazds et al. Citation2017; K. Henel, P. Mirski, unpubl. data). In turn, Konovalov et al. (Citation2019) documented with a camera the WtE’s occasional visits to the BS’s nest that did not lead to the destruction of the broods in Estonia. Indeed, one way these WtEs feed is by robbing the nests of large-sized birds (Cramp & Simmons Citation1980; Czubat et al. Citation2018). The authors have observed such behavior directly toward the white stork Ciconia ciconia chicks in the AF. Thus, it is likely that the WtE is able to kill chicks from BSs’ nests in the AF, but there is no indication that it is exerting strong pressure.
A direct accident of predation on adult BSs by the WtE has been reported in Germany (Langgemach & Henne Citation2001). In Belarus, BSs accounted for as much as 12.6% of the prey items noted in the WtE diet (Yurko Citation2016). In contrast, it needs to be conceded that the AF is somewhat different, with BSs constituting just 0.6% of the prey of WtEs. It is more typical of the WtE here to prey on white storks, which constituted 4.2% of its prey (Zawadzka et al. Citation2006). However, the recovering population of WtEs can also harm birds of prey. So far, it has been reported in the case of the black kite Milvus migrans and red kite Milvus milvus (Maciorowski Citation2017; Zawadzki et al. Citation2017) as well as the buzzard Buteo buteo (Kamarauskaitė et al. Citation2019).
The analysis of the composition of the diets of the AF’s WtEs shows that this raptor hunts mainly on lakes, than in open areas, and only sporadically within forests (Zawadzka et al. Citation2006). This indicates that BSs here can only fall prey to WtEs only accidentally. There have been two documented WtEs’ killings of young Estonian BSs with transmitters after they had left nests (Väli et al. Citation2021). All this data clarifies that the direct killings of adult birds or chicks by WtEs were not the main reason for the disappearance of occupied territories of the BS from the vicinity of WtEs’ nests. According to Creswell (Citation2011), the direct effect estimated through numbering individuals killed by a predator can only represent a relatively minor aspect of the overall population dynamics induced by a raptor. The most critical role can probably be played by fear of the predator – in our case, sufficient to “convince” BSs that they might not make safe use of space overlapping with the territories of the WtE. Storks seem to “fear” areas in the vicinity of the top avian predator, with the effect that their places of breeding are always removed to a safe distance capable of being termed the “safety distance”, created by the landscape of fear and, in our study, equal to 4.6 km. Fear of predators is an important factor shaping the behavior of animal species and, ultimately, its other characteristics, such as distribution and movement (Creel & Christianson Citation2008; Preisser & Bolnick Citation2008). High activity augments potential prey detectability and may result in higher probability encounters with mobile predators which in turn may increase the risk of being killing by predator (Salo et al. Citation2008).
The impact of habitat changes
Our study developed a model to indicate factors influencing BS population density. It turned out that the distance separating a BS’s nest from a WtE’s nest was a chief cause of the species distribution changes. According to the model, it is likely that this safety buffer around the BS’s occupied nests had the most significant impact on the BS’s selection of new places to breed and abandoning old ones. BSs engaged in a substantial level of avoidance of areas in the vicinity of WtEs, changing nests’ locations after a new WtEs appeared. However, predator pressure and persecution by WtEs were probably not the only reasons for BSs to experience declines in population and the changes in the location of occupied territories, as well as the creation of new nests, since factors relating to the habitat also proved essential to them, in particular, the share of areas accounted for by forest. The mixed model results confirm the importance of habitat quality in determining the population density among BSs. BSs show a preference for territories that have a high forest cover. This can be connected with a considerable extent of usability of such areas when it comes to foraging and the fact that WtEs use such areas of the forest only rarely for preying (Zawadzka et al. Citation2006). Moreover, in the area of high forest cover, BSs find much better conditions for choosing nest trees and hiding their nests (Zawadzki et al. Citation2020). Most waters in the study area are deep lakes unsuitable to be feeding grounds for wading BSs. The surfaces of open lakes represent typical places in which WtEs obtain prey and are thus avoided by BSs. The lack of a correlation between the length of watercourses and the BS’s density might be due to the possibility of birds foraging at a much greater distance from the nest than it was considered in our analysis, as indicated by Tucker and Heath (Citation1994) as well as Jiguet and Villarubias (Citation2004). This might mean that nests are located in safe places, even at the expense of being farther from optimal foraging grounds. Moreover, BSs feed along shallow streams and nearby small bodies of water (Drobelis Citation1993; Pugacewicz Citation2015; Olszewski et al. Citation2017). Our study could not demonstrate any significant relationships with the lengths of streams in areas occupied by BSs. Direct observations in the AF document how BSs can feed on small water-filled depressions on the floor of forest stands. The BSs’ preferences for a well-developed hydrographical network within forests have been documented in Lithuania (Drobelis Citation1993; Treinys et al. Citation2009). Marked declines in the population of the BS in the Białowieża Forest (eastern Poland) reflected a lowering of the water table and the resultant reduction in the abundance of food along streams and in the wet forest (Pugacewicz Citation2015). There is no data on the quantity of fish and amphibians in streams in the AF, though direct observations point to the drying up of shallow streams and wet forests during this long-term study.
However, changes in the numbers and distribution of the BSs might also have been caused by other factors. In populations with declining trends, reductions in numbers can also result from demographic factors, such as a lack of partners (Konovalov et al. Citation2019; Väli et al. Citation2021). In particular, disturbances caused by forest management, especially the intensity of logging as well as the tendency to lower the age of logging, are having a negative impact (Treinys et al. Citation2016; Banaś et al. Citation2019). On the other hand, Lõhmus et al. (Citation2005) showed that anthropogenic changes in the forest structure in Estonia were not responsible for such declines in population. Although direct effects of forest management on the habitat conditions for breeding populations of BSs in the AF were not assessed, results of our previous study had indicated that BSs require nesting trees that are much older than the ages of trees at the time of final cutting. Moreover, in recent decades, managed stands of the forest under study have been felled at successively lower age (Zawadzka et al. Citation2016; Zawadzki et al. Citation2020). However, the lowering the felling age could not be the deciding factor because it would negatively affect both the BS and the WtE. Both studied bird species require very old trees (older than the surrounding stands) for nesting, although they prefer different tree species and distinct forest surroundings (Zawadzki et al. Citation2020), which is why the population trends of the BS and the WtE would be similar, and yet they are opposite. There were no cases of direct occupation of the BS’s nests by the WtE. Moreover, changes related to forest management do not explain the changes in the distribution and concentration of BSs’ nests. The study in the AF did not include reproductive parameters and possible effects of breeding on changes in the population size or reasons for adult bird mortality other than the WtE. It cannot be excluded that taking these factors into account would slightly modify the results we obtained.
Concluding remarks
Our study confirms the hypotheses that the development of the WtE’s population in the AF was sufficient to affect the distribution of occupied nests of BSs. In turn, the distribution of BSs’ nests depends also on the forest habitat conditions, not only on the top raptor’s impact. The decline in the BS population and changes in nest distribution within the 30-years-long period was related mainly to the colonization of the forest by the WtE. We found that after the successive pairs of WtE had settled down, BSs left their nests nearby and occupied new territories at an average distance of at least 4.6 km from the initial WtE nests, defined as the “safety distance”. The reaction of storks to the territorial expansion of the WtE may have been due to the BSs’ fear of the WtE’s presence. The settlement of new pairs of the predator influenced the settlement of remote areas by the BS in order to avoid or minimize contact with the WtE. Measures to provide the active protection of the BS should mainly entail the improvement of the habitat conditions and saving the potential nesting stands, especially in areas away from the nests of the WtE. Given that it is impossible to reduce the population of the WtE, of particular importance is the preservation of patches of old, moist stands with old “veteran” trees. The most important factors identified to underpin changes in the distribution of BSs involved the degree of separation from the nests of WtEs, a high share of forest cover, and a limited area of open water. Together, these parameters generate the best model capable of explaining the properties of the distribution of BSs in the AF. It is advisable to continue monitoring the number and distribution of both bird species studied. Our results demonstrate how the appearance of one recolonizing species can take place at the expense of other species living in the same limited area, introducing changes to the use of scarce environmental resources and space. The emergence of large predators changes trophic and spatial relationships in the ecosystem. This is especially evident when one of the animals involved is a top predator species, such as the WtE.
Acknowledgements
We would like to express our gratitude to Adrian Bagnowski, Jarosław Borejszo, Joanna Harmuszkiewicz, Krzysztof Henel, Paweł Mirski, Dorota Piechowska, Wojciech Stankiewicz, Waldemar Sudnik, and Henryk Szuliński for their help in collecting data on the nests of black storks.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Banaś J, Zięba S, Bujoczek M, Bujoczek L. 2019. The impact of different management scenarios on the availability of potential forest habitats for wildlife on a landscape level: The case of the Black Stork Ciconia nigra (Linnaeus, 1758). Forests 10(5):362. DOI: 10.3390/f10050362.
- BirdLife International. 2021. IUCN red list for birds. Downloaded from. Available: http://www.birdlife.org. Accessed May 2021 14
- Bleicher S. 2017. The landscape of fear conceptual framework: Definition and review of current applications and misuses. PerrJ 5:e3772.
- Bokotej A, Dziubenko N. 2012. Badania i ochrona bociana czarnego Ciconia nigra L. na Ukrainie. Roczniki Bieszczadzkie 20:146–155.
- Chodkiewicz T, Chylarecki P, Sikora A, Ł W, Bobrek R, Neubauer G, Marchowski D, Dmoch A, Kuczyński L. 2019. The report under Article 12 of the Birds Directive in Poland for the period 2013-2018: Status, trends, threats. Biuletyn Monitoringu Przyrody 20:1–80.
- Chylarecki P, Chodkiewicz T, Neubauer G, Sikora A, Meer W, Woźniak B, Wylegała P, Ł Ł, Marchowski D, Betleja J, Bzoma S, Cenian Z, Górski A, Korniluk M, Moczarska J, Ochocińska D, Rubacha S, Wieloch M, Zielińska M, Zieliński P, Kuczyński L. 2018. Trends in bird numbers in Poland. Warszawa: GIOS.
- Clark PJ, Evans FC. 1954. Distance to nearest neighbour as a measure of spatial relationships in populations. Ecology 35(4):445–453. DOI: 10.2307/1931034.
- Cramp S, Simmons KLE. 1980. The birds of the Western Palearctic. Vol. 2. Oxford: Oxford University Press.
- Creel S, Christianson D. 2008. Relationships between direct predation and risk effects. Trends in Ecology & Evolution 23(4):194–201. DOI: 10.1016/j.tree.2007.12.004.
- Creswell W. 2011. Predation in bird populations. Journal of Ornithology 152(S1):251–263. DOI: 10.1007/s10336-010-0638-1.
- Czubat A, Sierakowski M, Szubart-Chodorowska E, Świerad R. 2018. Common buzzard Buteo buteo nestlings in nest of White-tailed Eagle Haliaeetus albicilla as an example of interspecific adoption. Ptaki Śląska 25:139–148.
- Dmitrenok MG, Pakul PA. 2018. The status of Black Stork population in Belarus Polesie. VII International Conference on Black Stork Ciconia nigra, Donana National Park, Spain. 28th-30th November 2018, Seville, Programme and Abstract Book, 10.
- Drobelis E. 1993. On the biology and protection of the Black Stork (Ciconia nigra L.) in Lithuania. Acta Ornithologica Lituanica 7-8:94–99.
- Heuck C, Herrmann C, Schabo DG, Brandl R, Albrecht J. 2017. Density-dependent effects on reproductive performance in a recovering population of White-tailed Eagles Haliaeetus albicilla. Ibis 159(2):297–310. DOI: 10.1111/ibi.12444.
- Horban I, Bumar H. 2006. Conservation biology of Black Stork Ciconia nigra in Ukrainian Polyssia. Biota 7(1–2):37–46.
- Jiguet F, Villarubias S. 2004. Satellite tracking of breeding black storks Ciconia nigra: New incomes for spatial conservation issues. Biological Conservation 120(2):153–160. DOI: 10.1016/j.biocon.2004.02.007.
- Kamarauskaitė A, Dementavičius D, Skuja S, Dagys M, Treinys R. 2019. Interaction between the White-tailed Eagle and Common Buzzard estimated by diet analysis and brood defence behaviour. Ornis Fenn 97(1):26–37.
- Konovalov A, Nellis R, Nellis R, Nurmla A, Sellis U, Ü V. 2019. Solitude at periphery: Lack of partners limits reproduction of the Black Stork (Ciconia nigra) at the margin of the distribution range. Ornis Fenn 96:13–23.
- Langgemach T, Henne E. 2001. Störche Ciconia ciconia, C. nigra und Kraniche Grus grus im Beutespektrum des Seeadlers Haliaeetus albicilla. Vogelwelt 122:81–87.
- Lõhmus A, Sellis U, Rosenvald R. 2005. Have recent changes in forest structure reduced the Estonian black stork Ciconia nigra population? Biodiversity and Conservation 14(6):1421–1432. DOI: 10.1007/s10531-004-9667-5.
- Lyly MS, Villers A, Koivisto E, Helle P, Ollila T, Korpimäki E. 2015. Avian top predator and landscape of fear: Responses of mammalian mezopredators to risk imposed by the golden eagle. Ecology and Evolution 5(2):503–514. DOI: 10.1002/ece31370.
- Maciorowski G. 2017. The occurrence and conservation of the black kite Milvus migrans in western Wielkopolska. Studia i Materiały CEPL 53:92–101.
- Olszewski A, Różycki A, Matusiak J. 2017. Habitat selection of nesting sites and types of nesting sites of black stork Ciconia nigra in the Kampinos National Park. Studia i Materiały CEPL 51:169–185.
- Pojer F, Kameníková J. 2018. The national Black Stork Census in the Czech Republic in 2014. VII International Conference on Black Stork Ciconia nigra, Donana National Park, Spain. 28th-30th November 2018, Seville, Programme and Abstract Book, 9.
- Preisser EI, Bolnick DI. 2008. The many faces of fear: Comparing the pathways and impacts of nonconsumptive predator effects of prey populations. PLoS One 3(6):e2465. DOI: 10.1371/journal.pone.
- Pugacewicz E. 2015. Black stork Ciconia nigra population decline in Białowieża Forest during 1985-2014. Dubelt 6-7:67–92.
- R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available: https://www.R-project.org/. Accessed January 2021 15
- Rebollo S, Martinez-Hesterkamp S, García-Salgado G, Pérez-Camacho L, Fernández-Pereira JM, Jenness J. 2017. Spatial relationship and mechanisms coexistence between dominant and subordinate top predators. Journal of Avian Biology 48(9):1226–1237. DOI: 10.1111/jav.01337.
- Rosenwald R, Lõhmus A. 2003. Nesting of the black stork (Ciconia nigra) and white-tailed eagle (Haliaeetus albicilla) in relation to forest management. Forest Ecology and Management 185(3):217–223. DOI: 10.1016/S0378-1127(03)00216-0.
- Salo P, Nordström M, Thomson RL, Korpimäki E. 2008. Risk induced by a native top predator reduces alien mink movements. Journal of Animal Ecology 77(6):1092–1097. DOI: 10.1111/j.1365-2656.2008.01430.x.
- Sikora A, Rohde Z, Gromadzki M, Neubauer G, Chylarecki P, eds. 2007. The atlas of breeding birds in Poland 1985-2004. Poznań: Bogucki Wydawnictwo Naukowe.
- Sokołowski AW. 2010. The Augustów Forest. Warszawa: CILP.
- Strazds M. 2015. Recent impact of DDT contamination on Black Stork eggs. Journal of Ornithology 156(S1):187–198. DOI: 10.1007/s10336-015-1244-z.
- Strazds M, Kuze J, Ose L. 2017. Impact of growing white-tailed eagle population on declining black stork population in Latvia. Abstracts of International conference Sea Eagle 2017 Conference, 5-7 October, Roosta, Estonia, 113.
- Strazds M, Ose L. 2018. New data on black stork nest predators in Latvia, VII International Conference on Black Stork Ciconia nigra, Donana National Park, Spain, 28th-30th November 2018, Seville, Programme and Abstract Book, 28.
- Tomiałojć L, Stawarczyk T. 2003. The avifauna of Poland. Distribution, numbers and trends. Polskie Towarzystwo Przyjaciół Przyrody ”pro Natura”. Wrocław.
- Treinys R, Dementavičius D, Mozgeris G, Skuja S, Rumbutis S, Stončius D. 2011. Coexistence of protected avian predators: Does a recovering population of White-tailed Eagle threaten to exclude other avian predators? European Journal of Wildlife Research 57(6):1165–1174. DOI: 10.1007/s10344-011-0529-7.
- Treinys R, Mozgeris G, Skuja S. 2016. Can intensified forestry be responsible for changes in habitat usage by the forest dwelling black stork? European Journal of Forest Research 135(6):1175–1186. DOI: 10.1007/s10342-016-1003-6.
- Treinys R, Stončius D, Augustis D, Skuja S. 2009. Breeding habitat of the black stork Ciconia nigra in Lithuania: Implications for Conservation Planning. Baltic Forestry 15(1):33–40.
- Tucker GM, Heath MF. 1994. Birds in Europe: Their conservation status. BirdLife Conservation Series No. 3. Cambridge, UK: BirdLife International.
- Väli U, Nellis R, Kaldma K, Vainus O, Sellis U. 2021. Must-toonecure arvukus, sigimisedukus ja ellujäämus Eestis aastatel 1991-2020. Hirundo 34(2):20–39.
- Yurko VV. 2016. Diet of the White-tailed eagle during the breeding season in the Polesski State Radiation-Ecological Reserve, Belarus. Raptor Research 32:21–31.
- Zawadzka D, Drozdowski S, Zawadzki G, Zawadzki J. 2016. The availability of cavity trees along an age gradient in fresh pine forest. Silva Fennica 50(3). Silva Fenn 50 article 3 id 1441.13p. DOI: 10.14214/sf.1441.
- Zawadzka D, Mizera T, Cenian Z. 2009. Population dynamics of the White-tailed Eagle Haliaeetus albicilla in Poland. Studia i Materiały CEPL 22:22–31.
- Zawadzka D, Zawadzki J. 1995. Preliminary characteristics of the Lake Wigry National Park avifauna. Notatki Ornitologiczne 36(3–4):297–309.
- Zawadzka D, Zawadzki J, Sudnik W. 2006. Population development, habitat requirements and ecology of the White-tailed Eagle Haliaeetus albicilla in the Augustów Forest. Notatki Ornitologiczne 47(4):217–229.
- Zawadzka D, Zawadzki G, Zawadzki J, Sołtys A. 2017. Population dynamic and reproductive parameters of the White-tailed eagle Haliaeetus albicilla in the Augustów Forest. Studia i Materiały CEPL 53:68–81.
- Zawadzki G, Zawadzka D, Sołtys A. 2017. Numbers and distribution of the black kite and red kite in Augustów Forest. Studia i Materiał y CEPL 53:82–91.
- Zawadzki G, Zawadzka D, Sołtys A, Drozdowski S. 2020. Nest sites selection by the white-tailed eagle and black stork – Implications for conservation practice. Forest Ecosystems 7(1):59. DOI: 10.1186/s40663-020-00271-y.
- Zieliński P, Profus P, Czuchnowski R. 2011. Present situation of the Black Stork (Ciconia nigra) in Poland. 8th conference of the European Ornithologists’ Union, Riga, 27-30 August 2011, Latvia.