Abstract
Scorpion venom plays an important role in scorpion predation, competition, communication, and defense. In this study, the species identification of Shandong scorpion and the analysis of venom extracted from male and female scorpion at the transcriptome level and their activity were compared. Whole de novo transcriptomes were performed on male and female scorpions and the sequences of cytochrome c oxidase subunit I were screened to identify the species. The scorpions collected were identified as Mesobuthus martensii and we have successfully annotated 16,726 (37.47%), 10,076 (22.57%), 10,878 (24.37%), and 10,187 (22.82%) unigenes with NR, Swissport, GO and KEGG database. A total of 17 kinds of toxins and 181 toxin-related unigenes were screened. There is no accurate identification of the differences between male and female scorpions at the molecular level and these need to be further explored. The difference ratio in toxins was 6.08%, and this number in unigenes was 0.78%. There are seventeen toxins and other toxins related genes were analyzed in male and female scorpions, among which the representative phospholipase, makatoxin toxin, and plancitoxin toxin were all related to the biological functions of scorpion martensii. EF chiral domain in phospholipase: helix turn helix is related to calcium channel toxin; The QWAKYGN base sequence contained in makatoxin toxin is α-toxin specific domain; It was found in plancitoxin I toxin that it contains two deoxyribonuclease II active sites, HEK, and DHSK. Since calcium channel toxins are associated with α- toxins belong to neurotoxins. Therefore, phospholipase and makatoxin are both toxins that act on the nervous system, while plancitoxin I toxin has deoxyribonucleotidase II activity and can degrade DNA in mammals. We constructed structure analysis including sequence alignment, 3D modeling, and phylogenetic analysis in phospholipase, makatoxin, and plancitoxin. There was little difference showed in GO and KEGG analysis and the differences ratio in unigenes annotated as toxins was much higher than that in all unigenes. What’s more, the evaluation of toxin activity showed that the toxicity of female scorpion was likely to be higher than that of male scorpion. The method can be used as a useful tool to evaluate the difference between male and female animals.
1. Introduction
As one of the most poisonous animals, scorpions, will attack the predators using their venom to protect themselves from being killed (Yuri, Citation2015). There were different toxin pools and peptides in their venom which was synthesized and secreted by their special glands (Santibáñez-López et al. Citation2015). After being stung by scorpion, the symptoms including dizziness, night sweats, convulsions, and dyspnea may occur and these symptoms may vary along the severity of the sting(Toshie et al. Citation2014). Mild injuries lead to local swelling, severe pain, blisters, necrosis, electrocution-like pain, headache, irritability, and nausea while the severe can cause anaphylactic shock, chest tightness, dyspnea, sweating, blood pressure dropping, and death. However, scorpion venom also contains potential therapeutic molecules, which also have a fatal impact on human beings (Louis Citation1980). Studies have found that scorpion venom is a kind of bioactive substance with complex components, including trimethylamine, taurine, betaine, etc.. According to the mode of their action, toxin proteins can be divided into two types: neurotoxin and cytotoxin. Neurotoxins are a class of short peptides composed of 50–70 amino acids, most of which are basic proteins (He et al. Citation2000), while cytotoxin has the function of direct hemolysis (Keusch & Mary Citation1975).
Mesobuthus martensii is widely distributed in China, especially in Henan, Hebei, Shandong, and Shanxi provinces (Mao et al. Citation2017). Morphologically, their cephalothrax are greenish-brown, and the anterior abdomens are composed of seven segments. Their backs are greenish-brown and the back abdomens are brown(Miller et al. Citation2010). They have positive tendency to temperature and humidity, and a negative tendency to strong light, sound, and vibration. Mesobuthus martensii is a kind of traditional Chinese medicine that has the function of wind, phlegm, collateral, and detoxification according to the theory of Traditional Chinese Medicine, owing to their strong toxicity (de Oliveira et al., Citation2015; Quintero-Hernandez et al. Citation2015). In addition, they play important roles in the treatment of many nervous system diseases, breast diseases, analgesia, bacteriostatic, ear diseases, facial paralysis, cerebrovascular injury, hemiplegia, migraine, tuberculosis, epilepsy, mastitis, breast fibroma, otitis media, and so on(Shi et al. Citation2016).
The characteristics of scorpion venom are also reflected in gender (Rodríguez-Ravelo et al. Citation2015). Since female scorpions have the mission and responsibility to breed offspring and protect their cubs, and in this process, they need a lot of nutrition to obtain energy and highly toxic scorpion venom that can protect themselves and their cubs, we infer that the toxicity of female scorpion venom may be higher than that of male scorpion venom. Therefore, we aim to provide a comparison of the expression level and toxin activity of male and female scorpions in Mesobuthus martensii and a study of Mesobuthus martensii venom and its related genes, so as to promote a better understanding of the differences between male and female scorpion venoms and lay a foundation for the discovery of new scorpion toxin components.
2. Materials and methods
2.1. Scorpion collection
In July 2019, Professor Liu Qing of Shanxi Agricultural University was entrusted to collection scorpions from Shandong Province. The scorpions were mailed back to Shanghai in August 2019.
2.2. Molecular identification of species
First, we extracted RNA with Trizol method. The total RNA quantity and purity were analysis of Bioanalyzer 2100 and RNA 1000 Nano LabChip Kit (Agilent, CA, USA) with RIN number >7.0. Poly(A) RNA is purified from total RNA (5ug) using poly-T oligo-attached magnetic beads using two rounds of purification. Following purification, the mRNA is fragmented into small pieces using divalent cations under elevated temperature. Then, the cleaved RNA fragments were reverse-transcribed to create the final cDNA library in accordance with the protocol for the mRNA-seq sample preparation kit (Illumina, San Diego, USA), the average insert size for the paired-endlibraries was 300 bp (±50 bp). And then we performed the paired-end sequencing on an IlluminaHiseq4000 at the (LC Sciences, USA) following the vendor’s recommended protocol and used the extracted RNA to obtain COI sequences and compare them with the database. COI (mtDNA cytochrome c oxidase subunit I) sequences were collected from the NCBI nucleotide database (https://www.ncbi.nlm.nih.gov/nuccore/).
As a catalytic subunit, cytochrome c oxidase subunit I (CO I) has been used as a common identification molecule because of its high conservatism. The gene ID number of CO I is screened in the NR database and according to its ID number, the mtDNA sequence and protein sequence of CO I gene were screened in the CDS database. The homologous segment sequence was selected for comparative analysis using the online tool BLASTn of NCBI. MEGA7.0 was used to calculate genetic distance based on K2P method. Next joining and Maximum likelihood were used to build the phylogenetic tree.
The evolutionary tree was constructed by the Neighbor-Joining method based on the General Time Reversible model. The bootstrap consensus tree inferred from 1,000 replicates was taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches (Felsenstein Citation1985). A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.8671)). The analysis involved 65 nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 413 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al. Citation2016).
2.3. Transcriptomics
2.3.1. RNA sequencing
Total RNA is isolated from the telson of scorpions. After all RNA extracted from the sample is qualified, the eukaryotic mRNA is enriched with magnetic beads connected with oligo (dT). The extracted mRNA is randomly interrupted into short fragments by a fragmentation buffer. Fragmented mRNA is used as a template, and a single-strand cDNA is synthesized using random hexamers. Then, buffers, dNTPs, RNaseH, and DNA polymerase I perform double-stranded cDNA synthesis. AMPure XP beads were used to purify the double-stranded product. T4 DNA polymerase and klenow DNA polymerase activity were used to repair the sticky ends of DNA to blunt ends. Base 3 was added at the 3 ‘end and a linker was added. AMPureXP beads were used for fragment selection, and then USER enzyme was used. The second strand of cDNA containing U was degraded and PCR amplified to obtain the final sequencing library. The library was from mRNA and using Illumina Novaseq™ 6000. The total RNA quantity and purity were analyzed through Bioanalyzer 2100 and RNA 1000 Nano LabChip Kit (Agilent, CA, USA) with RIN number >7.0. Next, the mRNA is fragmented into small pieces using divalent cations under elevated temperature. Then, the cleaved RNA fragments were reverse-transcribed to create the final cDNA library in accordance with the protocol for the mRNA-seq sample preparation kit (Illumina, San Diego, USA), the average insert size for the paired-end libraries was 300 bp (± 50 bp). And then we performed the paired-end sequencing on an Illumina Novaseq™ 6000 at the (LC Sciences, USA) following the vendor’s recommended protocol.
After cutting adapters and unqualified sequences are filtered out using Cutadapt to obtain valid data (clean data) and then processed. The next step of analysis is as follows: remove the adapter sequence from sequencing reads, then, perform a window quality scan on sequencing reads. The default scanning window is 6 bp. When the average quality value in the window is less than 20, the read is truncated from the beginning of the window to the end of 3. Remove poly A/T, remove sequences shorter than 100 bp after truncation, and remove sequences with truncated N content above 5%. Lastly, count the original sequencing quantity, the effective sequencing quantity, Q20, Q30, GC content, and conduct comprehensive evaluation.
Cutadapt and Perl scripts in house were used to remove the reads that contained adaptor contamination, low-quality bases and undetermined bases. Then sequence quality was verified using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), including the Q30 and GC-content of the clean data(Andrews et al. Citation2010).
2.3.2. Annotation of transcripts
De novo assembly of the transcriptome was performed with Trinity 2.4.0. Trinity groups transcribed into clusters based on shared sequence content. Such a transcript cluster is broadly referred to as a “gene”. The longest transcript in the cluster was chosen as the “gene” sequence (aka Unigene).
The strategy of mixed assembly of all samples is adopted to normalize all samples to get unigene (unigene is the only one after all genes in each sample are de redundant and de duplicated). Next, we will evaluate the assembly quality of these Unigene, including the length, GC content, and N50 of unigenes. N50 can be used as a standard to judge the splicing results of genome and transcriptome, which refers to the length of the assembled results when they reach half of the total length.
Unigenes were obtained for functional annotation of unigenes. The databases used for gene function annotation were the NCBI non-redundant (NR) (http://www.ncbi.nlm.nih.gov/protein/), SwissProt (http://web.expasy.org/docs/swiss-prot_guideline.html), Gene Ontology (GO, http://geneontology.org/), Kyoto Encyclopedia of Genes and Genome (KEGG, http://www.kegg.jp/), and evolutionary genealogy of genes: Nonsupervised Orthologous Groups (eggNOG, http://eggnog.embl.de/version_4.0.beta/) database.
2.3.3. Survival analysis
ICR mice (25 ± 2 g, provided by the Experimental Animal Center, Naval Medical University, Shanghai) were injected with PBS-diluted male and female scorpion venom (0.125 mg/kg~0.5 mg/kg, n = 10) through the tail vein, and then observed for 16 h. Dead mice were recorded. All animal experiments were approved by the Ethics Committee of Naval Medical University.
2.3.4. Blood biochemical analysis
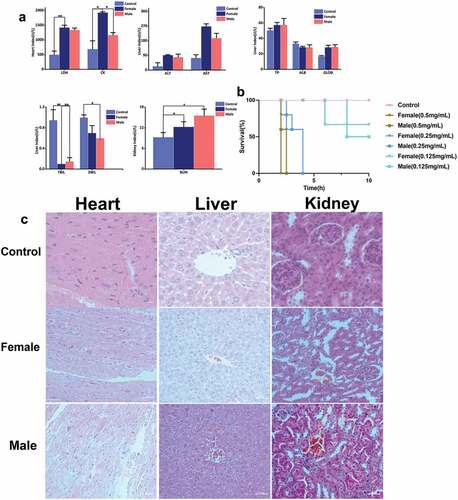
ICR mice were injected with 1 mL/kg of male and female scorpion venom. Blood samples were drawn from 5 survivors 6 h after injection. Meanwhile, blood samples in the negative group were taken from another five PBS-injected mice. Fresh blood samples were then immediately centrifuged at 2,000 g for 10 min, and the supernatant was taken for the determination of blood biochemical indicators, including cardiac indicators LDH, CK, and CK-MB, liver indicators ALT and AST, and kidney indicators sCre and BUN.
2.3.5. Pathological analysis
ICR mice were injected with 1 mL/kg scorpion venom and the actual amount of scorpion venom per mouse is about 300 μg. After 6 hours of injection, the surviving mice were dissected and the hearts, liver, and kidneys of the mice were removed. At the same time, the visceral sample negative group was taken from another five mice injected with PBS 6 hours ago. Put the viscera into the tissue fixation solution, take it out after soaking in the tissue fixation solution for 24 hours, and soak it in PBS buffer 10 min for 4 times. The tissue was trimmed to a thickness of about 5 mm with a plane, and then dehydrated according to the gradient alcohol, xylene transparent, waxed, embedded, trimmed, sliced, unfolded, and pasted, baked, he stained, etc.
3. Results
3.1. Species identification: morphological and molecular identification
The scorpions used in this study were collected in Shandong Province, China () which is one of the main distribution areas of Mesobuthus martensii. First, all the scorpions were divided into two groups according to the gender differences in morphological characteristic. The plier length, body length, trunk width, teeth number, telson length, and telson width were measured. The body length refers to the length from the mouth of the scorpion to the last segment of the tail (except the tip of the telson) and the telson is the whole hind abdomen of the scorpion. The limbs of the samples look like crab claws and connected to the head (). Preliminary statistics showed that the female scorpions were larger than the males with significant differences in plier length, body length, trunk width, and comb plate teeth and telson width (). Only the parameter of telson length () showed no significant difference between female and male samples. Therefore, we preliminarily concluded that the identification of male and female scorpion is related to body length, body width, clamp length, tail width, and the teeth number of comb plate.
Figure 1. Species identification: morphological and molecular identification. (a) The region of scorpions collected. The place marked by red is Shandong Province, China, where the samples were collected. (b) The morphological character of samples with different gender. (c) Pliers’ length distribution. (d) Body length distribution. (e) Trunk width distribution. (f) Teeth number distribution. (g) Telson length distribution. (h) Telson width distribution. (i) Phylogenetic tree constructed using mtDNA sequences of COI by using MEGA 7 with the Neighbor-Joining method. The sequencing TRINITY_DN28461_c1_g1 is marked with red circles and the arthropod are marked with yellow circles. The identity values are indicated on the right of each species name. (k) Phylogenetic tree constructed using the COI amino acid sequences of TRINITY_DN28461_c1_g1 and 6 other species using MEGA 7 with the Neighbor-Joining method. The sequencing TRINITY_DN28461_c1_g1 is marked with red circles and the arthropod are marked with yellow circles. The identity values are indicated to the right of each species name.
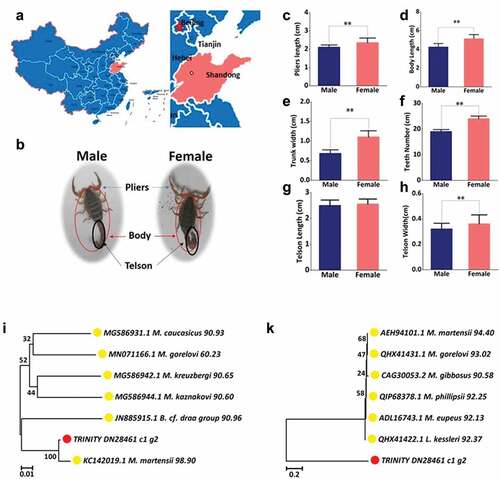
As a catalytic subunit, COI has been considered as a common recognition molecule due to its high conservation. Animals that share more than 97% COI sequence identity are generally considered as the same species. The most homologous sequence (98.90%) for COI was shown to be from arthropod, Mesobuthus martensii. COI nucleotide sequences were converted to protein sequences. The most identical protein sequence with 94.40% homology was also from Mesobuthus martensii. ().
3.2. Toxin activity
Our results show that mice injected with female scorpion venom tended to survive shorter than mice injected with male scorpion venom. The indexes of cardiac, renal, and liver function of mice injected with female scorpion venom were generally same with these indexes of mice injected with male scorpion venom. In the index of cardiac function, the lactate dehydrogenase and phosphocreatine kinase of mice injected with male and female scorpion venom increased at the same time. The concentrations of lactate dehydrogenase and phosphocreatine kinase in female scorpion venom treatment group were higher than those in male scorpion venom treatment group. The concentration of lactate dehydrogenase in the female scorpion venom treatment group was significantly different from that in the control group, and the concentration of creatine phosphokinase in the male and female scorpion venom treatment groups was significantly different from that in the control group. In the indexes of liver function, the concentrations of alanine aminotransferase and aspartate aminotransferase in female scorpion venom group were higher than those in male scorpion venom group, and both indexes increased. Total protein and globulin also showed an upward trend. The concentration of male scorpion venom treatment group was higher than that of female scorpion venom treatment group. On the contrary, albumin, total bilirubin, and direct bilirubin decreased, but the influence trend of scorpion venom on these three indicators was different between male and female scorpions. In terms of renal function indexes, the concentration of urea nitrogen in female and male scorpion venom group was significantly higher than that in the control group. At the same time, the urea nitrogen concentration of mice injected with male scorpion venom was higher than that of mice injected with female scorpion venom (). In addition, the survival rates of mice were inversely proportional to the concentration of scorpion venom. The higher the concentration of scorpion venom, the shorter the survival time. Notably, mice injected with female scorpion venom tended to survive shorter (). Also, the states of heart, liver, and kidney of mice injected with scorpion venom were different from that of mice injected with PBS. The bleeding points in the heart of mice injected with female scorpion venom were denser. The hepatic sinus became smaller and bleed after the toxic effect of male scorpion venom, and the hepatic sinus becomes smaller, and the structure of hepatocyte cord disappear after the toxic effect of female scorpion venom. In the renal pathological section, there were bleeding points in the glomerulus and congestion in the glomerulus after female toxicity ().
3.3. Quality control and overview of de novo transcriptome
Extraction of high-quality RNA has been proved to be a key step in the construction of transcriptome. Accordingly, the scorpions collected in Shandong Province were transported to the laboratory in Shanghai under adequate protection to ensure the high quality of RNA. Fortunately, the OD260/280 value of the extracted sample is 2.09, which meets the requirement of the construction. After the extraction, a series of subsequent steps including mRNA purification and fragmentation, cDNA synthesis and library construction, and final sequencing were completed.
The results of sequencing showed that almost all the values of the bases in the single-base quality distribution map exceeded 20, with a central wide plateau and two lower sides. The graph of the base content distribution showed that the ratios of A, T, C and G were parallel and close after a typically acceptable fluctuation in the initial stage due to the bias of the 6-bp random primers, indicating that the cDNA library and sequencing achieved good uniformity. A total of 35,882,884 to 53,769,744 raw reads with a GC content from 38.66% to 41.92% were obtained. After removing the adapters and low-quality sequences, a total of 35,579,172 to 53,278,296 clean reads were obtained with the valid percentage from 98.95% to 99.15% (). De novo assembly was then performed using the software, Trinity. Eventually, a total of 44,640 unigenes with a GC content of 34.34% were assembled from 31,876,587 bases after ruling out redundancy. With a BLASTX alignment e-value threshold of 10−5, we successfully annotated 10,878 (24.37%), 10,187 (22.82%), 11,563 (25.90%), 10,076 (22.57%), 14,030 (31.43%), and 16,726 (37.47%) unigenes with GO, KEGG, Pfam, Swissprot, eggNOG, and NR database (). The first three group of length distribution was 200–399, 400–599, and 600–799, and they accounted for 56.94%, 13.78%, and 6.46%, respectively. The total number of the unigenes with length less than 1,800 was 40,667 and it accounted for 91.09% of all the assembled unigenes (). In addition, the unigenes with a GC content from 31 to 33 were the most abundant group with several 6,383 which accounted for 14.29% of all (). Setting the fold change as 3/2 or 2/3 and FDR = 0.05 we screened a total of 350 (0.78%) differentially expressed unigenes, of which 160 genes were up-regulated and 190 were down-regulated. Interestingly, these up-regulated genes were all in female scorpions, while the down-regulated unigenes were all in male scorpions (). The cluster analysis showed that the four groups of female scorpions and the four groups of male scorpions were got together which indicated that the scorpions of different genders have good homogeneity ().
Figure 3. Quality control and overview of the transcriptome. (a) Number of unigenes and unigenes annotated in GO, KEGG, Pfam, Swissprot, eggNOG and NR database, (b) Length distribution of unigenes, (c) GC content distribution of unigenes. The abscissa of B and C are the unit of measurement. The primary ordinate of B and C correspond to the histogram representing the number of unigenes, while the secondary ordinate represent the cumulative percentage, (d) The number of up and down regulated unigenes with female vs. male, (e) Volcanic plot of differentially expressed unigenes, the abscissa represents log2 (fold change) (indicates the relative change trend) and the ordinate represents –log FDR (the negative logarithmic form of FDR represents the differential expression of genes, which can be well displayed on the ordinate). Each dot represents a unigenes, the red dot represents the up-regulated unigenes while the blue dot represents the down-regulated unigenes, and the grey dot represents the unigenes without significant difference, (f) Cluster heat map of the annotated unigenes. F1, F2, F3 and F4 represent the four groups of female scorpions and M1, M2, M3 and M4 represent the four male groups of scorpions.
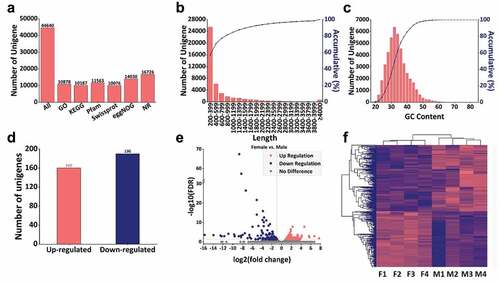
Table I. Overview of the sequencing quality control.
3.4. GO analysis
Transcriptome data results showed that 10,110 and 10,866 single genes were annotated from male and female transcriptome data, respectively. Gene ontology mainly includes three categories: Biological Process (BP), Cellular Component (CC) and Molecular Function (MF), and each describes the possible molecular function, cellular localization, and biological process involved in the gene product. According to our data results, in each functional category, the top 10 functions were selected for the next analysis.
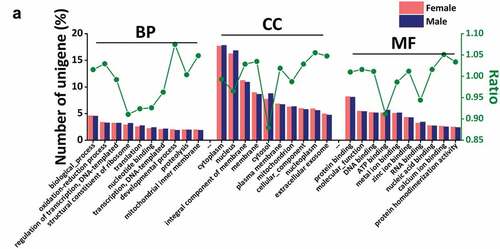
In BP, the three most abundant terms were biological process (female 464, 4.59% vs. male 491, 4.52%), oxidation-reduction process (female 340, 3.36% vs. male 355, 3.27%) and regulation of transcription, DNA-template (female 324, 3.20% vs. male 351, 3.23%). Among them, in biological process, oxidation-reduction process and developmental process, female scorpion contains slightly more functional genes than male scorpion. However, in structural constituent of ribosome, translation, nucleotide binding and transcription, DNA-template, the genes enriched in males were significantly higher than females. It shows the difference of gene expression level between male and female scorpions, and there are gender-specific genes, which only exist in female or male scorpions, such as makatoxin, plancitoxin.
Our results show that cellular component has the most abundant transcriptome data annotated in GO analysis, with more than 1000 genes related to cytoplasm and nucleus. Most of the unigenes associated with cytosol (0.88), nucleus (0.96) and mitochondrion (0.99) were significantly more abundant in males than females. The remaining genes related to membrane (1.03), nucleoplasm (1.06) and integral component of membrane (1.03) were slightly enriched in females than in males.
Molecular Function has a variety of binding functions, such as GTP binding, ATP binding, nucleoside binding, ubiquitin-protein ligase binding, protein binding, and DNA binding. Among them, the genes related to protein binding accounted for the largest number (826 female, 8.17% vs. male 879, 8.09%). And the ratio of the first 10 terms in MF was from 0.91 (ATP binding) to 1.05 (calcium ion binding) (). The difference of cell components may be related to protein binding, ATP binding, DNA binding, and RNA binding in molecular function; The site of DNA and RNA binding process is the nucleus, in which the base binding may have specific binding sites, such as plancitoxin can bind to specific binding sites to exert DNase II activity. ATP binding is to provide the site of ability, and the protein may bind to the membrane to form a pathway.
Figure 4. Gene Ontology (GO) analysis. (a)The top ten results of three GO terms: biological process (BP), cell composition (CC) and molecular function (MF). The abscissa is the annotated terms. The main ordinate corresponds to the histogram that indicates the ratio of unigenes in each term to the total unigenes identified, while the sub-ordinate represents the value of ratio folds in female vs. male. The word “ratio” means the ratio of proportion of annotated unigenes in female scorpions to the proportion of annotated unigenes in male scorpions in a certain term.
3.5. KEGG analysis
The results showed that genes related to the enrichment of metabolic and genetic information pathways accounted for more than half of the total number of genes. Overall, the number of genes related to metabolism was higher in females than in males, which result contrasts with genetic information pathway enrichment; Among them, the ability metabolism and amino acid metabolism in metabolism are more abundant in females than in males, this result was also reflected in ATP binding and protein binding in the GO analysis. Furthermore, the genes of nucleic acid metabolism and exogenous chemical degradation are more abundant in males than in female genes related to nucleic acid metabolism are also included in DNA-binding and RNA-binding moieties in MF (). Some studies have shown that the degradation of exogenous chemical substances may have a certain impact on biological detoxification. The genes enriched in genetic information processing of male scorpions are higher than those of female scorpions, mainly reflected in translation and folding, sorting, and degradation. This may be related to morphological differences or toxin effects between male and female scorpions (). Most of the genes enriched by environmental information processing were related to signal transduction, and the genes annotated in females were higher than those in males, but the differences were not significant. In addition, genes related to cellular processes were mostly annotated as transport and catabolism, and females were higher than males. Organismal systems have more enriched gene categories, and the genes annotated with immune system and endocrine system are significantly higher in males than in females. However, genes annotated in females were higher than males in functions such as circulatory system, environmental adaptation, and nervous system. Among the functional categories of human diseases, cancer, neurodegenerative disease, endocrine and metabolic disease, and infectious disease had the most enriched genes, and females were higher than males ().
Figure 5. KEGG analysis. (a) The annotated results of KEGG database in level 1, (b) Metabolism gene enrichment, Genetic information processing gene enrichment, Environmental information processing gene enrichment, Cellular Processes gene enrichment, Organism System gene enrichment, Human disease gene enrichment, (c) “Lysine degradation”, “oxidative phosphorylation” and “protein processing in the endoplasmic reticulum” gene enrichment. (Abscissa: functional annotation; ordinate: ratio of the proportion of annotated unigenes in female scorpions to the proportion of annotated unigenes in male scorpions).
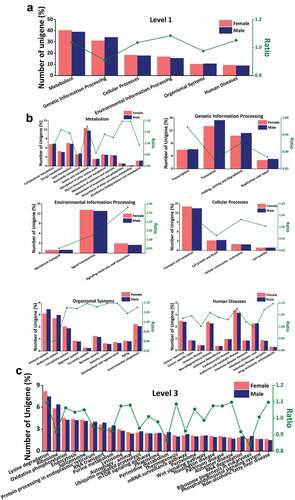
A total of 25 most abundant terms were chosen for further study. All their ratios were spread from 0.84 (Ribosome biogenesis in eukaryotes) to 1.10 (both Alzheimer disease and Non-alcoholic fatty liver disease). Among the genes enriched in lysine degradation, autophagy, Alzheimer’s disease and nonalcoholic fatty liver, females are significantly higher than males. Alzheimer’s disease and nonalcoholic fatty liver are caused by the pathological changes of brain nerve cells and simple fatty liver, respectively. In the process of lysine degradation and autophagy, it can pass through the function of blood–brain barrier, directly enter the brain tissue, affect the respiratory chain, and provide necessary energy sources for the repair of nerve cells and normal physiological activities. It also participates in the biosynthesis of carnitine to activate fat metabolism. Moreover, there are only two pathways in which males are higher than females, namely pyrimidine metabolism and ribosome biogenesis in eukaryotes. Most of these pathways are related to metabolism and human diseases, the other 10 items have little difference between male and female ().
3.6. Potential toxin screening
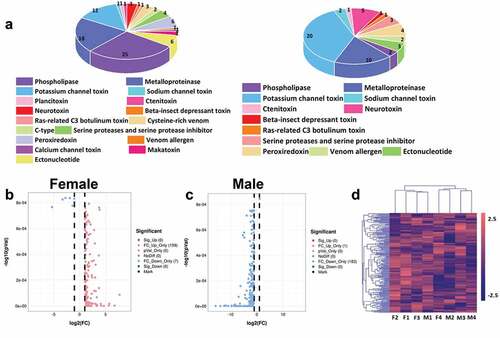
According to the blast annotation of NR and Swissport to transcriptome, a total of 17 kinds of toxins were screened from scorpion of Shandong Province and 181 toxin-related unigenes were screened. It mainly includes 48 metalloproteinase, 39 potassium channel toxins, 30 phospholipases, and other toxins (S , ). However, several different toxins are also contained. Among them, makatoxin, plancitoxin, and C-type were only specifically expressed in females and were not found in males (). Using the FDR3/2 or 2/3 and P-value 0.05 as the threshold, the up-regulated and down-regulated genes of scorpion venom of different genders have a tendency. The foldion in female scorpion venom is up-regulated, while that in male scorpion venom is the opposite (). Cluster analysis showed that the toxin homogeneity of different sexes was lower than that of all single genes ().
Figure 6. Toxins screening of scorpion. (a) The screened toxins in the transcriptome of collected male and female Mesobuthus martensii. Different colors represent different toxins, and different sizes represent the number of each toxin, (b) Volcanic map of the screened female scorpion toxin, (c) Volcanic map of the screened male scorpion toxin. The abscissas of B and C represent log2 (fold change) and the ordinate represents -log10 (P-value). Each dot represents a toxin unigenes, the red dot represents the up-regulated toxins while the blue dot represents the down-regulated toxins, and the grey dot represents the toxins without significant difference, (d) Cluster heat map of the annotated unigenes. F1, F2, F3 and F4 represent the four groups of female scorpions and M1, M2, M3 and M4 represent the four male groups of scorpions.
3.7. Makatoxin
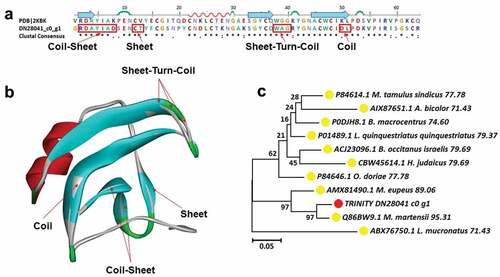
Makatoxin is an alpha toxin from Mesobuthus martensii and makatoxin-1 was reported to alter transmitter release of nitric oxide (Gong et al. Citation1997; Chen et al. Citation2000). Two kinds of makatoxin toxins, makatoxin-2, and makatoxin-3, have been screened in this study. In previous studies, makatoxin-1, and makatoxin-3, that is, the other two members of the makatoxin family except makatoxin-2, have been identified as toxins in Mesobuthus martensii. Therefore, I want to explore whether makatoxin-2 also belongs to Mesobuthus martensii and whether it is related to the gender difference of Mesobuthus martensii. The makatoxin-2 sequence TRINITY_DN28041_c0_g1 was chose for sequence alignment and 3D modeling. The main structural variations contain a coil-sheet in 1RDAYIAD9, a sheet in 11CT14, a sheet-turn-coil in 37WAG41 and a coil in 49DL52, respectively (). According to the results of phylogenetic tree analysis, Mesobuthus martensii was the closest relative to the target molecule, the makatoxin sequence TRINITY_DN28041_c0_g1. Their identity was 95.31% and the bootstrap was 97. The furthest relatives were Mesobuthus eupeus, Buthus occitanus israelis, and Hottentotta judaicus. All the 10 species identified as containing this toxin are scorpions, but they belong to different genera. Among these 10 species, the target species is Mesobuthus martensii. Therefore, it can be concluded that the toxin is scorpion venom ().
Figure 7. Structure analysis including sequence alignment, 3D modeling and phylogenetic analysis of makatoxin-2. (a) A putative sequences TRINITY_DN28041_c0_g1 was aligned with a model Makatoxin-2 (pdb ID: 2KBK). At the bottom of columns, asterisks (*) show conserved positions, colons (:) show conserved substitutions and points (.) show non-conserved substitutions. Grey line, green Bend, blue banded arrowhead, and red solenoid represent coil, turn, sheet and helix, respectively. Different fragments are framed by red lines, (b) 3D modeling was simulated using the template Makatoxin-2 (PDB ID: 2KBK) by Swiss-MODEL and viewed by Discovery Studio 4.5. The colors grey, green, blue, and red represent coils, turns, sheets and helices, respectively, (c) Phylogenetic tree constructed using TRINITY_DN28041_c0_g1 and 10 other sequences from different species using MEGA 7 with the Neighbor-Joining method. TRINITY_DN28041_c0_g1 was marked in red circle and the arthropod was marked in yellow circle.
3.8. Plancitoxin
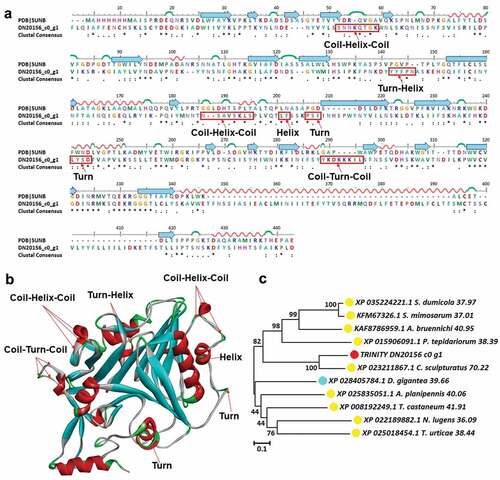
Plancitoxin is commonly found in poisonous organisms. However, this toxin was only found in female scorpions in our study (TRINITY_DN20156_c0_g1, plancitoxin-1-like isoform X1). The plancitoxin model (PDB ID: 5UNB) is used to perform sequence alignment and 3D modeling (). The main structural variations contain two coil-helix-coil in 51SNNKQTGK60 and 185NSAVSKLS196, two turns in 205PSF209 and 240LYSD245, a turn-helix in 141YYFPN147, a helix in 200LF203 and a coil-turn-coil in 288YKDKKKIL297, respectively. According to the results of phylogenetic tree analysis, Centruroides sculpturatus was the closest relative to the TRINITY_DN20156_c0_g1 and their identity was up to 70.22% (). The furthest relatives were Tribolium castaneum, Parasteatoda tepidariorum, and Stegodyphus dumicola and they all belong to arthropod animals.
Figure 8. Structure analysis including sequence alignment, 3D modeling and phylogenetic analysis of plancitoxin-1-like isoform X1. (a) A putative sequences TRINITY_DN20156_c0_g1 was aligned with a model plancitoxin-1-like isoform X1 (PDB ID: 5UNB). At the bottom of columns, asterisks (*) show conserved positions, colons (:) show conserved substitutions and points (.) show non-conserved substitutions. Grey line, green Bend, blue banded arrowhead, and red solenoid represent coil, turn, sheet and helix, respectively. Different fragments are framed by red lines, (b) 3D modeling was simulated using the template plancitoxin-1-like isoform X1 (PDB ID: 5UNB) by Swiss-MODEL and viewed by Discovery Studio 4.5. The colors grey, green, blue, and red represent coils, turns, sheets and helices, respectively, (c) Phylogenetic tree constructed using TRINITY_DN20156_c0_g1 and 10 other sequences from different species using MEGA 7 with the Neighbor-Joining method. TRINITY_DN20156_c0_g1 was marked in red circle, the arthropod was marked in yellow circle and the cnidarian was marked in blue circle.
4. Discussion
4.1. Contents of toxins in Mesobuthus martensii
Generally, we divide sodium channel toxins into three types: α - toxin, β - toxin, and insect-sensitive toxin. We have screened only one sodium channel toxin–meuna10, which belongs to an insect-sensitive toxin that acts on the corresponding overlapping sites of insect sodium channel (Shiomi et al., Citation2003).
Potassium channels are a subset of selective ion channels that conduct K+ions. Among ion channels, potassium channels form the most dense and diverse superfamily. These channels exist in living organisms from bacteria to humans. Their basic function in the human body is to set the resting potential and shape the action potential of nerves and muscles. Most potassium channels are primary α- Tetramers of subunits (heteropolymers are more common), which define their main properties and are usually supplemented by β- Subunit supplement. According to the recommendation of the International Union of Basic and Clinical Pharmacology (IUPHAR), there are 78 coding potassium channels in the human genome α. The subunit genes were divided into five groups: inward rectification (Kir), double pore domain (K2P), voltage gated (KV) and two groups of calcium activated potassium channels (KCa). Because these proteins have been identified as drug targets, people are increasingly interested in the development of potassium channel.
Scorpion toxin (abbreviated as KTx) provides>50% potassium channel ligand variability. They are composed of about 20–75 amino acid residues, usually containing 2–4 disulfide bonds. KTx has five structural folds: cysteine stable α- Helix/ β- Tablets. KTx normally suppresses the KV and KCa channels through hole plugging. According to their acts on potassium channels, the potassium channel toxins can be divided into nine types: Charybdotoxin (CTX), Noxiustoxin (NTX), Kaliotoxin (KTX), LTX1, Bmp05, P05, TSK, Mauxotoxin (MTX), P01, BmP01, and BTK-2(Valdivia et al. Citation1988; Strong Citation1990; Meera et al. Citation2000). However, only two subtypes of potassium channel toxins, α-KTX and γ-KTX, were isolated and purified from the crude venom of the North African scorpion Androyonus martetanicus maurtanicus, which blocked voltage-dependent potassium channels, including large conductance calcium activated potassium channels (BKCa).Some studies have shown that based on proteomic data, a large number of wild-type peptides and novel degradation peptides can play a role in processing K+channels. Some classical neurotoxins and degradation peptides have been proved to be effective potassium channel inhibitors, such as BmKKx2, BmKTX, BmKcug2, and BmKcug2-P1. In view of the close relationship between potassium channels and some diseases, more and more people believe that these channel blocking peptides can become the new drug leader of Kv channel diseases.
The calcium channel toxin is a short-chain scorpion venom polypeptide represented by imperatoxin. The peptide obtained by gel filtration and HPLC isolation and purification is composed of 33 amino acid residues, and 3 of them are cross-linked with two sulfur bonds. The Bmca-1 toxin was screened in this study(Mosbah et al. Citation2000). With the deepening of the research, ion channel toxins can be used as a tool to study the structure and function of target ion channels, reflecting the inestimable research value.
4.2. Differences in expression levels of male and female scorpion toxins
The difference between males and females in the overall animal level is reflected in their survival rate and biochemical indicators. We used mice to conduct simulation experiments to explore whether scorpion venom concentration affects the survival time of animals. The results showed that the survival rate was inversely proportional to the concentration and high concentrations of scorpion venom reduced the survival time of mice.
The influence of scorpion toxin and various enzymes on the human body is a multi-faceted problem. Relevant research was not only important for human emergency, but also play an important role in the treatment of some diseases. As a widely distributed biological toxin, scorpion venom has many physiological and pharmacological activities due to its complex components and properties. Modern research has proved that their components contain not only toxins but also trimethylamine, betaine, cholesterol, taurine, soft and stearic acid, lecithin, and many kinds of amino acids (Medicine, 220). In addition to its anticonvulsant, antiepileptic, analgesic, and sedative effects, its pharmacological effects are also reflected in antithrombotic and antitumor effects, but its mechanism of action is unknown.
When scorpion venom invades animals, they can damage the internal organs of animals. Mammals infected with female scorpion venom can affect heart and liver function, while male scorpion venom can impair kidney function. The creative phosphokinase was significantly increased, and the female scorpion venom increased more per unit time than the male scorpion venom. When myocardial infarction occurred, the concentration of creation phosphokinase in the serum was generally higher than aspartate aminotransferase and aspartate transaminase. The phenomenon of high specificity of hydrogenate may be related to acute myocardial infarction, and the venom of male and female scorpions make the whole animal develop the symptoms of acute myocardial infarction faster. The concentration of urea nitrogen is also increased. Scorpion venom can increase its concentration faster, and urea nitrogen is the main end product of human protein metabolism (Kavukcu et al. Citation2002). Usually, the kidney is the main organ for excreting urea, and urea can be reabsorbed in all tubules after filtration from the glomerulus, but the urine flow too fast will affect the reabsorption of urea. In the early stage of renal damage, blood urea nitrogen can be in the normal range, and when the glomerular filtration rate drops below 50%, the concentration of blood urea nitrogen will increase rapidly (Sabiullah Citation2019). Lactate dehydrogenase is widely present in the human body, and any organ or tissue disease may release lactate dehydrogenase into the blood. The relevant indicators of liver function are alanine aminotransferase and aspartate aminotransferase. In the early stage of hepatitis, due to the high content of aspartate aminotransferase in the liver, the content of serum aspartate aminotransferase is higher than alanine aminotransferase, but due to the slow clearance of alanine aminotransferase, in a short time, the content of alanine aminotransferase instead of aspartate aminotransferase. The recovery period is generally slower for alanine aminotransferase. The continuous increase of alanine aminotransferase and aspartate aminotransferase is often an indicator of chronic hepatitis, while the decrease in indicators such as total protein and albumin may be due to impaired liver function (Ekam & Udosen Citation2012), and the decrease in total protein may be caused by excess water in the body. Many Serum protein loss in various channels, such as hepatocellular lesions, impaired liver function, etc.; the reasons for the decrease in albumin concentration are roughly the same as for total protein (Louw & Visser Citation1978). Most of these biochemical indexes detected by female scorpion venom injected mice increased at a higher rate per unit time than male scorpions, and the corresponding symptoms of each index appeared earlier than male scorpion venom injected mice; while total bilirubin contrary to urea nitrogen, liver, and kidney damage may be more severe in the whole animal. Pathological sections showed that both male and female scorpions were toxic to mice, but their visceral changes were different. The toxicity of female scorpion was stronger than that of male scorpion. Combined with blood biochemical indexes, when male and female scorpion venoms are injected into mice at the same time, female scorpions will exert toxicity first and cause visceral damage. The toxicity received by mice invaded by different scorpion venoms is different, and the visceral damage of mice invaded by female scorpions is more serious.
We found that there were differences in the toxicity of scorpion venom between male and female at the overall animal level. Therefore, we tried to explore the differences at the transcriptome level. The toxin activity of male and female scorpions is different at the transcriptome level, which may be since female scorpions are generally larger than male scorpions in morphology, and their activities of toxin proteins required for daily predation, competition, and defense are higher than those of male scorpions. Female scorpions are responsible for the reproduction of the offspring of scorpions. They need more nutrients than male scorpions to supplement their daily energy needs and strengthen their own protection. Therefore, they need highly toxic venom to maintain a normal life. The scorpion must live on the back of the mother scorpion after it is born and can slowly climb down from the back of the mother scorpion until the age of 4–5 days to live on its own. Therefore, the female scorpion must also protect the young scorpion from the outside world (Ozkan et al. Citation2006). Makatoxin toxin is one of the newly discovered scorpion venoms of Mesobuthus martensii α-Toxin, which is gender specific and only exists in female scorpions. Known α-toxin is a neurotoxin, which shows that the neurotoxicity of female scorpions is higher than that of male scorpions. Plancitoxin generally exists in toxic organisms, especially in marine toxic organisms. Plancitoxin I was screened from the spider toxin transcriptome and the presence of this toxin in spiders was only based on transcripts. Its toxin composition is like scorpion venom and is composed of toxin proteins and enzymes, including metalloproteinase, phospholipase, serine protease, serine protease inhibitor, plancitoxin, etc. The amino acid sequence of plancitoxin I has high homology with mammalian deoxyribonucleotidase II (DNase II), and chromosomal DNA fragmentation occurs in rat hepatocytes after incubation with plancitoxin I. The base sequence of plancitoxin I screened by Mesobuthus martensii pincer scorpion toxin contains 151 HEK149 and 303DHSK306, two representative DNase II active sites, which can be inferred, Plancitoxin I in scorpion toxin has DNase II activity, which could degrade DNA.
4.3. Toxic difference between male and female Scorpions
It is mainly used to treat convulsions, apoplexy, hemiplegia, tetanus, encephalitis, leprosy, and other diseases (Zeng et al., Citation2010). There are 9 main toxin species in Mesobuthus martensii, they mainly include sodium toxin, potassium toxin, calcium toxin, chlorotoxin, phospholipase, antimicrobial peptide, lipolysis activation peptide, metal oxidase and hyaluronidase. Some of them act on ion channel nerves of mammalian, crustacean, and insect cell membranes, and can be divided into four types according to the receptor sites and electrophysiological effects(Fox et al., Citation1988). The improvised type refers to binding to the insect synaptosome membrane in a voltage-independent manner and plays an important role in the activation process (Luo & Bodnaryk Citation1987); the inhibitory type competes with the excitatory toxin, can depolarizes the axon membrane and blocks the evoked action potential (Kopljar et al. Citation2013); Type A slows down the inactivation process of sodium channels, prolonging the action potential, resulting in a high plateau potential, while type B activates sodium channels to keep them open or reopen at resting potentials, resulting in repeated action potential trains. The expression level of type A and type B toxin was positively correlated in all female samples and in more than half of male samples. There was no significant relationship between the species distribution of scorpion toxin and its gender.
In this study, the expression levels of proteins related to phospholipase and C-type were almost the same. The C-type toxins included cytochrome C-type heme lyase, CXXC type zinc finger protein, zinc finger CCHC type and RNA-binding motif-containing protein, nuclear factor 1 C-type, CXXC type zinc finger protein and nuclear factor 1(Gonzales & Neupert Citation1990; Chen et al., Citation1999). The expression level of C-type in female was slightly higher than that in male. The expression of 85/88 kDa calcium-independent phospholipase A2, phospholipase A2, calcium-independent phospholipase A2 gamma, basic phospholipase A2 pa-12c, cytosolic phospholipase A2, acid phospholipase A2, PA4, n-acyl-phosphatidylethanolamine hydrolyzing phospholipase D and putative were detected in our study. Among these toxin genes, the expression of phospholipase A2, basic phospholipase A2 pa-12c and putative phospholipase B in female was slightly higher than that in male. The amino acid sequence of plancitoxin I is highly homologous to mammalian DNase II and chromosomes appear in rat hepatocytes after incubation with plancitoxin I DNA fragmentation. These explain that plancitoxin I have DNase II activity. Previous research findings showed that the amino acid sequences from plancitoxin 1 showed high homology with mammalian DNase II and chromosomal DNA fragmentation appeared in rat liver cells after 3 hours incubation with plancitoxin 1(Shiomi et al. Citation2004; Ota et al. Citation2006). In addition, we only found the expression of phospholipase a-2-activating protein in male scorpions, and this protein was not annotated in female scorpions. Therefore, we can infer that the phospholipase a-2-activating protein is a male scorpion toxin, and the remaining toxins are shared by both male and female scorpions, and more of them are in female than in male.
4.4. Study and application of toxins in Mesobuthus martensii
It is found that scorpion venom is a kind of bioactive substance with a complex structure, and its components include scorpion toxin, trimethylamine, betaine, taurine, and palmitic acid (Shi et al. Citation2015). Scorpion toxin is the main substance that produces efficacy and is composed of toxic protein and enzyme. The toxins listed in this paper are scorpion neurotoxins, including α-scorpion neurotoxins, β-scorpion neurotoxins, and anti-insect toxins, while α-scorpion neurotoxins and β-scorpion neurotoxins belong to long-chain scorpion neurotoxins.
Mechanism of action α-scorpion neurotoxin can inhibit the inactivation of sodium channels and promote the closing and reopening of sodium channels. When synthetic peptide fragment containing sodium ion inactivation is applied to the inner side of cells, the fast-closing process of channels inhibited by α - scorpion neurotoxin or inactivated mutant channels can be restored. ß-scorpion neurotoxin can bind to the receptor site of a sodium ion channel with high affinity, which can facilitate the activation process of sodium current and reduce the peak sodium current. ß-scorpion neurotoxin can stably capture the specific extracellular link on the sodium channel, making it unable to reset, thus facilitating the process of sodium ion activation. Insect sensitive toxins are antagonists of sodium channels on the excitable cell membrane of insects, which can specifically bind to this channel and produce specific and selective lethal effects on insects. The biological insecticides with insecticidal ability can be obtained by recombining insect-sensitive toxin gene into organism(Loret et al. Citation1991; Landon et al. Citation2015). Studies have shown that a small amount of scorpion venom can significantly delay mouse carotid artery thrombosis and increase the content of PGI in plasma to promote dissolution.
5. Conclusion
In this work, a transcriptome analysis and bioinformatics analysis were carried out on male and female individuals of Mesobuthus martensii from Shandong Province. The samples of male and female scorpions have different morphology, and the conserved COI sequence is consistent with that of Mesobuthus martensii. A total of 44,640 unigenes were obtained from the transcriptome and among them, we successfully annotated 16,726 (37.47%), 10,076 (22.57%), 10,878 (24.37%), and 10,187 (22.82%) unigenes with NR, Swissprot, GO, and KEGG database, respectively. In addition, there was no significant difference in GO and KEGG between male and female scorpion. A total of 17 kinds of toxins were screened from scorpion of Shandong Province and 181 toxin-related unigenes were screened, including 48 metalloproteinase, 39 potassium channel toxin, 30 phospholipase, 11 neurotoxin, 11 ectonucleotide, 10 peroxiredoxin, 9 serine proteases and serine protease inhibitor, 4 sodium channel toxin, 3 venom allergen, 3 cysteine-rich venom, 3 C-type, 2 ras-related C3 botulinum toxin, 2 makatoxin, 2 tenitoxin, 2 Beta-insect depressant toxin, 1 calcium channel toxin and 1 plancitoxin. In this paper, we identified two toxins that exist only in female scorpion Mesobuthus martensii by transcriptome analysis and the toxicity of scorpion venom was verified at the whole animal level, The results showed that for the same concentration of scorpion venom, the toxicity of female scorpion was higher than that of male scorpion, and it had acute toxicity to the whole animal. In addition, the method in this study can be extended as an effective method to evaluate the difference of toxin between female and male poisonous animals.
Supplemental Material
Download MS Excel (26.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2022.2143584
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Andrews S, Gilley J, Coleman MP. 2010. Difference Tracker: ImageJ plugins for fully automated analysis of multiple axonal transport parameters. Journal of Neuroscience Methods 193(2):281–287. DOI:10.1016/j.jneumeth.2010.09.007.
- Chen X, Court D et al. 1999. Crystal structure of ERA: A GTPase-dependent cell cycle regulator containing an RNA binding motif. Proceedings of the National Academy of Sciences 96(15):8396–8401. DOI: 10.1073/pnas.96.15.8396.
- Chen Z, Reddy G, Hahin R. 2000. The isolation and purification of two peptides from the venom of Buthus martensii Karsch. Toxicon 38(12):1817–1832. DOI:10.1016/S0041-0101(00)00110-0.
- De Oliveira UC, Candido DM, Dorce VA et al. 2015. The transcriptome recipe for the venom cocktail of Tityus bahiensis scorpion. Toxicon 95:52–61. DOI: 10.1016/j.toxicon.2014.12.013.
- Ekam VS, Udosen EO. 2012. Total protein, albumin and globulin levels following the administration ofactivity directed fractions of Vernonia amygdalina during Acetaminophen induced hepatotoxicity in wistar rats. Global Journal of Pure & Applied Sciences 18.
- Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39(4):783–791. DOI:10.1111/j.1558-5646.1985.tb00420.x.
- Fox, J. A., Pfeffer, B. A., & Fain, G. L. 1988. Single-channel recordings from cultured human retinal pigment epithelial cells. The Journal of General Physiology 91(2): 193–222.
- Gong J, Kini RM, Gwee MC et al. 1997. Makatoxin I, a novel toxin isolated from the venom of the scorpion Buthus martensi Karsch, exhibits nitrergic actions. Journal of Biological Chemistry 272(13):8320–8324. DOI: 10.1074/jbc.272.13.8320.
- Gonzales DH, Neupert W. 1990. Biogenesis of mitochondrial c-type cytochromes. Journal of Bioenergetics & Biomembranes 22(6):753–768. DOI:10.1007/BF00786929.
- He XL, Deng JP, Wang M et al. 2000. Structure of a new neurotoxin from the scorpion Buthus martensii Karsch at 1.76 Å. Acta Crystallographica Section D Biological Crystallography 56(1):25–33. DOI: 10.1107/S0907444999014614.
- Kavukcu S, Türkmen M, Soylu A et al. 2002. Intestinal urea metabolism: Could the bacteria involved in nitrogen cycle lead to reutilization of intestinal urea nitrogen in uremic rabbits? Journal of Nutritional Science & Vitaminology 48(1):24. DOI: 10.3177/jnsv.48.24.
- Keusch GT, Mary J. 1975. The pathogenesis of Shigella diarrhea. V. Relationship of shiga enterotoxin, neurotoxin, and cytotoxin. Journal of Infectious Diseases 131(Supplement):S33. DOI:10.1093/infdis/131.Supplement.S33.
- Kopljar I, Labro AJ, Block TD et al. 2013. The ladder-shaped polyether toxin gambierol anchors the gating machinery of Kv3.1 channels in the resting state. Journal of General Physiology 141(3):359. DOI: 10.1085/jgp.201210890.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870–1874. DOI:10.1093/molbev/msw054.
- Landon C, Sodano P, Cornet B et al. 2015. Refined solution structure of the anti-mammal and anti-insect LqqIII scorpion toxin: Comparison with other scorpion toxins. Proteins: Structure, Function, and Genetics 28(3):360–374. DOI: 10.1002/(SICI)1097-0134(199707)28:3<360::AID-PROT6>3.0.CO;2-G.
- Loret EP, Martin-Eauclaire MF, Mansuelle P et al. 1991. An anti-insect toxin purified from the scorpion Androctonus australis hector also acts on the alpha- and beta-sites of the mammalian sodium channel: Sequence and circular dichroism study. Biochemistry 30(3):633. DOI: 10.1021/bi00217a007.
- Louis J. 1980. Contribution to the study of the scorpionidae of the Arabian grand maghreb. III. Study of the constituents of the venom of Androctonus mauretanicus (Pocock, 1902) from Morocco. Connecticut Medicine 38:65.
- Louw AI, Visser L. 1978. The synergism of cardiotoxin and phospholipase A2 in hemolysis. Biochimica et Biophysica Acta (BBA) - Biomembranes 512(1):163–171. DOI:10.1016/0005-2736(78)90227-4.
- Luo M, Bodnaryk RP. 1987. Synaptosomes and synaptosome membrane vesicles from the brain of Mamestra configurata: Application to voltage-dependent and ATP-dependent Ca2+ ion transport studies. Insect Biochemistry 17(6):911–918. DOI:10.1016/0020-1790(87)90028-X.
- Mao X, Liu H, Wen-Xin LI et al. 2017. The morphological and structural characteristics of Mesobuthus martensii. Chinese Journal of Zoology.
- Meera P, Wallner M, Toro L. 2000. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- and Ca 2+ -activated K + channel resistant to charybdotoxin and iberiotoxin. Proceedings of the National Academy of Sciences 97(10):5562–5567. DOI:10.1073/pnas.100118597.
- Miller FP, Vandome AF, Mcbrewster J et al. 2010. Mesobuthus Martensii. Alphascript Publishing.
- Mosbah A, Kharrat R, Fajloun Z et al. 2000. A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel. Proteins: Structure, Function, and Genetics 40(3):436–442. DOI: 10.1002/1097-0134(20000815)40:3<436::AID-PROT90>3.0.CO;2-9.
- Ota E, Nagashima Y, Shiomi K et al. 2006. Caspase-independent apoptosis induced in rat liver cells by plancitoxin I, the major lethal factor from the crown-of-thorns starfish Acanthaster planci venom. Toxicon 48(8):1002–1010. DOI: 10.1016/j.toxicon.2006.08.005.
- Ozkan O, Adiguzel S, Yakistiran S et al. 2006. Study of the relationship between Androctonus crassicauda (Oliver, 1807; scorpiones, buthidae) venom toxicity and telson size, weight and storing condition. Journal of Venomous Animals & Toxins Including Tropical Diseases 12(2):297–309. DOI: 10.1590/S1678-91992006000200011.
- Quintero-Hernandez V, Ramirez-Carreto S, Romero-Gutierrez MT et al. 2015. Transcriptome analysis of scorpion species belonging to the Vaejovis genus. PLoS One 10(2):e0117188. DOI: 10.1371/journal.pone.0117188.
- Rodríguez-Ravelo R, Batista CVF, Coronas FIV et al. 2015. Comparative proteomic analysis of male and female venoms from the Cuban scorpion Rhopalurus junceus. Toxicon 107:327–334. DOI: 10.1016/j.toxicon.2015.06.026.
- Sabiullah M. 2019. Estimation of serum creatinine, blood urea nitrogen and urine analysis in patients with diabetes to assess the renal impairments. International Journal of Advanced Biochemistry Research 3(2):01–04. DOI:10.33545/26174693.2019.v3.i2a.32.
- Santibáñez-López CE, Francke OF, Ureta C et al. 2015. Scorpions from Mexico: From species diversity to Venom complexity. Toxins (Basel) 8.
- Shi WT, Liu X, Bai ZT. 2016. Prescription formulation and application of traditional chinese medicine Scorpion. Journal of Yanan University.
- Shiomi K, Honma T et al. 2003. An epidermal growth factor-like toxin and two sodium channel toxins from the sea anemone Stichodactyla gigantea. TOXICON -OXFORD- 41(2):229–236. DOI: 10.1016/S0041-0101(02)00281-7.
- Shiomi K, Midorikawa S, Ishida M et al. 2004. Plancitoxins, lethal factors from the crown-of-thorns starfish Acanthaster planci, are deoxyribonucleases II. Toxicon 44(5):499–506. DOI: 10.1016/j.toxicon.2004.06.012.
- Shi L, Zhang T, Congying DU et al. 2015. Research progress on chemical constituents, pharmacological effects and clinical applications of Scorpio. Journal of Liaoning University of Traditional Chinese Medicine 10. DOI: 10.1186/s13020-015-0049-6.
- Strong PN. 1990. Potassium channel toxins. Pharmacology & Therapeutics 46:137–162.
- Toshie TOL, Ivo L, Abr?O CDV. 2014. Convulsive and neurodegenerative effects in rats of some isolated Toxins From The Tityus bahiensis Scorpion Venom. Journal of Toxins 2013:1–10.
- Valdivia HH, Smith JS, Martin BM et al. 1988. Charybdotoxin and noxiustoxin, two homologous peptide inhibitors of the K + (Ca 2+) channel. Febs Letters 226(2):280–284. DOI: 10.1016/0014-5793(88)81439-X.
- Yuri,UN. 2015. Animal venom studies: Current benefits and future developments. World Journal of Biological Chemistry 6(2): 28.
- Zeng XC, Corzo G, Hahin R. 2010. Scorpion Venom peptides without disulfide bridges. IUBMB Life (International Union of Biochemistry and Molecular Biology: Life) 57(1):13–21. DOI:10.1080/15216540500058899.