Abstract
Despite the growing interest in the evolutionary cytogenetics of squamates, chromosomal data are lacking for most taxa. We performed a preliminary molecular taxonomic analysis and a comparative cytogenetic study on Hemorrhois hippocrepis and Malpolon monspessulanus. We used a combination of standard karyotyping, Chromomycin A3/Methyl green staining, C-banding, Ag-NOR staining and NOR-FISH to provide the first karyotype description of H. hippocrepis and a re-description of the karyotype of M. monspessulanus, including chromosome markers, heterochromatin patterns and sex chromosome systems. Our results show that H. hippocrepis has 2 n = 36 chromosomes, with 16 macro- and 20 microchromosomes and NORs on the 6th pair. The 4th pair represents homomorphic (metacentric) ZW sex chromosomes, but the W chromosome is completely heterochromatic. Malpolon monspessulanus has 2 n = 44 chromosomes, with 20 macro- and 24 microchromosomes, NORs on the 6th telocentric pair. The 4th pair represents the sex chromosomes (ZZ/ZW), with a W chromosome smaller than the Z and completely heterochromatic. Comparing our cytogenetic data to those available from the literature, we note the occurrence and distribution of primitive and derived chromosomal characteristics and discuss the chromosome diversification in two snake clades belonging to Colubridae and Psammophiidae, respectively. We highlight that these two families followed different chromosome diversification pathways, characterised by a highly conserved karyotype structure in Colubridae and a higher chromosome variability in Psammophiidae, mostly driven by a progressive reduction of the chromosome number by means of chromosome fusions. We also provide cytotaxonomic insights supporting the distinction between M. monspessulanus and M. insignitus.
Introduction
Karyotype changes represent distinct markers that are helpful in detecting evolutionary trends or apomorphisms in the taxa studied (Noor et al. Citation2001; Rieseberg Citation2001; Dobigny et al. Citation2004; Olmo Citation2008).
Squamates display an extraordinary diversity in chromosome number and morphology, chromosome markers of varying number and location and in the occurrence of environmental and/or genetic sex determination, with the independent evolution of simple and multiple sex chromosome systems with either male or female heterogamety (Mezzasalma et al. Citation2017; Augstenová et al. Citation2018; Pallotta et al. Citation2017; Petraccioli et al. Citation2019; Sidhom et al. Citation2020). These characteristics make squamates extraordinary model organisms to explore the karyotype evolution and the diversification of sex chromosome systems in vertebrates (Olmo Citation2008; Gamble Citation2010; Rovatsos et al. Citation2015; Augstenová et al. Citation2018; Mezzasalma et al. Citation2021). In recent years, a growing number of studies have been performed to improve the knowledge on the chromosome evolution in squamates (Srikulnath et al. Citation2013; Rovatsos et al. Citation2015; Mezzasalma et al. Citation2016, Citation2020; Sidhom et al. Citation2020; Kostmann et al. Citation2021). However, raw karyotype data are still lacking for the majority of the squamate species currently described and the karyological diversification of many groups remains scarcely investigated or completely unexplored (Olmo & Signorino Citation2005; Mezzasalma et al. Citation2021).
Here, we performed a comparative cytogenetic analysis on the horseshoe whip snake Hemorrhois hippocrepis (Linnaeus, 1758) and the Montpellier snake Malpolon monspessulanus (Hermann, 1804). These two snake species have been largely studied with morphological and molecular methods (e.g. Utiger et al. Citation2002; Carranza et al. Citation2006; Machado et al. Citation2021). In contrast, no chromosome data are currently known for H. hippocrepis and the only available chromosome data on M. monspessulanus were reported by Matthey (Citation1931). We employed a combination of different staining, banding techniques and molecular cytogenetics, providing the first karyotype description of H. hippocrepis, a re-description of the karyotype of M. monspessulanus. We describe heterochromatin patterns in these two species, the occurrence and the karyotype localization of chromosome markers as well as the possible presence of differentiated sex chromosome systems. By comparing our data to those available from the literature on phylogenetically closely related species, we also discuss the occurrence and distribution of primitive and derived chromosomal characteristics and propose a scenario for the chromosome diversification of two snake clades of Colubridae and Psammophiidae, respectively.
Material and methods
Sampling
We analysed preserved tissues and cell suspensions of one male and one female of H. hippocrepis (from Guelmin, Morocco) and two males and two females of M. monspessulanus (from Essaouria, Morocco) hosted and preserved at the Dipartimento di Biologia Evolutiva e Comparata, Università degli Studi di Napoli Federico II, since 1972 (sample numbers CA0204-CA0209). All samples were used in a preliminary molecular analysis and the comparative cytogenetic study as described below.
Molecular analysis
We performed a preliminary molecular analysis using a fragment of about 550 bp of the mitochondrial 16S rRNA gene (16S), a widely used molecular marker in phylogenetically related species (see e.g. Nagy et al. Citation2003; Carranza et al. Citation2004; Alencar et al. Citation2016; Simoes et al. Citation2016), in order to obtain an accurate taxonomic identification of the examined samples. DNA was extracted following Sambrook et al. (Citation1989). For PCR amplifications, we used the same primers and parameters reported in Mezzasalma et al. (Citation2022). Amplicons were sequenced on an automated sequencer ABI 377 (Applied Biosystems, Foster City, CA, USA) using BigDye Terminator 3.1 (ABI). Chromatograms were checked and manually edited using Chromas Lite 2.6.6 and BioEdit 7.2.6.1 (Hall Citation1999) and compared with available homologous traits deposited in public repositories. The newly determined sequences were deposited in GenBank (accession numbers: OQ255883-OQ255888). The H. hippocrepis and M. monspessulanus samples showed no intraspecific nucleotide differences in the 16S sequence and had an intraspecific identity score of 99.5% and 99.2%, respectively, with homologous sequences of Moroccan specimens deposited in GenBank (AY643350 and AY643354).
Cytogenetic analysis
Chromosomes were obtained from tissue samples and cell suspensions using the air-drying method, as described in Mezzasalma et al. (Citation2019). The chromosome analysis was performed with standard karyotyping (5% Giemsa solution at pH 7 for 10 min) and different chromosome staining and banding methods. C-banding was performed following Sumner (Citation1972) and sequential C-banding + CMA3 + DAPI according to Mezzasalma and Odierna (Citation2021). Active nucleolus organizing regions (NORs) were identified following the Ag-NOR staining method described by Howell and Black (Citation1980), Chromomycin A3-methyl green staining (CMA3 /MG) as described in Sahar and Latt (Citation1980) and Fluorescence In Situ Hybridization (FISH) following Sidhom et al. (Citation2020), using as a probe the biotinylated 18S rRNA gene of Tarentola mauritanica (Linnaeus, 1758) amplified via PCR, in order to detect both active and inactive NORs. In brief, slides were dehydrated in ascending series of ethanol and, after denaturation in 70% formamide and 2x SSC for 2 min at 80°C, incubated overnight at 40°C with the hybridization mixture (10 ng/ml biotinylated 16 dUTP probe 0.1 µg/ml Escherichia coli DNA in 50% formamide and 2x SSC). After hybridization slides were washed twice in 1x SSC at 75°C and room temperature for 5 min, respectively, and cytochemical detection was performed using 5 µg/ml FITC-conjugated ExtrAvidin (Sigma) in 4x SSC + 1% BSA + 0.1% Tween 20, pH 7. After washing three times in 4x SSC and 0.1% Tween 20 for 10 min at 42°C, the detection of FISH signals was performed with ExtrAvidin FITC (Sigma Aldrich) counterstained with propidium iodide (PI) (200 ng/ml) in 2x SSC, pH 7, for 2 min at room temperature. Slides were then mounted with antifade solution (Sigma-Aldrich) and metaphase plates were scored and recorded with an optical and an epifluorescent microscope (Axioscope Zeiss) equipped with an image analysis system.
Karyogram reconstruction was performed after scoring at least 10 plates per sample and chromosomes were classified following Levan et al. (Citation1964).
Results
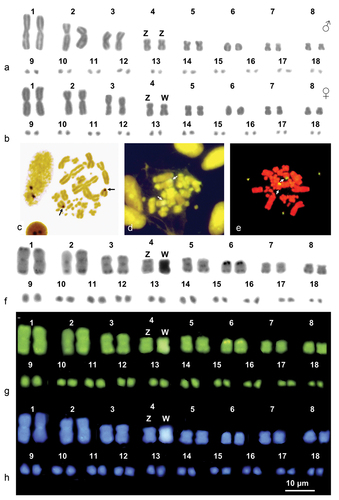
The horseshoe whip snake, H. hippocrepis, showed a karyotype of 2 n = 36 chromosomes, with 16 macro- and 20 microchromosomes. Among macroautosomes, pairs 1, 3, 5 and 7 are metacentric, pairs 2 and 8 are submetacentric and pair 6 is telocentric (Arm Number, AN = 50) (; ). The pair 4 corresponds to the ZZ/ZW sex chromosomes. Both the Z and the W chromosome are metacentric, but clearly distinguishable after C-banding. In fact, the Z is mostly euchromatic, showing only small centromeric and telomeric heterochromatic regions (negative to both fluorochromes), while the W is largely heterochromatic and strongly positive to CMA3 and DAPI (). All macroautosomes showed telomeric, centromeric and additional peritelomeric heterochromatic spots on the elements of the pair 6, corresponding to the NOR-associated heterochromatin. In fact, Ag-NOR-, CMA3/MG- and NOR-FISH all showed the occurrence of NORs on the pericentromeric region of the elements of the pair 6 (), respectively), which show a secondary constriction (). All microchromosomes are mostly euchromatic ().
Table I. Chromosome morphometric parameters of the studied samples of H. hippocrepis and M. monspessulanus. RL = Relative length (Chromosome length/total chromosome length*100); CI = Centromeric index (short arm length/chromosome length*100); m = metacentric; sm = submetacentric; t = telocentric.
Figure 1. Karyograms (a, b, f, g and h) and metaphase plates (c-e) of H. hippocrepis stained with Giemsa (a and b), Ag-NOR (c) (male), CMA3 /MG (d) (female), NOR-FISH (e) (female) and sequential C-banding + Giemsa (f) + CMA3 (g) + DAPI (h). Arrows indicate NOR-bearing chromosomes.
The western Montepellier snake, M. monspessulanus, had 2 n = 44 chromosomes, with 20 macro- and 24 microchromosomes (). The macrochromosomes are metacentric (pairs 1 and 8), submetacentric (pair 2), or telocentric (pairs 3, 5, 6, 7, 9, and 10) (; ). The pair 4 is homomorphic in the males and heteromorphic in the females, representing a ZZ/ZW sex chromosome system. The Z and W chromosomes are invariably metacentric, but the W is distinctively smaller than the Z (). Ag-NOR and CMA3/MG and NOR-FISH all showed NORs in the centromeric region of the telocentric chromosomes of pair 6 ()), where a secondary constriction occurs ().
Figure 2. Karyograms (a, b, f, g and h) and metaphase plates (c-e) of M. monspessulanus stained with Giemsa (a, and b), Ag-NOR (c) (male), CMA3 /MG (d) (male), NOR-FISH (E) (female) and sequential C-banding + Giemsa (f) + CMA3 (g) + DAPI (h). Arrows indicate NOR-bearing chromosomes.
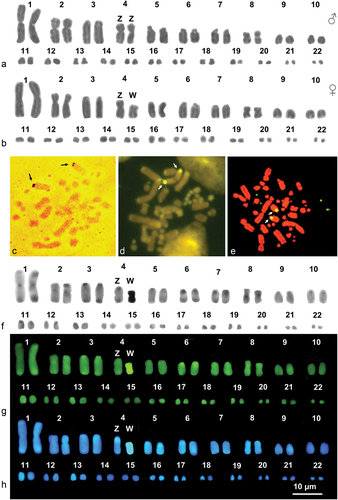
After C-banding, almost all macrochromosome pairs showed peritelomeric and telomeric heterochromatin and NOR-associated heterochromatin, CMA3 positive, was present on the telomeres of the chromosomes of the 6th pair ()). The microchromosomes are almost all euchromatic ()). The Z chromosome showed a small centromeric C-band, negative to either CMA3 and DAPI ()), while the W appeared fully heterochromatic and highly positive to both fluorochromes ()).
Discussion
The hypothesized ancestral snake karyotype (ASK) is composed of 2 n = 36 chromosomes, with 16 macro- and 20 microchromosomes and loci of NORs on a microchromosome pair (see e.g. Olmo & Signorino Citation2005; Oguiura et al. Citation2009; Rovatsos et al. Citation2015; Matsubara et al. Citation2016; Cole & Hardy Citation2019; Viana et al. Citation2019; Singchat et al. Citation2020). The ASK is highly conserved in various snake groups such as in Boidae and Colubridae, where many species are characterised by the same or very similar chromosome formula (see e.g. Olmo & Signorino Citation2005). Nevertheless, karyological diversity involving total chromosome number, macro-/microchromosomes ratio and chromosome morphology characterises many squamate and snake groups (Mezzasalma et al. Citation2014; Matsubara et al. Citation2016; Viana et al. Citation2019; Singchat et al. Citation2020).
In particular, the two snake species here studied present a combination of primitive and derived chromosome characteristics which are particularly evident when their karyotypes are compared to phylogenetically closely related species and to the ASK. Here, we discuss the cytogenetic results on H. hippocrepis and M. monspessulanus in the light of available karyological data (Matthey Citation1931; Gorman Citation1973; Branch Citation1980; Aprea et al. Citation2003; Mezzasalma et al. Citation2015, Citation2018; Abd Allah Citation2010; this study) and phylogenetic information (Pyron et al. Citation2013; Figueroa et al. Citation2016; Zaher et al. Citation2019) on closely related species. We highlight the occurrence and localization of different chromosomal states (number, morphology, heterochromatin distribution, and NOR localization) and rearrangements that can be considered plesiomorphic, synapomorphic and apomorphic in different taxa and describe an evolutionary scenario on the karyotype diversification in two clades of Colubridae and Psammophiidae, respectively ().
Figure 3. Phylogenetic relationships of Mediterranean whip snakes with available relative karyological data modified from .Pyron et al. (Citation2013), Figueroa et al. (Citation2016) and Zaher et al. (Citation2019). Chromosome data were collected from: (a) Mezzasalma et al. (Citation2015, Citation2018); (b) Matthey (Citation1931); (c) Gorman (Citation1973); (d) Branch (Citation1980); (e) this study; (f) Abd Allah (Citation2010)
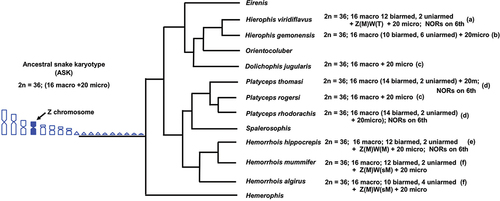
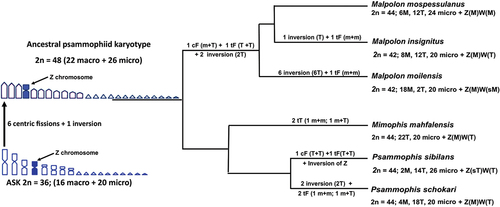
Figure 4. Phylogenetic relationships among Malpolon, Mimophis and Psammophis redrew from Pyron et al. (Citation2013), Figueroa et al. (Citation2016) and Zaher et al. (Citation2019), applied to available karyological data and the most parsimonious chromosome rearrangements occurred during specific diversification. cF = centric fusion; tF = tandem fusion; M = macrochromosome; m = microchromosome; T = telocentric macrochromosomes; sT = subtelocentric.
Hemorrhois hippocrepis belongs to a clade of Mediterranean colubrid whip-snakes that includes eight genera and only nine species of known karyotype (). The available karyological data suggest that the general configuration of the ASK is highly conserved in this clade in terms of total chromosome number, number of macro- and microchromosomes and morphology (2 n = 36 with 16 macro and 20 microchromosomes) (Matthey Citation1931; Gorman Citation1973; Mezzasalma et al. Citation2015; Branch Citation1980; Abd Allah Citation2010; this study) (). However, in species of different genera (H. hippocrepis, Platyceps rhodorachis, P. thomasi and Hierophis viridiflavus) NORs are located on the 6th macrochromosome pair (see ). This condition represents a synapomorphy of this clade of whip-snakes, which probably originated through a translocation of ribosomal cistrons from a microchromosome pair of the ASK. Furthermore, interspecific differences in chromosome morphology highlight the presence of several chromosome rearrangements in the group.
In the genus Hemorrhois, H. nummifer, H. hippocrepis and H. algirus differ in the number of meta-, submetacentric, subtelocentric and telocentric autosomes (Abd Allah Citation2010; present paper), suggesting the occurrence of multiple chromosomal inversions (). In addition, the homomorphic (metacentric) ZW sex chromosome pair in H. hippocrepis might be considered as an earlier differentiation stage than the heteromorphic ZW pair of H. nummifer and H. algirus, where the W element resulted submetacentric (Abd Allah Citation2010; present paper). In fact, a different morphology of the W chromosome in closely related species generally underlines the occurrence of different steps of the progressive diversification of the sex chromosome pair. In particular, sex chromosome pairs start their diversification as homomorphic autosomes which may progressively diverge in sequence content (mostly by heterochromatinization of the Y/W chromosome), morphology and dimensions, by means of addition/deletion of heterochromatin and chromosome rearrangements (e.g. Alam et al. Citation2018; Mezzasalma et al. Citation2019; Mezzasalma et al. Citation2021). However, in some cases the diversification of sex chromosomes may be less linear than that suggested only by morphology or differential heterocromatinization and future studies should focus on the examination of the genomic content of sex chromosomes (e.g. Kratochvíl et al. Citation2021; Mezzasalma et al. Citation2021).
Differently from Mediterranean whip-snakes, the psammophiid clade which includes the genera Malpolon, Mimophis and Psammophis show highly divergent karyotypes compared to the ASK (). The clade here considered includes six species with available karyotypes (Matthey Citation1931; Branch Citation1980; Aprea et al. Citation2003; Abd Allah Citation2010; this study), displaying 2 n = 42–44 chromosomes with a variable number of biarmed and telocentric chromosomes and representing the only available chromosome data for the whole family Psammophiidae (), which includes nine genera and more than 50 species (Uetz et al. Citation2022). Overall, the composition of the known Psammophiidae karyotypes is structurally similar to that of Lamprophiidae and Pseudoxyrhophiidae, sharing several characters such as similar total chromosome number, high number of telocentric elements and comparable macro-/microchromosome ratios (see Matthey Citation1931; Aprea et al. Citation2003; Abd Allah Citation2010; Mezzasalma et al. Citation2014; Augstenová et al. Citation2018; this study). Psammophiidae, Lamprophiidae and Pseudoxyrhophiidae probably share an ancestral condition of 2 n = 48 with 22 mostly telocentric macrochromosomes (excluding a metacentric Z sex chromosome) and 26 microchromosomes (see Aprea et al. Citation2003; Mezzasalma et al. Citation2014). Starting from the ASK, a karyotype of 2 n = 48 was probably inherited by different families of Elapoidea (see Zaher et al. Citation2019, for recent phylogenetic and systematic reassessment of snake taxa), mostly by means of chromosome fissions (see Aprea et al. Citation2003; Mezzasalma et al. Citation2014) (). Considering the hypothesised psammophiid ancestral karyotype of 2 n = 48, mainly composed of telocentric elements, it is possible to hypothesise that chromosome rearrangements in this group mostly involved a combination of chromosome inversions and fusions, leading to the formation of biarmed elements and to a reduction in the total chromosome number ().
In particular, in the genus Malpolon two fusions and two inversions likely produced the karyotype of 2 n = 44 of M. monspessulanus, while one additional tandem fusion and between one to six inversions occurred during the diversification of the karyotype of M. insignitus and M. moilensis (see ). In addition, M. monspessulanus, M. moilensis and M. insignitus have a different morphology in the W chromosome, which is metacentric in M. monspessulanus (but smaller than the Z), submetacentric in M. moilensis and telocentric in M. insignitus (Abd Allah Citation2010; present paper), probably representing different steps of sex chromosome diversification. Furthermore, the variable chromosome characters identified on multiple autosome pairs and the morphology of the W chromosome, provide further support to the taxonomic distinction between M. insignitus and M. monspessulanus, which was initially proposed based on molecular data (see Carranza et al. Citation2006). We also highlight that the karyotype of the Moroccan specimens of M. monspessulanus (this study) differs from that described by Matthey (Citation1931) for European specimens in the number of microchromosomes (24 and 22 microchromosomes, respectively). In consideration that Moroccan and European populations do not show a significant molecular distance (and together belong to the nominal subspecies M. m. monspessulanus) (Carranza et al. Citation2006; Machado et al. Citation2021), it appears plausible that the karyotype of the species previously reported by Matthey (Citation1931) was erroneously described.
In the genus Psammophis, the only two species with a known karyotype, P. schokari and P. sibilans, show the same total chromosome number (n = 22), but a different number of macro-, microchromosomes, telocentric and biarmed elements (). The karyotype of P. schokari shows 9 telocentric and 10 microchromosome pairs and probably originated from the psammophiid ancestral karyotype through two inversions and two tandem fusions (). In turn, the karyotype of P. sibilans, with 7 telocentric and 13 microchromosome pairs, likely originated from one inversion in the Z chromosome and two tandem fusions (). It is noteworthy that the subtelocentric Z chromosome of P. sibilans represents one of the few cases of morphological diversification (and chromosome rearrangement) of this chromosome in snakes. In fact, the Z chromosome is highly conserved in caenophidians, generally localized on the 4th metacentric chromosome pair and displays a similar sequence content in different taxa (Rovatsos et al. Citation2015; Matsubara et al. Citation2016; Cole & Hardy Citation2019; Viana et al. Citation2019; Singchat et al. Citation2020).
Similarly to the genus Psammophis, two tandem fusions in the primitive psammophiid karyotype would have likely generated the karyotype of the Malagasy Mimophis mahfalensis, which has 11 telocentric and 10 microchromosome pairs ().
Overall, the evidence gathered from our newly generated chromosome data and those available from the literature indicate that H. hippocrepis and M. monspessulanus have very different karyotype structures, which are highly representative of the families Colubridae and Psammophiidae. Starting from the ASK, these two families followed different pathways of chromosomal diversification, characterized by a highly conserved karyotype in Colubridae and by a progressive reduction of the chromosome number in Psammophiidae.
Acknowledgements
We thank the former Dipartimento di Biologia Evolutiva e Comparata of the Università degli Studi di Napoli Federico II which hosted the preserved cell suspensions and tissue samples and provided us with the study samples. We also thank Roland Daguerre for performing a linguistic revision on the last version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abd Allah AAE. 2010. Comparative karyological studies on some of Egyptian snakes. Master thesis. South Valley University, Zoology Department. Qena, Egypt.
- Alam SM, Sarre SD, Gleeson D, Georges A, Ezaz T. 2018. Did lizards follow unique pathways in sex chromosome evolution? Genes 9(5):239. DOI: 10.3390/genes9050239.
- Alencar LR, Quental TB, Grazziotin FG, Alfaro ML, Martins M, Venzon M, Zaher H. 2016. Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Molecular Phylogenetics and Evolution 105:50–62. DOI: 10.1016/j.ympev.2016.07.029.
- Aprea G, Odierna G, Andreone F, Glaw F, Vences M. 2003. Unusual karyotype in the Malagasy colubrid snake Mimophis mahfalensis. Amphibia-Reptilia 24(2):215–219. DOI: 10.1163/156853803322390471.
- Augstenová B, Mazzoleni S, Kratochvíl L, Rovatsos M. 2018. Evolutionary dynamics of the W chromosome in caenophidian snakes. Genes 9(1):5. DOI: 10.3390/genes9010005.
- Branch WR. 1980. Chromosome morphology of some reptiles from Oman and adjacent territories. The Journal of Oman Studies Special Report 2:333–345.
- Carranza S, Arnold EN, Pleguezuelos JM. 2006. Phylogeny, biogeography, and evolution of two Mediterranean snakes, Malpolon monspessulanus and Hemorrhois hippocrepis (Squamata, Colubridae), using mtDNA sequences. Molecular Phylogenetic and Evolution 40(2):532–546. DOI: 10.1016/j.ympev.2006.03.028.
- Carranza S, Arnold EN, Wade E, Fahd S. 2004. Phylogeography of the false smooth snakes, Macroprotodon (Serpentes, Colubridae): Mitochondrial DNA sequences show European populations arrived recently from NorthWest Africa. Molecular Phylogenetic and Evolution 33(3):523–532. DOI: 10.1016/j.ympev.2004.07.009.
- Cole CJ, Hardy LM. 2019. Karyotypes of six species of colubrid snakes from the Western Hemisphere, and the 140-million-year-old ancestral karyotype of Serpentes. American Museum Novitates 3926:1–14.
- Dobigny G, Ducroz JF, Robinson TJ, Volobouev V. 2004. Cytogenetics and cladistics. Systematic Biology 53(3):470–484. DOI: 10.1080/10635150490445698.
- Figueroa A, McKelvy AD, Grismer LL, Bell CD, Lailvaux SP. 2016. A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PLoS ONE 11(9):e0161070. DOI: 10.1371/journal.pone.0161070.
- Gamble T. 2010. A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sexual Development 4(1–2):88–103. DOI: 10.1159/000289578.
- Gorman GG. 1973. The chromosomes of the Reptilia, a Cytotaxonomic interpretation. In: Chiarelli AB, Capanne E, editors. Cytotaxonomy and vertebrate evolution. London: Academic press. pp. 349–424.
- Hall TA 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 41(41):95–98. DOI: 10.1021/bk-1999-0734.ch008.
- Howell WM, Black DA. 1980. Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: 1-step method. Experientia 36(8):1014–1015. DOI: 10.1007/BF01953855.
- Kostmann A, Kratochvíl L, Rovatsos M 2021. Poorly differentiated XX/XY sex chromosomes are widely shared across skink radiation. Proceedings of the Royal Society part B: Biological Sciences 288(1943):20202139. DOI: 10.1098/rspb.2020.2139.
- Kratochvíl L, Stöck M, Rovatsos M, Bullejos M, Herpin A, Jeffries DL, Peichel CL, Perrin N, Valenzuela N, Pokorná MJ. 2021. Expanding the classical paradigm: What we have learnt from vertebrates about sex chromosome evolution. Philosophical Transactions of the Royal Society of London Part B: Biological Sciences 376(1833):20200097. DOI: 10.1098/rstb.2020.0097.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas 52(2):201–220. DOI: 10.1111/j.1601-5223.1964.tb01953.x.
- Machado L, Harris DJ, Salvi D. 2021. Biogeographic and demographic history of the Mediterranean snakes Malpolon monspessulanus and Hemorrhois hippocrepis across the Strait of Gibraltar. BMC Ecology and Evolution 21(1):210. DOI: 10.1186/s12862-021-01941-3.
- Matsubara K, Nishida C, Matsuda Y, Kumazawa Y. 2016. Sex chromosome evolution in snakes inferred from divergence patterns of two gametologous genes and chromosome distribution of sex chromosome-linked repetitive sequences. Zoological Letters 2(1):19. DOI: 10.1186/s40851-016-0056-1.
- Matthey R. 1931. Chromosomes de reptiles sauriens, ophidiens, cheloniens. L’evolution de la formule chromosomiale chez les Sauriens. Revue suisse de Zoologie 38:117–186.
- Mezzasalma M, Andreone F, Aprea G, Glaw F, Odierna G, Guarino FM. 2017. Molecular phylogeny, biogeography and chromosome evolution of Malagasy dwarf geckos of the genus Lygodactylus (Squamata, Gekkonidae). Zoologica Scripta 46(1):42–54. DOI: 10.1111/zsc.12188.
- Mezzasalma M, Andreone F, Branch WR, Glaw F, Guarino FM, Nagy ZT, Odierna G, Aprea G. 2014. Chromosome evolution in pseudoxyrhophiine snakes from Madagascar: A wide range of karyotypic variability. Biological Journal of the Linnean Society 112(3):450–460. DOI: 10.1111/bij.12280.
- Mezzasalma M, Andreone F, Glaw F, Guarino FM, Odierna G, Petraccioli A, Picariello O. 2019. Changes in heterochromatin content and ancient chromosome fusion in the endemic Malagasy boid snakes Sanzinia and Acrantophis (Squamata: Serpentes). Salamandra 55(2):140–144.
- Mezzasalma M, Brunelli E, Odierna G, Guarino FM. 2022. First insights on the karyotype diversification of the endemic Malagasy leaf-toed geckos (Squamata: Gekkonidae: Uroplatus). Animals 12(16):2054. DOI: 10.3390/ani12162054.
- Mezzasalma M, Dall’Asta A, Loy A, Cheylan M, Lymberakis P, Zuffi MAL, Tomovíc L, Odierna G, Guarino FM. 2015. A sisters’ story: Comparative phylogeography and taxonomy of Hierophis viridiflavus and H. gemonensis (Serpentes, Colubridae). Zoologica Scripta 44(5):495–508. DOI: 10.1111/zsc.12115.
- Mezzasalma M, Di Febbraro M, Guarino FM, Odierna G, and Russo D. 2018. Cold-blooded in the Ice Age: “refugia within refugia”, inter-and intraspecific biogeographic diversification of European whipsnakes (Squamata, Colubridae, Hierophis). Zoology 127:84–94. DOI: 10.1016/j.zool.2018.01.005.
- Mezzasalma M, Guarino FM, Loader S, Odierna G, Streicher J, Cooper N. 2020. First karyological analysis of the endemic Malagasy phantom gecko Matoatoa brevipes (Squamata: Gekkonidae). Acta Herpetologica 15:137–141. DOI: 10.13128/a_h-8437.
- Mezzasalma M, Guarino FM, Odierna G. 2021. Lizards as model organisms of sex chromosome evolution: What we really know from a systematic distribution of available data? Genes 12(9):1341. DOI: 10.3390/genes12091341.
- Mezzasalma M, Odierna G. 2021. Sex chromosome diversification in the smooth snake Coronella austriaca (Reptilia, Serpentes). Acta Herpetologica 16(1):37–43. DOI: 10.36253/a_h-10418.
- Mezzasalma M, Visone V, Petraccioli A, Odierna G, Capriglione T, Guarino FM. 2016. Non-random accumulation of LINE1-like sequences on differentiated snake W chromosomes. Journal of Zoology 300(1):67–75. DOI: 10.1111/jzo.12355.
- Nagy ZT, Schmidtler JF, Joger U, Wink M. 2003. Systematics of dwarf snakes (Reptilia: Colubridae: Eirenis) and relatives, as inferred from DNA sequences and morphological data. Salamandra 39:149–168.
- Noor MA, Grams KL, Bertucci LA, Reiland J 2001. Chromosomal inversions and the reproductive isolation of species. Proceedings of the National Academy of Science USA 98(21):12084–12088. DOI: 10.1073/pnas.221274498.
- Oguiura N, Ferrarezzi H, Batistic RF. 2009. Cytogenetics and molecular data in snakes: A phylogenetic approach. Cytogenetic and Genome Research 127(2–4):128–142. DOI: 10.1159/000295789.
- Olmo E. 2008. Trends in the evolution of reptilian chromosomes. Integrative and Comparative Biology 48(4):486–493. DOI: 10.1093/icb/icn049.
- Olmo E, Signorino G. 2005. Chromorep: A reptile chromosomes database. Available: http://chromorep.univpm.it/. Accessed Nov 30 2022.
- Pallotta MM, Turano M, Ronca R, Mezzasalma M, Petraccioli A, Odierna G, Capriglione T. 2017. Brain Gene Expression is Influenced by Incubation Temperature During Leopard Gecko (Eublepharis macularius) Development. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 328(4):360–370. DOI: 10.1002/jez.b.22736.
- Petraccioli A, Guarino FM, Kupriyanova L, Mezzasalma M, Odierna G, Picariello O, Capriglione T. 2019. Isolation and characterization of interspersed repeated sequences in the common lizard, Zootoca vivipara, and Their Conservation in Squamata. Cytogenetic and Genome Research 157(1–2):65–76. DOI: 10.1159/000497304.
- Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13(1):93. DOI: 10.1186/1471-2148-13-93.
- Rieseberg LH. 2001. Chromosomal rearrangements and speciation. Trends in Ecology and Evolution 16(7):351–358. DOI: 10.1016/S0169-5347(01)02187-5.
- Rovatsos M, Vukíc J, Lymberakis P, Kratochvíl L 2015. Evolutionary stability of sex chromosomes in snakes. Proceedings of the Royal Society of London Series B, Biological Sciences. 282(1821):20151992. DOI: 10.1098/rspb.2015.1992.
- Sahar E, Latt SA. 1980. Energy transfer and binding competition between dyes used to enhance staining differentiation in metaphase chromosomes. Chromosoma 79(1):1–28. DOI: 10.1007/BF00328469.
- Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: A laboratory manual. 2nd 427 ed. New York: Cold Spring Harbor Lab Press.
- Sidhom M, Said K, Chatti N, Guarino FM, Odierna G, Petraccioli A, Picariello O, Mezzasalma M. 2020. Karyological characterization of the common chameleon (Chamaeleo chamaeleon) provides insights on the evolution and diversification of sex chromosomes in Chamaeleonidae. Zoology 141:125738. DOI: 10.1016/j.zool.2019.125738.
- Simoes BF, Sampaio FL, Douglas RH, Kodandaramaiah U, Casewell NR, Harrison RA, Hart NS, Partridge JC, Hunt DM, Gower DJ. 2016. Visual pigments, ocular filters and the evolution of snake vision. Molecular Biology and Evolution 33(10):2483–2495. DOI: 10.1093/molbev/msw148.
- Singchat W, Ahmad SF, Sillapaprayoon S, Muangmai N, Duengkae P, Peyachoknagul S, O’Connor RE, Griffin DK, Srikulnath K. 2020. Partial amniote sex chromosomal linkage homologies shared on snake W sex chromosomes support the ancestral super-sex chromosome evolution in amniotes. Frontiers in Genetics 11:948. DOI: 10.3389/fgene.2020.00948.
- Srikulnath K, Uno Y, Nishida C, Matsuda Y. 2013. Karyotype evolution in monitor lizards: Cross-species chromosome mapping of cDNA reveals highly conserved synteny and gene order in the Toxicofera clade. Chromosome Research 21(8):805–819. DOI: 10.1007/s10577-013-9398-0.
- Sumner AT. 1972. A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research 75(1):304–306. DOI: 10.1016/0014-4827(72)90558-7.
- Uetz P, Freed P, Aguilar R, Hošek J. 2022. The reptile database. Available: http://www.reptile-database.org. Accessed Jan 2023 17.
- Utiger U, Helfenberger N, Schätti B, Schmidt C, Ruf M, Ziswiler V. 2002. Molecular systematics and phylogeny of old and new world ratsnakes, Elaphe AUCT., and related genera (Reptilia, Squamata, Colubridae). Russian Journal of Herpetology 9:105–124.
- Viana P, Ezaz T, de Bello Cioffi M, Jackson Almeida B, Feldberg E. 2019. Evolutionary insights of the ZW sex chromosomes in snakes: A new chapter added by the Amazonian puffing snakes of the genus Spilotes. Genes 10(4):28. DOI: 10.3390/genes10040288.
- Zaher H, Murphy RW, Arredondo JC, Graboski R, Machado-Filho PR, Mahlow K, Montingelli GG, Quadros AB, Orlov NL, Wilkinson M, Zhang YP, Grazziotin FG. 2019. Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes (Squamata: Serpentes). PLoS ONE 14(5):e0216148. DOI: 10.1371/journal.pone.0216148.
