Abstract
Predators affect prey by killing them or inducing changes in their physiology and behaviour through a fear effect associated with predation risk. In birds, perceived predation risk influences reproductive decisions, such as the reduction of parental investment in offspring during both egg production and nestling rearing. Visual and vocal cues of predator presence have been widely used to test the direct effects of predation risk. However, few studies have examined the indirect cues of predator activity such as dead avian prey or their remains. In this study, for the first time, we experimentally studied whether piles of feathers, simulating the remains of avian prey, induce changes in the reproductive decisions of adult birds. Before and during egg laying, great tit, Parus major, pairs were exposed to piles of bright down and cover feathers from domestic goose (treatment), woodchips (procedural control), or were not exposed (control). Our experiment affected maternal investment in individual eggs, but did not influence other reproductive parameters. Females from the treatment group laid larger and more asymmetrical (pointed) eggs than control females. Moreover, females from the procedural control group laid larger eggs than those from the control group, but without differences in egg shape. However, the eggs from the treatment and procedural control groups did not differ. This indicates that great tit females can perceive feathers and woodchips as informative cues, such as potential predation risk or habitat suitability, or as novel items in the environment. Importantly, females respond to such cues by changing their maternal investment in eggs, which may result from an adaptive mechanism aimed at increasing offspring fitness in the face of specific environmental conditions experienced by a female. Our study contributes to the understanding of how female songbirds adjust their maternal reproductive investment in response to publicly available social and environmental cues.
Introduction
Predation constitutes a strong selective pressure and significantly determines the evolution of morphological, physiological, and behavioural traits such as flight initiation distance in prey (e.g. Agrawal et al. Citation1999; Caro Citation2005; Peluc et al. Citation2008; Coslovsky & Richner Citation2011; Giesing et al. Citation2011; Møller et al. Citation2017). The killing of prey and the ultimate ecological effect of predation are obvious. However, in addition to such direct, consumptive effects, predators may impose indirect, non-consumptive effects on the physiology and behaviour of their potential prey (e.g. Scheuerlein et al. Citation2001; Creel & Christianson Citation2008; Sheriff et al. Citation2009; Peacor et al. Citation2020). Variations in the behavioural and physiological responses to perceived predation risk may affect habitat selection, nest site choice, sociality, foraging activity and efficiency, mating, parental nest/brood attendance, and offspring behavior (Caro Citation2005; Lima Citation2009; Morosinotto et al. Citation2010; Santema et al. Citation2020). Furthermore, the non-consumptive effects of predation have been shown to affect the expression of life-history traits that are pivotal for fitness, i.e., the number and size of produced eggs or offspring (e.g. Lima Citation2009; Coslovsky & Richner Citation2011; Zanette et al. Citation2011; Dillon & Conway Citation2018; Dudeck et al. Citation2018; Possenti et al. Citation2019).
In birds, perceived predation risk influences female investment in eggs, both in terms of clutch size (Eggers et al. Citation2006; Morosinotto et al. Citation2010; Zanette et al. Citation2011) and egg quality (i.e. egg size and composition, Fontaine & Martin Citation2006; Morosinotto et al. Citation2019). In general, predation risk is expected to decrease maternal investment in eggs by producing smaller clutches (Travers et al. Citation2010; Hua et al. Citation2014; Dillon & Conway Citation2018) or eggs (Fontaine & Martin Citation2006), especially if predation concerns eggs or nestlings (Lima Citation2009; Hua et al. Citation2014). However, several field studies have found no effect of predation risk on the number of laid eggs (Coslovsky & Richner Citation2011; Possenti et al. Citation2019) and egg mass (Coslovsky & Richner Citation2011; Morosinotto et al. Citation2019), or even reported increase in egg mass (Zanette et al. Citation2011; Possenti et al. Citation2019). In addition to the effects of predation on maternal investment in eggs, the influence of predation risk on parental investment in hatched nestlings is commonly observed (e.g. Massaro et al. Citation2008; Lima Citation2009; Sofaer et al. Citation2013; Mutzel et al. Citation2019). The risk usually negatively affects parental care, such as reducing provisioning rate or brood attendance, which ultimately results in a decline in the number and/or condition of produced offspring (Coslovsky & Richner Citation2011; Zanette et al. Citation2011; Dudeck et al. Citation2018, but see Hua et al. Citation2014).
To date, the majority of studies on perceived predation risk in birds have been conducted by experimentally providing direct visual and/or auditory cues of predator presence, such as live predators, various types of predator models, the emission of predator calls with or without a predator’s dummy, comparing bird behaviour in places with and without a predator, and the use of olfactory stimuli (e.g. Coslovsky & Richner Citation2011; Amo et al. Citation2017; Carlson et al. Citation2017; Møller et al. Citation2017; Possenti et al. Citation2019; Santema et al. Citation2020). In contrast, few studies have examined the effects of perceived predation risk based on indirect visual cues of predatory activity resulting from the direct effects of predation, such as the presence of corpses or remains of avian prey (Ekner & Tryjanowski Citation2008; Peterson & Colwell Citation2014; Swift & Marzluff Citation2015 Citation2018; Carlson et al. Citation2017). For example, Swift and Marzluff (Citation2015) demonstrated that American crows, Corvus brachyrhynchos, use dead conspecifics to assess danger or new threats. This species responds to corpses by enhanced anti-predator behaviours (such as predator scolding and mobbing) and avoidance of risky areas. Given that the remains of prey may provide a key stimulus for adult birds to recognise potential threats associated with predator activity and generate specific behavioral responses to such danger (Peterson & Colwell Citation2014; Swift & Marzluff Citation2015; Carlson et al. Citation2017), it is possible that such cues also affect bird reproductive decisions.
Beside corpses, feather piles are visible remains of avian prey, killed by both avian and mammal predators. Such avian prey remains may provide useful heterospecific information on predator occurrence and activity. The Eurasian sparrowhawk, Accipiter nisus, the main avian predator of middle and small passerine birds, commonly plucks feathers from killed birds and leave piles of feathers, which are signs of predation and prey consumption (Newton Citation1986). Thus, the number and distribution of piles of feathers in a given area may, to some extent, provide information to potential avian prey regarding the level of predation risk. To the best of our knowledge, no study has examined whether piles of feathers remaining from avian prey affect the reproductive decisions of adult birds.
The aim of our study was to determine whether indirect visual signs of predator activity, such as piles of feathers, may be perceived by adult great tits, Parus major, and constitute reliable cues for them to change their reproductive decisions. For this purpose, we experimentally manipulated the level of perceived predation risk before and during egg laying by placing piles of white feathers (mostly down) from the domestic goose on the forest ground (treatment group). To separate the pure effect of feathers on reproductive output from the overall effects of the experiment (e.g., some disturbances in habitat due to appearing feathers), we created a procedural control group in which bright woodchips was used a substitute for the feathers. We also established a control group that was free of any treatment. We then examined how this predation risk experiment affected reproductive parameters, such as breeding pair abundance, laying date, clutch size, egg characteristics (size and shape), hatching success, and the number and condition of fledged offspring.
We hypothesised that adult birds perceive piles of feathers as signs of potential predation threats and use this information to adjust their reproductive decisions. Specifically, we predicted that adult birds would avoid areas with increased perceived predation risk (treatment group), which would result in the decline/absence of breeding pairs or some alterations in the date of breeding commencement. Avoiding areas with high predator pressure or changing egg-laying initiation has been previously documented as a response of adult birds to potential predation (Lima Citation2009; Mönkkönen et al. Citation2009). However, such responses to predation risk may go much further and ultimately alter reproductive decisions, most commonly by reducing parental investment in offspring (Zanette et al. Citation2011; Dillon & Conway Citation2018; Mutzel et al. Citation2019). Therefore, we expected that if birds did not avoid breeding under high perceived predation risk, they would decrease parental investment in eggs (i.e., a lower clutch size, smaller eggs, or lower hatching success) or nestlings (i.e., a reduction in fledgling number and/or deterioration of offspring condition). The latter is expected to be a carry-over effect of predation risk perceived during the early stage of breeding. In a statistical sense, we expected significant differences between the treatment and procedural control groups and between the treatment and control groups, with no difference between the procedural and control groups.
Materials and methods
Study area and species
The study was conducted in spring 2017 in a natural population of great tits occurring in the northern part of the Niepołomice Forest, in southern Poland (50°04` N, 20°21` E). The study area was located in a 60-year-old deciduous forest with poorly developed underwood, dominated by oaks, hornbeams, and limes. The great tit is a small passerine bird commonly used as a model species in behavioral studies, including research concerning social or public information use (Parejo et al. Citation2008) and the effects of predation risk on reproduction (e.g. Coslovsky & Richner Citation2011). In the deciduous part of the Niepołomice Forest, great tit females start egg-laying in April and lay 11 eggs in a clutch, on average. The incubation period for this species is approximately 13 days. Both parents feed nestlings that fledge within 15–18 days after hatching.
Experimental design and field procedures
The study area was divided into 12 plots, in which a similar number of wooden nest boxes (interior dimensions: 11 × 11 × 28 cm) were hung (mean ± SD: 21 ± 3 nest boxes per plot, range 18–26). The nest boxes were distributed in a 40 × 35 m grid (ca. 6.6 nest boxes per ha). They were deployed in autumn 2016 in an area where no nest boxes had been previously and no experiments had been conducted. Each plot was randomly assigned to one of three experimental groups: treatment, procedural control, and control. The number of nest boxes on plots assigned to the treatment, procedural control, and control groups were also similar (mean ± SD: 22 ± 3, 21 ± 3, and 21 ± 2 nest boxes per plot, respectively). In total, there were 85 nest boxes in the treatment plots, 85 in the procedural control plots, and 83 in the control plots.
On plots assigned to the treatment group, we laid down piles of feathers on the forest litter to simulate increased predator activity (Supplementary Figure S1). The feathers were placed on the ground in a shape resembling a circle with a diameter of approximately 50 cm. We used white feathers and down obtained from natural goose down feather pillows; thus, the cover feathers and down were absent of avian odors (which would attract mammal predators). In plots assigned to the procedural control group we used bright woodchips that were placed on the ground in the same manner as the feathers (Supplementary Figure S2). The woodchips were a substitute for white geese feathers to control the potential effect of changes in habitat made by laying out the feathers. More specifically, the effects of feather piles on bird behavior may not result from their presence themselves, but may also be caused by human presence and activity when putting the feathers on the ground. Thus, we expected to be able to separate these effects. The plots assigned to the control group were free of any experimental procedures. The piles of feathers and woodchips were placed at four randomly selected points every second day in each plot belonging to the treatment and procedural control groups, respectively. This experiment was performed in the morning, between 8 and 10 a.m. We ran the experiment from1 April until 20 April, i.e. during a period in which most great tits started building nests and laying eggs in the study area.
From 31 March, we started checking all nest boxes to detect nest building and determine laying dates and clutch sizes. In total, 25 pairs of great tits were bred in all plots (7, 8, and 10 in plots belonging to the treatment, procedural control, and control groups, respectively). Breeding pairs recorded in plots assigned to the treatment and procedural control groups did not differ in exposure time to experimental activities, that is, placing feathers and woodchips on the ground (Kruskal-Wallis rank-sum test: χ2 = 0.87, df = 1, P = 0.35). Exposure time was measured as the number of days from the date when nest building was observed for the first time up to the end of the experimental procedures. Thus, the time included only the period during which we actively conducted our experiment. However, piles of feathers and woodchips were still visible for approximately two weeks after the end of the experimental procedures, indicating that the potential effects of feathers (and woodchips) on breeding birds could have persisted much longer.
Egg measurements
Halfway through the incubation period, we determined the size and shape of all eggs laid in a clutch using digitized photos. Eggs were taken from a nest and placed on a flat surface with a black background. We then took photographs of the eggs at an angle of 90° to the egg’s long axis at a distance of approximately 30 cm using a Canon 450D camera and Canon EF-S 18–55 lens. Each photo contained a ruler (to the nearest 1 mm) that served as a scale bar for calculating the egg volume. Based on these images, we estimated egg volume and shape using an Egg Measurement Tool plug-in developed by Troscianko (Citation2014) for ImageJ software (Schneider et al. Citation2012). According to this method, egg shape is calculated as the deviation from a perfect ellipse, which provides a measure of egg asymmetry – pointedness (Troscianko Citation2014). The values of the egg shape index were multiplied by 1000 for increased readability. The higher the value of the shape index, the more pointed (less elliptical) the egg is. The modelling system for egg measurement proposed by Troscianko (Citation2014) provides egg volume estimation as a measure independent of egg shape. We found no correlation between egg volume and egg shape in our dataset (r = −0.06, P = 0.37; N = 256). In the studied population of great tits, the average egg volume (raw mean ± SE) was 1.80 ± 0.01 cm3, whereas the average egg shape index (raw mean ± SE) was 0.50 ± 0.02 (index = 0 means an ideal ellipse).
Nestling measurements
We regularly monitored incubating females around the expected day of hatching to detect the exact date at which nestlings hatched (hatching date = day 0). We then measured and ringed the nestlings when they reached the age of 14 days. Body mass was assessed using an electronic balance to the nearest 0.01 g, and the right tarsus length was measured using a caliper to the nearest 0.1 mm.
Statistical analyses
We used a chi-square test to examine the frequency of nest box occupancy by breeding great tits across experimental groups and to determine the frequency of nest abandonment among all three groups. We fitted general additive models (GAMs) with a Gaussian error distribution and an identity-link function to analyse the effects of predation risk on laying date and clutch size. To examine the effects of predation risk on the number of fledglings, we used the GAM with negative binomial error variance and a log-link function. In all GAMs, predation risk was treated as a categorical fixed factor (with three levels: treatment, procedural control, and control), laying date or hatching date (to control for the effects of seasonal changes), and clutch size (to control for the brood size effect) as covariates. Hatching success was examined using a generalised additive mixed model (GAMM) with a binomial error distribution and logit-link function. To analyze the effects of predation risk on egg volume and shape, as well as the body mass and tarsus length of 14-day-old nestlings, we fitted GAMMs with a Gaussian error distribution and identity-link function. All GAMMs included the manipulation of predation risk as a categorical fixed factor, and laying date or hatching date, clutch size, mean egg volume, egg shape per clutch (to test their effects on offspring condition), and tarsus length (to correct for body size in the analysis of nestling body mass) as covariates. The period in which females were exposed to experimental procedures was highly correlated with the laying date and hatching date (r = −0.84, P < 0.001 and r = −0.75, P = 0.002, respectively; N = 15). Since the laying date and hatching date appeared to have more biological sense in the analyses, the fact that the detected strong correlations allowed us to prevent testing the exposure time as an additional independent variable. In the GAMs and GAMMs, the predation risk factor was fitted as a linear predictor. We used a priori contrasts to determine the differences between the levels of this factor. The contrast estimates and P values based on the t-tests gathered from the models are presented in the text and tables. All covariates in the GAMs and GAMMs were fitted as thin plate regression splines to determine their potential non-linear effects on the dependent variables. To control spatial autocorrelation among great tit nests, we entered the longitude and latitude of each nest location as two covariates, which were then fitted as an interaction of thin-plate regression splines. As a result, part of the variation in the dependent variable was explained by geographical location, thus considering potential spatial gradients (differences) in habitat quality. Furthermore, for the GAMMs, we introduced nest identity as a random factor, which was fitted as a ridge penalty spline to account for the non-independence of eggs and nestlings from the same nest. We checked the assumptions for all models through visual inspection of the residual plots. To normalize the distribution of residuals in the model analysing egg shape, we used a square-root transformation of the raw data. The parameter estimates and statistics are presented from reduced (final) models after removing covariates that weakly contributed to explaining the variation in dependent variables (if P > 0.10). Both GAMs and GAMMs were performed using the mcgv and mgcViz packages (Wood Citation2017; Fasiolo et al. Citation2019) implemented in the R environment (R Core Team Citation2020). All statistical tests were two-tailed, and the significance level was set at P < 0.05.
Results
Effects of experiment on breeding phenology and reproductive parameters
The occupancy of nest boxes by the great tits did not differ among the experimental groups (χ22 = 0.72, P = 0.70; N = 253). In addition, birds that had started breeding abandoned their nests at similar frequencies in the treatment, procedural control, and control groups (χ22 = 0.07, P = 0.97; N = 25). We did not find any effect of the experiment on laying date, clutch size, hatching success, or number of fledged offspring (Supplementary Table S1). We found that the fledgling number was positively correlated with the clutch size (Supplementary Table S1, Supplementary Figures S3).
Effects of experiment on egg size and shape
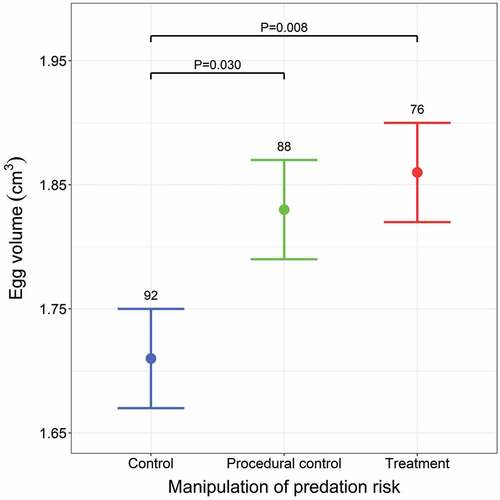
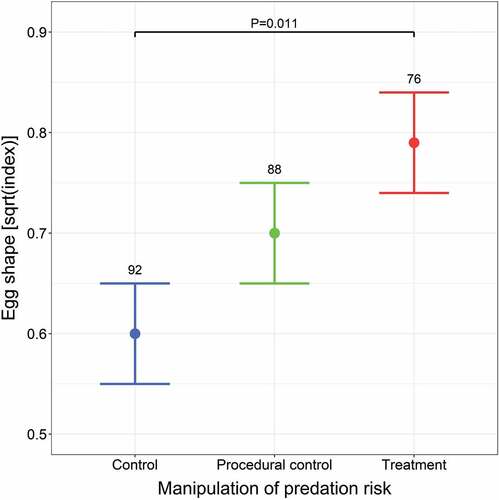
Our experimental manipulation affected both the size and shape of eggs (). Females from the treatment group laid larger eggs than females from the control group; however, there were no differences in egg volume between females from the treatment and procedural control groups (, ). Females from the procedural control group also had larger eggs than those from the control group (contrast estimate ± SE: 0.115 ± 0.053, P = 0.030; ). We also found a non-linear effect of clutch size on egg volume regardless of the experiment; egg volume increased abruptly until the clutch size reached eight and then slowly declined (Supplementary Figure S5). Furthermore, females from the treatment group laid more asymmetrical (pointed) eggs compared to females from the control group, in which eggs were more elliptical (, ). We did not detect these differences in the egg shape index between females from the treatment and procedural control groups (, ) or between females from the procedural control and control groups (contrast estimate ± SE: 0.099 ± 0.075, P = 0.19; ). Moreover, we found that the egg shape negatively correlated with the laying date, regardless of the experiment (, Supplementary Figure S5).
Table I. Results of generalised additive mixed models fitted with Gaussian error variance and identity-link function that examined a set of explanatory variables on the volume and square-root transformed shape of eggs. Predation risk (categorical variable with levels: treatment, control, and procedural control) was set as a linear predictor, with covariates as regression splines. Longitude and latitude were set as an interaction of regression splines to control for spatial autocorrelation of the data. Nest identity was introduced to models as a random factor (fitted as a ridge penalty spline). Reduced models are presented after removing covariates that weakly contributed to the explained variation in dependent variables (if P ≥ 0.10). Parameter estimates accompanied by SE in brackets for the intercept and linear predictor, and effective degrees of freedom (edf) for splines are given. Significance level for each explanatory variable is coded as *** (P < 0.001), ** (P < 0.01), * (P < 0.05), ’ (P < 0.10).
Effects of experiment on fledgling condition
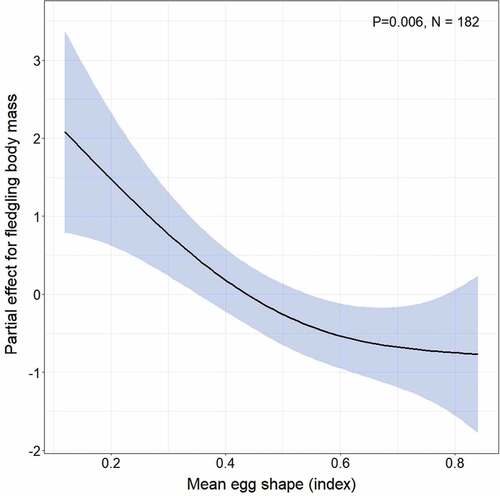
The experiment did not affect the body mass or tarsus length of the 14-day old nestlings (). Interestingly, we found that the mean egg shape in a clutch was a significant predictor of offspring body mass; the body mass of fledged nestlings decreased non-linearly as the value of mean egg asymmetry increased (, ). There was also a nonlinear but overall positive effect of tarsus length on fledgling body mass (, Supplementary Figure S6). Moreover, we found that the hatching date had a non-linear effect on fledgling tarsus length; tarsus length increased, flattened, and grew as the hatching date progressed (, Supplementary Figure S7).
Table II. Results of generalised additive mixed models fitted with Gaussian error variance and identity-link function that examined a set of explanatory variables on the body mass and tarsus length of fledged offspring. Predation risk (categorical variable with levels: treatment, control, and procedural control) was set as a linear predictor, with covariates as regression splines. Longitude and latitude were set as an interaction of regression splines to control for spatial autocorrelation of the data. Nest identity was introduced in the models as a random factor (fitted as a ridge penalty spline). Reduced models are presented after removing covariates that weakly contributed to explained variation in the dependent variables (if P ≥ 0.10). Parameter estimates accompanied by SE in brackets for the intercept and the linear predictor and effective degrees of freedom (edf) for splines are given. Significance level for each explanatory variable is coded as *** (P < 0.001), ** (P < 0.01), * (P < 0.05), ’ (P < 0.10).
Discussion
We experimentally demonstrated that piles of feathers and woodchips may be perceived as cues for potential threats or environmental stressors by adult great tits. The placement of feather piles and woodchips on forest litter affected the prenatal reproductive decisions of this species. Although our experiment influenced maternal investment in individual eggs, it had no effect on breeding occupancy, laying date, clutch size, and the number and condition of fledged nestlings. Females exposed to cues both before and during egg laying produced larger eggs than females not exposed to these treatments. Moreover, females from the treatment group laid more asymmetrical (pointed) eggs than the control females. Simultaneously, the volume and shape of eggs laid by females exposed to feathers did not differ from the eggs laid by females exposed to woodchips.
The observed lack of difference in maternal investment between the treatment and procedural control groups was unclear and unexpected, as we predicted that such a response would only be observed in the case of birds breeding on plots with piles of feathers, but not in plots with woodchips. Thus, it is likely that females from these two groups had to perceive both types of cues in a similar context, which translated into the parallel physiological responses of mothers, that is, similar investment in eggs. There are several possible explanations for why females that bred on plots with feathers and woodchips showed similar patterns in their reproductive investment. First, woodchips may not provide a neutral cue for birds, as originally assumed. In fact, birds that breed in managed forests with intensively logged trees (this concerns our study area) may experience significant stress associated with forest disruptions due to tree cutting, especially during the breeding season (Blumstein Citation2010; Leshyk et al. Citation2012; Messina et al. Citation2020). The presence of woodchips, as an effect of wood cutting, may be linked to birds as potential threats of nest loss, habitat disturbance, or human presence associated with logging. Second, the observed effect of feather and woodchip piles on maternal investment in eggs may result from the general response of birds to novel items in the environment (Crane & Ferrari Citation2017). If so, we cannot clearly determine whether piles of feathers indeed provide reliable cues about predation risk to adult birds, as the observed patterns may result from stress responses to a novel object (Tryjanowski et al. Citation2016; Goławski & Sytykiewicz Citation2021) rather than stress associated with potential predation. For these reasons, several authors argue that procedural controls should not be used in public social information experiments (Brumm & Slabbekoorn Citation2005; Seppänen et al. Citation2007; Szymkowiak et al. Citation2017); however, we do not agree with this statement. Third, the human itself, as a potential predator, may be an important factor affecting the behavior and reproduction of birds (Frid & Dill Citation2002). Previous studies have documented that forest birds avoid humans and/or alter their reproductive decisions because of regular human activity or presence (Lowe et al. Citation2014; Remacha et al. Citation2016; Hutfluss & Dingemanse Citation2019). Therefore, the occurrence of feathers or woodchips associated with the presence of humans may significantly enhance the physiological or behavioral responses of birds to these stimuli. Swift and Marzluff (Citation2015) showed that crows generated much stronger behavioral responses to dead conspecifics or their remains in the presence of predators (i.e., hawk or human) than dead crows presented alone. In our study, we personally put up piles of feathers and woodchips on the ground in the morning hours, when great tits had the opportunity to connect human presence with the distributed feathers and woodchips. Thus, we cannot exclude the possibility that our findings are a combined effect of human and feather or woodchip presence rather than a single effect of each cue. However, it seems unlikely that the observed effects on maternal reproductive decisions were related to human presence only, because all plots (including the control group) were visited at similar levels during nest box checking, with additional short visits associated only with the laying down of pile feathers and woodchips.
The fact that great tit females invested more in eggs when bred under stressful conditions is not an exceptional result, as previous research has observed similar patterns (Zanette et al. Citation2011; Possenti et al. Citation2019). Laying larger eggs (and also larger clutches) in response to potential threats may be an adaptive strategy, which allow females to mitigate the detrimental effects of adverse environmental conditions during breeding (Possenti et al. Citation2019). Larger eggs may allow the development of embryos to be less sensitive to cooling when incubation is interrupted by female escape due to potential threats appearing around the nest (e.g. predators or human disturbance; Gillooly et al. Citation2002). Basso and Richner (Citation2015) experimentally showed that great tit females exposed to predators increased the number of incubation sessions and recesses, and their eggs lost more mass over the incubation period than females not exposed to that threat. Importantly, larger eggs give larger avian offspring that grow fast and stay for a shorter period in a nest (Krist Citation2011). Thus, females and/or nestlings may benefit from enhanced investment in eggs in risky environments by shortening the period of nestling development, thereby decreasing the probability of brood detection (Briskie et al. Citation1999; Caro Citation2005). Moreover, larger offspring may also be less vulnerable to starvation when parental attendance at the nest is reduced due to the presence of possible predators (Rhymer Citation1988; Magrath Citation1991). Alternatively, the production of larger eggs by mothers experiencing an elevated death risk may be a manifestation of terminal investment. Females who perceive a high risk of being killed may invest more resources in current breeding attempts because of decreased chances of survival in the next reproductive event (Haapakoski et al. Citation2018; Sievert et al. Citation2019).
In addition to size, shape is an important property of avian eggs that may affect offspring development and remain under female control to some extent (Barta & Székely Citation1997). Our experiment supports this idea because of the differences in egg shape between the treatment and control groups. Interestingly, this effect was independent of egg size, as we found no relationship between egg size and shape (asymmetry). This result indicates that stressful conditions may affect egg production of a specific shape. To the best of our knowledge, this is the first study to show that a potential danger or uncertainty perceived by females may lead to a change in the shape of laid eggs. Egg shape is primarily associated with oviduct anatomy, especially the isthmus, a place where egg membranes are created (Barta & Székely Citation1997; Koyama et al. Citation2019). The tension of oviduct mussels and/or pressure of entrails on the oviduct may be responsible for determining specific egg shapes (Barta & Székely Citation1997; Koyama et al. Citation2019), which results from the physiological state of a female. The stress experienced by females during egg formation may affect the shape of the laid eggs. Previous research has shown that distinct physiological conditions in females may cause laying of eggs of various shapes (Cucco et al. Citation2012). Moreover, external environmental conditions, such as habitat quality and climate variability, may also influence egg shape in birds (Bańbura Citation2018; Duursma et al. Citation2018), likely through their impact on female conditions and physiology. The shape of eggs may also be changed by their composition, that is, the proportion of yolk, albumen, and shell in relation to the overall egg mass (Deeming Citation2018). Hence, we cannot exclude the possibility that the observed differences in egg asymmetry were driven by differences in egg composition, especially because we also found differences in egg size. The production of eggs with larger masses (sizes) in birds may be as a result of the increased deposition of only one egg component, in other words, a higher amount of yolk or albumen (Williams Citation1994).
We found no effect of our experiment on the number and condition of fledged nestlings, suggesting that the observed differences in egg characteristics between groups do not translate into offspring size at fledging. As we mentioned before, larger eggs should produce larger nestlings (although we did not measure nestling body mass shortly after hatching, still such correlation is expected, see Krist Citation2011) but potential inequalities in size between nestlings resulting from egg size differences may disappear with the advance of nestling development (Murphy Citation1985; Smith and Bruun Citation1998). This result also indicates that there was no carry-over effect of cues perceived by parents during egg production on subsequent parental care after hatching. This suggests that the feeding rate was not influenced by the environmental stressors experienced in the early stages of breeding. However, regardless of the experimental group, egg shape, but not size, affected the body mass of fledged offspring. Nestlings from broods containing elliptical eggs were heavier than those from broods containing asymmetrical (pointed) eggs. Previous studies have documented that the shape of bird eggs primarily affects the embryo death rate and hatching success but has no consequences for nestling development after hatching (Cucco et al. Citation2012; Alasahan and Copur Citation2016). In great tits, body mass at fledgling is a key fitness-related trait that predicts post-fledging offspring survival and recruitment to breeding populations (Both et al. Citation1999; Naef-Daenzer et al. Citation2001). However, we did not follow the fate of the birds that left the nest boxes.
Our study should be interpreted with caution, as some aspects may potentially influence the results and inferences. First, the sample size of breeding pairs was relatively low. This may affect the power of statistical tests, especially the analysis of reproductive parameters at the nest level (such as laying date, clutch size, and fledgling number), but also limit the range of potential between-nest variation in dependent variables. Second, our study was conducted on birds inhabiting nest boxes, but not on those in natural tree holes. In fact, hole-nesting birds breeding in natural cavities and nest boxes may differ in their reproductive output and/or offspring conditions (Janas et al. Citation2022; Sudyka et al. Citation2022). This fact did not depreciate our results but is important for their proper interpretation (e.g., this study should be compared with caution with studies conducted in birds inhabiting natural breeding sites). These limitations should be considered when other researchers use these results. Importantly, future studies should consider these problems when examining the effects of perceived predation risk on reproductive decisions in other populations and bird species.
In conclusion, our study indicates that female great tits are able to perceive the presence of feathers and woodchips in the environment. On the one hand, feathers and woodchips may play an informative role about potential predation risk or the habitat suitability, respectively. However, they can be treated as novel objects in the environment, resulting in stressful and/or uncertain conditions for breeding birds. Because of these cues, the physiology of females is probably affected in a way that alters prenatal maternal investment in eggs. However, differences in egg characteristics resulting from responses to environmental cues may influence nestling body mass at fledging only indirectly through changes in egg shape. The observed alteration of maternal investment in eggs may result from an adaptive mechanism aimed at increasing offspring fitness in the face of specific environmental conditions experienced by a female. Thus, our study demonstrates that birds exposed to publicly provided social and/or environmental cues may respond by plastically adjusting their reproductive decisions. Moreover, other than a control group, this study recommends the use of a proper procedural control to reliably differentiate between a pure effect of treatment and effects confounded by other factors.
Authors’ contributions
RM and PS conceived and planned the study; RM conducted the experiment and collected data in the field; RM performed statistical analyses, interpreted results, and wrote the first draft of the manuscript; PS contributed to the interpretation of the results and writing of the manuscript. Both authors have read and approved the final manuscript.
Ethical approval
The First Local Ethics Committee for Animal Experimentation in Kraków (Poland) approved the experimental procedures planned and conducted in our study (permit number: 49/2017). The Regional Directorate for Environmental Protection in Kraków (Poland) permitted this study of wild bird populations (permit number: OP-I.6401.45.2017.MMr).
Data accessibility
The dataset generated and analysed during the study is available as supplementary material.
Supplemental Material
Download MS Word (1.1 MB)Supplemental Material
Download MS Excel (52.2 KB)Acknowledgements
We thank the two anonymous Referees and Editors for their reviews and useful comments for improving our manuscript.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2181988
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agrawal AA, Laforsch C, Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401(6748):60–63. DOI:10.1038/43425.
- Alasahan S, Copur G. 2016. Hatching characteristics and growth performance of eggs with different egg shapes. Brazilian Journal of Poultry Science 18(1):1–8. DOI:10.1590/1516-635X1801001-008.
- Amo L, Tomás G, López-García A. 2017. Role of chemical and visual cues of mammalian predators in nest defense in birds. Behavioral Ecology and Sociobiology 71(3):49. DOI:10.1007/s00265-017-2281-9.
- Bańbura M, Glądalski M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2018. A consistent long-lasting pattern of spatial variation in egg size and shape in blue tits (Cyanistes caeruleus). Frontiers in Zoology 15(1):34. DOI:10.1186/s12983-018-0279-4.
- Barta Z, Székely T. 1997. The optimal shape of avian eggs. Functional Ecology 11(5):656–662. DOI:10.1046/j.1365-2435.1997.00136.x.
- Basso A, Richner H. 2015. Effects of nest predation risk on female incubation behavior and offspring growth in great tits. Behavioral Ecology and Sociobiology 69(6):977–989. DOI:10.1007/s00265-015-1910-4.
- Blumstein DT. 2010. Conservation and animal welfare issues arising from forestry practices. Animal Welfare 19(2):151–157. DOI:10.1017/S0962728600001408.
- Both C, Visser ME, Verboven N. 1999. Density-dependent recruitment rates in great tits: The importance of being heavier. Proceedings of the Royal Society of London B 266(1418):465–469. DOI:10.1098/rspb.1999.0660.
- Briskie JV, Martin PR, Martin TE. 1999. Nest predation and the evolution of nestling begging calls. Proceedings of the Royal Society of London B 266(1434):2153–2159. DOI:10.1098/rspb.1999.0902.
- Brumm H, Slabbekoorn H. 2005. Acoustic communication in noise. Advances in the Study of Behavior 35:151–209. DOI: 10.1016/S0065-3454(05.
- Carlson NV, Pargeter HM, Templeton CN. 2017. Sparrowhawk movement, calling, and presence of dead conspecifics differentially impact blue tit (Cyanistes caeruleus) vocal and behavioral mobbing responses. Behavioral Ecology and Sociobiology 71(9):133. DOI:10.1007/s00265-017-2361-x.
- Caro T. 2005. Antipredator defences in birds and mammals. Chicago: University of Chicago Press.
- Coslovsky M, Richner H. 2011. Predation risk affects offspring growth via maternal effects. Functional Ecology 25(4):878–888. DOI:10.1111/j.1365-2435.2011.01834.x.
- Crane AL, Ferrari MCO. 2017. Patterns of predator neophobia: A meta-analytic review. Proceedings of the Royal Society B 284(1861):20170583. DOI:10.1098/rspb.2017.0583.
- Creel S, Christianson D. 2008. Relationships between direct predation and risk effects. Trends in Ecology and Evolution 23(4):194–201. DOI:10.1016/j.tree.2007.12.004.
- Cucco M, Grenna M, Malacarne G. 2012. Female condition, egg shape and hatchability: A study on the grey partridge. Journal of Zoology 287(3):186–194. DOI:10.1111/j.1469-7998.2012.00902.x.
- Deeming DC. 2018. Effect of composition on shape of bird eggs. Journal of Avian Biology 49(1):jav–01528. DOI:10.1111/jav.01528.
- Dillon KG, Conway CJ. 2018. Nest predation risk explains variation in avian clutch size. Behavioral Ecology 29(2):301–311. DOI:10.1093/beheco/arx130.
- Dudeck BP, Clinchy M, Allen MC, Zanette LY. 2018. Fear affects parental care, which predicts juvenile survival and exacerbates the total cost of fear on demography. Ecology 99(1):127–135. DOI:10.1002/ecy.2050.
- Duursma DE, Gallagher RV, Price JJ, Griffith SC. 2018. Variation in avian egg shape and nest structure is explained by climatic conditions. Scientific Reports 8(1):4141. DOI:10.1038/s41598-018-22436-0.
- Eggers S, Griesser M, Nystrand M, Ekman J. 2006. Predation risk induces changes in nest-site selection and clutch size in the Siberian jay. Proceedings of the Royal Society B 273(1587):701–706. DOI:10.1098/rspb.2005.3373.
- Ekner A, Tryjanowski P. 2008. Do small hole nesting passerines detect cues left by a predator? A test on winter roosting sites. Acta Ornithologica 43(1):107–111. DOI:10.3161/000164508X345392.
- Fasiolo M, Nedellec R, Goude Y, Wood SN. 2019. Scalable visualisation methods for modern generalized additive models. arXiv.org, Available: https://arxiv.org/abs/1809.10632. Accessed Mar 2022 15.
- Fontaine JJ, Martin TE. 2006. Parent birds assess nest predation risk and adjust their reproductive strategies. Ecology Letters 9(4):428–434. DOI:10.1111/j.1461-0248.2006.00892.x.
- Frid A, Dill LM. 2002. Human-caused disturbance stimuli as a form of predation risk. Conservation Ecology 6(1):11. Available: https://www.ecologyandsociety.org/vol6/iss1/art11.
- Giesing ER, Suski CD, Warner RE, Bell AM. 2011. Female sticklebacks transfer information via eggs: Effects of maternal experience with predators on offspring. Proceedings of the Royal Society B 278(1712):1753–1759. DOI:10.1098/rspb.2010.1819.
- Gillooly J, Charnov E, West G, Savage VM, Brown JH. 2002. Effects of size and temperature on developmental time. Nature 417(6884):70–73. DOI:10.1038/417070a.
- Goławski A, Sytykiewicz H. 2021. How urban and rural birds respond to the colour of bird feeders? Journal of Ornitholofy 162(4):1193–1198. DOI:10.1007/s10336-021-01907-8.
- Haapakoski M, Hardenbol AA, Matson KD. 2018. Exposure to chemical cues from predator-exposed conspecifics increases reproduction in a wild rodent. Scientific Reports 8(1):17214. DOI:10.1038/s41598-018-35568-0.
- Hua F, Sieving KE, Fletcher Jr RJ, Wright CA. 2014. Increased perception of predation risk to adults and offspring alters avian reproductive strategy and performance. Behavioral Ecology 25(3):509–519. DOI:10.1093/beheco/aru017.
- Hutfluss A, Dingemanse N. 2019. Human recreation reduces clutch size in great tits Parus major regardless of risk-taking personality. Behavioral Ecology 30(6):1751–1760. DOI:10.1093/beheco/arz145.
- Janas K, Di Lecce I, Szulkin M, Sudyka J. 2022. Plumage colouration differs between offspring raised in natural cavities and nestboxes. bioRxiv 08:29.505638. DOI: 10.1101/2022.08.29.505638.
- Koyama H, Shi D, Fujimori T. 2019. Biophysics in oviduct: Planar cell polarity, cilia, epithelial fold and tube morphogenesis, egg dynamics. Biophysics and Physicobiology 16(1):89–107. DOI:10.2142/biophysico.16.0_89.
- Krist M. 2011. Egg size and offspring quality: A meta-analysis in birds. Biological Reviews 86(3):692–716. DOI:10.1111/j.1469-185X.2010.00166.x.
- Leshyk R, Nol E, Burke DM, Burness G. 2012. Logging affects fledgling sex ratios and baseline corticosterone in a forest songbird. Plos One 7(3):e33124. DOI:10.1371/journal.pone.0033124.
- Lima SL. 2009. Predators and the breeding bird: Behavioral and reproductive flexibility under the risk of predation. Biological Reviews of the Cambridge Philosophical Society 84(3):485–513. DOI:10.1111/j.1469-185X.2009.00085.x.
- Lowe A, Rogers AC, Durrant KL. 2014. Effect of human disturbance on long-term habitat use and breeding success of the European Nightjar, Caprimulgus europaeus. Avian Conservation and Ecology 9(2):6. DOI:10.5751/ACE-00690-090206.
- Magrath RD. 1991. Nestling weight and juvenile survival in the blackbird Turdus merula. Journal of Animal Ecology 60(1):335–351. DOI:10.2307/5464.
- Massaro M, Starling-Windhof A, Briskie JV, Martin TE. 2008. Introduced mammalian predators induce behavioural changes in parental care in an endemic New Zealand bird. Plos One 3(6):e2331. DOI:10.1371/journal.pone.0002331.
- Messina S, Edwards DP, Marasco V, Canoine V, Cosset CCP, Tomassi S, Benedick S, Eens M, Costantini D. 2020. Glucocorticoids link forest type to local abundance in tropical birds. Functional Ecology 34(9):1814–18250. DOI:10.1111/1365-2435.13586.
- Møller AP, Kwieciński Z, Tryjanowski P. 2017. Prey reduce risk-taking and abundance in the proximity of predators. Current Zoology 63(6):591–598. DOI:10.1093/cz/zow114.
- Mönkkönen M, Forsman JT, Kananoja T, Ylönen H. 2009. Indirect cues of nest predation risk and avian reproductive decisions. Biology Letters 5(2):176–178. DOI:10.1098/rsbl.2008.0631.
- Morosinotto C, Thomson R, Korpimäki E. 2010. Habitat selection as an antipredator behaviour in a multi-predator landscape: All enemies are not equal. Journal of Animal Ecology 79(2):327–333. DOI:10.1111/j.1365-2656.2009.01638.x.
- Morosinotto C, Thomson RL, Korpimäki E, Mateo R, Ruuskanen S. 2019. Maternal food supplementation and perceived predation risk modify egg composition and eggshell traits but not offspring condition. Journal of Experimental Biology 222(19):jeb201954. DOI:10.1242/jeb.201954.
- Murphy MT. 1985. Nestling eastern kingbird growth: Effects of initial size and ambient temperature. Ecology 66(1):162–170. DOI:10.2307/1941316.
- Mutzel A, Olsen AL, Mathot KJ, Araya-Ajoy YG, Nicolaus M, Wijmenga JJ, Wright J, Kempenaers B, Dingemanse NJ. 2019. Effects of manipulated levels of predation threat on parental provisioning and nestling begging. Behavioral Ecology 30(4):1123–1135. DOI:10.1093/beheco/arz060.
- Naef-Daenzer B, Widmer F, Nuber M. 2001. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. Journal of Animal Ecology 70(5):730–738. DOI:10.1046/j.0021-8790.2001.00533.x.
- Newton I. 1986. The sparrowhawk. London: Poyser Limited.
- Parejo D, Danchin E, Silva N, White JF, Dreiss AN, Avilés JM. 2008. Do great tits rely on inadvertent social information from blue tits? A habitat selection experiment. Behavioral Ecology and Sociobiology 62(10):1569–1579. DOI:10.1007/s00265-008-0586-4.
- Peacor SD, Barton BT, Kimbro DL, Sih A, Sheriff MJ. 2020. A framework and standardized terminology to facilitate the study of predation-risk effects. Ecology 101(12):e03152. DOI:10.1002/ecy.3152.
- Peluc SI, Sillett TS, Rotenberry JT, Ghalambor CK. 2008. Adaptive phenotypic plasticity in an island songbird exposed to a novel predation risk. Behavioral Ecology 19(4):830–835. DOI:10.1093/beheco/arn033.
- Peterson S, Colwell M. 2014. Experimental evidence that effigies reduce corvid occurrence. Northwestern Naturalist 95(2):103–112. DOI:10.1898/NWN13-18.1.
- Possenti CD, Bentz AB, Romano A, Parolini M, Caprioli M, Rubolini D, Navara K, Saino N. 2019. Predation risk affects egg mass but not egg steroid hormone concentrations in yellow-legged gulls. Current Zoology 65(4):401–408. DOI:10.1093/cz/zoy064.
- R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Availabe: https://www.r-project.org/. Accessed Mar 2022 15.
- Remacha C, Delgado JA, Bulaic M, Pérez-Tris J. 2016. Human disturbance during early life impairs nestling growth in birds inhabiting a nature recreation area. Plos One 11(11):e0166748. DOI:10.1371/journal.pone.0166748.
- Rhymer JM. 1988. The effect of egg size variability on thermoregulation of Mallard (Anas platyrhynchos) offspring and its implications for survival. Oecologia 75(1):20–24. DOI:10.1007/BF00378809.
- Santema P, Valcu M, Kempenaers B. 2020. Exposure to predator models during the fertile period leads to higher levels of extra‐pair paternity in blue tits. Journal of Animal Ecology 89(2):647–657. DOI:10.1111/1365-2656.13114.
- Scheuerlein A, Van’t Hof TJ, Gwinner E. 2001. Predators as stressors? Physiological and reproductive consequences of predation risk in tropical stonechats (Saxicola torquata axillaris). Proceedings of the Royal Society of London B 268(1476):1575–1582. DOI:10.1098/rspb.2001.1691.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9(7):671–675. DOI:10.1038/nmeth.2089.
- Seppänen JT, Forsman JT, Mönkkönen M, Thomson RL. 2007. Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88(7):1622–1633. DOI:10.1890/06-1757.1.
- Sheriff MJ, Krebs CJ, Boonstra R. 2009. The sensitive hare: Sublethal effects of predator stress on reproduction in snowshoe hares. Journal of Animal Ecology 78(6):1249–1258. DOI:10.1111/j.1365-2656.2009.01552.x.
- Sievert T, Haapakoski M, Palme R, Voipio H, Ylönen H. 2019. Secondhand horror: Effects of direct and indirect predator cues on behavior and reproduction of the bank vole. Ecosphere 10(6):e02765. DOI:10.1002/ecs2.2765.
- Smith H, Bruun M. 1998. The effect of egg size and habitat on starling nestling growth and survival. Oecologia 115(1–2):59–63. DOI:10.1007/s004420050491.
- Sofaer HR, Sillett TS, Peluc SI, Morrison SA, Ghalambor CK. 2013. Differential effects of food availability and nest predation risk on avian reproductive strategies. Behavioral Ecology 24(3):698–707. DOI:10.1093/beheco/ars212.
- Sudyka J, Di Lecce I, Wojas L, Rowiński P, Szulkin M. 2022. Nest-boxes alter the reproductive ecology of urban cavity-nesters in a species-dependent way. Journal of Avian Biology (In Press) 2022(11–12). DOI: 10.1111/jav.03051.
- Swift KN, Marzluff JM. 2015. Wild American crows gather around their dead to learn about danger. Animal Behaviour 109(11):187–197. DOI:10.1016/j.anbehav.2015.08.021.
- Swift KN, Marzluff JM. 2018. Occurrence and variability of tactile interactions between wild American crows and dead conspecifics. Philosophical Transactions of the Royal Society B 373(1754):20170259. DOI:10.1098/rstb.2017.0259.
- Szymkowiak J, Thomson RL, Kuczynski L. 2017. Interspecific social information use in habitat selection decisions among migrant songbirds. Behavioral Ecology 28(3):767–775. DOI:10.1093/beheco/arx029.
- Travers M, Clinchy M, Zanette L, Boonstra R, Williams TD. 2010. Indirect predator effects on clutch size and the cost of egg production. Ecology Letters 13(8):980–988. DOI:10.1111/j.1461-0248.2010.01488.x.
- Troscianko J. 2014. A simple tool for calculating egg shape, volume and surface area from digital images. Ibis 156(4):874–878. DOI:10.1111/ibi.12177.
- Tryjanowski P, Møller AP, Morelli F, Biaduń W, Brauze T, Ciach M, Czechowski P, Czyż S, Dulisz B, Goławski A, Hetmański T, Indykiewicz P, Mitrus C, Myczko Ł, Nowakowski JJ, Polakowski M, Takacs V, Wysocki D, Zduniak P. 2016. Urbanization affects neophilia and risk-taking at bird-feeders. Scientific Reports 6(1):28575. DOI:10.1038/srep28575.
- Williams TD. 1994. Intraspecific variation in egg size and egg composition in birds: Effects on offspring fitness. Biological Reviews 69(1):35–59. DOI:10.1111/j.1469-185X.1994.tb01485.x.
- Wood SN. 2017. Generalized Additive Models: An Introduction with R. 2nd ed. Boca Raton: Chapman and Hall/CRC.
- Zanette LY, White AF, Allen MC, Clinchy M. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334(6061):1398–1401. Available: https://www.jstor.org/stable/41352243.