Abstract
Palingenia Burmeister, 1839, is a small but intensively debated genus of mayflies due to the controversial taxonomy of the species belonging to it. Once widespread throughout Europe, Palingenia longicauda (Olivier, 1791), an iconic species of large pristine rivers, has lost most of its original range, but conservation efforts are greatly hampered by its morphology, which is similar to the sympatric P. fuliginosa (Georgi, 1802) and the peripatric P. sublongicauda Tshernova, 1949. Based on the revision of Soldán’s original collection, supplemented with fresh material from southeastern Europe, we analyzed 213 adult male specimens from 15 different locations from the presently known distribution area of Palingenia in Europe. Principal Component Analysis (PCA) based on the morphometry results of penis morphology and eye distances showed no significant differences among the analyzed populations. Further, the mtCOI barcode sequence data of 73 individuals from the known range of Palingenia species in southeastern Europe represented highly similar populations genetically, with no differentiation. These results show the presence of only one species in southeastern Europe, P. longicauda, thus disproving the distribution of P. fuliginosa in this region. The taxonomic status of P. sublongicauda remains doubtful, as no type material or fresh material from the locus typicus of the species was available for this study. A comprehensive revision of Palingenia, including additional species from Asia and the Indomalayan regions, is highly recommended to reevaluate the species composition of Palingenia and detect the most closely related taxa of the single P. longicauda in Europe.
Introduction
Biodiversity conservation highly benefits from species-level identification, because the species is the basic taxonomic unit with the highest biological information content (Kürzel et al. Citation2022). Palingenia Burmeister, 1839, is a small genus of the mayfly family Palingeniidae (Insecta, Ephemeroptera), yet these species are of important conservation value for the ecological integrity of large rivers. Members of the genus were less investigated in the past (except the once widely distributed P. longicauda), have controversial taxonomy or are facing extinction (some of them are possibly already extinct!) and showing important contractions of their original range (Bálint et al. Citation2012). Thus, a taxonomically well-supported strategy is recommended for the sustainable management of the remaining populations (Bálint et al. Citation2012; Dénes et al. Citation2022).
The genus contains among the largest extant mayflies in the world, ranging in body size between 25 and 40 mm and with forewings of length up to 30 mm. Taxonomically, they are clearly delimited from other genera within Palingeniidae (Demoulin Citation1965), and their major morphological characteristics were recently summarized by Bauernfeind and Soldán (Citation2012), as follows: adults light colored with dark markings on abdominal terga; mesonotal suture extremely stretched posteriorly; wings grey or brownish grey. Bifurcation of vein MA in the distal half of the wing; fore legs with 2 blunt claws in males, but with only a single one in female subimagines; forceps with 6–7 segments, the last segments reduced or vestigial; penis deeply cleft with widely separated tubular lobes, titillators absent; paracercus vestigial, but present in both sexes; larvae of the burrowing type, clypeus with 4–5 pointed processes, wider at the base than the apex, genae with 2–3 pointed processesl mandibles strong, protruding in dorsal view; mandibular tusks with long, conspicuous stout spines; tarsal claws without teeth, hooked in the fore legs, claws on the middle and hind legs slightly bent, as long as 1/2 of the tarsus; Gill I vestigial, gills II–VI consisting of an anterior and posterior branch with marginal fringes, the posterior part shorter and more rounded at the apex; branches of gills VII approximately equal in length and pointed apically; caudal filaments well developed, paracercus as long as about 1/3 of the cerci.
Only four species have Palearctic distribution (Demoulin Citation1965; Soldán & Landa Citation1986; Bauernfeind & Soldán Citation2012): P. anatolica Jacob, Citation1977, P. fuliginosa (Georgi, Citation1802), P. longicauda (Olivier, 1791) and P. sublongicauda Tshernova, Citation1949. Data on the Afrotropic P. apatris (Demoulin, Citation1965), and the Indomalayan P. orientalis Chopra, Citation1927, are considered doubtful or insufficiently known (Bauernfeind & Soldán Citation2012), as are the data on the locally distributed P. anatolica Jacob, Citation1977, which was described from the Asian part of Turkey by Jacob (Citation1977) based on a single male individual. No further data on the latter species nor fresh material were available to us during the present study.
Three species have been recorded within the geographical boundaries of Europe thus far. The type species of the genus, P. longicauda (Olivier, 1791), is probably the best known mayfly because of its spectacular mating flight and short adult lifespan (Russev Citation1987; Soldán Citation1997; Haybach Citation2007). A large number of papers contributed with detailed morphological descriptions of all life stages and discussed the Palearctic distribution of the species (Hagen Citation1859; Demoulin Citation1965; Jacob Citation1977; Soldán Citation1978, Citation1997; Russev Citation1987; Sartori et al. Citation1995; Kluge Citation1997; Andrikovics & Turcsányi Citation2001; Haybach Citation2007; Bálint et al. Citation2012). The most important characteristics of adult males, crucial for species identity, were suggested by Bauernfeind and Soldán (Citation2012) as follows: adults have top of the head pitch brown, distance between compound eyes approximately one-third of the eye width, no spots at the wing tips, legs yellowish, abdominal terga I–III yellowish brown, with diffuse triangular brownish spots in the middle, terga IV–VII light brown, darker in the middle, with a pair of diffuse oval light spots near the anterior margins, terga VIII–X with a pair of light divergent strips, penis lobes not expanded at the apex and diverging only slightly. Present distribution of the species in Europe is under revision (Vaida pers. com). Once distributed along all major rivers in Europe, from the Rhine to the Dniester, but also the Elbe, Vistula and Vardar basins, the species was considered extinct by the second half of the 20th century in most of its historic range but persisted in small enclaves in the Tisza (Tisa), Maros (Mureș), Raba, Prut and Danube rivers and the Danube Delta (Bálint et al. Citation2012; Munjiu Citation2017; Pavel Citation2019; Dénes et al. Citation2022). The type locality of the species was “bord de la Meuse”, Rotterdam, the Netherlands, where the species is now extinct, type material not known or probably lost (Bauernfeind & Soldán Citation2012).
The second European species of the genus, P. fuliginosa (Georgi, Citation1802), was named and described by Boeber and noticed by Georgi in 1802 from not only the Caucasus (see references in Bauernfeind & Soldán Citation2012), but also from Azerbaijan, Russia and Iran (Kasymov & Agaev Citation1986). The species has a controversial taxonomical history, as it was considered a junior synonym of P. longicauda for a long time but was redescribed by Tshernova (Citation1949) from the southern part of European Russia. It was also surprisingly mentioned by Landa (Citation1969) and Landa and Soldán (Citation1985) in sympatry with P. longicauda from the lower Latorica and upper Bodrog Rivers (Tisza River basin) in Slovakia. Later, Godunko & Kłonowska-Olejnik (Citation2003) also recorded some individuals from the Latorica (Latorca) River in Ukraine. The most important distinctive characteristics of P. fuliginosa were noticed in male morphological structures by Tshernova (Citation1949) and Soldán (Citation1978) and are as follows: top of the head greyish yellow, compound eyes almost touching, light spots present at the wing tips, legs yellowish at the femur, tibia and tarsi brownish, abdominal terga I–II with black dusting and a pair of light rounded spots in the middle, terga III–VII black with a pair of light v-shaped spots, terga VIII–X with light short diverged strips or spots, penis lobes slightly expanded first and then converging at the apex. Type material is not specified, most probably lost (Bauernfeind & Soldán Citation2012). The high variability of the morphological characteristics noticed in the Slovakian and Ukrainian populations of P. fuliginosa was also observed within the same populations (Soldán Citation1978; Martynov Citation2018).
Soldán (Citation1978) published a general revision of the European species of Palingenia, and it contained the most comprehensive identification keys to discriminate between the three European Palingenia species, based mainly on the Slovak populations (except for P. sublongicauda, which were most probably loaned from Tshernova, from southern Russia). This was subsequently adopted in all latter publications (Landa & Soldán Citation1985, Bauernfeind & Humpesch Citation2001; Derka Citation2003; Bauernfeind & Soldán Citation2012), thus creating great confusion for decades and maintaining the ambiguity of the taxon status of P. fuliginosa in Europe (Derka pers. com., Manko et al. in prep., Martynov Citation2018). Despite accepting the morphological variability of individuals, Soldán (Citation1978) argued for the presence of P. fuliginosa in Europe. The most important argument was the significantly different ecological preferences of the species, with nymphs preferring smaller rivers with unpolluted rapidly flowing water and high oxygen supply, thus being more sensitive to pollution than the lowland riverine P. longicauda.
The third European species, P. sublongicauda Tshernova, Citation1949, was described from the southern part of Russia (Tshernova Citation1949) and keyed by Soldán (Citation1978), with the following distinctive morphological characteristics: top of the head dark brown with occiput lighter, distance between compound eyes as long as one half of the eye width, no light spots at the wing tips, legs whitish yellow, femur the same color with tibia, abdominal terga I–II yellowish, without markings, terga III–X with diffuse patches in the middle or without markings, penis lobes widely separated and diverged with curved inner margin. Syntypes are presently deposited in the Zoological Institute of Russian Academy of Sciences, in SaintPetersburg, Russia (http://www.insecta.bio.spbu.ru/z/typi/typussublongicauda(Palingenia).htm).
The objective of the present paper was to reexamine the original collection of Soldán () in order to clarify the presence of these three Palingenia species in southeastern Europe. We used quantitative morphology and mitochondrial DNA sequences to test taxonomy hypotheses of the species based on integrative data and review the species distribution based on newly detected populations from southeastern Europe.
Materials and methods
Sampling and data collection
The type material of the three species (P. fuliginosa, P. longicauda and P. sublongicauda) recorded in Europe was not available during our investigations. However, the presence of P. fuliginosa in the studied area was reported by Soldán and Landa; therefore, we re-examined their reference collection of European Palingenia from the 1970s, deposited in the Institute of Entomology, Biology Centre CAS, České Budějovice, Czech Republic (). The collection contains hundreds of individuals of Palingenia labelled as P. longicauda and P. fuliginosa. Unfortunately, the collection does not contain individuals marked as P. sublongicauda, despite the fact that Soldán seems to have worked with individuals identified as P. sublongicauda in his revision (referring to 6 larvae, 3 males, 1 female and 1 subimago from the Volga River, from 1935, without any collection data) (Soldán Citation1978).
In our morphological revision, 174 specimens of male Palingenia were analyzed from 11 different sampling locations, representing 7 rivers (, ). The analyzed individuals from Soldán’s collection represented populations from the Latorica (Slovakia), Tisza (Slovakia) and the Danube (Bulgaria). The fresh Palingenia material was collected from the Danube (Hungary and Romania), Latorica (Slovakia), Tisza (HungarianSlovak border), Maros (Romania), Prut (Romania), Horyn (Ukraine) and Dniester (Ukraine). Molecular analysis included larvae material from five additional rivers: Raba River (Hungary), Tisza (Hungary), Bodrog (Hungary), Maros (Hungary) and Styr (Ukraine) (, ). All fresh material was conserved in 99% ethanol. The material of Landa and Soldán was conserved in denatured 70% ethanol.
Figure 2. Map showing the distribution of the analyzed sampling locations. The photo in the upper right corner shows a typical P. longicauda habitat with steep clay banks (Maros River, Nădlac, Arad county, photo: Vaida R.).
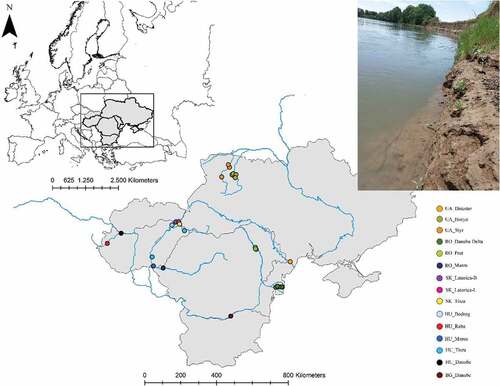
Table I. Palingenia material included in the present study. Individuals from the same river are considered as one population except for the Danube, where the middle Danube is treated as one population and the lower Danube (including the Danube Delta) is considered another one. NA – missing data due to reasons provided in the text.
Morphometry and statistical analyses
Linear morphometry was applied to quantify the possible morphological variability among the populations. In particular, the ratios of the characteristics were calculated as also used in Soldán’s (Citation1978) revision and are invariant for a particular measure of size (Mosimann Citation1970). Moreover, ratios are common in species descriptions, diagnoses and identification keys, and their use has a very long tradition in morphometric taxonomy (e.g. Winston Citation1999; Lestrel Citation2000).
Females were excluded from this study, as the majority of characteristics analyzed are located on the male genitalia. Larvae were also neglected in our quantitative morphology analyses, because the morphological criteria proposed by Soldán (Citation1978) to separate the two closely related species P. fuliginosa and P. longicauda were less obvious or presented high variability even in the same population and were not properly quantifiable.
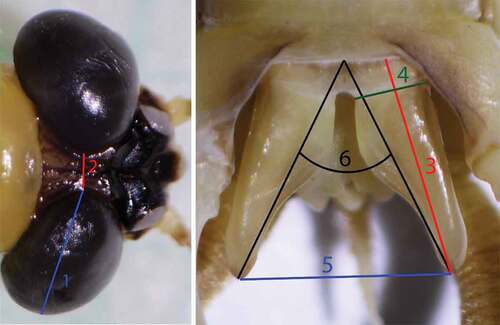
The morphometric characteristics analyzed in this study included the ratio between size and distance of the compound eyes, the ratio between the penis lobe length and the distance between penis lobe tips, the ratio between the penis lobe length and the base width, as well as the penis lobe angle were calculated to verify the differences described by Soldán (Citation1978). Measurements were made using photographs taken with a stereomicroscope equipped with a digital camera. Subsequent image analyses (measurements) of the obtained photographs were performed in ImageJ (ver. 1.53k, Schneider et al. Citation2012), as shown in , and then the given ratios were calculated. Some characteristics were not measured in each individual due to deformations caused by genital malformations and changes (mainly compound eye collapses) in older or incorrectly stored material.
Figure 3. Measured characteristics on male specimens of Palingenia (1 – size of compound eye, 2 – distance between compound eyes, 3 – penis lobe length, 4 – penis base width, 5 – distance between penis lobe tips, 6 – penis lobe angle).
Basic summary (univariate) statistical analyses of morphological measurements (median, mean, standard deviation, minimum, maximum) and Principal Components Analysis (PCA) were performed in the Past software (ver. 4.09; Hammer et al. Citation2001). In PCA, the between-groups eigenanalysis was used based on the group means, with the matrix normalizing all variables using division by their standard deviations, as different units were used for angles and for length (distance) measurements. Differences between sample medians of different populations of all measured morphological characteristics were analyzed using the Kruskal–Wallis test, commonly used as a test of equality of medians.
Molecular methods and data analyses
Tissue samples from 27 individuals were prepared and delivered according to the prescribed standards of the Canadian Centre for DNA Barcoding (CCDB, Biodiversity Institute of Ontario, University of Guelph), where DNA barcodes were obtained using the standard high-throughput protocol described in deWaard et al. (Citation2008). Specimen collection data, photographs, sequences, PCR, sequencing primers and trace files are available through the Barcode of Life Data Systems (BOLD; Sujeevan & Hebert Citation2007) under the project name Macrozoobenthos from Romanian freshwaters [ROMAC] (Supplementary Table 1). Three other sequences were also generated at the CCDB through the Barcoding Diptera from the Romanian freshwaters project [RODI] (Supplementary Table 1). Forty-three additional individuals were processed at the Interdisciplinary Research Institute on Bio–Nano–Sciences of Babeș–Bolyai University. Genomic DNA was extracted using a commercial kit (ISOLATE II Genomic DNA Kit, Bioline), and the mtCOI sequences were amplified using the standard LCO1490 and HCO2198 primer pair (Folmer et al. Citation1994) in a 50 µl volume at 42°C. Sequencing was performed by Macrogen Inc. (Europe). Sequences were verified at the NCBI website using a Basic Local Alignment Search Tool (BLAST) (Johnson et al. Citation2008) and deposited in the BOLD System under the project name EUPAL (accession numbers EUPAL001-23 – EUPAL043-23) (Supplementary Table 1).
The number of haplotypes and polymorphic sites (S), the haplotype (Hd) and nucleotide diversity (π) were calculated in DnaSp 6 (Rozas et al. Citation2017). A haplotype network was built by implementing the Median-Joining (MJ) algorithm in PopArt 1.7 (Leigh & Bryant Citation2015). Individuals were colored on the network based on the rivers they were collected from, in order to visualize the geographic distribution of the haplotypes. The p-distance between haplotypes and between populations of different rivers was calculated in Mega X (Kumar et al. Citation2018).
Results
Morphometry results
The results of the morphological analyses point to a relatively large variability between populations, with significant differences between sample medians of different populations in all analyzed morphological characteristics (). However, the differences were also large within the populations, often showing double or triple ratio values. This was observed, for example, in the ratio between length and distance of the penis lobes between several populations (Danube – Bulgaria, Danube delta – Romania, Dniester, Horyn – Ukraine, Latorica, Tisza – Slovakia, Maros – Romania), in the angle between the penis lobes (Danube Delta – Romania, Dniester, Horyn – Ukraine, Latorica, Tisza – Slovakia, Maros – Romania), or the ratio between distance and width of compound eyes (Horyn – Ukraine) ().
Table II. Results of the Kruskal–Wallis test of equality of medians.
Table III. Results of the univariate statistics of particular morphological characteristics of individuals collected in different sampling locations/different populations.
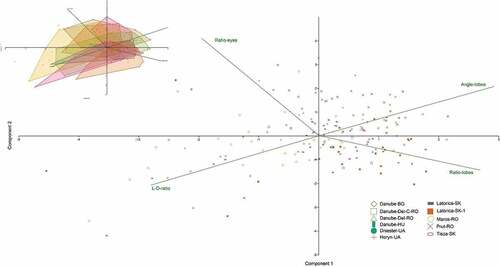
Principal component analysis used to explore the structure of the variation based on the sets of characteristics did not show any separation of the populations or species ().
Figure 4. Principal component analysis (PCA) biplot for morphometric characteristics (the percentage of total variance associated with PC1: 68.82%; PC2: 22.00%). Different colors and symbols represent different sampling locations. In the upper left corner, an identical graph is shown with the marking of polygons corresponding to different populations to show the overlap.
Molecular results
The 73 sequences showed 15 polymorphic sites leading to 15 haplotypes, with a haplotype diversity of Hd = 0.576 and a nucleotide diversity of π = 0.00252. Two haplotypes were common, corresponding to 46 and 13 individuals, and one haplotype was shared by two specimens collected at the same location. The other 12 haplotypes were unique, represented by only one individual (). The p-distance between haplotypes ranged between 0.159 and 1.297. When sequences were grouped based on different rivers, the p- distance showed values between 0.22 and 0.89 ().
Figure 5. Median-Joining haplotype network generated for the concatenated dataset using PopArt 1.7. Each circle represents a unique haplotype and circle size is proportional to the number of samples observed for that haplotype. The number of mutations is represented by hatch marks on the lines. Colors correspond to different rivers.
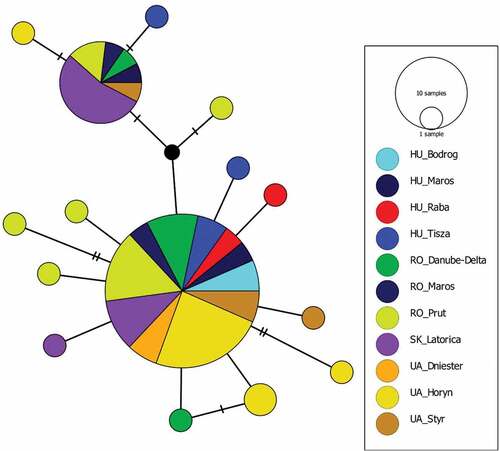
Table IV. P-distance (%) between populations of different rivers.
Discussion
Based on our morphometry results of penis morphology and eye distances of males, no significant differences support the clear separation of the analyzed populations and evidence of more than one Palingenia species (P. longicauda) in Europe.
Redescription of the European species belonging to the genus Palingenia (Soldán, Citation1978) was most probably based on the incorrect assumption of the occurrence of P. fuliginosa in Slovakia. Features listed by the author as distinctive in species identification were reanalyzed and proven to be based on the intraspecific variability observed in this study (.). Moreover, it is also important to note that some features are greatly influenced by the time at which individuals were fixed in ethanol after the subimagines had molted. This applies particularly, for example, to the angle between the lobes of the penis. In the case of subimagines, the angle between the lobes is very acute, and the penis lobes are essentially parallel. After molting, the angle increases until it stabilizes. If individuals are collected and fixed in the pre-stabilization period, the angle varies considerably. Thus, we consider the morphological differences mentioned by Soldán (Citation1978) between the males of the two species, P. longicauda and P. fuliginosa, highly doubtful and extremely variable, even within the same population, depending mostly on molting status of the examined individuals.
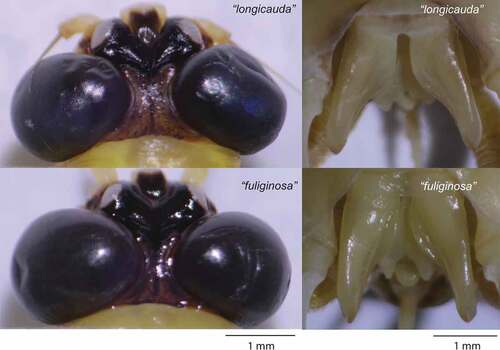
Figure 6. Differences in male genitalia structures and dimensions of compound eyes in individuals with highly similar mtCOI structures and sampled at the same time from a single population (Latorica, Slovakia). The specimens depicted here are morphologically close to the description and characteristics shown in Soldán’s revision as P. longicauda and P. fuliginosa (designated “longicauda” and “fuliginosa”, respectively).
Mitochondrial DNA analysis showed similar patterns to those observed in the previous molecular genetic studies of P. longicauda (Bálint et al. Citation2012; Dénes et al. Citation2022), with a low number of haplotypes present in the whole distribution area and several other private haplotypes present in different rivers. The MJ haplotype network did not show any evident or well-differentiated structures to indicate that the sequences could represent more than one taxonomic unit. The low p-distances, both when the haplotypes were compared (0.159‒1.297) and when the different river populations were compared (0.22 ‒ 0.89), are consistent with previously published intraspecific distance values for the Palingeniidae species (Webb et al. Citation2012: 1.6% maximum intraspecific and 12.7% minimum interspecific distances). These results support the micromorphology data, thus confirming the presence of a single Palingenia species, P. longicauda, in the south-eastern part of Europe.
According to Soldán (Citation1978), and consequently adopted by an important number of authors following (Andrikovics & Turcsányi Citation2001; Martynov Citation2018), the major argument for the presence of P. fuliginosa in Europe is the species’ stickling for different ecological demands compared to P. longicauda. Their nymphs were frequently collected in smaller rivers with unpolluted and rapidly flowing waters and high oxygen supply, while P. longicauda is present only at lower sectors of large rivers with a lower oxygen content. However, there were also some contributions that noticed the presence of the two morphotypes of Palingenia in the same river sector (ex. Bodrog in Hungary; Málnás et al. Citation2016) or even different species in the same sample (Bodrog in Slovakia) (Mišíková Elexová et al. Citation2015), which makes the above-mentioned argument questionable, and the presence of P. fuliginosa in the hydrographic basin of the middle sector of the Tisza River is highly doubtful. In contrast, these findings refute Soldán’s argument and indirectly support the presence of only a single species, P. longicauda, in the south-eastern part of Europe.
The taxonomic status of P. fuliginosa remains challenging; however, as the type material is missing, or its location is unknown, our repeated efforts to obtain fresh material from outside of Europe (from Iran, Armenia, Azerbaijan, for example) have failed.
The situation of the third European species, P. sublongicauda, is similar, as no adult male material was available to us prior to morphological investigation and no type material was available during the present study. Although the taxonomic status of these species is questionable, they cannot be challenged until a more comprehensive taxonomic revision of Palingenia, including additional species from Asia and the Indomalayan and Afrotropical regions, can be conducted.
The major contribution of the present paper is the first integrative analysis of morphology and molecular data of the Palingenia species recorded from south-eastern Europe. Beside our strong argument on the presence of only one, instead of three Palingenia species in Europe, further comprehensive sampling efforts are highly recommended, including not only an analysis of all species from the Palearctic area but also a revision on Indomalayan representatives, thus a well-supported taxonomic revision of the genus.
Our study also has important conservation implications, as all Palingenia species have high bio-indication value of the ecological integrity of medium-sized to large pristine rivers and are critically endangered in large part of the known distribution area (Russev Citation1987; Soldán et al. Citation2009; Bauernfeind & Soldán Citation2012). Thus, a taxonomically well-supported conservation effort is highly recommended for a species-focused sustainable management of these critically endangered taxa in the whole Palearctic area.
Supplemental Material
Download MS Word (22.7 KB)Acknowledgements
We thank Málnás Kristóf (Hungary) for providing individuals from the Hungarian part of the Danube and Pavel Sroka (University of South Bohemia, České Budějovice, The Czech Republic) for the opportunity to study and borrow material from thecollections of Soldán and Landa.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2191622
Additional information
Funding
References
- Andrikovics S, Turcsányi I. 2001. Tiszavirág [Palingenia longicauda]. Booklet of Tisza Club 10. Debrecen: Piremon Nyomda.
- Bálint M, Málnás K, Nowak C, Geismar J, Váncsa É, Polyák L, Lengyel S, Haase P. 2012. Species history masks the effects of human-induced range loss – unexpected genetic diversity in the endangered giant mayfly Palingenia longicauda. PLoS One 7(3):e31872–e31880. DOI: 10.1371/journal.pone.0031872.
- Bauernfeind E, Humpesch UH. 2001. Mayflies of central Europe – Identification and ecology (Insecta – Ephemeroptera). Wien: Verlag des Naturhistorischen Museums Wien.
- Bauernfeind E, Soldán T. 2012. The mayflies of Europe (Ephemeroptera). Denmark: Apollo Books, Ollerup.
- Chopra B. 1927. The Indian ephemeroptera (mayflies). Part I. The suborder Ephemeroidea: Families palingeniidae and polymitarcidae. Records of the Indian Museum 29(2):91–138.
- Demoulin G. 1965. Contribution а l’étude des Palingeniidae (Insecta, Ephemeroptera). Nova Guinea (Zoology) 33:305–344.
- Dénes AL, Vaida RM, Szabó E, Martynov AV, Váncsa É, Ujvarosi B, Keresztes L. 2022. Cryptic survival and an unexpected recovery of the long-tailed mayfly Palingenia longicauda (Olivier, 1791) (Ephemeroptera: Palingeniidae) in Southeastern Europe. Journal of Insect Conservation 26(5):823–838. DOI:10.1007/s10841-022-00425-z.
- Derka T. 2003. Súpis druhov vodných bezstavovcov (makroevertebrát) Slovenska Ephemeroptera [Checklist of Slovak aquatic macroinvertebrates – Ephemeroptera]. In: Šporka F, editor. Vodné bezstavovce (makroevertebráta) Slovenska, súpis druhov a autekologické charakteristiky. Slovak aquatic macroinvertebrates, checklist and catalogue of autecological notes. Bratislava: Slovenský hydrometeorologický ústav. pp. 33–36.
- deWaard JR, Ivanova NV, Hajibabaei M, Hebert PDN. 2008. Assembling DNA barcodes: Analytical protocols. In: Martin CC, editor. Methods in molecular biology: environmental genomics. Totowa, USA: Humana Press Inc. pp. 275–293.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5):294–299.
- Georgi JG. 1802. Nachträge für dessen Geographisch-physikalische und Naturhistorische Beschreibung des Russischen Reichs. Königsberg: Nicolovius.
- Godunko RJ, Klonowska-Olejnik M. 2003. A checklist of the Ukrainian mayflies (Ephemeroptera). Polskie Pismo Entomologiczne 72:203–210.
- Hagen H. 1859. Ueber das Vorkommen von Palingenia longicauda und Acanthaclisis occitanica in Preussen. Entomologische Zeitung 20:431–432.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9.
- Haybach A. 2007. Hinweise auf ein historisches Vorkommen von Palingenia longicauda (Olivier, 1791) in Bayern (Insecta: Ephemeroptera). Evidence for a historical occurrence of Palingenia longicauda (Olivier, 1791) in Bavaria (Southern Germany) (Insecta: Ephemeroptera). Lauterbornia 59:77–83.
- Jacob U. 1977. Palingenia anatolica n. sp. (Ephemeroptera, Palingeniidae) aus der Turkey. Entomologische Nachrichten 21(12):177–182.
- Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. 2008. NCBI BLAST: A better web interface. Nucleic Acids Research 3(Web Server):5–9. DOI:10.1093/nar/gkn201.
- Kasymov FG, Agaev NB. 1986. On the biology of ephemeron Palingenia fuliginosa (Ephemeroptera, Palingeniidae) from the Kura River. Doklady Akademii Nauk Azerbaidzhanskoi SSR 48(8):65–67.
- Kluge NJ. 1997. Order mayflies – Ephemeroptera. In: Tsalolikhin SJ, editor. Key to freshwater invertebrates of Russia and adjacent lands. Vol. 3. St. Petersburg: Zoological Institute RAS. pp. 176–220.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35(6):1547–1549. DOI:10.1093/molbev/msy096.
- Kürzel K, Kaiser S, Lörz AN, Rossel S, Paulus E, Peters J, Schwentner M, Arbizu PM, Coleman CO, Svavarsson J, Brix S. 2022. Correct species identification and its implications for conservation using haploniscidae (crustacea, isopoda) in icelandic waters as a proxy. Frontiers in Marine Science 8:795196. DOI: 10.3389/fmars.2021.795196.
- Landa V. 1969. Jepice-Ephemeroptera. Fauna CSSR 18:1–352.
- Landa V, Soldán T. 1985. Distributional patterns, chorology and origin of the Czechoslovak fauna of mayflies (Ephemeroptera). Acta Entomologica Bohemoslovaca 82:241–268.
- Leigh JW, Bryant D. 2015. Popart: Full-feature software for haplotype network construction. Methods in Ecology and Evolution 6(9):1110–1116. DOI:10.1111/2041-210X.12410.
- Lestrel PE. 2000. Morphometrics for the life sciences. Vol. 7. Singapore: World Scientific Publishing Company.
- Málnás K, Polyák L, Prill É, Hegedüs R, Kriska G, Dévai G, Horváth G, Lengyel S. 2016. Reappearance of Palingenia longicauda (Olivier, 1791) (Ephemeroptera, Palingeniidae) on the Hungarian Danube section – Range recovery of the species at the Rába-district. Folia Historico-Naturalia Musei Matraensis 40:21–25.
- Martynov AV. 2018. New records of some rare mayflies (Insecta, Ephemeroptera) from Ukraine. Ecologica Montenegrina 16:48–57. DOI: 10.37828/em.2018.16.6.
- Mišíková Elexová E, Ščerbáková S, Lešťáková M, Kováč V, Plachá M, Bitušík P, Očadlík M, Brúderová T, Vráblová Z, Haviar M. 2015. Výsledky monitorovania vodných útvarov povrchových vôd Slovenska, Zoznam taxónov, Vodná fauna, Bentické bezstavovce, mihule a ryby. Bratislava: Výskumný ústav vodného hospodárstva.
- Mosimann JE. 1970. Size allometry: Size and shape variables with characterizations of the lognormal and generalized gamma distributions. Journal of the American Statistical Association 65(330):930–945. DOI:10.1080/01621459.1970.10481136.
- Munjiu O. 2017. Distribution of endangered mayfly Palingenia longicauda (Olivier, 1791) (Ephemeroptera, Palingeniidae) on the territory of the Republic of Moldova. Lauterbornia 84:39–51.
- Pavel AB, Menabit S, Skolka M, Lupascu N, Pop IC, Opreanu G, Stanescu I, Scrieciu A. 2019. New data regarding the presence of two insect larvae species – Gomphus (Stylurus) flavipes (Odonata) and Palingenia longicauda (Ephemeroptera) – In the lower sector of the Danube River. Geo-Eco-Marina 25:253–264.
- Ratnasingham S, Hebert PDN. 2007. BOLD: The barcode of life data system. Molecular Ecology Notes 7(3):355–364. DOI:10.1111/j.1471-8286.2006.01678.x.
- Rozas J, Ferrer-Mata A, Sanchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sanchez-Gracia A. 2017. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution 34(12):3299–3302. DOI:10.1093/molbev/msx248.
- Russev B. 1987. Ecology, life history and distribution of Palingenia longicauda (Olivier) (Ephemeroptera). Tijdschrift voor Entomologie 130:109–127.
- Sartori M, Landolt P, Lubini V Ruffieux L. 1995. Biological studies of Palingenia longicauda (Olivier) (Ephemeroptera: Palingeniidae) in one of its last European refuges-Abiotic characteristics and description of the habitat. In: Corkum L H CJ, editors. Current Directions in Research on Ephemeroptera (Proc. 7th Int. Conf. Orono: Ephemeroptera. pp. 263–272.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9(7):671–675. DOI:10.1038/nmeth.2089.
- Soldán T. 1978. Revision of the genus Palingenia in Europe (Ephemeroptera, Palingeniidae. Acta entomologica bohemoslovaca 75:272–284.
- Soldán T. 1997. Mayflies (Ephemeroptera): One of the earliest insect groups known to man. In: Landolt P, Sartori M, editors. Ephemeroptera & Plecoptera. Biology-Ecology-Systematics. Fribourg: Mauron - Tinguely & Lachat, SA. pp. 511–513.
- Soldán T, Godunko RJ, Zahrádková S, Sroka P. 2009. Palingenia longicauda (Olivier, 1791) (Ephemeroptera, Palingeniidae): Do refugia in the Danube basin still work? Communications and Abstracts, SIEEC 21:81–84.
- Soldán T, Landa V. 1986. Life cycle of Palingenia fuliginosa (Ephemeroptera, Palingeniidae) in Czechoslovakia. In: Velthius HHW, editor. Amsterdam: Proc. 3rd European Congr. Entomol. pp. 143–146.
- Tshernova O. 1949. Kpoznaniyu roda Palingenia Burm. (Ephemeroptera, Palingeniidae). Entomologicheckoe Obozrenie 39(3–4):303–307.
- Webb JM, Jacobus LM, Funk DH, Zhou X, Kondratieff B, Geraci CJ, DeWalt RE, Baird DJ, Richard B, Phillips I Hebert PDN 2012. A DNA Barcode Library for North American Ephemeroptera: Progress and Prospects. Plos One 7(5):e38063. DOI:10.1371/journal.pone.0038063.
- Winston JE. 1999. Describing species: Practical taxonomic procedure for biologists. New York: Columbia University Press.

