 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Tetramorium Mayr, 1855 is a worldwide distributed and hyperdiverse ant genus consisting of almost 600 taxa manifesting various life history strategies. Species of the Tetramorium inquilinum species-group represents one of the most extreme forms of parasitism and consist of degenerate workerless social parasites of several Tetramorium species. So far, its members have been recorded in the Palearctic from montane to alpine zones and reveal an interesting disjunction in host selection observed between eastern-Mediterranean and western-Mediterranean species. We describe a fifth member of the Tetramorium inquilinum species-group: Tetramorium albenae Salata, van Delft & Borowiec sp. n. The species morphologically differs from the remaining members of the group by the combination of the following characters: dense and erect pilosity of appendages and the whole body, smooth head sculpture, predominantly smooth anepisternum and katepisternum, lack of blunt teeth on propodeum, and presence of distinct carianae on the dorsolateral margins of the propodeum. Tetramorium albenae Salata, van Delft & Borowiec sp. n. was collected in a lowland olive grove, a site so far not associated with the Tetramorium inquilinum species-group, from a nest of Tetramorium kephalosi (a new host species for the group). The data presented in this paper provide new insights into the habitat preferences of the species of the Tetramorium inquilinum species-group and extend our knowledge on the potential host species of these parasites.
http://zoobank.org/urn:lsid:zoobank.org:pub:62610BFE-7BF4-44E7-ADB5-3DD6A9B759C0
Introduction
Tetramorium Mayr, 1855 is a worldwide distributed and hyperdiverse ant genus consisting of 586 species and 9 subspecies (Bolton Citation2022). In ant taxonomy, an informal category between genus and species, “species group”, has been commonly used, especially within diverse genera, to organize species by region and/or their morphological similarities (e.g., Bolton Citation1980, Citation1987; Wilson Citation2003; LaPolla Citation2004; Radchenko & Elmes Citation2010; Hita Garcia & Fisher Citation2011; Salata & Fisher Citation2020; Schifani et al. Citation2022). This approach has been followed by the most informative taxonomic treatments of Tetramorium and facilitated revisionary work on this genus. Thanks to the comprehensive work of generations of taxonomists (e.g., Bolton Citation1976, Citation1977, Citation1980; Hita Garcia & Fisher Citation2011; Radchenko & Scupola Citation2015; Agavekar et al. Citation2017; Salata & Borowiec Citation2017; Wagner et al. Citation2017) the taxonomy of Tetramorium is currently in a relatively good condition.
Species of Tetramorium can be found in many habitats, from humid rainforests to arid deserts, and in different strata from the ground and leaf litter layer to the high forest canopy (Bolton Citation1980). Tetramorium also contains species manifesting various life history strategies with the majority being free-living taxa. However, a small group of its members is classified as social parasites, i.e. species exploiting the resources of colonies of other species (Forel Citation1874). Temporary social parasitism was reported for Tetramorium very recently (Wagner et al. Citation2021), while the most specialized form, i.e. inquilinism, has been documented within Tetramorium species since the 19th century. Inquilinism refers to permanent parasitism in which parasites do not enslave their host species and often do not produce a worker caste. In this relationship, “the host workers take over all husbandries and the parasite queen can invest all energy into the production of sexuals” (Buschinger Citation2009). The inquiline ant species occurring in the Palearctic region, due to their morphological distinctness, were originally recognized as separate genera: Anergates Forel Citation1874 (consisting of one species: Anergates atratulus (Schenck, Citation1852)) and Teleutomyrmex Kutter Citation1950 (consisting of four species: Teleutomyrmex schneideri; Citation1950, Teleutomyrmex kutteri; Tinaut, Citation1990, Teleutomyrmex buschingeri Lapeva-Gjonova, 2017 in Kiran et al. Citation2017, Teleutomyrmex seiferti Kiran & Karaman, 2017 in Kiran et al. Citation2017). However, based on the phylogenetic findings, both genera were recently synonymized under Tetramorium by Ward et al. (Citation2015).
The species formerly classified as Teleutomyrmex (henceforth referred to as the Tetramorium inquilinum species-group) are characterized by Bolton (Citation1976) by the following characters: females with blade-like mandibles that are edentate, except for the acute apical tooth; 10- or 11-segmented antennae with the second to fourth funicular segments showing a variable degree of fusion; shallowly transversely concave clypeus; present ocelli; compressed from side to side alitrunk with flight sclerites; absent metapleural glands; sessile petiole; postpetiole very broadly attached to first gastral segment; very strongly dorsoventrally flattened gaster with concave ventral surface; very reduced and non-functional sting. On the other hand, males have mandibles as females but much smaller; 10-segmented antennae with the second funicular antennomere being an elongate fusion-segment; ocelli and wings are present; gaster is downcurved and somewhat reflexed anteriorly; the remainder of the body appears pupoidal.
All four members of the Tetramorium inquilinum species-group are degenerate workerless social parasites of several Tetramorium species. Their females are found riding upon the queen of the host colony where they are efficiently tended and fed by the host workers. The males are pupoidal but possess wings (Bolton Citation1976; Kiran et al. Citation2017). Contrary to Tetramorium atratulum (Schenck Citation1852) (=Anergates atratulus (Schenck Citation1852) – a species native to almost the entire Palearctic), species of the Tetramorium inquilinum species-group have a rather narrow distribution range (Kiran et al. Citation2017).
Tetramorium inquilinum Ward et al., Citation2015 (=Teleutomyrmex schneideri Kutter Citation1950) was described from the Swiss Alps (Kutter Citation1950) and later recorded also from the French Alps, French Pyrenees, and the Cantabrian Mountains in Spain (Collingwood Citation1956; Buschinger Citation1985, Citation1987, Citation1999; Espadaler and Cuesta, Citation2006; Wegnez et al. Citation2015). Its record from Turkmenistan (Dlussky et al. Citation1990) was recently discussed and based on collected data refers to an undescribed species (Kiran et al. Citation2017). Thanks to the extensive work of Kutter (Citation1950), Brun (Citation1952), and Gösswald (Citation1953), its biology and anatomy are the most extensively studied within its species-group. Tetramorium kutteri (Tinaut, Citation1990) (=Teleutomyrmex kutteri Tinaut, Citation1990) is known only from Sierra Nevada and Sierra de Cazorla in Spain (Tinaut Citation1990; López and Martinez Citation2011). The two remaining species Tetramorium buschingeri (Lapeva-Gjonova, 2017 in Kiran et al. Citation2017) (=Teleutomyrmex buschingeri Lapeva-Gjonova, 2017 in Kiran et al. Citation2017) and Tetramorium seiferti (Kiran & Karaman, 2017 in Kiran et al. Citation2017) (=Teleutomyrmex seiferti Kiran & Karaman, 2017 in Kiran et al. Citation2017) are known from Eastern Rhodopes in Bulgaria and the eastern Black Sea range in Turkey respectively (Kiran et al. Citation2017). Both species have only been recorded once.
So far, all four described species have been recorded from montane to alpine zones (650 m to 2300 m in elevation). However, the lowest known locality recorded for this species-group belongs to the undescribed species collected in Farap in Turkmenistan (190 m a.s.l.) that was erroneously attributed by Dlussky et al. (Citation1990) to T. inquilinum (Kiran et al. Citation2017). Interestingly, there is also a disjunction in host selection observed between eastern-Mediterranean and western-Mediterranean species. Tetramorium kutteri and T. inquilinum parasitize on species of the Tetramorium caespitum species-group, while T. seiferti and T. buschingeri were collected from nests of the Tetramorium chefketi species-group (Kiran et al. Citation2017). Below, we describe a fifth member of the Tetramorium inquilinum species-group: Tetramorium albenae Salata, van Delft & Borowiec sp. n. The species was collected in a lowland olive grove, a site so far not associated with the Tetramorium inquilinum species-group, from a nest of Tetramorium kephalosi Salata & Borowiec, Citation2017 (a member of the Tetramorium semilaeve species-group).
Material and methods
Examined specimens are housed in the following collections:
BMNH – Natural History Museum, London, UK;
DBET – Department of Biodiversity and Evolutionary Biology, University of Wroclaw, Poland;
MHNG – Museum of Natural History, Geneva, Switzerland;
SMNG – Senckenberg Museum für Naturkunde (Staatliches Museum für Naturkunde), Görlitz, Germany;
UCDC – University of California, Davis.
Specimens were compared using standard methods of comparative morphology. All measurements were made in μm using a pin-holding stage, permitting rotations around X, Y, and Z axes. A Nikon SMZ18 stereomicroscope was used at a magnification of ×100 for each character. Photographs were taken using a Nikon SMZ 1500 stereomicroscope, Nikon D5200 camera, and Helicon Focus software. The activity of ants in captivity was documented with a Poco X3 Pro telephone camera with a clip-on Black Eye macro lens (×15). Images of type specimens are available online on AntWeb (www.AntWeb.org) and are accessible using the unique identifying specimen codes provided in the description sections.
Our recognition of species follows the biological species concept and species boundaries are based on comparative morphology and known geographic distributions of investigated taxa. Species described based on the single nest sample exhibit a distinct and unique set of morphological features allowing their separation from other Tetramorium species.
Pilosity inclination measurements follow Wilson (Citation1955): adpressed (0–5°) hairs run parallel, or nearly parallel to the body surface; decumbent hairs stand 10–15°; subdecumbent hair stands 30°; suberect hairs stand 35–45°; and erect hairs stand more than 45° from the body surface. The surface sculpturing glossary follows Harris (Citation1979).
Measurements (after Kiran et al. Citation2017, modified)
AOL – length of anterior ocellus; measured along the maximum diameter of the anterior ocellus;
AOW – width of ocellus; measured along the minimum diameter of the anterior ocellus;
CL – head length; measured in a straight line from the midpoint of the anterior clypeal margin to the mid-point of the posterior margin in full-face view (i.e., when both maximum head length in median line and maximum head width are positioned in visual plane);
ClyW – clypeal width; maximum width of clypeus measured in full-frontal view;
DLO – maximum distance between the inner margin of the posterior ocelli;
DFC – distance between frontal carinae at the level of the hind margin of scape joint capsule;
ECW – distance between the outer margins of the dorsal propodeal carinae at the point where the carinae begin to curve down along the caudal propodeal slope (this is the point where spines would be situated if there were any);
EL – maximum diameter of the eye over all structurally visible ommatidiae;
HFL – with the large diameter of the hind femur in the visual plane, maximum length of hind femur along its central axis;
HTL – with the large diameter of the hind tibia in visual plane, maximum length of hind tibia along its central axis;
MNW – maximum mesonotal width; measured as the maximum width of scutum in dorsal view;
PNW – pronotal width; measured as the maximum pronotum width in dorsal view;
PPW – postpetiole width; maximum width of the postpetiole in dorsal view;
PW – petiole width; maximum width of the petiole in dorsal view;
SL – scape length; maximum straight-line length of the scape excluding the articular condyle;
WL – mesosoma length; measured as diagonal length from the anterior end of the neck shield to the posterior margin of the propodeal lobe.
Indices
Study site
The Pelion (or Pilio) peninsula (Πήλιο in Greek, the etymology comes from the ancient Greek King Peleus (Πηλέας) of Fthia that ruled the area around the 12th c.BC.) expands northwest to southeast, close to central Greece in the administrative region of Thessaly. Its highest peak is at 1624 m (with an overall average altitude of 920 m) and it holds various habitats such as oak (Quercus), beech (Fagus), and pine (Pinus sylvestris) forests, while at lower altitudes, there are conformations of maquis, phrygana, and several crops (e.g. apples and olive orchards). It is a mountainous area with intense relief changes of high peaks and deep valleys of predominantly Mesozoic-Paleozoic rocks (Galanakis Citation1997). In particular, higher altitude regions undergo severe geological processes such as erosion from streams and heavy rain, landslides, karstification, and human activity. There are also several unexplored caves. A large part of the region is classified as a Natura2000 site (code GR1430008) with an area of 35,711 ha. Because of its lush vegetation, many beaches, a ski resort, and picturesque points, it is a popular tourist destination all year round.
Results
Synopsis of the Tetramorium inquilinum species-group
Tetramorium albenae Salata, van Delft & Borowiec n. sp.
Tetramorium buschingeri (Lapeva-Gjonova, 2017)
Teleutomyrmex buschingeri Lapeva-Gjonova, 2017: 151 (q.) in Kiran et al. Citation2017.
Tetramorium inquilinum; Ward et al., Citation2015
Replacement name for Tetramorium schneideri (Kutter, Citation1950) (junior secondary homonym of Tetramorium schneideri Emery, Citation1898)
Teleutomyrmex schneideri Kutter, Citation1950: 82 (q.m.)
Tetramorium kutteri (Tinaut, Citation1990)
Teleutomyrmex kutteri Tinaut, Citation1990: 202 (q.m.)
Tetramorium seiferti (Kiran & Karaman, 2017)
Teleutomyrmex seiferti Kiran & Karaman, 2017: 148 (q.m.) in Kiran et al. Citation2017.
Key to members of the Tetramorium inquilinum species-group (after Kiran et al. Citation2017, modified)
Carinae or teeth on dorsal surface of propodeum absent, dorsal profile of propodeum much shorter than the declivitous one. All lateral surfaces of mesosoma and petiole covered by a well-developed reticulate or alveolate microsculpture. Head length index CL/CW <0.945. Southern Balkans.T. buschingeri
–. Carinae or teeth on dorsal surface of propodeum present, dorsal profile of propodeum not much shorter than the declivitous one. Surfaces of lateral mesosoma and petiole only in patches covered by a reticulate or alveolate microsculpture or completely smooth. Head length index CL/CW >0.9452.
Dorsal surface of propodeum with indistinct carinae, teeth absent. Head surface smooth and shiny and katepisternum and anepisternum predominantly smooth and shiny. Greece.T. albenae
–. Dorsal surface of propodeum with blunt tooth. Head surface at least partially sculptured and/or katepisternum and anepisternum predominantly sculptured.3.
Scape long, SL/CS >1.00. Distance of frontal carinae clearly larger than petiolar width, DFC/PW >1.096. Size small, CW <464 µm. Scapes and tibiae with weaker, largely decumbent pilosity. Southern Iberia.T. kutteri
–. Scape shorter, SL/CS <1.00. Distance of frontal carinae not much larger than petiolar width, DFC/PW <1.096. Size larger, CW >464 µm. Scapes and tibiae with profuse erect or suberect pilosity.4
Ratio of distance between lateral ocelli and large diameter of complex eye larger: DLO/EL 0.93–1.11. Katepisternum with many long decumbent hairs, posterior corners of head posterior of the eyes smooth, absolute scape length larger: SL >457 µm. Anatolia.T. seiferti
–. Ratio of distance between lateral ocelli and large diameter of complex eye smaller: DLO/EL 0.70–0.80. Katepisternum without or only with a few decumbent hairs, posterior corners of head posterior of the eyes densely microreticulate, absolute scape length smaller: SL <457 µm. Alps, Pyrenees and Cantabrian Mountains.T. inquilinum
Species description
Tetramorium albenae Salata, van Delft & Borowiec n. sp.
Figures 1,2. Tetramorium albenae Salata, van Delft & Borowiec n. sp., holotype queen. Figure 1. Dorsal view. Figure 2. Lateral view.

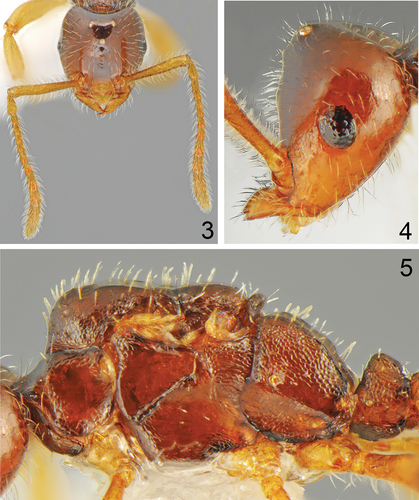
Figures 3-5. Tetramorium albenae Salata, van Delft & Borowiec n. sp., holotype queen. Figure 3. Head, full face view. Figure 4. Head, lateral view. Figure 5. Mesosoma, lateral view.
Figures 6-10. Ex situ pictures showing Tetramorium albenae riding the host gyne. Figure 6. Surrounded by host workers. Figure 7. Host workers collecting eggs from the gaster of the inquiline gyne (source of eggs uncertain). Mite near the hind leg of host gyne. Figures 8–10. Inquiline on host.

Figures 11,12. Tetramorium kephalosi Salata & Borowiec, gyne of host, the same specimen as in . Figure 11. Dorsal view. Figure 12. Lateral view.

Figure 13. Tetramorium albenae Salata, van Delft & Borowiec n. sp., habitat in locus typicus. Red arrow points the actual nest site.

Material examined
Type material.
Holotype
Dealate queen; Greece: Thessaly, Magnesia, 500 m NW of Stavrodromi (by Kala Nera-Milies road), 57 m, 39.30946/23.14404, 27 iv 2022, coll. J. & J. van Delft (DBET).
Other material.
Tetramorium albenae Salata, van Delft & Borowiec n. sp: 10 alate queens; the specimens hatched in the myrmicarium (DBET).
Tetramorium inquilinum Ward et al., Citation2015 (replacement name for Tetramorium schneideri (Kutter, Citation1950) junior secondary homonym of Tetramorium schneideri Emery, Citation1898): 2 syntype queens (CASENT0907569, CASENT0901024); Switzerland: Saas-Fee, 25.vii.1949. coll. H. Kutter (BMNH) [Syntype queens images examined, AntWeb, photos by Zach Lieberman and Ryan Perry, available on https://www.AntWeb.org]. One alate queen (CASENT0101582); France: Dautes-Alpes, Col de Granon, NW. Briançon, 44.95/6.61667, 2050 m, 26.vii.1994, coll. M. Sanetra (MHNG) [Queen images examined, AntWeb, photos by April Nobile, available on https://www.AntWeb.org].
Tetramorium kutteri (Tinaut, Citation1990): 1 paratype queen (ANTWEB1038039); Spain: Sierra Nevada, “Prados de Otero”, 2250 m, 27.vii.1982 (SMNG) [Syntype queen images examined, AntWeb, photos by Roland Schultz, available on https://www.AntWeb.org]. 1 alate queen (CASENT0106301); Spain: Andalusia, Cazorla, La Empanada, 37.92889/-2.78167, 1660 m, 27.viii.2010, coll. J.L. Reyez (UCDC) [Queen images examined, AntWeb, photos by Marek Borowiec, available on https://www.AntWeb.org].
Tetramorium seiferti (Kiran & Karaman, 2017): 1 paratype queen (ANTWEB1041295); Turkey: Artvin, Yusufeli, 3 km. NW Kinaliçam Village, 40.76, 41.5794, 1801 m., 25.vi.2013, 13/1592c coll. K. Kiran, C. Karaman & V. Aksoy (SMNG) [Syntype queen images examined, AntWeb, photos by Roland Schultz, available on https://www.AntWeb.org].
Type locality
Greece: Thessaly, Magnesia, Kala Nera-Milies, 57 m, 39.30946/23.14404.
Host species
Tetramorium kephalosi Salata & Borowiec, Citation2017
Differential diagnosis.
Tetramorium albenae n. sp. differs from T. kutteri in denser and erect pilosity of appendages and the whole body, smooth head sculpture, and lack of blunt teeth on propodeum; from T. buschingeri in presence of distinct carianae on the dorsolateral margins of the propodeum, smooth head sculpture and predominantly smooth anepisternum and katepisternum; from T. seiferti in almost entirely smooth anepisternum and katepisternum, less setae on katepisternum, smooth head sculpture and lack of teeth on propodeum; from T. inquilinum in smooth head sculpture, almost entirely smooth surfaces of anepisternum and katepisternum, and lack of teeth on propodeum.
Description.
Holotype. Measurements: AOL: 0.03; AOW: 0.02; DLO: 0.08; CL: 0.38; CW: 0.41; DFC: 0.11; ClyW: 0.19; SL: 0.41; EL: 0.09; WL: 0.73; HFL: 0.5; HTL: 0.36; PNW: 0.26; MNW: 0.21; ECW: 0.11; PW: 0.13; PPW: 0.26; CS: 0.40; CL/CW: 0.93; SL/CS: 1.04; ClyW/CS: 0.48; PNW/CS: 0.66; MNW/CS: 0.53; DLO/CS: 0.20; AOL/CS: 0.08; AOW/CS: 0.05; DFC/CS: 0.28; PW/CS: 0.33; PPW/CS: 0.66; HFL/CS: 1.27; HTL/CS: 0.91; EL/CS: 0.23; ECW/CS: 0.28.
Head.
In full-face view: sub-oval, not widening posteriorly, slightly wider than long (CL/CW 0.93); posterior emargination deep. Margins of the head with dense, suberect to erect pilosity; head dorsum with dense, and suberect to erect pilosity. Head smooth and shiny. Scape short with dense suberect to erect setae, when laid back, slightly exceeding the posterior head margin; funiculus 10-segmented, three apical segments forming a small club. Eyes well developed but small (EL/CS 0.23), ocelli well developed, distance of posterior ocelli rather large (DLO/CS 0.2). Anterior clypeal margin concave, posterior margin convex. Mandibles atrophied, largely triangular, and with pointed apex. Mesosoma. Shiny. Pronotum narrower than head (PNW/CS 0.66), anterolaterally with small angles; with sparse and indistinct rugulae, its lateral sides medially smooth. Mesoscutum, mesoscutellar disc and propodeum with denser and more distinct rugulae; scutoscutellar sulcus predominantly smooth and with few wrinkles; lower and upper metapleuron with distinct but sparse rugulae; katepisternum and anepisternum predominantly smooth with fine and sparse rugulae on edges. In lateral view scutum flat, placed higher than pronotum and propodeum. Dorsolateral margins of propodeum developed indistinct carinae, propodeal tooth or lobe absent. Dorsal surface of mesosoma with dense erect setae; katepisternum with a few short erect setae. Petiole. Shiny with dense rugulae; scale in profile triangular with convex apex; with a few short erect setae. Postpetiole. Smooth and shiny; broadly attached to gaster. Gaster. Shiny and smooth; dorso-ventrally flattened. Colour. Brown; antennae, legs, mandibles, gaster (except the first tergite), and area on head between eyes and mandibles brownish-yellow.
Note. The holotype queen was the only specimen of T. albenae that was collected in its natural habitat. This gyne, still attached to the host gyne, was later relocated to an artificial nest where she was laying eggs for several days. The holotype specimen was subsequently preserved in alcohol and its brood was left in the nest. After some time, the inquiline offspring hatched but shortly after they died, most likely due to suboptimal conditions in the nest. The hatched specimens had rudimentary wings, shorter and deformed legs and antennae, and their body sculpture appeared dull and denser than that of the holotype. Thus, we decided not to consider these specimens as paratypes and we did not include their morphological variability in the species description. Below we provide measurements of the 10 alate queens that were sclerotized enough to be point mounted.
Measurements (10q): AOL: 0.04 (0.03–0.04); AOW: 0.03 (0.02–0.04); DLO: 0.07 (0.06–0.08); CL: 0.37 (0.35–0.39); CW: 0.42 (0.40–0.43); DFC: 0.13 (0.11–0.15); ClyW: 0.17 (0.16–0.19); SL: 0.32 (0.29–0.36); EL: 0.09 (0.08–0.1); WL: 0.66 (0.61–0.71); HFL: 0.39 (0.33–0.51); HTL: 0.35 (0.29–0.46); PNW: 0.32 (0.29–0.33); MNW: 0.24 (0.18–0.27); ECW: 0.12 (0.10–0.15); PW: 0.12 (0.09–0.14); PPW: 0.27 (0.21–0.35); CS: 0.40 (0.38–0.41); CL/CW: 0.89 (0.83–0.93); SL/CS: 0.81 (0.73–0.90); ClyW/CS: 0.43 (0.40–0.48); PNW/CS: 0.80 (0.73–0.85); MNW/CS: 0.61 (0.48–0.67); DLO/CS: 0.18 (0.15–0.21); AOL/CS: 0.08 (0.05–0.11); AOW/CS: 0.08 (0.05–0.11); DFC/CS: 0.32 (0.28–0.39); PW/CS: 0.31 (0.23–0.35); PPW/CS: 0.68 (0.52–0.93); HFL/CS: 0.97 (0.86–1.28); HTL/CS: 0.88 (0.75–1.15); EL/CS: 0.22 (0.20–0.25); ECW/CS: 0.31 (0.25–0.40).
Etymology.
The species is named after Dr. Albena Lapeva-Gjonova who has made great contributions to the studies on Balkan ants.
Biology.
The type locality is situated at the border between the mountainous north and the hilly south of Pelion. The collecting site, situated at 57 m a.s.l., is a slightly south-southwest facing slope () situated approximately 50 m northwest from a small river (Miotiko) that flows through a narrow strip of Platanus-forest with many massive trees (). The nest was situated under a flat, fairly round stone with a diameter of approximately 30 cm. The stone lied 1 m from a low dry stone wall in an old and rocky olive grove. This part of the olive grove was lightly grazed by horses. The undergrowth consisted of a slightly ruderal, herb- and flower-rich grassland. Within some 5 m around the nest, olives, Quercus coccifera and many herbaceous plants were growing. Among them: Trifolium spp., Lotus sp., Bellis sp., Malva sp., yellow Asteraceae sp., white small Allium sp., Origanum vulgare, Geranium spp., Medicago sp., Vicia sp., Anagallis arvensis, Onobrychis sp., Urtica dioica, Plantago lanceolata, Sonchus sp., Orlaya sp.,Catapodium sp., Rubus sp. and Verbascum sp.
Immediately after turning the stone, the host gyne with the inquiline gyne attached on her gaster were spotted among numerous workers of the host species. Both specimens, along with numerous workers and brood, were collected with an insect aspirator and taken for observation in captivity. The nest was infested with mites that were also collected and are visible in the supplementary material. In captivity, the inquiline was riding upon the queen of the host colony (), regularly laying eggs and almost constantly tapping with her antennae on the thorax and legs of the host (Supplementary Materials 1–4). All eggs of the parasite were collected and taken care of by workers (). Later, the majority of its pupae died soon after they started moving. Subsequently, the inquilines that managed to develop into mature specimens were deformed and died shortly after they hatched.
Discussion
The extreme rarity of the members of the Tetramorium inquilinum species-group, combined with their small body size and cryptic biology, make them one of the most enigmatic species within the Tetramorium genus (Buschinger Citation1985, Citation1987; Tinaut Citation1990; Kiran et al. Citation2017). Such factors combined, additionally hinder studies on the infra- and interspecific variability of these taxa. As stated by Kiran et al. (Citation2017) the diversity of the Tetramorium inquilinum species-group is most likely severely underestimated and further studies should reveal new discoveries complementing the knowledge on the biology of these inquilines. The data presented in this paper provide new insights into the habitat and host preferences of the species of the Tetramorium inquilinum species-group, so far not observed within already described taxa.
The discovery of this species by two amateur ant enthusiasts emphasizes the value of citizen science and the existence of more popular field guides (e.g. Lebas et al. Citation2019) which lead to better knowledge of ants under a broader public.
Based on the literature records, the group has usually been associated with alpine and subalpine habitats. Tetramorium inquilinum was collected from altitudes ranging from 1660 m up to 2000 m (Collingwood Citation1956; Buschinger Citation1985, Citation1987, Citation1999; Espadaler and Cuesta, Citation2006; Wegnez et al. Citation2015). Similarly, T. kutteri is known only from two high altitude sites in Spanish mountains located above 2100 m a.s.l. (Tinaut Citation1990; López and Martinez Citation2011). The two most recently described taxa are also known only from montane habitats, i.e. T. buschingeri from a site located at 640 m a.s.l. and T. seiferti from a place situated at 1800 m a.s.l. (Kiran et al. Citation2017).
The only lowland site of a member of the Tetramorium inquilinum species-group was reported by Dlussky et al. (Citation1990) for an undescribed species found in Farap (190 m a.s.l.) in Turkmenistan, in a river plane at the margin of the Kara-Kum desert. The type locality of T. albenae (Pelion Peninsula) is located in a Mediterranean habitat at 57 m a.s.l. and it confirms that the members of this species group are not exclusively attached to alpine or montane habitats but can also be located in lowlands. This discovery extensively broadens the potential territory occupied by the inquiline Tetramorium species. However, both lowland sites (Farap in Turkmenistan and Pelion Peninsula in Greece) are located close to rivers that rise in high mountains. Farap is placed at the riverbank of Amu Darya – a river that rises in the Pamir Mountains. Similarly, the Greek site is close to the Miliotiko stream that rises in the Pelion Mountains. This could indicate that these two species are montane or alpine, but were transported downstream by waterbodies and later managed to invade host species or that they were transported downstream together with the host queen. However, it is also possible that these species are naturally present in the lowlands, but they have not been detected in such localities due to insufficient sampling and their rarity.
Tetramorium albenae also extends our knowledge on the potential host species of members of the Tetramorium inquilinum species-group. Kiran et al. (Citation2017) pointed out that there is a disjunction in host species selection between western and eastern Mediterranean inquilines. Tetramorium inquilinum and T. schneideri parasitize on species of the Tetramorium caespitum species-group, while T. seiferti and T. buschingeri were found in nests of species belonging to the Tetramorium chefketi species group. One should mention that members of the caespitum species-group were present at both the Bulgarian and Anatolian type localities. However, the holotype of T. albenae was collected in a nest of Tetramorium kephalosi – a member of the Tetramorium semilaeve species-group. This discovery proves that inquilines can parasitize on a wider group of taxa. In the Balkans, Tetramorium kephalosi is the most widespread and commonest member of the semilaeve species-group, inhabiting open habitats located from seaside to high mountain locations (Salata & Borowiec Citation2017). Thus, it appears as the most suitable host species for social parasites in this region. Unfortunately, due to the recently implemented taxonomic changes in the Palearctic Tetramorium, it is extremely difficult to assess the species identity of the host species of the inquiline collected in Turkmenistan.
The observations in captivity confirmed that T. albenae shares with T. inquilinum some behavioral patterns strongly associated with its biology. Gynes of both species are ectoparasites clinging to the backs of their host workers or queens (T. albenae was permanently attached to host queen’s gaster). They are almost completely inactive most of the time, and in the case of T. albenae, the holotype queen’s only observed activities were laying eggs and constantly tapping with her antennae on the thorax and legs of the host queen. The eggs of the parasite were collected and taken care of by workers. There was no aggressive behaviour expressed by host workers towards the inquiline queen. Additionally, no observation of the feeding behaviour was achieved, but because the queen was almost constantly attended by workers, we assume that it was fed by them.
In summary, the discovery of the new species T. albenae provides new insights into the biology and habitat preferences of the inquiline taxa grouped into the Tetramorium inquilinum species-group. We expect that further intensive studies in mountain areas and their surroundings will provide new records of this species-group and will help us to resolve questions focusing on their distributional pattern and evolution. Citizen scientists can play a relevant role in this undertaking.
Supplemental Material
Download MP4 Video (33.9 MB)Supplemental Material
Download MP4 Video (59.7 MB)Supplemental Material
Download MP4 Video (96.5 MB)Supplemental Material
Download MP4 Video (128.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2198548
Additional information
Funding
References
- Agavekar G, Hita Garcia F, Economo EP. 2017. Taxonomic overview of the hyperdiverse ant genus Tetramorium Mayr (Hymenoptera, Formicidae) in India with descriptions and X-ray microtomography of two new species from the Andaman Islands. PeerJ 5:50 pp. e3800. DOI: 10.7717/peerj.3800.
- Bolton B. 1976. The ant tribe Tetramoriini (Hymenoptera: Formicidae). Constituent genera, review of smaller genera and revision of Triglyphothrix Forel. Bulletin of the British Museum (natural history). Entomology 34:281–379.
- Bolton B. 1977. The ant tribe Tetramoriini (Hymenoptera: Formicidae). The genus Tetramorium Mayr in the Oriental and Indo-Australian regions, and in Australia. Bulletin of the British Museum (Natural History). Entomology 36:67–151.
- Bolton B. 1980. The ant tribe Tetramoriini (Hymenoptera: Formicidae). The genus Tetramorium Mayr in the Ethiopian zoogeographical region. Bulletin of the British Museum (Natural History). Entomology 40:193–384.
- Bolton B. 1987. A review of the Solenopsis genus-group and revision of Afrotropical Monomorium Mayr (Hymenoptera: Formicidae). Bulletin of the British Museum (Natural History). Entomology 54:263–452.
- Bolton B. 2022. An online catalog of the ants of the world. Available: https://antcat.org. Accessed Sep 2022 11.
- Brun R. 1952. Das Zentralnervensystem von Teleutomyrmex schneideri KUTT. (Hym. Formicid.). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 25:73–86.
- Buschinger A. 1985. New records of rare parasitic ants (Hym., Form.) in the French Alps. Insectes Sociaux 32(3):321–324. DOI: 10.1007/BF02224921.
- Buschinger A. 1987. Teleutomyrmex schneideri Kutter, 1950 and other parasitic ants found in the Pyrenees. Spixiana 10:81–83.
- Buschinger A. 1999. Wiederfund der sozialparasitischen Ameise Teleutomyrmex schneideri in der Schweiz. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 72:277–279.
- Buschinger A. 2009. Social parasitism among ants: A review (Hymenoptera: Formicidae). Myrmecological News 12:219–235.
- Collingwood CA. 1956. A rare parasitic ant (Hym., Formicidae) in France. Entomologists’ Monthly Magazine 92:197.
- Dlussky GM, Soyunov OS, Zabelin SI. 1990. Muravji Turkmenistana. Ashkhabad: Ylym Press. p. 273. (in Russian).
- Emery C. 1898. Beiträge zur Kenntniss der palaearktischen Ameisen. Öfversigt af Finska Vetenskaps-Societetens Förhandlingar 20:124–151.
- Espadaler X, Cuesta D. 2006. Teleutomyrmex schneideri Kutter, 1950 en España (Hymenoptera, Formicidae). Graellsia 62(2):261–262. DOI: 10.3989/graellsia.2006.v62.i2.69.
- Forel A. 1874. Les fourmis de la Suisse. Systématique, notices anatomiques et physiologiques, architecture, distribution géographique, nouvelles expériences et observations de moeurs. Neue Denkschriften der Allgemeinen Schweizerischen Gesellschaft für die Gesammten Naturwissenschaften 26:1–452.
- Galanakis D. 1997. Neotectonic and stratihraphic of the neogene-quaternary sediments of Almyros-Pagasitikos, Pilio, Oreoi-Trikeri and Maliakos basins. PhD Thesis, Aristotle University of Thessaloniki, Department of Geology, Section of Geology and Natural Geography. Thessaloniki. 261pp. In Greek. DOI: 10.12681/eadd/11195
- Gösswald K. 1953. Histologische Untersuchungen an der arbeiterlosen Ameise Teleutomyrmex schneideri Kutter (Hym. Formicidae). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 26:81–128.
- Harris RA. 1979. A glossary of surface sculpturing. Occasional papers in entomology. State of California Department of Food and Agriculture 28:1–31.
- Hita Garcia F, Fisher BL. 2011. The ant genus Tetramorium Mayr (Hymenoptera: Formicidae) in the Malagasy region — Introduction, definition of species groups, and revision of the T. bicarinatum, T. obesum, T. sericeiventre and T. tosii species groups. Zootaxa 3039(1):1–72. DOI: 10.11646/zootaxa.3039.1.1.
- Kiran K, Karaman C, Lapeva-Gjonova A, Aksoy V. 2017. Two new species of the “ultimate” parasitic ant genus Teleutomyrmex Kutter, 1950 (Hymenoptera: Formicidae) from the Western Palaearctic. Myrmecological News 25:145–155.
- Kutter H. 1950. Über eine neue, extrem parasitische Ameise. 1. Mitteilung. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 23:81–94.
- LaPolla JS. 2004. Acropyga (Hymenoptera: Formicidae) of the world. Contributions of the American Entomological Institute 33(3):1–130. PDF
- Lebas C, Galkowski C, Blatrix R, Wegnez P. 2019. Ants of Britain and Europe. A photographic guide. London: Bloomsbury Wildlife, 415 pp.
- López JR, Martinez AB. 2011. Nueva cita de Teleutomyrmex kutteri Tinaut, 1990 (Hym.: Formicidae) para la Península Ibérica. Boletín de la Sociedad Entomológica Aragonesa (S.E.A.) 49:206.
- Radchenko AG, Elmes GW. 2010. Myrmica ants (Hymenoptera: Formicidae) of the Old World. Fauna Mundi 3. Warsaw: Natura Optima Dux Foundation, 790 pp.
- Radchenko AG, Scupola A. 2015. Taxonomic revision of the striativentre species group of the genus Tetramorium (Hymenoptera, Formicidae). Vestnik Zoologii 49(3):219–244. DOI: 10.1515/vzoo-2015-0024.
- Salata S, Borowiec L. 2017. Species of Tetramorium semilaeve complex from Balkans and western Turkey, with description of two new species of (Hymenoptera: Formicidae: Myrmicinae). Annales Zoologici (Warsaw) 67(2):279–313. DOI: 10.3161/00034541ANZ2017.67.2.008.
- Salata S, Fisher BL. 2020. Pheidole Westwood, 1839 (Hymenoptera, Formicidae) of Madagascar – An introduction and a taxonomic revision of eleven species groups. ZooKeys 905:1–235. DOI: 10.3897/zookeys.905.39592.
- Schenck CF. 1852. Beschreibung nassauischer Ameisenarten - Jahrbuch des Vereins für Naturkunde im Herzogthum Nassau. Wiesbaden 8:1–149.
- Schifani E, Alicata A, Menchetti M, Borowiec L, Fisher BL, Karaman C, Kiran K, Oueslati W, Salata S, Blatrix R. 2022. Revisiting the morphological species groups of West-Palearctic Aphaenogaster ants (Hymenoptera: Formicidae) under a phylogenetic perspective: Toward an evolutionary classification. Arthropod Systematics and Phylogeny 80:627–648. DOI: 10.3897/asp.80.e84428.
- Tinaut A. 1990. Teleutomyrmex kutteri, spec. nov. A new species from Sierra Nevada (Granada, Spain). Spixiana 13:201–208.
- Wagner HC, Arthofer W, Seifert B, Muster C, Steiner FM, Schlick-Steiner BC. 2017. Light at the end of the tunnel: Integrative taxonomy delimits cryptic species in the Tetramorium caespitum complex (Hymenoptera: Formicidae). Myrmecological News 25:95–129.
- Wagner HC, Steiner FM, Schlick-Steiner BC, Csősz S. 2021. Mixed-colony records together with nest densities and gyne morphology suggest temporary social parasitism in Tetramorium (Hymenoptera: Formicidae). Zoologischer Anzeiger 293:190–201. DOI: 10.1016/j.jcz.2021.06.003.
- Ward PS, Brady SG, Fisher BL, Schultz TR. 2015. The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Systematic Entomology 40(1):61–81. DOI: 10.1111/syen.12090.
- Wegnez P, Ignace D, Lommelen E, Hardy M, Bogaert J, Nilsson C. 2015. Redécouverte de Teleutomyrmex schneideri Kutter, 1950 dans les Alpes françaises (Hymenoptera: Formicidae). – Bulletin de la Société royale belge d’Entomologie 151:52–57.
- Wilson EO. 1955. A monographic revision of the ant genus Lasius. Bulletin of the Museum of Comparative Zoology 113:1–201.
- Wilson EO. 2003. Pheidole in the New World. A dominant, hyperdiverse ant genus. Cambridge, Mass: Harvard University Press. pp. [ix] + 794.


