Abstract
An extreme radiation of hundreds of species of different groups of animals occurred in Lake Baikal, Siberia, Russia; among them, amphipods represent one of the most remarkable groups of invertebrates with about 350 endemic species. Amphipods host associated epibiont rotifers, and the aim of the study is to explore the possibility that bdelloid rotifers living as epibionts on amphipods in Lake Baikal coevolved with their hosts and diversified with species-specific host–epibiont associations. We sampled 148 individual amphipods belonging to 16 species and isolated all epibiont bdelloids from them, discovering that only one bdelloid species, Embata parasitica, lives associated with at least six amphipod species belonging to three different families. Similar to what is known in most other bdelloid species, the morphospecies Embata parasitica from Lake Baikal is likely to be a complex of cryptic species, as suggested by the high genetic diversity we found in one mitochondrial marker sequenced from several animals. Yet none of the divergent genetic lineages seemed to be associated to only one or a few amphipod species. In addition, nine bdelloid species were found living in the lake, increasing the known diversity of the area to 12 bdelloid species.
Introduction
Lake Baikal, located in Southern Siberia, Russia, is the deepest and most ancient lake of the world, containing the largest volume of fresh water of any water body (Logachev Citation2003). Hosting more than 2640 animal species, 60% of which are endemic, Lake Baikal is first among all lakes of the globe in terms of animal diversity (Timoshkin Citation2010–2011). It is home to one of the most spectacular evolutionary radiations in freshwater habitats (Sherbakov Citation1999; Brown et al. Citation2021), with hundreds of endemic species of fish (Kontula et al. Citation2003; Sideleva Citation2003), flatworms (Timoshkin et al. Citation2010), and other animals, including an endemic family of sponges (Khanaev et al. Citation2018). It also hosts an endemic freshwater seal (Pastukhov Citation1993; Palo & Väinölä Citation2006). The most unusual radiation in the lake is that of amphipods, with about 350 endemic species colonising different habitats and depths in the lake (Macdonald et al. Citation2005; Gurkov et al. Citation2019).
Lake Baikal represents an exception even for microscopic animals with almost no biogeographical patterns, like monogonont rotifers (Dumont Citation1983; Fontaneto Citation2019), a group that is notorious for having species mostly with broad, usually cosmopolitan distributions (Segers Citation2007; Fontaneto et al. Citation2012). A high proportion of species, 14%, is known to be endemic to the lake (Arov & Misharina Citation2018). For some genera, endemism is more extreme: half of the approximately 50 species of the genus Notholca Gosse, 1886 of the world are known only from the lake (Sheveleva et al. Citation1995), making this lake a unique hotspot of endemic diversity for monogonont rotifers.
Notwithstanding such a high interest in terms of the diversity and the biogeography of monogonont rotifers in Lake Baikal, almost nothing is known from the lake regarding bdelloid rotifers. Only three species of bdelloids have been reported so far from the area (Timoshkin Citation2001), namely Philodina acuticornis Murray, 1902, Philodina vorax (Janson, 1893), and Rotaria rotatoria (Pallas, 1766). Here we report on the first survey of bdelloid rotifers from the lake, with a special focus on epibiont species. Some bdelloid species are known to be strictly associated with crustaceans, mostly with isopods and amphipods (May Citation1989; Fontaneto & Ambrosini Citation2010). Thus, given the extremely high species diversity of amphipods in Lake Baikal, the expectation is that their associated epibiont bdelloid rotifers may have diversified into several species, coevolving with the different amphipod species, with patterns of host–symbiont coevolution in the frame of phylosymbiosis, the complex relationship of microscopic organisms living in association with their hosts (Lim & Bordenstein Citation2020). Congruent patterns of diversity between hosts and epibionts are already known in freshwater habitats, for example between branchiobdellidans and their freshwater crayfish hosts of the genus Austropotamobius, implying a strict host–epibiont association and thus coevolution (Šarić et al. Citation2018). Other known cases of coevolution of organisms with their hosts in aquatic systems can be found in the Daphnia–parasite system (Ebert Citation2008), in the phylosymbiosis between Hydra species and their microbiome (Rosenberg & Zilber-Rosenberg Citation2018), and in the holobiont associations known in corals and sponges (O’Brien et al. Citation2019), among other examples. In this study, we screen various species of amphipods, looking for patterns of phylosymbiosis in the occurrence of their associated epibiont bdelloid rotifers.
Very little is known about the ecology of epibiont and symbiont bdelloid rotifers (May Citation1989; Fontaneto & Ambrosini Citation2010), making any hypothesised scenario rather weak. In addition, the role and effect of rotifers and other microscopic metazoan living associated with amphipods are also poorly known (Bojko & Ovcharenko Citation2019).
Methods
Amphipods were collected in the area of Bolshiye Koty (WGS84 coordinates: 51°541′1.67″N, 105°4′7.61″E), to the north-east of Listvyanka, Irkutsk Oblast, Russia, on the western side of Lake Baikal in July 2016. Sediment samples were collected at the shore, by diving, and from dredges deployed from a boat. Depths at which samples were taken ranged from just below the surface, at less than 0.5 m, to about 30 m (), covering what should be the richest part of large ancient lakes in terms of biodiversity (Vadeboncoeur et al. Citation2011). All amphipods that were sampled were sorted and identified while alive using the identification key of Takhteev and Didorenko (Citation2015). Screening for epibiont bdelloid rotifers was performed under a stereomicroscope (from 10× to 50× magnification) and all epibiont bdelloids were removed from the host using mounted needles and pipette tips. Hosts were inspected repeatedly, until no new epibiont bdelloids were visible. Bdelloids were identified at the species level or at the highest possible taxonomic resolution according to Donner (Citation1965) using a compound microscope at 400× magnification. We searched for epibiont bdelloid rotifers also on any other large invertebrate that we found in the same samples: on sponges of the species Lubomirskia baikalensis (Pallas, 1776) and on caddisflies larvae (Trichoptera) from the lake, using the same procedure as that described for the amphipods; we looked for free-living bdelloids in water and sediment samples from the same areas where invertebrate hosts were collected. All sorting procedures were performed on living samples, close to the lake in the lab of the Baikal Biological Station of Institute of Biology, Irkutsk State University, in Bolshiye Koty.
Table I. Information on samples depending on substrate (epibiont on amphipod, with number of analysed individuals in parentheses; or on other invertebrates; or from the environment), host species in alphabetical order for epibiont samples, sample ID with sampling date, depth, and coordinates (WGS84 reference system). For each sample, occurrence of bdelloid species is reported, with the number of animals for the focal species Embata parasitica, and the number of sequenced individuals in parentheses.
To check that no epibionts were inadvertently removed from their hosts during the sampling and sorting procedure, all used equipment, vials, tubes, and liquids were inspected at the end of each working day to search for loosely attached epibionts that could have been detached, but no such animals were ever found.
We counted the number of epibionts on each individual host for each of the amphipod species and the other substrate. We used a chi-squared test in R 4.0.3 (R Core Team Citation2021) to assess whether the proportion of amphipod species hosting epibiont bdelloids was different between shallow waters at the shore and in deeper waters. No previous knowledge is available on epibiont bdelloids from amphipods in aphotic areas: thus, our aim was to test whether amphipods living on the shores and in deeper waters display any differences in their numbers of epibiont bdelloids, regardless of the species of host and epibiont. Given the small sample size in some of the events for the chi-squared test, we used Yates’s correction in the calculation of p values to avoid their overinflation.
Bdelloid rotifers were stored in ethanol and DNA was extracted by incubating each animal individually in 35 μL of Chelex (InstaGene Matrix, Bio-Rad) and proteinase K for 20 minutes, as is commonly done for bdelloids (Kaya et al. Citation2009). A fragment of a mitochondrial marker, cytochrome c oxidase subunit I (COI), was amplified for each individual using the Folmer primers (Folmer et al. Citation1994), with protocols commonly applied for rotifers (Mills et al. Citation2017; Cakil et al. Citation2021). Sequencing was performed in both directions and contigs obtained to be used for the following analyses. Additional data on COI of animals from the genus Embata was downloaded from GenBank (EF650596, EF650597, EF650608, JN660052, KM043189, KM043190). To expand the dataset to be used in comparison with the data from Lake Baikal, we also included sequences from epibiont Embata collected in New Caledonia during the Our Planet Reviewed New Caledonia expedition, a survey of aquatic diversity on the island (https://nouvellecaledonie.laplaneterevisitee.org/).
All sequences were aligned and checked using Mesquite 3.70 (Maddison & Maddison Citation2021) for the absence of indels and stop codons; primer regions still present in the sequences were trimmed. Uncorrected genetic raw distances were calculated between pairs of sequences to assess the level of genetic diversity. A phylogenetic reconstruction was estimated to obtain a visual representation of the evolutionary relationships between epibiont bdelloids using a maximum likelihood approach under a GTR+G + I evolutionary model in PhyML 3.0, with support values expressed as aLRT (approximate Likelihood-Ratio Test) (Guindon et al. Citation2010). As an outgroup for the phylogenetic reconstruction, we used sequences of Philodina citrina sampled in Lake Baikal during the survey.
Results
Out of the 33 samples we collected in the lake, focusing mostly on native endemic invertebrates (N = 25) to look for epibiont bdelloid rotifers, we confirmed the occurrence of Rotaria rotatoria, already known from the lake, and we increased the known diversity of Lake Baikal to 12 species, with nine new records (): Dissotrocha aculeata; Habrotrocha collaris; Philodina citrina; Philodina flaviceps; Philodina roseola; two undetermined species of Philodina, one on caddisflies larvae and one in the sponge Lubomirskia baikalensis; Pleuretra sulcata; and a high number of tardigrades of the species Grevenius baicalensis (Ramazzotti, 1966) (identified by Roberto Guidetti). Only one species of epibiont bdelloid, Embata parasitica, was found associated to the amphipods. We did not confirm the occurrence of two of the three previously known species for the lake, Philodina acuticornis and Philodina vorax.
We screened 148 individual amphipods in 23 samples from at least 16 species (10 determined to species level and six to genus level) of five families – Acanthogammaridae Garjajeff, 1901, Eulimnogammaridae Kamaltynov, 1999, Micruropodidae Kamaltynov, 1999, Ommatogammaridae Kamaltynov, 2009, and Pallaseidae Tachteev, 2001 (in addition to seven undetermined species) – and found 98 epibiont bdelloid rotifers identified as the morphospecies Embata parasitica (Table 1). No other epibiont metazoans were found. Occurrence of epibiont bdelloids was low, with on average 0.67 epibiont animals for each individual amphipod, colonising only six amphipod species of three families: Eulimnogammarus cyaneus, Eulimnogammarus marituji, Eulimnogammarus verrucosus, and Eulimnogammarus vittatus from Eulimnogammaridae, Gmelinoides fasciatus from Micruropodidae, and Pallasea sp. from Pallaseidae. The number of individual rotifers on each colonised amphipod ranged from 1 to 22. We found Embata parasitica on any part of the body of the host amphipods, on the ventral side, close to the leg base, on the antenna, around the mouth parts, close to the cloaca, or on the dorsal part. The amphipod species on which we could not find any epibiont bdelloid rotifers were Brandtia latissima lata, Brandtia sp., and Hyalellopsis sp. from Acanthogammaridae, Eulimnogammarus maackii and Eulimnogammarus viridis from Eulimnogammaridae, Micruropus wohlii from Micruropodidae, Ommatogammarus sp. from Ommatogammaridae, and Pallasea cancelloides from Pallaseidae. The occurrence of epibiont bdelloids in amphipods species at the shores (5 out of 12) was not different from that of amphipod species sampled in deeper waters, from 5 to 30 m (1 out of 11): no significant difference could be highlighted in the proportion of species colonised by epibiont bdelloid rotifers between shallow and deeper waters (X-squared1 = 3.16, p (Yates) = .1929).
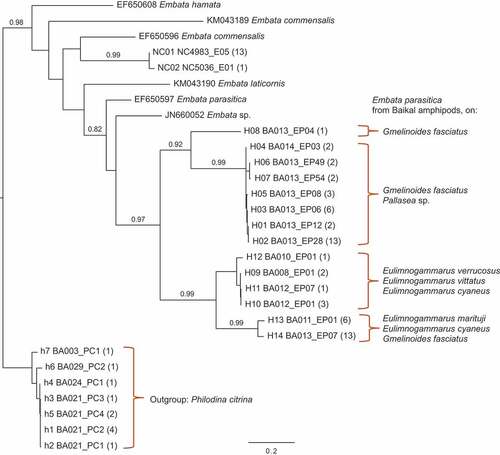
The phylogenetic reconstruction of the COI molecular divergence of the genus Embata was performed on 57 animals we sequenced out of the 98 we found in Lake Baikal (Supplementary Table S1). The mtDNA marker for the Baikal animals clustered together with the only known sequence available in GenBank for the same morphospecies (), E. parasitica (GenBank ID EF650597 from Italy), but with a high uncorrected genetic distance to it: from 16.1 to 22.0%. The sequenced individuals from Lake Baikal represented 14 COI haplotypes, which clustered in four main clades with within-clade molecular divergence up to 4.3% and between-clade diversity from 14.3% to 21.3%.
Figure 1. Phylogenetic reconstruction of the evolutionary relationships between all known and available COI sequences for the genus Embata according to maximum likelihood, with branch length proportional to the number of substitution sites of the scale bar. Support values, expressed as aLRT > 0.80, are reported above branches but not for short terminal branches. Samples downloaded from GenBank are reported with their GenBank accession number; samples from New Caledonia are marked with the prefix NC; samples from Baikal are marked with the prefix H for haplotypes of Embata parasitica, with the host amphipod species indicated for each clade; samples from Philodina citrina from Baikal, used as outgroup, are marked with the prefix h for haplotype. The number of individual animals having the same haplotype is reported in parentheses.
Discussion
Three species of bdelloid rotifers were previously known from Lake Baikal (Timoshkin Citation2001), and with our short survey we upgraded the known diversity of the lake to 12, with nine new records. The 10 taxa of bdelloid rotifers now known to the species level from the lake have a broad distribution around the globe (Segers Citation2007), with no evidence of the species being endemic to the lake. They are all common inhabitants of water bodies, usually found in any survey of lakes and ponds in the Palaearctic region (e.g. Donner Citation1965). Philodina vorax, previously cited from the lake but not confirmed in our survey, is a limno-terrestrial species (Donner Citation1965) and we did not look at samples from such habitats. Yet Pleuretra sulcata is usually a limno-terrestrial species too (Donner Citation1965), and we found it in sediments of Lake Baikal.
The apparent lack of endemic species is in striking contrast to what is known in the lake for monogonont rotifers, with several species that are indeed endemic to the lake (Sheveleva et al. Citation1995; Arov & Misharina Citation2018). Rotifers are notorious for having very low levels of endemic species worldwide (Dumont Citation1983; Fontaneto et al. Citation2012), with only one area of the world that seems to have endemic species for bdelloids: Antarctica (Garlasché et al. Citation2020). Yet the disparity in the proportion of endemic species between bdelloids and monogononts in Lake Baikal seems to go in the opposite direction to what is known in Antarctica. Whereas in Lake Baikal no endemic bdelloids are currently known and about 14% of the monogonont species are endemic (Arov & Misharina Citation2018), in Antarctica it seems that as much as one-third of the bdelloid species (~33%) and only about 8% of the monogonont species are endemic (Iakovenko et al. Citation2015; Garlasché et al. Citation2020). Such contrasting proportions could reflect a different history of diversification in Antarctica (e.g. Cakil et al. Citation2021) and in Lake Baikal between bdelloid and monogonont rotifers and surely deserves deeper study in both areas. No reliable inference can be supported at the moment with the limited knowledge we currently have on bdelloids from Lake Baikal. The comparison with Antarctica is also weak, given that Baikal is a lake and Antarctica a continent, but these are the only known areas in the world hosting high levels of endemic rotifers.
As expected from previous studies on freshwater crustaceans elsewhere (Donner Citation1965; May Citation1989; De Smet & Verolet Citation2016; Dražina et al. Citation2018), amphipods in Lake Baikal also hosted epibiont rotifers. Yet even though we explored amphipods from five families, only one morphospecies of bdelloid rotifer was found on different species of amphipods: morphologically it resembled Embata parasitica, a species that is already known to live as epibiont on amphipods and in general on freshwater arthropods in other parts of the world (Donner Citation1965; Fontaneto et al. Citation2004). Surprisingly, no other epibiont species of bdelloid rotifers was found on amphipods from Lake Baikal, even when other species of the same genus and of different genera are known to occur on amphipods elsewhere (Donner Citation1965; May Citation1989), and we focused on the littoral zones of the lake, where most of the diversity is expected (Vadeboncoeur et al. Citation2011). Bdelloids are known to be highly efficient dispersers and colonisers, due to their extreme resistance capabilities allowing them to desiccate and freeze and thus behave as dispersing-resistant propagules at any stage of their life cycle (Fontaneto Citation2019). Yet, contrary to the free-living species in the group, epibiont bdelloids are often unable to survive desiccation (Eyres et al. Citation2015; Nowell et al. Citation2018) and, therefore, they may be less prone to disperse than free-living bdelloids. Given the current and historical geographical isolation of Lake Baikal, one might speculate that the low diversity of epibionts is due to the difficulties epibiont species face in being dispersed to this area.
The single epibiont bdelloid species found on the endemic Baikal amphipods was not strictly associated with one species or one family of amphipods, but was found on several species, mostly of the genus Eulimnogammarus, and also on other genera from other families. Such a pattern is rather common in epibiont bdelloids, which can be found on different hosts (Donner Citation1965; May Citation1989). The lack of strong host–epibiont association and coevolution in rotifers could be due to a rather labile identification of cues for surface recognition and colonisation (Steinberg et al. Citation2002), and to the possibility for epibionts to survive on different hosts. Evidence from several groups of aquatic invertebrates suggests that many host–epibiont relationships are non-specific and generalist, making specialised and obligate epibionts quite rare (Wahl & Mark Citation1999; Harder Citation2009). Bdelloid rotifers living as epibionts on amphipods in Lake Baikal seem to follow such a general pattern, notwithstanding the impressive adaptive radiation of the hosts (Macdonald et al. Citation2005; Gurkov et al. Citation2019), potentially providing ample opportunities for divergence of the epibiont bdelloids. It is likely that host–symbiont relationships may produce stricter species-specific associations in parasites or obligate symbionts than in epibionts, as in the case of bdelloid rotifers on amphipods that we described in Baikal. Yet not enough is known on the ecology of epibiont bdelloids (May Citation1989) to allow any inference regarding the strength of the association with their hosts.
The highest abundance of epibionts was found on Gmelinoides fasciatus, with up to 22 bdelloids on one single host. Such a number, even if higher than those on other Baikal amphipods, is much lower than the numbers of epibiont bdelloids on the European waterlouse Asellus aquaticus (Linnaeus, 1758) (Isopoda), with hundreds of epibiont bdelloids on each host (Fontaneto & Ambrosini Citation2010), or to what can be found on gammarid amphipods, other crustaceans, molluscs or caddisfly larvae in Europe (May Citation1989; Fontaneto et al. Citation2004; Dražina et al. Citation2018; Ejsmont-Karabin & Karpowicz Citation2019). Gmelinoides fasciatus is known to be associated with habitats with higher trophic levels than other amphipods (Takhteev & Didorenko Citation2015) in the ultraoligotrophic Lake Baikal (Hampton et al. Citation2008): this preference of the host species may be the reason for a higher abundance of filter-feeding epibiont bdelloids. Interestingly, G. fasciatus is a species originally found only in Lake Baikal but nowadays representing a successful invasive species outside the lake (Pankova & Berezina Citation2007). Further studies on invasive populations could test whether the genetic lineages of E. parasitica associated with this amphipod species are spreading as invaders together with their host.
One reason for the low diversity and abundance of the single bdelloid species found epibiont on amphipods in Lake Baikal could be competition with other epibionts, for example the highly diverse and endemic unicellular Ciliophora (Yankowski Citation1982). Yet bdelloids are known to coexist with protists on the same host in other parts of the world (May Citation1989), with no evidence of competitive exclusion. Another reason could be that many Baikal amphipods begin to moult in late spring beginning of summer (Bazikalova Citation1941), with animals sampled in July representing newly moulted individuals, not yet fully colonised by all the potential epibiont species. Such a speculation to explain low diversity on newly moulted amphipods collected in July seems unlikely to be realistic, given that a reservoir for epibiont rotifers other than the amphipods would need to be hypothesised to allow bdelloids to recolonise the amphipod host. The assumption is not realistic because summer-reproducing amphipod species, such as E. cyaneus and G. fasciatus, have higher metabolic rates and moult more frequently in summer in comparison to E. verrucosus and E. vitttatus (Takhteev Citation2000; Jakob et al. Citation2016). If that were the case, we could expect more bdelloids on E. verrucosus and E. vittatus than on summer-reproducing species. In addition, bdelloids are known to be abundant on newly moulted crustaceans elsewhere (Fontaneto & Ambrosini Citation2010). Thus, no clear explanation can be put forward to explain the low diversity and abundance of epibiont bdelloids in the area that was sampled in Lake Baikal.
The other animals on which we looked for associated epibionts were caddisfly larvae (Trichoptera) on the shores. We found three epibiont species on them: Philodina citrina and Rotaria rotatoria, which are free-living animals occasionally found attached to other organisms (Donner Citation1965), and an undetermined species of Philodina, which may represent an additional epibiont species. We could not study these animals in detail in the field and we cannot confirm their identity. This also applies to another species of Philodina, found as an epibiont on the endemic Baikal sponge Lubomirskia baikalensis, for which no identification was possible during the survey. As a side note, the sponge hosted a high number of harpacticoid and cyclopoid copepods, and tardigrades identified as Grevenius baicalensis (Roberto Guidetti pers. comm.). Regarding the single individual of Philodina citrina found as an epibiont on caddisflies larvae (BA003), it could be considered an occasional coloniser of the host, as genetically it clustered closely to the free-living animals found in Lake Baikal (), with an uncorrected genetic distance to them between 2.5% and 4.0%, a COI distance that falls well within the same species in bdelloids (Gabaldón et al. Citation2017; Cakil et al. Citation2021).
Molecular divergence in COI was high within the morphospecies Embata parasitica found on Baikal amphipods, up to 21.3%. Such genetic distances are similar to those known for the same marker in other species complexes in monogonont and bdelloid rotifers (Gabaldón et al. Citation2017; Mills et al. Citation2017; Cakil et al. Citation2021). It is highly likely that a complex of at least four cryptic species is present in Lake Baikal, corresponding to the four divergent clades identified by the phylogenetic reconstruction (). Yet any inference about taxonomic identity based solely on one mitochondrial marker may be highly misleading in rotifers (e.g. Papakostas et al. Citation2016; Michaloudi et al. Citation2018) and further analyses on integrative taxonomy (sensu Schlick-Steiner et al. Citation2010) are needed. Interestingly, even at the level of the four mtDNA clades, taxonomic units would not be segregated on different amphipod hosts: different bdelloid clades were present on the same amphipod host and each bdelloid clade was not strictly associated with one amphipod host species ().
This study also represents the first investigation into the genetic diversity of the genus Embata. Five species, four of which were included in our phylogenetic analysis, are known in the genus, all associated to invertebrate hosts (Donner Citation1965). There could be potentially more species in the genus, among the four mtDNA clades in Lake Baikal and also in other parts of the world, as exemplified by the separate clade represented by the New Caledonian samples. Very few analyses on rotifer diversity have focused on epibiont animals like those of the genus Embata. Expanding this field of research could bring new insights on the host-associated biodiversity of microscopic animals (May Citation1989), as has been the case for the rotifers associated with freshwater isopods (Fontaneto & Ambrosini Citation2010) and for the surprising diversity of epibiont marine meiofauna associated with marine turtles (Ingels et al. Citation2020).
Permits
Sampling and sequencing from Russia were performed according to local regulations. Sampling and sequencing from New Caledonia were performed according to permits issued by the Province Sud and the Province Nord, including for Access and Benefit-Sharing (ABS) of the Nagoya Protocol.
Supplemental Material
Download MS Excel (14.4 KB)Acknowledgements
We give special thanks for the logistic support of Maxim Timofeyev for sampling in Lake Baikal, and to Ulf Jondelius, Oleksandr Holovachov, Philippe Bouchet and Nicolas Charpin for sampling in New Caledonia. Thanks to Roberto Guidetti for the identification of the tardigrades. Thanks to two anonymous reviewers for their comments that improved the first version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All new sequences were deposited in GenBank under accession numbers OP033973–OP034029 for Embata parasitica from Lake Baikal, OP034030–OP034043 for Embata sp. from New Caledonia, and OP033962–OP033972 for Philodina citrina from Lake Baikal.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2205875
Additional information
Funding
References
- Arov IV, Misharina EA. 2018. Modern state of knowledge and ways of genesis of the fauna of rotifers (Rotifera) of Lake Baikal. The bulletin of Irkutsk State University. Geoarchaeology, Ethnology and Anthropology Series 25:70–90.
- Bazikalova AJ. 1941. Materials on the study of the amphipods of Baikal. II. Reproduction. Izvestiya Academy of Sciences USSR 1:407–426.
- Bojko J, Ovcharenko M. 2019. Pathogens and other symbionts of the Amphipoda: Taxonomic diversity and pathological significance. Diseases of Aquatic Organisms 136(1):3–36. DOI:10.3354/dao03321.
- Brown KP, Gerber A, Bedulina D, Timofeyev MA. 2021. Human impact and ecosystemic health at Lake Baikal. Wiley Interdisciplinary Reviews: Water 8(4):e1528. DOI:10.1002/wat2.1528.
- Cakil ZV, Garlasché G, Iakovenko N, Di Cesare A, Eckert EM, Guidetti R, Fontaneto D. 2021. Comparative phylogeography reveals consistently shallow genetic diversity in a mitochondrial marker in Antarctic bdelloid rotifers. Journal of Biogeography 48(7):1797–1809. DOI:10.1111/jbi.14116.
- De Smet WH, Verolet M. 2016. Epibiotic rotifers of Gammarus pulex (L.) (Crustacea, Amphipoda), with descriptions of two new species and notes on the terminology of the trophi. Zootaxa 4107(3):301–320. DOI:10.11646/zootaxa.4107.3.1.
- Donner J. 1965. Ordnung Bdelloidea (Rotatoria, Radertiere). Berlin: Akademie Verlag. pp. 297.
- Dražina T, Korša A, Špoljar M, Maguire I, Klobučar GI. 2018. Epifauna of native and alien freshwater crayfish species (Crustacea: Decapoda): A host-specific community? Freshwater Science 37(3):593–604. DOI:10.1086/698764.
- Dumont HJ. 1983. Biogeography of rotifers. Hydrobiologia 104:19–30. DOI:10.1007/BF00045948.
- Ebert D. 2008. Host–parasite coevolution: Insights from the Daphnia–parasite model system. Current Opinion in Microbiology 11(3):290–301. DOI:10.1016/j.mib.2008.05.012.
- Ejsmont-Karabin J, Karpowicz M. 2019. Epizoic rotifers on Dreissena polymorpha in relation to biotic factors. Hydrobiologia 828(1):137–145. DOI:10.1007/s10750-018-3808-4.
- Eyres I, Boschetti C, Crisp A, Smith TP, Fontaneto D, Tunnacliffe A, Barraclough TG. 2015. Horizontal gene transfer in bdelloid rotifers is ancient, ongoing and more frequent in species from desiccating habitats. BMC Biology 13(1):1–17. DOI:10.1186/s12915-015-0202-9.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3:294–299.
- Fontaneto D. 2019. Long-distance passive dispersal in microscopic aquatic animals. Movement Ecology 7(1):1–10. DOI:10.1186/s40462-019-0155-7.
- Fontaneto D, Ambrosini R. 2010. Spatial niche partitioning in epibiont rotifers on the waterlouse Asellus aquaticus. Limnology and Oceanography 55(3):1327–1337. DOI:10.4319/lo.2010.55.3.1327.
- Fontaneto D, Barbosa AM, Segers H, Pautasso M. 2012. The ‘rotiferologist’ effect and other global correlates of species richness in monogonont rotifers. Ecography 35(2):174–182. DOI:10.1111/j.1600-0587.2011.06850.x.
- Fontaneto D, Segers H, Melone G. 2004. Epizoic rotifers (Rotifera: Monogononta, Bdelloidea) from the gill chambers of Potamon fluviatile (Herbst, 1785). Journal of Natural History 38(10):1225–1232. DOI:10.1080/0022293031000155197.
- Gabaldón C, Fontaneto D, Carmona MJ, Montero-Pau J, Serra M. 2017. Ecological differentiation in cryptic rotifer species: What we can learn from the Brachionus plicatilis complex. Hydrobiologia 796(1):7–18. DOI:10.1007/s10750-016-2723-9.
- Garlasché G, Karimullah K, Iakovenko N, Velasco-Castrillón A, Janko K, Guidetti R, Fontaneto D. 2020. A data set on the distribution of Rotifera in Antarctica. Biogeographia 35:17–25.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology 59(3):307–321. DOI:10.1093/sysbio/syq010.
- Gurkov A, Rivarola-Duarte L, Bedulina D, Fernández Casas I, Michael H, Drozdova P, Luckenbach T. 2019. Indication of ongoing amphipod speciation in Lake Baikal by genetic structures within endemic species. BMC Evolutionary Biology 19(1):1–16. DOI:10.1186/s12862-019-1470-8.
- Hampton SE, Izmest’eva LR, Moore MV, Katz SL, Dennis B, Silow EA. 2008. Sixty years of environmental change in the world’s largest freshwater lake – Lake Baikal, Siberia. Global Change Biology 14(8):1947–1958. DOI:10.1111/j.1365-2486.2008.01616.x.
- Harder T. 2009. Marine epibiosis: Concepts, ecological consequences and host defence. In: Flemming HC, Sriyutha Murthy P, Venkatesan R, Cooksey K, editors. Marine and Industrial Biofouling. Berlin: Springer. pp. 219–231.
- Iakovenko NS, Smykla J, Convey P, Kašparová E, Kozeretska IA, Trokhymets V, Janko K. 2015. Antarctic bdelloid rotifers: Diversity, endemism and evolution. Hydrobiologia 761(1):5–43. DOI:10.1007/s10750-015-2463-2.
- Ingels J, Valdes Y, Pontes LP, Silva AC, Neres PF, Corrêa GV, Dos Santos GA. 2020. Meiofauna life on loggerhead sea turtles-diversely structured abundance and biodiversity hotspots that challenge the meiofauna paradox. Diversity 12(5):203. DOI:10.3390/d12050203.
- Jakob L, Axenov‐Gribanov DV, Gurkov AN, Ginzburg M, Bedulina DS, Timofeyev MA, Pörtner HO. 2016. Lake Baikal amphipods under climate change: Thermal constraints and ecological consequences. Ecosphere 7(3):e01308. DOI:10.1002/ecs2.1308.
- Kaya M, Herniou EA, Barraclough TG, Fontaneto D. 2009. Inconsistent estimates of diversity between traditional and DNA taxonomy in bdelloid rotifers. Organisms Diversity and Evolution 9(1):3–12. DOI:10.1016/j.ode.2008.10.002.
- Khanaev IV, Kravtsova LS, Maikova OO, Bukshuk NA, Sakirko MV, Kulakova NV, Belikov SI. 2018. Current state of the sponge fauna (Porifera: Lubomirskiidae) of Lake Baikal: Sponge disease and the problem of conservation of diversity. Journal of Great Lakes Research 44(1):77–85. DOI:10.1016/j.jglr.2017.10.004.
- Kontula T, Kirilchik SV, Väinölä R. 2003. Endemic diversification of the monophyletic cottoid fish species flock in Lake Baikal explored with mtDNA sequencing. Molecular Phylogenetics and Evolution 27(1):143–155. DOI:10.1016/S1055-7903(02)00376-7.
- Lim SJ, Bordenstein SR. 2020. An introduction to phylosymbiosis. Proceedings of the Royal Society B 287(1922):20192900. DOI:10.1098/rspb.2019.2900.
- Logachev NA. 2003. History and geodynamics of the Baikal rift. Russian Geology and Geophysics 44:391–406.
- Macdonald III KS, Yampolsky L, Duffy JE. 2005. Molecular and morphological evolution of the amphipod radiation of Lake Baikal. Molecular Phylogenetics and Evolution 35(2):323–343. DOI:10.1016/j.ympev.2005.01.013.
- Maddison WP, Maddison DR 2021. Mesquite: A modular system for evolutionary analysis. Version 3.70. Available: http://www.mesquiteproject.org. Accessed June 2022 15.
- May L. 1989. Epizoic and parasitic rotifers. Hydrobiologia 186:59–67. DOI:10.1007/BF00048897.
- Michaloudi E, Papakostas S, Stamou G, Neděla V, Tihlaříková E, Zhang W, Declerck SA. 2018. Reverse taxonomy applied to the Brachionus calyciflorus cryptic species complex: Morphometric analysis confirms species delimitations revealed by molecular phylogenetic analysis and allows the (re) description of four species. PLoS One 13(9):e0203168. DOI:10.1371/journal.pone.0203168.
- Mills S, Alcántara-Rodríguez JA, Ciros-Pérez J, Gómez A, Hagiwara A, Galindo KH, Walsh EJ. 2017. Fifteen species in one: Deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 796(1):39–58. DOI:10.1007/s10750-016-2725-7.
- Nowell RW, Almeida P, Wilson CG, Smith TP, Fontaneto D, Crisp A, Barraclough TG. 2018. Comparative genomics of bdelloid rotifers: Insights from desiccating and nondesiccating species. PLoS Biology 16(4):e2004830. DOI:10.1371/journal.pbio.2004830.
- O’Brien PA, Webster NS, Miller DJ, Bourne DG. 2019. Host-microbe coevolution: Applying evidence from model systems to complex marine invertebrate holobionts. mBio 10(1):e02241–18. DOI:10.1128/mBio.02241-18.
- Palo JU, Väinölä R. 2006. The enigma of the landlocked Baikal and Caspian Seals addressed through phylogeny of phocine mitochondrial sequences. Biological Journal of the Linnean Society 88(1):61–72. DOI:10.1111/j.1095-8312.2006.00607.x.
- Pankova ES, Berezina NA. 2007. Predation rate and size selectivity of the invasive amphipod Gmelinoides fasciatus preying upon the native isopod Asellus aquaticus. Acta Zoologica Lituanica 17(2):144–150. DOI:10.1080/13921657.2007.10512826.
- Papakostas S, Michaloudi E, Proios K, Brehm M, Verhage L, Rota J, Declerck SA. 2016. Integrative taxonomy recognizes evolutionary units despite widespread mitonuclear discordance: Evidence from a rotifer cryptic species complex. Systematic Biology 65(3):508–524. DOI:10.1093/sysbio/syw016.
- Pastukhov VD. 1993. The Baikal Seal. Novosibirsk: Nauka.
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available: https://www.R-project.org/. Accessed June 2022 15.
- Rosenberg E, Zilber-Rosenberg I. 2018. The hologenome concept of evolution after 10 years. Microbiome 6(1):1–14. DOI:10.1186/s40168-018-0457-9.
- Šarić I, Klobučar G, Podnar M, Štambuk A, Maguire I. 2018. Molecular phylogeny of branchiobdellidans (Annelida: Clitellata) and their host–epibiont association with Austropotamobius freshwater crayfish. Invertebrate Systematics 32(1):55–68. DOI:10.1071/IS17028.
- Schlick-Steiner BC, Steiner FM, Seifert B, Stauffer C, Christian E, Crozier RH. 2010. Integrative taxonomy: A multisource approach to exploring biodiversity. Annual Review of Entomology 55:421–438. DOI:10.1146/annurev-ento-112408-085432.
- Segers H. 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 1564(1):1–104. DOI:10.11646/zootaxa.1564.1.1.
- Sherbakov DY. 1999. Molecular phylogenetic studies on the origin of biodiversity in Lake Baikal. Trends in Ecology and Evolution 14(3):92–95. DOI:10.1016/S0169-5347(98)01543-2.
- Sheveleva NG, Pomazkova GI, Melnik NG. 1995. Eco-taxonomical review of Rotatoria, Cladocera, Calanoida and Cyclopoida of Lake Baikal. Japanese Journal of Limnology 56(1):49–62.
- Sideleva VG. 2003. The endemic fishes of Lake Baikal. Leiden: Backhuys. pp. 270.
- Steinberg PD, De Nys R, Kjelleberg S. 2002. Chemical cues for surface colonization. Journal of Chemical Ecology 28:1935–1951. DOI:10.1023/A:1020789625989.
- Takhteev VV. 2000. Trends in the evolution of Baikal amphipods and evolutionary parallels with some marinemalacostracan faunas. Advances in Ecological Research 31:197–220.
- Takhteev VV, Didorenko DI. 2015. Fauna and ecology of amphipods of Lake Baikal: A training manual. Irkutsk: VB Sochava Institute of Geography SB RAS. pp. 116.
- Timoshkin OA. 2001. Index of animal species inhabiting Lake Baikal and its catchment area. Novosibirsk: Nauka.
- Timoshkin OA. 2010-2011. Main tendencies in research of ancient lake biodiversity: Most interesting recent discoveries in biodiversity of Lake Baikal. In: Timoshkin OA, editor. Index of anima species, inhabiting Lake Baikal and its catchment area. 2 Basins and channels in the South of East Siberia and North Mongolia, Vol. 2, Lake Baikal Book 2. Novosibirsk: Nauka. pp. 1421–1428.
- Timoshkin OA, Lukhnev AG, Zaytseva EP. 2010. First data on the endemic fauna of Turbellaria Proseriata (Platyhelminthes, Otomesostomidae) from Lake Baikal. Biology Bulletin 37(9):861–875. DOI:10.1134/S1062359010090013.
- Vadeboncoeur Y, McIntyre PB, Vander Zanden MJ. 2011. Borders of biodiversity: Life at the edge of the world’s large lakes. BioScience 61(7):526–537. DOI:10.1525/bio.2011.61.7.7.
- Wahl M, Mark O. 1999. The predominantly facultative nature of epibiosis: Experimental and observational evidence. Marine Ecology Progress Series 187:59–66.
- Yankowski AW. 1982. New genera of symbiothic protozoans of Baikal fauna. News on Baikal Fauna. Novosibirsk: Nauka. pp. 25–32.
