Abstract
We tested the climbing ability of two native Hungarian rodent species under laboratory conditions. One mouse species is the mound-building mouse (Mus spicilegus), which is associated with flat, arable environments; the other is the house mouse (Mus musculus), which occurs in many natural and artificial environments following human settlements. We sought answers to the following questions, whether each of these species can use the vertical dimension and whether there is a difference in climbing ability between individuals of different ages of the two species. From our results, we concluded that the climbing ability of mound-building mice is much lower than that of house mice, but young mound-building mice show a much greater inclination towards climbing than adult mound-building mice.
Introduction
Species belonging to the order of rodents are found worldwide, so they are excellently adapted to many environmental and topographical conditions. Some species are strongly tied to the ground, such as Guinea pigs and gerbils. In contrast, others have adapted to rocky environments, such as gundi species, and others to an arboreal lifestyle (squirrels) (Le Berre & Le Guelte Citation1993).
Homer’s (Citation1954) hypothesis is that the ability to climb is greatly influenced by the environmental conditions of the given species’ habitat. Field studies have shown that the subspecies of American wild deer mice (Peromyscus maniculatus) living in forested areas have better climbing ability (Smartt Citation1978; Holbrook Citation1979a Citation1979b) and regularly climb trees and shrubs, whereas desert and lowland deer mouse subspecies do not have climbing ability skills.
Thompson (Citation1990) studied two subspecies of the deer mouse (P.m. merram and P.m. sonoriensis). The morphology of the two subspecies is very similar, and they can be distinguished based on the color of the fur (Osgood Citation1909; Dice Citation1938). In laboratory conditions, it has been shown that the subspecies living in mountainous, forested areas have better climbing ability than individuals of the lowland subspecies (Thompson Citation1990).
Also belonging to the Peromyscus genus, the Florida mouse (Peromyscus floridianus) and the cotton mouse (Peromyscus gossypinus) are species found in the southeastern part of the United States of America, but with significantly different habitats. While the Florida mouse is a species limited to lowland, desert habitats (Hall & Kelson Citation1959; Layne Citation1963), the cotton mouse is widespread (Hall & Kelson Citation1959) and a well-adapted species showing excellent habitat tolerance (Hamilton Citation1943). Laboratory studies have shown that the cottonwood mouse living in woody habitats has the better climbing ability and longer tails than the Florida mouse found in flatlands (Layne Citation1970).
The results of Layne’s (Citation1970) studies are consistent with the hypothesis that spatial changes of natural selection affect behavior and morphology. The mouse species studied in forested areas have the better climbing ability and longer tails than their flatland counterparts.
The adaptation to the natural environment is also clearly visible in the examination of the desert rodent species living in the Sahara; those species that live in a more complex habitat, where there are trees and shrubs, have a better climbing ability compared to those species whose habitats are less diverse and whose vegetation is poorer (Le Berre & Le Guelte Citation1993). In his experiment on African rodents, Earl and Nel (Citation1976) monitored climbing ability as well as time to first climb and time spent on the climbing apparatus. As a result, he found that the species with the longest tail length (Thallomys paedulcus) showed a greater willingness to climb and spent more time on the climbing equipment than the two species with shorter tails (Praomys natalensis and Saccostomus campestris).
Two species of the Mus genus are found natively in Hungary, the house mouse (Mus musculus) and the mound-building mouse (Mus spicilegus). The mound-building mouse and the house mouse morphology are very similar (Demeter et al. Citation1995). In live conditions, during field tests, the two species can be safely separated based on tail diameter and relative tail length. The tail of house mice is thicker and longer in relation to their body than that of mound-building mice (Bárdos et al. Citation2022). The habitats of the two species differ significantly; the mound-building mouse occurs in lowland, agricultural areas, prefer abandoned fields, and avoids human settlements (Sokolov et al. Citation1998; Bihari Citation2004); on the other hand, the house mouse lives with an anthropogenic food source common species, extremely well-adapted and showing wide habitat tolerance.
In our study, we sought answers to the questions of whether there is a difference in the climbing ability of Hungarian mouse species with different habitat needs and morphology and whether there are differences in the climbing ability in the age groups within the species.
Materials and methods
The experiments were performed in the rodent house of the Hungarian University of Agriculture and Life Sciences (MATE) Kaposvár Campus. The university has its own mouse breed, where individuals of known ages, sexes, and backgrounds are kept. The current stock comes from several wild populations captured from different parts of the country. These studies were performed on laboratory-born offspring of wild animals. For the behavior test, 22 juvenile mound-building mice, 22 adult mound-building mice, 22 juvenile house mice, and 22 adult house mice were selected and both males and females were included in the test in equal proportions. Housing: Animals were housed in standard laboratory plastic rodent boxes at standard laboratory temperatures of 20–22°C. For littering, purified wood chips (LIGNOCELL J. Rettenmaier & Söhne GmbH) were used, complete rodent feed (SAFE® 132 Laboratory mouse feed) and water were available to the animals ad libitum.
The tools for the behavioral test were made by ourselves, which consists of a 27 cm high, 0,9 cm diameter rod that closes the 90-degree text with a 10 cm diameter round foot. Both the climbing stick and the sole were made of untreated wood, none of which were sanded smooth. Five climbing rods were used for the test in a circular plastic container with a diameter of 65 cm and a height of 55 cm. The bars were placed in the same shape for all mice ().
The selected mice were placed in the container one by one, and after each mouse, the storage and climbing rods were wiped with an alcohol swab so that the odor of each mouse did not affect the subsequent mice in the test. The total duration of the test is 15 minutes, of which 10 minutes is the “rest period”, which is used to allow the mouse to get used to and discover its new environment, and after the end of 10 minutes, a test time of 5 minutes was recorded. Whether a mouse climbed on the rod in 5 minutes or not, if it climbed, how many times it climbed, and in what seconds it was the first climb (). In addition, it was recorded during the climb whether or not the individual used its tail to climb. Mice were grouped by species and age group. Young mice were selected from 28 to 35 days of age while adult mice from around 500 days. Male and female mice used both for the test.
Statistical analysis
In the study, the duration of the first successful climb was analysed separately for the breed and age using the Survival function (Kaplan & Meier Citation1958) where the survival function (S (t)) estimates the probability (P) that the object of the experiment survives longer (T) than some specified time (t) in this study it means that the animal did not yet manage to climb the rod. Significant differences between the various survival function curves were determined by applying the Log-rank test (Kaplan & Meier Citation1958). Besides, the effects of breed and age on the duration of the first successful climb was also analysed applying Cox Proportional Hazard model (Cox Citation1972) (also called as Cox Regression) where the hazard is the probability that after a certain period the event would occur during the next time unit (i.e. the successful climb will occur in the next second). The Cox regression will provide the regression coefficients (b) for the breed and for the age and raising the coefficients to the power of the exponential constant (cca. 2.718) will provide the hazard ratio or the relative hazard.
The hazard ratio is constant for the whole examination period (5 minutes in this case). In the present study, the relative hazard shows the ratio of the probabilities that during the next unit of time a successful climb will occur in the examined breeds and in age groups, respectively (Woodward Citation2014). These statistical analyses were performed using the SAS 9.4 software package applying the LIFEREG procedures.
Results
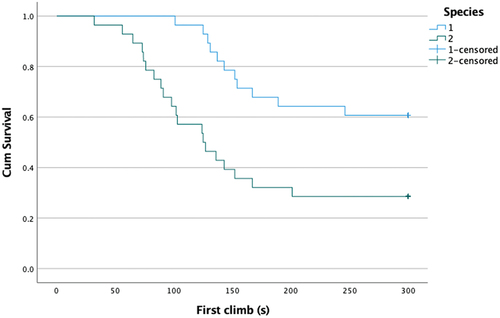
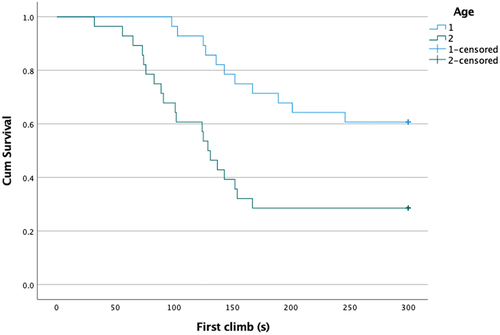
The curves of the survival function for different species and age groups are shown in and . The lower curves of a species or age group indicate that they climb the rod at a higher rate compared to animals of the other group. Based on the log-rank test, species and age also showed a significant difference (p < 0.05). () However, no significant difference was found between the sexes (p = 0.37).
shows that it took 101 seconds for the group of mound-building mice (group 1) to climb for the first time, while for house mice (group 2), the first climb took place in the 32nd second.
Among the mound-building mouse, 28 individuals did not climb the rod, which means that more than 63% of the mice showed no inclination to climb. In the case of house mice, on the other hand, only 10 individuals showed no inclination to climb during the test, i.e., less than 23% of the house mice did not climb the rod.
shows that both young mound-building mice and house mice (group 2) climbed the rod earlier than the adults (group 1). Furthermore, 25 of the adult mice did not climb the rod during the test, so more than 56% of the adult mice showed no inclination to climb. Among the young group, 11 individuals did not climb the rod, which means only 25% of the juvenile mice.
The Cox regression parameters estimated by the PHREG procedure are presented in .
Table I. Maximum Likelihood estimates of Cox regression coefficients (b) and Hazard ratios examining the effects of species and age of mice on the duration of their first successful climbing.
The estimated Cox regression coefficients were found to be significant, indicating a significant effect of species and age on the investigated trait. Based on the estimated hazard ratios, the probability that climbing occurs in the next time unit is four times more likely to occur in house mice than in mound-building mice, and three times more likely to occur in juvenile mice than in adults.
Discussion
The present study revealed that house mice, both adults and juveniles, show a greater tendency to climb than groups of mound-building mice. Furthermore, a difference between young and adult individuals can be discovered within groups of mound-building mice; young mound-building mice show a greater tendency to climb than adult mound-building mice.
We know from our previous studies (Bárdos et al. Citation2022) that the tails of house mice are relatively longer than those of mound-building mice. These results can be well paralleled with Homer’s (Citation1954) studies, according to which the deer mouse (Peromyscus maniculatus) with a longer tail shows a greater tendency to climb than the white-footed mouse (Peromyscus leucopus) with a shorter tail. In the case of the deer mouse, the researchers linked the longer tail and the greater tendency to climb to its habitat since the deer mouse lives in forested areas, where the ability to climb vertically is an advantage. The white-footed mouse, on the other hand, occurs mainly in grasslands, where, according to researchers, it has less need to climb, so its tail is also shorter than that of its forest companion (Homer Citation1954; King Citation1968).
The house mouse is a cohabitant species with a wide geographical distribution (Brown Citation1953) due to its excellent adaptability. Navigating human structures is essential to survival, which makes good climbing skills essential for the house mouse. In contrast to the house mouse, the mound-building mouse is a species associated with agricultural areas (Bihari Citation2004). In lowland agricultural areas covered mainly with herbaceous plants, the mound-building mouse, like the American white-footed mouse and the Florida mouse, which are also species associated with lowland habitats, have less need for good climbing ability due to their habitat (Homer Citation1954; Layne Citation1970).
The same phenomenon can be observed in many other rodent species, that species living in more complex habitats have longer tails and better climbing ability than species living in less vegetated habitats. Thallomys paedulcus, a rodent species native to Africa, showed a greater tendency to climb in an experiment than desert species with shorter tails (Praomys natalensis and Saccostomus campestris) (Earl & Nel Citation1976).
The overwintering strategy of house mice and mound-building mice is significantly different, the house mouse retreats to human structures in autumn (Carlsen Citation1993), but we know of mound-building mice that in autumn, several individuals build common mounds of earth and plant material (Simeonovska-Nikolova & Gerasimov Citation2000), the plant the filling consists mainly of spikelet inflorescence, which is chewed from the top of the plant.
Mound-building mice are cooperative in a unique way in the Mus genus (Simeonovska-Nikolova & Gerasimov Citation2000), and our studies revealed that within mound-building mice, young individuals have a better climbing ability than adults, so we consider it possible that young mound-building mice climb on the stems of monocots and chew off the spike inflorescence, which the adult mice incorporate into the mound. In order to prove this theory, field observations would be necessary during the construction of mounds by mice.
Institutional Review Board Statement: This research was approved by the Committee on the Ethics of Animal Experiments of Hungarian University of Agriculture and Life Sciences Kaposvár Campus (permit number: MATE KC MÁB/20-2/2022). The authors declare that all experiments were performed in accordance with approved guidelines and regulations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bárdos B, Kovács B, István N, Altbäcker V. 2022. In vivo classification of two closely related species of mice, mound-building mouse (Mus spicilegus) and house mouse (Mus musculus). Acta Agraria Kaposváriensis 26(1):27–35. DOI:10.31914/aak.2656.
- Bihari Z. 2004. A güzüegér (Mus spicilegus) életmódjának sajátságai és mezőgazdasági jelentősége. Növényvédelem 40:245–250.
- Brown RZ. 1953. Social behavior, reproduction, and population changes in the house mouse (Mus musculus L.). Ecological Monographs 23(3):218–240. DOI:10.2307/1943592.
- Carlsen M. 1993. Migrations of Mus musculus musculus in Danish farmland. Zeitschrift für Saugetierkunde 58:172–180.
- Cox DR. 1972. Regression Models and Life Tables. Journal of the Royal Statistical Society 34:187–220.
- Demeter A, Rácz G, Csorba G. 1995. Identification of house mice (Mus musculus) and mound-building mice (Mus spicilegus) using distance and landmark data. In: Marcus LF, Corti M, Loy A, Naylor G, Slice DE, editors. Advances in Morphometrics. New York: Plenum Press. pp. 359–369.
- Dice LR. 1938. Variation in nine stocks of the deermouse, Peromyscus maniculatus, from Arizona. Occasional Papers of the Museum of Zoology 375:1–19.
- Earl Z, Nel JAJ. 1976. Climbing behaviour in three African rodent species. African Zoology 11(1):183–192. DOI:10.1080/00445096.1976.11447523.
- Hall E, Kelson KR. 1959. The mammals of North America. Vol. 2. New York: Ronald Press.
- Hamilton WJ. 1943. The mammals of eastern United States. Ithaca, New York: Comstock Publishing Company. pp. 432.
- Holbrook SJ. 1979a. Habitat utilization, competitive interactions, and coexistence of three species of Cricetine rodents in east-central Arizona. Ecology 60(4):758–769. DOI:10.2307/1936613.
- Holbrook SJ. 1979b. Vegetational affinities, arboreal activity, and coexistence of three species of rodents. Journal of Mammalogy 60(3):528–542. DOI:10.2307/1380093.
- Homer BE. 1954. Arboreal adaptations of Peromyscus with special reference to use of the tail. Contributions from the Laboratory of Vertebrate Biology of the University of Michigan 61:1–84.
- Kaplan EL, Meier P. 1958. Nonparametric Estimation from Incomplete observations. Journal of the American Statistical Association 53(282):457–481. DOI:10.1080/01621459.1958.10501452.
- King JA. 1968. Biology of Peromyscus (Rodentia). Vol. 2. New York: American Society of Mammalogists.
- Layne JN. 1963. A study of the parasites of the Florida mouse, Peromyscus floridanus, in relation to host and environmental factors.
- Layne JN. 1970. Climbing behavior of Peromyscus floridanus and Peromyscus gossypinus. Journal of Mammalogy 51(3):580–591. DOI:10.2307/1378397.
- Le Berre M, Le Guelte L. 1993. Climbing abilities in four species of desert rodents. Tropical Zoology 6(2):237–241. DOI:10.1080/03946975.1993.10539224.
- Osgood WH. 1909. Revision of the mice of the American genus Peromyscus. Washington: Bureau of Biological Survey, North American Fauna 28:1–285.
- Simeonovska-Nikolova D, Gerasimov S. 2000. Seasonal changes of some population characteristics of Mus spicilegus Petenyi in North Bulgaria. Acta Zoologica Bulgarica 52:81–90.
- Smartt RA. 1978. A comparison of ecological and morphological overlap in a Peromyscus community. Ecology 59(2):216–220. DOI:10.2307/1936365.
- Sokolov VE, Kotenkova EV, Michailenko AG. 1998. Mus spicilegus. Mammalian Species 592(592):1–6. DOI:10.2307/3504484.
- Thompson DB. 1990. Different spatial scales of adaptation in the climbing behavior of Peromyscus maniculatus: Geographic variation, natural selection, and gene flow. Evolution 44(4):952–965.
- Woodward M. 2014. Epidemiology, study design and data analysis. 3rd ed. New York: CRC Press.