Abstract
The gradual conversion of forest stands to single-aged and single-species stands is resulting in the loss of natural roosts for many animal groups. The installation of bat boxes is one solution to compensate for the lack of natural roosting opportunities. The box models differ in their design and material, which to some extent can determine their suitability for bats. We investigated the occupancy of 187 boxes made of wood, ceramic and two sizes concrete with styrofoam; and the intra-seasonal (spring migration, breeding, mating/migration) thermal profile for each type of box. The environment of the boxes was defined by parameters that could directly affect the box’s thermal conditions (solar energy availability), or be related to sociality (distances, obstacles) or food availability (edge of forest, water). The box occupancy depended on the box type and the season: styrofoam-concrete boxes were preferred, with a higher occupancy during the mating and migration period (>75%), whereas the highest species richness occurred in ceramic boxes. Box types also differed significantly in their diurnal thermal profiles: the wooden boxes had inside temperatures similar to outside the box, whereas the styrofoam-concrete and with expanded clay aggregate (ceramic type) averaged 2°C higher. Overall, we found 6 bat species, although we concentrated our analysis on the most common species: Pipistrellus nathusii (88%). For this species, none of the parameters that could affect the box thermal condition had an impact on the occupancy, whereas the presence of obstacles and the distance to a water body, proved to be important. To ascertain that our results may be valid and be the result of differences in the box thermal properties, we tested the relationship of the box occupancy to the latitude, using data available in the literature. The occupancy of wooden boxes from the latter dataset significantly decreases with latitude, whereas for the concrete (with addition of sawdust or styrofoam) it increases, although this relationship is not significant and requires a larger sample.
Introduction
Despite the relatively high and slightly increasing forest cover ratio in the temperate zone of central Europe, around three-quarters of forests in the EU were reported as even-aged, of which only 14% had reached the mature phase (European Commission Citation2022). The increase in managed forest area comes at the expense of older stands – these are replaced by single-species and even-aged stands: in the temperate zone, managed forests are mainly conifer monocultures (European Commission Citation2022). This results in a substantial loss of biodiversity in plant, fungi and animal assemblages (Kirby et al. Citation1991). In the case of animals, species richness in the forest environment can be shaped not only by the diversity of trophic niches but also by the availability of different types of shelters. The abundance of natural cavities is often related to a forest’s age, the species composition of the trees and the management practices. Tree hollows and cavities are keystone microhabitats, used by a broad range of fauna for shelter and breeding (Larrieu et al. Citation2018). With the clearing of large, old trees or conversion of forest stands, the proportion of trees offering hollows decreases (Vesk et al. Citation2008). Cavity development through natural processes is slow, with cavity-bearing tree recruitment likely to take even more than 100 years (Andersson et al. Citation2018). Loss of natural shelters is one of the threats affecting a wide range of species, from invertebrates (Quinto et al. Citation2014), to birds (Van der Hoek et al. Citation2017), to mammals (Swihart et al. Citation2003). Within the latter group, bats constitute a significant element (Kunz et al. Citation2003).
Insectivorous bats are important pest consumers in forests: they prey on nocturnal invertebrates – inaccessible to other predators, which is of particular importance, both ecologically and economically, especially in managed forests (Charbonnier et al. Citation2014). In temperate zones, bats use tree hollows mostly during the active season as shelters for breeding, mating or as daily roosts (Ruczyński et al. Citation2010), but sometimes they are also found during hibernation, especially in areas with milder winters (Turbill & Geiser Citation2008; Gottfried et al. Citation2019). The availability of sufficient shelters may be crucial for the functioning of the population and its survival in the long term also due to the limited and interspecific detection of hiding places by bats (Ruczyński et al. Citation2007, Citation2009). The problem concerns in particular the “forest” bat species, especially those forming fission-fusion societies which use a number of shelters and change them regularly, and which therefore require a significant surplus of shelters (Kerth & König Citation1999; Kerth et al. Citation2006). The lack of shelters may result negatively in bats’ numbers, e.g. activity of bats is a few-times higher in old-growth stands, despite having the same level of feeding activity (Thomas Citation1988). Consequently, artificial shelters that mimic natural cavities – nest or roost boxes – are often used as a solution to compensate for the shortage of microhabitats for wildlife in human-disturbed landscapes where natural tree cavities have been depleted. The installation of boxes has led to positive conservation outcomes both for birds (Berthier et al. Citation2012) and for bats (Mayle Citation1990; Kowalski & Lesiński Citation1994). For several decades, bat boxes have been used in many countries, predominantly in temperate regions (see review: Rueegger Citation2016). The bat boxes can be used by bats as maternity roosts (Baranauskas Citation2009), during migration in spring (Petersons Citation2004), and in autumn (Jarzembowski Citation2003), and during mating (Rachwald Citation1992; Kasprzyk & Ruczyński Citation2001; Ciechanowski & Jarzembowski Citation2004). Although rarely observed, it is also possible to use the boxes even as winter shelters (Voigt et al. Citation2014). Whereas composed of a range of different box sizes and shapes, usually two types of material are used for the construction of the boxes: timber or plywood (mainly rectangular-shaped: Issel, Stebbings or Stratmann models); alternatively clay or cement mixed with various additives, such as sawdust, polystyrene or even rice chaff (mostly cylindrical-shaped) is used. These two main type of construction materials differ in their heat conductivity and capacity (Graczyk Citation1967; Bideguren et al. Citation2019; Brouwer & Henrard Citation2020), and therefore – their internal temperature, which is considered one of the most important selection criteria for bats (Kerth et al. Citation2001; Lourenço & Palmeirim Citation2004). Since bats are heterothermic mammals, temperature is one of the most important factors directly affecting phenology, feeding and reproduction (Dietz & Hörig Citation2011). Warm boxes might be preferred during pregnancy and the lactation period by females in temperate zones (Kerth et al. Citation2001), but above temperature 40°C rather avoided in the Mediterranean zone due to the detrimental effects of overheating (e.g. dehydration, heat stroke, or death) (Lourenço & Palmeirim Citation2004). The temperature inside the box can be affected not only by its construction material, but also by the colour of the box surface (Rhodes & Jones Citation2011), exposure to sunlight (Kerth et al. Citation2001), land cover (López-Baucells et al. Citation2017), or structures of the immediate environment, including the spatial structure of the tree crown; however, empirical data on these factors are not numerous (see: Rueegger Citation2016). Potentially, the same box type used on sites with different environmental conditions can unequally affect the roost microclimate, and, in consequence, the energy budget of individuals. Moreover, the problem of the diurnal temperature difference, between night and day, is often ignored when considering the thermal properties, whereas the box is a resting place only during the day and is empty at night (apart from the case of the maternity roost). In fact, it is not only thermal requirements that may be important in the non-random preferences of boxes – there may be also factors directly related to access to other resources, like specific foraging habitats providing abundant, preferred arthropods (Flaquer Citation2006; Froidevaux et al. Citation2021), or even the availability of water to drink, although the latter relationship one may expect to appear mostly in arid regions (Adams & Hayes Citation2008). In turn, the position of the box entrance above the ground level might be important as a factor that helps bats escape from predators (Boonman Citation2000). However, the vertical distribution of natural tree roosts is significantly wider than for artificial ones, such as boxes, and there is no clear empirical evidence that bat boxes need to be installed over a specific height (Pschonny et al. Citation2022).
The suitable placement of box installation within the landscape and species preferences are still under investigation, and, depending on the authors, also their recommended installation: whether along forest edges or pathways, in the forest interior or even near water (Long et al. Citation2006), which is more or less linked to access to food resources. In the temperate zone in Europe, the most common bat species inhabiting bat boxes comes from five genus: Myotis, Pipistrellus, Nyctalus, Plecotus and Barbastella (e.g. Lesiński et al. Citation2009; Rachwald et al. Citation2018; Wojtaszyn et al. Citation2021; Pschonny et al. Citation2022). Species-specific preferences for bat-box models are subtle, and to date no comprehensive analysis has been made (see Rueegger Citation2016). Until now, there has been a lack of explicitly studied underlying causes influencing the selection of some box models over others.
Although the managed pine forests of central Europe appear to be homogeneous – trees planted at equal distances result in equal crowns – the occupation of boxes, both between and within models, varies. Therefore, managed forests, with a uniform and repeatable spatial structure, provide an excellent opportunity to test hypotheses about the influence of biotic and abiotic factors on bat box preference. In this article, we test whether the difference in occupation results from the type of the box (affecting the thermal condition) or environmental factors around the shelter (affecting the access to the roost and the availability of food and water) in different phenological periods.
Material and methods
Study area
The study was conducted in Wdzydzki Landscape Park (WLP, Southern Pomerania Lakeland, northern Poland). The WLP covers an area of 1,783 ha, where 64% is covered by forests, 11% is water, 10% is arable land and the remaining 13% is for other types of use (e.g. built-up areas). The elevation varied from 140 to 160 m a.s.l., with large, sandy outwash plains and numerous postglacial lakes being predominant elements of the landscape. The climate is a humid continental one, a warm summer subtype (temperate), characterized by a late and short summer (65 days) with only 17 days with temperatures above 25°C, whereas the winter is long (92 days) with 73 days of snow cover (Woś Citation1996). The coldest month is January, with an average temperature of −2.1°C, and the warmest are July and August, with an average temperature of 16.8°C and 16.6°C, respectively. Scots pine Pinus sylvestris covers over 95% of the forested area, forming intensively managed, even-aged monocultures that represent mainly two communities: suboceanic fresh coniferous forest Leucobryo-Pinetum and dry non-coastal European Scots pine forest with lichens Cladonio-Pinetum (Leuschner and Ellenberg Citation2017). The average age of the stand during our research varies between 49 and 53 years and this age is systematically growing, but young stands are still dominant. Old trees, over 90 years old, occupy less than 5% of the area.
The bat-boxes
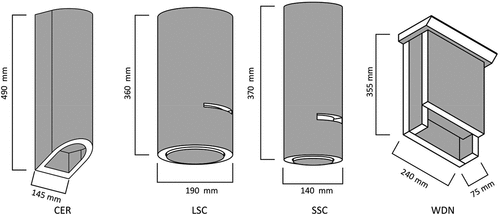
Boxes were installed in 2011 by the WLP employers for bat conservation purposes, mostly in the central part of the Landscape Park. Four bat-box types were placed: i) ceramic CER – half-cylindrical, made of concrete with an expanded clay aggregate (beige colour, dimensions of entrance: 10 cm × 1.5 cm; internal volume: 2500 cm3, mass: 7.2 kg); ii) large styrofoam-concrete LSC – cylindrical, made of a mix of concrete and styrofoam (brown colour, dimensions of entrance 10 cm × 1.5 cm; internal volume: 6000 cm3, mass: 6.5 kg); iii) small styrofoam-concrete SSC – cylindrical, made of a mix of concrete and styrofoam (brown colour, dimensions of entrance 10 cm × 1.5 cm; internal volume: 2750 cm3, mass: 4.6 kg); iv) wooden WDN – cubic, made of wood (non-painted wood, dimensions of entrance 20 cm × 2 cm, and with a 7 cm vertical board under the entrance; internal volume: 2500 cm3, mass: 4.0 kg). This type of bat box is most similar to the Issel model (Issel & Issel Citation1955) ().
Figure 1. Types of the bat boxes: ceramic (CER), large styrofoam-concrete (LSC), small styrofoam-concrete (SSC), wooden (WDN).
The boxes were hung in small groups: 4 up to 19 boxes (one group of 33 boxes) consisting of different types of boxes, at a height from 1.9 m to 3.4 m (mean 2.6 m). The average distance between box plots was 1863 ± 298 m (95%CI), while the average distances between boxes in the study plots were 26 ± 3 m (95%CI). All 187 bat-boxes, 46 CER, 41 LSC, 81 SSC and 19 WDN, were placed in pine stands, and the average age of the divisions (subareas) of the forest was 85 years (35–119, SD = 26) (The Forest Data Bank Citation2015).
Bat survey
The study was conducted in the third year after bat box installation, during the two next seasons: 2013 and 2014. To avoid the impact of manipulation on box occupancy, each box was inspected three times a year, during the following phenological periods: i) spring migration of bats, May (16–17.05.2013 and 5–6.05.2014; due to a cold April and in early May 2013, the first inspection in that year was carried out later); ii) nursing period: nursery colonies and lactation – beginning of July (6–7.07.2013 and 8–9.07.2014); iii) time of migration and mating season – the second part of August (22–23.08.2013, 24–26.08.2014). During every visit, the number of individuals and the species, their sex and the reproductive status of the bats were recorded. Only boxes with bats present inside during visits were considered as occupied, not those in which exclusive droppings were found. For analyses, the data of the 2 years were combined together. All contact with the bats was undertaken under permit from the Polish Regional Directorate of Environmental Protection in Gdańsk (RDOŚ-Gd-PNII.6401.58.2013.EK.1).
Thermal characteristics of the bat boxes
To describe the thermal characteristics of the bat-boxes, we used i-Button loggers (DS1921G-F5, Thermochron iButton, Maxim Integrated Products, USA, accuracy ±1 ◦C, resolution 0.5◦C). From each type of box, four boxes placed in a similar environment/surroundings were selected – making a total of 16 boxes. In each of these, a temperature logger was installed inside using a dedicated holder on a wire, in the middle of the box – without contact with the box walls and potentially bats (Tint) – and outside of the boxes in shadow, for recording the external temperature (Text). Temperatures were recorded during 2014 between 6 May and 26 August, with a sample rate of 60 min. Because the phenological periods are greatly varied in temperature, we used 14 days of every period to detect differences among the bat box models. Two-week periods were analysed, divided by seasons: i) spring migration – 2 weeks after 1st inspection; nursing period – 1 week before and 1 week after 2nd inspection; and migration and mating season – 2 weeks before 3rd inspection. The difference between the inside and outside temperature of each bat-box was calculated as the thermal capacity: Tcap (Tint – Text). These parameters show how much the temperature inside the box deviates from the ambient temperature: negative values indicate a lower temperature inside the box than outside, whereas positive values indicate a higher temperature than that prevailing in the surroundings. Due to the potentially different functions of the box: during the day as a resting shelter, and at night as a temporary or mating shelter, data were divided into these two periods. Temperature obtained from the nearest weather-station: Starogard Gdański (40 km to the east, ID: 253180030) was used to test differences between the Text and ambient temperature outside the forest.
Forest composition and structure
Although the forest community in which the bat boxes are installed is described as relatively uniform, we first tested whether the species composition in the subdivisions where the boxes are hung is homogeneous. We examined the proportion of each tree and shrub species in each layer: the undergrowth layer (up to 4 m), the understory layer (higher than the undergrowth, composed of trees of the same species as the tree layer but younger, not yet high enough as a tree layer) and the tree layer. The species of trees and shrubs found in the studied divisions were Scotch pine Pinus sylvestris, silver birch Betula pendula, European spruce Picea abies, European larch Larix decidua, common alder Alnus glutinosa, common oak Quercus robur, and European beech Fagus sylvatica; and the shrubs were common juniper Juniperus communis, alder buckthorn Frangula alnus, bird cherry Prunus padus, willow Salix sp., and rowan Sorbus aucuparia (The Forest Data Bank Citation2015). To test the homogeneity of the species composition between parts of the forest with different types of bat-boxes, Principal Components Analyses (PCA) were used. A one-way ANOVA of PC1 loadings showed significant differences between the box types (F = 3.315, DF = 3, 183; p-value: 0.0212), but these differences explained only 3.6% of the variation (adjusted R2 = 0.036), hence the forest communities were considered as homogeneous, and were omitted from further analyses.
The forest structure was described by the following parameters, corresponding to factors that potentially affect the bat box preferences: i) bat-box thermal conditions (= factors that affect solar access): height of the entrance above the ground (m), orientation of the entrance towards cardinal directions (in degrees, accuracy 45°), canopy cover above the bat-box location within a 10 m radius classified in one of five categories (none, <25%, 25–50%, 50–75%, >75), presence of obstacles (branches, undergrowth) in front of the entrance (45° to the right and left from the entrance) within a radius of 10 m (similarly categories as canopy cover; also an obstacle to accessing the box), distance to the nearest tree in front of the bat-box (45° to the right and left from the entrance; m); ii) competition or social interaction: distance to the nearest box (accuracy 0.1 m) as potentially alternative shelter, iii) foraging habitats and water resources: distances to the edge of the forest and to the nearest water body: a lake, a pond or a river. Spatial data: distance to the edge of the forest and distance to water bodies was taken from an orthophotograph (Open Geospatial Consortium – OGC).
Statistical analysis
The Generalised Linear Model (GLM) with binomial error distribution was used for testing whether a bat was present within a bat-box (occupied, non-occupied), in relation to the box type (WDN, CER, SSC, LSC) and the phenological period (spring, summer, migration). In addition, for the mating period, the average size of the groupings inhabiting the studied box types (GLM), and their sex-ratio (χ2) were tested. To test for which of the forest structure parameters modelled bats abundance (our case study: P. nathusii) in each of phenological periods we used GLM separately. To compare the circadian daily temperature profiles (Tcap = Tint-Text) and evaluate the differences among the bat-box models of each period (spring, summer, and migration), we first applied the PCA method for the data reduction: to reduce the diurnal temperature pattern (24 measurements), to a single point. Then, the resulting loadings were tested using linear methods. PCA output, PC1, was analysed using GLM. To test the bats occupancy (percentage) vs latitude, we used linear regression. All the analyses were carried out using RStudio, version 4.1.0 (R Core Team Citation2020).
Results
Species composition
During the 2 years of research, a total of 735 individuals, belonging to seven bat species, were recorded in the bat boxes. The most common species was Nathusius’ pipistrelle Pipistrellus nathusii (88.8% of recorded individuals), followed by the brown long-eared bat Plecotus auritus (4.8%), the greater mouse-eared bat Myotis myotis (4.2%), the common noctule Nyctalus noctula (1.8%), and solitary individuals: soprano pipistrelle P. pygmaeus, Daubenton’s bat M. daubentonii and pond bat M. dasycneme. In the CER model we noted all seven bat species, with the domination of P. nathusii (60.3%), followed by M. myotis, P. auritus, N. noctula with sporadic incidences of P. pygmaeus, M. daubentonii and M. dasycneme. In the remaining types of boxes, a superdomination of P. nathusii (96.4%–99.3%) was noted, with the limited presence of M. myotis (SSC and WDN) or P. auritus (LSC) – . Only single-species aggregation was found, mostly apparent mating groups (P. nathusii and M. myotis), and two maternity colonies: six and ~15 individuals of P. auritus were found in two ceramic boxes.
Table I. Species composition (numbers of individuals) of bats in the studied boxes found during the 2014 and 2015 years of monitoring. Types of the bat boxes: CER – ceramic, LSC – large styrofoam-concrete, SSC – small styrofoam-concrete, WDN – wooden.
Use of bat boxes
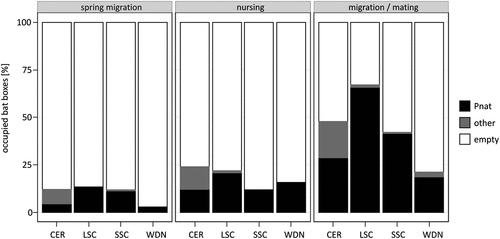
The presence of bats was recorded in 282 cases (25.1%). Box occupancy depended on the box type (χ2 = 23.93, df = 3, 1118, p≪0.00001), and the season of the year (χ2 = 143.5, df = 2,1118, p≪0.00001), with a nearly significant interaction of both factors (χ2 = 10.8, df = 6,1118, p = 0.095). Overall, the most frequently occupied boxes were ceramic (CER) and both styrofoam-concrete – large LSC and small SSC – types, whereas in the wooden boxes (WDN) bats were encountered much less frequently. The lowest occupation was recorded during the spring migration period (11.2%), with no significant differences between the models (χ2 = 4.40, df = 3, 370, p = 0.221). During the nursing period, the proportion of inhabited boxes was slightly higher (17.4%), whereas, in contrast, the SSC box type was used less frequently with nearly significant differences (χ2 = 7.68, df = 3, 370, p = 0.053). The period when the most occupied boxes were found during mating and migration: the second part of August (46.8%). Preferences were similar to those during the spring migration: apparently CER, LSC and SSC were inhabited to a high degree (42–67%), whereas WDN revealed the lowest occupancy (slightly over 21%) in comparison with the other types (χ2 = 26.1, df = 3, 370, p = 0.000009) (). During mating, the mean group size in LSC bat-box (4 ± 2.3), SSC (3 ± 1.8) and CER (4 ± 1.9) boxes was similar and slightly lower in WDN (2 ± 0.8), but not different between box type (two-way ANOVA: F = 1.22, d.f. = 3,185, p = 0.303) or years of study (F = 2.07, d.f.1,185, p = 0.15). In turn, sex-ratio differ between bat box models (χ2 = 8.43, df = 3, p = 0.038): CER = 34 vs 66 (M v F), LSC = 31 vs 69 (MvF), SSC = 35 vs 65 (M v F), WDN = 50 vs 50.
Figure 2. Proportion of bat-boxes used by bats in consecutive research periods. Data from the two years (2013–2014) were combined. Types of the bat boxes: CER – ceramic, LSC – large styrofoam-concrete, SSC – small styrofoam-concrete, WDN – wooden. Pnat – Pipistrellus nathusii, other – remaining bat species, empty – empty boxes.
Thermal characteristics of the bat-boxes
During the spring migration, the nursing period and migration/mating, altogether we recorded 2,784 temperature data points for each of the four bat-boxes types: CER, LSC, SSC and WDN and equally – outside the boxes (during research in 2014). The temperature outside of the boxes varied between 2°C and 17°C, with only a few readings reaching 20°C during the spring migration (mean: 11°C during the day, 8°C during the night), and between 12°C and 20°C, with a marginal share of measurements reaching 30°C in summer (mean: 21°C during the day, 18°C during the night) and between 5°C and 20°C, with a marginal share of measurements reaching 23°C in migration (mean: 15°C during the day, 12°C during the night). At corresponding time intervals, the average temperature from the bat box locations was 1.4°C lower than the data from the nearest weather station (average = 1.4 ± 2.4SD), with no differences between periods (ANOVA: d.f. = 2, 11,133, F = 1.895, p = 0.15).
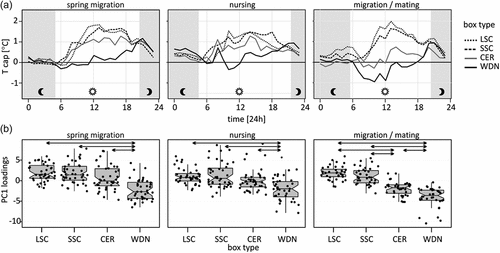
Temperatures inside of different bat-box models oscillated on average from Text within −1°C ÷ +2°C: the night temperature revealed a similar pattern in all models, from almost no differences in spring with a similar pattern in WDN and CER, following the slightly warmer SSC and warm LSC (). The daily temperature (sunlight time) differentiated the types of boxes much more: WDN heats up much slower (spring, summer), or is even cooler than Text (migration/mating), CER heats up to an average of 1°C above Text (spring, summer), whereas in migration it is just above Text. The two styrofoam-concrete boxes, LSC and SCC, showed a similar diurnal temperature pattern: they warm up fastest and the deviation from Ta reaches on average even 2°C. The circadian pattern of Tcap fully supports the differences among the box models (PCA loadings): WDN was different from the remaining boxes during the spring (ANOVA: F = 23.53, df = 3, 156, p≪0.0001) and during the summer (ANOVA: F = 12.97, df = 3, 156, p≪0.0001), whereas during migration WDN and CER were different both from LSC and SCC (ANOVA: F = 69.83, df = 3, 140, p≪0.0001). The differences among the bat-box models explained 29.8% of the variances in temperature during the spring migration, 18.4% during breeding and 59.1% during migration and mating.
Figure 3. Average temperature capacity Tcap bat-box for each box type by period (a), PC1 loadings of temperature daily pattern in four types of bat-boxes during (b). Arrows – results of significant post-hoc Tukey’s pairwise comparisons (p < 0.05). Types of the bat boxes: CER – ceramic, LSC – large styrofoam-concrete, SSC – small styrofoam-concrete, WDN – wooden.
Analysing the factors explaining the numbers of P. nathusii in boxes: 2.7% were in spring (F = 0.588, d.f. = 11, 175, p = 0.860), 4.7% in summer (F = 1.84, d.f. = 11, 175, p = 0.0503), and 15% in the migration period (F = 4.01, d.f. = 11, 175, p = 0.00003) (). The abundance of the P. nathusii strongly depended on the box model, but none of the factors potentially modelling the thermal conditions of the boxes has a significant effect. Similarly, the distance between boxes – the potential effect of competition or sociality – did not have any significance as well. In contrast, two factors were found to be significant: i) less obstacles in front of the entrance to the box showed a higher occupation; in turn, accessibility to the box, ii) the most preferred boxes were those ones near water resources – a factor related to food availability () ().
Figure 4. Relationship of the number of Nathusius’ Pipistrelle (Pipistrellus nathusii) inhabiting a particular type of box in spring migration, breeding and mating/migration periods, depending on the distance from the water reservoir. Line - linear model fitted to the data: black - LSC, grey - SSC. Types of the bat boxes: CER – ceramic, LSC – large styrofoam-concrete, SSC – small styrofoam-concrete, WDN – wooden.
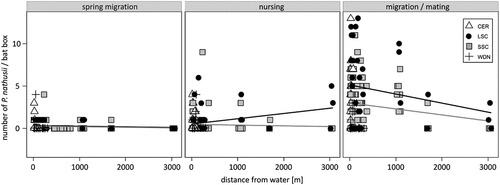
Table II. Results of the GLM explaining the P. nathusii numbers in relation to structure of forest characteristic in Wdzydzki LP, Poland, in the years 2013–2014. Factors: type – box design and material (CER – ceramic, SSC – small styrofoam-concrete, WDN – wooden), tree_front - distance to the nearest tree in front of the bat-box, canopy - canopy cover above the bat-box location, obstacles – presence of obstacles (branches, undergrowth) in front of the entrance, entr_orient – orientation of the entrance towards cardinal directions, edge – distance to the edge of the forest, water – distance to the nearest lake, pond or a river, box_near – the shortest distance to the nearest box. See material and methods section for details.
Discussion
This study is the first under natural conditions to show thermal differences between the three most common types of materials from which the bat boxes are constructed: wood, ceramics and styro-foam. Our results suggest that in managed forests with a homogeneous structure, bats clearly prefer two types of boxes, rather than select external conditions that can indirectly affect the thermal properties of the boxes. Bats – in this case P. nathusii – selected box localities close to water, and with a lower density of obstacles. The box occupation rate in WLP is exceptionally high (up to 70%) and could be an effect of young stands (an effect of lack of natural shelters like standing dead trees with tree hollows). This appears to confirm already known, negative relationship between the abundance of dead wood, treated as a proxy for availability of natural roosts, and occupation of bat boxes by N. noctula (Printz et al. Citation2021).
In the European temperate zone, bat boxes are mostly inhabited by typical tree-dwelling or eurytopic bat species: P. pipistrellus, P. nathusii, P. auritus, M. nattereri, N. noctula, with lower occupancy by other species from the same ecological group: M. daubentonii, M. bechsteinii, M. brandtii, N. leisleri and P. pygmaeus (Rueegger Citation2016; Pschonny et al. Citation2022). In some areas, the M. myotis – a species usually preferring attics or caves (Rodrigues et al. Citation2003) – also readily inhabits bat-boxes (Schmidt Citation2003; Wojtaszyn et al. Citation2021). In our study, the species composition does not differ from that typical of the lakelands of northern and central Poland: P. nathusii dominates, with a much smaller contribution of P. auritus (Kasprzyk & Ruczyński Citation2001; Ciechanowski et al. Citation2002; Ignaszak & Dzięgielewska Citation2009; Lesiński et al. Citation2009). Landscape composition may explain the superdomination of P. nathusii – study area is surrounded by the lakes, and wetlands are preferred habitat for this species (Flaquer et al. Citation2009; Ciechanowski Citation2015). Only the presence of M. myotis is slightly exceptional here – this species reached the northern limit of its range by 50 km further (Ruprecht Citation1971). In fact, the species composition of bats inhabiting the boxes is not stable and varies both phenologically (Lesiński et al. Citation2009) and geographically (Kowalski & Lesiński Citation1994; Rueegger Citation2016) – which in turn is often attributed to preferred habitats (López-Baucells et al. Citation2017). In our study, the high occupation of boxes in late summer is an effect of the appearance of bats migrating through the study area and using boxes as transitory and/or mating quarters. This pattern is typical for the Baltic countries and Central Europe, where P. nathusii is one of the most numerous migratory species (Jarzembowski Citation2003; Ciechanowski et al. Citation2016).
Apparent difference in species composition of bats between ceramic boxes and the remaining three other types of shelters require explanation, although their thermal properties did not provide any clues for that. Dimensions of the entrances to the CER boxes are also identical to that of styrofoam ones, while their volume is much smaller, yet the two largest bats in the sample (M. myotis and N. noctula) and the only long-eared one (P. auritus) clearly selected them. Entrance to CER is parallel to the longer axis of the box and allows to enter it directly from the rough surface of tree trunk below, providing an easy landing spot. On contrary, entrance to LSC and SSC is perpendicular to that axis and requires either landing directly on the front wall of the box or crawling across its smooth surface. We may only speculate what direct biomechanical, sensory or behavioural mechanism (ear size – see Taake & Hildenhagen Citation1989, mode of entrance detection – Ruczyński et al. Citation2009) stands behind that selection, but the larger, more massive bat species might not be so agile as to land directly on the box and bend their body down when entering, thus preferring CER with much easier access. Such explanation, however, does not cover similar preference in P. auritus, which body is about one-third smaller than N. noctula and M. myotis (Dietz et al. Citation2009).
Pregnant and lactating females reveal the most narrow habitat niche among bat individuals, while males and non-breeding females can thrive in sub-optimal habitats (Mackie & Racey Citation2007; Safi et al. Citation2007), and that trend applies to roost selection too (Istvanko et al. Citation2016). The reason for higher selectivity in reproductive females is their high energy demands (Racey & Speakman Citation1987), associated with their need to remain homeothermic, while non-reproductive individuals can benefit from diurnal torpor in the same time (Dietz & Kalko Citation2006). As in our sample, nursery roosts appeared only sporadically and none belong to the dominant species, P. nathusii, may one expect that the studied population consisted of generalistic individuals, with diminished roost selectivity. However, bats roosting in our boxes engaged in other energy-demanding activities, i.e. mating (Komar et al. Citation2022), which is additionally submitted to strong sexual selection and quality of occupied roost, might affect mate choice. P. nathusii is polygynic species and its multiple females may join a single territorial male at the same time and in the same roost, forming mating groups, which are often classified as harems (Ciechanowski & Jarzembowski Citation2004). Its mating system is, however, considered not harem, but resource-defence polygyny (Gerell-Lundberg & Gerell Citation1994), in which a quality of male territory should directly affect reproductive success. The distribution of size of mating groups in P. nathusii is, indeed, strongly right-skewed, meaning that the majority of potential mates is monopolised by the small minority of males, which one may expect to occupy the best roosts (Ciechanowski & Jarzembowski Citation2004). However, recent studies reveal that breeding system of P. nathusii also includes behaviours resembling more lek polygyny, where at least some males benefit from aggregation with neighbours roosting nearby, as they display advertisement vocalisations together (Jahelková & Horáček Citation2011). Thus, reproductive success, if we took the number of females in a mating group as a proxy, may not be related to individual roost quality at all (but rather the quality of display, i.e. song – Behr et al. Citation2006), yet it does not exclude the possibility that territorial males choose groups of boxes of particular characteristics to aggregate. Sex-ratio in P. nathusii, which is female-biased, indeed differs among bat-boxes, but in our example, it may be the result of avoiding wooden boxes by P. nathusii, but a sample was too small to test that hypothesis.
None of the parameters defined as structures that might affect the supply of solar energy and consequently temperature in the boxes were significant: the density of canopy, distance to the tree in front of the box and exposure of the box entrance, had no effect on the bat-box preferences by P. nathusii. The direct influence of the environment on the thermal conditions of the boxes mainly applies when the boxes are exposed to direct sunlight: orientation of the boxes towards cardinal directions (Lourenço & Palmeirim Citation2004), but a nonlinear relationship with hours of sunlight exposure was also found (Agnelli et al. Citation2011); also, the surface colours of the boxes were manipulated (Kerth et al. Citation2001). In fact, most of the research is focused on boxes mounted on buildings and/or even on poles (Hoeh et al. Citation2018; Fontaine et al. Citation2021), but this does not reflect the real conditions in the forest. However, the orientation of the entrance was found to significantly affect the occupation of bat boxes in European woodland condition at least in one study, where boxes directed to northwest appeared to be selected (Printz et al. Citation2021).
The density of the boxes (in our study: distance between boxes) – indicated as a factor important to offer opportunities for roost switching (Lewis Citation1995), or social interactions (Kerth & König Citation1999) – appeared to have no effect on the abundance/number of P. nathusii. The lack of these correlations seems to be fully understandable, since the intensively managed commercial forests are single-aged, single-species, and consequently strongly homogeneous, with severe shortage of natural shelters.
A factor not analysed by us due to low variance, but considered to potentially differentiate species, is the height at which the box hangs. The low height at which the boxes were hung – compared to the preferred shelters in the natural environment: mean height 19 m in primeval forest of eastern Poland (Ruczyński & Bogdanowicz Citation2005) or, at least, 9 m in small managed woodlands of the Netherlands (Boonman Citation2000), could explain low percentage of N. noctula. Noctules may, however, occupy bat boxes in significant numbers; in one German study conducted in sub-urban forests, they consisted 98% of all bats; unfortunately, authors provide information of only minimum height of box installation (2.5 m) (Printz et al. Citation2021) but it might suggest that this parameter had no significance at all. On the other hand, P. auritus in the forests of southeastern Poland is one of the most common bat species inhabiting the boxes, despite the similar height of the boxes as in our study (Wojtaszyn et al. Citation2021). In fact, those species reveal extreme plasticity in roosting behaviour, occupying hollow trees, bird and bat boxes, bridges and buildings to a similar extent (Swift Citation1998).
Finally, we found two factors significantly modelling the abundance of bats in occupied boxes: P. nathusii preferred shelters with a lower density of obstacles and located close to water; but both these relationships were only significant for the migration/mating period. This species has the fastest and least manoeuvrable flight within its genus in Europe, foraging at the largest distance from vegetation or other vertical obstacles (Baagøe Citation1987). Therefore, the density of obstacles can negatively affect the presence of P. nathusii by limiting the accessibility of the box, which may be particularly important during morning swarming (Naďo & Kaňuch Citation2013). Another explanation, in turn, is related directly to mating behaviour (Jahelková & Horáček Citation2011): males may avoid locations where the sounds of mating calls are suppressed by the surroundings.
The most important factor, however, was found to be the water distance. P. nathusii forages mainly on forest edges and over aquatic or wetland habitats (Flaquer et al. Citation2009; Ciechanowski Citation2015), and its diet contains to a large extent insect species whose larval development occurs in water or that swarms over it (Beck Citation1995; Vaughan Citation1997; Arnold et al. Citation2000; Kruger et al. Citation2014). During late summer and autumn, this species has an increased energy demand due to migration (even up to 2000 km: Petersons Citation2004), on the one hand, and mating behaviour (harems, vocalisation: Gerell-Lundberg & Gerell Citation1994) on the other, which may explain the choice of shelters as close as possible to feeding habitats.
One of the factors that could not be covered by our study, although mentioned by the other authors, is the species of tree at which the box was installed. Printz et al. (Citation2021) found that bats selected boxes installed on ash Fraxinus excelsior, while avoided those on black locusts Robinia pseudoacacia and Scotch pines. Even if these results were not a statistical artifact, most of the proposed explanations by those authors are highly improbable. Relating the box choice with the diversity and abundance of insects associated with particular tree species is especially unlikely, as the dominant species in that study, N. noctula, is an open-space aerial hawker that does not hunt inside a forest at all and is able to commute to foraging sites located several kilometres from daily roosts (Mackie & Racey Citation2007). Linking selection of boxes hanging on tree trunks with preferences of hollow trees belonging to the same species does not provide useful explanation as well, as only in the second case, properties of particular tree taxon may affect both roost abundance and quality (Ruczyński & Bogdanowicz Citation2008). Whatever the explanation of the phenomenon described by Printz et al. (Citation2021), we could not test that effect based on our sample. Our study was conducted in almost pure pine monocultures, where almost all boxes were installed at one species of tree, leaving bats with no choice regarding that element of roosting microhabitat.
And last but not least, in the investigated region (WLP), the occupancy of boxes is exceptionally one of the highest compared to other areas in Poland (see Appendix). Numerous factors have been suggested for the explanation to the variation of box occupation in the temperate zone, including the lack of natural cavities (Ciechanowski Citation2005; Printz et al. Citation2021; Pschonny et al. Citation2022), or box designs (see review: Rueegger Citation2016). However, for heterothermic organisms such as bats, microclimate requirements seem to be one of the most important factors (Kerth et al. Citation2001; Lourenço & Palmeirim Citation2004; Bartonička & Řehák Citation2007; Czenze et al. Citation2022). The temperature pattern inside the boxes in our research shows significant differences that are related to the material of the boxes: wooden boxes maintain a temperature close to the external (Text), whereas ceramic and styrofoam-concrete (both sizes) warm up on average to 2°C above Text, and gradually cool down during the night. Not irrelevant will be the fact that the temperature in the pine forest is on average about 1.4°C lower than the temperature recorded by the weather station. Therefore, non-wooden boxes provided a better thermal stability, especially during cold days in the spring and late summer and autumn. The selection of boxes featuring a higher heat capacity during the mating period may be connected with spermatogenesis: during this period, males reduce the use of torpor, maintaining a standard body temperature (Entwistle et al. Citation1998).
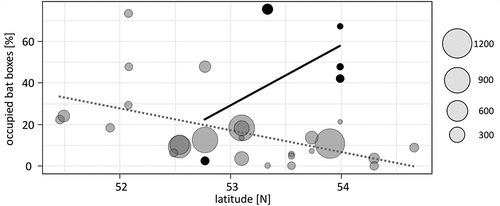
On the other hand, thermoregulatory behaviour seems to be species-specific – some species prefer lower roost temperatures than others (Otto et al. Citation2013). Therefore, the species-specific energy-saving strategy might explain to some degree the differentiation of the bat-box preferences. If the temperature was to be an essential factor in box occupancy, then we should expect different trends for boxes constructed from different materials, modelled by geographic location: a gradient connected with the ambient temperature. In Poland, the most popular boxes are the wooden ones (82%): bird S (29.6%), bird A (22.1%), Stratmann (21.9%), and Issel (13.1%), with marginal share of a crevice type – a special construction for the western barbastelle B. barbastellus (3.9%); whereas non-wooden: ceramic, mixed and concrete boxes are much less numerous, and account for up to 7.6% (over 7,500 boxes: Appendix, ). Thus, it resembles situation in most of the temperate zone countries (Rueegger Citation2016). In the analysis of 27 bat box locations, we found opposite trends for two types of material: box occupancy significantly decreased with increasing longitude for wooden construction (r = −0.554, r2 = 0.307, t = 3.40, p = 0.0022), and increased for the cement mixture construction – but this was not significant (r = 0.568, r2 = 0.332, t = 1.20, p = 0.318) (): the sample is small (only 6 locations), and requires additional data. Therefore, our results for the first time revealed that the thermal characteristics is a crucial factor behind the selection of particular box types by bats and how that parameter affects bat roost preferences in combination with factors associated with habitat and landscape structure. Variations from the trend can be modelled by various factors such as the forest type (deciduous, coniferous, mixed), elevation, or the model of the boxes within the broader category defined by material of the walls. Hence, more detailed analysis is required for a more comprehensive understanding. It seems that latitude should be taken into account as a factor that can directly affect the suitability of the particular box design for bats, when preparing the future conservation schemes. Whereas in the Mediterranean zone it may be important to avoid overheating, in colder climates, the properties of the boxes to compensate for heat loss will be more important (Bartonička & Řehák Citation2007; Bideguren et al. Citation2019). Already in southern Germany (48°N), N. noctula select wooden boxes over sawdust-concrete ones (Printz et al. Citation2021), fitting well in trend obtained solely from Polish data. This may indeed be even more important bearing in mind the currently modelled rapid climate change: improper artificial roosts may even become an ecological trap (Tillman et al. Citation2021), as bats may become exposed to lethal heat waves (Czenze et al. Citation2022).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adams RA, Hayes MA. 2008. Water availability and successful lactation by bats as related to climate change in arid regions of western North America. Journal of Animal Ecology 77(6):1115–1121. DOI: 10.1111/j.1365-2656.2008.01447.x.
- Agnelli P, Maltagliati G, Ducci L, Cannicci S. 2011. Artificial Roosts for bats: Education and research. The” Be a Bat’s Friend” project of the natural history museum of the University of Florence. Hystrix, the Italian Journal of Mammalogy 22(1):215–223. DOI: 10.4404/hystrix-22.1-4540.
- Andersson J, Domingo Gómez E, Michon S, Roberge JM. 2018. Tree cavity densities and characteristics in managed and unmanaged Swedish boreal forest. Scandinavian Journal of Forest Research 33(3):233–244. DOI: 10.1080/02827581.2017.1360389.
- Arnold A, Braun M, Becker N, Storch V. 2000. Contribution to the trophic ecology of Daubenton’s and Nathusius’ bats in the Upper Rhine valley (SW-Germany). Carolinea 58:257–263.
- Baagøe HJ. 1987. The Scandinavian bat fauna: Adaptive wing morphology, and free flight in the field. In: Fenton MB, Racey PA, Rayner JMV, editors. Recent advances in the study of bats. Cambridge, Mass: Cambridge University Press. pp. 57–74.
- Baranauskas K. 2009. The use of bat boxes of two models by Nathusius’ Pipistrelle (Pipistrellus nathusii) in Southeastern Lithuania. Acta Zoologica Lithuanica 19(1):3–9. DOI: 10.2478/v10043-009-0002-y.
- Bartonička T, Řehák Z. 2007. Influence of the microclimate of bat boxes on their occupation by the soprano pipistrelle Pipistrellus pygmaeus: Possible cause of roost switching. Acta Chiropterologica 9(2):517–526. DOI: 10.3161/1733-5329(2007)9[517:IOTMOB]2.0.CO;2.
- Beck A. 1995. Fecal analyses of European bat species. Myotis 32(33):109–119.
- Behr O, Helversen O, Heckel G, Nagy M, Voigt CC, Mayer F. 2006. Territorial songs indicate male quality in the sac-winged bat Saccopteryx bilineata (Chiroptera, Emballonuridae. Behavioral Ecology 17:810–817. DOI: 10.1093/beheco/arl013.
- Berthier K, Leippert F, Fumagalli L, Arlettaz R. 2012. Massive nest-box supplementation boosts fecundity, survival and even immigration without altering mating and reproductive behaviour in a rapidly recovered bird population. PLoS One 7(4):e36028. DOI: 10.1371/journal.pone.0036028.
- Bideguren GM, López-Baucells A, Puig-Montserrat X, Mas M, Porres X, Flaquer C. 2019. Bat boxes and climate change: Testing the risk of over-heating in the Mediterranean region. Biodiversity and Conservation 28(1):21–35. DOI: 10.1007/s10531-018-1634-7.
- Boonman M. 2000. Roost selection by noctules (Nyctalus noctula) and Daubenton’s bats (Myotis daubentonii). Journal of Zoology 251(3):385–389. DOI: 10.1111/j.1469-7998.2000.tb01089.x.
- Brouwer D, Henrard E. 2020. Too hot or not? The influence of colour and material on temperature and relative humidity in flat, single-chambered bat boxes in the Netherlands. Borculo: Ecologisch advies- & projectbureau NatuurInclusief.
- Charbonnier Y, Barbaro L, Theillout A, Jactel H. 2014. Numerical and functional responses of forest bats to a major insect pest in pine plantations. PLoS One 9(10):e109488. DOI: 10.1371/journal.pone.0109488.
- Ciechanowski M. 2002. Community structure and activity of bats (Chiroptera) over different water bodies. Mammalian Biology 67(5):276–285. DOI: 10.1078/1616-5047-00042.
- Ciechanowski M. 2005. Utilization of artificial shelters by bats (Chiroptera) in three different types of forest. Folia Zoologica 54(1):31–37.
- Ciechanowski M. 2015. Habitat preferences of bats in anthropogenically altered, mosaic landscapes of northern Poland. European Journal of Wildlife Research 61(3):415–428. DOI: 10.1007/s10344-015-0911-y.
- Ciechanowski M, Jakusz-Gostomska A, Żmihorski M. 2016. Empty in summer, crowded during migration? Structure of assemblage, distribution pattern and habitat use by bats (Chiroptera: Vespertilionidae) in a narrow, marine peninsula. Mammal Research 61(1):45–55. DOI: 10.1007/s13364-015-0249-6.
- Ciechanowski M, Jarzembowski T. 2004. The size and number of harems in the polygynous bat Pipistrellus nathusii (Keyserling and Blasius, 1839) (Chiroptera: Vespertilionidae). Mammalian Biology 69(4):277–280. DOI: 10.1078/1616-5047-00144.
- Czenze ZJ, Noakes MJ, Wojciechowski MS. 2022. Home is where the heat is: Thermoregulation of European bats inhabiting artificial roosts and the threat of heat waves. Journal of Applied Ecology 59(8):2179–2188. DOI: 10.1111/1365-2664.14230.
- Dietz C, Helversen O, Nill D. 2009. Bats of Britain, Europe and Northwestern Africa. A&C Black Publishers Ltd., London. pp. 400.
- Dietz M, Hörig A. 2011. Thermoregulation of tree-dwelling temperate bats—a behavioural adaptation to force live history strategy. Journal of Vertebrate Biology 60(1):5–16. DOI: 10.25225/fozo.v60.i1.a2.2011.
- Dietz M, Kalko EK. 2006. Seasonal changes in daily torpor patterns of free-ranging female and male Daubenton’s bats (Myotis daubentonii). Journal of Comparative Physiology B 176:223–231. DOI: 10.1007/s00360-005-0043-x.
- Entwistle AC, Racey PA, Speakman JR. 1998. The reproductive cycle and determination of sexual maturity in male brown long-eared bats, Plecotus auritus (Chiroptera: Vespertilionidae). Journal of Zoology 244(1):63–70. DOI: 10.1111/j.1469-7998.1998.tb00007.x.
- European Commission. 2022, February. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the regions new EU Forest Strategy for 2030. https://ec.europa.eu/environment/pdf/forests/swd_forest_strategy.pdf.
- Flaquer C, Puig-Montserrat X, Goiti U, Vidal F, Curcó A, Russo D. 2009. Habitat selection in Nathusius’ pipistrelle (Pipistrellus nathusii): The importance of wetlands. Acta Chiropterologica 11:149–155. DOI:10.3161/150811009X465767.
- Flaquer C, Torre I, Ruiz-Jarillo R. 2006. The value of bat-boxes in the conservation of Pipistrellus pygmaeus in wetland rice paddies. Biological Conservation 128(2):223–230. DOI: 10.1016/j.biocon.2005.09.030.
- Fontaine A, Simard A, Dubois B, Dutel J, Elliot KH. 2021. Using mounting, orientation, and design to improve bat box thermodynamics in a northern temperate environment. Scientific Reports 11(1):1–15. DOI: 10.1038/s41598-021-87327-3.
- The Forest Data Bank. A Database on Resources and Condition of Polish Forests. Available online: https://www.bdl.lasy.gov.pl/portal/en. Accessed April 2015.
- Froidevaux JS, Barbaro L, Vinet O, Larrieu L, Bas Y, Molina J et al. 2021. Bat responses to changes in forest composition and prey abundance depend on landscape matrix and stand structure. Scientific Reports 11(1):1–13. DOI: 10.1038/s41598-021-89660-z.
- Gerell-Lundberg K, Gerell R. 1994. The mating behaviour of the Pipistrelle and the Nathusius’ Pipistrelle (Chiroptera) – A comparison. Folia Zoologica 43:315–324.
- Gottfried I, Gottfried T, Zając K. 2019. Bats use larval galleries of the endangered beetle Cerambyx cerdo as hibernation sites. Mammalian Biology 95(1):31–34. DOI: 10.1016/j.mambio.2019.01.002.
- Graczyk R. 1967. New nesting boxes of sawdust-concrete as bats stay place. Roczniki Akademii Rolniczej w Poznaniu 38:15–19.
- Hoeh JPS, Bakken GS, Mitchell WA, O’Keefe JM. 2018. In artificial roost comparison, bats show preference for rocket box style. PLoS ONE 13(10):e0205701. DOI: 10.1371/journal.pone.0205701.
- Ignaszak K, Dziegielewska M. 2009. Wykorzystanie skrzynek drewnianych i trocinobetonowych przez nietoperze w Szczecinskim Parku Krajobrazowym „Puszcza Bukowa”. Studia i Materiały Centrum Edukacji Przyrodniczo-Leśnej 11(2 [21]):91–100.
- Issel B, Issel W. 1955. Versuche zur Ansiedelung von „Waldfledermäusen” in Fledermauskästen. Forstwissenschaftliches Centralblatt 74(7):193–204. DOI: 10.1007/BF01821576.
- Istvanko DR, Risch TS, Rolland V. 2016. Sex-specific foraging habits and roost characteristics of Nycticeius humeralis in north-central Arkansas. Journal of Mammalogy 97:1336–1344. DOI: 10.1093/jmammal/gyw102.
- Jahelková H, Horáček I. 2011. Mating system of a migratory Bat, Nathusius’ Pipistrelle (Pipistrellus nathusii): Different male strategies*. Acta Chiropterologica 13:123–137. DOI: 10.3161/150811011X578679.
- Jarzembowski T. 2003. Migration of the Nathusius’ pipistrelle Pipistrellus nathusii (Vespertilionidae) along the Vistula split. Acta Theriologica 48(3):301–308. DOI: 10.1007/BF03194170.
- Kasprzyk K, Ruczyński I. 2001. The structure of bat communities roosting in bird nest boxes in two pine monocultures in Poland. Folia Zoologica 50(2):107–116.
- Kerth G, Ebert C, Schmidtke C. 2006. Group decision making in fission–fusion societies: Evidence from two-field experiments in Bechstein’s bats. Proceedings of the Royal Society of London B: Biological Sciences 273(1602):2785–2790. DOI: 10.1098/rspb.2006.3647.
- Kerth G, König B. 1999. Fission, fusion and nonrandom associations in female Bechstein’s bats (Myotis bechsteinii). Behaviour 136(9):1187–1202. DOI: 10.1163/156853999501711.
- Kerth G, Weissmann K, König B. 2001. Day roost selection in female Bechstein’s bats (Myotis bechsteinii): A field experiment to determine the influence of roost temperature. Oecologia 126(1):1–9. DOI: 10.1007/s004420000489.
- Kirby KJ, Webster SD, Antczak A. 1991. Effects of forest management on stand structure and the quantity of fallen dead wood: Some British and Polish examples. Forest Ecology and Management 43(1–2):167–174. DOI: 10.1016/0378-1127(91)90083-8.
- Komar E, Fasel NJ, Szafrańska PA, Dechmann DKN, Zegarek M, Ruczyński I. 2022. Energy allocation shifts from sperm production to self-maintenance at low temperatures in male bats. Scientific Reports 12:2138. DOI: 10.1038/s41598-022-05896-3.
- Kowalski M, Lesiński G. 1994. Bats occupying nest boxes for birds and bats in Poland. Nyctalus (N.F.) 5(1):19–26.
- Krüger F, Clare EL, Symondson WO, Keišs O, Pētersons G. 2014. Diet of the insectivorous bat Pipistrellus nathusii during autumn migration and summer residence. Molecular Ecology 23(15):3672–3683. DOI: 10.1111/mec.12547.
- Kunz TH, Lumsden LF, Fenton MB. 2003. Ecology of cavity and foliage roosting bats. In: Kunz T, Fenton MB, editors. Bat ecology. Chicago: University of Chicago Press. pp. 3–89. DOI: 10.5281/zenodo.4655329.
- Larrieu L, Paillet Y, Winter S, Bütler R, Kraus D, Krumm F, Lachat T, Michel AK, Regnery B. 2018. Tree related microhabitats in temperate and Mediterranean European forests: A hierarchical typology for inventory standardization. Ecological Indicators 84:194–207. DOI: 10.1016/j.ecolind.2017.08.051.
- Lesiński G, Skrzypiec-Nowak P, Janiak A, Jagnieszczak Z. 2009. Phenology of bat occurrence in boxes in central Poland. Mammalia 73(1):33–37. DOI: 10.1515/MAMM.2009.014.
- Leuschner C, Ellenberg H. 2017. Ecology of Central European Forests. Vegetation Ecology of Central Europe. Vol. I. Springer Cham. pp. 972. DOI:10.1007/978-3-319-43042-3.
- Lewis SE. 1995. Roost fidelity of bats: A review. Journal of Mammalogy 76(2):481–496. DOI: 10.2307/1382357.
- Long R, Kiser W, Kiser S. 2006. Well-placed bat houses can attract bats to Central Valley farms. California Agriculture 60(2):91–94. DOI: 10.3733/ca.v060n02p91.
- López-Baucells A, Puig-Montserrat X, Torre I, Freixas L, Mas M, Arrizabalaga A, Flaquer C. 2017. Bat boxes in urban non-native forests: A popular practice that should be reconsidered. Urban Ecosystems 20(1):217–225. DOI: 10.1007/s11252-016-0582-9.
- Lourenço SI, Palmeirim JM. 2004. Influence of temperature in roost selection by Pipistrellus pygmaeus (Chiroptera): Relevance for the design of bat boxes. Biological Conservation 119(2):237–243. DOI: 10.1016/j.biocon.2003.11.006.
- Mackie I, Racey PA. 2007. Habitat use varies with reproductive state in noctule bats (Nyctalus noctula): Implications for conservation. Biological Conservation 140:70–77. DOI: 10.1016/j.biocon.2007.07.031.
- Mayle BA. 1990. A biological basis for bat conservation in British woodlands–a review. Mammal Review 20(4):159–195. DOI: 10.1111/j.1365-2907.1990.tb00111.x.
- Naďo L, Kaňuch P. 2013. Dawn swarming in tree-dwelling bats—an unexplored behaviour. Acta chiropterologica 15(2):387–392. DOI: 10.3161/150811013X679008.
- Otto MS, Becker NI, Encarnação JA. 2013. Cool gleaners: Thermoregulation in sympatric bat species. Mammalian Biology 78(3):212–215. DOI: 10.1016/j.mambio.2012.07.156.
- Petersons G. 2004. Seasonal migrations of north-eastern populations of Nathusius’ bat Pipistrellus nathusii (Chiroptera). Myotis 41(42):29–56.
- Printz L, Tschapka M, Vogeler A. 2021. The common noctule bat (Nyctalus noctula): Population trends from artificial roosts and the effect of biotic and abiotic parameters on the probability of occupation. Journal of Urban Ecology 7:juab033. DOI: 10.1093/jue/juab033.
- Pschonny S, Leidinger J, Leitl R, Weisser WW. 2022. What makes a good bat box? How box occupancy depends on box characteristics and landscape‐level variables. Ecological Solutions and Evidence 3(1):e12136. DOI: 10.1002/2688-8319.12136.
- Quinto J, Micó E, Martínez-Falcón AP, Galante E, Marcos-García MDLÁ. 2014. Influence of tree hollow characteristics on the diversity of saproxylic insect guilds in Iberian Mediterranean woodlands. Journal of Insect Conservation 18(5):981–992. DOI: 10.1007/s10841-014-9705-x.
- Racey PA, Speakman JR. 1987. The energy costs of pregnancy and lactation in heterothermic bats. Symposium of the Zoological Society of London 57:107–125.
- Rachwald A. 1992. Social organization, recovery frequency and body weight of the bat Pipistrellus nathusii from northern Poland. Myotis 30:109–118.
- Rachwald A, Gottfried I, Gottfried T, Szurlej M. 2018. Occupation of crevice−type nest−boxes by the forest dwelling western barbastelle bat Barbastella barbastellus (Chiroptera: Vespertilionidae). Folia Zoologica 67(3−4):231–238. DOI: 10.25225/fozo.v67.i3-4.a12.2018.
- R Core Team. 2020. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available: https://www.R-project.org/. Accessed May 2021 18.
- Rhodes M, Jones D. 2011. The use of bat boxes by insectivorous bats and other fauna in the greater Brisbane region. In: Law B, Eby P, Lunney D, Lumsden L, editors. The biology and conservation of Australasian bats. Sydney: Royal Zoological Society of New South Wales. pp. 424–442. DOI: 10.7882/FS.2011.043.
- Rodrigues L, Zahn A, Rainho A, Palmeirim JM. 2003. Contrasting the roosting behaviour and phenology of an insectivorous bat (Myotis myotis) in its southern and northern distribution ranges. Mammalia 67(3):321–335. DOI: 10.1515/mamm.2003.67.3.321.
- Ruczyński I, Bogdanowicz W. 2005. Roost cavity selection by Nyctalus noctula and N. leisleri (Vespertilionidae, Chiroptera) in Białowieża Primeval Forest, eastern Poland. Journal of Mammalogy 86(5):921–930. DOI: 10.1644/1545-1542(2005)86[921:RCSBNN]2.0.CO;2.
- Ruczyński I, Bogdanowicz W. 2008. Summer roost selection by tree-dwelling bats Nyctalus noctula and N. leisleri: A multiscale analysis. Journal of Mammalogy 89:942–951. DOI: 10.1644/07-MAMM-A-134.1.
- Ruczyński I, Kalko EK, Siemers BM. 2007. The sensory basis of roost finding in a forest bat, Nyctalus noctula. Journal of Experimental Biology 210(20):3607–3615. DOI: 10.1242/jeb.009837.
- Ruczyński I, Kalko EK, Siemers BM. 2009. Calls in the forest: A comparative approach to how bats find tree cavities. Ethology 115(2):167–177. DOI: 10.1111/j.1439-0310.2008.01599.x.
- Ruczyński I, Nicholls B, MacLeod CD, Racey PA. 2010. Selection of roosting habitats by Nyctalus noctula and Nyctalus leisleri in Białowieża Forest—adaptive response to forest management? Forest Ecology and Management 259(8):1633–1641. DOI: 10.1016/j.foreco.2010.01.041.
- Rueegger N. 2016. Bat boxes—a review of their use and application, past, present and future. Acta Chiropterologica 18(1):279–299. DOI: 10.3161/15081109ACC2016.18.1.017.
- Ruprecht AL. 1971. Distribution of Myotis myotis (Borkhausen, 1797) and representatives of the genus Plecotus Geoffroy, 1818 in Poland. Acta Theriologica 16(7):95–104. DOI: 10.4098/AT.arch.71-7.
- Safi K, König B, Kerth G. 2007. Sex differences in population genetics, home range size and habitat use of the parti-colored bat (Vespertilio murinus, Linnaeus 1758) in Switzerland and their consequences for conservation. Biological Conservation 137:28–36. DOI: 10.1016/j.biocon.2007.01.011.
- Schmidt A. 2003. Zum Orttsverhalten von Mausohren (Myotis myotis) ostbrandenburgicher Kiefernforste. Nyctalus (N.F.) 8:465–489.
- Swift SE. 1998. Long-eared bats. London: T & AD Poyser Ltd. pp. 182.
- Swihart RK, Gehring TM, Kolozsvary MB, Nupp TE. 2003. Responses of ‘resistant’ vertebrates to habitat loss and fragmentation: The importance of niche breadth and range boundaries. Diversity and Distributions 9(1):1–18. DOI: 10.1046/j.1472-4642.2003.00158.x.
- Taake KH, Hildenhagen U. 1989. Nine years’ inspections of different artificial roosts for forest-dwelling bats in Northern Westfalia: Some results. In: Hának V, Horaček I, Gaisler J, editors. European Bat Research 1987. Praha: Charles University Press. pp. 487–493.
- Thomas DW. 1988. The distribution of bats in different ages of Douglas-fir forests. The Journal of Wildlife Management 52(4):619–626. DOI: 10.2307/3800920.
- Tillman FE, Bakken GS, O’Keefe JM. 2021. Design modifications affect bat box temperatures and suitability as maternity habitat. Ecological Solutions and Evidence 2(4):e12112. DOI: 10.1002/2688-8319.12112.
- Turbill C, Geiser F. 2008. Hibernation by tree-roosting bats. Journal of Comparative Physiology B 178(5):597–605. DOI: 10.1007/s00360-007-0249-1.
- Van der Hoek Y, Gaona GV, Martin K. 2017. The diversity, distribution and conservation status of the tree‐cavity‐nesting birds of the world. Diversity and Distributions 23(10):1120–1131. DOI: 10.1111/ddi.12601.
- Vaughan N. 1997. The diets of British bats (Chiroptera). Mammal Review 27(2):77–94. DOI: 10.1111/j.1365-2907.1997.tb00373.x.
- Vesk PA, Nolan R, Thomson JR, Dorrough JW, Mac Nally R. 2008. Time lags in provision of habitat resources through revegetation. Biological Conservation 141(1):174–186. DOI: 10.1016/j.biocon.2007.09.010.
- Voigt CC, Lehnert LS, Popa-Lisseanu AG, Ciechanowski M, Estók P, Gloza-Rausch F et al. 2014. The trans-boundary importance of artificial bat hibernacula in managed European forests. Biodiversity and Conservation 23(3):617–631. DOI: 10.1007/s10531-014-0620-y.
- Wojtaszyn G, Lesiński G, Rutkowski T. 2021. Seasonal dynamics of occupation of bat boxes by bats in forests of south-western Poland. Acta Zoologica Bulgarica 73(3):431–436.
- Woś A. 1996. Zarys klimatu Polski. Poznań: Wydawnictwo Naukowe UAM. pp. 302.