Abstract
The Salmo trutta species complex includes threatened or intensively managed taxa. Domestic-Atlantic strains are widely farmed and massively introduced into the wild to support fisheries, although such practices seriously threaten native populations of Mediterranean brown trout through anthropogenic hybridization. Characterizing the distribution and genetic composition of wild populations across river catchments is crucial to identify conservation priorities and define appropriate management strategies. Here, we genotyped 586 brown trout at the diagnostic nuclear LDH-C1 gene and the mitochondrial D-loop fragment, to assess the conservation status of 33 sites in a protected area spanning river catchments from both the Adriatic and Tyrrhenian slopes in the northern Apennines (Italy). The results indicated a critical situation of widespread domestic-Atlantic introgression. Further analyses at 15 microsatellite loci on 159 individuals from 12 natural sites (along with 113 references from hatcheries) revealed similarity to hatchery stocks, higher genetic diversity and bottleneck signals in introgressed/exotic populations, consistently with relatively recent introductions of domestic-Atlantic individuals into wild sites. Conversely, the only native sites from a single river catchment on the Adriatic slope showed genetic distinctiveness, reduced diversity and demographic stability. We also found genetic evidence of a human-mediated introduction of allochthonous Mediterranean trout in a single wild site, as well as of a putative between-slopes translocation. We provide further insight into the occurrence and consequences of human manipulations on wild Mediterranean brown trout populations, contextually offering a reliable baseline for an ongoing conservation project aiming at preserving native populations of this endangered taxon.
Introduction
The brown trout is a complex of incipient species, referred to as Salmo trutta species complex, originally inhabiting freshwaters of the Palearctic region (Bernatchez Citation2001). In the Mediterranean area, the complex includes multiple morphotypes and ecotypes, subdivided into several genetically divergent evolutionary lineages, whose distribution results from an intricate phylogeographic history related to the Pliocene and Pleistocene climate fluctuations (reviewed in Lobón-Cerviá & Sanz Citation2018). In particular, the Italian Mediterranean trout, whose taxonomy is still controversial (Splendiani et al. Citation2019c), exhibits three native mitochondrial haplogroups – Adriatic (AD), Mediterranean (ME) and marmoratus (MA) (sensu Bernatchez Citation2001) – often coexisting within the same populations (e.g. Rossi et al. Citation2019, Citation2022; Splendiani et al. Citation2019b). Peninsular Mediterranean brown trout mostly inhabits mountain stretches of both the Tyrrhenian and Adriatic river basins. The remnant native populations are usually small, isolated and/or fragmented, and show low genetic diversity and pronounced differentiation (Fabiani et al. Citation2018; Splendiani et al. Citation2019b; Palombo et al. Citation2021; Rossi et al. Citation2022).
Mediterranean brown trout populations are threatened by multiple concurrent (anthropogenic) factors, such as habitat alteration and fragmentation, water pollution and abstraction, overfishing, poaching, climate changes, introgression and competition with alien species (Almodóvar et al. Citation2012; Ribeiro & Leunda Citation2012; Meraner & Gandolfi Citation2018; Carosi et al. Citation2020, Citation2022; Rossi et al. Citation2022). Such stressors are determining a dramatic decline of native populations throughout the Mediterranean basin (Aparicio et al. Citation2000; Vera et al. Citation2013), including the Italian Peninsula (Splendiani et al. Citation2016, Citation2019b). For these reasons, the Mediterranean trout is currently listed as “Critically Endangered” in the IUCN Red List of Italian Vertebrates (Rondinini et al. Citation2022) and included in the Annex II of the European Habitat Directive 92/43/CE among the species requiring the designation of Special Conservation Areas. According to the 4th Habitat Directive Monitoring Report (2013–2018 timespan), its populations show unfavourable-bad conservation status and a declining trend in Italy (Stoch & Grignetti Citation2021).
In particular, anthropogenic hybridization with allochthonous strains is a major threat to the long-term survival of Mediterranean-native brown trout populations. Indeed, massive introductions into the wild of hatchery trout of Atlantic origin, as well as of hybrids between native and domestic trout (Splendiani et al. Citation2019b), have been repeatedly performed since the 19th century to support fisheries and contrast local population demographic declines (Meraner & Gandolfi Citation2018). This eventually caused in-deep introgressive hybridization (Nonnis Marzano et al. Citation2003; Meraner & Gandolfi Citation2018), also at adaptive loci (Talarico et al. Citation2021), competition and spread of pathologies (Lobón-Cerviá & Sanz Citation2018). In turn, this has led to the extinction of entire local populations in many cases – for instance, Splendiani et al. (Citation2016) reported that <3% of Mediterranean brown trout populations from central Apennines preserved genetic integrity, while others showed various extents of introgression from Atlantic strains. In addition, the mixing of independently evolved lineages may erode gene pool architecture, eliminating original local adaptation (Lobón-Cerviá & Sanz Citation2018) or partial reproductive isolation, which seems to be at the basis of the native trout resistance with respect to the invasion process (Splendiani et al. Citation2019b).
In this context, mapping both genetic native and exotic diversity is crucial to identify conservation priorities and define appropriate management strategies, especially in protected areas since they play a crucial role in preserving the original variability of local Mediterranean trout populations and their adaptive potential, which is pivotal to dynamically respond to environmental and climate changes (Vera et al. Citation2013). The identification of Management Units should rely on the Evolutionary Significant Unit (ESU) approach, which accounts for the genetic distinctiveness of populations (Moritz Citation1994; Palsbøll et al. Citation2007) while overcoming taxonomic controversies (Splendiani et al. Citation2019c; Rossi et al. Citation2022).
In recent years, a number of European-funded projects provided for concrete actions targeting the conservation and recovery of Mediterranean trout populations in multiple Italian protected areas and Nature 2000 sites, such as the LIFE+ TROTA (LIFE12 AT/IT/0000940) and the LIFE STREAMS (LIFE18 NAT/IT/000931). These projects provide the opportunity to investigate the genetic makeup and population structure of local brown trout populations, identifying and characterizing, through an integrated genetic-demographic approach, residual native populations that preserve a high degree of genetic integrity. Indeed, remnant native populations play a crucial role in concrete conservation actions, representing a biodiversity reservoir from which to take wild spawners for supplemental breeding programmes. Contextually, investigating the presence and the origin of the alien genome allows identifying exotic populations to be successfully eradicated.
The Foreste Casentinesi, Monte Falterona and Campigna National Park (hereafter FCNP) is a protected area of 36,846 hectares, located in the Northern Italian Apennines (see below for further details, ) and involved in the above-mentioned LIFE STREAMS project along with other national/regional parks and Natura 2000 sites. Historical evidence reported the presence of Mediterranean brown trout populations in the Adriatic slope of the FCNP area since the Middle Ages (Nocita & Poggesi Citation2010 and references therein), while evidence for the Tyrrhenian slope dated back to the 17th century (Marcuccini Citation2021 and references therein). Similarly to elsewhere, trout were considerably appreciated for food purposes and thus fished, farmed and traded (Nocita & Poggesi Citation2010; Bottacci Citation2012), so that translocations between river catchments in the FCNP area can be reasonably assumed in historical times, despite the lack of explicit textual proof. More recently, i.e. in the last century, massive stocking activities were carried out to increase the abundance of wild populations, resulting in the occurrence of domestic Atlantic trout and hybrids (Baratti et al. Citation2006; Casali Citation2015).
Figure 1. Location and genetic makeup of 33 wild sampling sites in the northern Apennines (Italy) and four hatcheries (indicated in the bottom-right map). Pie charts, proportional to sample size, indicate the frequency and the geographic distribution of three genetic classes defined by combining information from the LDH-C1 genotype and the D-loop haplogroup: “native” = LDH-C1*100/*100 and an AD, ME or MA haplotype; “exotic” = LDH-C1*90/*90 and an AT haplotype; “hybrid/introgressed” = mixed genetic profiles. Site abbreviations are defined in ; the FCNP area is shown in pale green; dotted lines mark boundaries of major drainage basins; the purple rectangle indicates the study area in the bottom-right map.
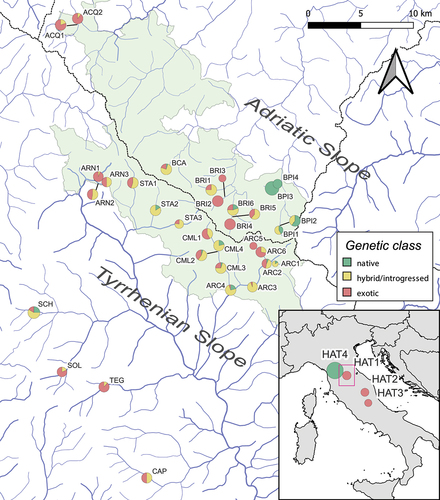
Table I. Detailed information on 33 sampling sites and 4 hatcheries of (Mediterranean) brown trout in northern Italy: river catchment and sampling locality; administrative region and protected area (FCNP = Foreste Casentinesi National Park); Apennine slope (Slope); elevation above the sea level (Elev); approximated geographic coordinates (datum = WGS84); sample size (N); sample size for STR-based analyses.
In this study, we applied multiple molecular markers to characterize wild brown trout populations in the FCNP and nearby areas. Specifically, we aimed at (i) mapping and quantifying domestic-Atlantic introgression across sites, and (ii) investigating genetic diversity, population structure and origin of populations. Our findings ultimately allow defining Management Units to support effective conservation actions (alien trout eradication, supplemental breeding) within the LIFE STREAMS project framework.
Materials and methods
Study area, sample collection and DNA extraction
During the summer of 2020 and 2021, we gathered 586 samples of wild brown trout from 33 sites in 12 river catchments, including tributaries, in the Tuscany and Emilia-Romagna regions (Italy). Sampling sites were located within the FCNP boundaries and the surroundings, an area including brooks and creeks flowing in either the Tyrrhenian or the Adriatic slope of the northern Apennines (, ), characterized by a seasonal water regime. Although the area is mostly made of flysch sediments, geology differs between the two slopes: erodible argillite and marly clays (“marnoso-arenacea” formation) prevail in the Adriatic side, so that waterfalls (often > 5 m) and jumps are frequent in major watercourses, which flow parallel to each other (Tramazzo, Montone, Rabbi and Bidente); conversely, compact sandstones (“macigno” formation) characterize the Tyrrhenian slope, resulting in a less interrupted morphology of watercourses, all flowing into the Arno drainage basin.
Individuals were captured by electrofishing and subsequently released after collecting a fin clip (authorization nos. 3327/2021 and 7951/2022). Additionally, we obtained 113 reference samples from hatcheries in the central and northern Apennines: 47 specimens from three hatcheries rearing domestic brown trout of Atlantic origin (HAT-1-2-3); 66 specimens from a small fish farm (HAT4) that semi-intensively breeds declared Mediterranean trout hailing from the Magra-Vara drainage basin, flowing into the northern Tyrrhenian Sea (, ). Fin clips were stored at −20°C in absolute ethanol until DNA extraction, which was carried out using DNeasy tissue kit (QIAGEN, Hilden, Germany) following the manufacturer’s protocol.
Molecular marker amplification and genotyping
For a basic assessment of Atlantic brown trout introgression in wild populations, collected samples were typed at the LDH-C1 nuclear gene following the RFLP-based procedure described in McMeel et al. (Citation2001), improved accordingly to Padula et al. (Citationin press). Such gene typically shows two diagnostic alleles: the LDH-C1*90 which is widespread across wild brown trout populations in Northern Europe and is fixed in European hatchery strains of Atlantic origin, and the LDH-C1*100 that occurs in native populations of Mediterranean catchments (McMeel et al. Citation2001).
Secondly, samples were typed at the mitochondrial Control Region (D-loop), which allows for distinguishing between major native (AD = Adriatic, ME = Mediterranean, MA = marmoratus) and exotic (AT = Atlantic) haplogroups in peninsular Italy. The D-loop was amplified as described in Cortey and García-Marín (Citation2002). PCR products were purified with A’SAP PCR clean up kit (ArcticZymes Technologies ASA, Tromsø, Norway) and then forward-sequenced using the BigDyeTM Terminator Cycle Sequencing chemistry (AppliedBiosystems, Foster City, USA). We visualized and manually corrected sequences in SeqScape v 3.0 software (Applied Biosystems, Foster City, USA). Sequence alignment (ClustalW algorithm) and the successive search against the NCBI database for haplogroup identification (sensu Bernatchez Citation2001) were performed in MEGA11 (Tamura et al. Citation2021).
To investigate the genetic structure and hybridization rates in the study area, we selected a subset of individuals from sites showing relatively lower levels of LDH-C1 and/or D-loop introgression from Atlantic strains, along with all Mediterranean/Atlantic reference samples, to be genotyped at 15 microsatellite loci (hereafter STR): Str60INRA and Str73INRA (Estoup et al. Citation1993); SsoSL417 (Slettan et al. Citation1995); MST85, MST543 and MST591 (Presa & Guyomard Citation1996); Ssa85 and Ssa197 (O’Reilly et al. Citation1996); Ssa408Uos and Ssa410Uos (Cairney et al. Citation2000); Omm1064 (Rexroad et al. Citation2002); SSsp2213 (Paterson et al. Citation2004); SSa103NVH (Thorsen et al. Citation2005); SsaD71 and SsaD190 (King et al. Citation2005). We set up 5 multiplexes of 3 loci each (Appendix S1). PCR reactions were performed in a total volume of 16 μL containing 5–15 ng of genomic DNA, 7 μl of QIAGEN Multiplex PCR Kit, 1.4 μl QSlol (QIAGEN Inc., Hilden, Germany), and 0.1–0.4 μl each 10 μM primer (see details in Appendix S1). Amplifications were carried out as follows: initial denaturation at 94°C for 15', followed by 35 cycles at 94°C for 30'', a multiplex-specific annealing temperature (Appendix S1) for 1’ 30'', 72°C for 60'', and a final elongation step at 72°C for 10'. Fragment analysis was conducted on an ABI 3730 DNA analyzer (Applied Biosystems) and genotypes were determined using GeneMapper 5.0 with GeneScan LIZ 500 size standard (Thermo Fisher Scientific, Waltham, MA, USA). A service facility (Bio-Fab Research s.r.l., https://www.biofabresearch.com/en/) accomplished all the above-described laboratory procedures.
Data analyses
We computed frequencies of LDH-C1 genotypes and D-loop haplotypes across sites and calculated the % of per-site exotic-Atlantic components (i.e., the LDH-C1*90 allele and the AT haplogroup). Then, we classified individuals into three genetic classes, by combining LDH-C1 and D-loop profiles: “native” for individuals showing LDH-C1 *100/*100 genotype and AD, ME or MA haplotype; “exotic” for LDH-C1 *90/*90 individuals carrying an AT haplotype; “hybrid/introgressed” for LDH-C1 heterozygotes or individuals with admixed LDH-C1/D-loop profiles.
The STR dataset was primarily tested for departure from Hardy–Weinberg expectations across sites, and for linkage disequilibrium between loci pairs (probability test and Markov chain method with default parameters), as implemented in GENEPOP of the Web (Rousset Citation2008) and applying the Bonferroni correction for multiple testing to p-values.
Indices of genetic variability were computed for both wild sites and hatcheries in GENALEX 6.5 (Peakall & Smouse Citation2012): the average allele number (An) and private alleles (Pa), the expected (He) and observed (Ho) heterozygosity. We also estimated the allelic richness (Ar), through the rarefaction procedure implemented in the POPGENEREPORT R-package (Adamack & Gruber Citation2014), to fairly compare diversity among populations with different sample sizes. Then, we evaluated the contribution of Atlantic strains introgression (LDH-C1*90 %) to genetic diversity (Ar and Ho) of wild sites – note that LDH-based introgression was preferred over D-loop-based introgression, as the former should be less biased by putative strain- and/or sex-dependent mechanisms such as mating preferences, fitness or migration (Hansen et al. Citation2000; Splendiani et al. Citation2019a). To address it, we used a linear model (LM function in R) and a generalized linear model with the Beta family (BETAREG package in R) to predict Ar and Ho, respectively, as the latter can range only between 0 and 1. In both cases, we fitted a quadratic regression, since higher diversity is expected when admixture between native and domestic lineage is maximized, namely at intermediate LDH-C1*90 frequency (Rossi et al. Citation2022).
We used multiple approaches to explore genetic structure and relationships among natural sites and reference hatchery stocks, as well as to deeply investigate the nature of examined individuals in wild populations. (1) We estimated genetic distances (Fst) between site pairs and tested their significance with 1000 permutations in ARLEQUIN 3.5 (Excoffier & Lischer Citation2010). (2) We visualized the above-mentioned Fst matrix of among-sites differentiation through a non-metric multidimensional scaling (NMDS) statistical procedure. A permutational multivariate analysis of variance (PERMANOVA) tested the statistical significance of the overall differentiation among four a priori-defined groups – Tyrrhenian sites, Adriatic sites, Atlantic hatcheries (HAT1-2-3) and the hatchery of Mediterranean trout (HAT4) – as well as pairwise differentiation, with 1000 permutations. Both NMDS and PERMANOVA were performed in PAST 4.04 (Hammer et al. Citation2001). (3) We performed a Bayesian admixture analysis in STRUCTURE v2.3.4 (Pritchard et al. Citation2000) with the following settings: admixture model testing both correlated and independent allele frequencies; 50,000 burn-in followed by 500,000 Monte Carlo Markov chains iterations; 10 runs for each K value in the range 1–16. Taking advantage of the STRUCTURE HARVESTER web routine (Earl & vonHoldt Citation2012), we inferred the optimal number of K clusters according to the ∆K method (i.e., the K corresponding to the highest rate of change in log-likelihood probability between successive K values; Evanno et al. Citation2005) and the visual inspection of the likelihood probability pattern for increasing K (Pritchard et al. Citation2000). Replicated runs for the chosen K values were collapsed in CLUMPAK (Kopelman et al. Citation2015). We also inspected the topology of STRUCTURE-generated trees showing relationships among inferred genetic clusters. (4) We run multiple AMOVAs in ARLEQUIN (Excoffier & Lischer Citation2010) to explore the partitioning of genetic variance (within populations and within/among groups) under the following grouping hypotheses: a single panmictic group; four geographic groups defined according to major geographic areas of origin (wild sites from the Tyrrhenian slope, wild sites from the Adriatic slope, hatcheries rearing exotic-Atlantic strains, hatchery of Mediterranean trout from Magra-Vara drainage basin); optimal genetic clusters as inferred by STRUCTURE analyses and after removing sites with an average >30% admixture from the analyses.
Finally, we tested for recent (substantial) population bottlenecks using the BOTTLENECK 1.2.02 software (Piry et al. Citation1999), applying the infinite allele model (IAM) and the two-phase mutation model (TPM, default settings: 70% stepwise microsatellite mutations and 30% multistep mutations) with 1,000 iterations and assessing the significance of a transitory heterozygote excess across loci with the one-tailed Wilcoxon test.
Results
The exotic-Atlantic LDH-C1*90 allele was found in all but two wild sites (BPI3, BPI4), mostly at high frequencies (i.e. > 80% in 16 sites) (). Relatively lower introgression rates emerged in Adriatic BPI sites, although sites from this slope did not show significantly different LDH-C1*90 frequencies compared to Tyrrhenian sites (two-sided Mann–Whitney test: U = 125, p = 0.868).
Table II. Genetic information obtained for 699 brown trout from the monitored 33 sampling sites and 4 hatchery stocks: LDH-C1 nuclear gene genotypes; LDH-C1-based introgression rate (i.e., frequency of the exotic Atlantic LDH-C1*90 allele); mitochondrial D-loop haplotypes; D-loop-based introgression (i.e., frequency of the exotic AT haplogroup); genetic classes obtained combining the individual LDH-C1 genotype and the D-loop haplotype (“native” = LDH-C1*100/*100 and native haplotype; “exotic” = LDH-C1*90/*90 and AT haplotype; “hybrid/introgressed” = mixed genotypes). The Apennine slope and the sample size (N) are also shown.
We obtained overall six D-loop haplotypes of 535–536 bp, all occurring in the NCBI database and associated with vouchers of S. trutta species complex (Appendix S2): two AD (Ad52, Ad-Tyrrh1); one ME (Me25); one MA (Ma2a); and two AT (At18, At63). The Me25 was the most frequent and widespread native haplotype in wild sites. AD haplotypes occurred in four Tyrrhenian sites (Ad-Tyrrh1 was shared with Mediterranean trout reared in HAT4), while the Ma2a haplotype (MA haplogroup) was particularly rare, being reported in only four individuals from three sites. The exotic AT haplotypes (the same found in Atlantic hatcheries) were detected, often at high frequencies, in all but three sites (ARC1, BPI3, BPI4) from both slopes with comparable frequencies (two-sided Mann–Whitney test: U = 125, p = 0.866) ().
Genetic classes obtained combining D-loop and LDH-C1 genotypes revealed widespread exotic (Atlantic) and hybrid individuals across the study area. BPI3 and BPI4 were the solely “pure Mediterranean” sites, as opposed to 19 sites entirely made up of Atlantic trout and/or their hybrids ().
Besides 113 reference samples, we genotyped at 15 STR a subset of 159 individuals chosen amongst 12 sites () showing a smaller introgression degree according to LDH-C1 and D-loop () – for such a reason, STR-based results reported below may be biased to some extent and they could not be entirely descriptive of the actual status of wild populations. The STR genotype dataset did not reveal systematic biases: linkage disequilibrium was significant (Bonferroni adjusted p < 0.05) in only 4 out of 1680 pairwise comparisons, all in HAT4, hence indicating no physical linkage between any STR loci (results not shown); 5 out of 240 tests indicated significant departures from Hardy-Weinberg expectations after Bonferroni correction, 3 of which were in HAT4 (Appendix S3).
Table III. Genetic diversity indices and results of demographic analyses for 12 brown trout sampling sites and 4 hatcheries (Site): observed (Ho) and expected (He) heterozygosity; the average number of alleles (An) and private alleles (Pa); allelic richness (Ar); probability (p) of a severe recent bottleneck as inferred by BOTTLENECK analysis according to IAM e TPM models. Standard errors (SE) are shown in parentheses when appropriate. The number of analyzed individuals at STR loci (NSTR) and the Apennine slope is also indicated for wild sites and the hatchery of Mediterranean trout.
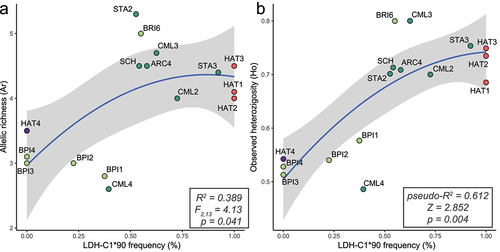
All STR loci were polymorphic, as we detected from 4 (Str60INRA) to 40 alleles (Omm1064) per locus. Genetic diversity (Ar, Ho, He) was roughly lower in Adriatic (BPI) than in Tyrrhenian sites (except for BRI6 and CML4 which revealed the opposite pattern) and Atlantic hatcheries. Maximum private alleles (Pa) were registered in SCH and BRI6. Per-site diversity metrics are summarized in . Performed models indicated that a higher population diversity occurred at intermediate LDH-C1*90 frequency, i.e. when the admixture between native and domestic lineages is maximized (). The frequency of the LDH-C1*90 allele significantly contributed to explaining both Ar (R2 = 0.389, F2,13 = 4.13, p = 0.041; ) and Ho (pseudo-R2 = 0.612, Z = 2.852, p = 0.004; ) across either sampling sites and hatcheries.
Figure 2. Relationship (quadratic regression) between the frequency of the domestic-Atlantic LDH-C1*90 allele and measures of genetic diversity – allele richness (a), observed heterozygosity (b), based on 15 STR loc. For each model, R-squared, statistics, p-value, and 95% confidence intervals (in grey) are shown. Site abbreviations refer to ; colours distinguish between Adriatic FCNP sites (pale green), Tyrrhenian FCNP sites (aquamarine), Atlantic hatcheries (pale red) and the Mediterranean hatchery (violet).
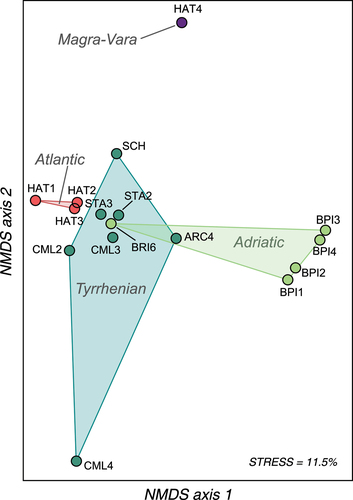
Pairwise genetic differentiation (Fst) was significant in all but four comparisons – namely, proximate sites within the Adriatic Bidente di Pietrapazza catchment (BPI1-BPI2, BPI3-BPI4), two Atlantic hatcheries (HAT2-HAT3) and the BRI6-HAT2 pair – and ranged between −0.01 and 0.39 (Appendix S4). The NMDS plot () depicting genetic relationships among sites indicated: (1) clear differentiation between Adriatic and Tyrrhenian sites, apart of BRI6 that was close to sites of the opposite slope; (2) similarity among Atlantic hatcheries which, in turn, were close to most Tyrrhenian sites; (3) strong divergence of CML4 and HAT4. The PERMANOVA analysis supported the overall differentiation among groups (F = 6.37, p = 0.0005), due to divergence between Adriatic and Tyrrhenian sites (F = 7.86, Bonferroni-adjusted p = 0.017); other tests between group pairs were not statistically significant (Bonferroni-adjusted p > 0.05).
Figure 3. Non-metric multidimensional scaling (NMDS) of the population-pairwise genetic distance (Fst) matrix based on 15 STR loci. Wild sites/hatcheries, which abbreviations refer to , are grouped according to their geographic area of origin and coloured accordingly: Tyrrhenian Apennine slope; Adriatic Apennine slope; Atlantic (hatchery strains); Magra-Vara drainage basin (Mediterranean hatchery).
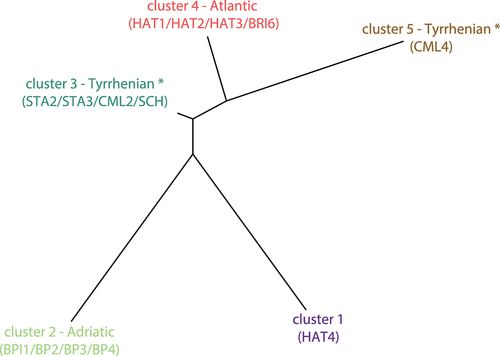
Evanno’s ∆K method strongly suggested three (K = 3) or, secondarily, five (K = 5) optimal clusters (), and the likelihood probability curve reached the plateau (Pritchard’s method) approximately at K = 5 (). Consequently, we discussed clustering options (K) in the range of 3–5. In the uppermost partitioning (K = 3; ) Adriatic sites (except BRI6) grouped together, Mediterranean trout from the Magra-Vara drainage basin (HAT4) constituted a distinct cluster, whereas Atlantic hatcheries, Tyrrhenian sites and BRI6 formed the third cluster. At increasing K values, a substructure emerged within the latter cluster: Tyrrhenian sites separated from Atlantic hatcheries and BRI6 at K = 4 (), while CML4 formed a cluster clearly distinguishable from other Tyrrhenian sites at K = 5 (). In all examined clustering options, ARC4 showed massive admixture with the “Adriatic cluster”, whereas some individuals of SCH revealed a large genome amount of Mediterranean trout from the Magra-Vara (i.e., the HAT4 cluster). The tree topology depicting relationships among 5 STRUCTURE-inferred clusters indicates coancestry, despite substantial divergence, between HAT4 and BPI sites, and between CML4 and Atlantic hatcheries; conversely, the cluster including most Tyrrhenian sites was in an intermediate position between Atlantic and Adriatic clusters (). The results above refer to the STRUCTURE analyses based on independent allele frequencies and the whole STR dataset. Anyhow, we gathered almost identical outputs after: setting correlated allele frequencies (results not shown); removing 18 full-siblings from HAT4, as detected by the COLONY software v. 2.0.0.6 (Wang Citation2009), to check for possible clustering biases due to either uneven sampling size (Puechmaille Citation2016) or family structuring within the stock (Anderson & Dunham Citation2008) (results not shown).
Figure 4. Results of the Bayesian clustering inference (independent allele frequencies) based on 15-STR genotypes of 272 brown trout from 12 wild sampling sites and four hatcheries in central-northern Apennines (Italy): the distribution of Evanno’s ∆K (a) and the log-likelihood probability (b) for increasing K values; barplots of individual assignment values according to the optimal clustering – K = 3 (c), K = 4 (d) and K = 5 (e). Site/hatchery abbreviations refer to .
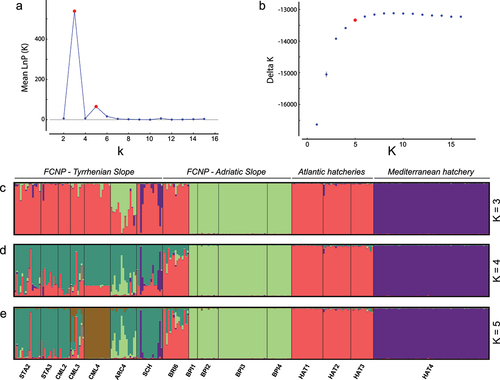
Figure 5. The distance tree generated by STRUCTURE and depicting genealogies among the 5 inferred genetic clusters (K = 5). Cluster colors refer to . Sites clearly assigned to a cluster (< 30% admixture) are indicated in parentheses – (*) note that “Tyrrhenian” refers to the geographic location of sites, rather than their genetic makeup (see text for details).
The AMOVA breakdown () indicated that differentiation within populations accounted for the majority of genetic variance, as expected for hypervariable STR loci. The variance explained by differences among and within geographic groups was comparable (around 11%). Conversely, partitioning based on optimal STRUCTURE-defined genetic clusters – and after removing substantially admixed sites that cannot unambiguously be assigned to a single cluster, i.e. ARC4 for K = 3, and ARC4 + CML3 for K = 5 – reduced the percentage of variance explained by within-group differences, while increasing the amount of variance accounting for the differentiation among groups.
Table IV. Results from AMOVA hierarchical analyses examining the partitioning of genetic (15 STR loci) variance according to different hypothesized structures: (1) no structuring with one panmictic population (no groups); (2) geographic differentiation with four groups defined according to major geographic areas of origin; (3) optimal genetic clustering (3 and 5 groups, after removing substantially admixed sites that cannot be clearly assigned to a cluster) as revealed by STRUCTURE analyses. The amount of variation (%) explained by differences among groups, among populations within groups, and within populations, along with the statistical significance of p-values (*** = p < 0.001; ** = p < 0.01; * = p < 0.05; ns = p ≥ 0.05) are given.
The BOTTLENECK IAM-based analysis detected significant population size reductions in all Tyrrhenian sites but ARC4, as well as in BRI6 and each hatchery stock. The bottleneck signal was confirmed by the TPM-based analysis in STA3, CML3 and BRI6 ().
Discussion
In this study, we mainly characterized the genetic diversity and population structure of Mediterranean brown trout populations of the FCNP and surrounding areas (northern Apennines, Italy), revealing that multiple anthropogenic manipulations have deeply modified the original genetic composition of wild populations. The research was conducted within the LIFE STREAMS project framework that aims at preserving the genetic integrity of native Mediterranean trout populations and recovering threatened ones in Italian protected areas and Natura 2000 sites.
The first-level genetic assessment, which relies on routinely used LDH-C1/D-loop diagnostic molecular markers (e.g. Splendiani et al. Citation2013; Berrebi et al. Citation2019; Rossi et al. Citation2019), showed extensive introgression from domestic-Atlantic strains in almost all wild sites, as well as several populations entirely made up of exotic trout (). Nuclear (LDH-C1-based) introgression rates correlated with mitochondrial (D-loop) ones across sites (Spearman rs = 0.76, p < 0.0001), although the former were more pronounced than the latter (Wilcoxon signed rank test: W = 215, p = 0.033). Similar findings were reported in previous studies (e.g. Rossi et al. Citation2022), probably as the consequence of mechanisms promoting Mediterranean female mating with Atlantic males, such as a different strain-dependent migratory behaviour, fitness and/or sexual selection (Hansen et al. Citation2000; Splendiani et al. Citation2019a).
Unfortunately, the observed overall bad conservation status of investigated FCNP populations was partly expected according to results reported in grey literature, which targeted other sites in the area (e.g. Casali Citation2015; technical reports of the T.R.O.T.A. project, see below). Indeed, despite stocking with hatchery strains of hybrid/Atlantic origin stopped around 10 years ago in the study area (Alberti, pers. comm.) – also corroborated by the occurrence of medium- to low-density, age-structured local populations (LIFE STREAMS unpublished data) –, releases have been reiterated in Tuscany (Nocita Citation2007) and in other Italian rivers since the middle of the 19th century to support fisheries (Meraner & Gandolfi Citation2018). This eventually caused the massive spread of Atlantic ancestry in native populations across the entire Italian peninsula (Nonnis Marzano et al. Citation2003; Splendiani et al. Citation2016; Fabiani et al. Citation2018; Berrebi et al. Citation2019; Rossi et al. Citation2019), even in protected areas (Splendiani et al. Citation2019b; Rossi et al. Citation2022), and substantially contributed to lead many of them towards the edge of extinction. Besides, hydrogeological features may have interplayed to generate the current scarcity of native populations in the FCNP area: Apennine watercourses flowing on impermeable rocks are particularly exposed to severe water flow fluctuations, which have likely caused cyclical reductions, or even extinctions, of local trout populations in the past. This could have ultimately favoured the recent establishment and the pervasive introgression of domestic-Atlantic lineages (Splendiani et al. Citation2013, Citation2016).
Patterns of population structure, genetic diversity and demographic inference gave additional evidence of the allochthonous/hybrid nature of most examined populations, especially from the Tyrrhenian slope, and provided further insight into their origin. At first, both the NMDS () and the uppermost STRUCTURE partitioning (K = 3; ) pointed to the similarity between Atlantic hatcheries and wild Tyrrhenian sites (along with BRI6). However, a finer genetic structure, further supported by AMOVA results, emerged at K = 5 (; ), suggesting some differentiation between wild sites (except BRI6) and hatcheries, but still pointing out the introgression/admixture with domestic-Atlantic trout. Such a subtle differentiation may result from the release of domestic trout of unsampled stocks and/or from the residual-native gene pool, extremely diluted with exotic genes – this latter hypothesis is consistent with the widespread occurrence of presumably local haplotypes (Me25, Ad52, Ma2a). Secondly, genetic diversity was generally higher, as found in previous studies investigating the effects of captive-bred ancestry in wild brown trout populations (Almodóvar et al. Citation2006; Prunier et al. Citation2022; Rossi et al. Citation2022). The contribution of hatchery strains to population polymorphisms, quantifiable as 39% for Ar and 61% for Ho, was suggested by the relationship between diversity metrics and the introgression degree (). Noticeably, the diversity of Atlantic stocks appeared higher than the diversity of native populations, confirming the remarkable polymorphism observed in European hatcheries rearing Atlantic strains, despite the expected genetic erosion (Berrebi et al. Citation2021). Thirdly, we detected signals of recent bottlenecks, although partially inconsistent between applied methods, in many introgressed populations (), likely suggesting a certain influence of the founder effect and genetic drift due to the release of a limited number of domestic individuals into the wild.
On the other hand, we were able to identify a few purely native or less introgressed sites, all within the Bidente di Pietrapazza (BPI) catchment in the Adriatic slope. These sites showed genetic and demographic patterns opposite to their hybrid/exotic counterparts, strengthening the hypothesis of their natural origin: population structure analyses (NMDS, PERMANOVA, STRUCTURE) indicated a strong differentiation from other wild sites, reference hatcheries and the domestic trout from the Magra-Vara drainage basin – even if the inbred/bottlenecked nature of the HAT4 stock may also contribute to the observed differentiation; the genetic diversity indices tended to be lower than the other sampled sites; BOTTLENECK analyses revealed demographic stability. Overall, similar patterns were documented in other wild Italian-native brown trout populations (Rossi et al. Citation2022), matching with the expectations for upstream populations that are constrained by both physical and ecological barriers (dams, river flow fluctuations, water temperature limits), and thus exposed to genetic consequences of population isolation and fragmentation (Paz-Vinas & Blanchet Citation2015; Sanz et al. Citation2019).
Adriatic sites of the Bidente di Pietrapazza were genetically unrelated to geographically close sites of the Tyrrhenian slope, thus suggesting that the Apennines have acted as a barrier to trout dispersal. However, contrary to all other Tyrrhenian sites, the lowest stretch of the Archiano stream (ARC4) revealed deep introgression from the Adriatic-native cluster coupled with the signal of demographic stability, raising interesting questions about its origin. A natural/semi-natural origin hypothesis would imply an ancient connection, through natural or anthropogenic river capture processes, between the currently separated Apennine drainage basins, a theory invoked to explain the distribution pattern of (genetic) diversity of freshwater fishes (Bianco Citation1994), such as the barbel in southern Apennines (Zaccara et al. Citation2019) or the marble trout in Slovenia (Berrebi et al. Citation2017). Here, despite a human-mediated river capture has been documented in the northern sector of the study area – the so-called “Taglio della Regina”, a canal presumably built during the Middle Ages to connect the Adriatic Acquacheta (ACQ) catchment to Tyrrhenian drainage basins (Gambi Citation1949; Veggiani Citation1972) –, a migration scenario appears improbable, as we did not find extensive introgression in Tyrrhenian river basins, but solely in a single site of the Archiano catchment. Alternatively, we may hypothesize recent-unauthorized and/or historical-episodic translocations from the Adriatic slope, as fish farming activities were carried on by local people and Camaldolesi Monks for food supply since the Middle Ages (Bottacci Citation2012).
It is worth mentioning that we also found persuasive indications of presumably recent anthropogenic manipulations involving allochthonous Mediterranean trout. Specifically, some individuals from the Scheggia stream (SCH) were assigned (or showed pervasive admixture) to the cluster of domestic trout of the Magra-Vara drainage basin (). The presence of the Ad-Tyrrh1 haplotype in SCH, which occurs in Liguria (LIFE STREAMS unpublished data) and is fixed in the inbred HAT4 stock (), provided further evidence for the allochthonous origin of many SCH individuals; we can easily rule out the hypothesis of a natural dispersion as the two drainage basins are separated by multiple impassable physical and ecological barriers. To our knowledge, the recent translocation of individuals from different drainage basins, as well as stocking activities with allochthonous Mediterranean trout for purposes other than biodiversity conservation have been occasionally investigated to date, although such practices may occur more frequently than reported (e.g. Caputo Barucchi Citation2003; Nocita & Poggesi Citation2010; Pinter et al. Citation2019) and despite their potentially deleterious effects on native populations. Indeed, the alteration of the (adaptive) genetic makeup of independently evolved populations may ultimately cause their viability to decrease (Pinter et al. Citation2019), particularly for the Mediterranean trout that often exhibits fine-scale population differentiation (Zaccara et al. Citation2015; Splendiani et al. Citation2019b; Palombo et al. Citation2021; Rossi et al. Citation2022).
The critical situation that emerged from this study calls for urgent conservation actions in the monitored area aimed at preserving any remnant native populations while eradicating allochthonous individuals. In particular, according to our findings, we may conclude that BPI sites, the only native in the study area, belong to a single genetically distinct (e.g. from domestic Mediterranean trout of the Magra-Vara drainage basin) Management Unit (Palsbøll et al. Citation2007). As such, they should be separately managed and accurately preserved (Moritz Citation1999). The BPI population could serve as a source of breeders for population enhancement and restocking plans, as already started within the framework of the European LIFE STREAMS project (https://www.lifestreams.eu/?lang=en), and the previous T.R.O.T.A. project (https://www.parcoforestecasentinesi.it/en/news/il-progetto-trota) funded on purpose by the FCNP. Appropriate stocking sites should be carefully chosen within the same drainage basin and among populations genetically compatible with the source one, namely belonging to the same Management Unit (Moritz Citation1999). Successful supplemental breeding programs should also account for the effective population size (Ne) of the donor population(s). In particular, Ne is rather small in BPI – Ne = 24 (95% confidence intervals = 14–42) according to COLONY estimates obtained setting analysis parameters as in Rossi et al. (Citation2022) and after cumulating 59 BPI individuals –, consistently with findings from other (isolated) populations of Mediterranean-native trout (e.g. Splendiani et al. Citation2019b; Rossi et al. Citation2022). Thus, we recommend the cautious use of wild spawners and their frequent replacement, to avoid further eroding genetic diversity of offspring and to mitigate other well-known issues associated with fish farming, such as domestication (Frankham et al. Citation2010).
In synthesis, this study highlights the relevance of a multilevel conservation genetic approach to investigate and clarify the origin and the makeup of wild populations of intensively managed taxa, such as salmonids, which could be extended to other areas and species subject to similar management issues.
Authors’ contribution
LT, NM, RC, CG and DA conceived and designed the study. DA, ADP, LC, MR, GT, CG, NM and ML did fieldwork and collected DNA samples. LT analysed the data and curated graphical outputs. LT wrote the manuscript with the contribution of AC. NM, RC, CG, ML, SD, DA, MR and LC revised the manuscript. DA, CP and SD provided financial support to the study. All authors read and agreed to the final version of the manuscript.
Supplemental Material
Download MS Word (67.2 KB)Acknowledgements
The authors gratefully thank Marco Carafa (Majella National Park), Alessandro Rosetti (Sibillini National Park), Antonio Perfetti and Paola Amprimo (Montemarcello-Magra Regional Park) for their contribution to DNA sampling and data sharing. We acknowledge the “Vivaio M. Petrolini” (Maresca - San Marcello Pistoiese) and the “Centro Ittiogenico Sperimentale e di Idrobiologia (CISI)” (L’Aquila) for providing samples from their hatcheries. Finally, we sincerely thank the associate Editor and the two anonymous Reviewers for their valuable comments and suggestions that greatly contributed to improve the quality of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2223222
Additional information
Funding
References
- Adamack AT, Gruber B. 2014. PopGenReport: Simplifying basic population genetic analyses in R. Methods in Ecology and Evolution Wiley/Blackwell (10.1111) 5:384–387 DOI: 10.1111/2041-210X.12158.
- Almodóvar A, Nicola GG, Ayllón D, Elvira B. 2012. Global warming threatens the persistence of Mediterranean brown trout. Global Change Biology 18:1549–1560. DOI: 10.1111/j.1365-2486.2011.02608.x.
- Almodóvar A, Nicola GG, Elvira B, García-Marín JL. 2006. Introgression variability among Iberian brown trout evolutionary significant units: The influence of local management and environmental features. Freshwater Biology 51:1175–1187. DOI: 10.1111/j.1365-2427.2006.01556.x.
- Anderson EC, Dunham KK. 2008. The influence of family groups on inferences made with the program structure. Molecular Ecology Resources 8:1219–1229. DOI: 10.1111/j.1755-0998.2008.02355.x.
- Aparicio E, Vargas MJ, Olmo JM, de Sostoa A. 2000. Decline of native freshwater fishes in a Mediterranean watershed on the Iberian Peninsula: A quantitative assessment. Environmental Biology of Fishes 59:11–19. DOI: 10.1023/A:1007618517557.
- Baratti M, Nonnis-Marzano F, Fratini S, Piccinini A, Patarnello T, Dessì-Fulgheri F, Gandolfi G. 2006. Caratterizzazione genetica delle popolazioni di Trota fario del Parco delle Foreste Casentinesi. Biologia Ambientale 20:237–240.
- Bernatchez L. 2001. The evolutionary history of brown trout (Salmo trutta L.) inferred from phylogeographic, nested clade, and mismatch analyses of mitochondrial DNA variation. Evolution 55:351–379. DOI: 10.1111/j.0014-3820.2001.tb01300.x.
- Berrebi P, Caputo Barucchi V, Splendiani A, Muracciole S, Sabatini A, Palmas F, Tougard C, Arculeo M, Marić S. 2019. Brown trout (Salmo trutta L.) high genetic diversity around the Tyrrhenian Sea as revealed by nuclear and mitochondrial markers. Hydrobiologia 826:209–231. DOI: 10.1007/s10750-018-3734-5.
- Berrebi P, Horvath Á, Splendiani A, Palm S, Bernaś R. 2021. Genetic diversity of domestic brown trout stocks in Europe. Aquaculture 544:737043. DOI: 10.1016/j.aquaculture.2021.737043.
- Berrebi P, Jesenšek D, Crivelli AJ. 2017. Natural and domestic introgressions in the marble trout population of Soča River (Slovenia). Hydrobiologia 785:277–291. DOI: 10.1007/s10750-016-2932-2.
- Bianco PG. 1994. L’Ittiofauna continentale dell’Appennino umbro-marchigano, barriera semipermeabile allo scambio di componenti primarie tra gli opposti versanti dell’Italia centrale. Biogeographia 17:427–485.
- Bottacci A. 2012. Cenni storici sulla Riserva naturale biogenetica di Camaldoli. In: Bottacci A, editor. La Riserva naturale biogenetica di Camaldoli. 1012-2012. Mille anni di rapporto uomo-foresta. Stia (Arezzo): Arti Grafiche Cianferoni. pp. 27–54.
- Cairney M, Taggart JB, Høyheim B. 2000. Characterization of microsatellite and minisatellite loci in Atlantic salmon (Salmo salar L.) and cross-species amplification in other salmonids. Molecular Ecology Blackwell Publishing Ltd 9:2175–2178.
- Caputo Barucchi V. 2003. Ricerche sulla biodiversità della trota fario (Salmo trutta L., 1758) nella Provincia di Pesaro e Urbino e nelle Marche. Pesaro, Italy: Quaderni dell’ambiente.
- Carosi A, Ghetti L, Padula R, Lorenzoni M. 2020. Population status and ecology of the Salmo trutta complex in an Italian river basin under multiple anthropogenic pressures. Ecology and Evolution 10:7320–7333. DOI: 10.1002/ece3.6457.
- Carosi A, Ghetti L, Soresina A, Lorenzoni M. 2022. Catch and release angling: Implications for the management and conservation of the Mediterranean trout in central Italy. Fisheries Research 150:106285. DOI: 10.1016/j.fishres.2022.106285.
- Casali L. 2015. Ricerca, genotipizzazione e recupero della trota autoctona all’interno del versante Romagnolo del Parco Nazionale delle Foreste Casentinesi. Università degli Studi di Urbino “Carlo Bo”.
- Cortey M, García-Marín JL. 2002. Evidence for phylogeographically informative sequence variation in the mitochondrial control region of Atlantic brown trout. Journal of Fish Biology 60:1058–1063. DOI: 10.1111/j.1095-8649.2002.tb02429.x.
- Earl DA, vonHoldt BM. 2012. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources 4:359–361. http://link.springer.com/10.1007/s12686-011-9548-7.
- Estoup A, Presa P, Krieg F, Vaiman D, Guyomard R. 1993. (CT)n and (GT)n microsatellites: A new class of genetic markers for Salmo trutta L. (brown trout). Heredity Nature Publishing Group 71:488–496. https://www.nature.com/articles/hdy1993167.
- Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software STRUCTURE : A simulation study. Molecular Ecology 14:2611–2620. DOI: 10.1111/j.1365-294X.2005.02553.x.
- Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10:564–567. DOI: 10.1111/j.1755-0998.2010.02847.x.
- Fabiani A, Gratton P, Zappes IA, Seminara M, D’Orsi A, Sbordoni V, Allegrucci G. 2018. Investigating the genetic structure of trout from the Garden of Ninfa (central Italy): Suggestions for conservation and management. Fisheries Management and Ecology 25:1–11. DOI: 10.1111/fme.12259.
- Frankham R, Briscoe D, Ballou J. 2010. Introduction to conservation genetics. Cambridge: Cambridge University Press.
- Gambi L. 1949. Di una catturetta fluviale in val Lamone. XIV Congresso Geografico Italiano 410–412.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9.
- Hansen MM, Ruzzante DE, Nielsen EE, Mensberg KLD. 2000. Microsatellite and mitochondrial DNA polymorphism reveals life-history dependent interbreeding between hatchery and wild brown trout (Salmo trutta L.). Molecular Ecology 9:583–594. DOI: 10.1046/j.1365-294x.2000.00898.x.
- King TL, Eackles MS, Letcher BH. 2005. Microsatellite DNA markers for the study of Atlantic salmon (Salmo salar) kinship, population structure, and mixed-fishery analyses. Molecular Ecology Notes 5:130–132. DOI: 10.1111/j.1471-8286.2005.00860.x.
- Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I. 2015. CLUMPAK: A program for identifying clustering modes and packaging population structure inferences across K. Molecular Ecology Resources 15:1179–1191. DOI: 10.1111/1755-0998.12387.
- Lobón-Cerviá J, Sanz N. 2018. Brown trout: Biology, ecology and management. Hoboken, NJ: John Wiley & Sons
- Marcuccini G. 2021. La scoperta della Lama «il più ameno e maestoso di tutti i luoghi» (Pietro Benci, 27 luglio 1821). In: Boattini A, Marcuccini G, Rossi A, editors. Alpe Appennina: Storia e storie fra Romagna e Toscana. Cesena: Raffaele Monti Editore. Vol. 3. pp. 9–30.
- McMeel OM, Hoey EM, Ferguson A. 2001. Partial nucleotide sequences, and routine typing by polymerase chain reaction-restriction fragment length polymorphism, of the brown trout (Salmo trutta) lactate dehydrogenase, LDH-C1*90 and *100 alleles. Molecular Ecology 10:29–34. DOI: 10.1046/j.1365-294X.2001.01166.x.
- Meraner A, Gandolfi A. 2018. Genetics of the genus Salmo in Italy: Evolutionary history, population structure, molecular ecology and conservation. In: Lobón‐Cerviá J, Sanz N, editors. Brown trout: Biology, ecology and management. Chichester, UK: John Wiley & Sons, Ltd. pp. 65–102. DOI: 10.1002/9781119268352.ch3.
- Moritz C. 1994. Defining ‘Evolutionarily significant units’. Trends in Ecology and Evolution 9:373–375.
- Moritz C. 1999. Conservation units and translocations: Strategies for conserving evolutionary processes. Hereditas 130:217–228. DOI: 10.1111/j.1601-5223.1999.00217.x.
- Nocita A. 2007. La fauna ittica del bacino dell’Arno. Biologia Ambientale 21:97–105.
- Nocita A, Poggesi M. 2010. Le introduzioni di pesci alieni nelle nostre acque: Tra necessità e gioco. In: Sznura F, editor. Fiumi e laghi della Toscana tra passato e presente - Pesca, memorie, regole. Provincia di Firenze, Firenze: Aska Edizioni. pp. 369–380.
- Nonnis Marzano F, Corradi N, Papa R, Tagliavini J, Gandolfi G. 2003. Molecular evidence for introgression and loss of genetic variability in Salmo (trutta) macrostigma as a result of massive restocking of Apennine populations (Northern and Central Italy). Environmental Biology of Fishes 68:349–356. DOI: 10.1023/B:EBFI.0000005762.81631.fa.
- O’Reilly PT, Hamilton LC, McConnell SK, Wright JM. 1996. Rapid analysis of genetic variation in Atlantic salmon (Salmo salar) by PCR multiplexing of dinucleotide and tetranucleotide microsatellites. Canadian Journal of Fisheries and Aquatic Sciences 53:2292–2298.
- Padula A, Greco C, Talarico L, Caniglia R, Antognazza CM, D’Antoni S, Lorenzoni M, Vanetti I, Zaccara S, Mucci N. (in press). A rapid and reliable detection procedure of Atlantic trout introgression at the diagnostic lactate dehydrogenase chain-1 gene. Aquaculture, Fish and Fisheries. DOI: 10.1002/aff2.124.
- Palombo V, De Zio E, Salvatore G, Esposito S, Iaffaldano N, Andrea MD. 2021. Genotyping of two Mediterranean trout populations in central-southern Italy for conservation purposes using a rainbow-trout-derived SNP array. Animals 11:1803. DOI: 10.3390/ani11061803.
- Palsbøll PJ, Bérubé M, Allendorf FW. 2007. Identification of management units using population genetic data. Trends in Ecology and Evolution 22:11–16. DOI: 10.1016/j.tree.2006.09.003.
- Paterson S, Piertney SB, Knox D, Gilbey J, Verspoor E. 2004. Characterization and PCR multiplexing of novel highly variable tetranucleotide Atlantic salmon (Salmo salar L.) microsatellites. Molecular Ecology Notes 4:160–162. DOI: 10.1111/j.1471-8286.2004.00598.x.
- Paz-Vinas I, Blanchet S. 2015. Dendritic connectivity shapes spatial patterns of genetic diversity: A simulation-based study. Journal of Evolutionary Biology 28:986–994. DOI: 10.1111/jeb.12626.
- Peakall R, Smouse PE. 2012. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics Oxford University Press 28:2537–2539. https://academic.oup.com/bioinformatics/article-lookup/doi/10.1093/bioinformatics/bts460.
- Pinter K, Epifanio J, Unfer G. 2019. Release of hatchery-reared brown trout (Salmo trutta) as a threat to wild populations? A case study from Austria. Fisheries Research Elsevier 219:105296. DOI: 10.1016/j.fishres.2019.05.013.
- Piry S, Luikart G, Cornuet J-M. 1999. BOTTLENECK: A computer program for detecting recent reductions in the effective population size using allele frequency data. Journal of Heredity 90:502–503. DOI: 10.1093/jhered/90.4.502.
- Presa P, Guyomard R. 1996. Conservation of microsatellites in three species of salmonids. Journal of Fish Biology 49:1326–1329.
- Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155:945–959. DOI: 10.1093/genetics/155.2.945.
- Prunier JG, Poulet N, Saint-pé K, Tissot L, Poulet N, Veyssière C, Blanchet S. 2022. Captive-bred ancestry affects spatial patterns of genetic diversity and differentiation in brown trout (Salmo trutta) populations. Aquatic Conservation: Marine and Freshwater Ecosystems in press 32:1529–1543. DOI: 10.1002/aqc.3826.
- Puechmaille SJ. 2016. The program STRUCTURE does not reliably recover the correct population structure when sampling is uneven: Subsampling and new estimators alleviate the problem. Molecular Ecology Resources 16:608–627. DOI: 10.1111/1755-0998.12512.
- Rexroad CE, Coleman RL, Hershberger WK, Killefer J. 2002. Rapid communication: Thirty-eight polymorphic microsatellite markers for mapping in rainbow trout. Journal of Animal Science 80:541–542. https://researchrepository.wvu.edu/faculty_publications.
- Ribeiro F, Leunda PM. 2012. Non-native fish impacts on Mediterranean freshwater ecosystems: Current knowledge and research needs. Fisheries Management and Ecology 19:142–156. DOI: 10.1111/j.1365-2400.2011.00842.x.
- Rondinini C, Battistoni A, Teofili C. 2022. Lista Rossa IUCN dei Vertebrati italiani 2022. Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica. Roma: Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica.
- Rossi AR, Petrosino G, Milana V, Martinoli M, Rakaj A, Tancioni L. 2019. Genetic identification of native populations of Mediterranean brown trout Salmo trutta L . complex (Osteichthyes: Salmonidae) in central Italy. The European Zoological Journal Taylor & Francis 86:424–431. DOI: 10.1080/24750263.2019.1686077.
- Rossi AR, Talarico L, Petrosino G, Crescenzo S, Tancioni L. 2022. Conservation genetics of Mediterranean brown trout in central Italy (Latium): A multi-marker approach. Water 14:937. DOI: 10.3390/w14060937.
- Rousset F. 2008. GENEPOP’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Molecular Ecology Resources Wiley/Blackwell (10.1111) 8:103–106. DOI: 10.1111/j.1471-8286.2007.01931.x.
- Sanz N, Araguas RM, Fernández-Cebrián R, Lobón-Cerviá J. 2019. Factors modelling population structure in brown trout Salmo trutta L.: Genetic monitoring of populations in Esva River (northwestern Spain). Hydrobiologia 837:117–131. DOI: 10.1007/s10750-019-3965-0.
- Slettan A, Olsaker I, Lie Ø. 1995. Atlantic salmon, Salmo salar, microsatellites at the SSOSL25, SSOSL85, SSOSL311, SSOSL417 loci. Animal Genetics 26:277–285. https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=3598642.
- Splendiani A, Fioravanti T, Ruggeri P, Giovannotti M, Carosi A, Marconi ML, Righi T, Nisi Cerioni P, Caputo Barucchi V. 2019a. Life history and genetic characterisation of sea trout Salmo trutta in the Adriatic Sea. Freshwater Biology 65:460–473. DOI: 10.1111/fwb.13441.
- Splendiani A, Giovannotti M, Righi TF, Cerioni PN, Lorenzoni M, Carosi A, La Porta G, Barucchi VC. 2019b. Introgression despite protection: The case of native brown trout in Natura 2000 network in Italy. Conservation Genetics Springer Netherlands 20:343–356. DOI: 10.1007/s10592-018-1135-y.
- Splendiani A, Palmas F, Sabatini A, Caputo Barucchi V. 2019c. The name of the trout: Considerations on the taxonomic status of the Salmo trutta L., 1758 complex (Osteichthyes: Salmonidae) in Italy. European Zoological Journal Taylor & Francis 86:432–442. DOI: 10.1080/24750263.2019.1686544.
- Splendiani A, Ruggeri P, Giovannotti M, Caputo Barucchi V. 2013. Role of environmental factors in the spread of domestic trout in Mediterranean streams. Freshwater Biology 58:2089–2101. DOI: 10.1111/fwb.12193.
- Splendiani A, Ruggeri P, Giovannotti M, Pesaresi S, Occhipinti G, Fioravanti T, Lorenzoni M, Nisi Cerioni P, Caputo Barucchi V. 2016. Alien brown trout invasion of the Italian peninsula: The role of geological, climate and anthropogenic factors. Biological Invasions Springer International Publishing 18:2029–2044. DOI: 10.1007/s10530-016-1149-7.
- Stoch F, Grignetti A. 2021. IV Report Direttiva Habitat: Specie animali. In: Ercole S, Angelini P, Carnevali L, Casella L, Giacanelli V, Grignetti A, La Mesa G, Nardelli R, Serra L, Stoch F, Tunesi L, Genovesi P, editors. Rapporti Direttive Natura (2013-2018). Sintesi dello stato di conservazione delle specie e degli habitat di interesse comunitario e delle azioni di contrasto alle specie esotiche di rilevanza unionale in Italia. ISPRA: Serie Rapporti 349/2021. pp. 39–68.
- Talarico L, Marta S, Rossi AR, Crescenzo S, Petrosino G, Martinoli M, Tancioni L. 2021. Balancing selection, genetic drift, and human mediated-introgression interplay to shape MHC (functional) diversity in Mediterranean brown trout. Ecology and Evolution 11:10026–10041. DOI: 10.1002/ece3.7760.
- Tamura K, Stecher G, Kumar S. 2021. MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution 38:3022–3027.
- Thorsen J, Zhu B, Frengen E, Osoegawa K, de Jong PJ, Koop BF, Davidson WS, Høyheim B. 2005. A highly redundant BAC library of Atlantic salmon (Salmo salar): An important tool for salmon projects. BMC Genomics BioMed Central Ltd 6:50.
- Veggiani A. 1972. Come si formò l’Acquacheta. Studi Romagnoli 23:35–47.
- Vera M, Garcia-Marin JL, Martinez P, Araguas RM, Bouza C. 2013. Identification and conservation of remnant genetic resources of brown trout in relict populations from Western Mediterranean streams. Hydrobiologia 707:29–45. DOI: 10.1007/s10750-012-1402-8.
- Wang J. 2009. A new method for estimating effective population sizes from a single sample of multilocus genotypes. Molecular Ecology 18:2148–2164. DOI: 10.1111/j.1365-294X.2009.04175.x.
- Zaccara S, Quadroni S, De Santis V, Vanetti I, Carosi A, Britton R, Lorenzoni M. 2019. Genetic and morphological analyses reveal a complex biogeographic pattern in the endemic barbel populations of the southern Italian peninsula. Ecology and Evolution 9:10185–10197. DOI: 10.1002/ece3.5521.
- Zaccara S, Trasforini S, Antognazza CM, Puzzi C, Britton JR, Crosa G. 2015. Morphological and genetic characterization of Sardinian trout Salmo cettii Rafinesque, 1810 and their conservation implications. Hydrobiologia Springer International Publishing 760:205–223.
