Abstract
Lampetra zanandreai (Vladykov, 1955) is a non-parasitic, freshwater lamprey endemic to the ancient Po basin. A few, mostly very dated studies have investigated some aspect of the biology of this lamprey, but surprisingly, despite it being considered a threatened species, information on its ecology is practically absent. Specifically, information about habitat preferences is generic and qualitative. Since most of the life cycle is spent in the fossorial larval stage, which is also the only one in which organisms feed, information about ecological requirements of ammocoetes is essential for any conservation strategy. In this study we provide the first data about physical habitat preferences for lamprey ammocoetes by analyzing their presence within sampled hydro-morphological units (HMUs), following the approach of habitat attribute description of the MesoHABSIM (MesoHABitat SImulation Model) methodology. To explore the relationship between lamprey presence and HMU characteristics, a random forest (RF) model was developed and tested using data collected in five stream reaches of the Po basin (NW Italy). The final parsimonious RF model performed well in terms of accuracy (95.2%) and true skill statistic (90.4%), allowing us to identify the most significant mesohabitat attributes for the considered species. Furthermore, in the Ghiandone River, where the highest density and number of individuals were found, a granulometric analysis of the riverbed material was carried out. Results showed that selected strains of sand and fine gravel, with low organic content, are preferred by ammocoetes. To our knowledge, this is the first study exploring the habitat preference of this endangered species, listed in Annex II of the European Habitats Directive.
Introduction
In 1955, Vladim Vladykov received lamprey samples from the Po Valley and realized that they belonged to a species new to science, distinctly separable from the well-known European river lamprey Lampetra fluviatilis (Linnaeus, 1758) on the basis of precise morphological characters, such as disposition and number of teeth on oral disc, number of trunk myomeres, coloration and body proportions. The ichthyologist named the species Lampetra zanandreai (Vladykov, Citation1955). Subsequent studies detected the species also in basins of central Italy, Slovenia, Croatia, and Bosnia and Herzegovina (Bianco Citation1992; Holcik & Mrakovic Citation1997; Tutman et al. Citation2009), an area corresponding to the ancient Po during the Wurm glacial period.
The Po brook lamprey is a non-parasitic lamprey with ammocoetes that reach greater dimensions than adults, in both length and weight. Ammocoetes probably live four or five years, then metamorphose to spend six to eight months in the adult stage. Larval stages are bottom filter feeders, with a digestive system that degenerates soon after metamorphosis (Bianco Citation1986). During the adult stage these lampreys do not feed, and energy for metamorphosis, gonadogenesis and spawning comes from body reserves: for these reasons, reproductive stages are distinctly smaller than their last larval stages. Metamorphosis takes place from August to October, reproduction occurs from January to June and adults expire after spawning (Bianco Citation1986).
Lampetra zanandreai is considered an endangered species, listed in Annexes II and V of the EEC (European Economic Community) Habitats Directive 92/43, in the Appendix II and III of the EEC Bern Convention and in the IUCN (International Union for Conservation of Nature) Red List of Threatened Species (Crivelli Citation2005; Freyhof Citation2011; IUCN Citation2018). The distribution of this species is considered discontinuous and declining, with a strong diminution over the past decades (Caputo et al. Citation2009). Very little is known about the ecology of this species, which has been the subject of few, mostly dated studies.
The Po brook lamprey has an enormous potential of interest as it is a very ancient species, with a basal position in the development line of vertebrates, a very interesting biogeographical distribution and a state of conservation that deserves attention and insights. As reported before, scientific studies on this lamprey have always been sporadic and mainly dedicated to specific biological aspects. In the first few decades after its discovery, studies focused on the description of biological characteristics of the species, considering for example biometry and morphology (Zanandrea Citation1961a), systematic position (Zanandrea Citation1961b), geographic distribution (Zanandrea Citation1955, Citation1958, Citation1962), endocrinology (Zanandrea Citation1956, Citation1965) and cytology (Bertolini Citation1965). More recently, other authors have focused mainly on genetic aspects (Tagliavini et al. Citation1994; Caputo et al. Citation2009, Citation2011).
Interestingly, almost nothing is known about the ecology of this species. For example, information about habitat preferences is quite generic and descriptive, with hardly any quantitative data available in the current literature, as pointed out by Negro et al. (Citation2021). Since most of the life cycle is spent in the larval stage, which is also extremely important because it is the only phase in which the organisms feed, any strategy to improve the conservation of the species must be based on a better knowledge of the habitat of the ammocoetes. Regarding the ecology of the species, one of the earliest indications can be found in Zanandrea (Citation1963), which reports that L. zanandreai occurs from the sea level to 600 m in elevation, inhabiting muddy or sandy riverbeds. Basically, all the other more or less recent studies have not added anything to this summary description, using more or less the same words. For example, Zerunian (Citation2002) reported that larval stages live in lowland rivers, with moderate water current, muddy or sandy bottom, and the same can be found in Bianco (Citation1986), Tutman et al. (Citation2009) and others. Even in an FAO (Food and Agriculture Organization) book (Renaud Citation2011) dedicated to lampreys, the ecological requirements of L. zanandreai are limited to a single word (i.e. Habitat: freshwaters).
To quantitatively describe and predict the distribution patterns of freshwater species, habitat suitability models (HSMs) have been increasingly used in river ecology (Yi et al. Citation2017). Such models are based on the use of habitat suitability criteria, which allows researchers to estimate suitable riverine patches for a target species (or group of species) according to local habitat attributes (e.g. water depth, current velocity, substrate composition, presence of covers, Ahmadi‐Nedushan et al. Citation2006). The definition of these suitability criteria (i.e. habitat preferences of target species) can be achieved by statistically analyzing empirical data collected in the field, to identify the habitat requirements of target species (e.g. Lamouroux et al. Citation1999; Mouton et al. Citation2011; Vezza et al. Citation2012). Among the different statistical techniques proposed in the literature for defining species habitat preferences (Ahmadi‐Nedushan et al. Citation2006), the random forest (RF) classification algorithm (Breiman Citation2001) is gaining prominence in ecology (Evans et al. Citation2011). The RF algorithm is a machine learning ensemble arising from the classification and regression trees (CART; Breiman et al. Citation1984) and the bagging (Breiman Citation1996) techniques. By combining the prediction of a large set of randomized trees fitted on the dataset, it produces results with high accuracy requiring little computational time and reduced tuning, and provides readily available outputs (Cutler et al. Citation2007; Evans et al. Citation2011).
RF has already been successfully applied to define habitat preferences of fish (Vezza et al. Citation2014) and macroinvertebrates (Vezza et al. Citation2016) in Italian rivers, by analyzing species distribution with respect to the mesohabitat attributes, as described in the MesoHABSIM approach (MesoHABitat SImulation Model, Parasiewicz Citation2007). The MesoHABSIM is a meso-scale HSM increasingly used at the European level for hydro-morphological impact assessment and definition of environmental flows (e.g. Vezza et al. Citation2017; Koutrakis et al. Citation2019; Virbickas et al. Citation2020). It enables river habitat description using an ecologically relevant spatial scale (i.e. the mesohabitat or hydro-morphological unit scale; Belletti et al. Citation2017), it is based on a robust and adequate hydro-morphological characterization of the river stretch, and it allows researchers to consider in the analysis a large range of biotic and abiotic environmental descriptors, not only at the point where the organism is observed.
The aim of this study is to provide the first quantitative description of Po brook lamprey ammocoetes’ habitat preferences, by developing the first meso-scale habitat suitability criteria for this species and by analyzing its favored substrate composition.
Material and methods
Study area
The study domain consisted of five hydro-morphologically homogeneous (Belletti et al. Citation2017) stream reaches distributed within four rivers of the Alpine catchments of the Po river basin (NW Italy, ).
Figure 1. Location of the surveyed reaches considered in the study. Surveyed stream reaches: 1 = Belbo River; 2–3 = Ghiandone River; 4 = Orco River; 5 = Pellice River.
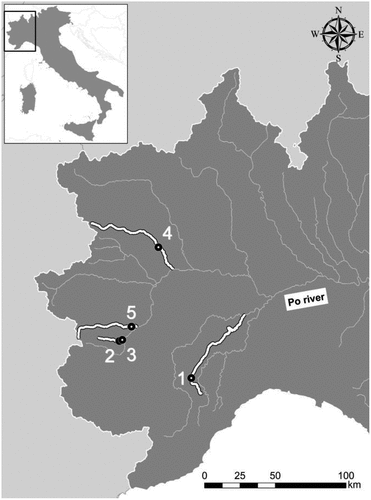
Reach 1 refers to the Belbo River, located in hilly areas of the southern Piemonte region. The reach is characterized by a single-thread channel, a high gradient, and a cobble-gravel substrate composition with some sand and silt. Reaches 2 and 3 were approximately 2 km apart and were both located in the Ghiandone River. The two reaches are pooled by gentle slopes, and are characterized by a similar sediment composition, which mostly consisted of fine gravel, sand and cobbles. Reach 4 was located in an unconfined reach of the Orco River, characterized by a pseudo-meandering morphology (Gurnell et al. Citation2014) with few secondary channels. In this reach river bed material comprised mainly cobbles and gravels with some sandy and silty patches located in the shallower marginal areas. Lastly, Reach 5 was located in the Pellice River, also characterized by a pseudo-meandering morphology with a gravel-sand sediment composition and a sequence of pools, riffles and bank-attached river bars. The main characteristics of the surveyed stream reaches are summarized in .
Table I. Name, location, characteristics and morphological classification of surveyed reaches. River morphology codes (according to Gurnell et al. Citation2014): C = confined; P = partly confined; U = unconfined; SS = straight-sinuous; M = meandering; S = sinuous; PM = pseudo-meandering. Reach width represents the mean value of the active river channel width in each surveyed reach.
Mesohabitat description and lamprey distribution
Between the autumn of 2019 and the summer of 2021, five field campaigns were performed in different seasons, in order to investigate the distribution of lamprey ammocoetes with respect to the physical habitat characteristics of the considered river reaches. In particular, within the five representative stream reaches (), 42 hydro-morphological units (HMUs) were identified and described following the MesoHABSIM approach (Parasiewicz Citation2007; Vezza et al. Citation2014). Surveys were carried out using the MapStream software (Vezza et al. Citation2017), by mapping each HMU in the QGIS environment by means of a rangefinder (Trupulse 360 R, Laser Technology, Inc., Centennial, CO, USA), a photographic tripod, a rugged field computer (Panasonic Toughbook FZ-M1, Panasonic Corporation, Kadoma, Japan) and multi-band RTK GNSS (Real-Time Kinematic Global Navigation Satellite System) receiver (Emlid Reach RS2, Emlid, Budapest, Hungary). HMU types included pool, glide, riffle and backwater, following the geomorphological unit classification reported by Belletti et al. (Citation2017). To cover the spatial variability of flow conditions and sediment composition in each HMU, between seven and 30 point measurements of water depth, mean water column velocity and substrate type were collected (Vezza et al. Citation2014). Seven measurements were empirically chosen as the smallest statistically relevant quantity (Parasiewicz Citation2007). Substrates consisted of 12 categories – gigalithal (rocks), megalithal (> 40 cm), macrolithal (20–40 cm), mesolithal (6–20 cm), microlithal (2–6 cm), akal (gravel > 0.2 cm), psammal (sand), pelal (silt and clay), detritus (organic matter), xylal (woody debris, roots), sapropel (dark anoxic mud), and phytal (submerged plants) – whereas covers consisted of nine categories – boulders, canopy shading, overhanging vegetation, exposed roots, submerged vegetation, emerging vegetation, undercut banks, woody debris, and shallow margins. HMU, cover types and longitudinal river connectivity were broken down into multiple variables in binary (no/yes) format, and measurements of depth and velocity were divided into frequency categories of 15 cm and 15 cm/s increments, respectively. The dominant substrate category was visually identified in each point measurement. Finally, the water surface mean gradient was recorded for each HMU. The total list of the collected habitat attributes is reported in .
Table II. The physical habitat attributes of the MesoHABSIM approach used to describe the hydro-morphological units (HMUs) in the surveyed reaches. For each habitat parameter the corresponding categories are expressed as reported in Vezza et al. (Citation2014, Citation2017).
Lamprey distribution data were collected by sampling every HMU with backpack electrofishing (i.e. two-pass removal method, Meador et al. Citation2003). To assure the direct association between HMUs and collected lampreys, sampled areas were isolated by using nets, and before release within the same sampled HMU, each lamprey was measured in terms of total length. The total number of captured lampreys was 78, which were classified into larvae, referred to as ammocoetes (n = 77), and adult (n = 1) life stages by looking at the enlargement and differentiation of the eye and differences in the head shape (Vladykov Citation1955). Due to a low number of observations of adults but mainly because the larval phase is prominent in the life cycle of the species, we focused on ammocoetes.
Data analysis
The association of HMU characteristics () with lamprey presence was explored using probabilistic models to investigate ammocoetes’ habitat preferences. In particular, the RF (Breiman Citation2001) classification algorithm was used to identify habitat attributes influencing lamprey distribution.
RF is an ensemble machine learning technique based on the aggregation of a large set of randomized classification trees. In RF, as implemented in R (library randomForest, version 4.6–7, Liaw & Wiener Citation2002), each tree is trained by selecting a random learning sample (i.e. bootstrap sample) from the original dataset. Selected observations (parent node) are then split into two further subsets (child nodes), depending on the value of a predictor randomly chosen from the variables space. Through a binary recursive partitioning technique, this process is repeated for each child node, which becomes the new parent node, as long as a minimum number of observations is reached in the last terminal nodes. The choice of the predictor for each partitioning (new branch of the trees) is oriented to reduce the Gini impurity, i.e. for maximizing class imbalance in the child nodes. The elements not included in each bootstrap sample are referred to as out-of-bag data (OOB, i.e. cross-validated accuracy estimates) for all trees. For classification RF models, once all trees are fully grown, the majority voting of the OOB elements across all trees determines the responsive class for each observation.
A binary absence/presence model was developed to distinguish between unsuitable and suitable habitats for lamprey ammocoetes. In this way the model was characterized by two boolean responsive classes (stated as 0/1), where 1 denoted a > 50% predicted probability of being a suitable HMU. As the responsive variable was a binary variable (fish absence/presence), we confined our attention to classification RF models. Among all surveyed HMUs, the prevalence (i.e. the frequency of occurrence of the target organism) was 0.26. Therefore, to address potential bias in the forest generation due to class imbalance, we developed and tested a model for which a random oversampling of the training dataset was first performed. In order to optimize the predictive performances of the RF model, minimizing the out-of-bag error (EOOB), a specific framework for variables selection and hyperparameters tuning was implemented. As EOOB increases when the number of predictors is significantly higher than optimal (Kohavi & John Citation1997), we tried to select a small (possibly minimal) number of variables providing the best possible classification result.
To assess the importance of a specific predictor we used a common wrapper for feature selection based on the RF named Boruta (Kursa & Rudnicki Citation2010). The Boruta algorithm is implemented in R as a package and allows the user to identify all relevant variables by providing as output a features ranking expressed in terms of importance. From this analysis, the most important predictors were chosen and, to avoid high correlation (Spearman’s rho correlation coefficient > 0.7) between selected predictors, were tested by means of the Spearman correlation matrix. The final parsimonious RF model was therefore characterized by 10 variables with high importance for classification results and low correlation. Hyperparameters generally refer to the RF settings which can be tuned by the user (e.g. number of decision trees). For our RF models, hyperparameters tuning involved (i) the number of decision trees for the forest (ntree), and (ii) the number of predictors randomly sampled as candidates at each node (mtry). To establish the suitable ntree value we followed the suggestion of Evans and Cushman (Citation2009), obtaining a number of trees for the final model of 600. The mtry parameter was established equal to the square root of the total number of selected predictors included in each model (Probst et al. Citation2019). For our final parsimonious model mtry was equal to 3. Finally, accuracy, sensitivity, specificity, and true skill statistic (TSS, Allouche et al. Citation2006) were used to assess the predictive performances of the RF model. The partial dependence plots (or response curve, PDPs), based on the RF results, provided a way to visualize the marginal effect of the selected independent variables on the fish distribution (Cutler et al. Citation2007), outlining the relationships between individual habitat attributes and the predicted probabilities of fish presence.
Granulometry
In order to better analyze the favored substrate for lamprey ammocoetes, we further collected streambed samples to characterize the sediment preferences of this species. On 16 March 2022, we carried out sampling with an electrofishing device in the Ghiandone stream in Reaches 2 and 3 (), where the highest density and number of individuals were assessed from the previous field campaigns. While an operator slowly moved the anode on the river bottom, the other two signaled with flags the points from which the lampreys emerged. In total, we considered 26 points in a 70 m-long stream reach. In each of these points we collected three sediment cores with cut-off 100 mL syringes (diameter: 3.6 cm, height: 15 cm – )). Sediment cores were transported to the laboratory, then oven-dried (24 h, 105°C) to assess dry mass. Samples were processed in a Bertel mechanical sieve shaker for 15 minutes, using four mesh sizes in order to obtain five granulometric ranges: > 2 mm (very fine gravel), 2.0–1.0 mm (very coarse sand), 1.0–0.5 mm (coarse sand), 0.5–0.2 mm (medium sand) and < 0.2 mm (fine sand and smaller) ()). For each granulometric fraction we performed a mass evaluation, weighting the fractions to the nearest 0.1 g, and a volumetric evaluation, inserting the fraction into a graduated cylinder containing a known water volume. To estimate the organic fraction in each sediment sample, after oven-drying as reported above, we placed sediments in a muffle furnace (500°C) for 4 h and reweighed them. The difference between weights indicated the organic fraction of the sediment as ash-free dry mass (AFDM). This value was the employed to assess the percentage organic content of each sediment sample (Logue et al. Citation2004).
Results
Mesohabitat description
In the field campaigns 42 mesohabitats were analyzed within the five selected river reaches. Most of the sampled HMUs were riffles (50% of total amount) or glides (26% of total amount). Pools and backwaters corresponded to 17% and 7% of the sample dataset, respectively. As we considered river reaches of different sizes (), HMU areas varied from a minimum of 6.7 m2 to a maximum of 950.5 m2. HMU water surface gradients were mostly lower than 2% (69% of total amount), except for reach 1, where a few mesohabitats exhibited gradient values up to 10%. Considering covers, the presence of shallow margins was identified in 20 HMUs, whereas boulders and submerged vegetation were identified in 18 and 17 mesohabitats, respectively. Overall, mesolithal (33% of the total sampled points) was the most frequent substrate category, followed by microlithal (26%), psammal (14%) and akal (10%). Finally, water depth and velocity values varied between 2 and 112 cm and between 0 and 210 cm/s, respectively.
Lamprey ammocoetes were found in 11 HMUs: five glides, two riffles, two pools, and two backwaters. Such mesohabitats were mostly characterized by shallow water depth (< 45 cm) and low flow velocities (< 45 cm/s), with a prevalent substrate constituted by psammal and akal. Overall, the highest number of lampreys (29) was assessed in a HMU classified as glide in Reach 3 (Ghiandone River). The largest density of individuals (0.48 ind./m2) was found in a backwater in Reach 2 (Ghiandone River).
Habitat preference analysis
The developed RF models permitted us to identify the most important mesohabitat physical attributes for lamprey ammocoetes presence within HMUs, providing the first meso-scale distribution model in the literature for the considered species. As the oversampled RF model produced a higher predictive performance, in the end this model alone was taken into account (). Cross validation of the final model demonstrated high predictive capability in detecting suitable mesohabitat for lamprey. In particular, model accuracy was assessed as 95.2%, sensitivity (i.e. true positive rate) was 96.8%, specificity (i.e. true negative rate) was 93.6%, and TSS was 90.4%.
Figure 3. Lampetra zanandreai ammocoetes RF model. Selected physical attributes (in order of importance) for the oversampled parsimonious model. The relationship between variables and probability of presence is reported using partial dependence plots (PDPs).
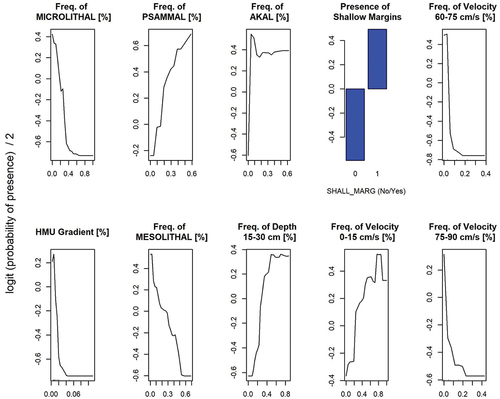
Considering predictive variables, the final parsimonious model was characterized by 10 mesohabitat attributes, presented in in the form of PDPs and sorted in terms of variable importance. The most significant attributes were microlithal, akal, and psammal substrate types. From the PDP graphs, microlithal substrate type was negatively correlated with the lamprey presence, whilst psammal and akal were positively correlated. Mesolithal showed a similar trend to microlithal, pointing out how coarser substrates exerted a negative influence on lamprey presence. The probability of the presence of lamprey ammocoetes also increased with (i) the presence of shallow margins in the HMUs, (ii) the proportion of low water depths (15–30 cm), and (iii) the proportion of low flow velocities (0–15 cm/s). Symmetrically, the proportion of higher velocities (i.e. 60–75 and 75–90 cm/s) and larger HMU gradients had a negative influence on the probability of lamprey presence.
Granulometry
We obtained the granulometric profile of the 25 samples collected and we firstly analyzed the relationships between weight and volume. For all granulometric classes we found a significant positive correlation (), so we decided to use mass as an indicator of sediment characteristics (following Holland et al. Citation2005).
Table III. Correlations between volume and mass of the different granulometric size classes.
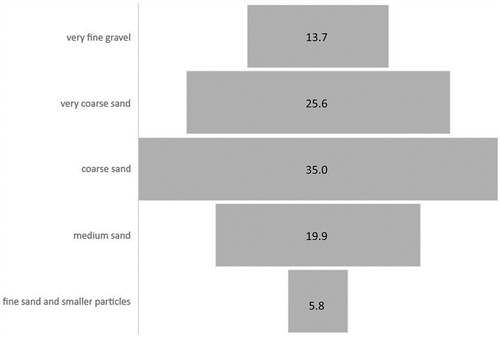
The average particle size mass profile consists of 34.98% (± 2.86 se, standard error) coarse sand, followed by 25.64% (± 2.51 se) very coarse sand, 19.87% (± 1.79 se) medium sand, 13.73% (± 1.86 se) very fine gravel and 5.77% (± 0.72 se) fine sand and smaller particles (). In general, most cores are largely composed of intermediate granules (from 2.0 to 0.5 mm). However, a certain tolerance in the particle size range is evident, also highlighted by : the lampreys were found in environments where the coarsest content (> 2 mm) is equal to 33.6% of the total but also in others in which the finest (<0.2 mm) reaches 15.6%. The organic content was 1.14% of sediment samples (mean value), with a minimum of 0.46% and a maximum of 3.42%.
Discussion
Studies on Italian cyclostomes have been practically at a standstill for about 50 years – that is, since the phase of conspicuous research activity stimulated mainly by Giuseppe Zanadrea ended (Zerunian Citation2002). While numerous aspects of the biology, phylogeny and morphology of this species are well known, virtually nothing is known about its ecological needs. To our knowledge, this is the first study focusing on the habitat requirements of Po Brook Lamprey.
The application of the MesoHABSIM approach allowed us to demonstrate that ammocoetes of this species can be found in different mesohabitats, such as pools, glides, riffles and backwaters. However, all the positive samples were characterized mainly by patches of fine sediment (psammal and akal), shallow water depth (< 45 cm), and low flow velocities (< 45 cm/s). Such results are consistent with Zanandrea (Citation1963), who reported that this species inhabits rivers with muddy or sandy bottoms. Furthermore, by comparing our findings with the habitat preferences reported in the literature for ammocetes of other European freshwater lampreys, a certain correspondence may be observed. In particular, Goodwin et al. (Citation2008) used the RF algorithm to understand the relationship between the presence of L. planeri and L. fluviatilis ammocoetes with respect to different environmental attributes at different scales (regional, catchment and microhabitat) in the Ballinderry River catchment (Northern Ireland). From their analysis it was possible to note that ammocoetes were more abundant in sediment patches characterized by very coarse sand (≥ 1.94 mm). Additionally, measured water depth (range 2–66 cm) and current velocity (range 0–75 cm) values where lamprey ammocoetes were found are in agreement with our data. Indeed, all specimens of Po brook lampreys we captured in the study sites inhabited HMUs characterized by water depth and velocity lower than 76 cm and 70 cm/s, respectively. Similarly, Taverny et al. (Citation2012), observed that, in the Dordogne River (France), L. planeri and L. fluviatilis ammocoetes preferred water depth below 50 cm, current velocity up to 50 cm/s and a sediment composition dominated by coarse and median sands (0.2–2 mm).
The use of the RF classification algorithm permitted us to better understand the relationship between L. zanandreai ammocoetes distribution and the mesohabitat attributes considered within the MesoHABSIM approach. In particular, the final parsimonious RF model highlighted that lamprey larvae generally preferred mesohabitats (HMU) characterized by shallow margins with a high proportion of (i) water depths in the range 15–30 cm, (ii) current velocities in the range 0–15 cm/s, and (iii) fine sediment (psammal and akal).
The high predictive performance (accuracy = 95.2% and TSS = 90.4%) obtained for the developed RF model confirmed the capabily of this technique for the definition of species distribution models in river ecology (Cutler et al. Citation2007). In particular, the current approach, which combined the MesoHABSIM methodology with a RF model, is in line with previous studies that pointed out how it can be considered suitable to define available habitat for freshwater fish and macroinvertebrates in rivers (Vezza et al. Citation2014, Citation2016). The present study contributes demonstrating the application potential for this approach. Indeed, this MesoHABSIM application can be seen as an extension of the approach for the definition of mesohabitat suitability criteria for Petromyzontidae. In addition, this study can serve as a basis for enhancing the preservation of the autochthonous Po brook lamprey, through the design of environmental flows and sediment river management, able to maintain specific habitat requirements (Vezza et al. Citation2017).
Ammocoetes of L. zanandreai inhabited depositional zones characterized by fine sediments, generally medium to coarse sand with smaller amounts of finer particles or small gravel. Interestingly, while in lowland running waters these kinds of substrata are often associated with moderate to elevated amounts of organic matter, in the cores positive for the presence of ammocoetes the organic component proved very small. This finding can explain the scattered distribution of the specimens. In fact, the occurrence of individuals is typically irregular in the stream reaches we examined, with large apparently suitable areas (judged by the presence of fine sediments) where no ammocetes were found. Probably the distribution of the organisms derives from a trade-off between the texture and composition of the substrate (which allow the larvae to burrow) and the organic substance content. An excessive accumulation of organic matter enhances bacterial biomass growth and therefore reduces oxygen diffusion at the interstitial level.
This study shows that lampreys select mesohabitat not only with precise velocity, depth and grain size ranges, but also with a low organic content. This implies that the chances of finding this species increase in rivers with better quality, in terms of ecological, chemical and hydrological status. Streams and rivers are increasingly threatened by factors acting at global (Piano et al. Citation2020) and local (Guareschi et al. Citation2014) scales. In particular, the synergic effects of these factors can be dramatically dangerous, because the reduction of flows can exacerbate the impacts of wastewater discharge, leading to an augmentation of fine organic matter deposition and a consequent diminution of lamprey habitat availability. Lampetra zanandreai is an ancient witness of the freshwater faunas of the past, an endemic and highly threatened species that becomes the symbol of an entire large ichthyological district, the Po basin: to protect and conserve Po Brook lamprey, we need a deeper knowledge of its environmental needs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmadi‐Nedushan B, St‐Hilaire A, Bérubé M, É R, Thiémonge N, Bobée B. 2006. A review of statistical methods for the evaluation of aquatic habitat suitability for instream flow assessment. River Research and Applications 22(5):503–523. DOI: 10.1002/rra.918.
- Allouche O, Tsoar A, Kadmon R. 2006. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). Journal of Applied Ecology 43(6):1223–1232. DOI: 10.1111/j.1365-2664.2006.01214.x.
- Belletti B, Rinaldi M, Bussettini M, Comiti F, Gurnell AM, Mao L, Nardi L, Vezza P. 2017. Characterising physical habitats and fluvial hydromorphology: A new system for the survey and classification of river geomorphic units. Geomorphology 283:143–157. DOI: 10.1016/j.geomorph.2017.01.032.
- Bertolini B. 1965. The structure of the liver cells during the life cycle of a brook-lamprey (Lampetra zanandreai). Zeitschrift für Zellforschung und Mikroskopische Anatomie 67:297–318. DOI: 10.1007/BF00339377.
- Bianco PG. 1986. Lethenteron zanandreai. In: Holcik J, editor. The freshwater fishes of Europe. Wiesbaden, Germany: Aula-Verlag. pp. 237–246.
- Bianco PG. 1992. Zoogeographical implications on a first record of Lethenteron zanandreai (Vladikov,1955) in central Italy. Ichthyological Exploration of Freshwaters 3:183–186.
- Breiman L. 1996. Bagging predictors. Machine Learning 24:123–140. DOI: 10.1007/BF00058655.
- Breiman L. 2001. Random forests. Machine Learning 45:5–32. DOI: 10.1023/A:1010933404324.
- Breiman L, Friedman JH, Olshen RA, Stone CJ. 1984. Classification and regression trees. New York, USA: Chapman & Hall,
- Caputo V, Giovannotti M, Nisi Cerioni P, Splendiani A, Marconi M, Tagliavini J. 2009. Mitochondrial DNA variation of an isolated population of the Adriatic brook lamprey Lampetra zanandreai (Agnatha: Petromyzontidae): Phylogeographic and phylogenetic inferences. Journal of Fish Biology 75:2344–2351. DOI: 10.1111/j.1095-8649.2009.02413.x.
- Caputo V, Giovannotti M, Nisi Cerioni P, Splendiani A, Tagliavini J, Olmo E. 2011. Chromosomal study of a lamprey Lampetra zanandreai (Vladykov, 1955) (Petromyzonida: Petromyzontiformes): Conventional and FISH analysis. Chromosome Research 19:481–491. DOI: 10.1007/s10577-011-9197-4.
- Crivelli AJ. 2005. Lethenteron zanandreai, IUCN Red List of Threatened Species, Versione 2020.2. IUCN, 2020.
- Cutler DR, Edwards Jr TC, Beard KH, Cutler A, Hess KT, Gibson J, Lawler JJ. 2007. Random forests for classification in ecology. Ecology 88(11):2783–2792. DOI: 10.1890/07-0539.1.
- Evans JS, Cushman SA. 2009. Gradient modeling of conifer species using random forests. Landscape Ecology 24(5):673–683. DOI: 10.1007/s10980-009-9341-0.
- Evans JS, Murphy MA, Holden ZA, Cushman SA. 2011. Modeling species distribution and change using random forest. In Predictive species and habitat modeling in landscape ecology. New York, NY: Springer. pp. 139–159.
- Freyhof J. 2011. Lampetra zanandreai (errata version published in 2018). The IUCN Red List of Threatened Species 2011: e.T6079.
- Goodwin CE, Dick JTA, Rogowski DL, Elwood RW. 2008. Lamprey (Lampetra fluviatilis and Lampetra planeri) ammocoete habitat associations at regional, catchment and microhabitat scales in Northern Ireland. Ecology of Freshwater Fish 17(4):542–553. DOI: 10.1111/j.1600-0633.2008.00305.x.
- Guareschi S, Laini A, Racchetti E, Bo T, Fenoglio S, Bartoli M. 2014. How do hydromorphological constraints and regulated flows govern macroinvertebrate communities along an entire lowland river? Ecohydrology 7:366–377. DOI: 10.1002/eco.1354.
- Gurnell M, Bussettini M, Camenen B, González Del Tánago M, Grabowski R, Hendriks D, Henshaw A, Latapie A, Rinaldi M, Surian N. 2014. REstoring rivers FOR effective catchment management (REFORM). Deliverable D2.1 Part I. A hierarchical multi-scale framework and indicators of hydromorphological processes and forms. Available: http://www.reformrivers.eu/system/files/D2.
- Holcik J, Mrakovic M. 1997. First record of Lethenteron zanandreai (Cyclostoma, Petromyzontidae) in the Adriatic basin of the Balkan peninsula and its zoogeographic consequences. Folia Zoologica 46:263–271.
- Holland GJ, Greenstreet SP, Gibb IM, Fraser HM, and Robertson MR. 2005. Identifying sandeel ammodytes marinus sediment habitat preferences in the marine environment. Marine Ecology Progress Series 303:269–282. DOI: 10.3354/meps303269.
- IUCN. 2018. The IUCN red list of threatened species. Version 2018-2. Available: www.iucnredlist.org. (Accessed Nov 2018 15).
- Kohavi R, John GH. 1997. Wrappers for feature subset selection. Artificial Intelligence 97(1–2):273–324. DOI: 10.1016/S0004-3702(97)00043-X.
- Koutrakis ET, Triantafillidis S, Sapounidis AS, Vezza P, Kamidis N, Sylaios G, Comoglio C. 2019. Evaluation of ecological flows in highly regulated rivers using the mesohabitat approach: A case study on the Nestos River, N. Greece Ecohydrology & Hydrobiology 19(4):598–609. DOI: 10.1016/j.ecohyd.2018.01.002.
- Kursa MB, Rudnicki WR. 2010. Feature selection with the Boruta package. Journal of Statistical Software 36:1–13. DOI: 10.18637/jss.v036.i11.
- Lamouroux N, Capra H, Pouilly M, Souchon Y. 1999. Fish habitat preferences in large streams of southern France. Freshwater Biology 42(4):673–687.
- Liaw A, Wiener M. 2002. Classification and regression by randomForest. R News 2(3):18–22.
- Logue JB, Robinson CT, Meier C, der Meer JRV. 2004. Relationship between sediment organic matter, bacteria composition, and the ecosystem metabolism of alpine streams. Limnology and Oceanography 49:2001–2010. DOI: 10.4319/lo.2004.49.6.2001.
- Meador MR, McIntyre JP, Pollock KH. 2003. Assessing the efficacy of single-pass backpack electrofishing to characterize fish community structure. Transactions of the American Fisheries Society 132(1):39–46. DOI: 10.1577/1548-8659(2003)132<0039:ATEOSP>2.0.CO;2.
- Mouton AM, Alcaraz-Hernández JD, De Baets B, Goethals PL, Martínez-Capel F. 2011. Data-driven fuzzy habitat suitability models for brown trout in Spanish Mediterranean rivers. Environmental Modelling & Software 26(5):615–622. DOI: 10.1016/j.envsoft.2010.12.001.
- Negro G, Fenoglio S, Quaranta E, Comoglio C, Garzia I, Vezza P. 2021. Habitat preferences of Italian freshwater fish: A systematic review of data availability for applications of the MesoHABSIM model. Frontiers in Environmental Science 9:634737. DOI: 10.3389/fenvs.2021.634737.
- Parasiewicz P. 2007. The MesoHABSIM model revisited. River Research and Applications 23(8):893–903. DOI: 10.1002/rra.1045.
- Piano E, Doretto A, Mammola S, Falasco E, Fenoglio S, Bona F. 2020. Taxonomic and functional homogenisation of macroinvertebrate communities in recently intermittent Alpine watercourses. Freshwater Biology 65:2096–2107. DOI: 10.1111/fwb.13605.
- Probst P, Wright MN, Boulesteix AL. 2019. Hyperparameters and tuning strategies for random forest. Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discovery 9(3):e1301.
- Renaud CB. 2011. Lampreys of the world. An annotated and illustrated catalogue of lamprey species known to date. FAO Species Catalogue for Fishery Purposes No. 5.
- Tagliavini J, Tizzi R, Conterio F, Mariottini P, Gandolfi G. 1994. Mitochondrial DNA sequences in three genera of Italian lampreys. Bolletino Zoologico 61:331–333. DOI: 10.1080/11250009409355903.
- Taverny C, Lassalle G, Ortusi I, Roqueplo C, Lepage M, Lambert P. 2012. From shallow to deep waters: Habitats used by larval lampreys (genus Petromyzon and Lampetra) over a Western European basin. Ecology of Freshwater Fish 21(1):87–99. DOI: 10.1111/j.1600-0633.2011.00526.x.
- Tutman P, Dulcic J, Glamuzina B. 2009. First record of Po Brook Lamprey, Lethenteron zanandreai (Cephalaspidomorphi: Petromyzontiformes: Petromyzontidae), in the Hutovo Blato wetland, Bosnia and Herzegovina. Acta Ichthyologica et Piscatoria 39:55–58. DOI: 10.3750/AIP2009.39.1.11.
- Vezza P, Ghia D, Fea G. 2016. Quantitative habitat models for the conservation of the endangered European crayfish Austropotamobius pallipes complex (Astacoidea: Astacidae). In: A global overview of the conservation of freshwater Decapod Crustaceans. Springer International Publishing. pp. 339–358.
- Vezza P, Parasiewicz P, Rosso M, Comoglio C. 2012. Defining minimum environmental flows at regional scale: Application of mesoscale habitat models and catchments classification. River Research and Applications 28(6):717–730. DOI: 10.1002/rra.1571.
- Vezza P, Parasiewicz P, Spairani M, Comoglio C. 2014. Habitat modeling in high‐gradient streams: The mesoscale approach and application. Ecological Applications 24(4):844–861. DOI: 10.1890/11-2066.1.
- Vezza P, Zanin A, Parasiewicz P. 2017. Manuale tecnico-operativo per la modellazione e la valutazione dell’integrità dell’habitat fluviale. ISPRA–Manuali e Linee Guida 154:1–102.
- Virbickas T, Vezza P, Kriaučiūnienė J, Akstinas V, Šarauskienė D, Steponėnas A. 2020. Impacts of low-head hydropower plants on cyprinid-dominated fish assemblages in Lithuanian rivers. Scientific Reports 10(1):1–14. DOI: 10.1038/s41598-020-78701-8.
- Vladykov VD. 1955. Lampetra zanandreai, a new species of lamprey from northern Italy. Copeia 3:215–223. DOI: 10.2307/1440464.
- Yi Y, Cheng X, Yang Z, Wieprecht S, Zhang S, Wu Y. 2017. Evaluating the ecological influence of hydraulic projects: A review of aquatic habitat suitability models. Renewable and Sustainable Energy Reviews 68:748–762. DOI: 10.1016/j.rser.2016.09.138.
- Zanandrea G. 1955. Isolamento geografico della Lampreda di ruscello nella pianura padana. Italian Journal of Zoology 22:2.
- Zanandrea G. 1956. Neotenia in Lampetra planeri zanandreai (Vladykov) e l’endocrinologia sperimentale dei Ciclostomi. Italian Journal of Zoology 23:413–427.
- Zanandrea G. 1958. Posizione sistematica e distribuzione geografica di Lampetra zanandreai Vladykov. Memorie del Museo Civico di Storia Naturale di Verona 6:207–237.
- Zanandrea G. 1961a. Ulteriori rilievi biometrici su Lampetra zanandreai (Vladykov). Italian Journal of Zoology 28:703–715.
- Zanandrea G. 1961b. Studies on European lampreys. Evolution 15:523–534. DOI: 10.2307/2406320.
- Zanandrea G. 1962. Rapporti tra l’alto e il medio versante adriatico d’Italia nella biogeografia delle Lamprede. Italian Journal of Zoology 29(2):727–734.
- Zanandrea G. 1963. Le lamprede della pianura padana e del rimanente versante adriatico d’Italia. Istituto poligrafico dello Stato. Bollettino Zoologico Piscicoltura Idrobiologia 26:153–180.
- Zanandrea G. 1965. Le lamprede della pianura padana e del rimanente versante adriatico d’Italia. Istituto poligrafico dello Stato.
- Zerunian S 2002. Pesci delle acque interne d’Italia. Quad. Cons. Natura 20 Min Ambiente IST NAZ Fauna Selvatica.