?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The stone moroko (Pseudorasbora parva) is an invasive species which spreads rapidly in European water bodies and occupies free ecological niches. In the years 2019–2020, monthly analyses of its population structure were carried out in the Wardynka River (western Poland), determining the age, size (TL and SL), body weight, growth, Fulton’s condition factor, and the parameters of the length–weight relationship. In addition, quantitative descriptions of the parasite communities were made. End-point PCR was used to verify the presence of Sphaerothecum destruens in the fish. The age structure of the population was dominated by fish at the age of 1+ to 3+, and the maximum age was 5 + . The TL of the fish ranged from 2.50 to 10.60 cm (average 6.24 cm), SL from 2.10 to 9.50 cm (average 5.20 cm), and body weight from 0.15 to 11.43 g (average 2.74 g). The average body weight and length of males were higher than in females. The average Fulton’s condition factor for the whole sample was 1.44 ± 0.24 and was similar for both sexes. The slope of the regression line (b > 3) indicates an allometric relationship between the length and weight of fish of both sexes. Back-calculated estimates of standard length fitted the von Bertalanffy growth function, although Taylor’s criterion showed that the asymptotic length (Linf) was overestimated. Comparison of the von Bertalanffy growth function parameters revealed differences between sexes. The presence of parasites not previously recorded in stone moroko in Poland was confirmed: Dactylogyrus squameus, Phyllodistomum elongatum, P. folium, Posthodiplostomum cuticola (metacercaria), and Bivalvia gen. sp. (glochidia). Electrophoresis of the end-point PCR product did not reveal any signs of amplification for either of the primer sets used. S. destruens was not detected in any of the analysed samples of stone moroko.
Introduction
Invasion by expansive alien species is one of the most important threats to biological diversity, due to the unpredictable effects of the appearance of a new species in the environment (IUCN Citation2000). Newly introduced (alien) species can become invasive and pose serious economic and ecological threats (Pimentel et al. Citation2000; Gozlan et al. Citation2005 Citation2010b; Rybczyk et al. Citation2020), causing changes in the habitats of native species (Rosecchi et al. Citation1993; Czerniejewski et al. Citation2020). Moreover, by competing with indigenous species they can affect density and biodiversity within the ecosystem (Pimentel et al. Citation2005). Pimentel et al. (Citation2000) estimated that the total losses in the global economy resulting from the presence of alien species amount to about 5% of annual production. According to Gozlan et al. (Citation2010a), as many as 625 species of freshwater fish have been introduced to water bodies outside their natural range. One way to fight invasive species is to eliminate them from the aquatic environment and prevent their return (Britton et al. Citation2008). However, in water bodies in which they cannot be eliminated, control and containment methods can be used to minimize their negative impact and further spread (Sorensen & Stacey Citation2004). Therefore it is essential to monitor the populations of these species and their habitats (Britton & Brazier Citation2006; Gozlan et al. Citation2010a).
The stone moroko Pseudorasbora parva (Temminck & Schlegel 1846) is one of the smallest invasive species in European water bodies. This fish has been recorded in standing water bodies, i.e. lakes, reservoirs, and fish ponds (Kapusta et al. Citation2008; Benzer & Benzer Citation2020; Piria et al. Citation2020; Davies & Britton Citation2021), as well as in watercourses (Carosi et al. Citation2016; Baek et al. Citation2022). Its body size makes its populations difficult to locate (Rosecchi et al. Citation1993; Gozlan et al. Citation2010b). It was introduced to Europe unintentionally together with stocking material for herbivorous fish species (grass carp Ctenopharyngodon idella, bighead carp Hypophthalmichthys nobilis, and silver carp Hypophthalmichthys molitrix). It was recorded for the first time in Europe in 1961, in southern Romania (in the Nucet fish farm in the Dambovita basin) and in Albania (Knezevič Citation1981; Bănărescu Citation1999), from which it spread to nearly all of Europe (Gozlan et al. Citation2010b). In Poland it was recorded for the first time in 1990, in fish ponds in the Barycz Valley near Milicz (Witkowski Citation1991), from which it spread together with stocking material not only to other fish ponds but also to natural surface waters (Witkowski Citation2002; Witkowski & Wiśniewolski Citation2005). The species is currently widespread in Poland, mainly in central and lowland regions. A few sites of this species are also known in northern Poland, where it colonizes fish ponds, small water bodies, lakes, and rivers, including small watercourses and drainage ditches (Czerniejewski et al. Citation2019a). Due to its very rapid spread, its negative impact on native hydrobionts, and the spread of infectious disease (Sphaerothecum destruens Arkush, Mendoza, Adkison & Hedrick, 2003 (Protozoa: Ichthyosporea), it is considered an “international pest species” in Europe (Welcomme Citation1992; Rosecchi et al. Citation1993; Gozlan et al. Citation2005; Pinder et al. Citation2005).
The state of infection of stone moroko by parasites and the pathogenic species Sphaerothecum destruens in Europe is not fully known. There have been reports of the occurrence of S. destruens in stone moroko in France (Charrier et al. Citation2016) and the Netherlands (Spikmans et al. Citation2020). As a host of S. destruens, stone moroko poses a potential threat to indigenous species of fish, both wild and farmed (Gozlan et al. Citation2005; Sana et al. Citation2018, Citation2020; Spikmans et al. Citation2020). Stone moroko populations in European countries are infected by parasites introduced from the natural sites of these fish, e.g. Dactylogyrus obscurus (Monogenea), as well as by parasites with a wide geographic range, acquired in ecosystems new to stone moroko, such as Trichodinella epizootica (Yuryshynets & Zaichenko Citation2015). Thus far eight parasite species have been recorded in stone moroko, four of which are new to this host: Trichodinella subtilis, Diplozoon paradoxum, Apatemon gracilis and Caryophyllaeus laticeps (Czerniejewski et al. Citation2019b). These species are recorded in Poland in indigenous fish species (Niewiadomska Citation2003; Pojmańska et al. Citation2007). Since the stone moroko appeared in Europe and Poland, it has played the role of a new, “additional” host in aquatic ecosystems, contributing to the development of populations of the above-mentioned parasite species. The occurrence of parasites may be associated with the growth and body condition of fish, which has been demonstrated in stone moroko in Poland (Czerniejewski et al. Citation2019b). Very little is known of the impact of parasites of stone moroko on its growth and body condition in European countries.
The aim of the study was to analyse and assess the population structure of stone moroko in the Wardynka River (Oder catchment area, north-western Poland) in an annual cycle, in terms of age, length, weight, Fulton’s condition factor, the length–weight relationship, growth, and infection by external and internal parasites. This study is part of a larger series on the ecology of P. parva populations in small rivers of the “Central Plains” European ecoregion, the results of which will be used to devise a management plan aimed at reducing the potential environmental damage caused by rapidly spreading populations of stone moroko.
Material and methods
Study site
The stone moroko population was monitored in the Wardynka River (Oder catchment area, north-western Poland, GPS: 53°08ʹ43.49”N, 15°34ʹ09.54”E) on the basis of monthly catches from May 2019 to October 2020 (). The mean depth of the river at the site of the catches was 0.1–0.4 m. The bottom at this location was covered with a layer of stones (15%), gravel (45%), sand (35%) and a small amount of mud (5%), diversified by depressions providing hiding places for fish. The hydrochemical parameters of the water at this site are presented in Kirczuk et al. (Citation2021). Fish were caught using an engine-powered electrofisher (Hans Grassl Gmbh IG, ELT60IIHI, Germany) on a 100 m stretch of the river, by wading across its entire width.
Length, weight and condition
A total of 683 stone moroko individuals were caught and transported to the laboratory, where they were measured (total length, TL, mm and standard length, SL, mm) to within 0.1 mm using an electronic calliper (115/B, Toolpack, Poland) and weighed to within 0.1 g using a digital balance (WLY 1/D, Radwag, Poland). Sex was determined on the basis of visual analysis of the gonads. The sex of the smallest fish, at the age of 0+, was not determined. Fulton’s condition factor was calculated according to the formula CF = 100*W/TL3 (Le Cren Citation1951), and parameters of the weight–length relationship (WLR) were determined. This relationship is calculated using the exponential equation W = a TLb, in which both variables are log-transformed to linearize the regression (log W = a + b × log TL), where a is the intercept of the regression line with the y axis, and b is the slope of the regression line. The slope (b) of the WLR relationship indicates whether it is isometric (b = 3) or allometric (b ≠ 3) (Froese Citation2006).
Age and growth
The age structure of the fish was determined by collecting 10 scales between the dorsal and caudal fins from above the lateral line of the fish (Rosecchi et al. Citation1993). The scales were cleaned by the alkaline immersion method (Huang et al. Citation2015), and age was determined by counting the annuli. Age and yearly increases in length were read using a Nikon Eclipse E600 stereomicroscope with an HD video camera and Lucia image analysis software (Nikon). The growth of fish was described using the von Bertalanffy equation (VBGR) (Ogle & Isermann Citation2017):
where: Lt is the length (cm) at time t (age in years), Linf is the standard length (cm) at time infinity (the predicted mean maximum length for the population), K is a growth constant which describes the rate at which Linf is attained (cm, year −1), t is age (years), and to is the time at which length = 0. The parameters of the equation were calculated in the R programming environment with the FSA, nlstools, magrittr, dplyr, and nnnet packages (Ogle Citation2016). To assess the accuracy of the von Bertalanffy growth rate (VBGR) we used Taylor’s criterion (Taylor Citation1962), which states that the asymptotic length is satisfactorily estimated when the maximum observed length represents approximately 95% of Linf. Because the parameters Linf and k are inversely correlated (Moreau et al. Citation1985), the index of growth performance φ’ (Munro & Pauly Citation1983) was calculated as follows:
where: Linf is the asymptotic standard length (in mm), and k is the rate at which the asymptotic length is approached.
Molecular detection of Sphaerothecum destruens
Samples of stone moroko (n = 58) were delivered to the laboratory and dissected, and pooled internal organs (kidney, liver, and spleen) were used for DNA extraction. The High Pure PCR Template Preparation Kit (Roche) was used to extract DNA, according to the manufacturer’s instructions. Spectrophotometric measurements using NanoDrop 2000 (Thermo Scientific) and agarose gel electrophoresis (1.5% agarose gel) were used to assess the quantity and quality of DNA extracts. DNA isolates were tested using end-point PCR to amplify the ITS1 and ITS2 or only ITS1 regions of Sphaerothecum destruens, as described by Gozlan et al. (Citation2009). Briefly, amplifications were conducted on the T100™ Thermal Cycler (Bio-Rad, USA) using the GoTaq PCR kit (Promega) according to the manufacturer recommendations, under the following conditions: 1 step of 5 min at 94°C followed by 35 cycles at 94°C for 30s, 56°C for 45s, 72°C for 90s, and final extension at 72°C for 7 min. The PCR results were assessed by 2% agarose gel electrophoresis.
Parasite communities of stone moroko
Parasitological examination of 124 stone moroko individuals was carried out in accordance with recommendations given by Klimpel et al. (Citation2019), Sepulveda and Kinsella (Citation2013), Pojmańska and Cielecka (Citation2001), Niewiadomska (Citation2010), Dzika (Citation2008), Grabda-Kazubska and Okulewicz (Citation2005), Prost (Citation1994), Moravec (Citation1994), and Lonc and Złotorzycka (Citation1994). The skin, fins, oral and nasal cavities, lens and vitreous body of the eye, gills, coelom, peritoneum, heart, swim bladder, gonads, intestine, gall bladder, urinary bladder and ureters, kidneys, and muscles were examined. Some of the fish were examined directly upon transport to the laboratory, while others (about 1/3) were frozen for further analysis. Parasites were fixed in 70–75% ethyl alcohol or identified to genus and species without fixation. Nematode (Nematoda) specimens were cleared in glycerine and Faure’s medium (Grabda-Kazubska & Okulewicz Citation2005), as were monogenetic trematodes (Monogenea) (Dzika Citation1987, Citation2008). Digenetic trematodes were stained with carmine alum, cleared in clove oil, and mounted in Canada balsam (Niewiadomska Citation2003). The presence of glochidia was noted in fresh specimens. Morphological examination of parasites was performed using the Zeiss StereoLumar v.12 stereoscopic microscope (5‒40×) and the ZEISS AxioScope.A1 biological light microscope (10‒400×) equipped with a Canon video camera and AxioVision Rel.4.8.2 image analysis software. Species identification was based on keys and original works (Bauer Citation1985, Citation1987; Moravec Citation1994; Kent et al. Citation2002; Niewiadomska Citation2003; Grabda-Kazubska & Okulewicz Citation2005; Niewiadomska & Pojmańska Citation2018).
Parasitological indices, i.e. prevalence, intensity of infection, and mean abundance, were calculated in accordance with terminology described by Bush et al. Citation1997. Parasitological indices were calculated from data obtained in spring (April–May), summer (June–August), and autumn (September–October). Parasitological examination was not performed on material obtained from November to March.
To characterize the parasite communities of stone moroko, i.e. the component community and infracommunity (terminology according to Bush et al. Citation1997), ecological indices were calculated for the entire study period: 1) the biodiversity index of the component community of parasites (Simpson’s diversity index), 2) the Berger–Parker dominance index defining the share of the dominant species in the component community, and the 3) Brillouin biodiversity index of infracommunities of parasites (Magurran Citation2004).
Ecological indices were calculated using Past v.2.11 software (Hammer et al. Citation2001).
Statistical analysis
Prior to the comparative statistical analyses, the normality of distributions of variables was tested using the Shapiro–Wilk test and homogeneity of variance by Levene’s test. The Mann–Whitney U test was used to compare data on the length (TL and SL), weight, and condition of fish (Sokal & Rohlf Citation2012). In addition, the significance of differences in numbers of males and females in each month (sex ratio) was estimated by the chi-square test with Yates’ correction.
Differences in the prevalence of parasites in different phenological seasons were compared using the chi-square test, and when the assumptions of the test were not met, Fisher’s exact test was applied. The non-parametric Mann–Whitney U test was used to compare differences in intensity of infection by parasites in phenological seasons. The relationship between the number of parasites and the length of fish (TL and SL) was examined by linear regression. A significance level of p ≤ 0.05 was adopted for the statistical analyses. Statistical computations were performed using the STATISTICA 12.0 PL software package (StatSoft, Polska).
Results
Sex structure
Among the fish caught (683 in total), a statistically significant disproportion was noted in the sex structure of the entire sample (χ2 = 24.8066, p < 0.01) and for individual months (). The proportion of males was statistically significantly higher in February, June, August, and December. The sex ratio for the entire sample of fish was 1:0.64.
Table I. Hydrochemical parameters of the Wardynka River at the site of the catches.
Length structure
The total length (TL) of the fish specimens ranged from 2.50 to 10.60 cm (average 6.24 cm), and the standard length (SL) ranged from 2.10 to 9.50 cm (average 5.20 cm). A statistically significant linear correlation was noted between TL and SL. The equation for the whole sample was TL = 0.1807 + 1.1645× SL (R2 = 0.98744, p < 0.001), and the equations for individual sexes were TLMale = 0.2292 + 1.1562× SL (R2 = 0.98, p < 0.001) for males and TLFemale = 0.1854 + 1.1642× SL (R2 = 0.98, p < 0.001) for females. In the case of juvenile fish (0+) of undetermined sex, the relationship is represented by the equation TL = 0.0214 + 1.1969× SL (R2 = 0.99, p < 0.001).
Males had statistically significantly greater total length (Mann–Whitney U test, Z = −6.9513, p < 0.001), standard body length (Z = −6.9048, p < 0.001), and individual weight (Z = −6.4122, p < 0.001) than females (). There were also significant differences in these parameters between sexes in individual months ().
Table II. Mean (± SD) total length (TL), standard length (SL) (in cm), and body weight (in g) of male, female and juvenile stone moroko individuals in the Wardynka River.
Figure 2. Standard length (SL, cm) (A) and individual body weight (W, g) of stone moroko caught in individual months. Values marked with different letters (a, b) indicate statistically significant differences between females (○) and males (∆) in a given month (Mann–Whitney U test, p < 0.05); mean ± SD.
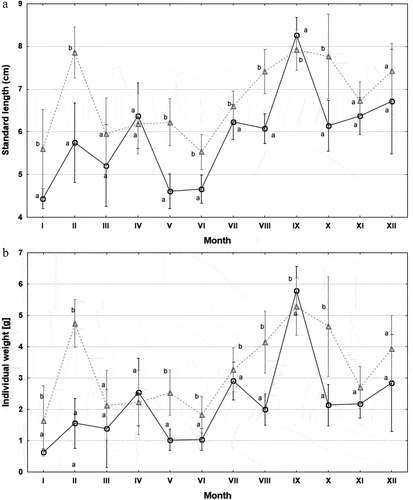
Statistically significant differences were also noted in individual months for the standard length (ANOVA, F = 27.0216, p < 0.001) and weight (ANOVA, F = 22.5189, p < 0.001) of the fish ().
Age structure
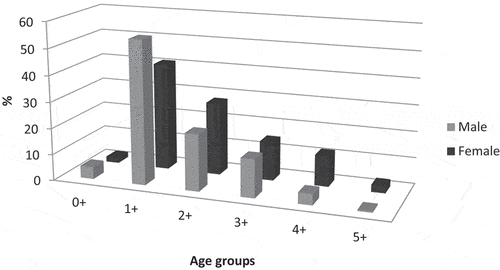
Six age groups were noted (0+ to 5+) among the 683 fish caught, with clear dominance of individuals in age groups 1+ (45.83%) and 2+ (23.78%). Fish in these groups accounted for 76.05% of females and 68.67% of males ().
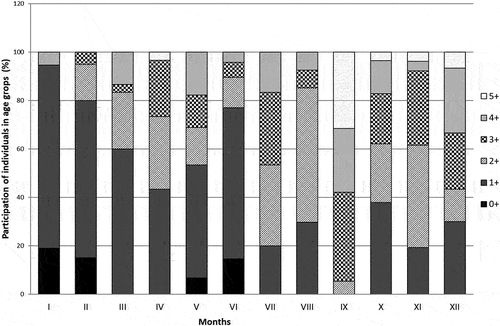
High monthly variation was observed in the percentages of individual age groups in the population structure (), which influenced the average length and individual weight of the fish in different months of the year ().
Figure 4. Percentage share of age groups in each month of the year in stone moroko from the Wardynka River.
presents the growth parameters of stone moroko based on the von Bertalanffy equation. Males of the species attained greater lengths than females in individual years of the study, as well as greater asymptotic maximum length. Greater growth in males was confirmed by the index of growth performance φ. Back-calculated standard length showed a good fit to the von Bertalanffy growth model. According to the Taylor criterion, however, the von Bertalanffy growth function describes growth well if the maximum observed length is about 95% of Linf. In both sexes the maximum observed lengths ranged from 89.85% to 92.23% of Linf (), which suggests that the asymptotic lengths determined by the von Bertalanffy model were slightly overestimated.
Table III. Estimation of von Bertalanffy growth function (VBGF) parameters for stone moroko in the Wardynka River.
Body condition
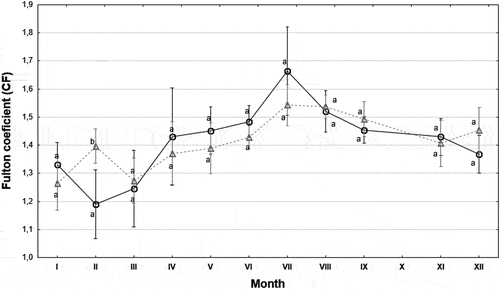
The mean value of Fulton’s condition factor for the entire sample of fish was 1.44 ± 0.24. Females (CFF = 1.44 ± 0.23) and males (CFM = 1.43 ± 0.22) had similar body condition (Mann–Whitney U test, Z = 0.0872, p = 0.93). The body condition of juveniles was CFJ = 1.47 ± 0.34. No significant differences in the condition factor were found between males and females in individual months, except for February (). It should be noted that the condition factors were highest (CF > 1.45) during the period from May to September. This was most likely because the water temperature was highest during this period, at > 16°C, close to the optimum temperature for the growth of the species.
Figure 5. Monthly fluctuations in the condition factor (CF) values of stone moroko from the Wardynka River. Values marked with different letters (a,b) indicate statistically significant differences in CF between females (○) and males (∆) in a given month (Mann–Whitney U test, p < 0.05); mean ± SD.
Analysis of correlations between the total length and individual weight of fish () revealed that the growth of stone moroko was allometric (b > 3.0), with a statistically significant (p < 0.001) and high correlation (R > 0.97).
Table IV. Parameters of the length–weight relationship (LogW = a + b logTL) of stone moroko from the Wardynka River.
The wide variation in the habitats of stone moroko populations contributes to disproportions in the size, growth, condition and population structures of the species, which suggests that it has a high capacity to adapt to environmental conditions.
Parasite fauna
The parasite community of stone moroko is represented by 9 taxa, including 5 identified to species. The parasites found were Chromista, Cilophora – 1 taxon determined to genus; Animalia: Platyhelminthes: Monogenea - 1 species, Trematoda – 3 species and 1 taxon identified as Subclass Digenea; Nematoda – 1 species and 1 taxon identified to family, and Mollusca – 1 taxon identified to family. Larvae of nematodes Anisakidae gen. sp., whose genus and species were not determined, were curled in a spiral, encysted and free ().
Table V. Parasites of stone moroko from the Wardynka River (2019–2020).
Prevalence for the entire study period was 58.87% (73 infected fish), while the average intensity of infection was 3, with a range of 1‒30 parasites per fish. Nematodes Pseudocapillaria (Pseudocapillaria) tomentosa had the highest prevalence and the highest intensity of infection (prevalence 37.90%, mean intensity of infection 2, range 1‒11). Prevalence of glochidia was lower (15.32%, mean intensity 3, range 1‒12). The lowest parameters of infection were found for Dactylogyrus squameus (prevalence 5.65%, mean intensity 2, range 1‒5), Phyllodistomum elongatum (prevalence 2.42%, mean intensity: 3, range 1‒5), and nematode larvae (Anisakidae gen. sp.) (prevalence 4%, mean intensity 11, range 1‒30). The remaining parasites, i.e. Trichodinella sp., Phyllodistomum folium and metacercariae of trematodes (Digenea gen. sp.), were noted in single fish, and the intensity of infection ranged from 1 to 2 parasites per fish. The most fish were infected in the spring, and the fewest in autumn ().
The prevalence of glochidia differed significantly between the spring and autumn (chi-square test = 8.112, Fisher exact p = 0.006) and between the summer and autumn (chi-square test = 12.437, p = 0.0004). The difference in the intensity of infection of stone moroko by P. tomentosa between summer and autumn was statistically significant (Mann–Whitney U test, p = 0.035) ().
The Simpson diversity index was 0.690, and the Berger–Parker dominance index was 0.459 and indicated the dominance of Pseudocapillaria (Pseudocapillaria) tomentosa in the parasite community of stone moroko. The average value of the Brillouin index was 0.0613 ± 0.152.
The number of parasite species and intensity of infection by parasites was shown to be weakly positively correlated with the body length of stone moroko. The correlation coefficient between the number of parasite species and standard length (SL) was 0.268, while the coefficient for the number of parasite species and total length (TL) was 0.237. The correlation coefficient for intensity of infection and standard length (SL) was 0.176, and the coefficient for intensity of infection and total length (TL) was 0.173.
End-point PCR showed no amplification signals for either assay used to detect S. destruens in any of the analysed samples of stone moroko.
Discussion
Stone moroko is an invasive species which is resistant to unfavourable environmental conditions and whose range is spreading in Europe. It is characterized by small body length, short longevity, early maturation, relatively low fecundity, multiple spawning and extended reproductive seasons (Gozlan et al. Citation2010a, Citation2010b; Kirczuk et al. Citation2021). These life-history traits, along with broad diet breath and environmental tolerance, appear to facilitate the invasion of freshwater fish in the Central European bioregion. The total length of the fish from the Wardynka River ranged from 2.50 to 10.60 cm (average 6.24 cm), and their body weight ranged from 0.15 to 11.43 g (average 2.74 g). The mean length, weight and growth parameter K were greater than those of some native and long-established populations. Alien species generally exhibit superior growth in water bodies to which they have been introduced than in their native water bodies (Grabowska et al. Citation2011). According to a study by Brandner et al. (Citation2013) on gobies, specimens from recently colonized areas were on average larger and heavier and had higher body condition than their conspecifics from long-established areas, where intraspecific competition is stronger and thus food choice is more limited.
Stone moroko should be continuously monitored at every new site of its occurrence not only for density and biological traits, but for parasitological characteristics as well. This is because its presence enables the spread of native and newly introduced parasites for which it is a vector. In the population inhabiting the Wardynka River, whose biological and population characteristics are described in this paper, parasites were recorded that had not previously been found in stone moroko in Poland: Dactylogyrus squameus, Phyllodistomum elongatum, P. folium, Posthodiplostomum cuticola (metacercaria), and Unionidae gen. sp. (glochidia). The trematode D. squameus was recorded in fish in Poland for the first time. Although the PCR analysis did not reveal the presence of S. destruens, parasites of stone moroko can have a negative effect on the local biodiversity and species richness of fish. The helminth fauna of invasive fish species reported from new territories is generally less rich than in their native areas (Torchin et al. Citation2003). However, transmission of parasites from invading to native species can occur, aiding the invasion process (Ondračková et al. Citation2004; Torchin & Mitchell Citation2004).
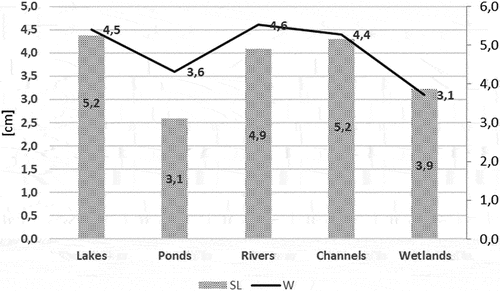
In fresh water stone moroko prefers shallow habitats, rich in food, with substantial cover by aquatic plants (Benzer Citation2019). According to literature data on the types of water bodies in which stone moroko is found, the highest average lengths (SL) and individual weights of fish have been noted in lakes, rivers, and large canals, while much lower values for these parameters have been recorded in ponds and wetlands with a smaller area (). A similar effect of water body size has been observed in other fish species (Pyrzanowski et al. Citation2020), but other authors have noted that the impact of the environmental conditions of the water body and the density of fish can be greater (Eckmann Citation2017; Teubner et al. Citation2019). The size (SL, W) of the stone moroko individuals from the Wardynka River was greater than in other non-native populations (Stavrescu-Bedivan et al. Citation2017; Piria et al. Citation2020; Rakauskas et al. Citation2021), but similar to that of some indigenous populations. For example, Piria et al. (Citation2020) reported that in the Hong (Hong Hu) and Huangpi Lakes in China, the average length (SL) of this species was 4.82 cm and 5.28 cm, respectively, and the individual weight was 2.25 g and 3.25 g. It should be noted that among Chinese populations, fish from the Yiluo River and Wujiang River stand out in this respect, with an average length of 6.07 cm and 6.60 cm, respectively, and individual weight of 9.95 g and 9.00 g. These differences in average SL and W between the population from the Wardynka River and other water bodies may be due to different age structure. In the Wardynka, fish at the age of 1+ and 2+ were dominant, as in many European water bodies (Stavrescu-Bedivan et al. Citation2017; Rakauskas et al. Citation2021), while older age groups predominated in the Yiluo River and Wujiang River.
Figure 6. Mean results for standard length (SL) and weight (W) of stone moroko (topmouth gudgeon) inhabiting various types of inland water bodies according to literature data (Kapusta et al. Citation2008; Carosi et al. Citation2016; Benzer & Benzer Citation2020; Piria et al. Citation2020; Davies & Britton Citation2021; Baek et al. Citation2022).
Literature data on the growth of various stone moroko populations indicate that the growth rate of the species varies significantly within its geographic range, as indicated by the growth parameters defined by the von Bertalanffy equation (). High variability in the growth of fish is a common phenomenon in species with a wide range of occurrence (Villeneuve et al. Citation2005; Grabowska & Przybylski Citation2015). The growth of stone moroko may be influenced by the thermal conditions of the water (Gaspar et al. Citation1999; Ding et al. Citation2019) and also depends on density, access to food and its abundance (Britton et al. Citation2007, Citation2008).
Table VI. Comparison of growth (in mm) characters of stone moroko in native and non-native areas.
The K parameter is responsible for the shape of the growth curve in fish defined by the von Bertalanffy equation (Pauly Citation1980). Analysis of the data in reveals that the highest values for the K parameter in stone moroko were obtained for short-lived populations with a lower Linf value. For example, the value of this parameter was higher for stone moroko in the Ushize River (Japan) and in a pond in England, in which the age of fish was 1+ and 2+ (Britton et al. Citation2007; Onikura & Nakajima Citation2013), than in the population from the Chabalang Wetland and the Wardynka River, in which the maximum length was 5+ (Ding et al. Citation2019; our own analysis).
The species structure and quantitative structure of parasites of stone moroko analysed in 2019–2020 differs in part from the structure determined in 2015–2016 (Czerniejewski et al. Citation2019a), except for the presence of protists of the genus Trichodinella and the nematode P. tomentosa. The trematode D. squameus had not previously been recorded in fish in Poland. It was originally described by Gusev (Citation1955), and was recorded for the first time in Central Europe in stone moroko in the Czech Republic and Slovak Republic by Ondračková et al. (Citation2004). According to Ondračková et al. (Citation2004), D. squameus was probably introduced by stone moroko to non-native water bodies during its expansion from native areas. Digenetic trematodes Phyllodistomum elongatum and P. folium were recorded in stone moroko for the first time in European water bodies. These trematodes have also not been recorded in stone moroko in their native water bodies. The hosts of P. elongatum are pea clams (Pisidium) or fingernail clams (Sphaerium), in which larval forms of these trematodes develop (Zhokhov Citation1987, Citation1991). The role of host for larval stages of P. folium can be played by zebra mussels Dreisena polymorpha (Peribáñez et al. Citation2011). The detection of only one metacercaria of P. cuticola and its atypical location in the body of the fish may indicate that stone moroko is an incidental host. The nematode larvae in the intestinal wall of stone moroko (Anisakidae gen. sp., larvae) were most similar in morphology and morphometry to third-stage larvae (L3) of Raphidascaris acus (Bloch, 1779). However, due to the absence of clearly visible morphological characters, it is not certain that the larvae belonged to this species. Several nematode species can be present in fish in larval form, located in the tissues of the internal organs, e.g. R. acus, Anguillicola crassus (Moravec Citation1994; Grabda-Kazubska & Okulewicz Citation2005), and Schulmanela petruschewskii (Schulman, 1948) (Moravec Citation1994). Unionidae gen. sp. (glochidia) have not previously been recorded in stone moroko in Poland. Stone moroko is described in the literature as a “poor host” for glochidia of the swan mussel Anodonta cygnea, in contrast with “good hosts” (e.g. Perca fluviatilis) (Huber & Geist Citation2017).
Invasive species of hosts, as a new element of the ecosystem, may be sensitive to parasites new to them, due to the lack of developed immune mechanisms against these parasites. The filter theory presented by Combes (Citation1995), which talks about the encounter filter and the match filter, may be important. In a new environment, the spectrum of parasites that can colonize a new host may be narrower than that of parasites that infect native species of hosts. On the other hand, parasites of invasive species may die in a new environment if the conditions of development outside the host are not optimal for the survival of their dispersion forms or if no intermediate hosts are present. Another aspect – parasites of an invasive host species that are alien to new geographic areas can acclimate and become invasive parasite species for native hosts (Pojmańska et al. Citation2007).
One example illustrating the introduction of parasites is the results of a study of Monkey goby parasites (Neogobius fluviatilis) in the lower Volga basin, during which, out of 19 species of parasites, two were considered to be introduced from other geographic regions (Bothriocephalus opsariichthydis and Nicolla skrjabini). The parasitic infestation parameters of the non-native species of goby in this study area differed from those of the native species of gobies by having a much lower number of parasitic species but a higher level of infection than the native species (Kvach et al. Citation2015).
Sphaerothecum destruens has not been recorded in stone moroko from the Wardynka River. The absence of this pathogen may be explained by two main factors. First, S. destruens may not yet have been transferred to the Wardynka River system, although its main host, stone moroko, is present in numerous water bodies across Poland and Europe (Czerniejewski et al. Citation2019a; Spikmans et al. Citation2020). Secondly, few P. parva were infected with the rosette agent in the catchment area. A large-scale study showed similar results in watercourses in France. Additionally, low water temperatures may restrict the proliferation, transmission and thus detection of S. destruens in fish samples and therefore lead to underestimation of its prevalence (Marine et al. Citation2022). To our knowledge, this is the first attempt to identify S. destruens in Poland. Further genetic analysis is needed to assess its potential spread and virulence in other native and invasive fish populations, as the pathogen represents a major ecological, health and economic threat to aquatic biodiversity and fish conservation (Combe & Gozlan Citation2018).
Acknowledgements
The authors kindly thank Professor Ewa Dzika for identifying the parasite species Dactylogyrus squameus
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Baek S-H, Park S-H, Kim J-H, Yoon J-H, Moon J-S, Kim D-H, Yoon J-D. 2022. Length–weight relations of 12 freshwater fish species (Actinopterygii: Cypriniformes) including two endangered species, Cobitis choii (Cobitidae) and Gobiobotia naktongensis (Cyprinidae), in the Geum River, South Korea. Acta Ichthyologica et Piscatoria 52(1):9–12. DOI: 10.3897/aiep.52.79067.
- Bănărescu P. 1999. Pseudorasbora parva (Temmnick et Schlegel 1846). In: Bănărescu P, editor. The freshwater fishes of Europe. 5.I. Cyprinidae 2/I. Wiesbaden: AULA Verlag. pp. 207–224.
- Bauer ON. 1985. Определитель паразитов пресноводных рыб фауны СССР. Часть II. [Key to parasites of freshwater fishes of the fauna of the USSR. Part II]. Leningrad: Izdatelstvo Nauka. pp 425.
- Bauer ON. 1987. Определитель паразитов пресноводных рыб фауны СССР. Часть III. [Key to parasites of freshwater fishes of the fauna of the USSR. Part III]. Izdatelstvo Nauka, Leningrad. pp 583.
- Benzer S. 2019. Morphometric features of topmouth gudgeon Pseudorasbora parva (Temminck & Schlegel, 1846) in the Hirfanlı Reservoir, Turkey. Acta Aquatica: Aquatic Sciences Journal 7(1):8–12. DOI: 10.29103/aa.v7i1.2030.
- Benzer S, Benzer R. 2020. Growth Properties of Pseudorasbora parva in Süreyyabey reservoir: Traditional and artificial intelligent methods. Thalassas. An International Journal of Marine Sciences 36(1):149–156. DOI: 10.1007/s41208-020-00192-1.
- Benzer S, Benzer R, Gül A. 2016. Artificial neural network applications for biological systems: The case study of Pseudorasbora parva. Developments in Science and Engineering. St. Kliment Ohridski University Press. pp 49–59. 144.
- Brandner J, Cerwenka AF, Schliewen UK, Geist J. 2013. Bigger is better: Characteristics of round gobies forming an invasion front in the Danube river. PLoS One 8(9):e73036 DOI: 10.1371/journal.pone.0073036. PMID: 24039854.
- Britton JR, Brazier M. 2006. Eradicating the invasive topmouth gudgeon, Pseudorasbora parva, from a recreational fishery in northern England. Fisheries Management and Ecology 13(5):329–335. DOI: 10.1111/j.1365-2400.2006.00510.x.
- Britton JR, Brazier M, Davies GD, Chare SI. 2008. Case studies on eradicating the Asiatic cyprinid Pseudorasbora parva from fishing lakes in England to prevent their riverine dispersal. Aquatic Conservation: Marine and Freshwater Ecosystems 18(6):867–876. DOI: 10.1002/aqc.919.
- Britton JR, Davies GD, Brazier M, Pinder AC. 2007. A case study on the population ecology of a topmouth gudgeon (Pseudorasbora parva) population in the UK and the implications for native fish communities. Aquatic Conservation: Marine and Freshwater Ecosystems 17(7):749–759. DOI: 10.1002/aqc.809.
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. 1997. Parasitology meets ecology on its terms: Margolis et al. revisited. The Journal of Parasitology 83(4):575–583. DOI: 10.2307/3284227.
- Carosi A, Ghetti L, Lorenzoni M. 2016. Status of Pseudorasbora parva in the Tiber River Basin (Umbria, central Italy) 20 years after its introduction. Knowledge and Management of Aquatic Ecosystems 417(22):11.
- Charrier A, Peudpiece M, Lesnel M, Daniel P. 2016. First report of the intracellular fish parasite Sphaerothecum destruens associated with the invasive topmouth gudgeon (Pseudorasbora parva) in France. Knowledge and Management of Aquatic Ecosystems 417(417):44. DOI: 10.1051/kmae/2016031.
- Combe M, Gozlan RE. 2018. The rise of the rosette agent in Europe: An epidemiological enigma. Transboundary and Emerging Diseases 65(6):1474–1481. DOI: 10.1111/tbed.13001.
- Combes C. 1995. Interactions durables. Ecologie et évolution du parasitisme. Paris: Masson, Editieur.
- Czerniejewski P, Linowska A, Brysiewicz A, Kasowska N. 2020. Body size, condition, growth rate and parasite fauna of the invasive Perccottus glenii (Actinopterygii: Odontobutidae) from small watercourse in the Vistula River basin, Poland. Journal of Water and Land Development 44(I–III):33–42. DOI: 10.24425/jwld.2019.127043.
- Czerniejewski P, Rybczyk A, Linowska A, Sobecka E. 2019b. New location, food composition, and parasitic fauna of the invasive fish Pseudorasbora parva (Temminck & Schlegel, 1846) (Cyprinidae) in Poland. Turkish Journal of Zoology 43(1):94–105. DOI: 10.3906/ZOO-1806-26.
- Czerniejewski P, Zatoń K, Kasowska N, Brysiewicz A. 2019a. Age structure, condition and length increase of the topmouth gudgeon (Pseudorasbora parva Schlegel 1842) in non-native populations of small rivers of Poland. Journal of Water and Land Development 40(1):113–118. DOI: 10.2478/jwld-2019-0012.
- Davies GD, Britton JR. 2021. Consistency in the life history traits of four invasive Pseudorasbora parva populations in Southern England. Journal of Applied Ichthyology 37(2):295–302. DOI: 10.1111/jai.14175.
- Ding H, Gu X, Zhang Z, Huo B, Li D, Xie C. 2019. Growth and feeding habits of invasive Pseudorasbora parva in the Chabalang Wetland (Lhasa, China) and its trophic impacts on native fish. Journal of Oceanology and Limnology 37(2):628–639. DOI: 10.1007/s00343-019-8004-5.
- Dzika E. 1987. Zmodyfikowana metoda zbierania drobnych Monogenea ze skrzeli ryb [A modified method for collecting tiny monogenea from the gills of fish]. Wiadomości Parazytologiczne 1:99–102.
- Dzika E. 2008. Pasożyty ryb Polski (klucze do oznaczania): Przywry monogeniczne – Monogenea [Fish parasites of Poland (keys for identification): Monogenea]. Warszawa (Warsaw): Polskie Towarzystwo Parazytologiczne. pp. 189.
- Eckmann R. 2017. The impact of density-dependant growth on whitefish production in re-oligotrophic lakes - a bioenergetics simulation study. Fundamental and Applied Limnology 189(3):249–256. DOI: 10.1127/fal/2016/0800.
- Froese R. 2006. Cube law, condition factor and weight–length relationships: History, meta‐analysis and recommendations. Journal of Applied Ichthyology 22(4):241–253. DOI: 10.1111/j.1439-0426.2006.00805.x.
- Gaspar MB, Ferreira R, Monteiro CC. 1999. Growth and reproductive cycle of Donax trunculus L., (Mollusca: Bivalvia) off Faro, southern Portugal. Fisheries Research 41(3):309–316. DOI: 10.1016/S0165-7836(99)00017-X.
- Gozlan RE, Andreou D, Asaeda T, Beyer K, Bouhadad R, Burnard D, Caiola N, Cakic P, Djikanovic V, Esmaelli R, Falka I, Golicher D, Harka A, Jeney G, Kovac V, Musil J, Nocita A, Nocita A, Poulet N, Virbickas T, Wolter C, Tarkan A, Tricarico E, Trichkova T, Verreychken H, Witkowski A, Zhang C, Britton J.R ZI. 2010b. Pan-continental invasion of Pseudorasbora parva: Towards a better understanding of freshwater fish invasions. Fish and Fisheries 11(4):315–340. DOI: 10.1111/j.1467-2979.2010.00361.x.
- Gozlan RE, Britton JR, Cowx I, Copp GH. 2010a. Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology 76(4):751–786. DOI: 10.1111/j.1095-8649.2010.02566.x.
- Gozlan RE, St-Hilaire S, Feist SW, Martin P, Kent ML. 2005. Disease threat to European fish. Nature 435(7045):1046. DOI: 10.1038/4351046a.
- Gozlan RE, Whipps C, Andreou D, Arkush K. 2009. Identification of a rosette-like agent as Sphaerothecum destruens, a multi-host fish pathogen. International Journal of Parasitology 39(10):1055–1058. DOI: 10.1016/j.ijpara.2009.04.012.
- Grabda-Kazubska B, Okulewicz A. 2005. Pasożyty ryb Polski (klucze do oznaczania). Nicienie – Nematoda [Fish parasites of Poland (determination keys) . Nematodes]. Warszawa: Polskie Towarzystwo Parazytologiczne. pp 168.
- Grabowska J, Pietraszewski D, Przybylski M, Tarkan AS, Lampart-Kałużniacka M ML. 2011. Life-history traits of Amur sleeper, Perccottus glenii, in the invaded Vistula River: Early investment in reproduction but reduced growth rate. Hydrobiologia 661(1):197–210. DOI: 10.1007/s10750-010-0524-0.
- Grabowska J, Przybylski M. 2015. Life-history traits of non-native freshwater fish invaders differentiate them from natives in the Central European bioregion. Reviews in Fish Biology and Fisheries 25(1):165–178. DOI: 10.1007/s11160-014-9375-5.
- Gusev AV. 1955. Monogenean parasites from fishes of 144 the Amur River. Tr. ZIN ASci. USSR 19:171–387.
- Hammer O, Harper DAT, Ryan PD. 2001. PAST: paleonthological statistics software package for education and data analysis palaeontol. Electron. 4–9. Available: http://palaeo-electronica.org. Accessed May 2022 17.
- Han XF, Li SH. 1995. The biology of Pseudorasbora parva in Baiyangdian Lake. Hebei Fisheries 2:3–6.
- Huang S, Wang Y, Zheng X, Wang W, Cao X. 2015. Comparative analysis of three methods of making scale specimens for small fish. Environmental Biology of Fishes 98(2):697–703. DOI: 10.1007/s10641-014-0299-7.
- Huber V, Geist J. 2017. Glochidial development of the freshwater swan mussel (Anodonta cygnea, L 1758) on native and invasive fish species. Biological Conservation 209:230–238. DOI: 10.1016/j.biocon.2017.02.030.
- IUCN. 2000. IUCN guidelines for the prevention of biodiversity loss caused by alien invasive species. Available: www.iucn.org/dbtw-wpd/edocs/Rep-2000-051.pdf.
- Kapusta A, Bogacka-Kapusta E, Czarnecki B. 2008. The significance of stone moroko Pseudorasbora parva (Temminck and Schlegel) in the small-sized fian assemblages in the littoral zone of the heated Lake Lichenskie. Archives of Polish Fisheries 16:49–62. DOI: 10.2478/s10086-008-0004-6.
- Kent ML, Bishop-Stewart JK, Matthews JL, Spitsbergen JM. 2002. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. comparative Medicine 52(4):354–358.
- Kirczuk L, Dziewulska K, Czerniejewski P, Brysiewicz A, Rząd I. 2021. Reproductive Potential of Stone Moroko (Pseudorasbora parva, Temminck et Schlegel, 1846) (Teleostei: Cypriniformes: Gobionidae) Inhabiting Central Europe. Animals 11(9):2627. DOI: 10.3390/ani11092627.
- Klimpel S, Kuhn T, Münster J, Dörge DD, Klapper R, Kochmann J. 2019. Techniques. In: Parasites of Marine Fish and Cephalopods. Cham: Springer. pp. 77–147. DOI: 10.1007/978-3-030-16220-7_4.
- Knezevič B. 1981. Pseudorasbora parva, new genus and species in Lake Skardar. Glasnik Republickogo Zarvoda Zastihi Za Prirode Muzeja 14:79–84.
- Kvach Y, Boldyrev V, Lohner R, Stepien CA. 2015. The parasite community of gobiid fishes (Actinopterygii: Gobiidae) from the Lower Volga River region. Biologia 70(7):948–957. DOI: 10.1515/biolog-2015-0108.
- Le Cren CD. 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the Perch (Perca fluviatilis). Journal of Animal Ecology 20(2):201–219. DOI: 10.2307/1540.
- Li HJ, Wang YP, Leng QL, Li XJ, Li XF, Yu TL, Huang B. 2017. Study on the age and growth of Pseudorasbora parva from Nanwan Lake upstream the Huaihe River. Acta Hydrobiologica Sinica 41(4):835–842.
- Lonc E, Złotorzycka J. 1994. Zajęcia praktyczne z parazytologii dla studentów biologii. Wrocław, Poland: Wydawnictwo Uniwersytetu Wrocławskiego.
- Magurran AE. 2004. Measuring biological diversity. Oxford, UK: Blackwell Publishing. pp 256.
- Marine C, Emira C, Amélie C, Bruno B, Georges MC, Chasserieau C, Foissy J-M, Gerard B, Gozlan Z, Guillouët J, Hérodet B, Laine M, Masseboeuf F, Mirkovic I, Nicolas D, Poulet N, Martin J-F, Gilles A, Elie GR. 2022. Towards unravelling the Rosette agent enigma: Spread and emergence of the co-invasive host-pathogen complex, Pseudorasbora parva - Sphaerothecum destruens. Science of the Total Environment 806:150427. DOI: 10.1016/j.scitotenv.2021.150427.
- Moravec F. 1994. Parasitic Nematodes of Freshwater Fishes of Europe. Dordrecht, the Netherlands: Kluwer Academic Publishers.
- Moreau J, Belaud A, Dauba F, Nelva A. 1985. A model for rapid growth evaluation in fishes: The case of the cyprinids of some large French rivers. Hydrobiologia 120(3):225–227. DOI: 10.1007/BF00045165.
- Munro JL, Pauly D. 1983. A simple method for comparing the growth of fish and invertebrates. FishByte, The WorldFish Center 1:5–6.
- Niewiadomska K. 2003. Pasożyty ryb Polski. Digenea [Polish fish parasites. Digenea]. Warsaw, Poland: Polskie Towarzystwo Parazytologiczne.
- Niewiadomska K. 2010. Przywry Trematoda. Część ogólna; część systematyczna ‒Aspidogastrea, Digenea: Strigeida. Łódź, Poland: Wydawnictwo Uniwersytetu Łódzkiego.
- Niewiadomska K, Pojmańska T. 2018. Przywry Trematoda część systematyczna Digenea: Plagiorchiida [Flukes Trematoda, systematic part Digenea: Plagiorchiida]. Łódź: Wydawnictwo Uniwersytetu Łudzkiego. pp 387.
- Ogle DH. 2016. Introductory fisheries analysis with R. Boca Raton, Florida: Chapman and Hall. CRC Press.
- Ogle DH, Isermann DA. 2017. Estimating age at a specified length from the von Bertalanffy growth function. North American Journal of Fisheries Management 37(5):1176–1180. DOI: 10.1080/02755947.2017.1342725.
- Ondračková M, Matejusova I, Šimková A, Gelnar M. 2004. New reports of dactylogyridean species (Monogenea) for Central Europe. Helminthologia 41:139–145.
- Onikura N, Nakajima J. 2013. Age, growth and habitat use of the topmouth gudgeon, Pseudorasbora parva in irrigation ditches on northwestern Kyushu Island, Japan. Journal of Applied Ichthyology 29(1):186–192. DOI: 10.1111/j.1439-0426.2012.02041.
- Pauly D. 1980. On the interrelationships between natural mortality, growth parameters and mean environmental temperature in 175 fish stocks. Journal du Conseil 39(2):175–192. DOI: 10.1093/icesjms/39.2.175.
- Peribánez MA, Ordovas L, Benito J, Benejam L, Gracia MJ, Rodellar C. 2011. Prevalence and sequence comparison of Phyllodistomum folium from zebra mussel and from freshwater fish in the Ebro River. Parasitology International 60(1):59–63. DOI: 10.1016/j.parint.2010.10.004.
- Pimentel D, Lach L, Zuniga R, Morrison D. 2000. Environmental and economic costs of non-indigenous species in the United States. BioScience 50(1):53–65. DOI: 10.1641/0006-3568(2000)050[0053:EAECON]2.3.CO;2.
- Pimentel D, Zuniga R, Morrison D. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52(3):273–288. DOI: 10.1016/j.ecolecon.2004.10.002.
- Pinder AC, Gozlan RE, Britton JR. 2005. Dispersal of the invasive topmouth gudgeon, Pseudorasbora parva in the UK: A vector for an emergent infectious disease. Fisheries Management and Ecology 12(6):411–414. DOI: 10.1111/j.1365-2400.2005.00466.x.
- Piria M, Mrkonjić Fuka M, Gavrilović A, Shiming W, Li L, Tanuwidjaja I, Tang R, Zhao H, Yang Q, Li D. 2020. Length-weight relationships and condition between invasive and native populations of topmouth gudgeon Pseudorasbora parva. 55th Croatian and 15th International Symposium on Agriculture, Vodice, 16-21 February 2020.
- Pojmańska T, Cielecka D. 2001. Tasiemce (Cestoda) związane ze środowiskiem wodnym. Łódź, Poland: Wydawnictwo Uniwersytetu Łódzkiego.
- Pojmańska T, Niewiadomska K, Okulewicz A. 2007. Pasożytnicze Helminty Polski. Gatunki, żywiciele, białe plamy [Parasitic Helminths of Poland. Species, hosts, white spots]. Monografie Parazytologiczne Nr 18. Warszawa: Polskie Towarzystwo Parazytologiczne. pp. 1–360.
- Prost M. 1994. Choroby ryb. Lublin: Polskie Towarzystwo Nauk Wewerynaryjnych.
- Pyrzanowski K, Zięba G, Przybylski M. 2020. Endangered weatherfish (Misgurnus fossilis) age and growth is affected by the size of the watercourses. Journal of Vertebrate Biology 69(1):1–12. DOI: 10.25225/jvb.19041.
- Rakauskas V, Virbickas T, Steponėnas A. 2021. Several decades of two invasive fish species (Perccottus glenii, Pseudorasbora parva) of European concern in Lithuanian inland waters; from first appearance to current state. Journal of Vertebrate Biology 70(4):21048. DOI: 10.25225/jvb.21048.
- Rosecchi E, Crivelli A, Catsadorakis G. 1993. The establishment and impact of Pseudorasbora parva, an exotic fish species introduced into Lake Mikri Prespa (north-western Greece). Aquatic Conservation: Marine and Freshwater Ecosystem 3(3):223–231. DOI: 10.1002/aqc.3270030306.
- Rybczyk A, Czerniejewski P, Keszka S, Janowicz M, Brysiewicz A, Wawrzyniak W. 2020. First data of age, condition, growth rate and diet of invasive Neogobius melanostomus (Pallas, 1814) in the Pomeranian Bay, Poland. Journal of Water and Land Development 47(X–XII):142–149. DOI: 10.24425/jwld.2020.135041.
- Sana S, Hardouin EA, Paley R, Zhang T, Andreou D. 2020. The complete mitochondrial genome of a parasite at the animal-fungal boundary. Parasites & Vectors 17;13(1):81 DOI: 10.1186/s13071-020-3926-5.
- Sana S, Williams C, Hardouin EA, Blake A, Davison P, Pegg J, Paley R, Zhang T, Andreou D. 2018. Phylogenetic and environmental DNA insights into emerging aquatic parasites: Implications for risk management. International Journal for Parasitology 48(6):473–481. DOI: 10.1016/j.ijpara.2017.11.002.
- Sepulveda MS, Kinsella JM. 2013. Helminth collection and identification from wildlife. Journal of Visualized Experiments 82:e51000. DOI: 10.3791/51000.
- Sokal RR, Rohlf FJ. 2012. Biometry: The principles and practice of statistics in biological research. 4th ed. New York: W. H. Freeman and Co. pp 937.
- Sorensen PW, Stacey NE. 2004. Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes. New Zealand Journal of Marine and Freshwater Research 38(3):399–417. DOI: 10.1080/00288330.2004.9517248.
- Spikmans F, Lemmers P, op den Camp HJ, van Haren E, Kappen F, Blaakmeer A, van der Veide G, van Langevelde F, Rsew L, van Alen TA. 2020. Impact of the invasive alien topmouth gudgeon (Pseudorasbora parva) and its associated parasite Sphaerothecum destruens on native fish species. Biological Invasions 22(2):587–601. DOI: 10.1007/s10530-019-02114-6.
- Stavrescu-Bedivan M, Aioanei TF, Vasile Scãeþeanu G. 2017. Length-weight relationships and condition factor of 11 fish species from the Timi river, western Romania. Agriculture and Forestry 63(4):281–285. DOI: 10.17707/AgricultForest.63.4.27.
- Taylor CC. 1962. Growth equation with metabolic parameters. Journal du Conseil Permanent International Pour l’Exploration de la Mer 27(3):270–286. DOI: 10.1093/icesjms/27.3.270.
- Teubner D, Klein R, Paulus M, Wesch C. 2019. Changes of fish growth in German rivers. Current Opinion in Environmental Science & Health 11:59–64. DOI: 10.1016/j.coesh.2019.06.004.
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, A.m K. 2003. Introduced species and their missing parasites. Nature 421(6923):628–630. DOI: 10.1038/nature01346.
- Torchin ME, Mitchell CE. 2004. Parasites, pathogens, and invasions by plants and animals. Frontiers in Ecology and Environment 2(4):183–190. DOI: 10.1890/1540-9295(2004)002[0183:PPAIBP]2.0.CO;2.
- Villeneuve F, Copp GH, Fox MG, Stakenas S. 2005. Interpopulation variation in growth and life-history traits of the introduced sunfish, pumpkinseed Lepomis gibbosus, in southern England. Journal of Applied Ichthyology 21(4):275–281. DOI: 10.1111/j.1439-0426.2005.00679.x.
- Welcomme RL. 1992. A history of international introductions of inland aquatic species. ICES Marine Science Symposia 194:3–14.
- Witkowski A. 1991. Pseudorasbora parva (Schlegel, 1842) (Cyprinidae, Gobioninae) nowy gatunek w polskiej ichtiofaunie [Pseudorasbora parva (Schlegel, 1842) (Cyprinidae, Gobioninae) a new species in the Polish ichthyofauna]. Przegląd Zoologiczny 35:323–331.
- Witkowski A. 2002. Introduction of fishes into Poland: Benefaction or plague. Nature Conservation 59:41–52.
- Witkowski A, Wiśniewolski W. 2005. Ryby i minogi Biebrzy, jej starorzeczy i dopływów [Fish and lampreys of the Biebrza, its oxbow lakes and tributaries]. In: W DA, Werpachowski C, red. Przyroda Biebrzańskiego Parku Narodowego. Osowiec-Twierdza: Wyd. Biebrzański Park Narodowy. pp. 247–255.
- Yuryshynets V, Zaichenko N. 2015. Comparative analysis of the Parasite Fauna of Pseudorasbora parva (Cyprinidae) in the native and non-native areas of its distribution. Hydrobiological Journal 51(5):101–109. DOI: 10.1615/HydrobJ.v51.i5.100.
- Záhorská E, Kováč V, Katina S. 2010. Age and growth in a newly-established invasive population of topmouth gudgeon. Central European Journal of Biology 5(2):256–261. DOI: 10.2478/s11535-010-0002-8.
- Zhokhov AE. 1987. New data on the developmental cycle and biology of the trematode Phyllodistomum elongatum (Fasciolata, Gorgoderidae). Parazitologiya 21:134–139. (In Russian).
- Zhokhov AE. 1991. Two types of Cercariae of the Trematode Phyllodistomum elongatum (Fasciolata: Gorgoderidae) from Pisidium amnicum Mollusks. Parazitologiya 25(1):63–68. (In Russian).