Abstract
Canthariphilous species are those arthropods attracted to cantharidin (CTD), a defensive compound produced by two beetle families (Meloidae and Oedemeridae). Although several species are known to be attracted to CTD, canthariphily was recently discovered in new species, suggesting that the list of canthariphilous species is still far from being complete. A systematic sampling focused to detect canthariphilous species has never been performed in Italy. The present research provides a list of seven canthariphilous species (Diptera: Ceratopogonidae, Anthomyiidae; Coleoptera: Anthicidae) from the Tolfa Mountains (Latium, Central Italy) resulting from a one-year sampling with CTD-baited and control traps. New species (Atrichopogon atriscapulus and A. tolfensis) were found to be attracted to CTD, and other species, already known as canthariphilous, were recorded for the first time in the Italian fauna (A. atriscapulus and A. meloesugans). A new scenario about the ecological significance of CTD in the sexual selection of canthariphilous species was speculated in A. meloesugans. Finally, a list of CTD-producing species occurring in the sample area was provided to suggest putative natural CTD sources.
1. Introduction
Arthropod species attracted to cantharidin (CTD) are defined as canthariphilous. CTD is a toxic terpene produced as defence compound by blister beetles (Coleoptera: Meloidae) and false blister beetles (Coleoptera: Oedemeridae) (Carrel & Eisner Citation1974; Carrel et al. Citation1986). Many arthropod species are known to be attracted to this toxic molecule (Hemp & Dettner Citation2001). The first associations between canthariphilous species and CTD-producing species date back to the nineteenth century and refer to members of antlike flower beetles (Coleoptera: Anthicidae) and fire-coloured beetles (Coleoptera: Pyrochroidae) feeding upon blister beetles and false blister beetles (e.g., Say Citation1827; Guyon Citation1848; de Diego Dm Citation1880; Chobaut Citation1895, Citation1897; Pic Citation1897). Later, other groups of insects attracted to both pure CTD or CTD-producing beetles were discovered, and canthariphily was recorded worldwide in about 300 species of Insecta (i.e., Coleoptera, Diptera, Hemiptera, and Hymenoptera) (Hemp & Dettner Citation2001). Recently canthariphily was also observed in new species even including a new class of Arthropoda (Arachnida: Opiliones) (e.g., Hashimoto & Hayashi Citation2014, Citation2016; Horiuchi et al. Citation2018; Kejval & Nardi Citation2018; Ramírez et al. Citation2021; Molfini et al. Citation2022), suggesting that the list of canthariphilous species is far from being complete.
The ecological significance of CTD in most of canthariphilous species is still unexplored or poorly documented. It has been proposed that some species might confuse CTD with analogous compounds driving aggregation or food searching (Dettner Citation1997; Tallamy et al. Citation1999; Hashimoto & Hayashi Citation2014). According to this hypothesis, the attraction to CTD might be merely accidental in some species, excluding an adaptive significance of this terpene in their ecology. Differently, other canthariphilous species can sequester CTD from producing beetles, and possibly use it as a deterrent against predators and parasites. Species able of sequestering CTD have been observed in some families of Coleoptera (Anthicidae, Cleridae, and Pyrochroidae) (Schütz & Dettner Citation1992; Frenzel & Dettner Citation1994; Holz et al. Citation1994; Eisner et al. Citation1996a, Citation1996b; Molfini et al. Citation2022) and Diptera (Anthomyiidae and Ceratopogonidae) (Frenzel & Dettner Citation1994). CTD sequestering has been also speculated in other Coleoptera (Cantharidae, Cerambycidae, Chrysomelidae, and Melyridae) (Islami & Nikbakhtzadeh Citation2009) and in Hemiptera (Miridae) (Ramírez et al. Citation2021). In particular, some species of Anthicidae, Pyrochroidae and Ceratopogonidae can transfer the ingested CTD to the eggs during oviposition, implying a role of CTD in offspring defence (Schütz & Dettner Citation1992; Frenzel & Dettner Citation1994; Holz et al. Citation1994; Eisner et al. Citation1996a, Citation1996b).
Although experimentally demonstrated in only a few species, it is generally assumed that males of several Anthicidae and Pyrochroidae have peculiar glands that allow them to present ingested CTD to females during courtship, thus being advantaged in sexual competition (Schütz & Dettner Citation1992; Holz et al. Citation1994; Eisner et al. Citation1996a). In some species of Anthicidae [especially in the tribe of Microhoriini (Anthicinae) and in the genus Notoxus Geoffroy, 1762 (Notoxinae)], these glands are localised in specialised elytral notches (also present in some genera of the basal blister beetle subfamily Eleticinae) (Abdullah Citation1965; Selander Citation1966; Bologna Citation1991; Kejval & Chandler Citation2020), while in Pyrochroidae these are localised within a peculiar cranial apparatus occurring in several genera of Pyrochroinae (e.g., Young Citation2019).
Some canthariphilous species are known to be distributed in the Italian peninsula, but a systematic sampling with CTD-baited traps to detect canthariphilous species has never been performed thus far in Italy.
This work aims contributing to assess the taxonomic diversity and phenology of the Italian canthariphilous fauna through a one-year sampling in a natural area where the presence of CTD producers (blister beetles and false blister beetles) is well ascertained: the EU Special Protection Area “Comprensorio Tolfetano-Cerite-Manziate” (IT6030005) in the province of Rome (Tolfa Mountains, Tolfa, Rome, Latium, Italy).
2. Material and methods
Sampling method involved two pairs of funnel traps (Horiuchi et al. Citation2018) placed in four sites characterised by different ecotones and along an altitudinal gradient, representative of the ecosystem heterogeneity of the Tolfa Mountains (site A: 42.058716N, 11.941148E, 48 m a.s.l., secondary pastures derived by Mediterranean sclerophyllous forests; - site B: 42.092617N, 11.974103E; 298 m a.s.l., secondary pastures derived by temperate oak forest; - site C: 42.181213N, 11.942283E, 443 m a.s.l., secondary clearing derived by temperate oak forest; - site D: 42.150367N, 11.908061E, 615 m a.s.l., secondary clearing derived by Apennine beech forests) ().
Figure 1. Sampling sites on the Tolfa Mountains (Rome, Latium, Italy). Orange dots indicate the location of the four sites (A, B, C, D). Each yellow dot indicates a pair of funnel traps (CTD-baited trap and control trap), two pairs of traps for each site were set (about 30 m apart). All the images are north-oriented. Satellite images were taken from Google Earth Pro.
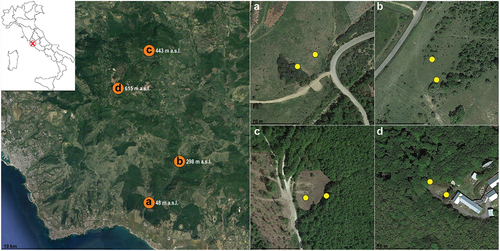
Each pair of traps consisted in one CTD-baited trap (0.5 ml of a 10−2M solution of synthetic CTD in acetone) and one control trap (0.5 ml of acetone) approximately 2 m apart (Hashimoto & Hayashi Citation2014). The distance between pairs at each site was about 30 m. In each site, one pair was placed at the ground level, while the other was suspended at about 1.5 m. Traps were active for 24 hours every two weeks from June 3rd, 2020, to June 15th, 2021, for a total of 24 surveys. After each sampling session, traps were capped with cotton wool and stored at −20°C to euthanize the sampled specimens. Specimens were then preserved in 70% ethanol for species identification. Binomial test implemented in R (R Core Team Citation2021) was used to test both the attraction to CTD of each taxon and differences in attraction between sexes. Only taxa with p-value < 0.05 or sampled more than three times in CTD-baited traps (without record in control traps) were identified at the species level. Difference in CTD attraction between sexes was tested only in species suspected to acquire CTD for offspring-defence and courtship (Hashimoto & Hayashi Citation2014). The material is deposited in the personal collections of the authors which identified the species (Nardi G., Anthicidae; Szadziewski R., Ceratopogonidae; Bologna M.A., Anthomyiidae and unidentified specimens). The diversity of canthariphilous species was described with the Shannon index H (Shannon & Weaver Citation1949) and the evenness index E (Pielou Citation1975) calculated as in Hashimoto and Hayashi (Citation2014). Faunistic records of blister beetles and false blister beetles, obtained from over 40 years of entomological samplings on the Tolfa Mountains (deposited in the collection of Bologna M.A.), were used to compile a list of CTD-producing species that might serve as CTD sources for canthariphilous species in the area ().
Table I. Checklist of Meloidae and Oedemeridae (in alphabetical order) recorded from Tolfa Mountains and close area (Latium, Italy) with the roughly phenology of adults. Species are proposed as putative natural sources of CTD for canthariphilous species. Diversity (H) and evenness (E) indices for canthariphilous species are reported at the top of the table for each sampling date. Table is divided and coloured as in to facilitate comparison.
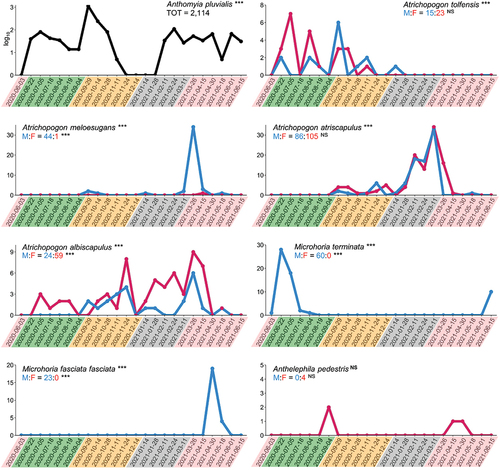
Figure 2. Total number of individuals collected for each species in CTD-baited traps during the one-year sampling on the Tolfa Mountains. Logarithmic scale (log10) is used for individuals of Anthomyia pluvialis (Anthomyiidae, Diptera) due to the broad range of variation. Male/female ratio is given for species belonging to Ceratopogonidae (Diptera) and Anthicidae (Coleoptera). Sampling dates are coloured to highlight the seasons (pink, spring; green, summer; yellow, autumn; grey, winter). Binomial test was used to test the occurrence in CTD-baited traps vs. control traps and differences in attraction between males and females. X-axis, sampling date (24 hours); y-axis, number of individuals; M, males (blue); F, females (purple); NS, p-value > 0.05; asterisks (***), p-value < 0.001.
3. Results and discussion
Baited and control traps collected 2,659 and 55 specimens, respectively, with seven species significantly attracted to CTD-baited traps (binomial test p-value < 0.001) (see Supplementary Material: Table S1). Canthariphilous species were identified in Diptera (Anthomyiidae and Ceratopogonidae) and Coleoptera (Anthicidae), as discussed below (). The diversity (H = 1.05) and evenness (E = 0.37) indices, calculated from the pooled frequencies of all canthariphilous species across all months, were consistent with those observed in a previous study conducted in central Japan (1.09 and 0.36, respectively; Hashimoto & Hayashi Citation2014). Values of H and E for each sampling session are shown in .
3.1. Diptera, Anthomyiidae
The 79% of specimens collected in CTD-baited traps were identified as belonging to the Anthomyia pluvialis complex (Diptera: Anthomyiidae) (Michelsen Citation1980) (N = 2,114) (), evidencing a conspicuous attraction of this taxon to CTD (only seven individuals were collected in control traps). However, although A. pluvialis is well known to be attracted to CTD (e.g., Görnitz Citation1937; Dettner Citation1997; Hemp & Dettner Citation2001) and individuals were observed lapping the body surface of death blister beetles (Bologna & Havelka Citation1985), CTD seems not having a role in offspring-defence and the ecological significance of canthariphily in this species remains unknown (Frenzel & Dettner Citation1994; Dettner Citation1997). The abundance of A. pluvialis strongly influenced the diversity indices of the canthariphilous community, and values increased to H = 2.23 and E = 0.86 when the taxon was excluded from the analysis.
3.2. Diptera, Ceratopogonidae
Canthariphily has been well documented in biting midges (Diptera: Ceratopogonidae), especially in the genus Atrichopogon Kieffer, 1906. During our sampling, four species of three Atrichopogon subgenera were collected in CTD-baited traps, i.e., subgenus Atrichopogon s. str.: A. tolfensis Szadziewski et al. Citation2022; subgenus Meloehelea Wirth, Citation1956: A. atriscapulus Kieffer, 1918 and A. meloesugans Kieffer, 1922; and subgenus Psammopogon Remm, 1979: A. albiscapulus Kieffer, 1918 (). Among these, canthariphily was previously observed in A. albiscapulus (Frenzel et al., Citation1998) and A. meloesugans (Wirth, Citation1980; Szadziewski et al., Citation2007), and described based on specimens from this sampling in A. tolfensis (Szadziewski et al., Citation2022). Nevertheless, this represented the first report of canthariphily for A. atriscapulus, leading the number of species included in the European list of canthariphilous Ceratopogonidae to nine (Szadziewski et al. Citation2022).
Adult females of some Atrichopogon spp. are known to feed on the haemolymph of both Meloidae and Oedemeridae, from which they sequester CTD (e.g., Wirth Citation1980; Frenzel & Dettner Citation1994; Szadziewski et al. Citation2007; Ciliberti et al. Citation2020; Hashimoto & Tateno Citation2022). Although males have reduced biting mouthparts, some evidence suggests that they can sequester CTD, but it is unclear how they obtain the compound (Frenzel & Dettner Citation1994). It has been proposed that males could intake CTD from the exuded haemolymph of blister beetles and false blister beetles (autohaemorrhaging is a defensive strategy adopted by several species of CTD producers; Fratini et al. Citation2021) or from the liquid faeces of female biting midges (Szadziewski & Elżbieta Citation2022).
According to our results, both males and females of A. tolfensis and A. atriscapulus were attracted to CTD-baited traps with no differences between sexes (p = 0.26 and p = 0.19 respectively), while females were significantly more attracted than males in A. albiscapulus (p < 0.001) (). Unexpectedly, almost only males of A. meloesugans were attracted to CTD-baited traps (p < 0.001), although females are well known to feed on CTD-producing species (especially on the blister beetle genus Meloe Linnaeus, 1758) (e.g., Szadziewski et al. Citation2007; Ciliberti et al. Citation2020).
As supposed for other species, females of A. meloesugans could not be particularly attracted to CTD itself, but other unknown signals, alone or in combination with CTD, might be involved in triggering the attraction to CTD-producing species (Dettner Citation1997; Molfini et al. Citation2022). Furthermore, the attraction of males to pure-CTD could suggest a mating preference for females that have ingested CTD from beetles. This might indicate a sexual selection of males towards such females. If confirmed, this hypothesis could represent a reversal of typical sex roles observed in other canthariphilous insects, where sexual selection is driven by females towards males that have ingested CTD (see paragraph Coleoptera, Anthicidae) (Schütz & Dettner Citation1992; Holz et al. Citation1994; Eisner et al. Citation1996a, Citation1996b). Additionally, it is possible that CTD acts as a kairomone, attracting males to CTD-producing species where females can also be found (Dettner Citation1997; Hashimoto & Hayashi Citation2016).
It is worth noting that interspecific interactions with CTD-producing beetles have never been documented in A. albiscapulus, A. atriscapulus and A. tolfensis, thus it is not possible to discern if these species confuse CTD with analogous compounds, or if this terpene plays an actual role in their ecology (Dettner Citation1997; Tallamy et al. Citation1999; Hashimoto & Hayashi Citation2014).
Adults of Ceratopogonidae were active for several months on the Tolfa Mountains (), suggesting a wide spectrum of putative CTD-producing species as CTD source (). In particular, adults of A. albiscapulus were active all over the year except for the late spring; A. atriscapulus was mainly collected during the winter season, with an activity period ranging from early autumn to early spring; A. tolfensis was collected during the summer-autumn period; and A. meloesugans showed its activity peak in the early spring, with two smaller peaks in both the early autumn and winter. The three peaks of activity observed in A. meloesugans might suggest interspecific interactions with several co-occurring Meloe species with different phenology (e.g., M. proscarabaeus Linnaeus, 1758, M. tuccia Rossi, 1792, and M. erythrocnemus Pallas, 1782 in the spring; M. autumnalis Olivier, 1792, M. mediterraneus Müller, 1925 in the autumn; M. mediterraneus Müller, 1925, M. ganglbaueri Apfelbeck, 1905, M. baudii Leoni, 1907 in the winter) (Bologna Citation1988, Citation1991).
This research also added two new species to the Italian fauna (A. atriscapulus and A. meloesugans), since only A. albiscapulus was already recorded in the Italian peninsula (Borkent et al. Citation2013) and A. tolfensis was newly described based on specimens from this sampling, currently representing an Italian endemite (Szadziewski et al. Citation2022). So far, in Europe, A. atriscapulus was exclusively reported in Poland and Lithuania (Szadziewski et al. Citation2007; Borkent et al. Citation2013) and A. meloesugans in Poland and Netherlands (Ciliberti et al. Citation2020), so this finding represents the third European record for both species.
3.3. Coleoptera, Anthicidae
Three species of Anthicidae [Microhoria fasciata fasciata (Chevrolat, 1834), M. terminata (Schmidt, 1842), and Anthelephila pedestris (Rossi, 1790)], were collected exclusively in CTD-baited traps (). Only males of M. f. fasciata and M. terminata were significantly attracted (p < 0.001), while the number of A. pedestris individuals (only four females) was too low to statistically assess an attraction. Although in literature both sexes of A. pedestris have been found to be attracted to CTD (Schütz & Dettner Citation1992), we collected only few females without a statistical significance, suggesting a lower attraction in this species than in the two Microhoria Chevrolat, 1877 species. However, broadly, these results confirmed previous observations, indeed canthariphily is commonly present in Microhoriini (which includes Microhoria), with only males attracted to CTD, and rarely observed in Formicomini (which includes Anthelephila Hope, 1833) with both sexes attracted (Hemp & Dettner Citation2001; Kejval & Chandler Citation2020).
Noteworthy, males of Microhoriini have modified elytral apex that contain putative CTD glands showed to females during courtship (Schütz & Dettner Citation1992; Kejval & Chandler Citation2020). Since in this group only males were attracted, our results are coherent with the hypothesis that CTD acts as a selective agent that increases male mating success in M. f. fasciata and M. terminata (Schütz & Dettner Citation1992; Kejval & Chandler Citation2020). Contrarily, males of A. pedestris have simple elytral apex and lower attraction to CTD, suggesting that this terpene likely does not play a key role in sexual selection of this species.
Despite their attraction to CTD, it is still unclear from which CTD-producing species M. f. fasciata and M. terminata sequester this compound. As far as we know, only M. f. fasciata was reported feeding on the blister beetle Meloe violaceus (Marsham, 1802) (Bucciarelli Citation1976), while M. terminata was only observed attracted to CTD (Hemp & Dettner Citation2001). According to our sampling in Central Italy, adults of Microhoria species have different phenology () which might result in different natural sources of CTD ().
4. Conclusions
This research represents the first systematic sampling specifically aimed at collecting and assessing the phenology of canthariphilous species in Italy. Our results were in line with previous findings, highlighting the presence of canthariphilous species in Anthomyiidae, Ceratopogonidae and Anthicidae and adding details on their phenology, with new records for the Italian fauna.
Unexpectedly, members of Pyrochroidae were not collected although the widespread of two canthariphilous species in Italy: Pyrochroa coccinea (Linnaeus, 1761) and P. serraticornis serraticornis (Scopoli, 1763) (Nardi & Bologna Citation2000; Scheffler Citation2013), whose presence in the sampling area has been recently confirmed for the latter (Molfini et al. Citation2023). Both species are known to be attracted to blister beetles and have been repeatedly observed feeding on Meloe spp. (Bologna & Havelka Citation1985; Lückmann Citation1999; Nardi & Bologna Citation2000; Lückmann & Niehuis Citation2009; Scheffler Citation2013). However, it is possible that individuals of P. s. serraticornis from Lückmann and Niehuis (Citation2009) should be attributed to the cryptic species P. bifoveata Molfini et al., Citation2023, which is also known to be canthariphilous (Molfini et al. Citation2023).
Much remains to be understood concerning the attraction to CTD of canthariphilous species, and why some of them seem not to be attracted to the pure compound (Molfini et al. Citation2022). Further faunistic samplings using additional traps to CTD-baited traps (e.g., traps baited with CTD-producing species or with CTD analogous produced by plants), together with behavioural and chemical analyses, could allow to better understand pattern of attraction of canthariphilous species, their hosts, the ecological role of CTD, and lead to the discovery of canthariphily in new taxa of arthropods.
Supplemental Material
Download MS Excel (87.2 KB)Acknowledgements
We greatly thank Dr. Tecla Gasperi (University of Roma Tre, IT) for having prepared the cantharidin solution used in the samplings. We also thank Dr. Giulia Scarparo (University of California Riverside, US) for her help in some sampling sessions. Finally, a symbolic thanks goes to the faithful 2003 Nissan Micra, which for years drives the first author and Giulia Scarparo to the exploration of Italian wilderness, and without which this sampling would not have been carried out; we hope your wreck becomes the 4 × 4 you have always deserved to be.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2227655
Additional information
Funding
References
- Abdullah M. 1965. Protomeloe argentinensis, a new genus and species of Meloidae (Coleoptera), with remarks on the significance of cantharidin and the phylogeny of the families Pyrochroidae, Anthicidae, Meloidae and Cephaloidae. Journal of Natural History 7(76):247–254. DOI: 10.1080/00222936408651465.
- Bologna MA. 1988. Note su Eurymeloe e revisione delle specie euromediterranee del gruppo rugosus. Fragmenta Entomologica 20:233–301.
- Bologna MA. 1991. Coleoptera Meloidae. Fauna d’Italia. XXVIII. Bologna: Calderini.
- Bologna MA, Havelka P. 1985. Nuove segnalazioni di attrazione della cantaridina dei Meloidae su Coleotteri e Ditteri. Bollettino Associazione Romana Entomologia 39:77–82.
- Borkent A, Dominiak P, Szadziewski R. 2013. Fauna Europaea: Ceratopogonidae. In: Beuk P, Pape T, editors. Fauna Europaea: Diptera, Nematocera. Fauna Europaea version 2017.06. https://fauna-eu.org/cdm_dataportal/taxon/7161829a-94f3-4c36-943d-0d15f8fa1f77. Accessed 10 Oct 2022.
- Bucciarelli I. 1976. Su alcuni Coleotteri Anticidi raccolti da M. e T. Cerrutti nelle isole di Creta, Corfù e Thasos (Grecia), con descrizione di una nuova specie di Microhoria (Coleoptera, Anthicidae). Fragmenta Entomologica 12:133–142.
- Carrel JE, Doom JP, McCormick JP. 1986. Identification of cantharidin in false blister beetles (Coleoptera, Oedemeridae) from Florida. Journal of Chemical Ecology 12(3):741–747. DOI: 10.1007/BF01012106.
- Carrel JE, Eisner T. 1974. Cantharidin: Potent feeding deterrent to insects. Science 183(4126):755–757. DOI: 10.1126/science.183.4126.755.
- Chobaut A. 1895. Note sur des Anthicus fairmairei Bris. trouves sur le corps d’un Meloe rugosus Marsh. (Col.). Bulletin de la Société Entomologique de France 1895:377–378.
- Chobaut A. 1897. Nouvelles observations sur le relations biologiques des Anthicides avec les vesicants. Bulletin de la Société Entomologique de France 2(4):74–78. DOI: 10.3406/bsef.1897.22003.
- Ciliberti P, d’Oliveira M, Slikboer L, Szadziewski R. 2020. An update of the Forcipomyiinae fauna of the Netherlands (Diptera: Ceratopogonidae). Nederlandse Faunistische Mededelingen 54:107–154.
- de Diego Dm S. 1880. Note on Meloe corallifer Germar. Actas de la Sociedad Española de Historia Natural 9:38–39.
- Dettner K. 1997. Inter- and intraspecific transfer of toxic insect compound cantharidin. In: Dettner K, Bauer G, Völk W, editors. Vertical food web interactions. Berlin: Springer. pp. 115–145. DOI: 10.1007/978-3-642-60725-7_8.
- Eisner T, Smedley SR, Young DK, Eisner M, Roach B, Meinwald J. 1996a. Chemical basis of courtship in a beetle (Neopyrochroa flabellata): Cantharidin as precopulatory “enticing” agent. PNAS 93(13):6494–6498. DOI: 10.1073/pnas.93.13.6494.
- Eisner T, Smedley SR, Young DK, Eisner M, Roach B, Meinwald J. 1996b. Chemical basis of courtship in a beetle (Neopyrochroa flabellata): Cantharidin as “nuptial gift”. PNAS 93(13):6499–6503. DOI: 10.1073/pnas.93.13.6499.
- Fratini E, Salvemini M, Lombardo F, Muzzi M, Molfini M, Gisondi S, Roma E, D’Ezio V, Persichini T, Gasperi T, Mariottini P, Di Giulio A, Bologna MA, Cervelli M, Mancini E. 2021. Unraveling the role of male reproductive tract and haemolymph in cantharidin-exuding Lydus trimaculatus and Mylabris variabilis (Coleoptera: Meloidae): A comparative transcriptomics approach. BMC Genomics 22(1):808. DOI: 10.1186/s12864-021-08118-8.
- Frenzel M, Dettner K. 1994. Quantification of cantharidin in canthariphilous Ceratopogonidae (Diptera), Anthomyiidae (Diptera) and cantharidin-producing Oedemeridae (Coleoptera). Journal of Chemical Ecology 20(8):1795–1812. DOI: 10.1007/BF02066223.
- Frenzel M, Havelka P, Brandl R. 1998. Morphological comparison of canthariphilic Atrichopogon (Diptera: Ceratopogonidae) from Europe and Central Africa – Implications for the evolution of canthariphily? Zoology: Analysis of Complex Systems 101:37.
- Görnitz K. 1937. Cantharidin als gift und anlockung smittel für Insekten. Arbeiten über Physiologie und angewandte Enomologie aus Berlin-Dahlem 4:116–157.
- Guyon G. 1848. Insectivorous Propensity of Notoxus monoceros. In: Newman E, editor. The Zoologist: A popular miscellany of natural history. VI. London: John Van Voorst. pp. 2000.
- Hashimoto K, Hayashi F. 2014. Cantharidin world in nature: A concealed arthropod assemblage with interactions via the terpenoid cantharidin. Entomological Science 17(4):388–395. DOI: 10.1111/ens.12074.
- Hashimoto K, Hayashi F. 2016. Cantharidin world on islands: Species diversity of canthariphilous arthropods in the Izu–Ogasawara Arc. Entomological Science 19(4):432–439. DOI: 10.1111/ens.12184.
- Hashimoto K, Tateno H. 2022. Holarctic canthariphilous biting midge Atrichopogon lucorum (Meigen, 1818) (Diptera: Ceratopogonidae) in Japan. Oriental Insects 56(4):489–500. DOI: 10.1080/00305316.2021.2023680.
- Hemp C, Dettner K. 2001. Compilation of canthariphilous insects. Beiträge zur Entomologie 51(1):231–245. DOI: 10.21248/contrib.entomol.51.1.231-245.
- Holz C, Streil G, Dettner K. 1994. Intersexual transfer of a toxic terpenoid during copulation and its paternal allocation to developmental stages: Quantification of cantharidin in cantharidin-producing oedemerids (Coleoptera: Oedemeridae) and canthariphilous pyrochroids (Coleoptera: Pyrochroidae). Zeitschrift für Naturforschung 49:856–864. DOI: 10.1515/znc-1994-11-1222.
- Horiuchi K, Hashimoto K, Hayashi F. 2018. Cantharidin world in air: Spatiotemporal distributions of flying canthariphilous insects in the forest interior. Entomological Science 21(3):306–314. DOI: 10.1111/ens.12310.
- Islami I, Nikbakhtzadeh MR. 2009. New records of canthariphily among beetles (Coleoptera) from Iran. Türkiye Entomoloji Dergisi 33:243–251.
- Kejval Z, Chandler DS. 2020. Generic revision of the Microhoriini with new species and synonymies from the Palaearctic Region (Coleoptera: Anthicidae). Acta Entomologica Musei Nationalis Pragae 60(1):95–154. DOI: 10.37520/aemnp.2020.007.
- Kejval Z, Nardi G. 2018. On some Notoxus (Coleoptera: Anthicidae) of the N. monoceros species-group. Klapalekiana 54:41–60.
- Lückmann J. 1999. Beobachtungen zur Anlockung von Pyrochroa coccinea (L.) durch Ölkäfer Ein Beitrag zur biologischen Bedeutung der Canthariphilie bei Feuerkäfem (Coleóptera: Meloidae, Pyrochroidae). Mitteilungen des Internationalen Entomologischen Vereins 24:137–143.
- Lückmann J, Niehuis M. 2009. Die Ölkäfer in Rheinland-Pfalz und im Saarland. Verbreitung, Phänologie, Ökologie, Situation und Schutz. Mainz: GNOR. pp. 480.
- Michelsen V. 1980. The Anthomyia pluvialis complex in Europe (Diptera, Anthomyiidae). Systematic Entomology 5(3):281–290. DOI: 10.1111/j.1365-3113.1980.tb00416.x.
- Molfini M, Mancini E, Bologna MA. 2023. Phylogeny of European Pyrochroa (Coleoptera, Pyrochroidae) reveals cryptic taxa and different glacial histories. Zoologica Scripta 52(1):58–69. DOI: 10.1111/zsc.12569.
- Molfini M, Stefanuto L, Gisondi S, Gasperi T, Di Giulio A, Mancini E, Bologna MA. 2022. New evidence of canthariphily: Tilloidea transversalis (Coleoptera: Cleridae) sequestering cantharidin from Lydus trimaculatus (Coleoptera: Meloidae). Journal of Insects Science 22:1–7. DOI: 10.1093/jisesa/ieac035.
- Nardi G, Bologna MA. 2000. Cantharidin attraction in Pyrochroa (Coleoptera: Pyrochroidae). Entomological News 111:74–75.
- Pic M. 1897. Sur les instincts carnassiers des Anthicides. Bulletin de la Société Entomologique de France 2(16):266–267. DOI: 10.3406/bsef.1897.22104.
- Pielou EC. 1975. Ecological Diversity. New York: John Wiley & Sons.
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available: https://www.R-project.org/.
- Ramírez GA, Henry TJ, Smith-Pardo AH. 2021. Eurycipitia clara (distant) (Hemiptera: Heteroptera: Miridae: Brycorinae): New distributions, first host records, descriptions of immatures, and notes on attraction to cantharidin. Proceedings of the Entomological Society of Washington 123(1):1–13. DOI: 10.4289/0013-8797.123.1.1.
- Say T. 1827. Description of new species of coleopterous insects inhabiting the United States. Journal of the Academy of Natural Sciences of Philadelphia 5:237–284.
- Scheffler I. 2013. Über die Körperkontaktbeziehung von einem Kardinalkäfer Pyrochroa coccinea (Linnaeus, 1761) zu einem Ölkäfer Meloe proscarabaeus Linnaeus, 1758. Märkische Entomologische Nachrichten 15:192–194.
- Schütz C, Dettner K. 1992. Cantharidin-secretion by elytral notches of male anthicid species (Coleoptera: Anthicidae). Zeitschrift für Naturforschung 47(3–4):290–299. DOI: 10.1515/znc-1992-3-420.
- Selander RB. 1966. A classification of the genera and higher taxa of the meloid subfamily Eleticinae (Coleoptera). The Canadian Entomologist 98(5):449–481. DOI: 10.4039/Ent98449-5.
- Shannon CE, Weaver W. 1949. The mathematical theory for communication. Urbana: University of Illinois Press.
- Szadziewski R, Dominiak P, Tothová A. 2007. European Atrichopogon biting midges of the subgenus Meloehelea (Diptera: Ceratopogonidae). Polish Journal of Entomology 76:267–284.
- Szadziewski R, Elżbieta S. 2022. Communication between males and females in flies of the biting midge family (Diptera: Ceratopogonidae). Kosmos 72(3):317–323. DOI: 10.36921/kos.2022_2889.
- Szadziewski R, Gwizdalska-Kentzer M, Bologna MA, Molfini M. 2022. A new canthariphilous species of the genus Atrichopogon Kieffer, 1906 from central Italy (Diptera: Ceratopogonidae). The European Zoological Journal 89(1):608–614. DOI: 10.1080/24750263.2022.2066209.
- Tallamy DW, Mullin CA, Frazier JL. 1999. An alternate route to insect pharmacophagy: The loose receptor hypothesis. Journal of Chemical Ecology 25(9):1987–1997. DOI: 10.1023/A:1021024420353.
- Wirth WW. 1980. A new species and corrections in the Atrichopogon midges of the subgenus Meloehelea attacking blister beetles (Diptera: Ceratopogonidae). Proceedings of the Entomological Society of Washington 82:124–139.
- Young DK. 2019. A new Pseudopyrochroa Pic, 1906 from Yunnan, China with a key to adult Pseudopyrochroa males from the Province and correction on type repository for Frontodendroidopsis pennyi Young (Coleoptera: Pyrochroidae: Pyrochroinae). Zootaxa 4695(2):182–188. DOI: 10.11646/zootaxa.4695.2.8.
