Abstract
The spionid genus Scolelepis is commonly divided into the subgenera Scolelepis (s. str.) and Parascolelepis. In the Black Sea, two species ascribed to the subgenus Parascolelepis have been reported so far – Scolelepis tridentata and Scolelepis cantabra. Both species are quite rare and when found are usually difficult to identify because of their fragility. To ascertain the identity of specimens from the Black Sea, the type-series of S. tridentata and non-type specimens of S. cantabra were examined. The examination of specimens from the Black Sea identified as Scolelepis tridentata revealed that they differ from the nominal species in several characters. Therefore, they are described herein as Scolelepis bellani n. sp., a species that most closely resembles S. korsuni from Arctic waters. Also, the occurrence of S. cantabra in the Black Sea is confirmed.
http://zoobank.org/urn:lsid:zoobank.org:pub:78FD82BA-084F-4213-A7A0-41C1EA6ACD44
http://zoobank.org/urn:lsid:zoobank.org:act:FF4E2A3C-7607-4402-9F79-42399574CCC5
Introduction
Scolelepis Blainville, 1828, is one of the most speciose and taxonomically difficult genera of spionid polychaetes (Blake et al. Citation2020). Currently, the genus numbers 91 species and is commonly divided into two subgenera (Sikorski & Pavlova Citation2015; Blake et al. Citation2020; Read & Fauchald Citation2023). The subgenus Scolelepis Blainville, 1828, includes species with blunt or conical, uni-, bi- or tridentate hooded hooks with falcate or straight shafts, while the subgenus Parascolelepis Maciolek, Citation1987 comprises species having sharp, multidentate hooks with curved shafts (Maciolek Citation1987). To this, Meißner and Götting (Citation2015) added the presence in Parascolelepis of papillated palpal sheaths as opposed to smooth sheaths fused to the palp bases in the subgenus Scolelepis (s. str.). Even though the recent phylogenetic analysis of the genus Scolelepis (s. lat.) made by Lee and Min (Citation2022b) supports the grouping of the genus into two subgenera, the species “traditionally” defined as Parascolelepis are scattered in both clades. Therefore, in the present paper only the genus level is used.
Members of Scolelepis typically inhabit sandy or mixed soft sediments in intertidal and shallow subtidal habitats. Currently, six species of the genus Scolelepis (s. lat.) have been reported in the Black Sea: Scolelepis cf. cirratulus (Delle Chiaje, Citation1829), S. foliosa (Audouin & Milne Edwards, Citation1833), S. mesnili (Bellan & Lagardère, Citation1971), S. neglecta Surugiu, Citation2016, S. tridentata (Southern, Citation1914) and S. cantabra (Rioja, Citation1918) (Kisseleva Citation2004; Surugiu Citation2016; Kurt Șahin et al. Citation2017; Surugiu et al. Citation2022). The identification of the latter two species often proved to be difficult because of the fragmentary nature of the material and some overlapping characters. Both species were formerly reported either as Nerinides or as Pseudomalacoceros, the invalid names of the genus Scolelepis. The records of S. tridentata and S. cantabra are confused and sometimes contradictory, not only in the Black Sea, but also in other parts of the world.
The main morphological characters of systematic importance of Scolelepis include: the shape of the prostomium, the presence or absence of the occipital tentacle, the presence or absence of notochaetae on chaetiger 1, the degree of fusion of branchiae to notopodial lamellae and the chaetiger on which these start to separate, the chaetiger on which neuropodial hooded hooks begin, the shape and the number of hooded hooks, the number and the arrangement of apical teeth of hooks, the ratio of lengths of hood to main fang, the presence or absence of a notch in posterior neuropodial postchaetal lamellae, the shape of the mid-body and posterior notopodial postchaetal lamellae, and the shape of the pygidium (Pettibone Citation1963; Light Citation1977; Blake Citation1983; Maciolek Citation1987; Imajima Citation1992; Sikorski Citation1994; Lee & Min Citation2022a).
Careful examination of material labeled “Nerinides cantabra” in the collection of the Grigore Antipa National Museum of Natural History, Bucharest, Romania (MGAB) revealed that it actually contained specimens belonging to two species preserved in the same vial. One of the species was identified as Scolelepis cantabra, while the second more closely resembled S. tridentata. To clarify the taxonomic identity of specimens from the Black Sea, the type series of S. tridentata (Southern, Citation1914), deposited in the National Museum of Ireland, Natural History of Dublin (NMINH), and available material of S. cantabra has been examined. Because of some inadvertencies found in subsequent descriptions of S. tridentata, the species is herein redescribed in detail and illustrated comprehensively to avoid future confusion. Due to several differences found in comparison to S. tridentata, the material originally identified in the Black Sea as Scolelepis tridentata is described as a new species.
Material and methods
Morphology
For light microscopy (LM) specimens were rinsed in distilled water and examined under stereo- or compound microscopes. To contrast some characters useful in species identification (e.g., shape of notopodial lamellae, occipital tentacle, caruncle extension, palpal papillae, shape of pygidium, etc.), methylene blue (MB) temporary staining was applied. For this, specimens were transferred first into distilled water for a while, then dipped for a few seconds into a saturated solution of MB in water and finally returned to distilled water for microscopical examination (the staining fades quickly if specimens are returned to alcohol).
Photographs of specimens were taken using a Leica DFC450C camera coupled to a Leica M205A stereomicroscope or to a Leica DM750 compound microscope. Before photography, specimens were stained with an aqueous solution of MB. Line drawings were prepared based on photographs using Adobe Illustrator.
For scanning electron microscopy (SEM) studies, selected specimens were transferred from 70% ethanol to distilled water for a few hours to allow the ethanol in the tissues to be replaced with distilled water. After cutting the specimens transversally into fragments, they were sonicated in distilled water for 0.5–3 min at 43 kHz to remove hoods from the hooks, dehydrated in a graded ethanol series (50%, 70%, 80%, 90%, 96% and 99.3%, leaving them for 10 minutes in each concentration) and then acetone, critical point dried (CPD) in carbon dioxide, mounted on stubs, coated with a thin layer (30 nm) of gold and examined with a scanning electron microscope at an accelerating voltage of 30 kV. SEM observations were carried out using a Vega Tescan SBH in the Electron Microscopy Laboratory, Faculty of Biology, Alexandru Ioan Cuza University of Iaşi.
The specimens of the newly described species were deposited in the collection of MGAB and NMINH. In the “Material examined” sections, complete specimens are indicated by “cs”, while anterior, middle, and posterior fragments are designated as “af”, “mf”, and “pf”, respectively. Information about samples is given below along with descriptions of specimens.
In addition to the freshly collected material, specimens deposited in various museum collections were examined. The following abbreviations were used for the museums and institutions that provided loans or lodged registered specimens:
| DBUA | = | Biological Research Collection of Marine Invertebrates, Departamento de Biologia, Universidade de Aveiro, Portugal |
| MGAB | = | Grigore Antipa National Museum of Natural History, Bucharest, Romania |
| MNHN | = | Muséum National d’Histoire Naturelle, Paris, France |
| NHMD | = | Natural History Museum of Denmark, Copenhagen, Denmark |
| NHMUK | = | Natural History Museum of United Kingdom, London, UK |
| NIBR | = | National Institute of Biological Resources, Incheon, South Korea |
| NMINH | = | National Museum of Ireland, Natural History, Dublin, Ireland |
| SMF | = | Senckenberg Museum, Frankfurt, Germany |
| ZMH | = | Zoologisches Museum Hamburg, Germany |
Morphometric analysis
A total of 72 specimens in optimal condition (i.e. specimens that presented all analysed morphological characters) belonging to six species of Scolelepis (17 individuals of S. tridentata from Ireland, Spain, and the Mediterranean coast of France, five individuals of S. papillosa (Okuda, Citation1937) from Korea (based on literature data), seven individuals of S. korsuni Sikorski, Citation1994 from the Atlantic coast of Norway, two individuals of S. quinquedentata (Hartmann-Schröder, Citation1965) from Chile, 35 individuals of Scolelepis bellani n. sp. from the Black Sea and six individuals of S. cantabra from Ireland, France, Portugal and the Black Sea) were analysed. The following eight variables were recorded (Table S1): the ratio between the body length in millimetres and the number of chaetigers (L/NC); body width (W); caruncle length as the number of the chaetigers to which it extends back (Ca); number of chaetigers to which branchiae are completely fused to notopodial postchaetal lamella (Br); number of chaetiger on which neuropodial hooded hooks start (VH); number of apical teeth in hooded hooks (NT); maximum number of hooks per neuropodium (MH); and the ratio of hood length to main fang height (H/F). Body width refers to the distance in mm between the distal most structures on the widest chaetiger seen on the anterior end in dorsal view (including parapodia but without chaetae). Maximum length and width (usually at chaetiger 10–14) of worms were measured using a calibrated ocular micrometer mounted on a stereomicroscope to the nearest 10 μm. Individuals with missing values were excluded from the multivariate statistical analysis (measurements and calculations are available in online supplementary material, Tables S1 and S2).
To take the different data types (continuous and discontinuous variables, ratios and proportions) into account, Gower’s similarity coefficient (Gower Citation1971) was chosen to calculate a similarity matrix. Non-metric Multi-Dimensional Scaling (nMDS) was subsequently employed to display the similarities of the different specimens. To test for the significance of differences between species, a series of non-parametric ANOSIM (analysis of similarities) tests was performed (Anderson Citation2001). Principal Component Analysis (PCA) was used to determine variability of characters and to identify characters that contribute most to the species differentiation. For this, the Principal Component scores were correlated (Spearman Rank Correlation Coefficient) with the measured character values of each individual. All multivariate statistical analyses were carried out with the PRIMER v 6.1.13 software package.
To discriminate between S. korsuni from Norway and S. bellani n. sp. from the Black Sea, a series of correlation analyses between the size of the worms (as an independent variable) and the morphological variables, that were revealed by multivariate analysis as most useful in discriminating between species, were carried out.
Taxonomic account
Family Spionidae Grube Citation1850
Genus Scolelepis Blainville, 1828, emend. Pettibone Citation1963
Type-species: Scolelepis squamata (O.F. Müller, Citation1806) as Lumbricus squamatus. By monotypy.
Scolelepis tridentata (Southern, Citation1914)
()
Figure 1. Scolelepis tridentata (Southern, Citation1914), syntype (NHMUK 1914.12.12.29): A. Anterior end, dorsal view; palps, left notopodial postchaetal lamellae of chaetiger 3, right parapodia of chaetigers 1 and 2, and right notopodial postchaetal lamellae of chaetigers 3 and 4 missing. B. Anterior end, lateral view; C. Notopodial limbate capillary chaetae, chaetiger 35. D. Neuropodial hook of chaetiger 34. E. Ventral inferior capillary chaeta, chaetiger 35. F. Alternating capillary chaeta, chaetiger 35. Scale bars: A, B = 0.5 mm; C = 0.1 mm; D–F = 50 μm.
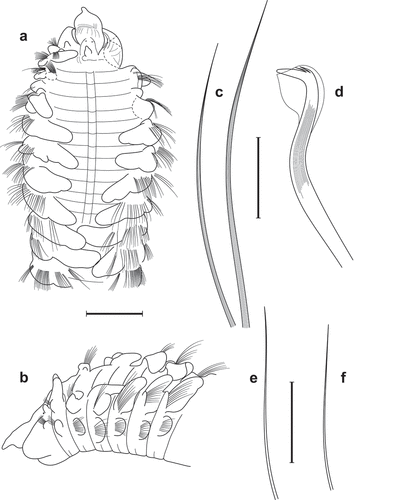
Figure 2. Scolelepis tridentata (Southern, Citation1914), syntypes (A–D – NMINH 1914.325.7; E – NMINH 1910.42.4): A. Parapodium of chaetiger 1, anterior view. B. Parapodium of chaetiger 2, anterior view. C. Parapodium of chaetiger 8, anterior view. D. Parapodium of chaetiger 21, anterior view. E. Parapodium of posterior chaetiger, anterior view. Scale bars: A–E = 0.2 mm.
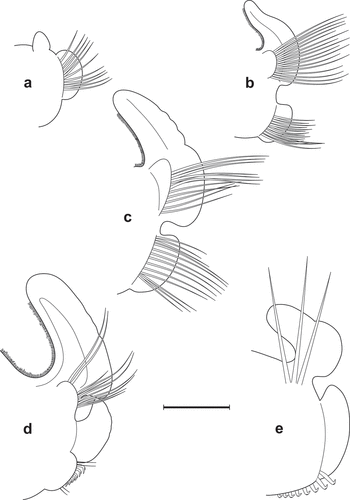
Figure 3. Scolelepis tridentata (Southern, Citation1914), syntype (NMINH 1914.325.7), LM micrographs, stained with MB: A. Pygidium, postero-latero-dorsal view; B, chaetigers 22–27, dorsal view. Scale bars: A = 0.25 mm, B = 0.2 mm.
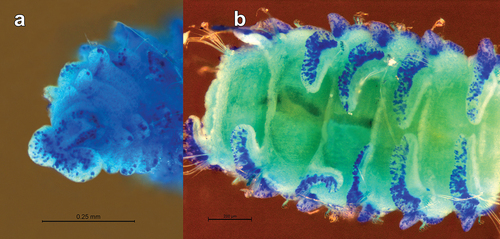
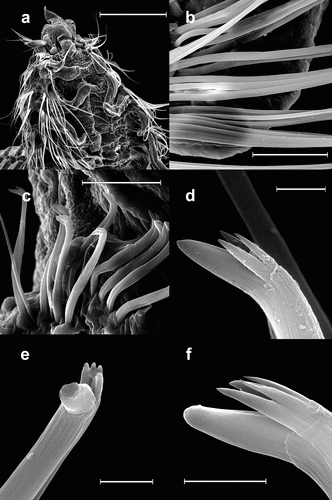
Figure 4. Scolelepis tridentata (Southern, Citation1914), syntype (NMINH 1914.325.7), SEM micrographs: A, Neuropodial hooks with hoods, lateral view; B, Neuropodial hook with three apical teeth, latero-apical view, hood removed; C, Neuropodial hooks with two and three apical teeth, latero-apical view, hoods removed; D, Neuropodial hook with two apical teeth, apical view, hood removed. Scale bars: A = 20 μm; B–D = 10 μm.
Nerinides tridentata Southern, Citation1914: 98–99, pl. X, fig. 23A–J.—McIntosh Citation1922: 20–21.—Fauvel Citation1927: 33, fig. 10f–l.
Scolelepis (Nerinides) tridentata.—Light Citation1977: 75–76, fig. 3c–e (paratypes only).
Scolelepis (Parascolelepis) tridentata.—Maciolek Citation1987: 33–34.—Hartmann-Schröder Citation1996: 337.
Pseudomalacoceros tridentata.—Parapar et al. Citation1992: 112, fig. 2C.
Material examined
Type material
Holotype: NE ATLANTIC: IRELAND, Blacksod Bay, Station W166, in Laminaria roots, Clare Islands Survey, coll. R. Southern, 20 Sep. 1910, complete specimen in alcohol (Industrial Methylated Spirits), NMINH 1914.325.1.—Paratypes: Same data as for holotype, one complete specimen in alcohol (IMS), NMINH 1914.325.2; one complete specimen broken into two fragments, NMINH 1914.325.3; one middle fragment, possibly dissected, NMINH 1914.325.4; one anterior fragment, NMINH 1914.325.5; one middle fragment, NMINH 1914.325.6; one specimen broken into an anterior end, three middle sections (one of them was used with permission for SEM) and a posterior end, NMINH 1914.325.7; same data as for holotype, one anterior end, NHMUK 1914.12.12.29.
Non-type material
IRELAND, Blacksod Bay, Station W137, on shore, Clare Islands Survey, coll. R. Southern, Mar. 1910, one complete specimen broken into two fragments in alcohol (IMS), NMINH 1910.42.4; Station W135, on shore, Clare Islands Survey, coll. R. Southern, Mar. 1910, one complete specimen broken into two fragments, NMINH 1910.42.5; Station W168, in Laminaria roots, Clare Islands Survey, coll. R. Southern, Sep. 1910, one dehydrated specimen and two middle sections, NMINH 1910.448.3; Station W160, on shore, in Laminaria roots, Clare Islands Survey, coll. R. Southern, Sep. 1910, one complete specimen, NMINH 1910.448.4.
SPAIN, NW Iberian Peninsula, Ría de Ferrol, Galicia, Batel, coll. J. Parapar, 26 Oct. 1988, No. 225, Ref. 225136261086-9, 4 anterior fragments; SW Iberian Peninsula, Huelva, coll. J.M. Viéitez, May 1988, 2 anterior fragments, poorly preserved.
WESTERN MEDITERRANEAN SEA, FRANCE, Golfe de Marseille, Carry-le-Rouet, Sta. E 3 M C 1 (I), Corer 1, in dead Posidonia, Carottier–KL, coll. & det. A. Willsie, 6 Sep. 1982, 1 af, SMF 16030; Carry-le-Rouet, Sta. E 3 M C 4 (II), Corer 4, in dead Posidonia, Carottier–KL, coll. H. Zibrowius, det. A. Willsie, 9 Sep. 1982, 1 af, SMF 16278; Plateau des Chèvres, Port Cros, Sta. 1 M 5 (I), in dead Posidonia, Carottier–KL, coll. H. Zibrowius, det. A. Willsie as Nerinides sp., 25 Sep. 1984, 1 af, SMF 16319.
Description
Holotype complete specimen, ca. 7.5 mm long, 0.681 mm wide for about 65 chaetigers (see also Taxonomic discussion). The longest complete paratype ca. 19 mm long, 1.5 mm wide for ca. 65 chaetigers. The largest incomplete paratype 2.0 mm wide, 5.4 mm long and with 19 chaetigers. Colour in alcohol pale tan or opaque off-white. Body broadest at anterior chaetigers 8–12, tapering to both ends. Anterior body flattened dorsally and rounded ventrally, posterior body more or less rounded in cross-section. Anterior segments short, mid-body and posterior segments longer.
Prostomium sharply or bluntly tapered anteriorly, extending posteriorly to middle of chaetiger 2 as a depressed caruncle (). Short, erect, stubby or conical occipital antenna near posterior margin of caruncle (between palp bases), usually directed forward. Two pairs of equal-sized, rounded, dark-brown eyes, arranged in a nearly straight transverse row or eyes absent. Palps missing in all examined specimens (see also Taxonomic discussion).
Peristomium well developed, separated from prostomium by a distinct furrow, without lateral wings, and folded longitudinally on ventral side ().
Primary and secondary dorsal transverse ciliated bands (TCB) in anterior chaetigers to around chaetiger 18 (); primary TCBs continuous with ciliation on inner edge of branchiae; secondary TCBs at the junction between chaetigers.
Chaetiger 1 with small, bluntly conical notopodial lamella and small, rounded neuropodial lamella and about 9–12 short neuropodial capillaries (, 2B); notopodial capillary chaetae absent (see Taxonomic discussion).
Branchiae from chaetiger 2 () and continuing to last chaetiger (). Branchiae meeting dorsally at around chaetigers 9–23 ().
Notopodial postchaetal lamellae broad, rounded, completely fused with branchiae through anterior and middle chaetigers (), folded along outer margin (), not reaching the tips of branchiae from around chaetigers 19–34 or completely fused to branchiae to posteriormost chaetigers; in far posterior chaetigers notopodial postchaetal lamellae relatively large, foliaceous (). Notopodial prechaetal lamellae low, rounded to conical, best developed in chaetigers 2–8, then gradually reduced in size ().
Neuropodial postchaetal lamellae rounded, auriculate anteriorly (), more elongate, lower and rectangular in subsequent chaetigers (), with an elongated superior lobe by chaetigers 12–14, situated dorsal to chaetae by chaetiger 16–21, triangular at first (), becoming more rounded in posterior chaetigers ().
Anterior chaetae all narrowly winged capillaries arranged in two rows. Notopodial capillaries longer, longitudinally striated, those of anterior row shorter (); capillaries of posterior notopodia arranged in indistinct double rows; additional dorsal superior fascicle of 3–5 long, thin capillaries. Neuropodial capillaries in distinct double rows: up to eight capillaries in anterior row, these short, broad, uniformly granulated; chaetae of posterior row longer, thinner, lacking granulations and an inferior bundle of 2–5 thin capillaries. Posterior capillaries slender, alimbate ().
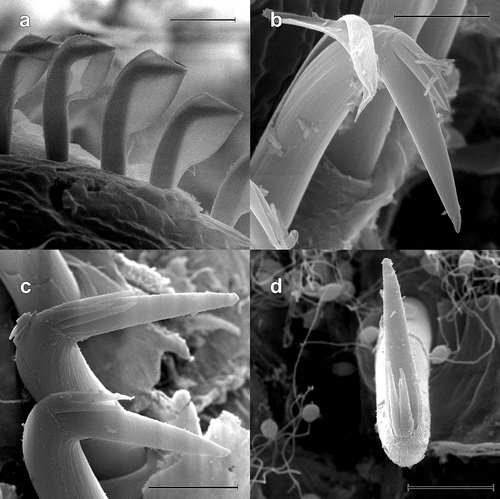
Neuropodial hooded hooks first present from chaetigers 15–17 in posterior row, numbering up to 16 per fascicle, accompanied by an anterior row of 0–7 slender alternating capillaries (in dorsalmost position) () and 2–4 ventral inferior slender capillaries (); number of alternating capillaries gradually diminishing towards posterior region. Hooded hooks with very long, sharp main fang bent at an almost right angle to shaft, surmounted by 2–3 small, closely applied apical teeth (); shaft gently curved (). Hoods short, 1.7–2.8 times as long as main fang (). Ventral sabre chaetae absent. Notopodial hooded hooks absent.
Pygidium cushion-like, with slightly bilobed ventral margin and dorsal anus ().
Staining pattern
Prostomium, peristomium, caruncle, occipital tentacle, branchial tips, margins of notopodial pre- and postchaetal lamellae, neuropodial postchaetal lamellae and pygidium more intensely stained (). Each segment has a broad band of stained speckles on the ventrum, more intense on anterior margin, leaving two whitish (without speckles) paramedian lines.
Habitat and ecology
The species inhabits the upper sublittoral and is found in Laminaria roots or in dead Posidonia.
Biology
Ovoid (180 × 279 µm) oocytes with honeycombed envelope are present in the coelom from chaetiger 17.
Taxonomic discussion
Southern (Citation1914) provided an exceptionally good description and illustrations for his new species. Unfortunately, the designated holotype is a specimen that seems to have regenerating the prostomium, peristomium and the first four chaetigers (judging from the smaller size of segments, branchiae and neuro- and notopodial lamellae). Palps were missing in all examined specimens. However, Southern (Citation1914) reported that “the tentacles [=palps] are short, thick and firmly adherent, of a deep chocolate colour”. To my knowledge, there are no subsequent descriptions of the palps of S. tridentata, but papillae at the bases of the palps are expected to be present. According to Southern (Citation1914), the notopodial lamella of chaetiger 1 is composed of two rounded lobes (see his Fig. 23C). However, it is likely that he misinterpreted the prechaetal lamella of chaetiger 2 as the second upper lobe. This was corrected by McIntosh (Citation1922), who showed that in chaetiger 1 there is “a small conical papilla or cirrus” [=notopodial postchaetal lamella]. Light (Citation1977) and Maciolek (Citation1987) re-examined some of the type specimens, but incorrectly reported notopodial capillaries as being present in chaetiger 1 and hooded hooks as having 3–5 (usually 4 or 5, re: Light Citation1977) apical teeth. Careful examination of the type material revealed that the original description was correct and that the notochaetae are absent in chaetiger 1 in all examined specimens. Also, close examination of hooded hooks under SEM revealed that these have only two or three teeth above the main fang (). Specimens of Scolelepis cf. tridentata reported from Northern California (Light Citation1977; Maciolek Citation1987; Blake Citation1996) do have notochaetae on chaetiger 1 and thus most likely belong to another, yet undescribed species. Fixing these errors will enable further correct identification of this species.
Scolelepis tridentata is closely related to S. papillosa (Okuda, Citation1937), S. quinquedentata (Hartmann-Schröder, Citation1965), S. texana Foster, Citation1971, S. towra Blake and Kudenov, Citation1978, and S. korsuni Sikorski, Citation1994. All these species lack notochaetae in chaetiger 1, have notopodial postchaetal lamellae completely fused with branchiae in anterior chaetigers, undivided neuropodial lamellae in posterior chaetigers and multidentate hooded hooks. However, S. tridentata can be distinguished from the other species by having the shortest lengths of the hoods as compared to the height of the main fang. Thus, this ratio is 1.9–2.8 in S. tridentata (Southern Citation1914: fig. 23j; Light Citation1977: fig. 3d; Parapar et al. Citation1992: fig. 2c; present study) vs. 3.1–4.6 in S. papillosa (Okuda Citation1937: fig. 2 f; Lee et al. Citation2021: fig. 4b), 3.2–6.5 in S. quinquedentata (Hartmann-Schröder Citation1965: fig. 190; Carrasco Citation1974: fig. 14), 3.4–4.3 in S. texana (Foster Citation1971: fig. 139; Maciolek Citation1987: fig. 10c), 3.3 in S. towra (Blake & Kudenov Citation1978: fig. 5e), and 3.0–6.2 in S. korsuni (Sikorski Citation1994: fig. 1 g; present study). In S. tridentata notopodial postchaetal lamellae are not distinctly separated from branchiae, but rather do not reach the tips of the latter in middle and posterior chaetigers. The same is true also for S. papillosa (Lee et al. Citation2021).
Apart from the ratio of lengths of hood to main fang, S. tridentata differs from S. papillosa only by having fewer hooded hooks per fascicle (10–16 in the former vs. 18–21 in the latter).
Occurrence and distribution
Atlantic coasts of Ireland (Southern Citation1914; Maciolek Citation1987), France (Fauvel Citation1927), and Spain (Parapar et al. Citation1992) and the Western Mediterranean Sea (present study).
Scolelepis bellani sp. nov.
()
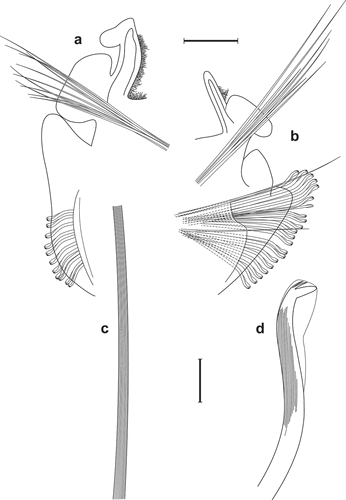
Figure 5. Scolelepis bellani n. sp., paratype (A–C, F – MGAB PLY0169), holotype (D – MGAB PLY0166), and voucher specimen (E – VS), LM micrographs, stained with MB: A. Anterior end, dorsal view, palps missing. B. Anterior end, lateral view, palps missing. C. Anterior end, frontal-dorsal view, palps missing. D. Middle body chaetigers (chaetigers 36–41), dorsal view, showing notopodial postchaetal lamellae divided into a superior flag-like process and inferior narrow part. E. Anterior end, lateral view, showing palps with papillate basal sheaths. F. Pygidium, dorsal view. Scale bars: A–D, F = 0.5 mm; E = 0.25 mm.
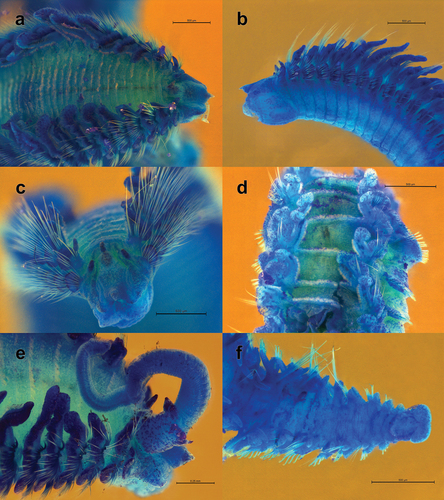
Figure 6. Scolelepis bellani n. sp., paratype (MGAB PLY0169): A. Parapodium of chaetiger 1, anterior view. B. Parapodium of chaetiger 2, anterior view. C. Parapodium of chaetiger 3, anterior view. D. Parapodium of chaetiger 14, anterior view. E. Parapodium of chaetiger 30, anterior view. Scale bars: A–E = 0.2 mm.
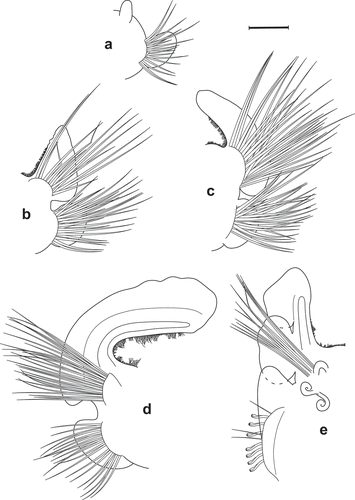
Figure 7. Scolelepis bellani n. sp., paratype (MGAB PLY0169): A. Parapodium of chaetiger 44, anterior view. B. Parapodium of posterior chaetiger, anterior view. C. Mid-part of a notopodial limbate capillary chaeta, chaetiger 30. D. Neuropodial hook of chaetiger 30. Scale bars: A, B = 0.2 mm; C, D = 20 μm.
Nerinides tridentata.—Vinogradov Citation1931: 7–9, fig. 2. Not Southern Citation1914
Nerinides cantabra.—Dumitrescu Citation1963: 186 (partim). Not Rioja Citation1918
Pseudomalacoceros tridentata.—Kisseleva Citation2004: 251–252, fig. 99. Not Southern Citation1914
Material examined
Type material
Holotype: BLACK SEA: UKRAINE, Grigorievsky Liman, Sta. 18, 46.601745°N, 31.020987°E, depth 0.3 m, temperature 22°C, salinity 15.54, dissolved oxygen 7.4 mg L–1, coll. I.A. Sinegub, 23 Jul. 2015, MGAB PLY0166.
Paratypes: Same data as for holotype, 1 cs + 4 af, MGAB PLY0167; Grigorievsky Liman, 46.6096°N, 31.0137°E, depth 0.3 m, fine compact sand with mud, coll. I.A. Sinegub, 12 and 29 Jul. 2013, det. O. Bondarenko as Nerinides tridentata, 3 af of which one as SEM stub, MGAB PLY0168; Gulf of Odessa, Cape Malyi Fontan, Sta. D90, 46.441667°N, 30.775000°E, depth 6.4 m, sand, pooled with Sta. 24, 46.3250°N, 30.7000°E, depth 11 m, temperature 17.6°C, dissolved oxygen 10.01 mg L–1, coll. A.P. Kurakin, Jul. 2012, 4 af + 1 mf + 1 pf, MGAB PLY0169; same data as for preceding, 3 af + 2 mf + 1 pf, NMINH 2023.3.1.
Additional material
BLACK SEA: ROMANIA, original label “Nerinides cantabra; Rioja, Citation1918, Marea Neagră”, 8 af, poorly preserved, MGAB PLY0170; UKRAINE, Dzharylhach Island, marine side, 46.013833°N, 32.904056°E, midlittoral zone in the excavated pit (higher than edge of the water), medium sand mixed with broken shells, coll. M.O. Son, 21 Aug. 2015, 3 af, MGAB PLY0171; CRIMEA, Karkinit Bay, 45.86469°N, 33.51307°E, depth 0.6–0.8 m, coll. N. Boltachova, 24 Aug. 2008, identified as Scolelepis tridentata, 3 af, MGAB PLY0172; Sevastopol Bay, 44.61977°N, 33.54409°E, depth 17 m, coll. N. Boltachova, 10 Nov. 2006, identified as Scolelepis tridentata, 3 af + 1 mf, MGAB PLY0173; SEA OF AZOV, RUSSIA, Taman Bay, Sta. 33–2, 45.29050°N, 36.806033°E, depth 4.5 m, coll. & det. V. Syomin as Scolelepis sp., 10 Jul. 2013, det. as Scolelepis korsuni by V. Radashevsky in 2016, 3 very small af of which one was lost during the study, MGAB PLY0174; Taman Bay, Sta. 43–2, 45.338252°N, 36.781862°E, coll. & det. V. Syomin as Scolelepis cf. cantabra, 9 Aug. 2012, 1 af, MGAB PLY0175; Taman Bay, Sta. 3–2, 45.27933°N, 36.95785°E, depth 1.8 m, coll. & det. V. Syomin as Scolelepis cf. cantabra, 3 Jul. 2013, 1 af + 2 mf, MGAB PLY0176; Taman Bay, Sta. 3–3, same data as for preceding, 1 af with palps, VS personal collection; Taman Bay, Sta. 3–4, same data as for preceding, 2 af, MGAB PLY0177.
TYRRHENIAN SEA: ITALY: Campania, N. of Napoli, Gulf of Gaeta, Licola, 1966–1968, leg. J. Dörjes, 1 af, SMF 12914.
Comparative material
Scolelepis korsuni. Holotype: NORTH SEA: NORWAY: Lille Frigg II, Sta. 4–1, 59.96167°N, 2.395556°E, depth 108 m, Van-Veen grab (0.1 m2), muddy sand, coll. Akvaplan-niva, 11 May 1992, det. A. Sikorski, 1 Dec. 1992, NHMD 108731 (POL-000948); non-type material: NOWEGIAN SEA: NORWAY: Gullfaks oil field, station 4–2, 61.19725°N, 2.302004°E, depth 211 m, coll. A. Sikorski, 5 Jun. 2002, 1 cs + 1 af + 1 mf + 2 palps, MGAB PLY0178; Mosjøen, Vefsnfjorden, station 4, 65.85503°N, 13.17673°E, depth 107 m, coll. A. Sikorski, 30 Jun. 2006, 2 af, MGAB PLY0179; Breisundet 17, Ref. 2–1, 67.419967°N, 13.884983°E, depth 42 m, coll. A. Sikorski, 24 Jan. 2017, 1 cs + 1 af, MGAB PLY0180.
Scolelepis quinquedentata. Holotype: CHILE, Isla Mocha, Sta. 75, black fine sand with small amount of detritus, depth 26 m, 10.2°C and 1.84 ml L–1 O2, 11 Mar. 1960, 1 af, ZMH P-14928. Paratypes: same data as for holotype, 4 af, poorly preserved, ZMH P-14929.
Scolelepis cantabra. NE ATLANTIC: PORTUGAL, continental shelf off Cascais, Guia, Sta. G6, 38.678333°N, 9.471667°W, 40 m depth, Jun. 1998, Smith-McIntyre grab, RV Andrómeda/Auriga, cruise GUIA98, 1 af, DBUA 0170.02; shelf off Cascais, Guia, Sta. G18, 38.666667°N, 9.436667°W, 40 m depth, Jun. 1998, Smith-McIntyre grab, RV Andrómeda/Auriga, cruise GUIA98, 1 af, DBUA 0190.01; Sado estuary, Sta. S4, 38.472933°N, 8.780000°W, 8 m depth, Feb. 1997, Smith-McIntyre grab, RV Mestre Costeiro, cruise TOXSADO97, 1 af and 1 mf, DBUA 0191.01; continental shelf off Aveiro, Sta. S16, 40.636550°N, 8.773150°W, 12 m, Dec. 2002, Smith-McIntyre grab, RV Andrómeda/Auriga, cruise SIMRIA02, 1 af, DBUA 2022.01; IRELAND, Blacksod Bay, Fishery Station W166, sand, Clare Islands Survey, coll. R. Southern, 16–23 Sep. 1910, det. as Nerine longirostris, 1 af, poorly preserved, NMINH 910.448.5; FRANCE, Dinard, 1893, coll. de M. le Baron de St. Joseph, No. 20–1911, det. as Nerine longirostris Qfg., re-det. as Nerinides cantabra by N. Maciolek (?), 1 af + 1 mf, MNHN-IA-PNT 143; Dinard, St. Vaast, 1882, coll. de M. le Baron de St. Joseph, No. 20–1911, det. as Nerine longirostris Qfg., 1 af + 3 mf, MNHN-IA-PNT 144; Dinard, 1893, coll. de M. le Baron de St. Joseph, No. 20–1911, det. as Nerine longirostris Qfg., 68c, 1 af, MNHN-IA-PNT 145; Tatihou, May 1895, leg. & det. as. Nerine foliosa by P. Fauvel, 1 af + 1 mf, MNHN-IA-PNT 146; BLACK SEA: ROMANIA, original label “Nerinides cantabra Rioja, Citation1918, Marea Neagră”, 3 af, poorly preserved, MGAB 30.288.
Description
Holotype incomplete specimen, ca. 9.0 mm long, 2.2 mm wide for 41 chaetigers. Longest complete specimen 8.5 mm long, 1.5 mm wide for 60 chaetigers; longest incomplete specimen 22 mm for about 61 chaetigers; largest specimen 3.1 mm wide (at chaetiger 17) for 64 chaetigers. Colour in alcohol opaque off-white, without pigmentation. Body very fragile, broad, flattened anteriorly, narrow and almost cylindrical in cross section in mid-body and posterior segments. Anterior segments short, mid-body segments longer.
Prostomium pointed anteriorly, terminating posteriorly in narrow caruncle reaching anterior margin of chaetiger 2, posterior part of caruncle pointed and raised as short, stubby, anteriorly directed occipital antenna (). Two pairs of small, indistinct, equal, dark-brown oval eyes, arranged in a wide trapezium or in a nearly straight transverse row just in front of palp bases, with anterior eyes set further apart; sometimes eyes absent. Palps (absent in holotype) very caducous, reaching at most chaetiger 20. Edges of basal sheaths papillated; papillae triangular, pointed, 3–9 in number on lateral and posterior sides of palp bases, unequal in size, the largest in posterior most position ().
Figure 8. Scolelepis bellani n. sp., paratype (MGAB PLY0168), SEM micrographs: A. Anterior end, dorsal view, palps missing. B. Neuropodial capillary chaetae of chaetiger 4. C. Neuropodial hooded hooks of chaetiger 28, left latero-ventral view (dorsal side is to the left and the anterior end is down). D, Neuropodial hook with three apical teeth, latero-apical view, hood removed. E, Neuropodial hook with four apical teeth, frontal view, hood removed. F, Neuropodial hook with four apical teeth, latero-apical view, hood removed. Scale bars: A = 0.5 mm; B, C = 50 μm; D–F = 5 μm.
Peristomium distinct from chaetiger 1, inflated, without lateral wings ().
Metameric dorsal ciliated organs as primary TCBs, continuous with ciliation along the inner edge of branchiae; anterior 14–30 chaetigers (22 in holotype) with additional secondary TCBs located at the junction between chaetigers (). Low dorsal transverse folds present between the branchiae on middle chaetigers ().
Chaetiger 1 with short, tapering, cirriform notopodial lamellae and larger, auricular neuropodial lamellae; capillary chaetae present in neuropodia only ().
Branchiae from chaetiger 2 (), best developed by chaetiger 10–20, then gradually decreasing in size, continuing until last five segments ().
Notopodial postchaetal lamellae of anterior chaetigers attain full size by chaetigers 5–6, very broad, with entire folded outer margin, with glandular cells, fused to branchiae along entire length in first 15–30 chaetigers (). At around chaetiger 15–31 (27 in holotype) a notch divides notopodial lamella into superior rounded lobe and broad, inferior elongated lobe (). In subsequent chaetigers notch deepens and enlarges, separating both lobes completely. Superior lobe gradually diminishes in size to an apical, rounded, flag-like process; inferior lobe becomes elongate and narrow (). In posterior chaetigers notopodial postchaetal lamellae foliaceous (). Notopodial prechaetal lamellae low and rounded, best developed in chaetigers 5–17 ().
Neuropodial postchaetal lamellae wide, auricular in anteriormost chaetigers (). In following chaetigers lamellae become narrower and more elongate, not notched, with superior lobe projecting upward ().
Anterior parapodia with only capillary chaetae in both rami, all broad, narrowly limbate, arranged in double rows (); capillaries of anterior row shorter, broader, with moderately and uniformly granulated cores in direct light and fibrous in reflected light; capillaries of posterior row longer, thinner, lacking granulations (); notopodial capillaries 1.5 times longer than neuropodial ones; additional superior and inferior fascicle of long, thin capillaries without granulations. Notopodial capillaries very long in posterior chaetigers ().
Neuropodial hooded hooks present from chaetigers 15–28 (24 in holotype) in posterior row, numbering up to 23 per row (up to 15 in holotype), accompanied by 1–3 slender alimbate capillaries in dorsal part (). Hooded hooks with bluntly tipped main fang, forming a wide angle (~110°) to shaft, surmounted by three () or four slender apical teeth (); shaft long, gently curved, without constriction. Hood elongated, 4–5 times the length of main fang (). Ventral sabre chaetae absent. Notopodial hooded hooks absent.
Pygidium a slightly bilobed ventral pad; anus as dorsal vertical slit. One or two prepygidial achaetous segments ().
Staining pattern
Occipital tentacle, caruncle, notopodial lamella of chaetiger 1 and tips of palpal papillae most intensely stained (). Blue pigmentation on margins of both notopodial and neuropodial postchaetal lamellae, intense blue elongate spots at posterior ventral base of each neuropodial postchaetal lamella (). Prostomium and peristomium with uniformly dispersed speckles (). Ventrally peristomium with longitudinal rows of blue speckles. Each segment presenting ventrally two speckled transverse strips, interrupted mid-ventrally, with anterior strip longer and more intense than the posterior one (). Palpal sheaths with dispersed speckles ().
Etymology
This species is named in honour of Dr Gérard Bellan, Centre d’Océanologie de Marseille, France, in recognition of his extensive studies of the polychaete fauna of the Mediterranean Sea. I dedicate this species to him also as a moral amend for mistakenly synonymyzing his species Scolelepis mesnili (Bellan & Lagardère, Citation1971) with S. squamata (Surugiu Citation2016), which subsequently was confirmed as a distinct species based on molecular markers (Surugiu et al. Citation2022).
Gender: Masculine
Type locality
Black Sea, Ukraine, Grigorievsky Liman, 46.601745°N, 31.020987°E, depth 0.3 m.
Habitat and ecology
Species inhabits coarse sand between algae, gravely sand, sand with shell debris, fine sand and muddy sand at 0–30 m depth (Vinogradov Citation1949; Caspers Citation1951; Dumitrescu Citation1957; Citation1963; present study).
Biology
Scolelepis bellani n. sp. is gonochoristic. Ovoid (123 × 197 µm) oocytes with reticulated envelope from chaetiger 26–38.
Morphometric analysis
The non-metric Multi-Dimensional Scaling (nMDS) plot () provided a fairly good representation of differences between species (2D Stress value = 0.16). There appears to be a good segregation of S. cantabra, S. papillosa, S. quinquedentata, and S. tridentata. However, S. bellani n. sp. and S. korsuni are morphologically poorly separated.
Figure 9. Results of the morphometric multivariate analysis of individuals belonging to six species of Scolelepis: A. Non-metric multidimensional scaling (nMDS) plot. B. Principal Component Analysis (PCA) plot. S. tridentata (green upward-pointing triangles), S. papillosa (blue circles), S. korsuni (blue downward-pointing triangles), S. quinquedentata (pink squares), S. bellani n. sp. (red diamonds), and S. cantabra (grey asterisks). Holotypes represented by hollow symbols.
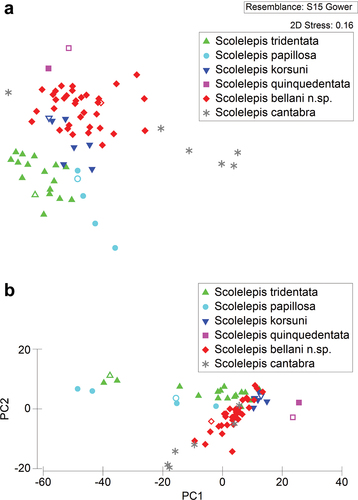
The Principal Component Analysis (PCA), whose first principal component (PC1) accounted for 75.0% of the total variability in the data set, the second principal component (PC2) for 15.5% and the third principal component (PC3) for 8.4%, gave similar results (). The PCA plot of the first two principal components showed that S. quinquedentata forms a distinct group from remaining species and has the highest PC1 scores, which generally correspond to the fact that all branchia are not completely fused with the notopodial postchaetal lamellae. In contrast, S. tridentata and S. papillosa, which have branchiae completely fused with the notopodial lamellae to the last segments, have the lowest PC1 scores. The remaining species, in which notopodial lamellae separate from the branchiae at a certain body segment, are placed in the middle position of the PCA plot.
The ANOSIM analysis resulted in a global R-value of 0.751 (p < 0.001), indicating that there are statistically significant differences between groups of a priori defined morphospecies. Subsequent pairwise tests established that there are strong and highly significant differences (p < 0.001) between some species (e.g., between S. bellani n. sp. and S. cantabra, between S. tridentata and S. cantabra, between S. bellani n. sp. and S. tridentata, and between S. tridentata and S. korsuni) (). However, the morphometric differences between S. bellani n. sp. and S. korsuni are weakest, but still significant (R = 0.349, p = 0.003).
Table I. Results of ANOSIM pairwise comparisons between analysed species of Scolelepis, showing R statistics and p values.
The Spearman Rank Correlation Coefficient between the Principal Component’s scores and the measured character values of specimens revealed that the segment on which notopodial lamellae starts separation from branchiae (Br), the maximum number of hooks per row (MH), and the size of worms expressed as the width of anterior chaetigers (W) are the most important characters in discriminating between analysed species (rS > 0.50 at p < 0.01). Also, fairly good discriminating characters in distinguishing between analysed species (along PC2) are the first hook-bearing segment (VH) and the ratio of hood length to main fang height (H/F) (rS > 0.55 at p < 0.01) (Table S2).
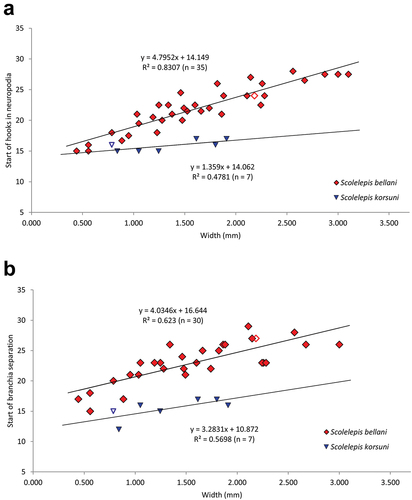
Therefore, to discriminate between S. bellani n. sp. and S. korsuni scatter plots of the body width (W) against segment on which notopodial lamellae start splitting from branchiae (Br), and the first hook-bearing segment (VH) were constructed. The maximum number of hooks per row (MH) was not taken into account because it gradually increases towards the posterior end and most of the analysed specimens lacked posterior parts. The scatter plots show that both the splitting between branchiae and notopodial lamellae (Br) and the neuropodial hooks (VH) appear earlier in S. korsuni than in S. bellani ().
Figure 10. Scatter plots of relationships between: A. The body width (W) and the number of the first chaetiger bearing neuropodial hooded hooks (VH). B. The body width (W) and the number of the first chaetiger on which notopodial postchaetal lamella splits from branchia (Br) (red diamonds – Scolelepis bellani n. sp.; blue downward-pointing triangles – Scolelepis korsuni). Holotypes represented by hollow symbols.
Taxonomic discussion
S. bellani n. sp. is most closely related to S. tridentata from Ireland, S. papillosa from Korea, S. quinquedentata from Chile, and S. korsuni from the North Sea. All these species are characterized by a pointed prostomium, presence of an occipital antenna, palps with papillated basal sheaths, the absence of notochaetae in chaetiger 1, posterior neuropodia without ventral papilla, and a bilobed pygidium.
However, S. bellani n. sp. can be distinguished from S. tridentata in that it has a superior flag-like process on mid-body notopodial postchaetal lamellae (absent in S. tridentata) and branchiae are separated from notopodial lamellae from chaetigers 25–30 (completely fused or only weakly separated to end of body in S. tridentata). Also, in S. bellani n. sp., the angle between the main fang and shaft of the hooks is larger than 100°, while in S. tridentata it is around 90–100°. Finally, in S. tridentata neuropodial hooded hooks have 2–3 apical teeth (rather than the 3–4 in S. bellani n. sp.), and an inferior fascicle of neurochaetae is present (rather than absent as in S. bellani n. sp.). The main fang of hooks is thinner, with a sharply pointed tip in S. tridentata (), while in S. bellani n. sp. it is shorter and has a blunt tip (). Unfortunately, this latter feature can only be seen under SEM.
Differences between S. bellani n. sp. and S. papillosa are, in general, similar to those between S. bellani n. sp. and S. tridentata. However, in S. papillosa the ratio of the length of the hood to the height of the main fang is closer to that of S. bellani n. sp. (3–4.6 times in the former vs. 4–5 times in the latter).
S. quinquedentata can be separated from S. bellani n. sp. by its hooks having very long hoods, ca. 5–6 times the length of the main fang (see Taxonomic discussion for S. tridentata). Also, all branchae in S. quinquedentata are distally free from the notopodial postchaetal lamellae.
S. bellani n. sp. is very similar morphologically to S. korsuni (). Nevertheless, the two species differ in the following characters: (1) eyes are usually absent in S. korsuni (eyes are usually present in S. bellani n. sp.); (2) neuropodial hooded hooks have the median tooth situated below the lateral ones in S. korsuni (the median unpaired tooth is situated on the top and the two side-by-side below in S. bellani n. sp.); (3) neuropodial hooks begin on chaetigers 14–18 in S. korsuni (hooks begin on chaetigers 15–28 in S. bellani n. sp.); and (4) branchial separation starts on chaetigers 12–19 in S. korsuni (versus chaetigers 15–29 in S. bellani n. sp.). However, small individuals of S. bellani n. sp. could not be differentiated from S. korsuni (). There are also differences in the ecology of the two species. S. korsuni lives in full strength marine waters at 95–450 m depth and temperatures below 7°C, while S. bellani n. sp. lives in brackish waters at 0–30 m depth, where temperatures may raise to more than 22°C.
In the Black Sea, this species was also confused with Scolelepis cantabra. Fauvel (Citation1927) even considered Nerinides tridentata as juveniles of Nerinides cantabra. Like S. bellani n. sp., S. cantabra also has an occipital tentacle, chaetiger 1 with only neurochaetae, and mid-body branchiae with a flag-like end. Differences between S. bellani n. sp. and S. cantabra consist in the shape of hooks, the number of apical teeth on the hooks, and the shape of the prostomium.
Occurrence and distribution
Black Sea: Sevastopol – Uchkuyevka (Kisseleva Citation1981, as Nerinides tridentata; Boltachova et al. Citation2006, as Pseudomalacoceros tridentata); southern Crimea (Kisseleva & Slavina Citation1963, as Nerinides tridentata) – Lis’ya Bay (Kisseleva Citation1992, as Nerinides tridentata), Karadag region (Vinogradov Citation1931; Citation1949, as Nerinides tridentata), Caucasian coast (Kisseleva & Slavina Citation1966, as Nerinides tridentata), Dzharylhach Bay and the brackish water lagoons Sasyk, Shagani, Alibey, Burnas, and Shabolat (Vinogradov & Losovskaya Citation1963, as Nerinides tridentata), Romanian coast (Dumitrescu Citation1957; Citation1963; Băcescu et al. Citation1965, as Nerinides cantabra, in part), Bulgarian coast – Varna Bay (Caspers Citation1951; Marinov Citation1957, as Nerinides tridentata; Vorobyova & Bondarenko Citation2009, as Pseudomalacoceros tridentata); Sea of Azov (Kisseleva Citation1987, as Nerinides tridentata; Syomin Citation2011, as Pseudomalacoceros cantabra); and Tyrrhenian Sea – Gulf of Gaeta (present study). Most probably reports of S. tridentata from the Sea of Marmara (Gillet & Ünsal Citation2000; Çinar et al. Citation2011) belong to this species.
Concluding remarks
The Black Sea is characterized by a very low species richness because of the reduced surface water salinity (on average 17–18 psu) and the presence of hydrogen sulphide in deep waters (about 87% of its volume is anoxic). The number of species living in the Black Sea is estimated to represent only 20–25% of those known in the Mediterranean Sea (see references in Marin & Antokhina Citation2022). Most of the Black Sea species, however, have immigrated from the neighbouring Mediterranean Sea during the last 7–9 thousands of years, when the last connection between these seas has been established through the Bosphorus and the Dardanelles straits. Therefore, most of the Black Sea species are of Mediterranean origin (Surugiu Citation2008). Despite being one of the most studied seas, recent descriptions of new species in the Black Sea (Surugiu & San Martín Citation2017; Marin & Antokhina Citation2022; Mureșan et al. Citation2022; Agannemone & Micali Citation2023; this study) indicate a yet uncovered cryptic diversity. With the advent of molecular techniques, the rate of the descriptions of new species in the Black Sea is presumed to increase rapidly. However, it is very likely that species originally described from the Black Sea are also present in the Mediterranean Sea as in the Black Sea there are virtually no endemic species (Surugiu Citation2008).
Scolelepis cantabra is an enigmatic species. Though this study confirms its presence in the Black Sea, it is still unclear whether the species is still present in the Black Sea or not, because there are no other reports since 1960s. Scolelepis cantabra seems to be is also very rare in other European seas, the only most recent confirmed findings being those from the coasts of Portugal. Its large size and occurrence in shallow subtidal might be conducted to its overcollection in some areas by recreational fishermen to be used as bait (Tarik Meziane, pers. comm.). Accordingly, its status should be evaluated through the IUCN Red List categories and criteria (IUCN Citation2012).
Supplemental Material
Download MS Excel (12.9 KB)Supplemental Material
Download MS Excel (23.1 KB)Acknowledgements
I am much indebted to Nancy Maciolek, Danny Eibye-Jacobsen and to two anonymous reviewers for critical appraisal of the earlier version of the manuscript and whose valuable comments and suggestions greatly improved the quality of the paper. I would like to thank Melanya Stan (MGAB), Dieter Fiege (SMF), Adrian Glover (NHMUK), Emma Sherlock (NHMUK), Nigel T. Monaghan (NMINH), Paolo Viscardi (NMINH), Amy Geraghty (NMINH), and Danny Eibye-Jacobsen (NHMD) for arranging the loans of the material used in this study. Elena Lisitskaya and Natalia Boltachova (Kovalevsky Institute of Marine Biological Research RAS, Sevastopol, Crimea), Vitaly Syomin (Shirshov Institute of Oceanology of Russian Academy of Sciences, Gelendjik, Russia), Olena Bondarenko and Mikhail Son (Institute of Marine Biology of the NAS of Ukraine, Odessa, Ukraine), and Andrey Sikorski (Akvaplan-niva AS, Tromsø, Norway) are kindly acknowledged for providing specimens for the present study. I thank Geon Hyeok Lee (Inha University, Korea) for providing measurements of specimens of Scolelepis papillosa from Korea. I am also very grateful to Ascensão Ravara (DBUA), Martin Schwentner (ZMH), and Tarik Meziane (MNHN) for facilitating the study of their museum’s polychaete collection. Many thanks to Irina Gostin, Maria-Magdalena Fusu and Ștefan Mihăiță Olaru for their assistance with SEM and LM studies. This research was partially supported by the German Academic Exchange Service personal research fellowship (DAAD No. 57378441) and by the European Commission SYNTHESYS+ Transnational Access grant (FR-TAF_Call3_042). Financial support was also provided by the Romanian Ministry of Research, Innovation and Digitization, within Program 1 – Development of the national RD system, Subprogram 1.2 – Institutional Performance – RDI excellence funding projects, Contract no. 11PFE/30.12.2021.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2229855.
Additional information
Funding
References
- Agannemone F, Micali P. 2023. Cerithiopsis corinae n. sp. (Gastropoda: Cerithiopsidae) from the Black Sea. Bollettino Malacologico 59(1):113–120. DOI: 10.53559/BollMalacol.2022.21.
- Anderson M. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46. DOI: 10.1111/j.1442-9993.2001.01070.pp.x.
- Audouin JV, Milne Edwards H. 1833. Classification des Annélides et description de celles qui habitent les côtes de la France. Cinquème Famille. Ariciens. Annales des sciences naturelles, Paris, sér. 1, 29:388–412.
- Băcescu M, Gomoiu M-T, Bodeanu N, Petran A. 1965. Studii asupra variaţiei vieţii marine în zona litorală nisipoasă de la Constanţa. Ecologie marină 1:7–138.
- Bellan G, Lagardère F. 1971. Nerine mesnili, n. sp. Spionidien méconnu des plages sableuses de la Province Lusitaniene. Bulletin de la société Zoologique de France 96(4):571–579.
- Blake JA. 1983. Polychaetes of the family Spionidae from South America, Antarctica, and adjacent seas and islands. Biology of the Antarctic Seas XIV. Antarctic Research Series Washington 39:205–288. DOI: 10.1029/AR039p0205.
- Blake JA. 1996. Family Spionidae Grube, 1850. Including a review of the genera and species from California and a revision of the genus Polydora Bosc, 1802. In: Blake JA, Hilbig B, Scott PH, editors. Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel. Vol. 6. Santa Barbara, CA: The Annelida. Part 3 — Polychaeta: Orbiniidae to Cossuridae. Santa Barbara Museum of Natural History. pp. 81–223.
- Blake JA, Kudenov JD. 1978. The Spionidae (Polychaeta) from southeastern Australia and adjacent areas with a revision of the genera. Memoirs of the National Museum of Victoria 39:171–280. DOI: 10.24199/j.mmv.1978.39.11.
- Blake JA, Maciolek NJ, Meißner K. 2020. Spionidae Grube, 1850. In: Purschke G, Westheide W, Böggemann M, editors. Handbook of Zoology: Annelida, Pleistoannelida: Vol. 2. Sedentaria II. Berlin: De Gruyter. pp. 1–103. DOI: 10.1515/9783110291681-001.
- Boltachova NA, Mazlumyan SA, Kolesnikova EA, Makarov MV. 2006. Long-term changes of the shallow sea benthos near Sevastopol (The Black Sea). Ekologiya Morya 72:5–13. [in Russian].
- Carrasco FD. 1974. Spionidae (Polychaeta) provenientes de la Bahia de Concepcion y lugares adyacentes. Boletin de la Sociedad de Biologia de Concepción 48:185–201.
- Caspers H. 1951. Quantitative Untersuchungen über die Bodentierwelt des Schwarzen Meeres im bulgarischen Küstenbereich. Archiv für Hydrobiologie 45(1–2):1–192.
- Çinar ME, Dagli E, Açik S. 2011. Annelids (Polychaeta and Oligochaeta) from the Sea of Marmara, with descriptions of five new species. Journal of Natural History 45(33–34):2105–2143. DOI: 10.1080/00222933.2011.582966.
- de Blainville H. 1828. Dictionnaire des Sciences naturelles, dans lequel on traite méthodiquement des différents êtres de la nature, considérés soit en eux-mêmes, d’après l’état actuel de nos connaissances, soit relativement à l’utilité qu’en peuvent retirer la médicine, l’agriculture, le commerce et les arts. Suivi d’une biographie des plus célèbres naturalistes. Vol. 57. Strasbourg & Paris: F.G. Levrault. p. 628.
- Delle Chiaje S. 1829. Memorie sulla storia e notomia degli animali senza vertebre del Regno di Napoli. Vol. 4. Napoli: Stamperia della Societá Tipografica. pp. i–viii + 1–214, plates 50–69.
- Dumitrescu E. 1957. Contribuţii la studiul polichetelor din Marea Neagră, litoralul românesc. Buletin ştiinţific. Secţia de biologie şi ştiinţe agricole (Seria zoologie) 9(2):119–130.
- Dumitrescu E. 1963. Polychètes marins de la zone littorale roumaine (1 à 20 m de profondeur). Travaux du Muséum d’Histoire Naturelle „Grigore Antipa” 4:181–192.
- Fauvel P. 1927. Polychètes sedentaires. Addenda aux Errantes, Archiannélides, Myzostomaires. Faune de France. Vol. 16. Paris: Paul Lechevalier. pp. 494.
- Foster NM. 1971. Spionidae (Polychaeta) of the Gulf of Mexico and the Caribbean Sea. Studies on the Fauna of Curaçao and Other Caribbean Islands 36:1–183.
- Gillet P, Ünsal M. 2000. Résultats de la campagne océanographique du “Bilim”: Annélides polychètes de la Mer de Marmara, du Bosphore et des régions prébosphoriques de la Mer Noire (Turquie). Mesogee 58:85–91.
- Gower JC. 1971. A general coefficient of similarity and some of its properties. Biometrics 27(4):857–871. DOI: 10.2307/2528823.
- Grube AE. 1850. Die Familien der Anneliden. Archiv für Naturgeschichte, Berlin 16(1):249–364.
- Hartmann-Schröder G. 1965. Die Polychaeten des Sublitorals. Teil II. In: Hartmann-Schröder, G. and Gerd Hartmann, Zur Kenntnis des Sublitorals der chilenischen Küste unter besonderer Berücksichtigung der Polychaeten und Ostracoden. (Mit Bemerkungen über den Einfluss sauerstoffarmer Strömungen auf die Besiedlung von marien Sedimenten.). Mitteilungen aus dem Hamburgischen zoologischen Museum und Institut 62:59–305.
- Hartmann-Schröder G. 1996. Annelida, Borstenwürmer, Polychaeta. 2nd ed. Die Tierwelt Deutschlands, 58. Jena: Neubearbeitete Auflage, Gustav Fischer Verlag. pp. 648.
- Imajima M. 1992. Spionidae (Annelida, Polychaeta) from Japan, 8. The genus Scolelepis. Bulletin of the National Science Museum Tokyo (Japan). Series A. Zoology 18:1–34.
- IUCN. 2012. IUCN Red List Categories and Criteria: Version 3.1. 2nd ed. Gland and Cambridge: IUCN. pp. iv + 32.
- Kisseleva MI. 1981. Benthos of the soft bottoms of the Black Sea. Kiev: Naukova Dumka, pp. 168. [in Russian].
- Kisseleva MI. 1987. Changes in the composition and distribution of polychaete worms in the Azov Sea. Gidrobiologicheskii Zhurnal 23:40–45. [in Russian].
- Kisseleva MI. 1992. Development of benthos in the sand biotope of the Lis’ya Bay (south-eastern coast of Crimea). Ekologiya Morya 40:50–55. [in Russian].
- Kisseleva MI. 2004. Polychaetes (Polychaeta) of the Azov and Black Seas. Apatity: Kola Science Centre of the Russian Academy of Science Press. pp. 409. [in Russian].
- Kisseleva MI, Slavina OY. 1963. Benthic biocoenoses of southern Crimea. Trudy Sevastopolskoy Biologicheskoy Stantsii 16:176–191. [in Russian].
- Kisseleva MI, Slavina OY. 1966. Quantitative distribution of macrobenthos near the Caucasian coast. In: Vodyanistsky VA, editor. Benthos distribution and biology of benthic animals in Southern Seas. Kiev: Naukova Dumka. pp. 55–74. [in Russian].
- Kurt Şahin G, Dağli E, Sezgin M. 2017. Spatial and temporal variations of soft bottom polychaetes of Sinop Peninsula (southern Black Sea) with new records. Turkish Journal of Zoology 41(1):89–101. DOI: 10.3906/zoo-1510-15.
- Lee GH, Lee H, Min G. 2021. DNA barcoding of Scolelepis (Parascolelepis) papillosa (Annelida, Spionidae) in Korea, with additional taxonomic notes. Animal Systematics, Evolution and Diversity 37:349–353. DOI: 10.5635/ASED.2021.37.4.028.
- Lee GH, Min G. 2022a. Two new Scolelepis species (Annelida: Spionidae) from the Yellow Sea in Korea. Zootaxa 5092(2):221–237. DOI: 10.11646/zootaxa.5092.2.5.
- Lee GH, Min G-S. 2022b. A new polychaete, Scolelepis (Parascolelepis) brunnea sp. nov. (Annelida: Spionidae), from Korea. Zoological Science 39(5):500–506. DOI: 10.2108/zs220031.
- Light WJ. 1977. Spionidae (Annelida: Polychaeta) from San Francisco Bay, California: A revised list of nomenclatural changes, new records, and comments on related species from the northeastern Pacific Ocean. Proceedings of the Biological Society of Washington 90(1):66–88.
- Maciolek NJ. 1987. New species and records of Scolelepis (Polychaeta: Spionidae) from the east coast of North America. Bulletin of the Biological Society of Washington 7:16–40.
- Marin IN, Antokhina TI. 2022. A new symbiotic scale worm (Polychaeta: Polynoidae) living in association with burrowing callianassid shrimps in the Black Sea. Zoosystematica Rossica 31(2):272–285. DOI: 10.31610/zsr/2022.31.2.272.
- Marinov T. 1957. Beitrag zur Kenntnis unserer Schwarzmeer Polychätenfauna. Arbeiten aus der Biologischen Meersstation in Varna 19:105–119. [in Bulgarian with German summary].
- McIntosh WC. 1922. I. Notes from the Gatty Marine Laboratory, St. Andrews. 1. On new and rare Polychaeta, Gephyrea, etc., from various regions. 2. Recent additions to the British marine Polychaeta (continued). Annals and Magazine of Natural History 9(49): 1–30, plates I–III. DOI: 10.1080/00222932208632638. 1–30, plates I–III.
- Meißner K, Götting M. 2015. Spionidae (Annelida: ‘Polychaeta’: Canalipalpata) from Lizard Island, Great Barrier Reef, Australia: The genera Malacoceros, Scolelepis, Spio, Microspio, and Spiophanes. Zootaxa 4019(1):378–413. DOI: 10.11646/zootaxa.4019.1.15.
- Müller OF, Abildgaard PC, Vahl M, Holten JS, Rathke J. 1806. Zoologia Danica seu Animalium Daniae et Norvegiae rariorum ac minus notorum. Descriptiones et historia. Vol. IV. Atlas. Havniae [Copenhagen]: N. Christensen.
- Mureșan M, Moțoc R, Menabit S, Teacă A, Begun T. 2022. A new species of free-living nematodes (Desmodorida, Desmodoridae) in the Romanian Black Sea waters. Diversity 14(11):933. DOI: 10.3390/d14110933.
- Okuda S. 1937. Spioniform polychaetes from Japan. Journal of the Faculty of Science, Hokkaido University, Ser. 6, Zoology 5(3):217–254.
- Parapar J, Besteiro C, Urgorri V. 1992. Nuevas aportaciones al conocimiento de los anélidos poliquetos en el litoral gallego (N.O. Peninsula Ibérica). Nova Acta Científica Compostelana (Bioloxía) 3:109–123.
- Pettibone MH. 1963. Revision of some genera of polychaete worms of the family Spionidae, including the description of a new species of Scolelepis. Proceedings of the Biological Society of Washington 76:89–104.
- Read G, Fauchald K (Ed.). 2023. World Polychaeta Database. Scolelepis Blainville, 1828. Availabe: https://www.marinespecies.org/aphia.php?p=taxdetails&id=129623. Accessed Feb 2023 8
- Rioja E. 1918. Adiciones a la fauna de anélidos del Cantábrico. Revista de la Real Academia de Ciencias Exactas, Físicas y Naturales de Madrid 17:54–79.
- Sikorski AV. 1994. New Arctic species of Scolelepis (Polychaeta, Spionidae). In: Dauvin J-C, Laubier L, Reish DJ, editors. Actes de la 4ème Conférence internationale des Polychètes. Vol. 162. Paris: Mémoires du Muséum National d’Histoire Naturelle. pp. 279–286.
- Sikorski AV, Pavlova LV. 2015. New species of Scolelepis (Polychaeta, Spionidae) from the Norwegian coast and Barents Sea with a brief review of the genus. Fauna Norvegica 35:9–19. DOI: 10.5324/fn.v35i0.1666.
- Southern R. 1914. Clare Island Survey. Archiannelida and Polychaeta. Proceedings of the Royal Irish Academy 31(47):1–160.
- Surugiu V. 2008. Zoogeographical origin of the polychaete fauna of the Black and Azov seas. Cahiers de Biologie Marine 49(4):351–354.
- Surugiu V. 2016. On the taxonomic status of the European Scolelepis (Scolelepis) squamata (Polychaeta: Spionidae), with description of a new species from southern Europe. Zootaxa 4161(2):151–176. DOI: 10.11646/zootaxa.4161.2.1.
- Surugiu V, San Martín G. 2017. Taxonomic contribution to the genus Sphaerosyllis (Annelida: Syllidae: Exogoninae) in the Black Sea. Zootaxa 4329(3):281–291. DOI: 10.11646/zootaxa.4329.3.6.
- Surugiu V, Schwentner M, Meißner K. 2022. Fixing the identity of Scolelepis squamata (Annelida: Spionidae) – Neotype designation, redescription and DNA barcode sequences. Systematics and Biodiversity 20(1):1–24. DOI: 10.1080/14772000.2021.2003906.
- Syomin VL. 2011. Ecology of polychaetes of the Sea of Azov and of the estuaries of the Russian part of its coast. Abstract of PhD thesis of biol. sc. Murmansk: Murmansk Marine Biological Institute. pp. 25. [in Russian].
- Vinogradov KA. 1931. Quelques additions a la faune des polychètes de la Mer Noire. Trudy Karadagskoy Biologicheskoy Stantsii 4:5–21. [in Russian].
- Vinogradov KA. 1949. To the fauna of the bristle worms (Polychaeta) of the Black Sea. Trudy Karadagskoy Siologicheskoy Stantsii 8:1–84. [in Russian].
- Vinogradov KA, Losovskaya GV. 1963. Polychaeta of the north-western part of the Black Sea. Naukovi Zapiski Odeskoi Biolgichnoi Stantsii 5:3–11. [in Ukrainian].
- Vorobyova LV, Bondarenko OS. 2009. Meiobenthic bristle worms (Polychaeta) of the western Black Sea shelf. Journal of Black Sea/Mediterranean Environment 15:109–121.
