?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Trichopodus, the genus of dwarf gourami fish, is the oldest genus in the Osphronemidae family, with six valid species, three of which are found in Sumatra. In this study, the status, distribution, and morphometrics of Trichopodus leerii, T. pectoralis, and T. trichopterus from Sumatra were observed and analyzed. Although these species can be distinguished based on phenotypic characteristics, it is still possible to misidentify them in small or preserved samples. In this study, analyses were carried out on specimens in the collection of the Museum Zoologicum Bogoriense, combined with literature on the species status. The results showed that T. trichopterus and T. pectoralis were in the least concern category. T. trichopterus had the widest distribution, followed by T. pectoralis. T. leerii had the smallest distribution and is in a near-threatened conservation status. Further analysis revealed that 23 of the 25 measured morphological characteristics were informative, with the first three axes of a principal component analysis on morphometric data explaining 51.28% of the total variance in the sample. The three species were distinguished using a combination of pectoral fin length, eye diameter, pelvic fin length, and anterior snout width. Cluster analysis showed that there is consistency in the morphometrics of the species from various sample locations, where the same species are in the same clade. This research contributes to our understanding of the status and distribution of the species in genus Trichopodus and employs morphometric analysis to provide a practical key to classifying them in Sumatra.
Introduction
The dwarf gourami fish belongs to the genus Trichopodus, which was formerly included in the genus Trichogaster (Paepke, Citation2009; Töpfer & Schlindler Citation2009; Supiwong et al. Citation2010). It is a tropical freshwater labyrinth fish (Regan Citation1909). Trichopodus is the oldest genus in the family Osphronemidae, which spread across Southeast Asia (Berra Citation1981). Trichopodus is closely related to Trichogaster; both of these species have long, thread-like pelvic fins known as “feelers”, which function to feel the environment (Nelson Citation1994). Trichopodus has a shorter dorsal fin as a juvenile, but it grows longer as an adult (Paepke Citation2009; Töpfer & Schlindler Citation2009). The gourami fish also show sexual dimorphism in body size (Dorado et al. Citation2010; Boonanuntanasarn et al. Citation2020). In nature, gourami fish can live in lowland wetlands (Gustiano et al. Citation2015), swamps and canals (Kottelat et al. Citation1993; Haryono & Agus Citation2000; Haryono Citation2012), and lakes (Haryono Citation2010). These fish are also found in paddy fields, ditches, and rivers with dense vegetation, as well as in shallow waters with slow or stagnant currents or seasonally flooded habitats (Smith Citation1945; Mohsin Citation1983; Roberts Citation1989; Rainboth Citation1996).
Currently, this group of gouramis has been successfully bred in non-Asian countries due to the increased trade as ornamental fish (Ng & Tan Citation1997). Aside from being an ornamental fish, this gourami also has economic value as a food fish (Nguyen et al. Citation2019; Gustiano et al. Citation2021; Kurniawan et al. Citation2021). There are six valid species in the Trichopodus genus, namely T. leerii, T. microlepis, T. pectoralis, T. trichopterus, T. cantoris, and T. poptae (Low et al. Citation2014). In Indonesia, there are four species of gourami fish, identified as T. leerii, T. pectoralis, T. poptae, and T. trichopterus (Low et al. Citation2014; Tan et al. Citation2019; Ahmadi Citation2021). Trichopodus poptae is the lates described which is only reported from Kalimantan. The snakeskin gourami, T. pectoralis, is a commercial species that has economic value as a food fish (Yoonpundh & Little Citation1997; Nguyen Citation2014; Nguyen et al. Citation2019; Gustiano et al. Citation2022). Meanwhile, the other three species have economic value as ornamental fish.
Although sometimes considered “classical”, the biometric characterization is still a powerful method to determine different taxa. In many cases, biometrics can be used to distinguish between different taxa in the field. Over the last decade, many gourami families and fish in general have been subjected to biometric analyses to provide accurate taxonomic and key identifications (Sneath & Sokal Citation1973; Sokal & Rohlf Citation1980; Roberts Citation1992; Eviota et al. Citation2016; Jumawan et al. Citation2016; Ahmadi Citation2021; Gustiano et al. Citation2021; Perdana et al. Citation2021; Nur et al. Citation2022b). There is currently no information on the status, distribution, and morphometric differences among Trichopodus species found in Sumatra, Indonesia. As a result, the purpose of this research is to gain a better understanding of the species’ status, distribution, morphometric variation, and distinct character, which can be used as practical keys.
Materials and methods
The specimens used for this study form part of the Museum Zoologicum Bogoriense (MZB) collection. All specimens are categorized by type, location, and quantity. Meanwhile, gravid females and deformed fish were not used for morphometric analysis, but the location of the fish was recorded. A total of 84 fish samples were selected for morphometric analysis.
Status and distribution
The conservation status of each species was validated and confirmed through the online data in the IUCN Red List database www.iucnredlist.org. While the local distribution (LD) was calculated based on Muchlisin et al. (Citation2015): LD = x 100, where: Ni.st = The number of locations where fish were found; N.st = The total number of locations. LD analysis was carried out based on specimen data available at the Zoologicum Bogoriense Museum.
Morphometric analysis
Morphometric measurements were performed on 19 samples of T. leerii, 15 samples of T. pectoralis, and 50 samples of T. trichopterus. A total of 25 morphometric characteristics were measured on the 84 specimens of Trichopodus spp. (). The specimens were measured using a digital caliper (Insize 1108–200 made in China). The data were then transformed using the following formula: Mtrans = M/SL x 100, where M is the original measurement result, Mtrans is the transformation result, and SL is the standard length. Four characteristics (PFL, ED, PeFL, and SNW) were transformed using HL for further analysis.
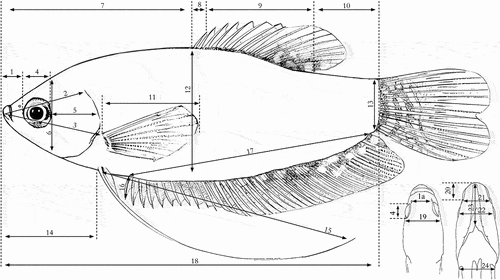
Figure 1. Morphometric characteristics measured in fish samples Trichopodus spp.: 1. Snout length (SnL); 1a. Anterior snout width (SNW); 2. Head length (HL); 3. Prepectoral length (PrePL); 4. Eye diameter (ED); 5. postorbital length (POL); 6. head depth (HD); 7. Predorsal length (PrDL); 8. dorsal spine length (DSpL); 9. Dorsal fin base length (DFBL); 10. Postdorsal length (PoDL); 11. Pectoral fin length (PFL); 12. Body depth (BD); 13. Caudal peduncle depth (CPD); 14. Prepelvic length (PrPL); 15. Pelvic fin length (PeFL); 16. Anal spine length (ASL); 17. Anal fin length (AFL); 18. Standard length (SL); 19. Head width (HW); 20. Lower jaw length (LWJ); 21. Mouth width (MW); 22. Interorbital length (IOL); 23. Distance snout to isthmus (DstL); 24. Body width (BW).
Data analysis
The morphometric data were analyzed using univariate analysis (one-way ANOVA) and multivariate analysis (Principal Component Analysis, PCA) using the SPSS version 26.0 and PAST version 4.03. Before running PCA on the covariance matrix, data measurements were log transformed to reduce the effect of non-normality. To determine morphological similarities among individuals, a hierarchical cluster analysis based on morphometric data was performed using the unweighted pair-group method with arithmetic average algorithm (UPGMA).
Results
Species composition, distribution, and conservation status
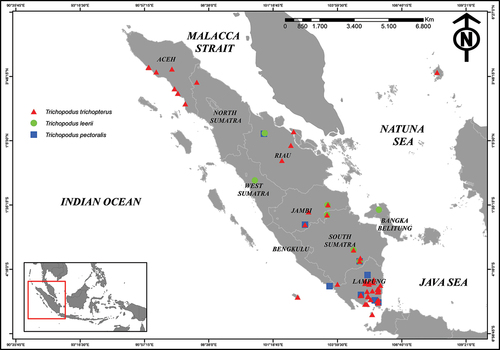
A total of 338 samples of Trichopodus spp. consisting of T. leerii (n = 19), T. pectoralis (n = 16), and T. trichopterus (n = 294) were dispersed across Sumatra ( and ). The results showed that T. trichopterus was widely distributed at 49 sites covering 7 provinces (LD 76.56%), followed by T. pectoralis (LD 12.50%), and T. leerii, which had the lowest distribution and was only found in seven locations (LD 10.94%) (). Based on the IUCN Red List database, among three species of gourami found in Sumatra, one is categorized as a Near Threatened species (T. leerii), while the two other species (T. pectoralis and T. trichopterus) in the Least Concern category ().
Table I. Types and locations of specimen collection Trichopodus spp. at the Museum Zoologicum Bogoriense from Sumatra, Indonesia.
Table II. Status and distribution of Trichopodus spp. in Sumatra Waters, Indonesia.
Morphometric Trichopodus spp.
Trichopodus leerii had a standard length that ranged between 46.30 and 72.10 mm (average 59.24 ± 7.83 mm), T. pectoralis ranged from 82.24 to 151.80 mm (average 110.73 ± 19.12 mm), T. trichopterus, from 30.42 to 82.77 mm (average 53.52 ± 11.94 mm). The ANOVA test showed significant variations for individual characteristics among the investigated Trichopodus species (p < 0.01) (). However, T. pectoralis and T. trichopterus display high morphological similarity across 11 morphometric characteristics, namely SnL, HL, PrePL, PoDL, BD, CPD, PrPL, PeFI, AFL, IOL, and BW. Overall, there are only four different characteristics, namely SNW, POL, ASL, and HW among the three species ().
Table III. Result of univariate ANOVA applied to a total 24 morphometric characteristics of Trichopodus spp. specimens collected from Sumatra waters.
Table IV. The mean value ± SD of the morphometric character of Trichopodus spp. from Sumatra waters.
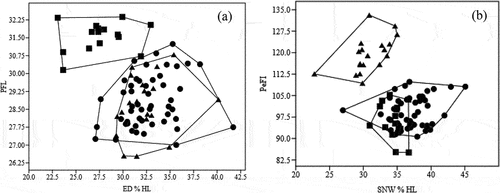
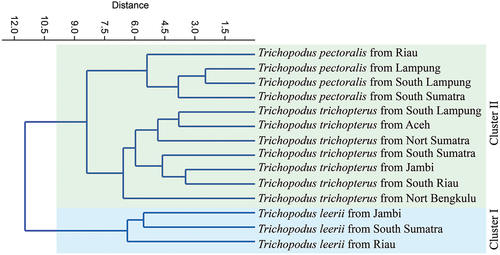
Principal Component Analysis (PCA) generated three functions (see ). PC1 had an eigenvalue of 5.77, explaining 24.02% of the total variance. The major characteristics that contributed to this function are POL (Postorbital Length), CPD (caudal peduncle depth), PeFI (pelvic fin length), HW (head width), IOL (interorbital distance), and BW (body width). These factors are those related to head, body form and fins. PCA 2 had an eigenvalue value of 4.31, which explains 17.98% of the total variance, and the contributing characteristics are SNW (anterior snout width), PrePL (Prepectoral length), HD (head depth), LJL (lower jaw length), and DStI (distance snout to isthmus). PCA 3 had an eigenvalue value of 2.22, which explains 9.26% of the total variance, with the contribution of BD (body depth) characteristics (). T. leerii was distinguished from two other species by plotting ED%HL and PFL characteristics against PeFL characteristics, whereas T. pectoralis was distinguished from two other species by plotting SNW%HL characteristics against PeFL (). On the basis of morphometric data, a dendrogram of the species was derived by the unweighted pair group (UPGMA) cluster analysis. The UPGMA cluster analysis based on the Euclidean distances between group centroids showed that the three species produced two major clusters. T. leerii (cluster I) and T. pectoralis and T. Trichopterus (cluster II) ().
Table V. Eigenvalue, percentage of variance, and percentage of cumulative variance for three character factors on Trichopodus spp. from Sumatra waters.
Discussions
A total of three species of Trichopodus were recorded during the study, namely T. trichopterus, T. pectoralis, and T. leerii (). Based on the total number of specimens, T. trichopterus was most predominant (76.56%) and had a wider local distribution, followed by T. pectoralis (12.50%) and T. leerii (10.94%). The results showed that the number of fish samples varies depending on the habitat types. Areas of river where water flows have fewer fish samples compared to stagnant waters. The findings indicated that the quantity of fish samples changes according to the kind of habitat. Compared to stagnant waters, areas of rivers where water is flowing have less fish samples. This shows that T. trichopterus is highly adaptable in various types of waters, which is reflected in a larger distribution and population number compared to the other two species (Haryono Citation2012; Huwoyon & Gustiano Citation2013; Gustiano et al. Citation2022). Nur et al. (Citation2022a) reported the exact same phenomenon on Betta spp. from Aceh waters. Trichopodus leerii, on the other hand, has a limited distribution and has been labelled as “near threatened”, therefore that it is essential to maintain this species in nature even if there are more samples of T. leerii than T. pectoralis.
Figure 5. Representative specimens of each species (a) T. leerii; (b) T. pectoralis; (c) T. trichopterus.

Based on the MZB collection, T. trichopterus lives in various habitats. For example, in Aceh, this fish was found in paddy field and river, while in South Lampung and Riau, it was found in swamps and river. Thus, this study also shows that T. trichopterus is more adaptable than the other two species. This distinctive adaptability is probably related to the presence of a breathing apparatus (labyrinth), which enables oxygen (O2) absorption directly from the atmosphere (Mendez‐Sanchez & Burggren Citation2019; Degani et al. Citation2021). T. leerii has been discovered in four provinces, namely West Sumatra at Teluk Bangka Lake, Lubuk Lampan, and Lempuing River in South Sumatra; at most of the rivers in Jambi and Riau Province. Trichopodus pectoralis has been discovered in four provinces, namely South Sumatra, Lampung, Riau and Jambi. In three provinces the species were found in the rivers except in Lampung where the species found in swamp.
Gustiano et al. (Citation2021) found that closely related species have a significant amount of phenotypic similarities in terms of biometrics. Based on this study, it may be assumed that T. pectoralis and T. trichopterus, which share numerous morphometric characteristics, are likely to have a closer kinship. Three of the species under investigation share the same diploid number and fundamental number, which is 46 when all the chromosomes are telocentric. The chromosome numbers for T. leerii, T. pectoralis, and T. trichopterus’ (large-medium-small karyotypes) are 16–28-2, 20–26-0, and 10–30-6, respectively (Supiwong et al. Citation2021). Additionally, all species have a single pair of the marker chromosomes that display the NOR positions, but there are variations in their locations and pairs, such as pairs nos. 1, 7, 2, and 1, respectively. Most species, except for T. trichopterus, have NOR sites near the centromere in an interstitial region. Telomeric NORs are expressed in T. trichopterus. Constitutive heterochromatin blocks occurred at the centromeric/pericentromeric regions of all the chromosomes in T. leerii, whereas in T. pectoralis and T. trichopterus, they formed at interstitial sites in addition to the centromeres of many chromosomal pairs.
The same species were in the same cluster in this study despite coming from different places and habitat types, demonstrating the consistency of the results (). Based on morphometric findings, genetic research carried out by other experts revealed conclusions that reinforced one another. One could claim that genetic analysis findings and morphometric data are consistent. Genetic diversity results in morphological changes, mostly in body shape, as a result of interactions between genetic and environmental factors, according to Hebert et al. (Citation2003) and Crispo (Citation2008). Barlow (Citation1961) asserts that this is true particularly in the early phases of growth. Numerous non-genetic factors, such as those connected to water conditions like temperature, turbidity, water currents, geography or habitat, and dietary habits, can contribute to the presence of this variance. The water’s depth can also play a significant role in significantly influenced by the differences in sex, predators, populations, physiology, and food sources (Langerhans et al. Citation2004).
Conclusion
Regarding distribution, T. trichopterus is the widest, where it was found in 49 locations covering 7 provinces (LD 76.56%), followed by T. pectoralis (LD 12.50%), and T. leerii with the smallest one, found in only seven locations (LD 10.94%). Status of T. leerii is the near-threatened category, while the other two species belong to the least concern category. Plotting anterior snout width (SNW) characteristics against pelvic fin length (PeFL) was successful in differentiating T. leerii from 2 other species, while eye diameter (ED) and pectoral fin length (PFL) characteristics were successful in separating T. pectoralis from two other species. The three species, which come from different locations, are frequently seen together in one cluster. The research showed that the findings from morphometrics and genetics are consistent.
Authors’ contribution
There is equal contribution from each author. FMN wrote the initial draft after gathering and analyzing the facts. RG planned the study, examined and interpreted data, edited the writing, and completed the manuscript. H developed the project and contributed samples, and completed the manuscript. AWP edited the writing, and completed the manuscript.
Acknowledgements
The authors thanks to Dr. Bayu Adjie, Director of Research Center for Biosystematics and Evolution, National Research and Innovation Agency (BRIN) for his support and financing of the project. Mr. Sopian Sauri for his assistance in preparing materials. Thanks to Martin Wilkes for proofreading the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmadi A. 2021. Morphometric characteristic and condition factor of snakeskin gourami (Trichogaster pectoralis) from Sungai Batang Swamp, Indonesia. Iranian Journal of Ichthyology 8(1):19–29.
- Barlow GW. 1961. Causes and significance of morphological variation in fishes. Systematic Zoology 10(3):105–117. DOI: 10.2307/2411595.
- Berra TM. 1981. An atlas of distribution of the freshwater fish families of the world. USA: University of Nebraska Press.
- Boonanuntanasarn S, Jangprai A, Na-Nakorn U. 2020. Transcriptomic analysis of female and male gonads in juvenile snakeskin gourami (Trichopodus pectoralis). Scientific Reports 10(1):5240. DOI: 10.1038/s41598-020-61738-0.
- Crispo E. 2008. Modifying effects of phenotypic plasticity on interactions among natural selection, adaptation and gene flow. Journal of Evolutionary Biology 21(6):1460–1469. DOI: 10.1111/j.1420-9101.2008.01592.x.
- Degani G, Veksler-Lublinsky I, Meerson A. 2021. Markers of genetic variation in Blue gourami (Trichogaster trichopterus) as a model for labyrinth fish. Biology 10(3):228. DOI: 10.3390/biology10030228.
- Dorado EL, Anthony M, Torres JAC, Barrion AA, Amparado R, Gorospe JG, Demayo CG. 2010. Sexual dimorphism in body shapes of the three-spotted gourami, Trichogaster trichopterus (Pallas, 1770) of Lake Buluan. Research Journal of Fisheries and Hydrobiology, 5(2): 111-118.
- Eviota MP, Jumawan JC, Joseph CCD, Samson NL. 2016. Geometric morphometric analysis of three spot gourami, Trichopodus trichopterus in Masao River, Butuan City, Mindanao, Philippines. Aquaculture, Aquarium, Conservation & Legislation 9(5):1011–1019.
- Gustiano R, Iskandariah I, Ath-Thar MF, Huwoyon GH, Radona D. 2022. Domestication of Snakeskin Gourami (Trichopodus pectoralis Regan, 1910) in Indonesia: characterization, bioreproduction and early development. Pakistan J. Zool. 1-8. DOI: 10.17582/journal.pjz/20220111140146.
- Gustiano R, Kurniawan K, Haryono H. 2021. Optimizing the utilization of genetic resources of indonesian native freshwater fish. Asian Journal of Conservation Biology 10(2):189–196. DOI: 10.53562/ajcb.67022.
- Gustiano R, Kusmini I, Ath-thar M. 2015. Introduction to genetic resources of Indonesian freshwater fishes for aquaculture development. Bogor: IPB University Press.
- Haryono H. 2010. Panduan lapang ikan perairan lahan gambut. Jakarta: LIPI Press.
- Haryono H. 2012. Fish fauna of Central Kalimantan peatland waters in rainy season. Jurnal Iktiologi Indonesia 12(1):83–91.
- Haryono H, Agus HT. 2000. Study on the peat mining impact on fish diversity in Bengkalis, Riau. Berita Biologi 5(3):323–330.
- Hebert PDN, Alina C, Shelley LB, Jeremy RD. 2003. Biological identifications through DNA barcodes. Proceedings of the Royal Society of London B: Biological Sciences 270(1512):313–321. DOI: 10.1098/rspb.2002.2218.
- Huwoyon GH, Gustiano R. 2013. Peningkatan produktivitas budidaya ikan di lahan gambut. Media Akuakultur 8(1):13–22. DOI: 10.15578/ma.8.1.2013.13-21.
- Jumawan JH, Asuncion DQ, Joseph CC, Abastillas SO, Moreno MP, Hewe JG, Etaoc JD, Arguilles JO, Cabuga Jr CC, Velasco JP. 2016. Assessment study using fluctuating asymmetry in the body shapes of Trichopodus trichopterus as bio-indicator of stress in Ubod-Ubod Creek, Baan, Butuan City, Agusan del Norte, Philippines. Aquaculture, Aquarium, Conservation & Legislation 9(1):71–80.
- Kottelat M, Whitten A, Katikasari S, Wirjoatmodjo S. 1993. Ikan air tawar Indonesia bagian Barat dan Sulawesi. Jakarta: Periplus Edition.
- Kurniawan K, Gustiano R, Kusmini II, Prakoso VA. 2021. Genetic resources preservation and utilization of Indonesian native freshwater fish consumption. Ecology. Environment and Conservation 27(1):227–233.
- Langerhans RB, Layman CA, Shokrollahi AM, DeWitt TJ. 2004. Predator‐driven phenotypic diversification in Gambusia affinis. Evolution 58(10):2305–2318. DOI: 10.1111/j.0014-3820.2004.tb01605.x.
- Low BW, Tan HH, Britz R. 2014. Trichopodus poptae, a new anabantoid fish from Borneo (Teleostei: Osphronemidae). Ichthyological Exploration of Freshwaters 25:69–77.
- Mendez‐Sanchez JF, Burggren WW. 2019. Hypoxia‐induced developmental plasticity of larval growth, gill and labyrinth organ morphometrics in two anabantoid fish: The facultative air‐breather Siamese fighting fish (Betta splendens) and the obligate air‐breather the blue gourami (Trichopodus trichopterus). Journal of Morphology 280(2):193–204. DOI: 10.1002/jmor.20931.
- Mohsin M. 1983. Freshwater fishes of Peninsular Malaysia. Selangor: Serdang: UPM Press.
- Muchlisin Z, Akyun Q, Halim A, Rizka S, Sugianto S, Fadli N, Siti-Azizah M. 2015. Ichthyofauna of Tripa peat swamp forest, Aceh province, Indonesia. Check List 11:1. DOI: 10.15560/11.2.1560.
- Nelson J. 1994. Fishes of the world. New York: John Wiley & Sons Inc.
- Ng PK, Tan H. 1997. Freshwater fishes of Southeast Asia: Potential for the aquarium fish trade and conservation issues. Aquarium Sciences and Conservation 1(2):79–90. DOI: 10.1023/A:1018335617835.
- Nguyen TDP. 2014. Snakeskin gourami in the Mekong. Aqua Culture Asia Pacific 10(4):24–27.
- Nguyen PM, Pham XM, Van Linh NT. 2019. Several aspects influencing to production of dry-salted snakeskin gourami (Trichogaster pectoralis). Oriental Journal of Chemistry 35(2):773. DOI: 10.13005/ojc/350238.
- Nur FM, Batubara AS, Fadli N, Rizal S, Siti-Azizah MN, Muchlisin ZA. 2022a. Diversity, distribution, and conservation status of Betta fish (Teleostei: Osphronemidae) in Aceh waters, Indonesia. The European Zoological Journal 89(1):142–151. DOI: 10.1080/24750263.2022.2029587.
- Nur FM, Batubara AS, Fadli N, Rizal S, Siti-Azizah MN, Muchlisin ZA. 2022b. Elucidating species diversity of genus Betta from Aceh waters Indonesia using morphometric and genetic data. Zoologischer Anzeiger 296:129–140. DOI: 10.1016/j.jcz.2021.12.004.
- Paepke HJ. 2009. The nomenclature of Trichopodus pectoralis Regan, 1910; Trichopodus cantoris Sauvage, 1884 and Osphronemus saigonensis Borodin, 1930 (Teleostei: Perciformes: Osphronemidae). Vertebrate Zoology 59(1):53–60. DOI: 10.3897/vz.59.e30949.
- Perdana AW, Batubara AS, Nur FM, Syahril A, Muchlisin ZA. 2021. Morphometric analysis of three species gourami group (Osphronemidae) from Aceh waters, Indonesia. IOP Conference Series: Earth and Environmental Science 674(1):012087. DOI: 10.1088/1755-1315/674/1/012087.
- Rainboth W. 1996. Fish of the Cambodian Mekong. Italy: FAO species identification field guide for fishery purpose. FAO Rome.
- Regan CT. 1909. The Asiatic fishes of the family Anabantidae Proceedings of the Zoological Society of London Proceedings of the Zoological Society of London, B. 79(4):767–787. DOI: 10.1111/j.1469-7998.1910.tb06972.x.
- Roberts T. 1992. Systematic revision of the Southeast Asian anabantoid fish genus Osphronemus, with descriptions of two new species. Ichthyol Explor Freshwat 2:351–360.
- Roberts TR. 1989. The freshwater fishes of Western Borneo (Kalimantan, Barat, Indonesia). Memoirs of the California Academy of Sciences 14:1–210.
- Smith HM. 1945. The freshwater fishes of Siam, or Thailand. Bulletin of the United States National Museum 188:9 pls.
- Sneath PH, Sokal RR. 1973. Numerical taxonomy. San Francisco: The principles and practice of numerical classification.
- Sokal RR, Rohlf FJ. 1980. Biometry. USA: W.H. Freeman and Co.
- Supiwong W, Tanomtong A, Kenthao A, Seetapan K, Kaewsri S, Sanoamuang LO. 2010. Standardized karyotype of the three-spot gourami, Trichogaster trichopterus (Perciformes, Belontidae) from Thailand by conventional and Ag-NOR staining technique. The Nucleus 53(3):103–107. DOI: 10.1007/s13237-011-0016-2.
- Supiwong W, Wongchantra P, Thongnetr W, Mingkwan B, Chaiyasan P, Pinmongkhonkul S, Pinthong K, Tanomtong A. 2021. Comparative cytogenetic analysis of fishes in the genus Trichopodus (Osphronemidae) in Thailand. Biodiversitas Journal of Biological Diversity 22:7. DOI: 10.13057/biodiv/d220757.
- Tan MP, Amornsakun T, Siti Azizah MN, Habib A, Sung YY, Danish-Daniel M. 2019. Hidden genetic diversity in snakeskin gourami, Trichopodus pectoralis (Perciformes, Osphronemidae), inferred from the mitochondrial DNA CO1 gene. Mitochondrial DNA Part B 4(2):2966–2969. DOI: 10.1080/23802359.2019.1662741.
- Töpfer J, Schlindler I. 2009. On the type species of Trichopodus (Teleostei: Perciformes: Osphronemidae). Vertebrate Zoology 59(1):49–51. DOI: 10.3897/vz.59.e30948.
- Yoonpundh R, Little D. 1997. Trends in the farming of the Snakeskin gourami (Trichogaster pectoralis) in Thailand. NAGA, The WorldFish Center. 20(3/4). 18–20.