Abstract
In medicolegal forensic entomology, the estimation of the minimum postmortem interval and any other evaluation depend on the accuracy of the species identification. Although the most common species received a lot of attention in the last years, other species in less common families still remain poorly investigated and sometimes the immature stages, especially puparia, are not described. The finding of several specimens of Heleomyza serrata (Linnaeus, 1758) (Diptera: Heleomyzidae) during an experiment with rabbit cadavers provided the opportunity to describe the puparium of this species. Molecular analysis based on the COI region enabled the evaluation of the monophyly of the genus and on the other side highlighted the usefulness of this sequence for the identification of this taxon. In general, this work offers a morphological and molecular overview about a species that has been underestimated in forensic investigations being potentially present on human and other animals’ cadavers during the cold seasons.
Introduction
Diptera and Coleoptera are considered the most important taxa colonizing bodies after death, with Diptera being the first colonizer of a body. Among Diptera, species in the families Calliphoridae, Sarcophagidae, Muscidae, Fanniidae, Piophilidae and Phoridae are constantly reported from human and animal cadavers during the decomposition process all over the world (Tomberlin & Adler Citation1998; Schroeder et al. Citation2003; Vanin et al. Citation2008; Grisales et al. Citation2010), whereas other families are reported only sporadically from bodies discovered in specific habitat and/or seasons, e.g.: Trichoceridae, Milichidae, Sphaeroceridae (Smith Citation1986; Giordani et al. Citation2019b; Giordani & Vanin Citation2020). Among the species that are only occasionally found on humans, animals and in traps deployed for forensic purposes or for studying the entomofauna associated with decomposing bodies, the family Heleomyzidae must be mentioned (Vanin Citation2012; Farinha et al. Citation2014; Sessa et al. Citation2019). It is also worth mentioning that their occurrence on cadavers seems to be particularly relevant in the cold season. (Hwang & Turner Citation2005; Martin‐Vega & Baz Citation2013). Members of this family are often underestimated because of the lack of tools for the identification of their immature stages and for this reason reported only at the family level, as already highlighted for several families of potential forensic interest. Moreover, because of the presence of puparia of this family in funerary archaeoentomological context, the possibility of their correct identification based on morphological characters (DNA may be too degraded in these contexts) may provide useful information for archaeological reconstruction.
The family Heleomyzidae consists of flies that range from minute to large in size (1.2–12 mm) and from yellow to dark grey or brown in colour. Their wings often show distinctly longer bristles mixed with shorter bristles along the leading edge of the costal vein.
The family includes approximately 600 species that occur in all the biogeographical regions of the world with the exception of Antarctica; 153 European species are known (Woźnica Citation2006; Lo Giudice & Woźnica Citation2013; SoszyńskaMaj & Woźnica Citation2016). According to Gorodkov (Citation1984), the Palearctic Heleomyzidae are divided into three subfamilies: Suilliinae, Heteromyzinae and Heleomyzinae (Gorodkov Citation1984; Papp Citation1998; SoszyńskaMaj & Woźnica Citation2016). The description of puparia of species in this family is very limited despite the very interesting study by Rotheray (Citation2012). Among Heteromyzinae, Heleomyza serrata (Linnaeus, 1758) [Syn: Musca serrata Linnaeus, 1758; Helomyza geniculata Zetterstedt, 1838] was reported from excrements, bird nests, fungi, dung, chicken manure, decomposing plant remains and cadavers (Gill Citation1962; Skidmore Citation1962; Garnett & Foote Citation1966; Krivosheina Citation2008; Carles-Tolrá Citation2011). Heleomyza serrata, as many other heleomyzids, is a cold-adapted species, active in winter and often collected on the surface of snow in Poland (Soszyńska Citation2004; Soszyńska-Maj & Woźnica Citation2012) and Scandinavia (Hågvar & Greve Citation2003) and it has often been found in caves in Norway, Austria and Poland (Kjærandsen Citation1993; Christian & Spötl Citation2010; Østbye & Lauritzen Citation2013). Adults are often confused with Heleomyza captiosa (Gorodkov, Citation1962) as highlighted by Chandler (Citation1998), from which it can be separated by the analysis of the male genitalia or by the shape of spermathecae (for more details see Gorodkov Citation1962; Soszyńska-Maj & Woźnica Citation2016).
In order to improve knowledge about this species and to help in its identification, morphological descriptions of adults and puparia are provided and discussed, together with molecular analyses useful for identification.
Materials and methods
Puparia were collected in 2017 from completely skeletonized rabbits exposed in 2014 on a layer of sand a few centimetres deep used in a decomposition experiment as previously reported (Tuccia et al. Citation2019).
The dead rabbits (2.75–3.50 kg) were placed in plastic boxes on the roof of the Science Building at the University of Huddersfield (West Yorkshire, UK, 53°38′36.5″ N 1°46′40.1″ W).
Puparia were stored at room temperature in jars covered with laboratory paper until adult emergence.
A Keyence VHX-S90BE digital microscope, equipped with Keyence VH-Z250R and VH-Z20R lens and VHX-2000 Ver. 2.2.3.2 software (Keyence, Japan) was used for the observation of both adults and puparia. To improve the pictorial archive available for H. serrata, air-dried cleaned puparia were observed with a FEI QUANTA 650 FEG scanning electron microscope (SEM) (Thermo Scientific, USA). Images were directly digitized from the SEM.
Adult identification was provided by Dr Andrzej J. Woźnica (Instytut Biologii, Uniwersytet Przyrodniczy we Wrocławiuand – Poland).
Three adult flies were used for molecular analyses. Manufacturer’s instructions of QIAamp® DNA Mini kit were followed. The amount of proteinase K was increased from 20 to 40 μl, previous extractions showed better results with this amount. Each specimen was submerged and incubated overnight at 56 °C into the extraction buffer preventing morphological destruction. The commercial kit “Quant-iT™ Qubit™ dsDNA High-Sensitivity Assay Kit” (Invitrogen, United States) was used on a Qubit® 3.0 Fluorometer (Invitrogen, United States) for DNA quantification. The universal primers LCO-1490 and HCO-2198 designed by Folmer et al. (Citation1994) were used for the amplification of the mitochondrial COI Barcoding region. A total reaction volume of 20 μl was prepared using Promega GoTaq® Flexi Polymerase protocol: 4 μl of Colourless GoTaq Flexi Buffer (5×), 2 μl of MgCl2 (25 mM), 0.5 μl of each primer (10 pmol/μl), 0.5 μl of dNTPs Mix (10 mM), 0.25 μl GoTaq DNA Polymerase (5 u/μl) and 2 μl of DNA template. The reactions, including positive and negative controls, were assembled under a Purair PCR-36 laminar flow cabinet (AirScience®, Florida, USA) in order to prevent cross contaminations between samples.
The thermal cycler BioRad C1000 (Bio-Rad Laboratories, Inc.) was used to perform the amplification setting the following program: initial heat activation step at 95 °C for 10 min, 35 cycles of 95 °C for 1 min, 49.8 °C for 1 min, 72 °C for 1 min and a final extension step at 72 °C for 10 min. Positive and negative DNA templates were used as controls for the reaction. The quality of the reactions was qualitatively assessed by 1.5% (w/v) agarose gel electrophoresis stained with Midori Green Advanced DNA Stain (Geneflow Ltd., Lichfield, UK.) Amplicons were purified using QIAquick PCR Purification Kit® (Qiagen, Hilden, Germany) following the manufacturer’s instructions, eluted in 40 μl of EB and sequenced by Eurofins Genomics (Ebersberg, Germany).
The newly produced sequences have been deposited in GenBank (). Sequences of COI gene from Heleomyzidae species were downloaded from BOLD online system and included in the phylogenetic analyses (). Sequences were aligned with Clustal Omega (Sievers et al. Citation2011). The phylogenetic trees were built using the Neighbour Joining method on MEGA 7.0 (Kumar et al. Citation2016). Fifty sequences of 563 bp were used for the phylogenetic analysis. Drosophila melanogaster Meigen, 1830 (Diptera, Drosophilidae) was used as an outgroup.
Table I. GenBank codes of COI sequences produced in this work.
Table II. COI sequences of Heleomyzidae downloaded from BOLD and included in the analyses.
Results
Morphological analysis
Adults
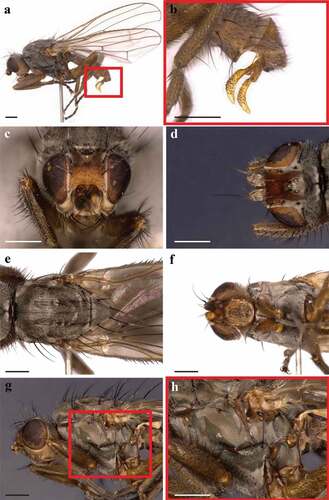
In the analysed samples, adults body length was 4.3 ± 0.3 mm (N = 7) while the length of the wings was 4.9 ± 0.3 mm (). Males show long L shape gonostyli (). Adults are grey flies with an orange frons and the eye height is more than double the gena height. The head shows a grey ocellar triangle with two divergent ocellar setae and two converging postvertical bristles (). Two outer vertical bristles, three pairs of fronto-orbital bristles and numerous minute scattered bristles in the frons are present. Lunula is bare. The hind part of the head is grey (). The lower part of the head and the gena are yellowish-light brown with one strong black vibrissa and two additional smaller bristles (). The thorax has four dorsocentral bristles, one short prescutellar acrostichal bristle, one humeral bristle, one posthumeral bristle, two notopleural bristles and two post-alar bristles (). Prosternum is shiny grey with four to six lateral bristles (). The katepisternum has one strong posterodorsal black bristle and six small anterior setulae (). The scutellum has four long and strong black marginal setae (). This species can be distinguished from Heleomyza captiosa (Gorodkov, Citation1962), a species also present in the UK, using the male genitalia or by the shape of the spermathecae.
Figure 1. Heleomyza serrata adult (male). Lateral view (a), magnification of male genitalia (red box 1a)(b), head chaetotaxy in frontal view (c), head chaetotaxy in dorsal view (d), thorax chaetotaxy in dorsal view (e), prosternum (f), thorax chaetotaxy in lateral view (g) and magnification of the katepisternum (red box 1g)(h). Observation performed using a Keyence VHX-S90BE digital microscope (scale bar 500 µm).
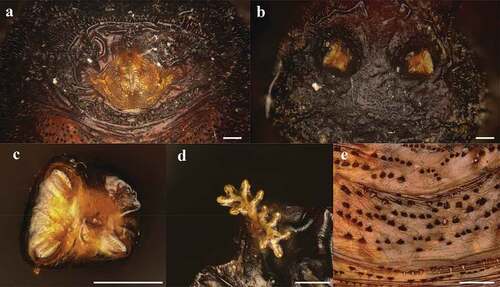
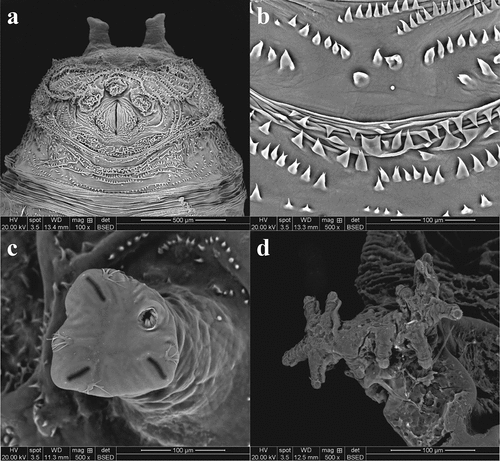
Puparia
The average length of the puparia, in the analysed sample, was 5.24 ± 1.14 mm (N = 20). Puparia are yellowish-brown in colour, wrinkled and covered by spines with anterior and posterior spiracles clearly detectable (). The anal plate is light brown and has short expansions upward directed (wings). The post and subanal papillae are present (). The posterior spiracles are allocated on two parallel tubercules. The slits are radiate, and the scar is in upper position (). Dorsal muscle scars are well defined and visible in SEM observation (). Anterior spiracles in the analysed samples have a branch shape with 11–13 prospiracular lobes (). The ventral welt of abdominal segment seven is made up of small flame-shaped spines, some of which bifid, placed in disorganized rows (). Some rows are directed toward the anal plate, some other toward the anterior part.
Figure 2. Heleomyza serrata puparium. Ventral (a), dorsal (b) and lateral (c) views. Observation performed using a Keyence VHX-S90BE digital microscope (scale bar 500 μm).

Molecular analysis
The total amount of DNA extracted from the adults ranged from 460 to 1290 ng. A phylogenetic reconstruction was performed to confirm the morphological identification.
The analysis of the sequences obtained from the analysed specimens confirmed the morphological identification as Heleomyza serrata. All the sequences obtained an extremely high value of identity with the sequences of the same species present in the databases (100–97.58%, 0.0 E). The NJ analysis of 50 sequences of 563 bp belonging to different genera (Borboropsis Czerny, 1902, Heleomyza Fallen, 1810, Suillia Robineau-Desvoidy, 1830, Tephrochlamys Loew, 1862 and Trixoscelis Rondani, 1856) in the Heleomyzidae family brought about a phylogenetic tree with well-supported clades at both genus and species levels (). However, the low level of bootstrap at the basal nodes of the tree does not allow for any statement about the relationship between and among genera.
Figure 5. Phylogenetic tree of Heleomyzidae family. Neighbour joining method analysis of 563 bp sequence of the COI gene. The green spots and the number at each node indicate the bootstrap support. Sequences from this study are reported in red.
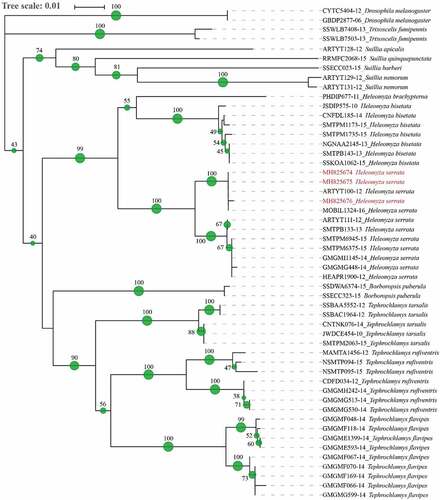
All the sequences of H. serrata cluster together, and the sequences obtained from this study (MH825676, MH825675 and MH825674) branch together with two sequences of H. serrata coded in BOLD as MOBIL1324-16 and ARTYT100-12 from Alaska and Canada.
Discussion and conclusion
Time since death and cadaver displacement from the primary scene are among the information that insects can provide from a crime scene (Amendt et al. Citation2007, Citation2011; Gennard Citation2007; Tomberlin & Benbow Citation2015) as long as the correct species identification is provided. Extensive literature is available about the morphology of adults and larvae of insects of sanitary, medical, veterinary, agricultural and forensic interest (Barros de Carvalho & Antunes de Mello-Patiu Citation2008; Rochefort et al. Citation2015; Szpila et al. Citation2015) while puparia are still an under examined subject except for few taxa of forensic interest (Giordani et al. Citation2018, Citation2019a, Citation2019b, Citation2023; Giordani & Vanin Citation2020; Tuccia et al. Citation2021).
Although puparia's general shape and size allow family-level identification, these features are not sufficient to reach the species level. Some forensic scientists do not feel confident in the use of morphological characters for identification of immatures (Amendt et al. Citation2011; Grzywacz et al. Citation2017) preferring to rear the immatures until fully develop of the adults or relying on the molecular method. The high probability of encountering empty puparia or dead pupae, and the consequent impossibility to rear the specimens to adults, is a limitation of this strategy, in addition to the time and space required. On the other hand, molecular databases may lack information or contain misidentified species sequences, thereby failing in the identification of numerous taxa of forensic interest (Vanin Citation2008). This is particularly evident for “secondary families” not largely investigated. The synergy between the two approaches – morphological and molecular – is therefore the only way to reach a reliable identification result for many species. To do that, open-access, well-illustrated keys need to be available for non-specialists to rely on easily recognizable characters (Grzywacz et al. Citation2017) and it is also vital the use of DNA extraction methods that prevent the morphological destruction of the specimens. A method to perform DNA extraction preserving the morphology on 3rd instar larvae of Calliphoridae, Muscidae and Phoridae has been reported (Tuccia et al. Citation2016), but nothing has been published yet about empty puparia.
From a morphological point of view, the images reported in the paper show peculiar shapes and structures of the spiracles, of the spinose bands and also of the anal region of the puparium that can be considered and used, as in other taxa, for example in the genus Hydrotaea (family Muscidae) and in the family Piophilidae, as robust diagnostic characters for the puparia identification (Giordani et al. Citation2019a, Citation2023).
Some important and inclusive molecular studies have been performed for numerous families of insects of forensic interest (Kits et al. Citation2013; Marshall et al. Citation2015; Zajac et al. Citation2016) but, to the knowledge of the authors, none for Heleomyzidae. A high number of sequences are available in GenBank for the Heleomyzidae family but unfortunately referring to a few species, reflecting the absence of phylogenetic work. The lack of sequences of H. captiosa in BOLD system and NCBI GenBank prevents the possibility to differentiate the two species and it underlines the actual weakness of the molecular approach in poorly investigated families.
It is worth mentioning that the phylogenetic reconstruction shows the monophyly of the analysed genera (Tephrochlamys, Borboropsis, Heleomyza and Suilla) and a well-supported differentiation of co-generic species. Additional sequences are needed to discuss the phylogenetic relationships among and between the taxa of this family.
This work offers a morphological and molecular overview of H. serrata that has been under-evaluated or non-identified in forensic contexts, especially in cold seasons. Further studies of habitat preference and the rate of development at different temperatures are fundamental to make this species useful in medico-legal and veterinary forensic entomology.
Acknowledgements
The authors are grateful to Dr Andrzej J. Woźnica (Instytut Biologii, Uniwersytet Przyrodniczy we Wrocławiu – Poland) for the species identification.
The work of G. Giordani was funded by the Leverhulme Trust Doctoral Scholarship program, and the results here presented are part of her PhD Thesis at the University of Huddersfield http://eprints.hud.ac.uk/id/eprint/34893/; https://core.ac.uk/download/pdf/200757534.pdf.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc HN, Hall MJ. 2007. Best practice in forensic entomology-standards and guidelines. International Journal of Legal Medicine 121(2):90–104. DOI: 10.1007/s00414-006-0086-x.
- Amendt J, Richards CS, Campobasso CP, Zehner R, Hall MJ. 2011. Forensic entomology: Applications and limitations. Forensic Science, Medicine and Pathology 7(4):379–392. DOI: 10.1007/s12024-010-9209-2.
- Barros de Carvalho CJ, Antunes de Mello-Patiu C. 2008. Key to the adults of the most common forensic species of Diptera in South America. Revista Brasileira de Entomologia 52(3):390–406. DOI: 10.1590/S0085-56262008000300012.
- Carles-Tolrá M. 2011. Some dipterans collected on winter cadavers in La Rioja (Spain):(Diptera: Phoridae, Heleomyzidae and Sphaeroceridae). Boletín de la Sociedad Entomológica Aragonesa 48:147–150.
- Chandler PJ. 1998. Checklists of insects of the British Isles. Handbooks for the Identification of British Insects 12:1–234.
- Christian E, Spötl C. 2010. Karst geology and cave fauna of Austria: A concise review. International Journal of Speleology 39(2):71–90. DOI: 10.5038/1827-806X.39.2.3.
- Farinha A, Dourado CG, Centeio N, Oliveira AR, Dias D, Rebelo MT et al. 2014. Small bait traps as accurate predictors of dipteran early colonizers in forensic studies. Journal of Insect Science 14(77):77. DOI: 10.1673/031.014.77.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5):294–299.
- Garnett W, Foote B. 1966. Notes on the biology of certain heleomyzid flies of eastern North America (Diptera: Heleomyzidae). Journal of the Kansas Entomological Society 39:552–555.
- Gennard D. 2007. Forensic entomology: An introduction. Chichester: Wiley. pp. 224.
- Gill GD. 1962. The heleomyzid files of America north of Mexico (Diptera: Heleomyzidae). Proceedings of the United States National Museum 113(3465):495–603. DOI: 10.5479/si.00963801.113-3465.495.
- Giordani G, Grzywacz A, Vanin S. 2019a. Characterization and identification of Puparia of Hydrotaea Robineau-Desvoidy, 1830 (Diptera: Muscidae) from forensic and archaeological contexts. Journal of Medical Entomology 56(1):45–54. DOI: 10.1093/jme/tjy142.
- Giordani G, Tuccia F, Floris I, Vanin S. 2018. First record of Phormia regina (Meigen, 1826) (Diptera: Calliphoridae) from mummies at the Sant’Antonio Abate Cathedral of Castelsardo, Sardinia, Italy. PeerJ 6:e4176. DOI: 10.7717/peerj.4176.
- Giordani G, Tuccia F, Martín-Vega D, Angell CS, Pradelli J, Vanin S. 2023. Morphological and molecular characterization of puparia of Piophilidae species of forensic relevance. Medical and Veterinary Entomoly 37(2):339–358. DOI: 10.1111/mve.12635.
- Giordani G, Tuccia F, Zoppis S, Vecchiotti C, Vanin S. 2019b. Record of Leptometopa latipes (Diptera: Milichiidae) from a human cadaver in the Mediterranean area. Forensic Sciences Research 4(4):341–347. DOI: 10.1080/20961790.2018.1490473.
- Giordani G, Vanin S. 2020. Description of the puparium and other notes on the morphological and molecular identification of Phthitia empirica (Diptera, Sphaeroceridae) collected from animal carcasses. Egyptian Journal of Forensic Sciences 10(1):13. DOI: 10.1186/s41935-020-00187-2.
- Gorodkov K. 1962. Revision of the Palaearctic species of the genus Leria R.-D. (Diptera, Helomyzidae). Entomologicheskoe Obozrenie 41:643–671.
- Gorodkov KB. 1984. Family Heleomyzidae. In: L SAP, editor. Catalogue of Palaearctic Diptera. Amsterdam: Elsevier Science. pp. 15–45.
- Grisales D, Magnolia R, Villegas S. 2010. Insects associated with exposed decomposing bodies in the Colombian Andean coffee region. Revista Brasileira de Entomologia 54(4):637–644. DOI: 10.1590/S0085-56262010000400016.
- Grzywacz A, Hall MJ, Pape T, Szpila K. 2017. Muscidae (Diptera) of forensic importance-an identification key to third instar larvae of the Western Palaearctic region and a catalogue of the muscid carrion community. International Journal of Legal Medicine 131(3):855–866. DOI: 10.1007/s00414-016-1495-0.
- Hågvar S, Greve L. 2003. Winter active flies (Diptera, Brachycera) recorded on snow-a long-term study in south Norway. Studia Dipterologica 10:401–421.
- Hwang C, Turner BD. 2005. Spatial and temporal variability of necrophagous Diptera from urban to rural areas. Medical and Veterinary Entomology 19(4):379–391. DOI: 10.1111/j.1365-2915.2005.00583.x.
- Kits JH, Marshall SA, Skevington JH. 2013. Phylogeny of the Archiborborinae (Diptera: Sphaeroceridae) based on combined morphological and molecular analysis. PloSOne 8(1):e51190. DOI: 10.1371/journal.pone.0051190.
- Kjærandsen J. 1993. Diptera in mines and other cave systems in southern Norway. Entomologica Fennica 4(3):151–160. DOI: 10.33338/ef.83761.
- Krivosheina N. 2008. Macromycete fruit bodies as a habitat for dipterans (Insecta, Diptera). Entomological Review 88(7):778–792. DOI: 10.1134/S0013873808070038.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33:1870–1874. DOI: 10.1093/molbev/msw054.
- Lo Giudice G, Woźnica AJ. 2013. An updated checklist of the Italian Heleomyzidae (Diptera: Sphaeroceroidea). Genus 24:439–458.
- Marshall SA, Skevington JH, Kelso S, Zhou C. 2015. A redefinition and review of the genus Myrmolimosina Marshall (Diptera: Sphaeroceridae), with morphological and molecular assessments of new species from Mexico and Guatemala. The Canadian Entomologist 147:696–701. DOI: 10.4039/tce.2014.88.
- Martin‐Vega D, Baz A. 2013. Sarcosaprophagous Diptera assemblages in natural habitats in central Spain: Spatial and seasonal changes in composition. Medical and Veterinary Entomology 27(1):64–76. DOI: 10.1111/j.1365-2915.2012.01028.x.
- Østbye E, Lauritzen S-E. 2013. A checklist of invertebrates from Norwegian caves and mines. Fauna Norvegica 33:35–51. DOI: 10.5324/fn.v33i0.1585.
- Papp L. 1998. Families of Heleomyzoidea. In: László P, Béla D, editors. Contributions to a Manual of Palaearctic Diptera. Budapest: Science Herald. pp. 425–455.
- Rochefort S, Giroux M, Savage J, A. Wheeler T. 2015. Key to forensically important Piophilidae (Diptera) in the Nearctic Region. Canadian Journal of Arthropod Identification 27:1–37.
- Rotheray GE. 2012. Morphology of the puparium and breeding sites of eight species of Heleomyzidae (Diptera). Journal of Natural History 46(33–34):2075–2102. DOI: 10.1080/00222933.2012.707241.
- Schroeder H, Klotzbach H, Puschel K. 2003. Insects’ colonization of human corpses in warm and cold season. Legal Medicine 5(Suppl 1):S372–374. DOI: 10.1016/S1344-6223(02)00135-9.
- Sessa F, Varotto E, Salerno M, Vanin S, Bertozzi G, Galassi FM et al. 2019. First report of Heleomyzidae (Diptera) recovered from the inner cavity of an intact human femur. Journal of Forensic and Legal Medicine 66:4–7. DOI: 10.1016/j.jflm.2019.05.021.
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology 7:539. DOI: 10.1038/msb.2011.75.
- Skidmore P. 1962. Notes on the Helomyzidae of Lancashire and Cheshire, including records from other parts of North West England. Entomologist 95:226–236.
- Smith KGV. 1986. A Manual of Forensic Entomology. Ithaca: Cornell University Press. pp. 205.
- Soszyńska A. 2004. The influence of environmental factors on the supranivean activity of flies (Diptera) in Central Poland. European Journal of Entomology 101:481–490. DOI: 10.14411/eje.2004.068.
- Soszyńska-Maj A, Woźnica AJ. 2012. Comments on the biology, systematics and distribution of Scoliocentra (Leriola) nigrinervis (Wahlgren, 1918) in Poland and Europe (Diptera: Heleomyzidae). Dipteron. Bulletin of the Dipterological Section of the Polish Entomological Society 28:23–28.
- Soszyńska-Maj A, Woźnica AJ. 2016. A case study of Heleomyzidae (Diptera) recorded on snow in Poland with a review of their winter activity in Europe. European Journal of Entomology 113:279–294. DOI: 10.14411/eje.2016.035.
- Szpila K, Richet R, Pape T. 2015. Third instar larvae of flesh flies (Diptera: Sarcophagidae) of forensic importance - critical review of characters and key for European species. Parasitology Research 114:2279–2289. DOI: 10.1007/s00436-015-4421-3.
- Tomberlin JK, Adler PH. 1998. Seasonal colonization and decomposition of rat carrion in water and on land in an open field in South Carolina. Journal of Medical Entomology 35:704–709. DOI: 10.1093/jmedent/35.5.704.
- Tomberlin JK, Benbow ME. 2015. Forensic Entomology: International Dimensions and Frontiers. Boca Raton: CRC Press. pp. 468.
- Tuccia F, Giordani G, Cattaneo C, Mazzarelli D, Vanin S. 2021. First record of Physyphora alceae (Preyssler, 1791)(Diptera, Ulidiidae) from a forensic case in Northern Italy: Description of immature stages, DNA barcoding and phylogenetic analysis. The European Zoological Journal 88:1071–1083. DOI: 10.1080/24750263.2021.1981469.
- Tuccia F, Giordani G, Vanin S. 2016. A combined protocol for identification of maggots of forensic interest. Science & Justice 56:264–268. DOI: 10.1016/j.scijus.2016.04.001.
- Tuccia F, Zurgani E, Bortolini S, Vanin S. 2019. Experimental evaluation on the applicability of necrobiome analysis in forensic veterinary science. MicrobiologyOpen 8:e828.
- Vanin S. 2008. Utilità e limiti dell’identificazione tramite DNA delle specie di insetti di interesse forense in Italia e Europa. Rivista Italiana di Medicina Legale 30(6):1403–1411.
- Vanin S. 2012. Carrion breeding fauna from a grass snake (Natrix natrix) found in an artificial nest. Lavori Società Veneziana di Scienze Naturali 37:73–76.
- Vanin S, Tasinato P, Ducolin G, Terranova C, Zancaner S, Montisci M et al. 2008. Use of Lucilia species for forensic investigations in Southern Europe. Forensic Science International 177(1):37–41. DOI: 10.1016/j.forsciint.2007.10.006.
- Woźnica AJ. 2006. Three new species of the genus Suillia Robineau-Desvoidy, 1830 from the Neotropical region (Diptera: Heleomyzidae). Annales Zoologici 56:657–665.
- Zajac BK, Martin-Vega D, Feddern N, Fremdt H, E Castro CP, Szpila K et al. 2016. Molecular identification and phylogenetic analysis of the forensically important family Piophilidae (Diptera) from different European locations. Forensic Science International 259:77–84. DOI: 10.1016/j.forsciint.2015.12.024.