Abstract
Analysis of the qualitative signature of fatty acids in tissues of organisms is an increasingly utilized tool in studies of trophic ecology in aquatic and terrestrial ecosystems. Here, we studied a colonial waterbird, the black-headed gull Chroicocephalus ridibundus, nesting in three colonies. We aimed to investigate whether fatty acid composition of its eggs is affected by the location of the colony (reflecting the spatial variation in habitat composition and, in turn, female diet at the time of egg formation), and the egg laying sequence (reflecting the temporal variation in female diet). We found that the composition of fatty acids in eggs of black-headed gulls differed among colonies, but not among subsequent eggs within the clutch. We interpret these results in the context of spatial differences in the diet of females breeding in different colonies and a lack of temporal variation in the diet of individual females. The pattern of the ratio of omega-3 to omega-6 fatty acids reflected the contribution of aquatic/terrestrial prey to their diet, and was consistent with the area of water courses around the colonies. High levels of omega-6:omega-3 can promote inflammation and thus lead to increased susceptibility to antigens. Further studies with prey sampling are required to reconstruct the diet composition of females during egg formation in more detail.
Introduction
Fatty acids (FAs), along with glycerol, are the most important component of lipids, and their main tasks are to provide energy, build cell membranes (phospholipids) and stabilize internal organs in animals (Williams & Buck Citation2010). In vertebrates three groups of FAs can be detected: saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), which can both be synthesized directly in the organism, and polyunsaturated fatty acids (PUFAs), which are sourced only from the diet. PUFAs are divided into omega-3 (alpha-linolenic acid (ALA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA)) and omega-6 (linoleic acid (LA), gamma-linolenic acid (GLA), arachidonic acid (AA)). The proportion of FAs in organisms play an important role in various physiological processes, and in consequence is vital for individual fitness (Romieu et al. Citation2008; Isaksson Citation2015; Twining et al. Citation2016).
Composition of the FAs in avian tissues and eggs, is species- and habitat-dependent, and may also differ among individuals in relation to their age, sex or physiological status (Dannenberger et al. Citation2013; Lohner et al. Citation2013; Piaskowska et al. Citation2015; Pedrazzoli et al. Citation2017). This composition can additionally be disturbed by various environmental stressors, including physical (e.g. temperature, radiation leading to lipid peroxidation), chemical (free radicals, hydrolysis of ester linkages) (Lei et al. Citation2012; Filimonova et al. Citation2016; Lee et al. Citation2018), and biological (e.g. viral, bacterial, protozoans and fungal infections) factors (Desbois & Smith Citation2010; Ferronato & Prandini Citation2020). But the composition of PUFAs, which are not synthesized in the organism, largely reflects its diet (Williams & Buck Citation2010). Thus, analysis of FA composition in organisms is an increasingly utilized tool in studies of trophic ecology in aquatic and terrestrial ecosystems. It has been proved to be a useful technique in delineating spatial and temporal variability in diets, identifying the consumption of key species, and providing quantitative estimates of diet composition (Iverson et al. Citation2007; Williams & Buck Citation2010; Twining et al. Citation2018).
High concentrations of PUFAs from the omega-3 group in tissues of consumers are related to high consumption of aquatic organisms, but omega-6 FAs are abundant in terrestrial food, e.g. in anthropogenic remains and in pollens and seeds of oily plants (rape, hemp, maize). Thus, the proportion of omega-3 to omega-6 FAs in an animal’s organism can indicate whether it fed on aquatic or terrestrial food (Laurich et al. Citation2019), and can even show if fish or grass seeds were the dominant food (Speake et al. Citation1999; Decrock et al. Citation2001; Surai et al. Citation2001). However, in omnivorous species, especially when the diet is supplemented with anthropogenic food (e.g. bread), it becomes difficult to assess the transfer of the FAs from food (Klasing Citation1998; Speake et al. Citation1999). The ratio of PUFA omega-6 to omega-3 is also considered to be an indicator of the proportion of anthropogenic food in the diet (Lopes et al. Citation2022), and of the diet-induced susceptibility to inflammation, because these FAs generally differ in their influence on inflammatory reactions and oxidative stress in animals (Larsson et al. Citation2004; Romieu et al. Citation2008; Isaksson Citation2015). For example, in mammals, a high omega-6:omega-3 ratio (above 3) induces not only an inflammatory reaction but also the production of pro-oxidants, and thus potentially increases oxidative stress in animals living in cities (Simopoulos Citation2002; Kiecolt-Glasera et al. Citation2013).
In this study we investigated FA composition in eggs of a colonially breeding omnivorous waterbird, the black-headed gull Chroicocephalus ridibundus, feeding mainly on earthworms, insects, fish, molluscs and crustaceans, plants, and anthropogenic food waste in urban areas (Creutz Citation1963; Bakke Citation1972, Vernon Citation1972; Hanssen Citation1982a, Citation1982b; Cuendet Citation1983; Kitowski et al. Citation2017; Indykiewicz P. – unpublished data). Females adopt a mixed strategy to gain nutrients for egg production. Some nutrients are endogenous reserves the females had acquired before breeding, whereas others are obtained near the breeding colony (Klaassen et al. Citation2004; Stephens et al. Citation2009). Given that in the studied area black-headed gulls usually spend a relatively long time (on average 3–4 weeks, up to 6 weeks) near their colonies before egg laying, we expect that the FAs accumulated in their eggs reflect mainly local food and environmental conditions (Kitowski et al. Citation2017).
We aimed to investigate factors affecting FA composition in eggs of black-headed gulls breeding in three colonies. We considered as factors location of the colony (reflecting the spatial variation in habitat composition and, in turn, in female diet at the time of egg formation), and the egg laying sequence (reflecting the temporal variation in female diet). We used qualitative analysis of FA signatures, which has been successfully employed to delineate spatial and temporal shifts in diets of seabirds (e.g. Wang et al. Citation2009). Egg yolk lipids are rich in triglycerides, and their FA composition reflects the diet of the female at the time of egg formation (Noble et al. Citation1996; Leskanich & Noble Citation1997).
Given the availability of various foraging grounds in the vicinity of the three studied colonies, we expect that exploration of different foraging grounds might affect the diet composition. For example, we expect a higher contribution of anthropogenic food (bread, garbage) in the diet of birds from the colony surrounded by most urban areas compared with the other colonies. Considering that one of the colonies is surrounded by more water bodies and wetlands than the others, we expected the highest contribution of aquatic food in the diet of birds in this colony. In the colony surrounded by more agricultural land we expect a considerable contribution of soil prey, like earthworms, in the diet. We expect these potential differences in food availability to be reflected in the FA composition of eggs.
Considering a relatively long period of egg formation and egg laying, which is on average 1.4–1.5 days (Indykiewicz Citation2001) or even 2 days (Cramp & Perrins Citation1983), we expect that any temporal variation in the female diet would be reflected in differences in composition of FAs between subsequent eggs. In a previous study (Kitowski et al. Citation2017), which partly included the same colonies, significant intra-clutch differences in concentrations of some elements in eggshells were found, suggesting a temporal variation in the accessibility of some food types.
Material and methods
Study area
We collected eggs of black-headed gulls in three breeding colonies in Northern Poland. They differed in the habitat structure of the potential foraging grounds (, ). The colony at Bydgoszcz (320 breeding pairs) is situated in an urban zone of Bydgoszcz City (350,000 inhabitants) on a small islet at the eutrophic Brda River (53°07.136′N, 18°06.318′E), which offers high availability of food close to the colony. The area around this colony was highly developed, with factories, service buildings and a railway line located nearby. The colony at Koronowo (130–150 breeding pairs) is situated on a small islet (53°20.069′N, 17°57.884′E) in the northern part of Koronowskie Lake (1560 ha), an artificial reservoir established in the late 1960s. The lake is used for recreation, and seasonal holiday houses are located near the island. It is situated 0.1 km from the dam of Koronowskie Lake, surrounded by green areas, and 2.5 km from the centre of the Koronowo town (12,000 inhabitants). Finally, the colony at Skoki Duże (800–1300 breeding pairs) is situated on two sandy islets overgrown with grass on a deep, oligotrophic artificial water body in a functioning gravel pit (52°36.399′N, 19°23.643′E). The colony is surrounded by agricultural areas and a small deciduous woodland ().
Figure 1. Location of the colony: KOR - Koronowo, BYD - Bydgoszcz, SKO - Skoki Duże. Main habitat types according to the corine land cover CLC2012 model, level 1; https://land.copernicus.eu/pan-european/corine-land-cover/clc-2012). Natura 2000 SPAs – boundaries of Special Protection areas under the EU Birds Directive.
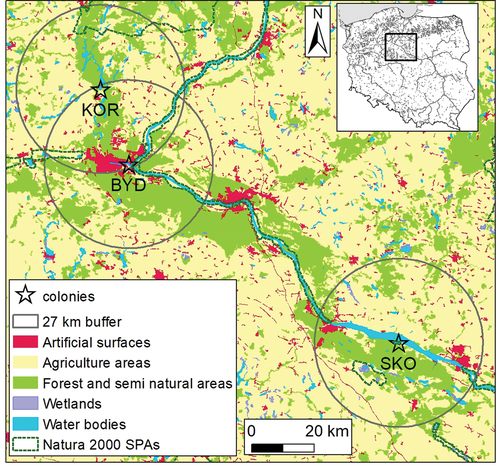
Table I. Habitat structure, i.e. relative abundance [%] of each habitat type (according to CORINE Land Cover CLC 2012 - Program monitoring land cover changes in 2006-2012 coordinated by the European Environment Agency (CORINE land cover CLC2012 model), level 1; source: https://land.copernicus.eu/pan-european/corine-land-cover/clc-2012) in buffers within a 27 km radius from each black-headed gull colony (this distance = the maximum distance of foraging flights of Global Positioning System (GPS) -tracked individuals from Bydgoszcz and Koronowo; Jakubas et al. Citation2020); chi-square statistical test of independence (χ2), level of statistical significance (p) – results of inter-colony comparison of particular habitat types (χ2 tests of independence performed on raw data from all three colonies in km2, df = 2).
To characterize potential foraging grounds of black-headed gulls breeding in particular colonies, we analysed habitat composition within buffers with a radius of 27 km around each colony (). This is the maximum flight distance from the colony recorded for GPS Global Positioning System (GPS)-tracked individuals breeding at Bydgoszcz and Koronowo (Jakubas et al. Citation2020). We determined main habitat types based on the level 1 data from CORINE Land Cover - Program coordinating natural information on land cover forms (CORINE) Land Cover (CLC) 2012, version 18_5. CLC uses a minimum mapping unit of 25 ha, and land cover classes are grouped in a three-level hierarchy with an ascending number of land cover classes from 5 to 44 (https://land.copernicus.eu/pan-european/corine-land-cover/clc-2012); we used level 1, which distinguishes five types of land cover. We extracted data from the CORINE model and produced all maps using ArcMap/ArcGIS 10.3.1 (Environmental Systems Research Institute).
We found that the habitat structure around the studied colonies differed significantly (). The vicinity of the colony at Skoki Duże was characterized by a significantly lower contribution of agricultural areas and a significantly larger proportion of water bodies and wetlands than the two other studied colonies. The area around the Bydgoszcz colony had the lowest proportion of agricultural areas and the greatest coverage by forest and semi-natural areas compared to the other colonies. Areas around the Bydgoszcz and Koronowo colonies were characterized by a relatively large proportion of artificial surfaces (buildings, roads, pavements) and smaller areas of water bodies and wetlands than in Skoki Duże ().
Fieldwork
We visited colonies between 7 April and 2 May 2015 (Julian days 97–122) and collected 24 eggs in the colony at Bydgoszcz, 24 at Koronowo and 27 at Skoki Duże. Eggs were collected from three-egg clutches, which predominated in the studied colonies, and constituted 74.9% of clutches at Bydgoszcz, 68.7% at Koronowo, and 83.2% at Skoki Duże in 2015. The mean clutch size standard deviation (±SD) was 2.86 ± 0.48 eggs/nest (N = 187) at Bydgoszcz, 2.76 ± 0.51 eggs/nest (N = 131) at Koronowo, and 2.97 ± 0.41 eggs/nest (N = 161) at Skoki Duże. We collected freshly laid eggs because some yolk FAs, such as arachidonic acid (20:4n-6), are preferentially incorporated into the developing embryo (Groscolas et al. Citation2003).
Laboratory analyses
After initial preparation and lyophilisation using a Martin Christ Alpha 1–4 LD Plusfreeze-dryer, we extracted lipids from the yolk. We obtained FA esters according to PN-EN ISO 12966-2:2011. We analysed the ester samples using an Agilent Technologies type 7890B gas chromatograph with an MSD 5977A detector and an HP-88 capillary column measuring 60 m × 0.25 mm × 0.25 µm. We set the initial temperature for the analysis to 70°C, the final temperature to 210°C for 20 min, the injector temperature to 250°C, split to 1:100, the transfer line temperature to 250°C, the helium flow rate to 1.2 mL/min, and the volume of the injected sample to 1 µL. From the results obtained we calculated the percentage content of FAs in the sample. Using an Agilent Technologies type 7890B gas chromatograph to identify FAs, we were not able to satisfactorily separate acids C20:3n3, C20:4n6 and C22:1n9 from one another, nor C22:0 and C20:3n6 from each other. Thus, we present them jointly in .
Table II. Fatty acid composition [%] in subsequent eggs (A – first, B – second, C – third) of black-headed gulls breeding in the studied colonies.
Table III. Sources of variability in fatty acid compositions in eggs of black-headed gulls breeding in the studied colonies (log-transformed) (AD = average dissimilarity, CO = relative contribution), according to a SIMPER analysis with Bray–Curtis similarity measure; only elements with a contribution of >5% are presented.
We investigated the relative abundance of the following FAs: myristic acid (C14:0), pentadecanoic acid (C15:0), palmitic acid (C16:0), palmitoleic acid (C16:1), margaric acid (C17:0), stearic acid (C18:0), oleic acid (C18:1n9), linoleic acid (C18:2n6) (LA), arachidic acid (C 20:0), a-linolenic acid (C18:3n3) (ALA), gadoleic acid (C20:1), icosadienoic acid (C20:2), eicosapentaenoic acid (C20:5n3) (EPA), docosahexaenoic acid (C22:6n3) (DHA) and two unseparated groups of FAs: the first being eicosatrienoic acid (C20:3n3), arachidonic acid (C20:4n6) and gadoleic acid (C22:1n9), and the second being docosanoic acid (C22:0) and gamma-linolenic acid (C20:3n6) (GLA). All the listed FAs summed up to 100%. A similar method of identifying FAs was applied by e.g. Surai and Speake (Citation2008), Serré et al. (Citation2022) and Lopes et al. (Citation2022).
Statistical analyses
To investigate whether FA composition in eggs was affected by colony and/or egg laying sequence, we performed the following multivariate statistics, on all FAs:
Multivariate two-way permutation multivariate analysis of variance (PERMANOVA) (non-parametric Multivariate (or Multiple) Analysis of Variance (MANOVA) based on the Bray–Curtis measure) (Anderson Citation2001) with fixed factors (colony and egg laying sequence) and their interaction as explanatory variables; to further investigate the effects of the statistically significant predictor, we used one-way PERMANOVA as a post hoc test;
The similarity percentage breakdown (SIMPER) procedure to assess the average percentage contribution of individual factors to the dissimilarity between objects (here, FAs) in a Bray–Curtis dissimilarity matrix (Clarke Citation1993).
Then, after finding the factor significantly affecting FA composition (here, the colony), we used a principal component analysis (PCA) to reduce the number of variables (here, FAs) into a few new factors, each representing a group of FAs with significantly correlated concentrations. Since the composition of all FAs was measured in the same units (%), we did the PCA on a variance–covariance matrix.
Finally, we also tested differences in particular FA levels (which were characterized by the highest dissimilarity in SIMPER) among the significant factor levels (here, colonies) using univariate tests (Kruskal–Wallis and post hoc Mann–Whitney U/Wilcoxon tests).
We performed all multivariate analyses on log (x + 1)-transformed data. We also compared the ratio of omega-3 (C18:3n3, C20:5n3, C22:6n3) to omega-6 (C18:2n6) FAs, which we used as an indicator of the relative contribution of aquatic vs terrestrial prey in the diet (Arts & Wainman Citation1999; Meyer et al. Citation2003), using Wilcoxon test.
We performed PERMANOVA, SIMPER, and PCA analyses using the PAST 3.0 software (Hammer et al. Citation2001). We expressed results as similarities in non-metric multidimensional scaling (nMDS) and Analysis of similarities is a non-parametric statistical test widely used in the field of ecology (ANOSIM) and as dissimilarities in SIMPER. We performed unimodal tests in the R software (R Core Team Citation2022) using the ggpubr package (Kassambara Citation2019).
Results
The composition of the most abundant FAs in egg yolk was generally similar across all sampled groups (). Saturated fatty acids constituted the greatest proportion among all FAs in the examined samples. Their median proportion in different breeding colonies was within 40.06–42.29% for palmitic acid (C16:0). For stearic acid (C18:0), this measure was within 10.37–13.07%. We found a relatively small proportion (0.67–0.74%) of myristic acid (C14:0). Oleic acid (C18:1n9), which represents MUFAs, occurred in a proportion of 24.87–26.50% in eggs of the studied gulls, being the second largest constituent after C16:0 ().
The main PUFAs in gull eggs appeared to be C18:3n-3 (ALA), C18:2n-6 (LA), C20:3n3, C20:4n6, C22:1n9, for which the median contents in eggs of gulls from the studied colonies were, respectively, 0.23–0.42%, 4.31–6.35%, 6.04–9.42%, 0.12–0.68% and 2.41–3.53%. C20:5n-3 (EPA) and 22:6n-3 (DHA) also occurred, but in smaller amounts. The contribution of C20:5n3 (EPA) ranged from 0.05% to 1.02% in all studied colonies ().
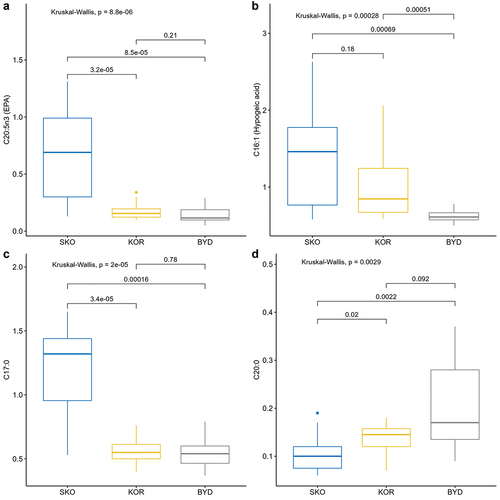
We found that the relative concentrations of all FAs combined were significantly affected only by the colony (multivariate two-way PERMANOVA, similarity measure: Bray–Curtis, F2,44 = 15.58, p = .0001). Other effects, such as the egg laying sequence, and interaction between the colony and the egg laying sequence, were not significant (p = .424 and .927, respectively). Concentrations of all FAs differed significantly among all studied colonies (p < .006). The SIMPER analysis showed that C20:5n3, C16:1, C17:0, C20:2, and C20:0 contributed the most (33%, 9.7%, 9.3%, 8.5% and 8.3%, respectively) to the pattern of inter-colony dissimilarity observed in FA composition (). This pattern was in general similar to the most pronounced differences in C20:5n3 contribution (20–36% of inter-colony dissimilarity). Additionally, dissimilarities in C20:0 composition greatly contributed to differences between the colonies at Koronowo and Bydgoszcz ().
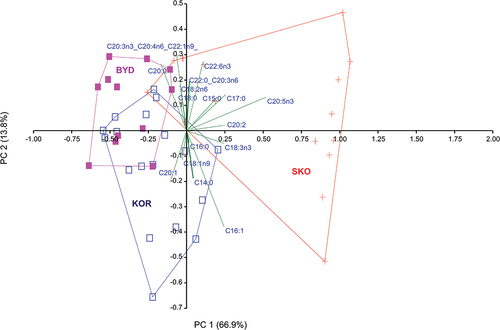
PCA of data on FA composition in various colonies revealed that the first axis, which explained 66.9% of the total variance, was correlated most with EPA C20:5n3 (r = 0.66). The second axis, which explained 13.8% of the total variability, was correlated with C16:1 (r = −0.48), and with C20:3n3_C20:4n6_C22:1n9 (r = 0.41) (). Most samples from the Skoki Duże colony clustered in a different position than those from the two other colonies. Convex hulls including samples from Koronowo and Bydgoszcz partly overlapped ().
Figure 2. Principal component analysis biplot of fatty acid composition in eggs of black-headed gulls breeding in the studied colonies. The analysis was based on log(x + 1)-transformed data for each fatty acid composition. Convex hulls include all samples from each colony: SKO – Skoki Duże (red), KOR – Koronowo (blue), BYD – Bydgoszcz (purple).
Univariate analyses of the FA composition characterized by the highest inter-colony dissimilarity in the SIMPER analysis revealed a significantly higher contribution of EPA (C20:5n3) in eggs from the colony at Skoki Duże compared to the colonies at Koronowo and Bydgoszcz. Values at Bydgoszcz and Koronowo were similar (). In the case of palmitoleic acid (C16:1), we found a significantly lower contribution at Bydgoszcz compared to two other colonies. The contribution of this FA was similar at Koronowo and Skoki Duże (). The proportion of C17:0 at Skoki Duże was significantly higher than at two other colonies. The signature of this FA was similar at Bydgoszcz and Koronowo (). The signature of C20:0 at Skoki Duże was significantly lower than at the other two colonies, but similar at Bydgoszcz and Koronowo ().
Figure 3. Composition of the fatty acids with the highest inter-colony dissimilarity in a SIMPER analyses: EPA – C20:5n3 (A), hypogeic acid – C16:1 (B), C17:0 (C), C20:0 (D). Colony abbreviations: SKO – Skoki Duże, KOR – Koronowo, BYD – Bydgoszcz. Box plots show the median (line inside the box), the first (25%) and the third (75%) quartiles (box), the lowest and the highest values within the 1.5 interquartile range (whiskers), and the outliers (dots). Values above lines indicate p values for the post hoc Wilcoxon test.
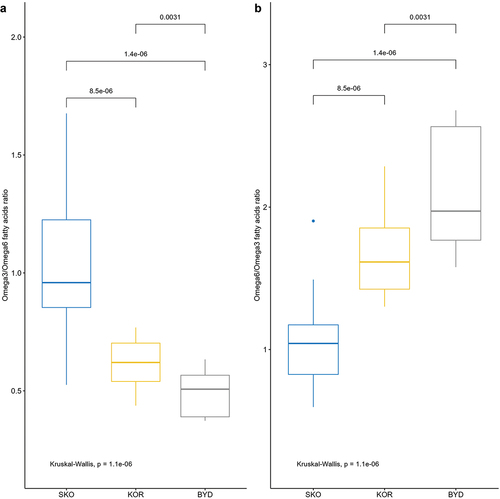
We found that the ratio of omega-6/omega-3 FAs differed significantly among eggs from the studied colonies, with the highest ratio at Skoki Duże and the lowest at Bydgoszcz ().
Figure 4. The ratio of: A – omega-3/omega-6, and B – omega-6/omega-3 fatty acids in eggs of black-headed gulls breeding in three colonies. Colony abbreviations: SKO – Skoki Duże, KOR – Koronowo, BYD – Bydgoszcz. Box plots show the median (line inside the box), the first (25%) and third (75%) quartiles (box), the lowest and the highest values within a 1.5 interquartile range (whiskers), and outliers (dots). Values above lines indicate p values for the post hoc Wilcoxon test.
Discussion
Our study revealed that FA composition in eggs of black-headed gulls differed between the colonies, reflecting the differing habitat composition of the foraging grounds in the vicinity of breeding colonies, and in turn their different diet composition. In contrast, the composition of FAs did not differ between subsequent eggs in each clutch, suggesting a similar female diet throughout the whole period of egg formation.
The qualitative content of FAs in eggs laid by black-headed gulls in the three studied colonies was generally close to the content recorded in other gull species (), including herring gulls Larus argentatus and lesser black-backed gulls Larus fuscus (Speake et al. Citation1996; Serré et al. Citation2022), and in other waterbirds, such as American white pelican Pelecanus erythrorhynchos, double-crested cormorant Phalacrocorax auritus, and also great skua Catharacta skua and northern gannet Morus bassanus (Surai et al. Citation2001).
Table IV. Mean concentration (mg/g) of fatty acids and the ratio of omega-3 to omega-6 in eggs of selected species of waterbirds which include fish in their diet. Data sources: Athis study, BSpeake et al. (Citation1996), CSerré et al. (Citation2022), DSurai et al. (Citation2001). Colony abbreviations: SKO – Skoki Duże, KOR – Koronowo, BYD – Bydgoszcz.
Our results showed, for the first time in a species of the family Laridae , no significant differences in the content of FAs between subsequent eggs laid by the female. This is an important result as this issue is poorly studied in wild birds and requires further research (Surai & Speake Citation2008). Such studies have been conducted so far only on a few bird species, mostly passerines, such as the great tit Parus major, blue tit Cyanistes caeruleus, house sparrow Passer domesticus and tree sparrow Passer montanus, and conclusions were inconsistent even for a single species. Bourgault et al. (Citation2007), Isaksson et al. (Citation2017) and Mentesana et al. (Citation2019) showed that in species that lay more than one egg in a clutch, the content of FAs changed with the sequence of egg laying. We consider that the main reason for a lack of differences in the FA content in subsequently laid eggs of black-headed gulls, as in the great tit (Toledo et al. Citation2016), are uniform environmental conditions that these birds experienced in each colony, mainly a relatively large stability of food supply during egg formation and laying by the females.
Moreover, the habitat structure around each colony that we studied most likely supported females with food that provided sufficient amounts of most (12 out of 16) of the identified FAs located in subsequently laid eggs (). Good examples are stearic acid (C18:0) and oleic acid (C18:1n9), which are supplied to birds mostly in seeds and leaves of grass and other plants (Surai & Speake Citation2008), and from earthworms (Sampedro et al. Citation2006). As grass forms the smallest part of the black-headed gull’s diet (Hanssen Citation1982a, Citation1982b; von Blotzheim Citation1999), the females in the studied colonies probably did not experience any difficulty obtaining sufficient amounts of food containing the mentioned FAs.
However, for some FAs, such as C16:1, C17:0, C20:0 and C20:5n3 (EPA), we found differences in their content in eggs laid in different colonies, which probably reflects inter-colony variation in the habitat structure of feeding areas, and thus the accessibility of certain types of food. An example is EPA (C20:5n3), which is abundant in earthworms (Sampedro et al. Citation2006), which usually predominate in the diet of black-headed gulls (Jirsik Citation1945; Creutz Citation1963; Vernon Citation1969; Hanssen Citation1982a, Citation1982b). But in eggs from Skoki Duże we noted a several times greater proportion of this acid than in eggs from the other two colonies, which suggests a considerable contribution of fish in the diet of birds from Skoki Duże. Three arguments support this assumption. Firstly, fish especially of the family Cyprinidae, which are the most abundant at the studied area (Kapusta Citation2022), are an exceptionally rich source of the long-chain polyunsaturated omega-3 acids, especially of EPA, but also of the palmitoleic acid C16:1n7 and of DHA C22:6n3 (Taşbozan et al. Citation2013). Secondly, in carp, palmitoleic acid occurs in greater concentrations than EPA, which we also found in the yolk of eggs laid in the colonies we studied () (Gunstone et al. Citation1978; Henderson & Tocher Citation1987; Cowey Citation1988, Kmínková et al. Citation2001; Sahena et al. Citation2009; Parzanini et al. Citation2020). Thirdly, in eggs laid by black-headed gulls at Skoki Duże, we found the greatest ratio of omega-3 to PUFA omega-6 (), which is an indication of the proportion of aquatic prey in the diet (Twining et al. Citation2016). This ratio corresponded with the gradient in the proportion of water bodies in areas around colonies, which was the largest at Skoki Duże and the smallest in Bydgoszcz (). Birds from the colony at Skoki Duże foraged on the local reservoir located within the Special Protection Area for Birds Natura 2000 – Żwirownia Skoki (PLB040005), at the Włocławek Reservoir in the Middle Vistula River (created after the construction of a dam in Włoclawek), and in several nearby lakes within SPA Natura 2000 – Błota Rakutowskie (PLB040001), within Gostynińsko-Włocławski Landscape Park with over 40 lakes, including the largest Rakutowskie Lake (301 ha) () where fish are readily available (Kakareko Citation2000; Kukuła Citation2003; Kakareko et al. Citation2009; Błażejewski et al. Citation2022; Kapusta Citation2022). A high contribution of fish in the diet of black-headed gulls from this colony has been confirmed by observations (PI – unpublished data) and by the ratio of omega-3 to omega-6 (mean 1.03) close to that in typical piscivores such as the northern gannet and the great skua (1.0 and 1.5, respectively, Surai et al. Citation2001). Fish also constituted the most important quantitative (20% of the mass of food) component of food harvested by black-headed gulls in colonies in eastern Germany (Creutz Citation1963), Denmark (identified in the stomachs of 14% individuals), and Norway (10–18% of pellets) (Hanssen Citation1982b). At the Wangerooge Island (East Frisian Islands), fish was identified in the food of 31% of studied individuals (Lorch et al. Citation1982).
The next most abundant FA in eggs of black-headed gulls from Skoki Duże was the heptadecanoic acid C17:0. But this result is difficult to interpret in the context of the diet because convincing evidence has shown that the level of this acid is not directly related to the consumed food; rather the endogenous synthesis of C17:0 affects its level in the organism (Jenkins et al. Citation2017).
Significantly higher content of the icosanoic acid C20:0 n6 in eggs of black-headed gulls from Bydgoszcz as compared to those from Skoki, located within the Natura 2000 area, can be interpreted in light of the fact that easily available anthropogenic food, including poultry, sausages, eggs, animal fat and red meat, is the main source of this acid for birds in urban areas (Tallima & El Ridi Citation2018; Li et al. Citation2022). Using anthropogenic waste from meat and fish processing factories, harbours, garbage dumps and sewage treatment plant by gulls has been reported by many authors (Felton Citation1969; Vernon Citation1969; Camphuysen Citation1993; Del Hoyo et al. Citation1996). Access to this type of food enables gulls to reduce time and energy expenditure on feeding (Hickling Citation1954; Hanssen Citation1982a, Citation1982b; Richards Citation1990; Bellebaum Citation2005; Maciusik et al. Citation2010).
The highest ratio of omega-6 to omega-3 in our study was found in the yolk of eggs of females from urban areas (Bydgoszcz: 2.12), and the lowest ratio was found in the colony located in the Special Protected Area Natura 2000 (Skoki Duże: 1.06), which can be the consequence of spatial differences in habitat structure and in the level of anthropogenic pressure. Similar differences in the proportion of FAs (omega-6/omega-3) between birds from urban and suburban areas were found in other gulls, i.e. in yellow-legged gulls Larus michahellis and lesser black-backed gulls Larus fuscus (Pais de Faria et al. Citation2021; Lopes et al. Citation2022), as well as in passerines such as great tit and blue tit (Isaksson et al. Citation2017). High levels of omega-6:omega-3 may have negative consequences for chicks, leading to their increased susceptibility to antigens, through promoting a state of inflammation (Calder et al. Citation2007, Citation2009; Romieu et al. Citation2008; Cherian et al. Citation2009). Moreover, the described differences can also be caused by the differences in FA content between anthropogenic and natural food sources, which has been reported earlier (e.g. by CitationAndersson et al. Citation2015).
To conclude, we found that the fatty acid composition in eggs of black-headed gulls differed among three colonies, which reflected the varying habitat composition of foraging grounds in the vicinity of the breeding colonies, and in turn different diet composition, in terms of the contribution of fish prey. We have demonstrated the potential of FA signatures as a technique providing valuable insight into the diet of female birds during egg formation. However, analyses based solely on FA signatures of consumer tissues cannot give precise information about ingested food. Studies including FA signatures of main food items are required to reconstruct the diet composition of the consumer in more detail.
Acknowledgements
The study was performed with permissions from the Regional Directorate of Environmental Protection in Bydgoszcz (WPN.6401.1.257.2015.MO from 15 December 2015).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Australian Ecology 26:32–46. DOI: 10.1111/j.1442-9993.2001.01070.pp.x.
- Andersson MN, Wang HL, Nord A, Salmon P, Isaksson C. 2015. Composition of physiologically important fatty acids in great tits differs between urban and rural populations on a seasonal basis. Frontiers in Ecology and Evolution 3:93. DOI: 10.3389/fevo.2015.00093.
- Arts MT, Wainman BC. 1999. Lipids in freshwater ecosystems. New York: Springer Science and Business Media. p. 319. DOI: 10.1007/978-1-4612-0547-0.
- Bakke TA. 1972. Food of the Common gull, Larus canus L., and the black-headed gull, Larus ridibundus L., at Sola airport, Rogaland County [in Norwegian]. Fauna 25:197–204.
- Bellebaum J. 2005. Between the herring gull Larus argentatus and the bulldozer: Black-headed gull Larus ridibundus feeding sites on a refuse dump. Ornis Fennica 82:166–171.
- Błażejewski M, Król J, Kakareko T, Mierzejewska K, Hliwa P. 2022. Daily and seasonal dynamics of littoral zone fish communities in the lowland Włocławek reservoir (central Poland), with a special emphasis on alien invasive gobies. Journal of Limnology 81:2059. DOI: 10.4081/jlimnol.2022.2059.
- Bourgault P, Thomas DW, Blondel J, Perret P, Lambrechts MM. 2007. Between-population differences in egg composition in blue Tits (Cyanistes caeruleus). Canadian Journal Zoology 85:71–80. DOI: 10.1139/z06-189.
- Calder PC. 2007. Immunomodulation by omega-3 fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids 77(5–6):327–335. DOI: 10.1016/j.plefa.2007.10.015.
- Calder PC. 2009. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 91(6):791–795. DOI: 10.1016/j.biochi.2009.01.008.
- Camphuysen J. 1993. Scavenging seabirds behind fishing vessels in the Northeast Atlantic. With emphasis on the Southern North Sea. Nederlands Instituut voor Onderzoek der Zee 1–80.
- Cherian G, Bautista-Ortega J, Goeger DE. 2009. Maternal dietary n-3 fatty acids alter cardiac ventricle fatty acid composition, prostaglandin and thromboxane production in growing chicks. Prostaglandins, Leukotrienes and Essential Fatty Acids 80(5–6):297–303. DOI: 10.1016/j.plefa.2009.02.006.
- Clarke KR. 1993. Non-parametric multivariate analysis of changes in community structure. Australian Journal of Ecology 18:117–143. DOI: 10.1111/j.1442-9993.1993.tb00438.x.
- Cowey CB. 1988. The nutrition of fish: The developing scene. Nutrition Research Reviews 1(1):255–280. DOI: 10.1079/NRR19880017.
- Cramp S, Perrins CM. 1983. Handbook of the birds of Europe, the middle East and North Africa: The birds of the Western Palearctic. Volume 3 - Waders to gulls. Oxford: Oxford University Press.
- Creutz G. 1963. Ernahrungsweise und Aktionsradius der Lachmowe (Larus ridibundus L.). Beitrage zur Vögelkde 9:3–58.
- Cuendet G. 1983. Earthworm Ecology. London: From Darwin to Vermiculture Chapman and Hall. pp. 415–424. DOI: 10.1007/978-94-009-5965-1_36.
- Dannenberger D, Nuernberg G, Nuernberg K, Hagemann E. 2013. The effects of gender, age and region on macro-and micronutrient contents and fatty acid profiles in the muscles of roe deer and wild boar in Mecklenburg-Western Pomerania (Germany). Meat Science 94:39–46. DOI: 10.1016/j.meatsci.2012.12.010.
- Decrock F, Groscolas R, McCartney RJ, Speake BK. 2001. Transfer of n-3 and n-6 polyunsaturated from yolk to embryo during development of the king penguin. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 280:R843–R853. DOI: 10.1152/ajpregu.2001.280.3.R843.
- Del Hoyo J, Elliot A, Sargatal J. 1996. Handbook of the birds of the World. Vol. 3. Barcelona, Spain: Hoatzin to Auks. Lynx Edicions.
- Desbois AP, Smith VJ. 2010. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Applied Microbiology Biotechnolgy 85:1629–1664. DOI: 10.1007/s00253-009-2355-3.
- Felton C. 1969. Black-headed gulls following boats at night. British Birds 62(3):117.
- Ferronato G, Prandini A. 2020. Dietary supplementation of inorganic, organic, and fatty acids in pig: A review. Animals 10(10):1740. DOI: 10.3390/ani10101740.
- Filimonova V, Goncalves F, Marques JC, De Troch M, Goncalves AM. 2016. Fatty acid profiling as bioindicator of chemical stress in marine organisms: A review. Ecological Indicators 67:657–672. DOI: 10.1016/j.ecolind.2016.03.044.
- Groscolas R, Fréchard F, Decrock F, Speake BK. 2003. Metabolic fate of yolk fatty acids in the developing king penguin embryo. American Journal Physiology: Regulatory, Integrative and Comparative Physiology 285:R850–R861. DOI: 10.1152/ajpregu.00105.2003.
- Gunstone FD, Wijesundera RC, Scrimgeour CM. 1978. The component acids of lipids from marine and freshwater species with special reference to furan-containing acids. Journal of the Science Food Agriculture 29:539–550. DOI: 10.1002/jsfa.2740290608.
- Hammer O, Harper DAT, Ryan PD. 2001. PAST paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9.
- Hanssen OJ. 1982a. Komparativ naeringsokologi hos hettemåke Larus ridibundus L. og fiskemåke L. canus L. i skjaergården sor for Fredrikstad, Ostfold [in Norwegian]. Thesis, Zoological Museum, University of Oslo, Norway.
- Hanssen OJ. 1982b. Feeding ecology of black-headed and Common gull in SE Norway [in Norwegian]. Fauna 35:154–161.
- Henderson RJ, Tocher DR. 1987. The lipid composition and biochemistry of freshwater fish. Progress in Lipid Research 26(4):281–347. DOI: 10.1016/0163-7827(87)90002-6.
- Hickling RAO. 1954. The wintering of gulls in Britain. Bird Study 1(4):129–148. DOI: 10.1080/00063655409475800.
- Indykiewicz P. 2001. Gulls and terns. Ecology of the breeding colony of Larus ridibundus and Common tern Sterna hirundo in Myślęcinek [in Polish]. Pub. NICE, Bydgoszcz.
- Isaksson C. 2015. Urbanisation, oxidative stress and inflammation: A question of evolving, acclimatizing or coping with urban environmental stress. Functional Ecology 29:913–923. DOI: 10.1111/1365-2435.12477.
- Isaksson C, Andersson MN, Nord A, von Post M, Wang HL. 2017. Species-Dependent effects of the urban environment on fatty acid composition and oxidative stress in birds. Frontiers in Ecology and Evolution 5:44. DOI: 10.3389/fevo.2017.00044.
- Iverson SJ, Springer AM, Kitaysky AS. 2007. Seabirds as indicators of food web structure and ecosystem variability: Qualitative and quantitative diet analyses using fatty acids. Marine Ecology Progress Series 352:235–244. DOI: 10.3354/meps07073.
- Jakubas D, Indykiewicz P, Minias P, Kowalski J, Ciek T. 2020. Inter-colony variation in flight characteristics of black-headed gulls Chroicocephalus ridibundus during the incubating period. Ecology and Evolution 10:5489–5505. DOI: 10.1002/ece3.6291.
- Jenkins B, de Schryver E, van Veldhoven PP, Koulman A. 2017. Peroxisomal 2-hydroxyacyl-CoA lyase is involved in endogenous biosynthesis of heptadecanoic acid. Molecules 22:1718. DOI: 10.3390/molecules22101718.
- Jirsik SC. 1945. The importance of the black headed gull (Larus ridibundus L.) in the economy of agriculture and fisheries. Acta Societatis Scientarum Naturalium Moraviae 17:28.
- Kakareko T. 2000. The ichtiofauna of lower Vistula River: The state art and the program of research. In Hydrobiology of the Lover Vistula River between Wyszogród and Toruń. Acta Universitatis Nicolai Copernici, Limnological Papers 21:85–90.
- Kakareko T, Płąchocki D, Kobak J. 2009. Relative abundance of Ponto-Caspian gobiids in the lower Vistula River (Poland) 3-4 years after their appearance. Journal of Applied Ichtyology 25:647–651. DOI: 10.1111/j.1439-0426.2009.01301.x.
- Kapusta A. 2022. The use of ichthyofauna monitoring for fish stock assessment and fishery management [in Polish]. Inst. Ryb. Śródlądowy in Olsztyn. https://wmodr.pl/files/i5B3JTLQ6IMoG9YeOouc0mU2dkvOJIFRB8sZrwgK.pdf Accessed Dec 2022 10.
- Kassambara A. 2019. Ggpubr R package: Ggplot2-based publication Ready Plots. https://rpkgs.datanovia.com/ggpubr/.
- Kiecolt-Glasera JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, Malarkey WB, Hwang BS, Blackburn E, et al. 2013. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behavior and Immunity 28:16–24. DOI: 10.1016/j.bbi.2012.09.004.
- Kitowski I, Indykiewicz P, Wiącek D, Jakubas D. 2017. Intra-clutch and inter-colony variability in concentrations of elements in eggshells of the black-headed gull Chroicocephalus ridibundus in N Poland. Environmental Science and Pollution Research 24(11):10341–10353. DOI: 10.1007/s11356-017-8635-z.
- Klaassen M, Baarspul T, Dekkers T, van Tienen P. 2004. The relationship between carbon stable isotope ratios of hatchling down and egg yolk in black-headed gulls. Journal of Field Ornithology 75(2):196–199. DOI: 10.1648/0273-8570-75.2.196.
- Klasing KC. 1998. Comparative avian nutrition. Wallingford, UK: CAB International. DOI: 10.1079/9780851992198.0000.
- Kmínková M, Winterová R, Uéera J. 2001. Fatty acids in lipids of carp (Cyprinus carpio) tissues. Czech Journal Food Science 19:177–181. DOI: 10.17221/6604-CJFS.
- Kukuła K. 2003. Structural changes in the ichthyofauna of Carpathian tributaries of the River Vistula caused by anthropogenic factors. Acta Hydrobiologica 4(Suppl.):1–63.
- Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. 2004. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. The American Journal of Clinical Nutrition 79:935–945. DOI: 10.1093/ajcn/79.6.935.
- Laurich B, Drake C, Gorman OT, Irvine C, MacLaurin J, Chartrand C, Hebert CE, et al. 2019. Ecosystem change and population declines in gulls: Shifting baseline considerations for assessing ecological integrity of protected areas. Journal of Great Lakes Research 45(6):1215–1227. DOI: 10.1016/j.jglr.2019.08.009.
- Lee MC, Park JC, Lee JS. 2018. Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquatic Toxicology 200:83–92. DOI: 10.1016/j.aquatox.2018.04.016.
- Lei A, Chen H, Shen G, Hu Z, Chen L, Wang J, et al. 2012. Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnology for Biofuels 5(1):1–11. DOI: 10.1186/1754-6834-5-18.
- Leskanich RO, Noble RC. 1997. Manipulation of the n-3 polyunsaturated fatty acid composition of avian eggs and meat. World`s Poultry Science Journal 53:155–183. DOI: 10.1079/WPS19970015.
- Li Z, Hong T, Zhao Z, Gu Y Guo Y, Han J. 2022. Fatty acid profiles and nutritional evaluation of fresh sweet-waxy corn from three regions of China. Foods 11:2636. DOI:10.3390/foods11172636.
- Lohner S, Fekete K, Marosvölgyi T, Decsi T. 2013. Gender differences in the long-chain polyunsaturated fatty acid status: Systematic review of 51 publications. Annals of Nutrition and Metabolism 62(2):98–112. DOI: 10.1159/000345599.
- Lopes CS, Antunes RCC, Paiva VH, Gonçalves AMM, Correia JJ, Ramos JA, et al. 2022. Fatty acids composition in yellow-legged (Larus michahellis) and lesser black-backed (Larus fuscus) gulls from natural and urban habitats in relation to the ingestion of anthropogenic materials. Science of the Total Environment 809:15109. DOI: 10.1016/j.scitotenv.2021.151093.
- Lorch HJ, Schneider R, Loos-Frank B. 1982. Parasitologische Untersuchungen nestiunger Lachmöwen (Larus ridibundus) in Brutkolonien des Binnenlandes and der Kiiste. Journal für Ornithologie 123:29–39. DOI: 10.1007/BF01644147.
- Maciusik B, Lenda M, Skórka P. 2010. Corridors, local food resources, and climatic conditions affect the utilization of the urban environment by the black-headed gull Larus ridibundus in winter. Ecological Research 25(2):263–272. DOI: 10.1007/s11284-009-0649-7.
- Mentesana L, Isaksson C, Goymann W, Andersson MN, Trappschuh M, Hau M. 2019. Female variation in allocation of steroid hormones, antioxidants and fatty acids: A multilevel analysis in a wild passerine bird. Journal of Avian Biology 50(2019):1. DOI:10.1111/jav.01859.
- Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe Peter RC, et al. 2003. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 38(4):391–398. DOI: 10.1007/s11745-003-1074-0.
- Noble RC, Speake BK, McCartney R, Foggin CM, Deeming DC. 1996. Yolk lipids and their fatty acids in the wild and captive ostrich (Struthio camelus). Comparative Biochemistry and Physiology 113(4):753–756. DOI: 10.1016/0305-0491(95)02097-7.
- Pais de Faria J, Vaz PT, Lopes CS, Calado JG, Pereira JM, Veríssimo SN, Paiva VH, Gonçalves AM, Ramos JA. 2021. The importance of marine resources in the diet of urban gulls. Marine Ecology Progress Series 660:189–201. DOI: 10.3354/meps13599.
- Parzanini C, Colombo SM, Kainz MJ, Wacker A, Parrish CC, Arts MT. 2020. Discrimination between freshwater and marine fish using fatty acids: Ecological implications and future perspectives. Environmental Reviews 28:546–559. DOI: 10.1139/er-2020-0031.
- Pedrazzoli M, Dal Bosco A, Castellini C, Ranucci D, Mattioli S, Pauselli M, Roscini V, et al. 2017. Effect of age and feeding area on meat quality of wild boars. Italian Journal of Animal Science 16(3):353–362. DOI: 10.1080/1828051X.2017.1292114.
- Piaskowska N, Daszkiewicz T, Kubiak D, Janiszewski P. 2015. The effect of gender on meat (Longissimus lumborum muscle) quality characteristics in the Fallow Deer Dama dama L. Italian Journal of Animal Science 14:3845. DOI: 10.4081/ijas.2015.3845.
- R Core Team. 2022. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/.
- Richards A. 1990. Seabirds of the northern hemisphere. Limpsfield, UK: Dragon’s World Ltd.
- Romieu I, Castro-Giner F, Kunzli N, Sunyer J. 2008. Air pollution, oxidative stress and dietary supplementation: A review. The European Respiratory Journal 31:179–196. DOI: 10.1183/09031936.00128106.
- Sahena F, Zaidul ISM, Jinap S, Saari N, Jahurul HA, Abbas KA, Norulaini NA. 2009. Pufas in fish: Extraction, fractionation, importance in health. Comprehensive Reviews in Food Science and Food Safety 8(2):59–74. DOI: 10.1111/j.1541-4337.2009.00069.x.
- Sampedro L, Jeannotte R, Whalen JK. 2006. Trophic transfer of fatty acids from gut microbiota to the earthworm Lumbricus terrestris L. Soil Biology and Biochemistry 38(8):2188–2198. DOI: 10.1016/j.soilbio.2006.02.001.
- Serré S, Irvine C, Williams K, Hebert CE. 2022. Lake Superior herring gulls benefit from anthropogenic food subsidies in a prey–impoverished aquatic environment. Journal of Great Lakes Research 48(5):1258–1269. DOI: 10.1016/j.jglr.2022.08.008.
- Simopoulos AP. 2002. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine and Pharmacotherapy 56(8):365–379. DOI: 10.1016/s0753-3322(02)00253-6.
- Speake BK, McCartney RJ, Feast M, Maldjian A, Noble RC. 1996. The relationship between the fatty acid profiles of the yolk and the embryonic tissue lipids: A comparison between the lesser black-backed gull (Larus fuscus) and the pheasant (Phasianus colchicus). Comparative Biochemistry and Physiology 115B(4):493–499. DOI: 10.1016/S0305-0491(96)00188-5.
- Stephens PA, Boyd IL, McNamara JM, Houston AI. 2009. Capital breeding and income breeding: Their meaning, measurement, and worth. Ecology 90(8):2057–2067. DOI: 10.1890/08-1369.1.
- Surai PF, Bortolotti GR, Fidgett AL, Blount JD, Speake BK. 2001. Effects of piscivory on the fatty acid profiles and antioxidants of avian yolk: Studies on eggs of the gannet, skua, pelican and cormorant. Journal of Zoology 255(3):305–312. DOI: 10.1017/S0952836901001406.
- Surai PF, Speake BK. 2008. The natural fatty acid compositions of eggs of wild birds and the consequences of domestication. In: De Meester F, Watson R, editors. Wild-type food in health promotion and disease prevention. pp. 121–137.
- Tallima H, El Ridi R. 2018. Arachidonic acid: Physiological roles and potential health benefits – a review. Journal of Advanced Research 11:33–41. DOI: 10.1016/j.jare.2017.11.004.
- Taşbozan O, Gökçe MA, Çelík M, Tabakoglu SS, Küçükgülmez A, Başusta A. 2013. Nutritional composition of Spiny Eel (Mastacembelus mastacembelus) caught from the Aattürk dam Lake in Turkey. Journal of Applied Biological Sciences 7(2):78–82.
- Toledo A, Andersson MN, Wang HL, Salmón P, Watson H, Burdge GC, Isaksson C, et al. 2016. Fatty acid profiles of great tit (Parus major) eggs differ between urban and rural habitats, but not between coniferous and deciduous forests. The Science of Nature 103(7–8):55. DOI: 10.1007/s00114-016-1381-0.
- Twining CW, Brennab JT, Lawrenceb P, Shipleya JR, Tollefsonc TN, Winklera DW. 2016. Omega-3 long-chain polyunsaturated fatty acids support aerial insectivore performance more than food quantity. Proceedings of the National Academy of Sciences USA 113(39):10920–10925. DOI: 10.1073/pnas.1603998113.
- Twining CW, Shipley JR, Winkler DW, Jeyasingh P. 2018. Aquatic insects rich in omega‐3 fatty acids drive breeding success in a widespread bird. Ecology Letters 21(12):1812–1820. DOI: 10.1111/ele.13156.
- Vernon JDR. 1969. Black-headed gulls taking olives. British Birds 62:43.
- Vernon JDR. 1972. Feeding habitats and food of the black-headed and common gulls. Part 2-food. Bird Study 19(4):173–186. DOI:10.1080/00063657209476341.
- von Blotzheim UNG. 1999. Handbuch der Vögel Mitteleuropas. Band 8. Charadriiformes. Teil 3. Wiesbaden: Aula-Verlag.
- Wang SW, Iverson SJ, Springer AM, Hatch SA. 2009. Spatial and temporal diet segregation in northern fulmars (Fulmarus glacialis) breeding in Alaska: Insights from fatty acid signatures. Marine Ecology Progress Series 377:299–307. DOI: 10.3354/meps07863.
- Williams CT, Buck CL. 2010. Using fatty acids as dietary tracers in seabird trophic ecology: Theory, application and limitations. Journal of Ornithology 151(3):531–554. DOI: 10.1007/s10336-010-0513-0.
