 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A specimen of female tub gurnard (Chelidonichthys lucerna) caught as bycatch in commercial saithe, Pollachius virens, fisheries near the Shetland Islands was identified as a new definitive host for the cestode Bothriocephalus scorpii (Bothriocephalidea) sensu stricto. It was also the first Cestoda species recorded in tub gurnard. However, as shown in our study, the identification of complex species such as Bothriocephalus scorpii based only on external characteristics had to be supplemented with molecular marker analysis to determine the taxonomic status of the specimen. Therefore, to successfully identify the parasite specimen, DNA was extracted from it, and a partial sequence of lsrDNA was amplified and the PCR product was subjected to 2 × 300 bp PE sequencing with 10,000× coverage on MiSeq. A BLAST search using the 346 bp sequence showed 100% similarity to the lsrDNA sequence of B. scorpii deposited in GenBank under accession number AF286942. Relatedness between the sequence of B. scorpii found in our study and the 20 most similar records from GenBank was identified using a phylogenetic tree constructed under maximum likelihood. Our study proves that next-generation sequencing of partial lsrDNA supplemented the morphological identification of the parasite specimen and can be applied in future parasitological studies.
Tub gurnard (Chelidonichthys lucerna) is a nectobenthic fish distributed widely in the eastern Atlantic, the Mediterranean, and the Black Sea. It also occurs sporadically from the western to the southeastern Baltic Sea (Lithuania) (Dainys et al. Citation2017). The status of the species in the HELCOM area is data deficient (Więcaszek et al. Citation2011), and data on parasite fauna of C. lucerna are scarce (Suppl. Table S1) and include only 20 taxa, mainly copepods. Therefore, the full host spectrum of many cestode taxa (mainly marine) and their actual host specificity has yet to be fully described because of the insufficiently identified taxonomical status in many cases (Kuchta & Scholz Citation2007).
Bothriocephalus scorpii is a parasite of fish species that inhabit the northern Pacific and Atlantic oceans (Kuchta et al. Citation2008) and the Baltic and Mediterranean seas (Markowski Citation1935; Davey & Peachey Citation1968; De Groot Citation1971; Renaud et al. Citation1984; Sulgostowska et al. Citation1998; Škeříková et al. Citation2004). B. scorpii is regarded as a complex species (Blend & Dronen Citation2003), and to date adult form has been recorded in the intestine of more than 50 genera and almost 100 nominal species of marine fish from different, distantly related families and orders (Renaud et al. Citation1984; Kuchta et al. Citation2008; Polyakova Citation2020). They include, inter alia, brill (Scophthalmus rhombus) and turbot (Scophthalmus maximus) (Davey & Peachey Citation1968; De Groot Citation1971; Sulgostowska et al. Citation1998; Skrzypczak & Rolbiecki Citation2015); round goby (Neogobius melanostomus) (Rokicki & Rolbiecki Citation2002); red cod (Pseudophycis bacchus) (McKinnon & Featherston Citation1982), longspined bullhead (Taurulus bubalis; referred to as Cottus bubalis); and striped sea snail (Liparis liparis) (Jones Citation1975). The aim of the present paper was to supplement knowledge about tub gurnard endoparasitic fauna and to identify species of the parasite considered sibling species using Next-Generation Sequencing (NGS) on a low-quality sample.
A female tub gurnard specimen was caught as bycatch during a commercial catch of saithe (Pollachius virens L., 1758) near the Shetland Islands (60°24’N and 02°41’E) at a depth of 128–132 m with a bottom trawl on March 9, 2018. The specimen measured 49.10 cm total length (TL). Body wet weight (W) was 1,210.9 g, gonad weight was 27.0 g, and liver weight was 36.4 g. Fulton’s condition factor (K) and the hepatosomatic factor (HSI) were calculated using the following equations:
Next, prey items were collected from the stomach and identified based on species-related characters of sagittal otoliths (Härkönen Citation1986) using a Nikon SMZ 1000 microscope coupled with ToupViev 3.7 image analysis software (Nikon, Tokyo, Japan). Parasitological examination of the gills, mouth, internal organs (oesophagus, stomach, intestine, pyloric caeca, liver, heart, spleen, gallbladder, swim-bladder, and gonads), and the muscles revealed the presence of 46 cestode specimens in the intestine alone. All parasite specimens were preserved in bulk in 70% EtOH. Subsequently, single individuals (n = 10) were placed on glass slides, covered with glycerine, stained with carmine alum, and fixed in Canadian balm (Pojmańska & Cielecka Citation2001). Morphological characters of all the specimens were assessed using an Olympus B× 50 microscope with a camera and AxioVs40 V 4.8.2.0 software (Olympus, Tokyo, Japan).
To perform molecular identification of the specimens collected, a mix (n = 10) of parasite specimens were air dried under ambient conditions for 10 min. DNA extraction and subsequent quality and quantity assessment were performed according to the methodology published in Piasecki et al. (Citation2022). Due to the low DNA concentration (<5 ng/μl) and poor sample quality (signs of degradation were apparent on agarose gel), several primer pairs in different configurations and extensive optimization (e.g., adjusted PCR conditions, reamplification, nested PCR, and semi-nested PCR) failed to amplify the COI, lsrDNA, or ssrDNA regions (Suppl. Table S2) on both individual specimens and a pooled sample of 10 individuals. Amplification was partially successful only with the ZX-1 and 1500 R primers for lsrDNA under conditions recommended by Caira et al. (Citation2014). Sanger sequencing revealed only partial lsrDNA sequences (approximately 450 bp of an expected 1,100 bp) because of background noise (multiple reads), and these sequences were not used for specimen identification. Therefore, the NGS approach was used to confirm the parasite species. Based on the partial sequence of lsrDNA, an additional ZX-1-R primer was designed using Geneious 11.1.5 (Kearse et al. Citation2012), and primers ngs-ZX-1F and ngs-ZX-1-R were synthesized with overhangs (Suppl. Table S2). The 346 bp PCR product was amplified under the same conditions, and a library was created using NextEra XT DNA Library Preparation Kit (Illumina, San Diego, CA, USA) following the manufacturer’s instructions. The product was then subjected to 2 × 300 bp PE sequencing with 10,000× coverage on MiSeq (Illumina, San Diego, CA, USA) using a MiSeq Reagent Kit v3 (600-cycle) (Illumina, San Diego, CA, USA). Cutadapt v3.0 (Martin Citation2011) was used to remove adaptor sequences and to filter low quality (q > 25) sequences, and the reads were aligned using the fastq-join algorithm. Clustering was performed using the QIIME (Caporaso et al. Citation2010) and UCLUST algorithms (100% sequence identity) (Edgar Citation2010). After the filtering procedure, the most abundant cluster (63788 identical sequences) was used to generate the resulting sequence, which was compared against BLAST, and the first 20 records were downloaded. Next, the sequences were aligned to calculate percentage similarity among sequences, and a phylogenetic tree was constructed under the maximum likelihood (ML) criterion (General-Time-Reversible, GTR model, 10000 bootstrap) using the PhyML 3.0 plugin in Geneious 11.1.5 5 (Guindon et al. Citation2010; Kearse et al. Citation2012). The resulting ML tree was visualized using Interactive Tree of Life v3 (Letunic & Bork Citation2016).
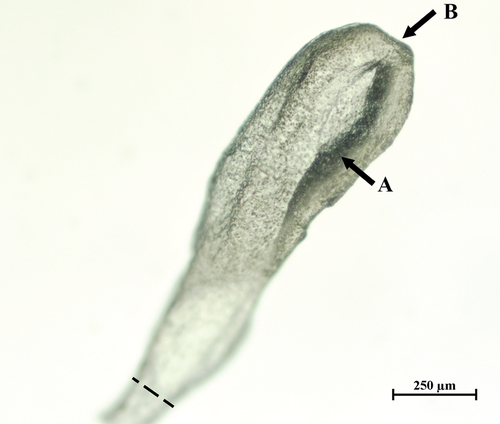
The parasites found in the intestine of the tub gurnard were initially identified as adult forms of Bothriocephalus scorpii (Müller 1776) Cooper, Citation1917 (Bothriocephalidea) based on species-specific descriptions published in Yamaguti (Citation1959), Jones (Citation1975), Bray et al. (Citation1994) and Pojmańska and Cielecka (Citation2001). The parasite was mature and had complete external strobilisation, and individuals were up to 950 mm in length. All the following corresponded to the species descriptions: the shape of the proglotides; the scolex with an apical disc and shallow bothria; the presence of two sets of reproductive organs per proglottid; the number (32–60 per one set) and position of the testes, which form two lateral bands that continue along the strobila, a pear-shaped cirrus sac, the vas deferens forms numerous loops, with the right or left-cirrus sac, lack of seminal receptacle, uterine duct in loops strongly developed; the position and kidney-shaped ovary cortical vitelline follicles (). Apart from a slight reddening of the intestinal mucosa, no harmful effects of parasites on the host were identified. Surprisingly, the presence of 46 parasite specimens in the digestive tract of the fish examined did not affect its condition status. Fulton’s condition factor K (1.02) and HSI (3.01) values were markedly higher than those in a population studied in northeast Portugal (Rodrigues et al. Citation2019) and slightly higher than values reported in studies conducted on specimens from the North Sea (Mahé et al. Citation2018).
Figure 1. Scolex of Bothriocephalus scorpii specimen found in a tub gurnard. Explanations:
The distribution of tub gurnard overlaps with the distribution of B. scorpii and its intermediate hosts. To date, three intermediate hosts have been described in the life cycle of B. scorpii. Markowski (Citation1935) and Marcogliese (Citation1995), after Protasova (Citation1977), reported that Eurytemora affinis affinis (Poppe 1880) (Copepoda, Calanoida) is the first intermediate host of B. scorpii. However, the development of the genus Eurytemora is limited to brackish habitats, while Solonchenko (Citation1985) reports Acartia (Acartiura) clausi Giesbrecht, 1889; therefore, other marine calanoids can probably also serve as intermediate hosts of this cestode, which suggests its wide distribution and a greater number of definitive hosts than that documented to date. The second intermediate hosts of B. scorpii (classified based on its morphology) are fish of the genera Nerophis, Pomatoschistus, Gasterosteus, and Pungitius (Protasova Citation1977) and many others including those of the genus Gadus (Yamaguti Citation1959). Tub gurnard are opportunistic predators and their feeding habits depend on size. Small fish typically prey on crustaceans (Aphipoda, Decapoda), while fish (max. length of 18 cm) predominate in stomach of larger individuals (Stagioni et al. Citation2012). The stomach content analysis of tub gurnard revealed skeleton of gadoid fish (7 cm of length) and two sagittal otoliths, which were identified as remains of poor cod (Trisopterus minutus L., 1758). Bothriocephalus scorpii was previously noted in poor cod, although it was described as a first or second intermediate host (Blend & Dronen Citation2003). Therefore, B. scorpii could get into the stomach of tub gurnard with its prey. However, we found adult forms of B. scorpii in the intestine of tub gurnard.
According to previous knowledge, B. scorpii sensu stricto only infects (as the definitive host) Myoxocephalus scorpius (L., 1758) from the North Sea (Verneau et al. Citation1997; Škeříková et al. Citation2004; Waeschenbach et al. Citation2007). Recently, Scorpaena porcus L., 1758 from the Black Sea was included in the group of definitive hosts (Polyakova Citation2020). Chelidonichthys lucerna is the third fish species recognized as a member of this group. All the species mentioned are phylogenetically related since they belong to the order Scorpaeniformes (currently included in Perciformes) (Froese & Pauly Citation2021). The standard molecular approach to parasite systematics (Caira et al. Citation2014; Brabec et al. Citation2015; Waeschenbach & Littlewood Citation2017) did not reveal the identity of the samples collected. In our study, Sanger sequencing of lsrDNA, ssrDNA, and COI amplicons produced low quality reads with overlapping fluorescence peaks even when different primer combinations were tested under carefully adjusted PCR conditions. Therefore, we used the NGS method to sequence a 346 bp lsrDNA fragment that eventually facilitated successfully identifying the parasite found in the tub gurnard. A high quality 346 bp lsrDNA fragment obtained using NGS was successfully used to identify a parasite found in the tub gurnard. Based on the sequence, we assigned the specimen to B. scorpii with 99.38% similarity between the sequence obtained and the lsrDNA fragment of B. scorpii deposited in GenBank under accession number AF286942 (, ). The partial lsrDNA sequence of B. scorpii was deposited in GenBank under accession number OL742642.
Figure 2. Phylogenetic tree of Bothriocephalus scorpii specimens (denoted as B. scorpii_PL_1) found in C. lucerna from the North Sea. A phylogenetic tree was constructed with the Maximum Likelihood method (GTR model, 10000 bootstrap samples).

Table I. Percentage of bases that are identical between selected species (sub-branch) of Bothriocephalus genus.
Additionally, we proved that the partial sequence of lsrDNA with high sequencing coverage together with morphological examination can be used to identify parasite species. NGS was used previously in a parasitological study to annotate and characterize the complete mitochondrial genome and nuclear rRNA operons of Schyzocotyle acheilognathi, and the method showed promising applicability (Brabec et al. Citation2016). Applying NGS enriches the array of standard molecular methods used to identify parasite species unambiguously; however, in the present paper, we used NGS just to overcome Sanger sequencing limitations (complicated chromatograms). Despite the great advantage of molecular methods over the morphological assignment of cestode species, the dissimilarity expressed by a percentage of nucleotide difference does vary within families. For instance, a study on phyllobothriid cestodes (Cestoda: Tetraphyllidea, currently referred to as Phyllobothriidea; Caira et al. Citation2014) showed that variation within species was between 0% and 0.74% (Randhawa et al. Citation2007). The differences observed in our study among sequences of B. scorpii from the North Sea (Shetland Islands) (OL742642), the North Sea off St. Abbs Head (AF286942), and the Pacific Ocean in the vicinity of Nuggets and Kaka points, Catlins Region, South Island, New Zealand (KY909259) suggested the geographic origin of the parasite. Our sequence was more like that of the parasite found in a shorthorn sculpin caught in the North Sea when compared to that of the parasite found in a sole caught in the Pacific Ocean near New Zealand (Waeschenbach et al. Citation2007; Anglade & Randhawa Citation2018). The other explanation could be host–parasite interactions, which can influence the strength and specificity of selection under different environmental conditions (Wolinska & King Citation2009).
Supplemental Material
Download MS Word (22.3 KB)Acknowledgements
The authors are very grateful to Wiesław Szaszkiewicz for providing the tub gurnard specimen for parasitic study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2264911.
References
- Akmirza A. 2001. The samples from metazoon parasites detected in fish around Gokçeada. Proceedings of the I Congress of National Aegean Islands, Gokçeada, Turkey. pp. 85–96.
- Akmirza A. 2013. Monogeneans of fish near Gökçeada, Turkey. Turkish Journal of Zoology 37:441–448. DOI: 10.3906/zoo-1205-4.
- Alaş A, Öktener A, Türker Çakır D. 2015. Bomolochus bellones and caligus diaphanus with morphologic characters from Turkey. Proceedings of 9. International Symposium of Fish Parasites, 31 August - 4 September, Valencia, Spain. pp. 187.
- Anglade T, Randhawa H. 2018. Gaining insights into the ecological role of the New Zealand sole (Peltorhamphus novaezeelandiae) through parasites. Journal of Helminthology 92(2):187–196. DOI: 10.1017/S0022149X17000323.
- Bartoli P, Gibson DI, Bray RA. 2005. Digenean species diversity in teleost fish from a nature reserve off Corsica, France (Western Mediterranean), and a comparison with other Mediterranean regions. Journal of Natural History 39(1):47–70. DOI: 10.1080/00222930310001613557.
- Blend CK, Dronen NO. 2003. Bothriocephalus gadellus n. sp. (Cestoda: Bothriocephalidae) from the beardless codling Gadella imberbis (Vaillant) (Moridae) in the southwestern Gulf of Mexico, with a review of species of Bothriocephalus Rudolphi, 1808 reported from gadiform fishes. Systematic Parasitology 54(1):33–42. DOI: 10.1023/A:1022102111388.
- Brabec J, Kuchta R, Scholz T, Littlewood DTJ. 2016. Paralogues of nuclear ribosomal genes conceal phylogenetic signals within the invasive Asian fish tapeworm lineage: Evidence from next generation sequencing data. International Journal for Parasitology 46(9):555–562. DOI: 10.1016/j.ijpara.2016.03.009.
- Brabec J, Waeschenbach A, Scholz T, Littlewood DTJ, Kuchta R. 2015. Molecular phylogeny of the Bothriocephalidea (Cestoda): Molecular data challenge morphological classification. International Journal for Parasitology 45(12):761–771. DOI: 10.1016/j.ijpara.2015.05.006.
- Bray RA, Jones A, Andersen KI. 1994. Order Pseudophyllidea Carus, 1863. In: Khalil LF, Jones A, Bray RA, editors. Keys to the cestode parasites of vertebrates. Wallingford, UK: CAB International. pp. 205–247.
- Caira JN, Jensen K, Waeschenbach A, Olson PD, Littlewood DTJ. 2014. Orders out of chaos–molecular phylogenetics reveals the complexity of shark and stingray tapeworm relationships. International Journal for Parasitology 44(1):55–73. DOI: 10.1016/j.ijpara.2013.10.004.
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Gonzalez Pena A, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7(5):335–336. DOI: 10.1038/nmeth.f.303.
- Carreras-Aubets M, 2013. Parasites of three fish species of commercial interest from the north – western Mediterranean Sea: Mullus barbatus, Spicara maena and Trachinus draco (Osteichthyes, Perciformes). Use as tags of environmental condition. Doctoral thesis, University of Barcelona. pp. 1–207.
- Dainys J, Pūtys Ž, Bacevičius E, Shiao JC, Iizuka Y, Jakubavičiūtė E, Ložys L. 2017. First record of tub gurnard, Chelidonichthys lucerna (Linnaeus, 1758), from the south-eastern Baltic Sea (Lithuania). Journal of Applied Ichthyology 33(6):1223–1225. DOI: 10.1111/jai.13491.
- Davey JT, Peachey JE. 1968. Bothriocephalus scorpii (Cestoda: Pseudophyllidea) in turbot and brill from British coastal waters. Journal of the Marine Biological Association of the United Kingdom 48:335–340. DOI:10.1017/S0025315400034524.
- De Groot SJ. 1971. Bothriocephalus scorpii (Müller) (Cestoda: Pseudophyllidea) in turbot Scophthalmus maximus (L.) and brill S. rhombus (L.) from the southern North Sea. Journal of Fish Biology 3:147–149. DOI:10.1111/j.1095-8649.1971.tb03658.x.
- Demirkale İ, Özak AA, Yanar A, Boxshall GA. 2014. Caligus solea n. sp. (Copepoda: Caligidae) parasitic on the common sole Solea solea (Linnaeus) from the north-eastern Mediterranean off the Turkish coast. Systematic Parasitology 89(1):23–32. DOI: 10.1007/s11230-014-9505-4.
- De Sterck T, 2019. De prevalentie en lokalisatie van Anisakidae in Belgische consumptievis. Master thesis, Ghent University.
- Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461. DOI: 10.1093/bioinformatics/btq461.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3:294–299.
- Froese R, Pauly D, editors. 2021. FishBase. World Wide Web electronic publication, 08. Available: www.fishbase.org,version.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology 59(3):307–321. DOI: 10.1093/sysbio/syq010.
- Härkönen T. 1986. Guide to the otoliths of the bony fishes of the Northeast Atlantic. Danbiu ApS. biological consultants, p. 256.
- Herrera-Cubilla A, Raibaut A. 1990. Acanthochondria triglae n. sp., copépode parasite des fosses nassales de poissons Triglidae. Crustaceana 59:82–88. DOI:10.1163/156854090X00327.
- Heuch PA, Øines Ø, Knutsen JA, Schram TA. 2007. Infection of wild fishes by the parasitic copepod Caligus elongatus on the south east coast of Norway. Diseases of Aquatic Organisms 77:149–158. DOI:10.3354/dao01833.
- Jones A. 1975. The morphology of Bothriocephalus scorpii (Muller) (Pseudophyllidea, Bothriocephalidae) from littoral fishes in Britain. Journal of Helminthology 49:251–261. DOI:10.1017/S0022149X00026250.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649. DOI: 10.1093/bioinformatics/bts199.
- Kuchta R, Scholz T. 2007. Diversity and distribution of fish tapeworms of the “Bothriocephalidea” (Eucestoda). Parassitologia 49(3):129–146.
- Kuchta R, Scholz T, Bray RA. 2008. Revision of the order Bothriocephalidea Kuchta, Scholz, Brabec & Bray, 2008 (Eucestoda) with amended generic diagnoses and keys to families and genera. Systematic Parasitology 71(2):81–136. DOI: 10.1007/s11230-008-9153-7.
- Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Research 44:W242–W245. DOI:10.1093/nar/gkw290.
- Mahé K, Bellamy E, Delpech J, Lazard C, Salaun M, Vérin Y, Coppin F, Travers-Trolet M. 2018. Evidence of a relationship between weight and total length of marine fish in the North-eastern Atlantic Ocean: Physiological, spatial and temporal variations. Journal of the Marine Biological Association of the United Kingdom 98(3):617–625. DOI: 10.1017/S0025315416001752.
- Marcogliese DJ. 1995. The role of zooplankton in the trans-mission of helminth parasites to fish. Reviews in Fish Biology and Fisheries 5:336–371. DOI:10.1007/BF00043006.
- Markowski S. 1935. Űber den entwicklungszyklus von Bothriocephalus scorpii (Müller, 1776). Bulletin L’Académie Polonaise des Science Letter Series B 3:1–17.
- Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal 17(1):10–12. DOI: 10.14806/ej.17.1.200.
- McKinnon AD, Featherston DW. 1982. Location and means of attachment of Bothriocephalus scorpii (Muller) (Cestoda: Pseudophyllidea) in red cod, Pseudophycis bacchus (Forster in Bloch & Schneider), from New Zealand waters. Marine & Freshwater Research 33(3):595–598. DOI: 10.1071/MF9820595.
- Öktener A, Alaş A, Tüker D. 2016. First record of Caligus diaphanus Nordmann, 1832 from Turkish marine habitats. Boletim do Instituto de Pesca Sao Paulo 42:203–208. DOI:10.20950/1678-2305.2016v42n1p203.
- Öktener A, Trilles JP. 2004. Three new parasitic copepod species for the parasite fauna of marine fish of Turkey. Journal of the Black Sea / Mediterranean Environment 10:71–80.
- Piasecki W, Barcikowska D, Panicz R, Eljasik P, Kochmański P. 2022. First step towards understanding the specific identity of fish muscle parasites of the genus Sarcotaces (Copepoda: Philichthyidae)—New species and first molecular ID in the genus. The International Journal for Parasitology: Parasites and Wildlife 18:33–44. DOI:10.1016/j.ijppaw.2022.03.008.
- Pojmańska T, Cielecka D. 2001. Tasiemce (Cestoda) związane ze środowiskiem wodnym. Łódź, Poland: Wydawnictwo Uniwersytetu Łódzkiego.
- Polyakova TA. 2020. Fish cestodes of the Karadag nature reserve an adjacent water area of the Black Sea. Marine Biological Journal 5(1):50–63. DOI: 10.21072/mbj.2020.05.1.06.
- Protasova EN. 1977. Cestodes of fish – Bothriocephalata. In: Ryzhikov KM, editor Principles of cestodology 8. Moscow, Russia: Nauka. p. 298.
- Raibaut A, Combes C, Benoit F. 1998. Analysis of the parasitic copepod species richness among Mediterranean fish. Journal of Marine Systems: Journal of the European Association of Marine Sciences and Techniques 15(1–4):185–206. DOI: 10.1016/S0924-7963(97)00079-1.
- Ramdane Z, Trilles J. 2010. New Algerian parasitic copepods. Bulletin of the European Association of Fish Pathologists 30(2):41–47.
- Randhawa HS, Saunders GW, Burt MDB. 2007. Establishment of the onset of host specificity in four phyllobothriid tapeworm species (Cestoda: Tetraphyllidea) using a molecular approach. Parasitology 134(9):1291–1300. DOI: 10.1017/S0031182007002521.
- Renaud F, Gabrion C, Romestand B. 1984. The Bothriocephalus scorpii complex (Mueller, 1776). Differentiation of species of parasites of the turbot (Psetta maxima) and the brill (Scophthalmus rhombus). Study of protein fractions and antigen complexes. Annales de Parasitologie Humaine et comparee 59(2):143–149. DOI: 10.1051/parasite/1984592143.
- Rodrigues J, Feijó D, Rocha A, Erzini K, Correia AT, 2019. Age, growth and reproductive biology of the tub gurnard (Chelidonichthys lucerna) in North-East Portugal. Frontiers in Marine Science Conference Abstract: XX Iberian Symposium on Marine Biology Studies (SIEBM XX). DOI: 10.3389/conf.fmars.2019.08.00158.
- Rokicki J, Rolbiecki L. 2002. Colonization of the round goby, neogobius melanostomus (Gobiidae) by parasites in the new environment of the Gulf of Gdańsk (Southern Baltic). Wiadomosci Parazytologiczne 48(2):197–200.
- Škeříková A, Hypša V, Scholz T. 2004. A paraphyly of the genus Bothriocephalus Rudolphi, 1808 (Cestoda: Pseudophyllidea) inferred from internal transcribed spacer–2 and 18S ribosomal DNA sequences. The Journal of Parasitology 90(3):612–617. DOI: 10.1645/GE-3302.
- Skrzypczak M, Rolbiecki L. 2015. Helmintofauna of turbot Scophthalmus maximus (Linnaeus, 1758) from the southern Baltic Sea including new data. Polish Journal of Veterinary Sciences 18(3):599–605. DOI: 10.1515/pjvs-2015-0077.
- Solonchenko AI. 1985. Development of larval stages of Bothriocephalus scorpii. Parasitology and pathology of marine organisms of the world ocean. NOAA Technical Reprot NMFS 25:83–84.
- Stagioni M, Montanini S, Vallisneri M. 2012. Feeding of tub gurnard Chelidonichthys lucerna (Scorpaeniformes: Triglidae) in the north-east Mediterranean. Journal of the Marine Biological Association of the United Kingdom 92:605–612. DOI:10.1017/S0025315411000671.
- Sulgostowska T, Szostakowska B, Myjak P. 1998. Helminth fauna of flounder Platichthys flesus (L.) and turbot Scophthalmus maximus (L.) from the Gulf of Gdańsk. Acta Ichthyologica et Piscatoria 28:69–79. DOI:10.3750/AIP1998.28.2.07.
- Verneau O, Renaud F, Catzeflis F. 1997. Evolutionary relationships of sibling tapeworm species (Cestoda) parasitizing teleost fishes. Molecular Biology and Evolution 14(6):630–636. DOI: 10.1093/oxfordjournals.molbev.a025802.
- Waeschenbach A, Littlewood T. 2017. A molecular framework for the Cestoda. In: Caira JN, Jensen K, editors Planetary biodiversity inventory (2008–2017): Tapeworms from vertebrate bowels of the earth. Lawrence, KS: University of Kansas, Natural History Museum, Special Publication No. 25. pp. 431–451.
- Waeschenbach A, Webster BL, Bray RA, Littlewood DTJ. 2007. Added resolution among ordinal level relationships of tapeworms (Platyhelminthes: Cestoda) with complete small and large subunit nuclear ribosomal RNA genes. Molecular Phylogenetics and Evolution 45(1):311–325. DOI: 10.1016/j.ympev.2007.03.019.
- Więcaszek B, Sobecka E, Dudko S, Keszka S. 2011. New and ‘visiting’ fish species collected off the western coast of Poland (Baltic Sea) in 2007–2008 with a description of their parasite fauna. Oceanologia 53(1):163–179. DOI: 10.5697/oc.53-1.163.
- Wolinska J, King KC. 2009. Environment can alter selection in host–parasite interactions. Trends in Parasitology 25(5):236–244. DOI: 10.1016/j.pt.2009.02.004.
- Yamaguti S. 1959. Systema helminthum. Volume II. The cestodes of vertebrates. New York, USA: Interscience Publishers Inc.
