Abstract
A new species of annelid, Alitta yarae sp. nov., is described from material collected in southern Brazil. This species was collected mostly from fishing buoys but also in muddy substrates of the following estuaries: Paraná Estuary Complex, Guaratuba, and Babitonga Bay. Alitta yarae sp. nov. belongs to a group of species with pennant-shaped posterior dorsal ligules. The most similar species of this group is A. succinea, which shares glandular structures from posterior dorsal ligules on lower edge only and having yellow-amber mandibles. However, the two species differ in the extension of the bare space between areas VI and VII–VIII, which is reduced in A. yarae sp. nov. and wider in A. succinea (respectively shorter than or equal to the palpophore), and the paragnath arrangement. Molecular analysis recovered ~20% genetic distance from the closest species A. succinea and no pattern of segregation among the estuaries studied. Finally, both the morphological and the molecular analyses strongly support the designation of a new species.
http://zoobank.org/urn:lsid:zoobank.org:act:2575CF46-57C9-47AE-A4C1-235189F6C107
Introduction
Nereididae Blainville, 1818 is a family of annelid polychaetes widespread in a variety of environments (e.g. shallow and deep sea, or freshwater), that includes several species well studied for ecological, biological and developmental research (e.g. Platynereis dumerilii (Audouin & Milne Edwards, 1833), Alitta succinea (Leuckart, 1847)), as well as commercial species, important as fish baits and food supply (e.g. Hediste diversicolor (O.F. Müller, 1776)) (Bakken et al. Citation2022). The morphological identification of several species belonging to this family is often confusing because of a high intraspecific variation of the characters (e.g. in the pharynx and the parapodia), variable colour patterns, and the presence of cryptic/pseudocryptic species (Glasby et al. Citation2013). The relatively recent integration of the traditional morphological identifications with DNA barcoding (Hebert et al. Citation2003) helped unveil the presence of potential species complexes. Indeed, in several annelid families, an increasing number of studies indicate that the high intraspecific heterogeneity and the cosmopolitan distribution usually assigned to a single species name actually corresponds to complexes of cryptic or pseudocryptic species (e.g. Nygren et al. Citation2018; Simon et al. Citation2019; Teixeira et al. Citation2020). DNA barcoding proved to be a valuable tool to both delimit and assist in the identification of cryptic nereidid species as well (e.g. Virgilio et al. Citation2009; Sampieri et al. Citation2021; Teixeira et al. Citation2022).
Among these species complexes stands A. succinea, which has a putative widespread distribution, and displays high tolerance to several environmental stressors (e.g. Kuhl & Oglesby Citation1979; Neuhoff Citation1979; Gillet et al. Citation2011; Ghasemi et al. Citation2013). Originally described from Helgoland, Germany (North Sea), this species inhabits brackish-water environments, marshes, lagoons and estuaries in tropical and temperate regions (Rioja Citation1946; Villalobos-Guerrero & Carrera-Parra Citation2015). The morphological delineation of this species has been complex, with misleading descriptions that combined morphological features of putative specimens of this species from diverse regions. Recently, Villalobos-Guerrero and Carrera-Parra (Citation2015), in an extensive work, redescribed A. succinea based on topotype specimens, reinstated A. acutifolia (Ehlers, 1901), a congeneric species, and the most morphologically similar, and provided CO1 sequences of both species. Although similar, A. succinea and A. acutifolia differ in at least 29 morphological characters, including paragnath arrangement, parapodial features and glandular distribution, and display molecular divergences also (Villalobos-Guerrero & Carrera-Parra Citation2015). Moreover, with this study, by reinstating A. acutifolia, the authors rejected the hypothesis of A. succinea occurrence along the Central American Pacific shores.
Although a few studies including molecular data have been carried out in Brazil, the presence of A. succinea sensu stricto was already confirmed (e.g. Alves et al. Citation2023). Specimens commonly identified as A. succinea have been recorded from Maranhão (North-East Brazil) to Rio Grande do Sul States (South Brazil), in either hard or soft substrates, mostly in poly- and euhaline environments, but overlooking potential morphological differences reported (e.g. Ribeiro et al. Citation2018). Data from the grey literature suggest the presence of distinct genetic populations of A. succinea, supported also by the paragnath arrangement, although this has never been published. Therefore, in this study, we aimed to describe a new species of Alitta inhabiting the oligo- and mesohaline sectors of selected southern Brazilian estuaries, previously commonly identified as A. succinea, by integrating the morphological description with the molecular data. Due to the presence along the coast of A. succinea sensu stricto, and its similarity to A. yarae sp. nov., a morphological comparison between these two species and A. acutifolia is provided.
Materials and methods
Animal collection and sampling area
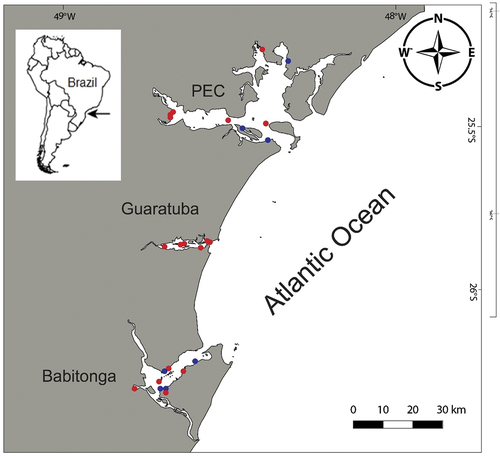
Specimens were collected in three large estuarine systems along the southern Brazilian coast: the Paranaguá Estuarine Complex (PEC), Guaratuba Bay and Babitonga Bay (; Suppl. Table S1). The PEC is one of the largest (612 km2) and best preserved coastal areas along the southern American coast, despite increasing port and tourist activities in the last several decades. Around 40 km south of the PEC, Guaratuba Bay is found; it is ~15 km long (E–W axis) and 5 km wide (N–S axis), with a surface area of 50.19 km2 (Bigarella Citation2001; Marone et al. Citation2006). Babitonga Bay, ~40 km south of Guaratuba Bay, is an estuarine complex in the north of the Santa Catarina state, with a 160 km2 surface area (IBAMA Citation1998). The three bays share a microtidal regime, predominantly semidiurnal, with spring tides that may reach up to 2 m in the inner estuarine sector (Marone et al. Citation2006). Salinity fluctuations are high (~0–35 psu), with horizontally and vertically highly stratified waters (Marone et al. Citation2005, Citation2006). Three sectors can be recognised: euhaline (30–35 psu) near the inlets, polyhaline (20–30 psu) and mesohaline (5–20 psu) in the middle, and the inner oligohaline sector (0–5 psu), which displays greater riverine influence (Lana & Bernardino Citation2018).
Figure 1. Map of the study area. Red dots mark the areas in which Alitta yarae sp. nov. occurred; blue dots mark the areas where A. succinea was collected. PEC = Paranaguá Estuarine Complex.
Animals were collected using a shovel from the intertidal mudflats or by scraping the fishing buoys. Animals were sampled under the authorisation of the “Sistema de Autorização e Informação em Biodiversidade” (SISBIO, permit # 36255–1/73,627–1). In the laboratory, animals were relaxed in MgCl2, then fixed in 96% ethanol, changing the solution at least once after 1 hour, and stored at −20°C.
Morphological analysis
The specimens were analysed under light microscopy using a Leica MZ stereomicroscope and a Leica DM 6000 B microscope. Photographs were taken with a Leica M205C stereomicroscope and a Leica DMC5400 camera connected to the Leica LAS software with the Z-stack option. As the specimens were fixed with a totally everted proboscis, the routine systematics procedure involved the dissection of only the parapodia and mandibles. Parapodia from the left side of chaetigers 2, 20, 45, 65, and 75 were mounted in glycerol and photographed in both anterior and posterior views. Measurements were made from the photographs using ImageJ software, following Villalobos-Guerrero and Carrera-Parra (Citation2015). The measurements included total length (TL), length and width of chaetiger 15 (L15, W15), and number of chaetigers, with sample size (n), mean (μ) and range (ran). For scanning electron microscopy (SEM), the specimens were mounted on a metallic stub, sputter-coated with gold (2 nm), and examined under a Phenom Pro X.
For taxonomic nomenclature, we followed the main revisions of the Alitta genus (Villalobos-Guerrero & Carrera-Parra Citation2015; Villalobos-Guerrero & Bakken Citation2018) and the paragnath morphology adopted by Bakken et al. (Citation2009) and Bakken and Wilson (Citation2005). The type material was deposited at the Museu Nacional – Universidade Federal do Rio de Janeiro (UFRJ), and at the Museu de Diversidade Biológica, Zoologia, Instituto de Biologia, Universidade Estadual de Campinas (IB-UNICAMP).
DNA isolation, amplification and alignment
For molecular analysis, data were generated in three laboratories (in Norway, Poland and Brazil) using different protocols. Total DNA was isolated from either one central segment or a parapodium, using either QuickExtractTM kit (Lucigen) following the manufacturer’s protocol, phenol chloroform extraction (Hillis et al. Citation1996), or NaOH extraction adapting the protocol of Wang et al. (Citation1993). The NaOH protocol consisted of the following: (1) addition of 300 µL of 50 mM NaOH to a vial containing the annelid tissue previously reduced to small fragments; (2) incubation at 95°C for 60 min; (3) addition of 300 µL of Tris–HCl 1 M, pH 8; (4) centrifugation at 12,000 rpm for 5 min. Amplifications of both the mitochondrial cytochrome c oxidase subunit I (CO1) and 16S rRNA gene, and the nuclear 28S rRNA gene, were carried out using several primer sets. Primers used and related polymerase chain reaction (PCR) programs are listed in Suppl. Table S1. Reagents and reaction volumes used are described in detail in Suppl. file S1. Raw sequences were trimmed in Geneious R11 (https://www.geneious.com) and aligned using MAFFT (Katoh & Standley Citation2013) therein implemented, and inspected for eventual stop codons and indels to prevent inclusion of pseudogenes in the analyses. To confirm their identities, each sequence was searched on the Basic Local Alignment Tool (BLAST) provided by National Centre for Biotechnology (https://blast.ncbi.nlm.nih.gov/Blast.cgi). DNA barcode data were uploaded in the dataset “DS-ALYAPEC” (doi: dx.doi.org/10.5883/DS-ALYAPEC) in the Barcode of Life Data System (BOLD; Ratnasingham & Hebert Citation2007). A second dataset was created also joining the sequences of Alitta already present in BOLD (DS-ALITTAW, doi:dx.doi.org/10.5883/DS-ALITTAW).
Estimates of genetic diversity
The divergence within CO1 sequences of the two putative Alitta morphospecies was calculated using the Kimura two-parameter model (K2P; Kimura Citation1980), BOLD aligner, and complete deletion, as implemented in BOLD in Distance Summary function. Number of haplotypes (H), haplotype diversity (Hd) and nucleotide diversity (π) were calculated with the program DNASP 5.10 (Librado & Rozas Citation2009) to estimate the genetic diversity for each species using a shortened alignment of 581 bp (Suppl. file S2).
Molecular-based species delineation
Molecular operational taxonomic units (MOTUs) were defined applying three methods of molecular-based species delineation. The assemble species by automatic partitioning (ASAP) species delineation tool was run on a web interface (https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html; Puillandre et al. Citation2021), using the default settings (i.e. JC69). This tool, based on barcode gap detection (i.e. breaks between the distribution of intraspecific and interspecific distances of the barcode region), sorts the sequences into hypothetical species (Puillandre et al. Citation2012). Then, CO1 sequences were submitted to the Barcode Index Number (BIN) system implemented in BOLD. This approach clusters barcode sequences algorithmically to calculate MOTUs showing high concordance to species (Ratnasingham & Hebert Citation2013). Finally, we applied the Bayesian Poisson tree processes (bPTP) tree-based method (Zhang et al. Citation2013). The bPTP method incorporates the number of substitutions in the model of speciation and assumes the probability that a substitution gives rise to a speciation event follows a Poisson distribution. The branch lengths of the input tree are supposed to be generated by two independent Poisson process classes, one corresponding to speciation and the other to coalescence (Zhang et al. Citation2013). For the input phylogenies (CO1 and 16S) the maximum likelihood (ML) method was used through PhyML (Guindon et al. Citation2010). For the CO1 all the sequences from the DS-ALITTAW dataset were downloaded, all sequences shorter than 500 bp were excluded, and the remainder were collapsed to single haplotypes (i.e. 37 sequences) to avoid redundancy in the analysis (Suppl. file S3). For 16S all the sequences available were used (i.e. 12 sequences) of which, two sequences of A. succinea were already public (Suppl. file S4). Branch support was inferred from 1000 bootstraps. The best substitution model (HKY85 + I for CO1 and GTR for 16S) was tested with the SMS routine in PhyML using both Akaike and Bayesian information criteria (respectively AIC and BIC) as optimality criteria (Lefort et al. Citation2017). The trees were edited with FigTree (Rambaut Citation2010). Species delimitation analyses were done on the bPTP web server (available at http://species.h-its.org/) with 2 × 105 (results not shown). The results of the ML PTP were also considered for the CO1 and presented with the PhyloMap-PTP, a visualisation combining (principal coordinate analysis, PTP, and species tree mapping.
To visualise the molecular diversity of the two MOTUs, a haplotype network was computed using the shortened alignment of CO1 with the TCS method (Clement et al. Citation2000) in the software Popart (v. 1.7). The genetic differentiation among the estuaries for each MOTU, Fst estimations, were estimated using standard haplotype frequencies in Arlequin 3.5 (Excoffier & Lischer Citation2010). The significance of pairwise Fst values was determined by performing 1023 permutations between locations, under the null hypothesis of no differentiation. Analysis of molecular variance (AMOVA) was also performed with similar settings in Arlequin 3.5. Fu’s Fs (Fu Citation1997) and Tajima’s D (Tajima Citation1989) were computed for each species in Arlequin 3.5 (Excoffier & Lischer Citation2010) to test the departure from neutrality. Finally, the Geographic Distance Correlation tool (Blagoev et al. Citation2016), using K2P, BOLD Aligner and complete deletion as implemented in BOLD, was applied to assess the correlation between genetic and geographical distances.
Results and discussion
Taxonomic section
Family Nereididae de Blainville, 1818
Subfamily Nereidinae de Blainville, 1818
Alitta Kinberg, 1865
Type species: Nereis virens Sars, 1835, by monotypy.
Alitta yarae sp. nov.
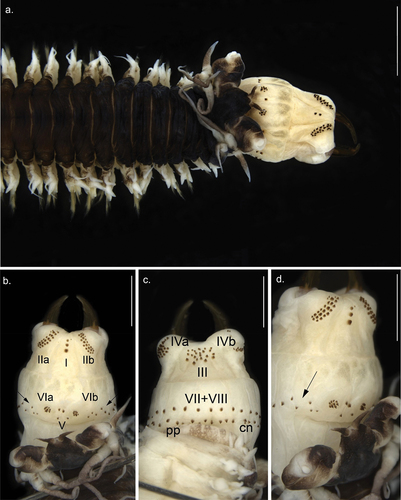
Figure 2. Anterior view of Alitta yarae sp. nov. (a) paratype (MNRJP 007662): anterior end, dorsal view. (b–d) Holotype (MNRJP 007659): (b) dorsal view, (c) ventral view, (d) lateral view. Arrows point to fleshy pads between areas VI and VII–VIII bearing paragnaths. Scale bars: A = 2 mm; B–D = 1 mm. cn, conical paragnath, pp, pyramidal paragnath.
ZooBank: urn:lsid:zoobank.org:act:2575CF46-57C9-47AE-A4C1-235189F6C107
Etymology
The specific name refers to the ancient Brazilian myth of Yara (Mother of the Waters), a beautiful brown-skinned mermaid from the Amazon River. In old Tupi-Guarani, Yîara means “Lady of the Lake”. The name was chosen because of the occurrence of this species in the oligohaline sectors of the bays.
Material examinedType material
Holotype: Southern Brazil, Paranaguá Estuarine Complex, Antonina Bay, 25.435712°S, 48.705104°W, 28 September 2021, coll. R. Castro Álvarez, fouling communities in buoys, holotype (MNRJP 007659). –Paratypes (13): Same data as the holotype, MNRJP 007657 (DNA voucher, BOLD: ESPBR166–23), MNRJP 007658 (DNA voucher, BOLD: ESPBR167–23), MNRJP 007660 (1 spec), MNRJP 007661 (1 spec), MNRJP 007662 (1 spec), MNRJP 007663 (1 spec), MNRJP 007664 (1 spec), MNRJP 007665 (1 spec), MNRJP 007666 (1 spec), MNRJP 007667 (1 spec), MNRJP 007668 (1 spec), MNRJP 007669 (1 spec), MNRJP 007670 (1 spec), MNRJP 007671 (8 spec).
Description based on an atokous specimen
Holotype (MNRJP 007659) complete with 93 chaetigers (n = 12, u = 78.5 ± 14.45, ran = 56–94); TL = 51.5 mm (n = 12, u = 42.96 ± 8.08, ran = 24–51.5); L15 = 0.6 mm (n = 12, u = 0.7 ± 0.14, ran = 0.5–1); W15 = 1.5 (n = 12, u = 1.69 ± 0.44, ran = 1.3–3).
Prostomium anteriorly complete (), 1.5 times wider than long (L = 1.55 mm, W = 3.0 mm); mid-dorsal groove running from the anterior end of prostomium to the middle of the anterior and posterior eyes. Palpophores suboval (), 1.1 times longer than wide (L = 1.2 mm, W = 1.1 mm), equaling 0.36 times the prostomium width. Antennae slightly separated (), separation 0.5 times the antennal diameter, short (L = 0.7, W = 0.2 mm), conical, not exceeding the palp tip, reaching half of the prostomium, anteriorly directed.
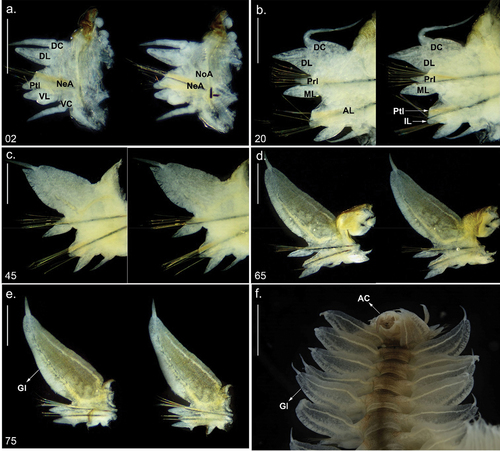
Figure 3. Parapodia and posterior end of Alitta yarae sp. nov. (a–e) Holotype (pMNRJP 007659) chaetiger; (f) paratype (MNRJP 007661), posterior end, dorsal view. Scale bars: a–e = 0.5 mm; f =1 mm. AC = anal cirrus, AL = neuroacicular ligule, DC = dorsal cirrus, DL = dorsal ligule, Gl = glandular edge, IL = inferior lobe, ML = median ligule, NeA = neurociculae; NoA = notoaciculae, Prl = notopodial prechaetal lobe, Ptl = neuropodial postchaetal lobe, VC = ventral cirrus, VL = ventral ligule.
Eyes with a trapezoidal arrangement, posterior ones 0.25 times longer than the anterior ones, reddish, oval, both pairs well separated; lens evident, whitish. Anterior pair is as wide as the antennal width; lenses rounded, anterolaterally displaced, covering 30%. The posterior pair is three-quarters of the antennal diameter; lenses are rounded, whitish, covering 70%. Nuchal organs are embedded, slightly longer than the posterior eyes.
Tentacular cirri pattern: postero-dorsal cirri 1.75 times longer than the antero-dorsal cirri; postero-ventral cirri 1.5 times longer than the antero-ventral cirri. Antero-dorsal cirri reaching chaetiger 4, antero-ventral cirri same length as the palpophore. Postero-dorsal cirri reaching chaetiger 9, postero-ventral cirri extending slightly over the palpostyle. Dorsal cirrophores wrinkled, subcylindrical, longer than the ventral ones; postero-dorsal cirrophores up to 3 times longer than antero-ventral cirrophores.
Pharynx bearing yellow-amber jaws, with two longitudinal channels; teeth broken, 3–4 complete (n = 39, μ = 9.3 ± 0.3, ran: 8–11) (). Paragnaths all conical, except some pyramidal in VII−VIII. Paragnaths without plate-like basement. Maxillary ring: I = 4 (n = 42, μ = 3.3 ± 0.4, ran: 1–9), in a longitudinal line (); IIa = 25 (n = 42, μ = 20.0 ± 1.2, ran: 12–29), IIb = 24 (n = 42, μ = 20.7 ± 1.2, ran: 11–29), two or three irregular curved rows with small-slender and large-coarse cones, the latter anteriorly placed (); III = 22 (n = 42, μ = 34.5 ± 1.8, ran: 16–43), four irregular rows in a rectangular group (sometimes three irregular rows forming a trapezoid; ); IVa = 10 (n = 42, μ = 23.4 ± 1.2, ran: 16–34), IVb = 26 (n = 42, μ = 23.9 ± 1.1, ran: 18–34), crescent-shaped, tapering anteriorly (). Oral ring: V = 4 (n = 42, μ = 2.0 ± 0.3, ran: 0–4), when present, two longitudinal or transversal cones (), or three cones forming a triangle; VIa = 9 (n = 41, μ = 8.2 ± 0.5, ran: 5–13), VIb = 8 (n = 41, μ = 8.5 ± 0.7, ran: 6–18), arranged in circular group with concentric cone (); VII−VIII = 49 (n = 42, μ = 52.6 ± 1.8, ran: 41–66), two rows with cones and pyramidal, anterior row continuous with alternating combination of cones and pyramidal (). Bare space between VI and VII−VIII reduced, as wide as one-third of palpophore ().
Anterior parapodia are one-fifth of the anterior body width; posterior parapodia are equal to or longer than the posterior body width. Notopodia and neuropodia are distinct ().
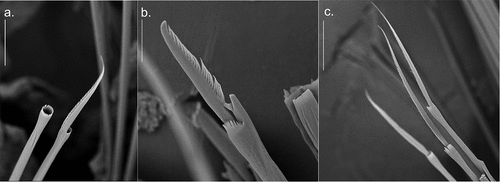
Figure 4. Detail of Alitta yarae sp. nov. chaetae from central segments (S.E.M. = scanning electron microscope; SM): (a) homogomph spiniger from notochaetae; (b) heterogomph falciger from neurochaetae; (c) heterogomph spiniger from neurochaetae. Scale bars: a = 30 µm; b, c = 50 µm.
Dorsal cirri basally attached in first anterior chaetigers, medially in middle chaetigers, and distally in posterior chaetigers; cirriform, 1.2 times length and 0.5 times width of dorsal ligule in anterior chaetigers, 0.5 times length and 0.1 times width in medium chaetigers, and 0.2 times length and 0.1 times width in posterior chaetigers (). Dorsal cirri inserted in one-fourth of the ligule in the first two chaetigers (), two- to three-fifths in the anterior chaetigers (), subterminal in mid-body chaetigers () and terminal in the last 24–27 posterior chaetigers ().
Dorsal ligule triangular in the anterior chaetigers, elliptical in medium-body chaetigers and pennant-like in the posterior chaetigers (); a ventral glandular edge observed on posterior chaetigers (); anterior chaetigers with dorsal ligules 1.3 times length and 0.72 times width of medium ligule; medium chaetigers 1.6–2 times length and 2.3–2.7 times width, and 3.7–4 times length and 3.7–4.5 times width in posterior chaetigers (). Dorsal ligule with rounded ventral surface from medium chaetigers onwards ().
Notopodial pre-chaetal lobe papilliform in chaetigers 3–4, conical in the following anterior chaetigers, and smaller and papilliform in posterior chaetigers (). Lobe narrower than the medium ligule, nearly two-thirds in the most anterior chaetigers, one-third in medium and posterior chaetigers; nearly two-thirds length in most anterior chaetigers, one-third in medium posterior chaetigers.
Median ligule not exceeding dorsal ligule throughout; conical in anterior chaetigers, lanceolate from chaetiger 22, thin and conical in last posterior chaetigers ().
Neuracicular ligule shorter than ventral ligule throughout; anterior chaetigers with ventral ligule 0.85 times length and 2.3 times width of ventral ligule; medium chaetigers 0.6 times length and 2.3 times width; and 0.4 times length and 0.5–0.7 times width in posterior chaetigers (). Neuropodial postchaetal lobe conical, almost reaching ventral ligule extension, decreasing in size from medium chaetigers onwards, varying from one-third to two-thirds ventral ligule extension ().
Inferior lobe rounded, exceeding neuropodial aciculae ().
Ventral ligule oblong along the body; anterior chaetigers with ventral ligules equal in length and 0.7 times width of medium ligule; medium chaetigers equal in length and 0.7 times width, and 1.7 times length and 0.7 times width in posterior chaetigers ().
Ventral cirri emerging from the basis of the ventral ligule, digitiform in the first chaetigers, decreasing gradually in size in posterior chaetigers; subtriangular and reaching basis of ventral ligule in last posterior chaetigers ().
Pygidium bearing two long anal cirri, as long as the last six chaetigers. Anus terminal, striated ().
Notoacicula in first two chaetigers thinner than neuroaciculae, equaling width of neuroacicula on the following chaetigers.
Notochaetae homogomph spinigers present in all chaetigers (). Spiniger blades of different sizes, bearing long teeth, with long blades on upper spinigers. More or less same size as neuropodial spinigers on anterior and medium chaetigers, decreasing abruptly in size on posterior chaetigers.
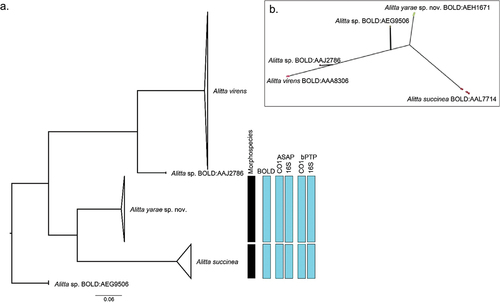
Figure 5. Molecular-based species delineation. (a) maximum likelihood (ML) phylogeny based on cytochrome c oxidase subunit I (CO1), with information regarding the different Molecular operational taxonomic units (MOTU) delineation methods. (b) PhyloMap visualisation of Poisson tree partitions (PTP) species delimitation result. Variance explained by first and second axes: 80.35% and 16.04%, respectively. The circles represent the different haplotypes used for the analysis; the colour is relative to the result of the PTP delimitation. The complete ML phylogeny, without collapsed clades, is accessible in Suppl. file S5.
Neurochaetae in upper fascicle include homogomph spinigers and heterogomph falcigers (). Spinigers of different sizes, decreasing blade extension in lower spinigers, long blades throughout, longer than those from lower fascicle. Falcigers are different in size, terminal tendon the same length as the other teeth.
Neurochaetae in lower fascicle include heterogomph spinigers () and heterogomph falcigers. Spinigers of different sizes, longer blades in the uppermost, decreasing gradually in size in last posterior chaetigers. Falcigers are differently sized; terminal tendon is same length as the other teeth; anterior chaetigers with shorter blade than medium chaetigers, decreasing in size on posterior chaetigers.
Live colouration
Dark brown on dorsal surface, with orange dorsal stripes over parapodia basis. Pygidium and anal cirri brown, less pigmented.
Preserved colouration. General body colouration dark brown on dorsum, bearing oblique lateral stripes on first anterior chaetigers and small pale points near the parapodia basis ()). Ventral surface colourless, whitish (). Palps, prostomium and cirrophores are darkly pigmented also (). Parapodia with coral ventral glandular integument in dorsal ligule.
Habitat: Fouling communities in buoys, intertidal mudflats. Oligo- and mesohaline sectors of the estuaries.
Type locality: Antonina Bay, Estuarine Complex of Paranaguá, Brazil.
Distribution: Southern Brazil.
Remarks
The main morphological differences among species of Alitta with pennant-shaped dorsal ligules (i.e. A. succinea, A. acutifolia, and A. yarae sp. nov.) are summarised in . Alitta yarae is most similar to A. succinea in having expanded dorsal ligules with glandules only on the inferior edge, having yellow-amber mandibles, and long-blade notopodial homogomph spinigers. The two species differ, however, in the following features: (1) extension of bare space between areas VI and VII–VIII, reduced in A. yarae sp. nov. and wider in A. succinea (respectively, shorter than or equal to the palpophore). (2) Dorsal cirri size in anterior chaetigers, 1.2 times length of dorsal ligule in A. yarae sp. nov. and same length in A. succinea. (3) Dorsal cirri size in posterior chaetigers, 0.2 times length of dorsal ligule in A. yarae sp. nov., and 0.3 times length in A. succinea. (4) Dorsal cirrus is terminal on last 24–27 posterior chaetigers in A. yarae sp. nov., whereas in A. succinea it is terminal on last 18–22 chaetigers. (5) Ventral surface of dorsal ligule in posterior part of the body, rounded in A. yarae sp. nov., and straight in A. succinea. (6) Paragnath distribution in A. yarae sp. nov. is II = 11–26, III = 11–22, IV = 10–31, V = 0–4, and VII−VIII = 40–64, compared with II = 19–22, III = 32–37, IV = 22–25, V = 1–3, and VII−VIII = 50–55. So far, A. yarae sp. nov. has been found only in three southern Brazilian estuaries, while A. succinea has a wider distribution along the Brazilian coast and the Atlantic Ocean (e.g. Sampieri et al. Citation2021; Alves et al. Citation2023). Concerning the distribution along the estuaries, in the bays where both species were found (i.e. PEC and Babitonga Bay), they never co-occurred in the same sampling site or sector, and A. yarae sp. nov. seemed to be restricted to the oligo- and mesohaline sectors (; ). These observations are in accordance with Mucciolo et al. (Citation2021), who found that Alitta yarae sp. nov. (referred to in the paper as Alitta sp.), seemed to better tolerate and regulate its cell volume in response to lower salinities. However, when A. succinea is not present (i.e. Guaratuba Bay), A. yarae sp. nov. occupies both the oligohaline sectors and the polyhaline ones, confirming the euryhalinity of this species, already pointed out by Mucciolo et al. (Citation2021).
Table I. Comparison of Alitta yarae sp. nov. with the most similar congeneric species. DC = dorsal cirrus, DL = dorsal ligule.
Table II. Species, process ID, amplified loci, habitat details, and geographical coordinates for the annelid specimens used in this work.
Molecular-based species delineation
The CO1 barcode was successfully sequenced from 41 specimens (32 for Alitta yarae sp. nov., and nine for A. succinea), while only for A. yarae sp. nov. we sequenced nine 16S and two 28S (. The initial BLAST search of their CO1 barcode sequences retrieved either A. succinea specimens sequenced by Villalobos-Guerrero and Carrera-Parra (Citation2015) with > 99% identity, or various nereidid public sequences with less than 85% identity, suggesting a different species for the latter. The high similarity of the public sequences with those retrieved from Brazil confirm the presence of this supposedly widespread species in the study area.
The mean K2P distance between species was 20.56%, which is several times higher than the common threshold for species (i.e. ~3%; Hebert et al. Citation2003; Teixeira et al. Citation2022). The distance within species was lower in A. yarae sp. nov. (mean: 0.39%, max: 0.9%) compared to A. succinea (mean: 1.05%, max: 2.66%). Alitta yarae sp. nov. displayed a general Hd of 0.813, with values between 0.800 and 0.905 for the different bays. Alitta succinea showed a similar general Hd (i.e. 0.833) but a higher difference between the sites (i.e. 0.500 and 1.000); however, the number of specimens was fewer than 10 per bay. The nucleotide diversity was below 0.005 for A. yarae sp. nov. while for the PEC A. succinea had a value of 0.015 ().
Table III. Genetic diversity of the species found in the study area. Abbreviations: n, Number of sequences; hap, number of haplotypes; Hd, haplotype diversity; π, nucleotide diversity. The table includes Tajima’s D, Fu’s FS and their respective p values.
Moreover, all the molecular species delimitations (i.e. BINs, ASAP, bPTP, PhyloMap-PTP) performed on both CO1 and 16S always recovered two different MOTUs (). The ML phylogeny recovered three well-supported clades (bootstrap >94%) belonging to A. virens, A. succinea, and A. yarae sp. nov. and two singletons (). The CO1 and 16S phylogenies were virtually identical (Suppl. files S5–S6).
Figure 6. Haplotype network based on CO1 for Alitta succinea and A. yarae sp. nov. Each haplotype is represented by a circle, the size of which corresponds to the number of haplotypes as indicated in the displayed scale. Colours indicate the geographic location of the haplotype. Numbers in parentheses refer to the relative haplotype (see Suppl. Table S3).
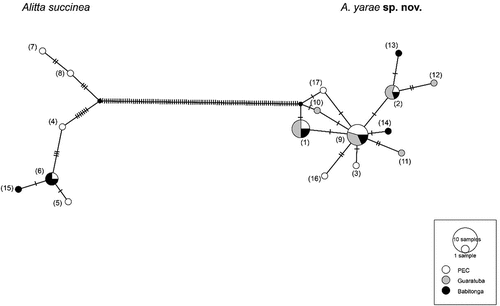
The haplotype network was split into two clades with > 100 mutations of difference, further supporting the molecular delimitations. Alitta yarae sp. nov. shared haplotypes among the three estuaries, presenting also unique haplotypes for each one. On the other hand, A. succinea was not found in Guaratuba Bay, and one out of six haplotypes was shared between the remaining bays, while two haplotypes occurring in the PEC displayed > 10 mutations’ difference from the closest other sequence (). Neither the Fst nor the AMOVA (Suppl. Table S2) returned significant results, indicating the lack of any spatial segregation, as also suggested by the shared haplotypes. The neutrality tests were both significantly negative only for A. yarae sp. nov., suggesting recent population expansion and rejecting the null hypothesis of equilibrium (). Moreover, the correlation of the genetic and the geographic distances was not significant, supporting the general pattern of high connectivity among the estuaries studied. The high abundance of rare haplotypes for A. succinea, together with its disjunct presence – limited to the bays with active ports – suggest that multiple introductions have occurred via shipping. In fact, ports are well known to act as “sinks” for non-indigenous species, which are constantly supplied with new propagules from international shipping (Gollasch Citation2006; Seebens et al. Citation2013). We must underline that there was possibly undersampling, and more haplotypes may be present for both species (especially for A. succinea); however, our results provide an important baseline for future studies involving more genes and specimens. Future studies should also address the status of A. succinea as invasive, introduced or already naturalised (Darling & Carlton Citation2018) to determine whether there is any risk to the native fauna, such as A. yarae sp. nov.
This study supports the recent belief that true cosmopolitan species may rarely occur nowadays without anthropogenic action. In fact, although the presence of A. succinea was confirmed in this study for the estuaries with international ports, it is possible that several records ascribed to it along the Brazilian coast are actually other species, such as A. yarae sp. nov. Furthermore, the presence of a congeneric species possibly native to the study area, together with the widespread and aggressive A. succinea, shows once again the risk posed by the taxonomic impediment of losing unknown species before their discovery, and the need for more integrative studies.
Supplemental Material
Download MS Word (17.2 KB)Supplemental Material
Download MS Word (14 KB)Supplemental Material
Download MS Word (16.4 KB)Supplemental Material
Download MS Word (93.4 KB)Supplemental Material
Download MS Word (1 MB)Supplemental Material
Download MS Word (12.7 KB)Supplemental Material
Download MS Word (14.5 KB)Supplemental Material
Download MS Word (13.9 KB)Supplemental Material
Download MS Word (12.8 KB)Acknowledgements
The authors are grateful to Prof. Paulo Lana and honour him with this manuscript. He supervised the PhD of SM and RCA, and they thank him for the constructive discussions, moments and experiences shared, and for always supporting them. The authors are also grateful to Prof. Nataliya Budaeva from the Department of Natural History, University Museum of Bergen, for her financial support to perform both morphological and molecular studies of the species in Norway. The authors thank Louise Lindblom from the University of Bergen, for her help with molecular work, and the Department of Invertebrate Zoology and Hydrobiology, University of Lodz, for their facilities and their scientific and technical assistance with SEM. The authors also thank Prof. Emanuel Maltempi de Souza and Valter Baura from the Department of Biochemistry of the University Federal do Paraná for assisting in the molecular work and for the access to their laboratories in Brazil. The authors thank Prof. Maria Angelica Haddad for her continuous efforts in the study of the Brazilian fouling community that contributed to the discovery of this species. Finally, the authorsthank the reviewers, including Prof. Torkild Bakken, for their detailed and constructive comments that considerably improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2269199
Additional information
Funding
References
- Alves PR, Halanych KM, Silva EP, Santos CSG. 2023. Nereididae (Annelida) phylogeny based on molecular data. Organisms Diversity & Evolution 23(3):529–541. DOI:10.1007/s13127-023-00608-9.
- Bakken T, Glasby CJ, Santos CSG, Wilson RS. 2022. Nereididae Blainville, 1818. In: Purschke G, Böggemann G, Westheide W, editors. Handbook of zoology online. Volume 4 Pleistoannelida, Errantia II. Germany: De Gruyter GmbH. pp. 259–307.
- Bakken T, Glasby CJ, Wilson RS. 2009. Zoosymposia 2: A review of paragnath morphology in Nereididae (Polychaeta). Zoosymposia 2(1):305–316. Available: www.mapress.com/zoosymposia/.
- Bakken T, Wilson RS. 2005. Phylogeny of nereidids (Polychaeta, Nereididae) with paragnaths. Zoologica Scripta 34(5):507–547. DOI:10.1111/j.1463-6409.2005.00200.x.
- Bigarella JJ. 2001. Contribuição ao Estudo da Planície Litorânea do Estado do Paraná. Brazilian Archives of Biology and Technology jubilee 65–110. DOI:10.1590/S1516-89132001000500005.
- Blagoev GA, deWaard JR, Ratnasingham S, deWaard SL, Lu L, Robertson J, Telfer AC, Hebert PDN. 2016. Untangling taxonomy: A DNA barcode reference library for Canadian spiders. Molecular Ecology Resources 16(1):325–341. DOI:10.1111/1755-0998.12444.
- Clement M, Posada D, Crandall KA. 2000. TCS: A computer program to estimate gene genealogies. Molecular Ecology 9(10):1657–1659. DOI:10.1046/j.1365-294x.2000.01020.x.
- Darling JA, Carlton JT. 2018. A framework for understanding Marine cosmopolitanism in the anthropocene. Frontiers in Marine Science 5:293. DOI:https://doi.org/10.3389/fmars.2018.00293.
- Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10(3):564–567. DOI:10.1111/j.1755-0998.2010.02847.x.
- Fu Y. 1997. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147(2):915–925. DOI:10.1093/genetics/147.2.915.
- Ghasemi AF, Taheri M, Jam A. 2013. Does the introduced polychaete Alitta succinea establish in the Caspian Sea? Helgoland Marine Research 67(4):715–720. DOI:10.1007/s10152-013-0356-1.
- Gillet P, Surugiu V, Vasile R, Metais I, Mouloud M, Simo P. 2011. Preliminary data on population dynamics and genetics of Alitta succinea (Polychaeta: Nereididae) from the Romanian coast of the Black Sea. Italian Journal of Zoology 78(sup1):229–241. DOI:10.1080/11250003.2011.593347.
- Glasby CJ, Wei N-WV, Gibb KS. 2013. Cryptic species of Nereididae (Annelida: Polychaeta) on Australian coral reefs. Invertebrate Systematics 27(3):245. DOI:10.1071/IS12031.
- Gollasch S. 2006. Overview on introduced aquatic species in European navigational and adjacent waters. Helgol Mar Res 60:84–89. DOI:10.1007/s10152-006-0022-y.
- Guindon S, Dufayard J, Lefort V, Anisimova M, Hordijk WOG, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3 .0. Systematic Biology 59(3):307–321. DOI:10.1093/sysbio/syq010.
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003. Biological identifications through DNA barcodes. Proceedings of the Royal Society of London: Series B: Biological Sciences 270(1512):313–321. DOI:10.1098/rspb.2002.2218.
- Hillis DM, Mable BK, Larson A, Davis SK, Zimmer EA. 1996. Nucleic acids IV: Sequencing and cloning. In: Hillis DM, Moritz C, Mable BK, editors. Molecular systematics. 2nd ed. Sunderland, Massachusetts: Sinauer. pp. 321–381.
- IBAMA. 1998. Proteção e controle de ecossistemas costeiros: manguezal da Baía de Babitonga. Brasilia, Brazil: IBAMA.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30(4):772–780. DOI:10.1093/molbev/mst010.
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16(2):111–120. DOI:10.1007/BF01731581.
- Kuhl DL, Oglesby LC. 1979. Reproduction and survival of the pileworm Nereis succinea in higher Salton Sea salinities. The Biological Bulletin 157(1):153–165. DOI:10.2307/1541084.
- Lana PC, Bernardino AF. 2018. Brazilian estuaries. A benthic perspective (Lana PC, Bernardino AF, editors). Cham, Switzerland: Springer. DOI:10.1007/978-3-319-77779-5.
- Lefort V, Longueville J-E, Gascuel O. 2017. SMS: Smart model selection in PhyML. Molecular Biology and Evolution 34(9):2422–2424. DOI:10.1093/molbev/msx149.
- Librado P, Rozas J. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451–1452. DOI:10.1093/bioinformatics/btp187.
- Marone E, Machado EC, Lopes RM, Silva ETD. 2005. Land-ocean fluxes in the Paranaguá Bay estuarine system, southern Brazil. Brazilian Journal of Oceanography 53(3–4):169–181. DOI:10.1590/S1679-87592005000200007.
- Marone E, Noernberg MA, Dos Santos I, Lautert LF, Andreoli OR, Buba H, Fill HD. 2006. Hydrodynamic of Guaratuba Bay, PR, Brazil. Journal of Coastal Research 3:1879–1883.
- Mucciolo S, Desiderato A, Leal SM, Mastrodonato M, Lana P, Freire CA. 2021. Variability in the degree of euryhalinity of neotropical estuarine annelids. Journal of Experimental Marine Biology and Ecology 544:151617. DOI:10.1016/j.jembe.2021.151617.
- Neuhoff HG. 1979. Effects of seasonally varying factors on a Nereis succinea population (Polychaeta, Annelida). Marine Ecology Progress Series 1:263–268. DOI:10.3354/meps001263.
- Nygren A, Parapar J, Pons J, Meißner K, Bakken T, Kongsrud JA, Oug E, Gaeva D, Sikorski A, Johansen RA, Hutchings PA, Lavesque N, Capa M, Chiang T-Y. 2018. A mega-cryptic species complex hidden among one of the most common annelids in the North East Atlantic. PLOS ONE 13(6):e0198356. DOI:10.1371/journal.pone.0198356.
- Puillandre N, Brouillet S, Achaz G. 2021. ASAP: Assemble species by automatic partitioning. Molecular Ecology Resources 21(2):609–620. DOI:10.1111/1755-0998.13281.
- Puillandre N, Lambert A, Brouillet S, Achaz G. 2012. ABGD, automatic barcode gap discovery for primary species delimitation. Molecular Ecology 21(8):1864–1877. DOI:10.1111/j.1365-294X.2011.05239.x.
- Rambaut A. 2010. FigTree v1.3.1. Edinburgh: Institute of Evolutionary Biology, University of Edinburgh. https://tree.bio.ed.ac.uk/software/figtree/.
- Ratnasingham S, Hebert PDN. 2007. BARCODING: Bold: The barcode of life data system. Molecular Ecology Notes 7(3):355–364. Available: http://www.Barcodinglife.org.
- Ratnasingham S, Hebert PDN. 2013. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 8(7):e66213. DOI:10.1371/journal.pone.0066213.
- Ribeiro RP, Alves PR, de Almeida ZDS, Ruta C. 2018. A new species of Paraonis and an annotated checklist of polychaetes from mangroves of the Brazilian Amazon coast (Annelida, Paraonidae). ZooKeys 740:1–34. DOI:10.3897/zookeys.740.14640.
- Rioja E. 1946. Estudios anelidológicos. 15. Nereidos de agua salobre de los esteros del litoral del Golfo de México. Anales del Instituto de Biología, Universidad Nacional Autónoma de México 17:205–214.
- Sampieri BR, Vieira PE, Teixeira MAL, Seixas VC, Pagliosa PR, Amaral ACZ, Costa FO. 2021. Molecular diversity within the genus Laeonereis (Annelida, Nereididae) along the west Atlantic coast: Paving the way for integrative taxonomy. PeerJ 9:e11364. DOI:10.7717/peerj.11364.
- Seebens H, Gastner MT, Blasius B. 2013. The risk of marine bioinvasion caused by global shipping. Ecol Lett. 16:782–790. DOI:10.1111/ele.12111.
- Simon CA, Sato-Okoshi W, Abe H. 2019. Hidden diversity within the cosmopolitan species Pseudopolydora antennata (Claparède, 1869) (Spionidae: Annelida). Marine Biodiversity 49(1):25–42. DOI:10.1007/s12526-017-0751-y.
- Tajima F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123(3):585–595. DOI:10.1093/genetics/123.3.585.
- Teixeira MAL, Langeneck J, Vieira PE, Hernández JC, Sampieri BR, Kasapidis P, Mucciolo S, Bakken T, Ravara A, Nygren A, Costa FO. 2022. Reappraisal of the hyperdiverse Platynereis dumerilii (Annelida: Nereididae) species complex in the Northern Atlantic, with the description of two new species. Invertebrate Systematics 36(11):1017–1061. DOI:10.1071/IS21084.
- Teixeira MAL, Vieira PE, Pleijel F, Sampieri BR, Ravara A, Costa FO, Nygren A. 2020. Molecular and morphometric analyses identify new lineages within a large Eumida (Annelida) species complex. Zoologica Scripta 49(2):222–235. DOI:10.1111/zsc.12397.
- Villalobos-Guerrero TF, Bakken T. 2018. Revision of the Alitta virens species complex (Annelida: Nereididae) from the North Pacific Ocean. Zootaxa 4483(2):201–257. DOI:10.11646/zootaxa.4483.2.1.
- Villalobos-Guerrero TF, Carrera-Parra LF. 2015. Redescription of Alitta succinea (Leuckart, 1847) and reinstatement of A. acutifolia (Ehlers, 1901) n. comb. based upon morphological and molecular data (Polychaeta: Nereididae). Zootaxa 3919(1):157. DOI:10.11646/zootaxa.3919.1.7.
- Virgilio M, Fauvelot C, Costantini F, Abbiati M, Backeljau T. 2009. Phylogeography of the common ragworm Hediste diversicolor (Polychaeta: Nereididae) reveals cryptic diversity and multiple colonization events across its distribution. Molecular Ecology 18(9):1980–1994. DOI:10.1111/j.1365-294X.2009.04170.x.
- Wang H, Qi M, Cutler AJ. 1993. A simple method of preparing plant samples for PCR. Nucleic Acids Research 21(17):4153–4154. DOI:10.1093/nar/21.17.4153.
- Zhang J, Kapli P, Pavlidis P, Stamatakis A. 2013. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29(22):2869–2876. DOI:10.1093/bioinformatics/btt499.
