Abstract
Here we describe a new species of Ochthebius (Cobalius) from marine rockpools of NW Sicily: O. (C.) senczuki sp. n. This species was first discovered on the basis of molecular evidence, but its taxonomic distinction has been subsequently supported by morphological characters. Ochthebius (C.) senczuki sp. n. shows a clear genetic diversity from the known related species, with an average COI p distance ranging from 5.6% to 10.7%; it exhibits only small morphological differences, mostly limited to dorsal punctation, pronotal and elytral outer edge, and (in males) to the mobile part of the median lobe of the aedeagus. The origin of O. (C.) senczuki sp. n. is estimated to lie in the Late Miocene (~9 Mya); the ancestors of this taxon probably remained isolated from populations of the related taxa of the Ochthebius (Cobalius) subinteger and O. (Cobalius) adriaticus complexes during cycles of regressive and transgressive marine phases that occurred in the SW Mediterranean during Late Miocene and Pliocene. The detailed state of the art of members of the O. (Cobalius) adriaticus complex throughout the Central and Eastern Mediterranean rocky coastal areas is also preliminarily discussed.
http://zoobank.org/urn:lsid:zoobank.org:pub:CBFA5063-2FBE-488B-8497-E5DA7DCA9361
Introduction
Rocky shores and marine coastal rockpools frequently host a specialized aquatic microfauna (Antonini et al. Citation2010; Audisio et al. Citation2010; Sabatelli et al. Citation2016, Citation2018, Citation2021a, Citation2021b; Rosenfeld et al. Citation2019; Vecchioni et al. Citation2019, Citation2021) mostly found in the supralittoral zone. Marine rockpools are extreme habitats, filled by splashes of sea water during high tides or storms, or by rain, and are highly variable in size, water depth, elevation above sea level, distance from the sea water front, average temperature, relative salinity, and the local composition and density of microalgal flora (Underwood & Skilleter Citation1996; Firth & Williams Citation2009; Firth et al. Citation2014). Among the invertebrates adapted to the harsh and ever-changing environment of the supratidal zone, several members of the beetle family Hydraenidae, genus Ochthebius, are known to inhabit (at both larval and imaginal stages) the hypersaline marine rock pools along the east Atlantic, Mediterranean, Macaronesian and Southern Africa coasts (Jäch Citation1989, Citation1993; Delgado & Soler Citation1995, Citation1997; Urbanelli et al. Citation1996; Turner Citation2004; Audisio et al. Citation2010; Antonini et al. Citation2010; Sabatelli et al. Citation2013, Citation2016, Citation2018, Citation2021a, Citation2021b; Jäch & Delgado Citation2017; Ribera & Foster Citation2018; Ribera & Cieslak Citation2019; Villastrigo et al. Citation2020a, Citation2020b, Citation2022a, Citation2022b; Mirón-Gatón et al. Citation2022; Velasco et al. Citation2022), Australia (Perkins Citation2007; Villastrigo et al. Citation2019), East Asia and the west coast of North America (Jäch & Delgado Citation2014; Villastrigo et al. Citation2019). The genus Ochthebius Leach, 1815 (Coleoptera: Hydraenidae: Octhebiinae) includes more than 500 aquatic or semi-aquatic species worldwide, mostly distributed in Palearctic, Nearctic, and Oriental regions, with a still increasing number of new species recently discovered in almost all continents (Audisio et al. Citation2010; Jäch & Delgado Citation2017; Yoshitomi et al. Citation2019; Villastrigo et al. Citation2020b; Bilton Citation2021). Within this widespread genus, in the Atlantic–Mediterranean coastal areas two lineages are recorded as regular and nearly obligate colonizers of marine rockpools, i.e. members of the subgenus Cobalius Rey, 1869, and members of the “O. quadricollis group” (as recently considered by Sabatelli et al. (Citation2016) and Villastrigo et al. (Citation2019, Citation2020a, Citation2022b)), within the species-rich and highly diversified nominal subgenus Ochthebius s.str.
Recently, Sabatelli et al. (Citation2016, Citation2021b) and Villastrigo et al. (Citation2020b) detected the presence of a remarkable number of new NE Atlantic and Mediterranean cryptic taxa of Cobalius and of the “Ochthebius quadricollis group”, clearly evidencing in both groups the presence of at least four types of “hidden” biodiversity, frequently combined with one another: (1) true cryptic species, morphologically indistinguishable, although separated by high genetic divergence based on mitochondrial markers (e.g. COI mean p-distance >6–7%) and difficult to discover without availability of molecular data; (2) morphologically distinct but exceptionally rare species, in most cases confused among thousands of specimens of common similar taxa, and then almost impossible to discover without massive sampling in each explored locality; (3) species with very limited geographic ranges, almost impossible to discover without an extended, complete, and careful geographic coverage of the rocky coastal areas of the whole Mediterranean basin and of the NE Atlantic, including small islands and Macaronesia; (4) species apparently specialized only to peculiar microhabitats represented by ecologically different types of marine rockpools or of other coastal salt habitats, such as below boulders in gravel and among coarse sands in the intertidal zone (Villastrigo et al. Citation2020b), or along the shores of salt coastal wetlands (Bennas et al. Citation2013) and isolated salt ponds, or even in backwaters of coastal saline streams (A. Millàn, pers. comm., 2016).
This situation is particularly evident in members of the subgenus Cobalius, which usually exhibit higher levels of ecological specialization and geographic endemization (Villastrigo et al. Citation2020b; Sabatelli et al. Citation2021b; Mirón-Gatón et al. Citation2022; Velasco et al. Citation2022), the latter condition probably associated with a reduced dispersal ability and demographic success (Velasco et al. Citation2022), if compared with the frequently syntopic members of the “O. quadricollis group”.
In this general scenario, as a result of recent additional research on the Ochthebius of the marine rockpools in southern Italy by Sabatelli et al. (Citation2021b), we report here the discovery of a new morphologically and molecularly distinct species of Cobalius, O. (C.) senczuki sp. n. from NW Sicily. The new species was first detected only based on molecular evidence, but its taxonomic distinction has been subsequently supported by morphological characters. In the same study (Sabatelli et al. Citation2021b), using the Bayesian relaxed phylogenetic approach, the possible origin of this new species was estimated in the Late Miocene (~9 Mya). The present paper is therefore devoted to formally describing this new species of Cobalius, and to briefly discussing the state of the art of the interesting clade of Cobalius (to which our new Sicilian species could be also related) including O. (C.) adriaticus from Central and Eastern Mediterranean areas and its problematic subspecies and/or related cryptic species.
Materials and methods
Abbreviations
Abbreviations of museum institutions are as follows:
BCP—D. Bilton’s collection, University of Plymouth, UK
CAR-MZUR—P. Audisio’s collection, currently housed in the Zoological Museum, Sapienza Rome University, Rome, Italy
Measurements, drawings and photographs
Color photographs of the new species herein described () were taken in the Inter-departmental Laboratory of photo stereomicroscopy of the Sapienza Rome University (Department of Environmental Science), using a Leica® IC80 HD digital camera mounted on a Leica® M205C stereomicroscope, with a PLANAPO® 1.0× lens. The high-definition images were acquired through the LAS® v. 4 acquisition program and then processed with the Helicon Focus® 7 software package.
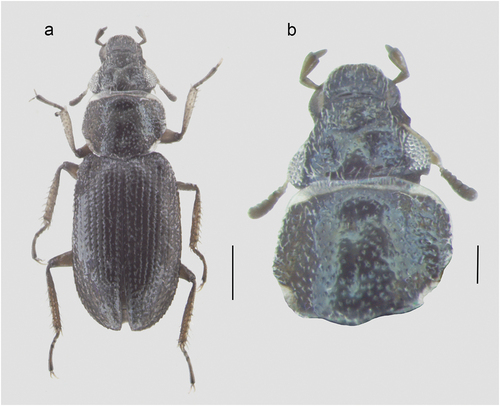
Figure 1. Holotype of O. (Cobalius) senczuki sp. n.; (a) dorsal habitus; (b) head and pronotum. Scale bars: a = 0.3 mm; b = 0.1 mm.
Measurements of adult external anatomical features were made using a digital camera mounted on a WILD® MZ8 stereomicroscope (40–80×), and the image processing software package WINVISION® (Delta Sistemi®, Rome). Photos of the male genitalia of the new species () were obtained using a ZEISS®Axio Zoom V16 microscope with an APO Z® 1.5× lens, acquired through the ZEN® Toolkit acquisition software and then processed with the Helicon Focus® 7 software package. The drawing of the male genitalia of the new species () was made with a drawing tube mounted on an a B×50OLYMPUS® upright microscope (200–1000×). Measurements of tarsal, antennal, and genitalic characters were made using the same device and software.
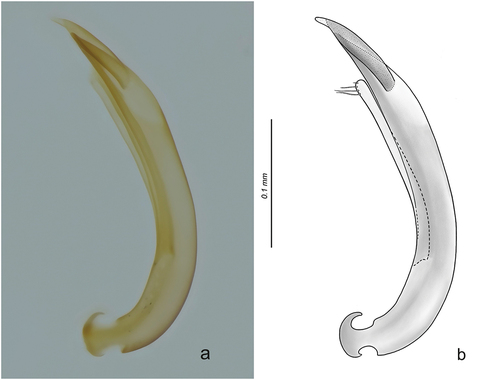
Figure 2. Aedeagus of O. (Cobalius) senczuki sp. n.; (a) (male holotype from Riserva Naturale Orientata dello Zingaro, Sicily, Italy), ZEISS® Axio Zoom V16 microscope photo, lateral view; (b) accurate drawing of the same (BX50 OLYMPUS® upright microscope, 400×), better evidencing the structure of the distal mobile lobe of the aedeagus (drawn slightly darker than reality, to better highlight its structure) and of a paramere. Scale bar: 0.1 mm.
Taxonomy: species description
Ochthebius (Cobalius) senczuki sp. n.
()
Type locality
ITALY: Sicily, Riserva Naturale dello Zingaro (Zingaro Nature Reserve), Trapani province, 38°05′17″N, 12°48′21″E ().
Type material
Holotype male (CAR-MZUR) Italy, Sicily, Riserva Naturale dello Zingaro (Trapani province), Cala Capreria, 38°05′17″N, 12°48′21″E, 19 May 2021, S. Sabatelli & P. Audisio leg., with red holotype label. Aedeagus dissected and mounted in Euparal® on the card bearing the specimen. Not used for DNA extraction and sequencing, but for microscope photos (). Paratypes: same locality data as for holotype, 4 November 2015, S. Sabatelli leg., 1 ♂ (CAR-MZUR); ibidem, 22 May 2013, G. Senczuk leg., 1 ♂ (CAR-MZUR); ibidem, S. Sabatelli leg., 4 December 2015, ♂ (CAR-MZUR); Italy, Sicily, Terrasini (Palermo province), 38°09′15″N, 13°09′28″E, 7 June 2015, S. Sabatelli & P. Audisio leg., 2 ♂♂, 1 ♀ (CAR-MZUR, BCP). Five additional male specimens from Sicily, Riserva Naturale dello Zingaro, 22 May 2013, G. Senczuk leg., not included in the type series, have been used for molecular analyses (Sabatelli et al. Citation2021b), their male genitalia having been previously dissected and preserved for morphological comparisons.
Diagnosis
A small-sized Ochthebius (Cobalius) (body length: 1.68–1.90 mm) similar in general shape to the more closely related species of the O. (Cobalius) subinteger species complex (: O. (Cobalius) subinteger and O. (Cobalius) lejolisi), but characterized by other traits shared with members of the O. (Cobalius) adriaticus complex (), i.e. (, ) more flattened pronotum and elytra, dorsal surface between punctures rather smooth and shining, pronotal punctures separated from one another by ca. 2× their diameter, dark dorsal coloration (blackish to brown-blackish with barely distinct metallic hues: ), lateral edges of pronotum very simple, with only barely distinct microscopical and blunt tegumental teeth directed outward, lateral edges of elytra with smaller, shorter and blunter tegumental teeth directed outward, and slightly distinct male genitalia (). See for a list of the main characters useful to distinguish the new species from the likely more related Mediterranean taxa. Additionally, as reported by Sabatelli et al. (Citation2021b), this new species exhibits a genetic COI mean p-distance (Table S1 and accession numbers MW369112–MW369174; MW369083–MW369085) between 5.6% and 10.7% from Italian (including Sicilian) and W European populations of the related O. subinteger and O. lejolisii and of ca. 6.0–7.5% from the perhaps less closely related and markedly larger members of the O. adriaticus complex, including a syntopic population only tentatively referred to O. adriaticus (see also discussion below).
Table I. Genus Ochthebius Leach 1815, subgenus Cobalius Rey, 1886: list (in roughly phylogenetic order) of 18 included putative species, with recognized synonymies or homonymies (indented), as well as data on their geographic distribution. Refer to the main text for the here proposed re-evaluation at the specific rank of the three supposed subspecies of O. adriaticus throughout the Mediterranean. All known species are associated with marine rockpools, particularly those shallow (2–10 cm deep), small (20–70 cm wide) and positioned relatively farther from the sea (ca. up to 10 m high and inland from the sea), with the exception of the isolated O. serratus, present in southern Spain and northern Morocco in salty coastal wetlands and along backwaters of coastal salty streams.
Table II. Comparative table of the main diagnostic characters of Ochthebius (Cobalius) senczuki sp. n. In relation to other members of the more closely related Mediterranean species or species complexes (the subinteger and adriaticus complexes) of Cobalius. O. (C.) celatus, which based on external morphology should be attributed to the O. (C.) subinteger complex (Jäch Citation1989), exhibits quite distinct male genitalia more recalling those of members of the O. (C.) adriaticus complex (Jäch Citation1989; Villastrigo et al. Citation2020b), as well as a molecular positioning fully confirming its closer genetic relationships with the latter complex (Villastrigo et al. Citation2020b; Sabatelli et al. Citation2021b). For these combined reasons it is here considered separately from both the subinteger and adriaticus complexes. For our estimated values of COI genetic distances among the same taxa, see table S1 herein.
Description
Habitus (male holotype) as in . Length: 1.70 mm; width: 0.62 mm. Body color uniformly shiny dark brown, with pale brown appendages. Frons without distinct microreticulation, shiny (only feeble traces of isodiametric meshes close to the eyes), and with two distinct symmetrical and elongate foveae between eyes; with sparse setiferous punctures associated with short, thick silvery setae. Surface of clypeus and labrum shiny, with very sparse punctation and with setae shorter and finer than on frons; anterior margin of labrum evenly arcuated, in the middle with a barely distinct sinuosity. Eyes large, prominent. Without ocelli.
Pronotum () hexagonal, lateral margins rounded anteriorly, markedly and abruptly narrowed posteriorly, almost indistinctly, obtusely and irregularly serrated along its outer lateral edges; surface smooth and shiny, with very sparse small punctures and irregular, larger setiferous pits of irregular shape and different diameters, less dense on disk, smaller and denser laterally, separated from one another by 1–2 diameters. Anterior angles roundly obtuse, posterior ones distinct but widely obtuse; with a narrow hyaline band at anterior and posterior margins, nearly as wide as antennal club.
Elytra () elongate, rather parallel-sided, oval distad; lateral margins rather narrowly flattened, regularly and very feebly arched, serrated through their length, with very short but distinct and sharp, spaced denticles, oriented posteriorly. Surface with rather regular series of shallow setiferous punctures, with short, fine and recurved silvery setae; surface uniformly smooth and shiny, with small and irregular rugosities around punctures. Normally developed hind wings.
Legs () elongate and slender, tibiae with rows of strong, rather long spine-like setae along their outer edge, and with a couple of long, exceptionally thin and barely visible natatorial setae.
Ventral surface. Surface of metaventrite dull, with very small and very dense setiferous punctures, separated from one another by 0.5–1 diameters, and with small meshes and distinct microreticulation between punctures. Setae rather long and silvery-golden. Abdominal ventrites i–v densely pubescent, setae silvery-golden, nearly as long as those on metaventrite, but slightly longer and more distinct close to their posterior margins. Surface with a very distinct dull microreticulation.
Aedeagus (, lateral view) with main piece evenly curved, of uniform width, apex pointed. Distal lobe tubular, finger-like, only moderately sclerotized, with apex regularly narrowed, moderately pointed and hyaline distad. Parameres inserted near the basal third of the median lobe, not reaching its apex.
Variation
Body length: 1.70–1.90 mm, aedeagus with moderately variable mobile distal piece.
Etymology
Named after our friend and colleague Gabriele Senczuk (zoologist at the University of Molise, Italy), who first collected this species during a herpetological survey in SW Sicily.
Distribution
Only known from the extreme NW portion of Sicily, along rocky coastal areas between the SW Palermo Province and the NW Trapani province.
Habitat
All known specimens of the new species from both Riserva Naturale dello Zingaro and Terrasini were collected in small (ca. 15–50 cm wide) and shallow (ca. 2–15 cm deep) marine rockpools (together with a few specimens of O. sp. cfr. adriaticus and several specimens of the common O. quadricollis), between ca. 2 and 5 m a.s.l., on calcareous rocks ().
Comments on the state of the art of taxonomy and evolutionary relationships of members of the Ochthebius (Cobalius) adriaticus species complex
As discussed above, O. (C.) senczuki sp. n. was collected in the company of a few specimens morphologically referable to a putative population of O. adriaticus. But the latter exhibits a genetic COI mean p-distance between 3.6% and 4.4% from other Italian populations of the “true” O. adriaticus Reitter, Citation1886 (including those from E Sicily, the previously westernmost known populations of this taxon, if we exclude our unexpected record from W Sicily, Zingaro: see Sabatelli et al. Citation2021b and discussion below). This data allows us to hypothesize the possible existence of a further distinct and allopatric cryptic species of this complex in the same locality of the extreme NW Sicily. This situation has suggested to us to deepen some aspects, at the moment still rather problematic, of the evolutionary, biogeographical and taxonomic relationships involving the whole complex of species, widely distributed in most parts of the Central and Eastern N Mediterranean (Jäch Citation1989; Villastrigo et al. Citation2020b, Citation2022). In addition to the probably related O. (C.) senczuki sp. n. and the possible second new syntopic species just mentioned, the relations between the presumed oriental subspecies of O. adriaticus are in fact unclear and confusing.
The latter typical present-day subspecies (O. adriaticus s.str.) was described (Reitter Citation1886) from Croatia (Pula, Istria County), and it was later reported from most of the Adriatic and Jonian coasts of Italy, Slovenia, Croatia, Albania, NW Greece, and E Sicily (Jäch Citation1989). In the same paper Reitter (Citation1886) also published as a distinct species O. (C.) pleuralis from the Near East (“Syrien”, probably a locality in the coastal areas of the present-day SE Turkey, Syria, Lebanon or Israel); further reports of this taxon are from Israel and from Crete Island (S Greece: Villastrigo et al. Citation2022). This taxon has long been considered a synonym or an eastern subspecies of O. adriaticus (Jäch Citation1989). A third possible subspecies of O. adriaticus was later described by Pretner (Citation1929) from Peloponnese (S Greece) under the name O. (C.) adriaticus moreanus, and several records of this taxon were later reported from eastern continental Greece, Crete Island, and Samos Island (close to the Aegean coasts of W Turkey) (Jäch Citation1989; Villastrigo et al. Citation2022). It is interesting to highlight the recently recorded presence in sympatry in Crete of both O. adriaticus moreanus and O. adriaticus pleuralis (Villastrigo et al. Citation2022; presence confirmed also by our research team: unpublished data, June 2023), which strongly suggests that these two taxa, as well as very likely the allopatric O. adriaticus s.str., should represent distinct biological species, despite their rather low respective genetic distances (Villastrigo et al. Citation2020b, Citation2022). We have therefore introduced this tentative taxonomic framework in our above, where we decided to formalize (subject to further molecular data that may eventually modify our conclusions) the new taxonomic state of these three taxa. A clearer and more complete scenario for this puzzling species complex could be likely obtained from further molecular and morphological analyses carried out on populations of O. (Cobalius) adriaticus s.l. from coastal sites in the N Mediterranean, from western Sicily to Israel, to better clarify the occurrence and co-occurrence of the different known and unknown members of this group, as very appropriately discussed by Jäch (Citation1989).
Supplemental Material
Download ()Acknowledgements
We thank Dr. G. Senczuk (University of Molise, Campobasso), colleague and friend, who contributed to collecting many specimens from Sicily used in the present study, as well as our colleagues G. Sabella (University of Catania, Sicily) and F. Marrone (University of Palermo, Sicily). We are also grateful to our colleague and friend A. Millàn (Universidad de Murcia, Spain) for valuable material and useful information (2016) on Ochthebius (Cobalius) serratus in southern Spain and northern Morocco. Finally, two anonymous reviewers provided useful suggestions that have certainly helped to improve the quality of this paper. Also, we owe thanks to Prof. M. Iberite, Dr. C. Moricca and Dr. Aleida Ascenzi (Sapienza University of Rome) for their helpfulness and support in using the Leica® M205C stereomicroscope and ZEISS Axio Zoom V16 microscope.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2270989
Additional information
Funding
References
- Antonini G, Audisio P, Mancini E, de Biase A, Tronci C, Rossetti G, Trizzino M. 2010. Molecular phylogeography of two Italian sibling species of Calobius (Coleoptera, Hydraenidae, Ochthebiinae) inhabiting Mediterranean marine rock-pools. Marine Biology 157(2):371–381. DOI: 10.1007/s00227-009-1324-9.
- Audisio P, Trizzino M, de Biase A, Rossetti G, Mancini E, Antonini G. 2010. Molecular and morphological evidence of a new sibling species of Calobius (Coleoptera: Hydraenidae) of the C. quadricollis complex from peninsular Italy. Italian Journal of Zoology 77(1):29–37. DOI: 10.1080/11250000902845738.
- Bennas N, Benamar L, Millán A. 2013. Los ambientes salinos mediterráneos Un “Hot Spot” de endemismo de biodiversidad acuática: caso de Marruecos. Libro de actas del III Congreso internacional de geologia y mineria ambiental para el ordenamiento territorial y el desarrollo. Cardona – 2013. pp. 137–150.
- Bilton DT. 2021. Differentiation of South African coastal rock pool Ochthebius is associated with major ocean currents (Coleoptera: Hydraenidae). Acta Entomologica, Musei Nationalis Pragae 61(1):253–260. DOI: 10.37520/aemnp.2021.015.
- d’Orchymont A. 1932. Les rockpools submarines et leur population entomologique. Notes sur certains sous-genres d’Ochthebius. Bulletin et Annales de la Société Entomologique de Belgique LXXII:41–52.
- d’Orchymont A. 1940. Palpicornia des Iles Atlantiques. Mémoires du Musée royal d’histoire naturelle de Belgique 2(20):1–86.
- Delgado JA, Soler AG. 1995. Descripción de la larva de Ochthebius (Cobalius) subinteger Mulsant & Rey, 1861 (Coleoptera, Hydraenidae). Graellsia 51:121–128. DOI: 10.3989/graellsia.1995.v51.i0.402.
- Delgado JA, Soler AG. 1997. Morphology and chaetotaxy of larval Hydraenidae (Coleoptera) III: The genus Calobius Wollaston, 1854. Aquatic Insects 19:165–175. DOI: 10.1080/01650429709361651.
- Firth LB, Schofield M, White FJ, Skov MW, Hawkins SJ. 2014. Biodiversity in intertidal rock pools: Informing engineering criteria for artificial habitat enhancement in the built environment. Marine Environmental Research 102:122–130. DOI: 10.1016/j.marenvres.2014.03.016.
- Firth LB, Williams GA. 2009. The influence of multiple environmental stressors on the limpet Cellana toreuma during the summer monsoon season in Hong Kong. Journal of Experimental Marine Biology and Ecology 375(1–2):70–75. DOI: 10.1016/j.jembe.2009.05.011.
- Ghosh J, Saini J, Gupta D, Ghosh SK, Chandra K. 2022. A catalogue of Indian Hydraenidae (Insecta: Coleoptera). Zootaxa 5087(4):558–570. DOI: 10.11646/zootaxa.5087.4.4.
- Hansen M. 1998. World catalogue of Insects. Vol. 1. Hydraenidae (Coleoptera). Stenstrup: Apollo Books. pp 169.
- Hernando C, Villastrigo A, Ribera I. 2017. A new species of Micragasma J. Sahlberg, 1900 (Coleoptera: Hydraenidae) from Crete. Aquatic Insects 38(4):185–196. DOI: 10.1080/01650424.2017.1384024.
- Jäch MA. 1989. Revision of the Palearctic species of the genus Ochthebius LEACH II. The subgenus Cobalius REY (Hydraenidae, Coleoptera). Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen 41:41–51.
- Jäch MA. 1993. Revision of the Palaearctic species of the genus Ochthebius XI. The subgenus Calobius Wollaston, 1854 (Insecta: Coleoptera: Hydraenidae). Reichenbachia 30:4–7.
- Jäch MA, Delgado JA. 2014. Revision of the Palearctic species of the genus Ochthebius leach XXIX. The Asian species of the O. vandykei group (Coleoptera: Hydraenidae). Koleopterologische Rundschau 84:81–100.
- Jäch MA, Delgado JA. 2017. Revision of the Palearctic species of the genus Ochthebius leach, 1815 XXXII. Ochthebius (Cobalius) biltoni sp. n. From Sicily (Italy). Koleopterologische Rundschau 87:85–88.
- Mirón-Gatón JM, Botella-Cruz M, García-Meseguer AJ, Millán A, Velasco J. 2022. Discordant pattern between realised and fundamental saline niches in two supralittoral Ochthebius species (Coleoptera: Hydraenidae). Ecological Entomology 2022:1–11. DOI: 10.1111/een.13220.
- Mulsant ME, Rey C. 1861. Description d’un espèce nouvelle d’Ochthebius et la larve de cet insecte. Mémoires de la Société des Sciences Naturelles de Cherbourg 20:181–189.
- Perkins PD. 2007. A revision of the Australian intertidal water beetle genus Hughleechia Perkins (Coleoptera: Hydraenidae). Zootaxa 1527:17–29. DOI: 10.11646/zootaxa.1527.1.2.
- Pretner E. 1929. Neue Hydrophiliden aus dem östlichen Mittelmeergebiet. Coleopterologisches Centralblatt 3(5/6):199–205.
- Reitter E. 1886. Über die Ochthebius-Arten aus der Gruppe des O. Lejolisii Muls. Wiener Entomologische Zeitung 5:156–157. DOI:10.5962/bhl.part.20590.
- Ribera I, Cieslak A. 2019. New data on Ochthebius (Cobalius) algicola Wollaston from Madeira, with other records of interest. Latissimus 43:25–27.
- Ribera I, Foster GN. 2018. Report of Frank Balfour-Browne’s collecting in Gran Canaria and Madeira (1932-1933), with the description of Ochthebius (Cobalius) lanthanus sp. nov. (Coleoptera, Hydraenidae). Zootaxa 4524(1):65–76. DOI: 10.11646/zootaxa.4524.1.4.
- Rosenfeld S, Blaustein L, Kneitel J, Duchet C, Horwitz R, Rybak O, Polevikov A, Rahav E. 2019. The abundance and larval performance of Aedes phoeniciae in supralittoral rock-pools. Hydrobiologia 846(1):181–192. DOI: 10.1007/s10750-019-04063-6.
- Rosenhauer WG. 1856. Die Thiere Andalusiens. Erlangen: Verlag Theodor Blaesnig. pp. 429.
- Sabatelli S, Audisio P, Antonini G, Solano E, Martinoli A Trizzino M. 2016. Molecular ecology and phylogenetics of the water beetle genus Ochthebius revealed multiple independent shifts to marine rockpools lifestyle. Zoologica Scripta 45(2):175–186. DOI: 10.1111/zsc.12141.
- Sabatelli S, Audisio P, Di Giulio A. 2021a. Larval morphology of the water beetle Ochthebius balfourbrownei (Coleoptera: Hydraenidae) from marine rockpools of Cape Verde Islands. The European Zoological Journal 88(1):659–668. DOI: 10.1080/24750263.2021.1913248.
- Sabatelli S, Audisio P, Trizzino M, Di Giulio A. 2013. Description of the larva of Ochthebius capicola (Coleoptera: Hydraenidae) from marine rock-pools of South Africa. Zootaxa 3683:280–288. DOI: 10.11646/zootaxa.3683.3.4.
- Sabatelli S, Mancini E, Audisio P. 2018. Taxonomical and bionomical notes on the Sicilian endemic water beetle Ochthebius (Cobalius) biltoni (Coleoptera: Hydraenidae). Fragmenta Entomologica 50:75–76. DOI: 10.4081/fe.2018.289.
- Sabatelli S, Ruspantini P, Cardoli P Audisio P. 2021b. Underestimated diversity: Cryptic species and phylogenetic relationships in the subgenus Cobalius (Coleoptera: Hydraenidae) from marine rockpools. Molecular Phylogenetics and Evolution 163:107243. DOI: 10.1016/j.ympev.2021.107243.
- Sählberg J. 1900. Coleoptera Mediterranea et Rosso-Asiatica Nova vel Minus Cognita Itineribus Annis 1895–1896 et 1898–1899 collecta. Öfversigt af Finska Vetenskaps-Societetens Förhandlin-gar 42:174–208.
- Turner CR. 2004. Ochthebius (s.str.) capicola (Coleoptera) re-discovered in the Cape Peninsula, South Africa. African Journal of Aquatic Science 29(2):271–273. DOI: 10.2989/16085910409503820.
- Underwood AJ, Skilleter GA. 1996. Effects of patch-size on the structure of assemblages in rock pools. Journal of Experimental Marine Biology and Ecology 197(1):63–90. DOI: 10.1016/0022-0981(95)00145-X.
- Urbanelli S, Sallicandro P, De Vito E, Colonnelli E, Bullini L. 1996. Molecular re-examination of the taxonomy of Ochthebius (Calobius) (Coleoptera: Hydraenidae) from the Mediterranean and Macaronesian Regions. Annals of the Entomological Society of America 89:623–636. DOI: 10.1093/aesa/89.5.623.
- Vecchioni L, Arculeo M, Cottarelli V, Marrone F. 2021. Range-wide phylogeography and taxonomy of the marine rock pools dweller Tigriopus fulvus (Fischer, 1860) (Copepoda, Harpacticoida). Journal of Zoological Systematics & Evolutionary Research 59:839–857. DOI:10.1111/jzs.12457.
- Vecchioni L, Marrone F, Rodilla M, Belda EJ, Arculeo M. 2019. An account on the taxonomy and molecular diversity of a marine rock-pool dweller, Tigriopus fulvus (Copepoda, Harpacticoida). Ciencias Marinas 45(2):59–75. DOI: 10.7773/cm.v45i2.2946.
- Velasco J, Mirón-Gatón JM, García-Meseguer AJ, Botella-Cruz M. 2022. Life cycle differences between two coexisting species of supratidal rockpools: Ochthebius quadricollis Mulsant, 1844 and Ochthebius lejolisii Mulsant & Rey, 1861 (Coleoptera, Hydraenidae). In: Suplementos del Boletín de la Asociación española de Entomología, N° 4: 15-XI-2022 - Advances in aquatic and subterranean beetles research: a tribute to Ignacio Ribera. pp. 131–136. DOI: 10.1111/een.13220.
- Villastrigo A, Arribas P, Ribera I. 2020a. Irreversible habitat specialization does not constrain diversification in hypersaline water beetles. Molecular Ecology 29(19):3637–3648. DOI: 10.1111/mec.15593.
- Villastrigo A, Bilton DT, Abellàn P, Millán A, Ribera I, Velasco J. 2022a. Cryptic lineages, cryptic barriers: Historical seascapes and oceanic fronts drive genetic diversity in supralittoral rockpool beetles (Coleoptera: Hydraenidae). Zoological Journal of the Linnean Society 2022 XX:1–17. DOI: 10.1093/zoolinnean/zlac032.
- Villastrigo A, Hernando C, Millán A. 2022b. The Ochthebius (Coleoptera, Hydraenidae) from western palaearctic supratidal rockpools. Boletín de la Asociación española de Entomología 4(Suppl. 4):100–108.
- Villastrigo A, Hernando C, Millán A, Ribera I. 2020b. The neglected diversity of the Ochthebius fauna from Eastern Atlantic and Central and western Mediterranean coastal rockpools (Coleoptera, Hydraenidae). Organisms Diversity & Evolution 20(4):785–801. DOI: 10.1007/s13127-020-00463-y.
- Villastrigo A, Jäch MA, Cardoso A, Valladares LF, Ribera I. 2019. A molecular phylogeny of the tribe Ochthebiini (Coleoptera, Hydraenidae, Ochthebiinae). Systematic Entomology 44(2):273–288. DOI: https://doi.org/10.1111/syen.12318.
- Wollaston TV. 1871. On additions to the Atlantic Coleoptera. Transactions of the Entomological Society of London 1871:203–314.
- Yoshitomi H, Karube H, Hayashi M. 2019. A new species of the genus Ochthebius (Coleoptera, Hydraenidae) from the Ogasawara Islands, Japan, with a description of the larva. ZooKeys 855:95. DOI: 10.3897/zookeys.855.35677.

