?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The Leuser Ecosystem Area (LEA) is one of the largest conservation areas in Southeast Asia. However, the explorations of ichthyofauna at LEA are still relatively scarce compared to other vertebrates fauna. We report the first ichthyofauna studies of the northern river of LEA in Aceh Province. This study aimed to determine the composition, diversity, biometric condition, potency, and conservation status of freshwater fish in Merbau River, LEA, Indonesia. Fish sampling was carried out at six research stations using selective gill nets, throwing net, hook, tray net, and scoop net. A total of 434 individual fish belonging to 21 species, nine families and six orders were collected from the sampling location. Cyprinidae was the predominant family obtained, namely 42.9%. Based on their conservation status, 85.7% of collected fish were categorized as Least Concern, 9.5% as Data Deficient, and 4.8% as Near Threatened. A total of 14 fish have the potential as consumption fish, two species have the potential as ornamental fish, and five others have the potential both as consumption and ornamental fish. All fish species collected had negative allometric growth patterns with growth coefficient values (b) ranging from 1.17–2.51. The diversity and evenness index of fish was categorized as moderate (H’ = 2.52) with stable conditions (E = 0.85).
1. Introduction
The Leuser Ecosystem Area (LEA) is one of the largest conservation areas (reaches 2.6 million ha) in Southeast Asia, comprising with high biodiversity of both flora and fauna (Nuribadah Citation2022). The composition of flora in LEA was recorded at 4000 species, where around 113 species were categorized as endemic (Ardi et al. Citation2021). Meanwhile, 739 species of fauna were also reported, including mammals, aves, reptiles, amphibians, fish, and other invertebrates (Djufri Citation2015). In addition, LEA has become the last natural habitat for endemic Sumatran fauna, for instance, orangutan sumatera (Pongo abelii), Sumatran elephant (Elephas maximus sumatrensis), Sumatran tiger (Panthera tigris sumatrae), and Sumatran rhinoceros (Dicerorhinus sumatrensis) (Djufri Citation2015). Unfortunately, the explorations of ichthyofauna in LEA are still relatively scarce compared to other vertebrates fauna.
An inventory of ichthyofauna is crucial for demonstrating variety, investigating imported and invasive fish, assessing the potential of fish, and contributing to the search for new species. Therefore, this information provided a valuable reference for constructing conservation policies for aquatic ecosystems (Shen et al. Citation2019; Mariac et al. Citation2022). Besides having economic value, fish is also susceptible to water pollution, potentially becoming a bio-indicator (Chovanec et al. Citation2003; Darwall & Vie Citation2005; Laffaille et al. Citation2005; Sarkar et al. Citation2008). Recently, the excessive level of anthropogenic activities, such as water pollution, habitat fragmentation, and the existence of invasive species, can reduce and threaten ichthyofauna biodiversity, especially endemic and native fish (Latuconsina Citation2020).
Temporarily, ichthyofauna studies in LEA were investigated by Kreemer (Citation1922), followed by Wirjoatmodjo (Citation1987), Hadiaty and Mun’im (Citation1998), Defira and Muchlisin (Citation2004), Hadiaty (Citation2005), Haryono (Citation2006), Muchlisin et al. (Citation2015), Mardianti et al. (Citation2018), and Maghfiriadi et al. (Citation2019). The covering areas were the Alas River, Lembang River, Bahorok River, Tripa Peat Swamp, and Kluet River (Wirjoatmodjo Citation1987; Defira & Muchlisin Citation2004; Hadiaty Citation2005; Haryono Citation2006; Muchlisin et al. Citation2015; Mardianti et al. Citation2018; Maghfiriadi et al. Citation2019). Based on the previous studies, the Lembang River was reported to have the highest composition of fish species, followed by Alas River, Bahorok River, and Kluet River, which were 53, 52, 32, and 23 species, respectively. A previous study by Hadiaty (Citation2005) reported several new species, including Osteochilus jeruk, Osteochilus serokan, Nemacheilus tuberigum, Mystus punctifer, Hemibagrus caveatus, and Kryptopterus piperatus. In contrast, Maghfiriadi et al. (Citation2019) reported two introduced and invasive fish in the Alas River at LEA, namely Oreochromis niloticus (native from the northern of Africa and the Levante area) and Pterygoplichthys pardalis (native from the Amazon River basin).
The Merbau River is one of the rivers in the LEA that has not been studied for ichthyofauna. In contrast to the previous studies, which were dominant in the southwest region of the KEL, this river is located in the northern part of the LEA. Its upstream was administratively located in Tenggulun Subdistrict, while the estuary was in the Simpang Kiri Subdistrict, Aceh Tamiang Regency. Even though it is protected, illegal land conversion into palm plantations around the Merbau watershed has been massively occurring since 2000. Several adverse effects of this activity, mainly for the aquatic environment, were reported, including water pollution, erosion, and sedimentation.
Since 2014, the vegetation restoration program has been conducted around the Merbau River watershed. Around 230 hectares of palm plantation area were successfully restored until 2017 (Mongabay Citation2017). Referring to a similar program, Maghfiriadi et al. (Citation2019) reported a positive effect between the vegetation restoration and rehabilitation program as well as increased fish composition (from 9 to 20 species) in the Alas River. Therefore, this study aims to describe the composition, diversity, biometric condition, potency, and conservation status of freshwater fish in Merbau River, LEA. The results might provide further reference and scientific information to prevent biodiversity loss.
2. Material and methods
Fish collection and identification
Fish sampling was carried out at six locations along the Merbau River with different habitat typologies. The location and characteristics of each station are presented in and . Fish were collected using gears, including throwing nets, gill nets, rods, and scoops. As many as three gill nets (50 × 1 m) with different mesh size were set at 09.00–15.00 Indonesian Western Time (WIB) and lifted every two hours. The mesh sizes used were 0.5, 1 and 2 inch, and the set distance between the nets was 50 m. Meanwhile, the throwing nets were spread 15 times at each station. Fish sampling was conducted for three days in each location. Furthermore, the collected fish were counted and sorted by species. The morphometric data, including total weight, total length (TL), standard length (SL), and fork length (FL), were measured with a digital scale and caliper at an accuracy of 0.01 g and 0.01 mm. For further identification, each fish species was preserved using 4% formalin and transferred into a labeled bottle. Identification of taxonomic groups was conducted using standard fish keys provided by Allen et al. (Citation1990), Kottelat and Whitten (Citation1996), Rachmatika (Citation2004), Tan and Kottelat (Citation2009), Official Fishbase website https://www.fishbase.se/, as well as Eschmeyer’s Catalog of Fishes (www.calacademy.org). Information related to local fish names was obtained from community interviews. Voucher specimens were transferred to 70% ethanol and deposited at the Laboratory of Ecology, Department of Biology, Faculty of Science and Technology, Universitas Islam Negeri Ar-Raniry (Lots ARY16102 to ARY16122, ).
Figure 1. Location of the study sites (sampling locations with green dot, Merbau River indicated with red line).
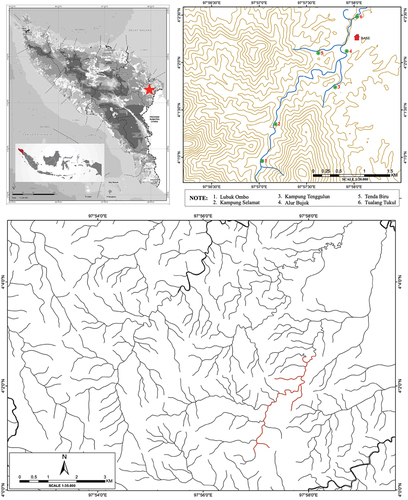
Figure 2. Sampled localities in the Merbau River, LEA. (A) Lubuk Ombo, (B) Kampung Selamat, (C) Kampung Tenggulun, (D) Alur Bujok, (E) Tenda Biru, (F) Tualang Tukul. Photographs by Siti Maulizar.

Table I. Description of research stations.
Table II. Taxonomic classification, local name, distribution pattern, conservation status and potency of fishes in Merbau River, LEA, Indonesia.
Diversity indices
Diversity indices, including the Shannon-Wiener diversity index (H’), the Simpson evenness index (E), the Simpson dominance index (C), and the Sorensen similarity index (SI) between sampling areas, were measured. The measurement of the Shannon-Wiener diversity index (H’) was calculated as follows Shannon and Wiener (Citation1963):
where H′ = Shannon – Weiner index, pi = the proportion of density of ith species (pi = ni/N), “ni” is the density of each species in the sample, and “N” is the density of individuals of all species. The Simpson evenness index (E) was calculated as follows:
where, E = Evenness index, H = Diversity index, Hmax = ln S, S = Number of species found. The Simpson dominance index (C) was calculated as follows:
where C = The Simpson dominance index, pi = the proportion of density of ith species (pi = ni/N), “ni” is the density of individuals of each species in the samples, and “N” is the density of individuals of all species. The Sorensen similarity index (SI) was calculated as follows:
Where A = the number of all fish species in location A, B = the number of all fish species in location B, and C = the number of common species (present in both locations A and B).
Length-weight relationship, length-length relationship, and condition factor
Length-weight relationship of fish was calculated using the following equation: W = aLb. Where W = fish total weight (g), L = fish total length (mm), a = intercept, and b = fish growth coefficient. The relationship between total (TL), standard (SL), and fork length (FL) was measured using a simple linear regression equation, namely TL = a + b*SL and TL = a+ b*FL (Rosli & Isa Citation2012). The condition factor (K) was calculated using the formula: K = W × 100/L3 (Ricker Citation1973). Only fish species with at least three individuals were counted and examined.
Potency and conservation status
The potency of fish was divided into consumption and ornamental categories. Fish consumption was identified based on interviews with local communities regarding its taste, availability, and price. Meanwhile, ornamental fish was determined by referring to the color of the scales, fins, morphology, body color patterns, and behavior (Muchlisin Citation2013; Maghfiriadi et al. Citation2019). The conservation status was evaluated based on the IUCN (International Union for Conservation of Nature) website https://www.iucnredlist.org/.
3. Results
Species composition
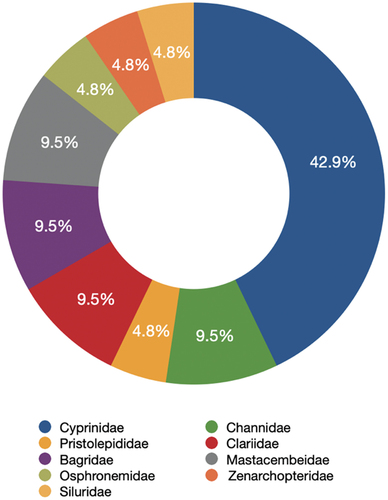
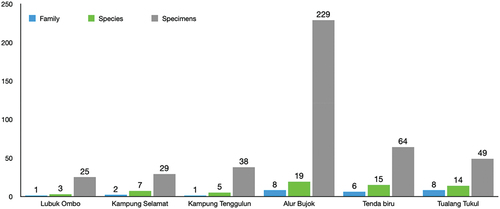
This study reported 21 species (434 individuals) of fish belonging to six orders and nine families spatially distributed randomly among six stations (, ). Osteochilus vittatus was the predominant species collected (70 individuals), followed by Hemibagrus sabanus (46 individuals), Rasbora sumatrana (36 individuals), and Cyclocheilichthys apogon (30 individuals). In contrast, Zenarchopterus dispar was the least species collected, with only one individual. The families of Cyprinidae was the most represented family (nine species; 42.9%). Each of Clariidae, Channidae, Bagridae, and Mastacembelidae family has two species (9.5%) representation; likewise, each of Osphronemidae, Siluridae, Zenarchopteridae, and Prestolepidae has one species (4.8%) representation in the fish composition (). Based on the sampling stations, Alur Bujok has the highest family, species, and specimens contribution, with eight families, 19 species, and 229 specimens. Meanwhile, Lubuk Ombo has the lowest values, with a single family, three species, and 25 specimens ().
Figure 3. Fish species from Cyprinidae. (1) Crossocheilus cobitis; (2) Cyclocheilichthys apogon; (3) Hampala macrolepidota; (4) Labiobarbus leptocheilus; (5) Mystacoleucus obtusirostris; (6) Osteochilus vittatus; (7) Oxygaster anomalura; (8) Rasbora sumatrana; (9) Striuntius lateristriga. Scale bar = 50 mm.

Figure 4. Fish species from Channidae, Prestolepididae, Clariidae, Siluridae, Bagridae, Mastacembelidae, Osphronemidae, Zenarchopteridae. (10) Channa lucius; (11) Channa striata; (12) Prestolepis fasciata; (13) Clarias batrachus; (14) Clarias meladerma; (15) Silurichthys phaiosoma; (16) Hemibagrus sabanus; (17) Mystus nigriceps; (18) Macrognathus maculatus; (19) Macrognathus aculeatus; (20) Trichopodus trichopterus; (21) Zenarchopterus dispar. Scale bar = 50 mm.

Species diversity
The diversity and evenness indexes of fish species in the Merbau River at LEA were categorized as medium (H’ = 2.52) and stable (E = 0.85). The Alur Bujok has the highest diversity (H’ = 2.55; medium), while Tenda Biru has the highest evenness index values (E = 0.92; stable community). In contrast, Lubuk Ombo has the lowest diversity (H’ = 0.78; low category) and evenness index value (E = 0.71; unstable community) (). The dominance index of fish was categorized as low (C = 0.07). The highest and lowest values were detected in Kampung Tenggulun (C = 0.74; medium category) and the Alur Bujok (C = 0.07; low category) (). Furthermore, the composition of fish in the Alur Bujok tended to be similar to Tenda Biru and Tualang Tukul, with the similarity index values ranging from 75.86–84.85%. The lowest was observed between Kampung Tenggulun and Tualang Tukul, with a value of 10.52% ().
Table III. Diversity indices of fishes according to sampling stations in Merbau River.
Table IV. Similarity index (%) of fishes in Merbau River.
Length-weight relationship, length-length relationship, and condition factor
All fish collected had negative allometric patterns with b coefficient values ranging from 1.17–2.51 (). The highest and lowest value of b was observed in Osteochilus vittatus and Trichopodus trichopterus. Based on the total length-standard relationship, the highest and lowest coefficient a (slope) value was recorded in Crossocheilus cobitis (TL = 38.72 + 0.84 SL) and Macrognathus maculatus (TL = 3.24 + 1.03 SL). However, Macrognathus maculatus and has the highest value of the coefficient of b (TL = 3.24 + 1.03 SL), while Hampala macrolepidota and Striuntius lateristriga have the lowest value (TL = 37.06 + 0.68 SL) (). Based on the total length-fork relationship, the highest and lowest coefficient a (slope) value was recorded in Hemibagrus sabanus (TL = 24.84 + 0.87 FL) and Oxygaster anomalura (TL = 0.61 + 1.02 FL). Silurichthys phaiosoma has the highest value of the coefficient of b (TL = 10.49 + 1.11 FL), while Hampala macrolepidota (TL = 20.14 + 0.82 FL) and Striuntius lateristriga (TL = 20.13 + 0.82 FL) have the lowest value (). The highest and lowest condition factor was recorded in Osteochilus vittatus (K = 1.47) and Hemibagrus sabanus (K = 0.78) ().
Table V. Estimated parameters from length–weight relationship of fishes in Merbau River. a: intercept; b: slope of the linear regression; Cl: confidence limits; R2: coefficient of determination; K: condition factor.
Table VI. Estimated parameters from relationship between total length (TL), standard length (SL) and fork length (FL) of fishes in Merbau River.
Potency and conservation status
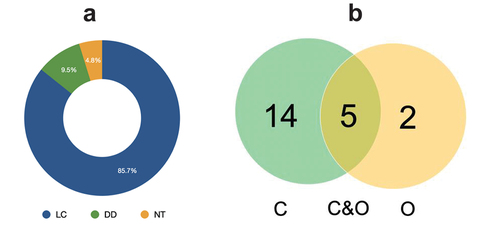
A total of 24% of fish species collected have the potential to be utilized both as consumption fish and ornamental fish, 9% have the potential as ornamental fish, and 67% have the potential as consumption fish (). Some consumption fish with high economic value, including Hampala macrolepidota (4.60–6.57 USD/kg), Macrognathus aculeatus, and Macrognathus maculatus (5.52–7.88 USD/kg), and Osteochilus vittatus (2.63–3.94 USD/kg). Trichopodus trichopterus and Zenarchopterus dispar were potentially ornamental due to their color patterns, unique body shapes, and varied patterns ( number 20–21). The conservation status of fish in the Merbau River was divided into three categories, namely Least concern (18 species), Data deficient (2 species), and Near Threatened (1 species) (, ).
4. Discussion
As many as 12 of 21 fish species in the Merbau River were also detected in the rivers located south-west of LEA (Alas River, Lembang River, Bahorok River, Kluet River, and rivers around Tripa Peat Swamp). These species include Osteochilus vittatus, Cyclochtheilichthys apogon, Rasbora sumatrana, Mystacoleucus marginatus, Hampala macrolepidota, Channa striata, Channa lucius, Silurichthys phaiosoma, Mystys nigriceps, Macronagthus maculatus, Macronagthus aculeatus, and Trichogaster trichopterus (Defira & Muchlisin Citation2004; Hadiaty Citation2005; Muchlisin et al. Citation2015; Mardianti et al. Citation2018; Maghfiriadi et al. Citation2019). Meanwhile, four fish species were recorded in the northern part of LEA (Bahorok River), including Mystacoleucus marginatus, Mystys nigriceps, Channa striata, and Trichogaster trichoperus (Haryono Citation2006). Interestingly, Striuntius lateristriga, Oxygaster anomalura, Crossocheilus cobitis, Pristolepis fasciata, Clarias meladerma, Silurichthys phaiosoma, and Zenarchopterus dispar were first reported in the river of LEA.
The number of fish orders found in the Merbau River (6) were still lower than other rivers in the LEA including Lembang River (13), Kluet River (12), Tripa Peat Swamp (10), Bahorok River (9), and Alas River (8) (Defira & Muchlisin Citation2004; Hadiaty Citation2005; Haryono Citation2006; Muchlisin et al. Citation2015; Mardianti et al. Citation2018; Maghfiriadi et al. Citation2019). Cypriniformes, Anabantiformes, Siluriformes, Synbranchiformes, and Beloniformes were also found in the rivers in the South West of LEA. Meanwhile, only Cypriniformes, Siluriformes, Anabantiformes, and Synbranchiformes were similar to river in northern of LEA (Haryono Citation2006).
The composition of fish species in aquatic ecosystems might be influenced by several factors, such as physicochemical water parameters and food availability (Gebrekiros Citation2016; Burgos-Aceves et al. Citation2019). Differences in habitat characteristics between the LEA’s west-south and northern rivers might be the main factors influencing the variation in fish composition. The south-west area was reported to consist of more rivers with more varied environmental conditions, including large rivers, tributaries, small channels, and estuaries (Defira & Muchlisin Citation2004; Hadiaty Citation2005; Muchlisin et al. Citation2015; Maghfiriadi et al. Citation2019). Additionally, the vegetation around the rivers in the south-west of the LEA was still relatively natural and well-maintained. In contrast, the environmental conditions of rivers in the northern part tend to be more homogeneous and accompanied by many land conversions around the river. Degradations in the forest cover areas in the Aceh Tamiang Regency watersheds that might be responsible for sedimentation and water quality changes. The shrinking of fish composition due to the high level of sedimentation has been reported in other rivers, including the Ganges, India (Vass et al. Citation2010), Sironko (Turyahabwe et al. Citation2022), and rivers in the Alpine (Scheurer et al. Citation2009).
Cyprinidae was the predominant family in the Merbau River, with 42.9% of the total fish found. This percentage was relatively high compared to other rivers of LEA, such as the Lembang and Ketambe River (14%) (Hadiaty Citation2005) and the Bahorok River (31%) (Haryono Citation2006). However, it was still lower compared to the Kluet River (57%) (Mardianti et al. Citation2018) and Alas River (50%) (Defira & Muchlisin Citation2004; Hadiaty Citation2005; Maghfiriadi et al. Citation2019). The dominance of the Cyprinidae family has also been reported in several other rivers in Indonesia, including the Enim River, South Sumatra (Hamidah Citation2004), rivers in the Tesso Nilo area, Riau (Rachmatika et al. Citation2006) and Kampar Kanan River, Riau (Aryani Citation2015)
Hubert et al. (Citation2015) stated that Cyprinidae was the dominant fish family in the Indonesian freshwater ecosystem, especially in areas on the Sunda shelf. However, the Cyprinidae family tends to be sensitive to the alteration of the aquatic environment. Previous research revealed a decrease in Cyprinidae family composition due to water pollution in rivers, for instance, the Karnataka River, India, and Pahang River, Malaysia (Shetty et al. Citation2015; Rashid et al. Citation2018).
Osteochilus vittatus was the predominant fish caught, which was 70 individuals. This species was also reported recorded in other LEA rivers, such as Alas River (Maghfiriadi et al. Citation2019), Kluet River (Mardianti et al. Citation2018), and rivers around Tripa Peat Swamp (Muchlisin et al. Citation2015). The high population has also been reported in other waters in Indonesia, such as the Kepari and Emperas River, West Kalimantan (Siska et al. Citation2020), Benanga reservoir, East Kalimantan (Jusmaldi et al. Citation2020), and Lake Talaga, Central Sulawesi (Muryanto & Sumarno Citation2016). The abundance of Osteochilus vittatus might be influenced by its ability to spawn throughout the year and its adaptability toward the fluctuations of water conditions (Putri et al. Citation2015; Madihah et al. Citation2021). Zenarchopterus dispar appears to be the most diminutive species which only has one individual. The presence was first reported in LEA and only detected at Kampung Selamat Station, Merbau River. The river around this station had specific characteristics, such as the relatively high torrent, river basins/bottoms, substrate dominated by gravel and sand, and fern vegetation around the river. The characteristics of this habitat and the minor abundance of Zenarchopterus dispar were almost similar to other river locations where this type of fish was discovered.
Based on locations, the highest composition of fish species was found in Alur Bujok (19 species), followed by Tenda Biru (15 species) and Tualang Tukul (14 species). The similarity index (IS) analysis showed that the composition of fish in these stations was similar (IS value = 75.86–84.85%). Alur Bujok was also the location with the highest diversity index. High species composition and similarity were closely related to the characteristics of the habitat and its location, which was included in the downstream area of the river. Power et al. (Citation2013) and Humphries et al. (Citation2020) stated that the downstream area of the river was broader and accompanied by highly abundant feed availability, resulting in increasing fish species and populations. A high composition of fish species in the downstream area was also reported in the Alas River (Maghfiriadi et al. Citation2019).
Analysis of the diversity index showed that the Merbau River was categorized as moderate (2.52). Fish diversity index in the moderate category was also reported in Kluet River (H’ = 1.52–2.30) (Mardianti et al. Citation2018) and the Alas River (H’ = 1.43–2.03) (Defira & Muchlisin Citation2004). Based on the evenness index, the Merbau River ecosystems are included in the stable category (E = 0.91). The stable community category was also reported in Alas River (E = 0.93) (Defira & Muchlisin Citation2004) and Tripa Peat Swamp (E = 0.81) (Muchlisin et al. Citation2015).
Information regarding the conservation status of fish in the LEA has not been widely reported. The availability of data related to the conservation status in LEA waters was only reported in the Alas River (Maghfiriadi et al. Citation2019). Based on the IUCN conservation status category, most Merbau River fish were considered the least concerned at 85.7%. These results are similar to the Alas River (LEA) and several others in Indonesia, such as Unda River (Indrawan et al. Citation2021), Way Sindalapai River (Juriani et al. Citation2020), and Cimanuk River (Herawati et al. Citation2020). The lack of information related to the conservation status of fish is inseparable from reduced attention to ichthyological studies. Hadiaty et al. (Citation2019) reported that limited funds, research support facilities, and the difficulty of adapting to various ethnic groups and cultures are the major obstacle that hinders the diversity assessment of ichthyofauna. Therefore, further studies are needed to keep the information updated regarding the potential of fish switching/changing status, e.g., Zenarchopterus dispar (the least abundance fish).
As many as 14 species of fish collected have the potential to be used as consumption fish, five species of fish are bi-potential as both consumption and ornamental fish, and two other species have potential only as ornamental fish. Compared to other rivers in LEA, the number of fish that have the potential to be consumed from the Merbau River was higher than the Alas River (16 species) but similar to the rivers around Tripa Peat Swamp (18 species) (Muchlisin et al. Citation2015), and the Bahorok River (20 species). Ornamental fish from the Merbau River was still lower than those in Bahorok River, Alas River, and Rawa Tripa Peat Swamp consisting of 20, 12, and 20 species, respectively (Haryono Citation2006; Muchlisin et al. Citation2015; Maghfiriadi et al. Citation2019). Studies related to the potential of each type of fish are essential for the optimal utilization of fishery resources while paying attention to aspects of sustainability (Rochette et al. Citation2014; Su et al. Citation2020).
Information regarding biometric conditions (length to weight, length to length, and conditional factors) is needed in order to examine the general health of fish populations and assist in estimating the recruitment potential in population dynamics studies (Hossain et al. Citation2012; Kumar et al. Citation2013; Lim et al. Citation2013). The results showed that the growth patterns of all fish collected are in the negative allometric category. The dominance of fish with these patterns is also found in several other rivers, including the Torsa River, India (Koushlesh et al. Citation2017) and the Basistha River, India (Borah et al. Citation2017). Osteochilus vittatus is one type of fish that tends to have a fluctuating growth pattern. In this study, Osteochilus vittatus has a negative allometric growth pattern which is similar to Lake Telaga (Putri et al. Citation2015) and the waters of Rawa Pening (Rochmatin et al. Citation2014). In contrast, Osteochilus vittatus collected from the Benanga Reservoir, East Kalimantan showed allometric positive growth pattern (Jusmaldi et al. Citation2020). Differences in fish growth patterns between habitats can be influenced by several factors, such as the availability of fish feed, competition between populations, and the level of gonad maturity (Muchlisin et al. Citation2010; Batubara et al. Citation2019).
5. Conclusions
The study of ichthyofauna in the Merbau River recorded 21 species of fish belonging to six orders and nine families, where Cyprinidae was the most dominant family at 42.9%. Analysis of the diversity index shows that the river belongs to the moderate diversity index (H’ = 2.52) and stable condition (E = 0.85). From the classifications, five species have potential both as consumption and ornamental fish, 14 species as consumption fish, and two species was included in the ornamental category. The conservation status of fish in the Merbau River was categorized into three categories: Least concern (85.7%), Data deficient (9.5%), and Near Threatened (4.8%). The further study related to fisheries biology and population dynamics in the LEA is necessary to support sustainable management program.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allen G, Coates D, Kaiola P, Burgess W. 1990. Studies on freshwater fishes of New Guinea and Northern Australia. Western Australian Museum 206p.
- Ardi R, Yasin A, Iswandari A, Nugroho FA. 2021. Jenis-jenis Pohon Asli Taman Nasional Gunung Leuser. Sumatera Utara: Balai Besar Taman Nasional Gunung Leuser.
- Aryani N. 2015. Native species in Kampar Kanan River, Riau Province Indonesia. International Journal of Fisheries and Aquatic Studies 25:213–217.
- Batubara AS, Muchlisin ZA, Efizon D, Elvyra R, Irham M. 2019. Length-weight relationships and condition factors of the naleh fish, Barbonymus gonionotus (Pisces, Cyprinidae) harvested from Nagan Raya Waters, Indonesia. Vestnik zoologii 53(1):75–82. DOI: 10.2478/vzoo-2019-0008.
- Borah S, Bhattacharjya BK, Saud BJ, Yadav AK, Debnath D, Yengkokpam S, Das P, Sharma N, Singh NS, Sarma KK. 2017. Length–weight relationship of six indigenous fish species from Deepor beel, a Ramsar site in Assam, India. Journal of Applied Ichthyology 33(3):655–657. DOI: 10.1111/jai.13348.
- Burgos-Aceves MA, Lionetti L, Faggio C. 2019. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Science of the Total Environment 670:1170–1183. DOI:10.1016/j.scitotenv.2019.03.275.
- Chovanec A, Hofer R, Schiemer F. 2003. Fish as bioindicators. In: Markert B, Breure A Zechmeister H, editors. Bioindicators and biomonitors: Principles, concept and applications. Amsterdam: Elsevier. pp. 639–676.
- Darwall WRT, Vie JC. 2005. Identifying important sites for conservation of freshwater biodiversity: Extending the species based approach. Fisheries Management and Ecology 12(5):287–293. DOI: 10.1111/j.1365-2400.2005.00449.x.
- Defira CN, Muchlisin ZA. 2004. Fish population in the Alas River, Soraya Research station, Leuser Ecosystem area, Simpang Kiri, Aceh Singkil Regency. Jurnal Ilmiah MIPA 7(1):61–67.
- Djufri D. 2015. Leuser Ecosystem of Aceh Province as a natural laboratory for the study of biodiversity to find the raw materials of drugs. Prosiding Seminar Nasional Masyarakat Biodiversitas Indonesia 1(7):1543–1552.
- Gebrekiros ST. 2016. Factors affecting stream fish community composition and habitat suitability. Journal of Aquaculture and Marine Biology 4(2):76. DOI: 10.15406/jamb.2016.04.00076.
- Hadiaty RK. 2005. The biodiversity of fish in Squaq Balimbing and Ketambe Gunung Leuser National Park, Sumatera. Jurnal Biologi Indonesia 3(9):379–388.
- Hadiaty RK, Mun’im A. 1998. The biodiversity of fish in Ketambe Research Station Gunung Leuser National Park, Aceh. Bogor: Puslitbang Biologi-LIPI.
- Hadiaty RK, Rahardjo MF, Allen GR. 2019. Ichthyofauna in small islands and coral reef and new freshwater species in Indonesian waters. Jurnal Iktiologi Indonesia 19(1):167–186. DOI: 10.32491/jii.v19i1.446.
- Hamidah A. 2004. Fish diversity in River enim, Muara enim Regency, South Sumatera province. Jurnal Iktiologi Indonesia 4(2):51–55.
- Haryono H. 2006. The fish fauna of Bukit Lawang waters areas in Gunung Leuser National Park. Jurnal Iktiologi Indonesia 6(2):111–119.
- Herawati T, Sidik RAR, Sahidin A, Herawati H. 2020. Fish community structure in the downstream of Cimanuk River West Java province in rainy season. Jurnal Perikanan UGM 22(2):113–122. DOI: 10.22146/jfs.47655.
- Hossain MY, Rahman MM, Abdallah EM. 2012. Relationships between body size, weight, condition and fecundity of the threatened fish Puntius ticto (Hamilton, 1822) in the Ganges River Northwestern Bangladesh. Sains Malaysiana 41(7):803–814.
- Hubert N, Wibowo A, Busson F, Caruso D, Sulandari S, Nafiqoh N, Pouyaud L, Rüber L, Avarre JC, Herder F, Hanner R, Keith P, Hadiaty RK. 2015. DNA barcoding Indonesian freshwater fishes: Challenges and prospects. DNA Barcodes 3(1):144–169. DOI: 10.1515/dna-2015-0018.
- Humphries P, King A, McCasker N, Kopf RK, Stoffels R, Zampatti B, Price A. 2020. Riverscape recruitment: A conceptual synthesis of drivers of fish recruitment in rivers. Canadian Journal of Fisheries and Aquatic Sciences 77(2):213–225. DOI: 10.1139/cjfas-2018-0138.
- Indrawan GS, Wiradana PA, Wijaya IMS, As-Syakur AR, Syahputra MRR, Wijana IMS. 2021. Checklist, ecological index, and conservation status of Aquatic fauna communities in the Unda River and around Jumpai Beach, Klungkung Regency, Bali province. Jurnal Bumi Lestari 21(1):9–17. DOI: 10.24843/blje.2021.v21.i01.p02.
- Juriani J, Susanto GN, Kanedi M, Suratman S. 2020. The diversity of freshwater fish species in Way Sindalapai River, Liwa Botanical Garden West Lampung. Jurnal Ilmiah Biologi Eksperimen dan Keanekaragaman Hayati 7(1):54–61. DOI: 10.23960/jbekh.v7i1.17.
- Jusmaldi J, Hariani N, Wulandari NA. 2020. Length-weight relationship and condition factor of bonylip barb (Osteochilus vittatus Valenciennes, 1842) in benanga reservoir, east kalimantan. Berita Biologi 19(2):127–139. DOI: 10.14203/beritabiologi.v19i2.3806.
- Kottelat M, Whitten T. 1996. Freshwater fishes of western Indonesia and Sulawesi. Hong Kong: Periplus Edition.
- Koushlesh SK, Sinha A, Kumari K, Borah S, Chanu TN, Baitha R, Das SK, Gogoi P, Sharma SK, Ramteke RH, Das BK. 2017. Length weight relationship and relative condition factor of five indigenous fish species from Torsa River West Bengal India. Journal of Applied Ichthyology 34(1):169–171. DOI: 10.1111/jai.13518.
- Kreemer J. 1922. Atjeh. Algemeen samenvaltend overzicht van land en volk van Atjeh en onder hoorigheden. Leiden: E.J. Brill.
- Kumar K, Lalrinsanga PL, Sahoo M, Mohanty UL, Kumar R, Sahu AK. 2013. Length-weight relationship and condition factor of Anabas testudineus and Channa species under different culture systems. World Journal of Fish & Marine Sciences 5(1):74–78.
- Laffaille P, Acou A, Gullouet J, Legault A. 2005. Temporal change in European eel, Anguilla anguilla, stock in a small catchment after installation of fish passes. Fisheries Management and Ecology 12(2):123–129. DOI: 10.1111/j.1365-2400.2004.00433.x.
- Latuconsina H. 2020. Ekologi Ikan Perairan Tropis: Biodiversitas, Adaptasi, Ancaman, dan Pengelolaanya. Yogyakarta: Universitas Gadjah Mada Press. p. 564.
- Lim LS, Chor WK, Tuzan AD, Malitam L, Gondipon R, Ransangan J. 2013. Length-weight relationships of the pond-cultured spotted barb (Puntius binotatus). International Research Journal of Biological Sciences 2(7):61–63.
- Madihah M, Andriani S, Nisa SA, Wibowo I, Sumarsono SH. 2021. Reproductive performance and vitellogenin gene expression on female Bonylip barb (Osteochilus vittatus) during its reproductive cycle under culture conditions. Agriculture and Natural Resources 55(4):557–568.
- Maghfiriadi F, Zulfahmi I, Paujiah E, Sarong MA. 2019. Ichthyofauna of Alas River, around Soraya Research station, Leuser Ecosystem area, subulussalam, Aceh. Jurnal Iktiologi Indonesia 19(3):361–374. DOI: 10.32491/jii.v19i3.502.
- Mardianti M, Nasir M, Devira CN. 2018. Diversity of fish species in the Kluet River, South Aceh Regency. Prosiding Seminar Nasional Biotik 5(1):216–221.
- Mariac C, Duponchelle F, Miranda G, Ramallo C, Wallace R, Tarifa G, Garcia-Davila C, Ortega H, Pinto J, Renno JF. 2022. Unveiling biogeographical patterns of the ichthyofauna in the Tuichi basin, a biodiversity hotspot in the Bolivian Amazon, using environmental DNA. PloS One 17(1):e0262357. DOI: 10.1371/journal.pone.0262357.
- Mongabay.co.id. 2017. Robohnya Sawit Ilegal di Hutan Lindung Aceh Tamiang [The Collapse of Illegal Palm Oil in the Aceh Tamiang protected forest]. Available: https://www.mongabay.co.id/2017/07/03/robohnya-sawit-ilegal-di-hutan-lindung-aceh-tamiang/. Accessed October 2020 25.
- Muchlisin ZA. 2013. Potency of freshwater fishes in Aceh waters as a basis for aquaculture development program. Jurnal Iktiologi Indonesia 13(1):91–96.
- Muchlisin ZA, Akyun Q, Rizka S, Fadli N, Sugianto S, Halim A, Azizah S. 2015. Ichthyofauna of Tripa Peat Swamp forest, Aceh province, Indonesia. Check List 11(2):1–9. DOI: 10.15560/11.2.1560.
- Muchlisin ZA, Musman M, Siti Azizah MN. 2010. Length‐weight relationships and condition factors of two threatened fishes, Rasbora tawarensis and poropuntius tawarensis, endemic to Lake Laut Tawar, Aceh province, Indonesia. Journal of Applied Ichthyology 26(6):949–953. DOI: 10.1111/j.1439-0426.2010.01524.x.
- Muryanto T, Sumarno D. 2016. Observation of eating habits of nilem fish (Osteochilus vittatus) caught by gill nets in Lake Talaga, Donggala Regency, Central Sulawesi. Buletin Teknik Litkayasa Sumber Daya dan Penangkapan 12(1):51–54.
- Nuribadah N. 2022. The existence of the Aceh Government in reducing Aceh’s forest damage. Asia-Pacific Journal of Public Policy 8(1):25–35. DOI: 10.52137/apjpp.v8i1.121.
- Power ME, Holomuzki JR, Lowe RL. 2013. Food webs in Mediterranean rivers. Hydrobiologia 719(1):119–136. DOI: 10.1007/s10750-013-1510-0.
- Putri MRA, Sugianti Y, Krismono K. 2015. Some biological aspects of Bonylip Barb, (Osteochillus vittatus) in Lake Talaga, Central Sulawesi. BAWAL Widya Riset Perikanan Tangkap 7(2):111–120. DOI: 10.15578/bawal.7.2.2015.111-120.
- Rachmatika I. 2004. Fish fauna of the Gunung Halimun National Park, West Java. Jakarta: Binamitra.
- Rachmatika I, Munim A, Dewantoro GW. 2006. Fish diversity in the Tesso Nilo area, Riau with notes on rare, cryptic species. Treubia 34:59–74.
- Rashid ZA, Amal MNA, Shohaimi S. 2018. Water quality influences on fish occurrences in Sungai Pahang, Maran District, Pahang, Malaysia. Sains Malaysiana 47(9):1941–1951. DOI: 10.17576/jsm-2018-4709-01.
- Ricker WE. 1973. Linear regressions in fishery research. Journal of the Fisheries Research Board of Canada 30(3):409–434. DOI: 10.1139/f73-072.
- Rochette J, Unger S, Herr D, Johnson D, Nakamura T, Packeiser T, Proelss A, Visbeck M, Wright A, Cebrian D. 2014. The regional approach to the conservation and sustainable use of marine biodiversity in areas beyond national jurisdiction. Marine Policy 49:109–117. DOI: 10.1016/j.marpol.2014.02.005.
- Rochmatin SY, Solichin A, Saputra SW. 2014. The growth aspects and reproduction of nilem (Osteochilus hasselti) in Rawa Pening, District of Tuntang, Semarang Regency. Management of Aquatic Resources Journal 3(3):153–159.
- Rosli NAM, Isa MM. 2012. Length weight and length-length relationship of long snouted catfish, Plicofollis argyropleuron (Valenciennes, 1840) in the Northern Part of Peninsular Malaysia. Tropical Life Sciences Research 23(2):59–65.
- Sarkar UK, Pathak AK, Lakra WS. 2008. Conservation of freshwater fish resources of India: New approaches, assessment and challenges. Biodiversity and Conservation 17(10):2495–2511. DOI: 10.1007/s10531-008-9396-2.
- Scheurer K, Alewell C, Bänninger D, Burkhardt-Holm P. 2009. Climate and land-use changes affecting river sediment and brown trout in alpine countries—A review. Environmental Science and Pollution Research 16(2):232–242. DOI: 10.1007/s11356-008-0075-3.
- Shannon CE, Wiener W. 1963. The mathematical theory of communities. Urbana: University of Illinois Press. p. 117.
- Shen Y, Hubert N, Huang Y, Wang X, Gan X, Peng Z, He S. 2019. DNA barcoding the ichthyofauna of the Yangtze River: Insights from the molecular inventory of a mega‐diverse temperate fauna. Molecular Ecology Resources 19(5):1278–1291. DOI: 10.1111/1755-0998.12961.
- Shetty A, Venkateshwarlu M, Muralidharan M. 2015. Effect of water quality on the composition of fish communities in three coastal rivers of Karnataka, India. International Journal of Aquatic Biology 3(1):42–51.
- Siska YH, Anwari MS, Yani A. 2020. Diversity of freshwater fish species in Kepari River and Emperas River, Kepari Village, Sungai Laur Sub-District, Ketapang Regency. Jurnal Hutan Lestari 8(2):299–309. DOI: 10.26418/jhl.v8i2.39827.
- Su S, Tang Y, Chang B, Zhu W, Chen Y. 2020. Evolution of marine fisheries management in China from 1949 to 2019: How did China get here and where does China go next? Fish and Fisheries 21(2):435–452. DOI: 10.1111/faf.12439.
- Tan HH, Kottelat M. 2009. The fishes of the Batang Hari drainage, Sumatra, with description of six new species. Ichthyological Exploration of Freshwaters 20(1):13–69.
- Turyahabwe R, Mulinya C, Shivoga WA. 2022. Relationships between land use, habitat quality, physicochemical water quality and fish communities in the Sironko River Catchment, a mountainous tropical stream flowing into the Lake Kyoga in Eastern Uganda. Lakes & Reservoirs: Research & Management 27(2):e12406. DOI: 10.1111/lre.12406.
- Vass KK, Mondal SK, Samanta S, Suresh VR Katiha PK. 2010. The environment and fishery status of the River Ganges. Aquatic Ecosystem Health and Management 13(4):385–394. DOI: 10.1080/14634988.2010.530139.
- Wirjoatmodjo S. 1987. The river ecosystem in the forest area at Ketambe, Gunung Leuser National Park, Aceh, Indonesia. Advances in Limnology 28:239–246.